Supramolecular assemblies of multifunctional microgels for biomedical applications
Jingxia
Zheng
a,
Canjie
Zhu
a,
Xun
Xu
b,
Xinwei
Wang
b and
Jun
Fu
 *a
*a
aKey Laboratory of Polymeric Composite and Functional Materials of Ministry of Education, Guangdong Functional Biomaterials Engineering Technology Research Centre, Guangzhou Key Laboratory of Flexible Electronic Materials and Wearable Devices, School of Materials Science and Engineering, Sun Yat-sen University, Guangzhou Higher Education Mega Centre, 132 Waihuan Road East, Panyu, Guangzhou 510006, China. E-mail: fujun8@mail.sysu.edu.cn
bState Key Laboratory of Polyolefins and Catalysis, Shanghai Research Institute of Chemical Industry, Shanghai 200062, China
First published on 22nd May 2023
Abstract
Biomedical materials with outstanding biochemical and mechanical properties have great potential in tissue engineering, drug delivery, antibacterial, and implantable devices. Hydrogels have emerged as a most promising family of biomedical materials because of their high water content, low modulus, biomimetic network structures, and versatile biofunctionalities. It is critical to design and synthesize biomimetic and biofunctional hydrogels to meet demands of biomedical applications. Moreover, fabrication of hydrogel-based biomedical devices and scaffolds remains a great challenge, largely due to the poor processibility of the crosslinked networks. Supramolecular microgels have emerged as building blocks for fabrication of biofunctional materials for biomedical applications due to their excellent characteristics, including softness, micron size, high porosity, heterogeneity and degradability. Moreover, microgels can serve as vehicles to carry drugs, bio-factors, and even cells to augment the biofunctionalities to support or regulate cell growth and tissue regeneration. This review article summarizes the fabrication and the mechanism of supramolecular assemblies of microgels, and explores their application in 3D printing, along with detailed representative biomedical applications of microgel assemblies in cell culture, drug delivery, antibacterial and tissue engineering. Major challenges and perspectives of supramolecular microgel assemblies are presented to indicate future research directions.
10th anniversary statementCongratulations on the 10th anniversary of the Journal of Materials Chemistry family. I am grateful to have had twenty papers published in Journal of Materials Chemistry B covering topics from implantable polymers, tough hydrogels, tissue engineering scaffolds, soft actuators, and hydrogel-based flexible electronics. All these research articles and reviews have been well acknowledged by readers, thanks to the outstanding efforts by the editorial team and the volunteer referees. I am honored to have served as an Advisory Board Member since 2017. It provides me with great opportunities to make more contributions to this journal and the community. This review article introduces an emerging field of microgel-based 3D printing for biomedical materials. This important concept proposes to utilize microgels as functional building blocks to construct multifunctional and high performance biomedical devices. Supramolecular chemistry plays critical roles in spontaneously and efficiently assembling microgels together. This supramolecular strategy can be readily applied to integrate microgels loaded with drugs, factors, peptides, enzymes, and even cells into soft chips, organoids, and artificial biotissues. I hope this review will be of broad interest to readers in the field of biomedical materials and engineering. |
1. Introduction
Biomaterials play an extremely important role in biomedical engineering through integration and interactions with other functional substances such as cells, growth factors, drugs and genes.1 Biocomposites can be used as a 3D matrix for cell or drug delivery, by protecting them from unwanted mechanical damage.2 Moreover, biomaterials with optimal biochemical and mechanical properties can promote cell survival and retention, and promote cell-mediated tissue matrix production and vascularization,3 which has been widely used to promote cell delivery and tissue regeneration.4,5 Supramolecular assembly is prevalent in biomedical systems that organize biomacromolecules into hierarchical structures and biotissues.6,7 The generation of supramolecular materials assembled by transient cross-linking facilitates engineering of multifunctional, self-assembled, stimuli-responsive biomaterials.8Hydrogels have broad application prospects as biomaterials due to the high water content, adjustable physicochemical properties and structural similarity to the natural extracellular matrix (ECM).9 However, the tight nanometer-scale cross-linking network of traditional block hydrogels is unfavorable for the penetration of cells and blood vessels, and it is hard to process or damage the biological activity and endogenous healing performance during extruding. To tackle this problem, many types of hydrogels have been processed into microgels via a variety of fabrication techniques,10 including mechanical crushing,11–13 emulsion polymerization,14–17 microfluidic and microchannel,18–21 and templating methods.22–24 By using various assembly principles including jamming,25–27 chemical bonding,28–30 or non-covalent interactions,31–33 microgels have been processed into assemblies with many excellent characteristics, including softness, micron size, high porosity, heterogeneity and degradability.9,34
Supramolecular microgels are made of a variety of materials, which mainly include host–guest molecules such as cyclodextrin, Cucurbit[8]uril, adamantane, azobenzene, and ferrocene, synthetic polymers such as polyethylene glycol (PEG), poly(N-isopropyl acrylamide) (PNIPAAM), and natural polysaccharides, like hyaluronic acid, sodium alginate, etc. The structures of supramolecular microgel assemblies determine multifunctional performance and biomedical applications. Supramolecular microgel assemblies are cross-linked by non-covalent interactions, which are weak and dynamic, and result in beneficial properties, including self-healing, shear thinning, injectability, or printability.12,30,35 The micron size of microgels allows for minimal invasive injection. Most microgels are amiable to protect cells during injection and transportation, and are ideal candidates for 3D printing bioinks to fabricate artificial tissues and organs. Therein, collective dynamic supramolecular interactions help maintain the stability of printed scaffolds. Moreover, microgels with different formulations like materials, composition, size and contents can be used as modules to create multifunctional materials and constructs. Microgel scaffolds are usually porous, which is conducive to cell invasion, proliferation and migration.36,37 What's more, multi-stimuli responsiveness is another important feature of supramolecular microgel assemblies, which respond to a variety of physical or biochemical stimuli38–40 like temperature, light, magnetic/electric field, mechanical force, pH, and redox, etc. Responsive microgels serve as intelligent carriers for bioactive molecules such as drugs, proteins, carbohydrates and DNA. Based on the micro porous structures and multifunctional characteristics of supramolecular microgel assemblies, they are suitable for broad biomedical applications, including cell transplantation,41 drug delivery,42–44 antibacterials,45 biocatalysis,46 and tissue engineering,34,36etc.
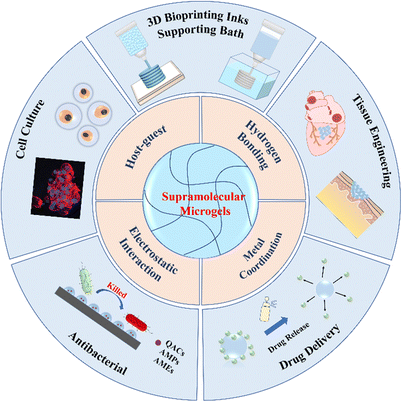
In this review article, we discuss the latest advances of supramolecular assemblies of multifunctional microgels for biomedical applications. Firstly, we simply introduce the fabrication of microgels. Next, we introduce the mechanism of supramolecular assemblies of microgels, including host–guest interactions, hydrogen bonding, metal coordination and electrostatic interaction, etc. Then, we briefly describe 3D printing of supramolecular microgels, and summarize in detail representative applications of microgel assemