Open Access Article
Open Access ArticleBibliometric study on the application of manganese dioxide in environmental catalysis worldwide from 1991 to 2021
Yaoguang
Guo
 a,
Qianqian
Chen
a,
Xiaohu
Sun
a,
Yujing
Liu
a,
Jie
Guan
*a,
Xiaojiao
Zhang
a,
Nuo
Liu
a,
Xiaoyi
Lou
*b,
Yingshun
Li
c and
Xiangwen
Zhang
d
a,
Qianqian
Chen
a,
Xiaohu
Sun
a,
Yujing
Liu
a,
Jie
Guan
*a,
Xiaojiao
Zhang
a,
Nuo
Liu
a,
Xiaoyi
Lou
*b,
Yingshun
Li
c and
Xiangwen
Zhang
d
aShanghai Collaborative Innovation Centre for WEEE Recycling, School of Resources and Environmental Engineering, Shanghai Polytechnic University, Shanghai 201209, China. E-mail: guanjie@sspu.edu.cn
bLaboratory of Quality Safety and Processing for Aquatic Product, East Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shanghai 200090, China. E-mail: huoxingmayi@126.com
cShanghai Xin Jinqiao Environmental Protection Co., Ltd, Shanghai 201201, China
dSafety Production Association of Pudong New Area, Shanghai, 201201, China
First published on 4th January 2023
Abstract
Since the 21st century, manganese dioxide (MnO2) has been attracting increasing attention in the environmental and energy fields due to its excellent catalytic oxidation properties. To better grasp the development and trend of MnO2 in the field of environmental catalysis, the published literature studies in the Science Citation Index Expanded database in the Web of Science Core Collection from 1991 to 2021 with a total of 1133 articles and reviews were analyzed by using visualization software of CiteSpace and VOSviewer. The results show an exponential growth in the number of papers related to MnO2 in the environmental catalysis field, with China, USA, India, South Korea and Australia providing the main drivers, while China being the most active country, with Applied Catalysis B-Environmental, Environmental Science & Technology, Catalysis Today and Chemical Engineering Journal being the most important sources for publishing relevant research. At present, a more complete theoretical framework and research methods have been formed for MnO2 environmental catalysis worldwide, but the research network is too centralized and the frontier branches are few. The catalytic research on MnO2 has been expanded from the macroscopic level to the microscopic scale. Structure–activity relationship, density functional theory, catalytic oxidation and mechanism have become the frontier of research. The present study is of significance for better understanding and supporting further research on the MnO2 environmental catalytic process.
Environmental significanceAs an important environment-benign transition metal oxide, MnO2 has a wide range of promising applications in electrode materials, electrochromism, catalysis, biosensors, etc. To date, there are more than 1000 published papers related to MnO2 in environmental catalysis according to the Web of Science (WoS), one of the largest databases of peer-reviewed academic literature from Clarivate Analytics. However, there are rarely reports on analyzing the current status of MnO2 research in the field of environmental catalysis from a bibliometric perspective. As the environmental and energy fields continue to evolve, MnO2 environmental catalysis has become a new hot research frontier. Therefore, an analysis of the current state of this field is essential, and bibliometrics can provide a new approach to identify the evolution of research hotspots and frontiers in this topic. The results of the present study are of significance for better understanding and supporting further research on MnO2 catalytic processes, and add new aspects to the field of environmental science. |
1. Introduction
Since Antonsson et al.1 selectively formed substituted cyclopentane derivatives in the presence of Pd(OAc)2-MnO2-benzoquinone as the catalyst in 1986, manganese dioxide (MnO2) has been capturing more and more attention worldwide. As an important environment-benign transition metal oxide, MnO2 has the advantages of abundant reserves, diverse morphology, rich crystalline forms and controllable grain sizes, and has a wide range of promising applications in electrode materials,2–4 electrochromism,5 catalysis,6–8 biosensors,9–11etc.To date, there are more than 4000 published papers related to MnO2 in catalysis according to the Web of Science (WoS), one of the largest databases of peer-reviewed academic literature from Clarivate Analytics. Among them, there are more than 1000 papers on the application of MnO2 in environmental catalysis. As the environmental and energy fields continue to evolve, MnO2 research in the field of environmental catalysis has become a new hot research frontier. However, there are rarely reports that analyze the current status of MnO2 research in the field of environmental catalysis from a bibliometric perspective. Therefore, an analysis of the current state of this field is essential, and bibliometrics can provide a new approach to identify the evolution of research hotspots and frontiers in this topic.
Bibliometrics is the quantitative analysis of the development of a research topic using mathematical and statistical methods,12 which has been adopted by many disciplines for its macroscopic research advantages of objectivity, quantification, and modeling.13 A research field can be analyzed by using visualization methods and mapped knowledge domain analysis to evaluate the current research situations, and evolutionary trajectory and predict the trend of a research field.12
In this study, literature data including titles, authors, institutions, journals, keywords, and references are processed by bibliometric methods, and these literature data can directly produce citation networks, co-occurrence networks, and coupling networks for further analysis. Data sources and research methods are described firstly. Following this, publication trends, source journal analysis, author contributions and collaborations, keyword co-occurrence analysis, co-citation analysis, and research frontiers and hotspots are comprehensively analyzed. The present study is significant for providing new insights into further research on MnO2 environmental catalysis.
2. Data and methods
2.1 Data retrieval
Data were retrieved from the Web of Science Core Collection (WoS CC). The searches are limited to the SCI-Expanded database with strict review criteria and collecting peer-reviewed scientific papers, which ensures the high quality and representativeness of the selected papers used for this study.14 “MnO2” and “Manganese Dioxide” as search terms are connected by the Boolean operator “OR”, and the search results “cataly*” and “environment*” are connected by the Boolean operator “AND” to filter the documents. In addition, the language is further limited to English, and the document type is restricted to “article” and “review”. In order to collect all relevant papers, the time span of the search was set to “all years”, from 1950 to the date of the search (December 31, 2021). Finally, the titles and abstracts of all publications were manually reviewed to remove the irrelevant ones, and a total of 1133 relevant papers were obtained, which were written by 5107 authors from 72 countries/regions and spanned 1087 institutions.2.2 Methods
Bibliometrics has covered structural, dynamic, evaluative, and predictive scientometrics.15 After data collected from the WoS Core Collection, including the number of publications, authors, institutions, countries/territories, citations, etc., the analysis was performed by tabulation and visual mapping. In addition, knowledge domain maps of author contributions and collaborations, journal co-citations, and keyword co-occurrence were created using the VOSviewer software package.12,16 Reference co-citation knowledge domain maps and keyword timelines were created using the Citespace software package.163. Results and discussion
3.1 Yearly quantitative distribution of the literature
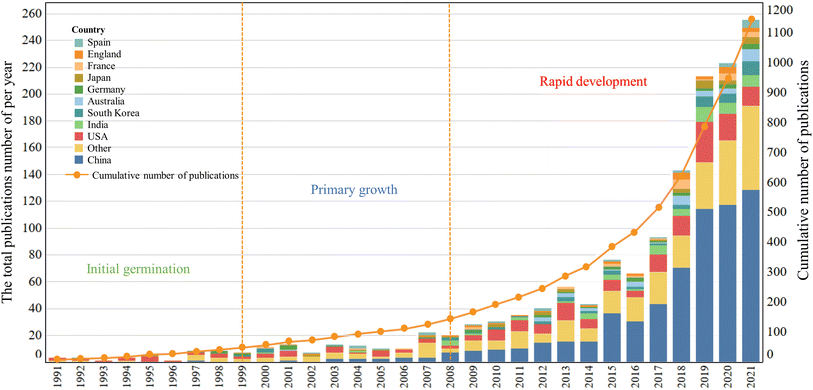
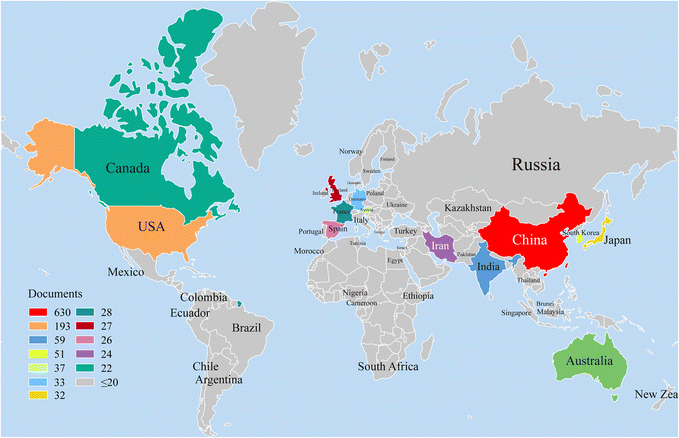
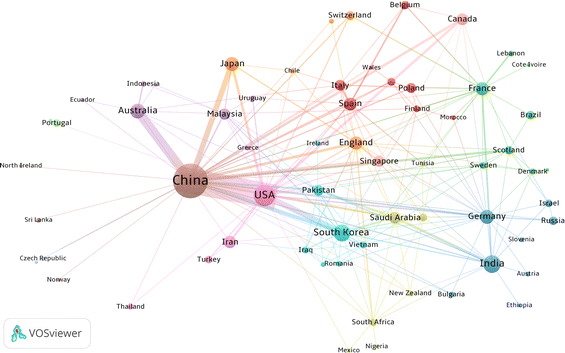
Statistical analysis of the publication year provides a clear picture of the trend of scientific output, development and maturity of a research field. Fig. 1 shows the output distribution of MnO2 in catalysis research based on time series. In general, from 1991, when the first article was published, to 2021, MnO2 has been active in the field of environmental catalysis, with increased published articles each year, which can be roughly divided into three stages: (1) the initial stage (1991–1999): the total publications (TP) increased slowly with the year, from 1 in 1991 to 7 in 1999, with an average of 6 publications per year. (2) the primary growth stage (2000–2008): the number of total publications in this period peaked in 2007 with 15 papers, and the lowest annual number of publications was 6 in 2002 though. Between 2000 and 2008, the average annual number of papers was 12, which is higher than the average annual publication in the initial phase, but the overall research progress is still relatively slow, and more papers are expected to be published in the following years. (3) The rapid development stage (2009–2021): the number of papers published per year increased sharply during this period, from 23 published in 2009 to 185 published in 2021, with 82.4 average annual publications.Annual publications from the top 11 countries are also presented in Fig. 1. It can be seen that the research on MnO2 environmental catalysis in China has been increasing year by year since 1997, while the number of published papers in the USA, India, South Korea, Australia, Germany, Japan, France, England and Spain is showing a fluctuating growth trend. Moreover, the cumulative number of publications showed an exponential growth.
3.2 Source journal analysis
Journals are the most important source of scholarly communication and dissemination of scientific results, and journal analysis can be conducted to identify influential journals in the field. The retrieved results show that 1133 papers have been published in 309 journals, covering research areas such as catalytic chemistry, environmental chemistry, engineering and materials, and energy chemistry. Table 1 lists the top 11 prolific journals that published more than 17 articles related to MnO2 environmental catalysis, and they are all included in SCI/SCIE. The Applied Catalysis B-Environmental (ACB-E) is a professional journal, which publishes experimental, theoretical and computational research related to new technologies and catalysts for catalytic combustion. With 174 papers, about 15.4% of the total were published in ACB-E, the most prolific journal reflecting the prevalence of catalytic research in the environmental catalysis. The second-ranked journal is Environmental Science & Technology (EST, 57, 5.0%), which focuses on chemical engineering, environmental engineering, and materials synthesis and processing. The third-ranked one is Catalysis Today (CT 36, 3.2%), whose broad scope is catalytic chemistry. At the same time, contaminant elimination is one of the hot topics related to MnO2 environmental catalysis, with the related journals Environmental Science and Pollution Research (ESPR, 26, 2.3%), and Journal of Hazardous Materials (JHM, 21, 1.9%). In addition, research on MnO2 in the field of environmental catalysis is also closely related to surface and interface reactions, and the related journals include ACS Applied Materials & Interfaces (ACS AMI, 18, 1.6%). In general, the core journals are almost related to this topic, specializing in different aspects of MnO2 in the field of environmental catalysis.| Rank | Source Journal | NPa | h | TCc | CPPd | IFe |
|---|---|---|---|---|---|---|
| a Number of papers. b h-index. c Total citation. d Citations per paper. e 2021 impact factor. | ||||||
| 1 | Applied Catalysis B-Environmental | 174 | 274 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869 869 |
102.7 | 24.319 |
| 2 | Environmental Science & Technology | 57 | 425 | 5336 | 93.6 | 11.357 |
| 3 | Catalysis Today | 36 | 221 | 2053 | 57.0 | 6.562 |
| 4 | Chemical Engineering Journal | 31 | 248 | 1714 | 55.3 | 16.744 |
| 5 | Environmental Science and Pollution Research | 26 | 132 | 329 | 12.7 | 5.19 |
| 6 | Journal of Hazardous Materials | 21 | 307 | 975 | 46.4 | 14.224 |
| 7 | Journal of Environmental Sciences | 20 | 109 | 673 | 33.7 | 6.796 |
| 8 | Journal of Environmental Chemical Engineering | 19 | 90 | 313 | 16.5 | 7.968 |
| 9 | ACS Applied Materials & Interfaces | 18 | 255 | 802 | 44.6 | 10.383 |
| 10 | Science of the Total Environment | 18 | 275 | 519 | 28.8 | 10.753 |
| 11 | Energy & Environmental Science | 17 | 376 | 4073 | 239.6 | 39.714 |
3.3 Author contribution and collaboration
| Rank | Author | Country | Institution | NPa | TLSb | TCc | CPPd | h |
|---|---|---|---|---|---|---|---|---|
| a Number of papers. b Total link strength. c Total citation. d Citations per paper. e h-index. | ||||||||
| 1 | Zhang P | China | Tsinghua University | 19 | 41 | 1755 | 92.4 | 52 |
| 2 | Li J | China | Tsinghua University | 18 | 44 | 2464 | 136.9 | 74 |
| 3 | Wang S | Australia | Curtin University | 14 | 25 | 2710 | 193.6 | 131 |
| 4 | He H | China | Chinese Academy of Sciences | 12 | 24 | 550 | 45.8 | 80 |
| 5 | Ma J | China | Harbin Institute of Technology | 12 | 24 | 635 | 52.9 | 31 |
| 6 | Suib, S l | USA | University of Connecticut | 12 | 12 | 748 | 62.3 | 91 |
| 7 | Sun H | Australia | Edith Cowan University | 12 | 22 | 2161 | 180.1 | 90 |
| 8 | Wang J | China | Huazhong Normal University | 12 | 22 | 1098 | 91.5 | 24 |
| 9 | Peng Y | China | Tsinghua University | 9 | 29 | 435 | 48.3 | 55 |
| 10 | Rong S | China | Nanjing University of Science and Technology | 9 | 16 | 546 | 60.7 | 13 |
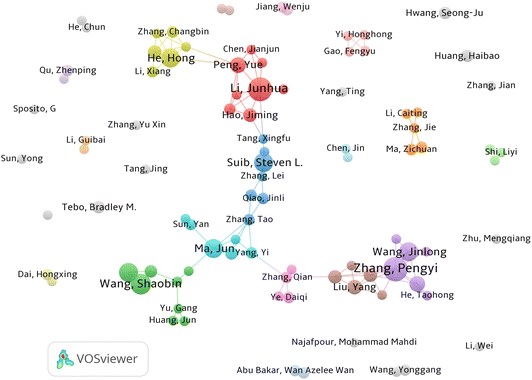
The total link strength (TLS) obtained from VOSviewer can effectively reveal the relationship between the number and frequency of co-authors. Each node represents an author, and the size of the node represents the number of co-authored papers. The link between two nodes represents the collaboration between them, and a larger link width means a closer collaboration between authors. Different colors represent different author cooperation clusters. The results in Fig. 2 show several clusters of authors working closely together, such as Zhang P (Tsinghua University), Wang S (Curtin University), Li J (Tsinghua University) and Suib S L (University of Connecticut). However, there is no close connection within different clusters, which indicates that the field is currently relatively independent in terms of international collaboration.
| Rank | Institution | Country | NPa | Pb | TLSc | TCd | CPPe |
|---|---|---|---|---|---|---|---|
| a Number of papers. b Proportion%. c Total link strength. d Total citation. e Citations per paper. | |||||||
| 1 | Chinese Academy of Sciences | China | 94 | 8.3% | 86 | 5725 | 60.9 |
| 2 | Tsinghua University | China | 64 | 5.6% | 49 | 5659 | 88.4 |
| 3 | University of Chinese Academy of Sciences | China | 32 | 2.8% | 40 | 1701 | 53.2 |
| 4 | Harbin Institute of Technology | China | 26 | 2.3% | 6 | 1830 | 70.4 |
| 5 | University of Connecticut | USA | 21 | 1.9% | 11 | 1416 | 67.4 |
| 6 | Wuhan University of Technology | China | 18 | 1.6% | 17 | 1079 | 59.9 |
| 7 | Dalian University of Technology | China | 17 | 1.5% | 6 | 571 | 33.6 |
| 8 | Fudan University | China | 17 | 1.5% | 10 | 2316 | 136.2 |
| 9 | Sun Yat-sen University | China | 14 | 1.2% | 7 | 891 | 63.6 |
| 10 | University of Science and Technology of China | China | 14 | 1.2% | 15 | 882 | 63.0 |
3.4 Co-citation analysis
The word co-citation was first proposed by the American intelligence scientist Henry Small, and it refers to the relationship between two papers when they are simultaneously referenced by a later published paper.23 At the same time, the highly co-cited journals represent the core journals at the forefront of current research. To identify core journals and knowledge bases relevant to this study, co-citation analysis of source journals and publications was performed using the VOSviewer tool.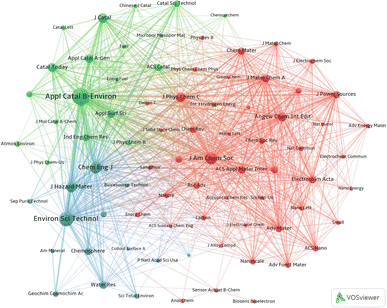 | ||
| Fig. 6 Mapped knowledge domains for journal co-citation in the field of MnO2 environmental catalysis. | ||
From Fig. 6, ACB-E is the largest node among all journals, indicating that ACB-E is the most cited journal along with other journals. This is not only related to the influence of the journal, but also to the number of articles published in the journal, with ACB-E ranking first among all journals in terms of the number of publications (Table 1). In the green cluster, CT and Journal of Catalysis (JC) are also widely cited, which shows that journals related to environmental catalysis generally have a broader impact. In addition, the connecting lines among ACB-E, JHM and Chemical Engineering Journal (CEJ) are thicker than the other lines, indicating a higher frequency of co-citation among these three journals.
The blue color in Fig. 6 is concentrated in the engineering and technology, with the representative journals of EST and CEJ. In terms of co-citation intensity, EST, JHM and CEJ have a close co-citation relationship with other journals.
The red cluster covers mainly chemical sciences-related journals, including Journal of the American Chemical Society (JACS), Angewandte Chemie-International Edition (ACIE) and Chemical Society Reviews (CSR). The node of links in the green cluster is greater than in the other clusters, suggesting that research on MnO2 in environmental catalysis is more widespread.
| Rank | Title | Journal | Author | Year | Citations | IF (2021) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts-A review | Catalysis Today | Li et al. | 2011 | 772 | 6.562 | 27 |
| 2 | Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia | Applied Catalysis B: Environmental | Kapteijn, et al. | 1994 | 732 | 24.319 | 28 |
| 3 | MnOx–CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: effect of preparation method and calcination temperature | Applied Catalysis B: Environmental | Tang, et al. | 2006 | 663 | 24.319 | 29 |
| 4 | Manganese oxides with rod-, wire-, tube-, and flower-like morphologies: highly effective catalysts for the removal of toluene | Environmental Science & Technology | Wang et al. | 2012 | 571 | 11.357 | 30 |
| 5 | Defect-engineered ultrathin delta-MnO2 nanosheet arrays as bifunctional electrodes for efficient overall water splitting | Advanced Energy Materials | Zhao et al. | 2017 | 568 | 29.698 | 34 |
| 6 | Persulfate activation on crystallographic manganese oxides: mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants | Environmental Science & Technology | Zhu et al. | 2019 | 517 | 11.357 | 35 |
| 7 | The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds | Applied Catalysis B: Environmental | Santos, et al. | 2010 | 504 | 24.319 | 36 |
| 8 | Surface characterization studies of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3 | Applied Catalysis B: Environmental | Ettireddy, et al. | 2007 | 470 | 24.319 | 37 |
| 9 | Gas phase ozone decomposition catalysts | Applied Catalysis B: Environmental | Dhandapani, et al. | 1997 | 464 | 24.319 | 38 |
| 10 | In situ X-ray absorption spectroscopy investigation of a bifunctional manganese oxide catalyst with high activity for electrochemical water oxidation and oxygen reduction | Journal of the American Chemical Society | Gorlin, et al. | 2013 | 417 | 16.383 | 39 |
| 11 | Different crystallographic one-dimensional MnO2 nanomaterials and their superior performance in catalytic phenol degradation | Environmental Science & Technology | Saputra, et al. | 2013 | 363 | 11.357 | 31 |
| 12 | Electrosynthesis, functional, and structural characterization of a water-oxidizing manganese oxide | Energy & Environmental Science | Zaharieva, et al. | 2012 | 362 | 39.714 | 40 |
| 13 | Removal of volatile organic compounds by single-stage and two-stage plasma catalysis systems: a review of the performance enhancement mechanisms, current status, and suitable applications | Environmental Science & Technology | Chen et al. | 2009 | 350 | 11.357 | 41 |
| 14 | Co-doping a metal (Cr, Fe, Co, Ni, Cu, Zn, Ce, and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperatures | Applied Catalysis B: Environmental | Thirupathi, et al. | 2011 | 345 | 24.319 | 42 |
| 15 | Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions | Applied Catalysis B: Environmental | Saputra, et al. | 2013 | 339 | 24.319 | 43 |
| 16 | Interactions of manganese with the nitrogen cycle: alternative pathways to dinitrogen | Geochimica et Cosmochimica Acta | Luther, et al. | 1997 | 302 | 5.921 | 44 |
| 17 | 3D-hierarchically structured MnO2 for catalytic oxidation of phenol solutions by activation of peroxymonosulfate: structure dependence and mechanism | Applied Catalysis B: Environmental | Wang et al. | 2015 | 300 | 24.319 | 45 |
| 18 | Catalytic decomposition of gaseous ozone over manganese dioxides with different crystal structures | Applied Catalysis B: Environmental | Jia et al. | 2016 | 299 | 24.319 | 46 |
| 19 | The effect of manganese vacancy in birnessite-type MnO2 on room-temperature oxidation of formaldehyde in air | Applied Catalysis B: Environmental | Wang et al. | 2017 | 294 | 24.319 | 47 |
| 20 | The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds | Applied Catalysis B-Environmental | Santos, et al. | 2010 | 443 | 24.319 | 36 |
The most cited paper is “Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts-A review” written by Li et al., with 772 citations. This study reviews two types of the low-temperature catalyst (LTC), the metal oxide catalyst and metal exchanged zeolite catalyst. For industrial flue gas and exhaust gas of diesel engines, it is of great significance to develop LTC for selective catalytic reduction of NOx with ammonia (NH3-SCR). At present, V2O5,24 Fe2O3 (ref. 25) and MnOx (ref. 26) are mainly used as active components in LTC research. Among them, MnOx are the most active components for NH3-SCR of NO at low temperatures. MnO2 exhibited the best catalytic activity in the temperature range of 100–300 °C, but its low N2 selectivity required further improvement. In addition, efforts need to be made on detailed mechanisms of SO2 poisoning and H2O suppression effect on Mn based metal oxides at low temperatures. At the same time, the synergistic effect of composite oxides is also a hot topic in recent years.27
Part of the research in the above review has been documented in a paper published in 1994 titled “Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia”, which was the second highly cited article. This study reported that manganese oxides with different crystallinity, oxidation state and specific surface area were used in NH3-SCR. The highest SCR activity per unit surface area is exhibited by MnO2, followed by Mn5O8, Mn2O3, Mn3O4 and MnO, in that order. The temperature-programmed reduction (TPR) experiments indicate a relation between the SCR process and surface reactive oxygen species.28
The third highly cited paper focuses on catalytic oxidation. The complete oxidation of formaldehyde by MnOx–CeO2 mixed oxides prepared by sol–gel, co-precipitation and modified coprecipitation method is reported.29 It is worth noting that half of the number of the top 20 highly cited articles are on catalytic oxidation, which fully illustrates that catalytic oxidation is a hot area of current research in the complete oxidation of formaldehyde,29 toluene elimination,30 phenol degradation,31etc. Due to the advantages of high catalytic activity, strong stability and low cost, the non-noble metal catalyst MnO2 has been widely used in Volatile Organic Compound (VOC) elimination. In order to improve its catalytic activity, researchers are now using doping modification and structural modulation to compensate for its weak electron transfer ability and low specific surface area.32 Although a large number of reports have been conducted for catalytic oxidation of VOCs, there are still some specific areas that need our further attention for the development of industry and the need of environmental protection, for example, the removal of halogenated VOCs and nitrogen-containing VOCs, and the combination of experimental and theoretical calculations to gain insight into the kinetics and mechanism of the catalytic oxidation reaction of VOCs on MnO2 surfaces. In addition, how to improve the hydrothermal stability of catalysts in industrial applications is also a focus of future research.
As can be seen in Table 4, MnO2 has also attracted great attention in the electrocatalytic technology due to its excellent hydrogen/oxygen evolution reaction performance. In addition, hydrogen energy is regarded as a green, efficient and renewable new source with the most potential to replace fossil fuels,33 so MnO2 catalytic HER technology might be promising to solve the energy crisis. In order to further enhance the electrocatalytic properties of MnO2, researchers have made many useful explorations, such as doping,48 construction of defective vacancies49 and compounding with other active nanomaterials.50
3.5 Keyword co-occurrence analysis
The objective of keyword co-occurrence analysis is to study the core content and structure of a specific field and thus to reveal the research frontiers in that field. A total of up to 5330 keywords were extracted from the Web of Science core database for the analysis of MnO2 environmental catalysis. We selected keywords with a frequency of 22 or more occurrences for visual analysis and obtained a total of 83 keywords, and the results in Fig. 7 show that a total of 4 clusters were obtained, where a node represents a keyword, and the node size represents the frequency of the keyword's occurrence, and the density of the connecting line between the nodes represents the strength of the co-occurrence between the keywords.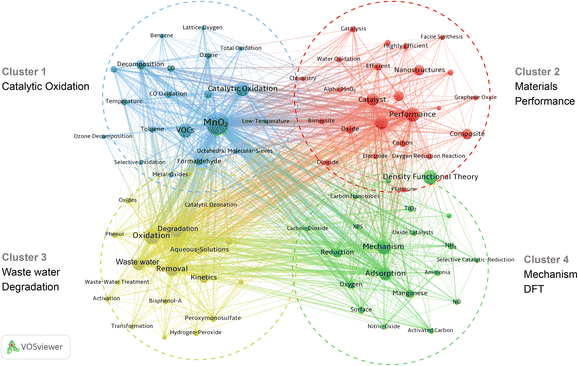 | ||
| Fig. 7 Keyword co-occurrence knowledge domain map of manganese dioxide in the field of environmental catalytic research. | ||
Cluster 1 (Blue): the most frequent keyword in the blue cluster is “MnO2”, which is connected to 82 nodes out of 83 nodes in total in Fig. 7. The blue cluster mainly spreads out around the nodes of “Catalytic Oxidation”, “Low-Temperature” and “VOCs”. VOCs, such as toluene and formaldehyde, are of widespread concern as a major cause of global air pollution.51 Low-temperature catalytic oxidation technology can convert VOCs into CO2 and water, which is recognized as one of the blue, efficient and environmentally friendly treatment means.52 MnO2 has inherent advantages, such as multiple valence states (Mn2+, Mn3+, and Mn4+) and crystal structures (α-MnO2, β-MnO2, γ-MnO2, and δ-MnO2). Moreover, MnO2 is excellent in the significant adsorption of oxygen and low-temperature self-reduction;30 especially, α-MnO2 with a [2 × 2] tunnel structure is considered as one of the ideal catalysts for VOC removal.53
Cluster 2 (Red): among the 83 keywords, “Performance” is well correlated with “MnO2” and is also the largest node in the red cluster. This cluster focuses on the preparation of catalyst materials and their catalytic performance. Zeng et al.54 reviewed the progress of catalytic oxidation of formaldehyde based on materials prepared by different methods, such as MnO2 loaded noble metals, structural regulation of MnO2, and MnO2 and composites of MnO2 with other non-noble metal materials. The influence of material synthesis methods on the catalytic performance was revealed. In addition, the regulation of interfacial structure is also one of the research hotspots for catalytic activity. Duan et al.55 prepared a bi-component MnO2 and Mn3O4 supported Pt catalyst by interfacial modulation in the in situ liquid-phase reduction strategy, which showed excellent catalytic activity for toluene oxidation and could achieve complete mineralization of toluene at 160 °C. This report reveals that the modulation of the interfacial structure in the bi-component manganese oxide supported Pt catalysts is a feasible way to improve the catalytic oxidation performance of toluene.
Cluster 3 (Yellow): the largest node is “Degradation”, with 72 nodes connected, including “Oxidation”, “Waste water”, “Removal”, etc., within cluster 3. This cluster mainly reflects the subject of heterogeneous catalysis. He et al.56 investigated the structure–activity relationship of MnO2 and the mechanism of catalytic ozone oxidation in “Aqueous Solution”. Gan et al.57 prepared β-MnO2/kenaf carbon fiber (KCF) composites for the degradation of “Bisphenol-A” (BPA) in water via catalytic oxidation.
Cluster 4 (Green): the largest node is “Density Functional Theory” (DFT), with 48 nodes linked, which indicates that the use of computer simulation techniques to study the catalytic mechanism is becoming an auxiliary research tool. Zhou et al.48 synthesized nano-hybridized MnO2 catalysts via α-MnO2 nanotubes, and DFT calculations showed that the surface of pristine α-MnO2 (111) could promote the adsorption and activation of O2, while the surface of hybridized MnO2 (111) contributed to the absorption/decomposition of the product H2O.
3.6 Research frontier identification
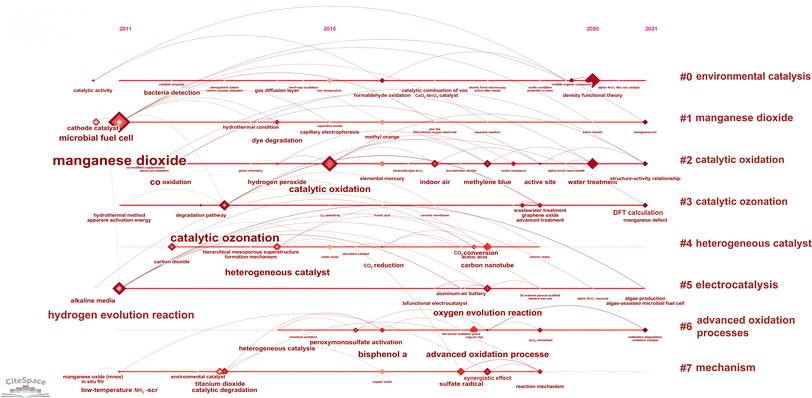
The timeline view can clearly reflect the results of the different clusters over time.12 The X-axis is the year of publication and the Y-axis represents the different keyword clusters. Fig. 8 shows the entire timeline of MnO2 environmental catalysis from 2011 to 2021. A keyword represents a node, i.e., the larger node represents the stronger keyword burst, that is to say, the connection between keywords indicates a co-occurrence relationship between each other.16 A total of eight clusters were obtained with the evolution of representative keywords under each cluster (Fig. 8).From 2011, many keywords appeared in the timeline, including manganese dioxide, hydrogen evolution reaction, and low-temperature NH3-SCR. By 2015, the keywords, such as catalytic oxidation, low temperature, CO2 selectivity, and noble metal, appeared. In 2020, the keywords, such as density functional theory and water treatment, appeared. In 2021, structure–activity relationship, DFT calculation, and manganese defect as the three key words emerged.
The top 15 keywords in terms of citation burst strength obtained by CiteSpace software are shown in Table 5. It can be seen that the keyword with the highest citation burst strength is density functional theory. From 2019 to 2021 there were strong citation bursts for the three keywords, i.e., structure–activity relationship, density functional theory and water treatment. Based on the strong citation bursts and the impact of keywords, it can be predicted that overall water splitting, density functional theory, and water treatment will remain research hot words for MnO2 environmental catalysis in the future. As shown in Fig. 8, the emerging keywords in 2021, such as DFT calculation and structure–activity relationship, might represent that themes, such as performance, catalytic oxidation and mechanism, are also the research frontier in the field of MnO2 environmental catalysis (Fig. 7). As is known, the morphology has a crucial influence on the catalytic performance of MnO2.30,58–61
4. Conclusion
A bibliometric analysis of published papers related to MnO2 environmental catalysis in the WOS core database is conducted to obtain a knowledge map through information visualization analysis. To date, MnO2 environmental catalysis research shows the following characteristics:(1) MnO2 environmental catalysis research can be divided into three stages: the initial stage (1991–1999), the primary growth stage (2000–2008), and the rapid development stage (2009–2021). China is the most active country in this field, and the disciplinary categories indicate that MnO2 environmental catalysis is a multidisciplinary field based on catalytic chemistry, environmental chemistry, engineering and materials, and energy chemistry. Relevant institutions and authors in China, the USA, India, South Korea, and Australia are the main driving forces of MnO2 environmental catalysis research. However, from the analysis of author cooperation, the current national cooperation of this field is relatively independent, and global cooperation should be further strengthened.
(2) In terms of co-citation, ACB-E, EST, and JACS have a close co-citation relationship with other journals, which is mainly determined by the annual publication papers and the influence of the journal. According to the literature co-citation analysis, electrocatalysis, catalytic oxidation, AOPs, and SCR are the research spots. In addition, the keyword analysis points out that catalytic oxidation, materials and performance, waste water degradation, mechanism and DFT research themes can also be worthy of attention.
(3) By analyzing the research frontiers, 8 categories are classified, including environmental catalysis, manganese dioxide, catalytic oxidation, catalytic ozonation, heterogeneous catalyst, electrocatalysis, advanced oxidation processes, and mechanism. The themes of structure–activity relationship, density functional theory, catalytic oxidation, and mechanism are also the research frontier in the field of MnO2 environmental catalysis research.
Author contributions
Yaoguang Guo: investigation, writing original draft, and funding acquisition. Qianqian Chen: investigation, visualization, and writing original draft. Xiaohu Sun: methodology, and investigation. Yujing Liu: methodology, and investigation. Jie Guan: resources, funding acquisition and supervision. Xiaojiao Zhang: software. Nuo Liu: methodology, and investigation. Xiaoyi Lou: writing – review & editing, and supervision. Yingshun Li: methodology. Xiangwen Zhang: editing and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present work was financially supported by Shanghai Natural Science Foundation (20ZR1421100), Natural Science Foundation of China (52070127, and 52270129), and the Central-Public interest Scientific Institution Basal Research Fund (2019T14). Dr Guo also thanks the financial support of Science and Technology Development Fund of Pudong New Area (PKJ2021-C01, and PKJ2022-C07).References
- T. Antonsson, A. Heumann and C. Moberg, J. Chem. Soc., Chem. Commun., 1986, 518–520 RSC.
- J.-J. Huang, Y.-X. Zhang and J.-X. Zhang, J. Electron. Mater., 2021, 50, 6535–6544 CrossRef CAS.
- A. Kumar, A. Thomas, A. Gupta, M. Garg, J. Singh, G. Perumal, E. Sujithkrishnan, P. Elumalai and H. S. Arora, J. Energy Storage, 2021, 42, 103100 CrossRef.
- Y. Xu, Y. Yan, W. Lu, S. Yarlagadda and G. Xu, ACS Appl. Energy Mater., 2021, 4, 10639–10645 CrossRef CAS.
- D. Ma, A. Lee-Sie Eh, S. Cao, P. S. Lee and J. Wang, ACS Appl. Mater. Interfaces, 2021, 14, 1443–1451 CrossRef PubMed.
- A. Gowrisankar and T. Selvaraju, Langmuir, 2021, 37, 5964–5978 CrossRef CAS PubMed.
- A. Shuai, S. Li, W. Yang, Y. Yang, Y. Deng and C. Gao, Corros. Sci., 2020, 170, 108679 CrossRef.
- S. Yang, H. Yang, J. Yang, H. Qi, J. Kong, Z. Bo, X. Li, J. Yan, K. Cen and X. Tu, Chem. Eng. J., 2020, 402, 126154 CrossRef CAS.
- S. Lin, H. Cheng, Q. Ouyang and H. Wei, Anal. Methods, 2016, 8, 3935–3940 RSC.
- L. Xue, N. Jin, R. Guo, S. Wang, W. Qi, Y. Liu, Y. Li and J. Lin, ACS Sens., 2021, 6, 2883–2892 CrossRef CAS PubMed.
- N. Sohal, B. Maity and S. Basu, ACS Appl. Bio Mater., 2021, 4, 5158–5168 CrossRef CAS PubMed.
- H. Liu, R. Hong, C. Xiang, C. Lv and H. Li, Fuel, 2020, 262 Search PubMed.
- D. Yu, Z. Xu, Y. Kao and C.-T. Lin, IEEE Trans. Fuzzy Syst., 2018, 26, 430–442 Search PubMed.
- J. Liu, J. Li and C. Fan, J. Loss Prev. Process Ind., 2020, 63, 104030 CrossRef.
- H. Tan, J. Sun, W. Wenjia and C. Zhu, Int. J. Hum.-Comput. Interact., 2021, 37, 297–307 CrossRef.
- H. Tan, J. Li, M. He, J. Li, D. Zhi, F. Qin and C. Zhang, J. Environ. Manage., 2021, 297, 113382 CrossRef CAS PubMed.
- Y. Zhang, H. Zheng, P. Zhang, X. Zheng and Q. Zuo, J. Hazard. Mater., 2021, 408, 124917 CrossRef CAS PubMed.
- T. Shao, P. Zhang, L. Jin and Z. Li, Appl. Catal., B, 2013, 142–143, 654–661 CrossRef CAS.
- H. Zhang, X. Zheng, T. Xu and P. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 17532–17542 CrossRef CAS PubMed.
- W. Shan, Y. Yu, Y. Zhang, G. He, Y. Peng, J. Li and H. He, Catal. Today, 2021, 376, 292–301 CrossRef CAS.
- B. Bai, Q. Qiao, H. Arandiyan, J. Li and J. Hao, Environ. Sci. Technol., 2016, 50, 2635–2640 CrossRef CAS PubMed.
- D. Wang, Q. Chen, X. Zhang, C. Gao, B. Wang, X. Huang, Y. Peng, J. Li, C. Lu and J. Crittenden, Environ. Sci. Technol., 2021, 55, 2743–2766 CrossRef CAS PubMed.
- H. Small, J. Am. Soc. Inf. Sci., 1973, 24, 265–269 CrossRef.
- H.-m. Long, Y.-d. Zhang, T. Yang, L.-x. Qian and Z.-w. Yu, J. Iron Steel Res. Int., 2021, 29, 1176–1184 CrossRef.
- C. Jin, Y. Zhou, S. Han and W. Shen, J. Phys. Chem. C, 2021, 125, 26031–26038 CrossRef CAS.
- E. Akbari, S. M. Alavi, M. Rezaei and A. Larimi, Int. J. Energy Res., 2021, 46, 6292–6313 CrossRef.
- J. Li, H. Chang, L. Ma, J. Hao and R. T. Yang, Catal. Today, 2011, 175, 147–156 CrossRef CAS.
- F. Kapteijn, L. Singoredjo, A. Andreini and J. A. Moulijn, Appl. Catal., B, 1994, 3, 173–189 CrossRef CAS.
- X. Tang, Y. Li, X. Huang, Y. Xu, H. Zhu, J. Wang and W. Shen, Appl. Catal., B, 2006, 62, 265–273 CrossRef CAS.
- F. Wang, H. Dai, J. Deng, G. Bai, K. Ji and Y. Liu, Environ. Sci. Technol., 2012, 46, 4034–4041 CrossRef CAS PubMed.
- E. Saputra, S. Muhammad, H. Sun, H. M. Ang, M. O. Tadé and S. Wang, Environ. Sci. Technol., 2013, 47, 5882–5887 CrossRef CAS PubMed.
- X. Liu, Y. Wang, F. Liu, C. Zhao, H. Liu and S. Lin, Prog. Chem., 2019, 31, 1159 CAS.
- J. O. Abe, A. P. I. Popoola, E. Ajenifuja and O. M. Popoola, Int. J. Hydrogen Energy, 2019, 44, 15072–15086 CrossRef CAS.
- Y. Zhao, C. Chang, F. Teng, Y. Zhao, G. Chen, R. Shi, G. I. N. Waterhouse, W. Huang and T. Zhang, Adv. Energy Mater., 2017, 7, 1700005 CrossRef.
- S. Zhu, X. Li, J. Kang, X. Duan and S. Wang, Environ. Sci. Technol., 2019, 53, 307–315 CrossRef CAS PubMed.
- V. P. Santos, M. F. R. Pereira, J. J. M. Órfão and J. L. Figueiredo, Appl. Catal., B, 2010, 99, 353–363 CrossRef CAS.
- P. R. Ettireddy, N. Ettireddy, S. Mamedov, P. Boolchand and P. G. Smirniotis, Appl. Catal., B, 2007, 76, 123–134 CrossRef CAS.
- B. Dhandapani and S. T. Oyama, Appl. Catal., B, 1997, 11, 129–166 CrossRef CAS.
- Y. Gorlin, B. Lassalle-Kaiser, J. D. Benck, S. Gul, S. M. Webb, V. K. Yachandra, J. Yano and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 8525–8534 CrossRef CAS PubMed.
- I. Zaharieva, P. Chernev, M. Risch, K. Klingan, M. Kohlhoff, A. Fischer and H. Dau, Energy Environ. Sci., 2012, 5, 7081 RSC.
- H. L. Chen, H. M. Lee, S. H. Chen, M. B. Chang, S. J. Yu and S. N. Li, Environ. Sci. Technol., 2009, 43, 2216–2227 CrossRef CAS PubMed.
- B. Thirupathi and P. G. Smirniotis, Appl. Catal., B, 2011, 110, 195–206 CrossRef CAS.
- E. Saputra, S. Muhammad, H. Sun, H.-M. Ang, M. O. Tadé and S. Wang, Appl. Catal., B, 2013, 142–143, 729–735 CrossRef CAS.
- G. W. Luther, B. Sundby, B. L. Lewis, P. J. Brendel and N. Silverberg, Geochim. Cosmochim. Acta, 1997, 61, 4043–4052 CrossRef CAS.
- Y. Wang, H. Sun, H. M. Ang, M. O. Tadé and S. Wang, Appl. Catal., B, 2015, 164, 159–167 CrossRef CAS.
- J. Jia, P. Zhang and L. Chen, Appl. Catal., B, 2016, 189, 210–218 CrossRef CAS.
- J. Wang, J. Li, C. Jiang, P. Zhou, P. Zhang and J. Yu, Appl. Catal., B, 2017, 204, 147–155 CrossRef CAS.
- Z. Ye, T. Li, G. Ma, Y. Dong and X. Zhou, Adv. Funct. Mater., 2017, 27, 1704083 CrossRef.
- Y. Zhao, J. Zhang, W. Wu, X. Guo, P. Xiong, H. Liu and G. Wang, Nano Energy, 2018, 54, 129–137 CrossRef CAS.
- K.-L. Yan, X. Shang, W.-K. Gao, B. Dong, X. Li, J.-Q. Chi, Y.-R. Liu, Y.-M. Chai and C.-G. Liu, J. Alloys Compd., 2017, 719, 314–321 CrossRef CAS.
- H. Huang, Y. Xu, Q. Feng and D. Y. C. Leung, Catal. Sci. Technol., 2015, 5, 2649–2669 RSC.
- M. Wen, G. Li, H. Liu, J. Chen, T. An and H. Yamashita, Environ. Sci.: Nano, 2019, 6, 1006–1025 RSC.
- T. Uematsu, Y. Miyamoto, Y. Ogasawara, K. Suzuki, K. Yamaguchi and N. Mizuno, Catal. Sci. Technol., 2015, 6, 222–233 RSC.
- X. Zeng, C. Shan, M. Sun, T. He and s. Rong, Prog. Chem., 2021, 33, 2245 CAS.
- X. Duan, Z. Qu, C. Dong and Y. Qin, Appl. Surf. Sci., 2020, 503, 144161 CrossRef CAS.
- Y. He, L. Wang, Z. Chen, B. Shen, J. Wei, P. Zeng and X. Wen, Sci. Total Environ., 2021, 785, 147328 CrossRef CAS PubMed.
- L. Gan, X. Fang, L. Xu, L. Wang, Y. Wu, B. Dai, W. He and J. Shi, Mater. Des., 2021, 203, 109596 CrossRef CAS.
- R. Yang, Y. Fan, R. Ye, Y. Tang, X. Cao, Z. Yin and Z. Zeng, Adv. Mater., 2021, 33, 2004862 CrossRef CAS PubMed.
- Y. Dong, H. Yang, K. He, S. Song and A. Zhang, Appl. Catal., B, 2009, 85, 155–161 CrossRef CAS.
- J. Luo, H. T. Zhu, H. M. Fan, J. K. Liang, H. L. Shi, G. H. Rao, J. B. Li, Z. M. Du and Z. X. Shen, J. Phys. Chem. C, 2008, 112, 12594–12598 CrossRef CAS.
- J. Luo, C. Hu, X. Meng, J. Crittenden, J. Qu and P. Peng, ACS Sustainable Chem. Eng., 2017, 5, 2255–2264 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |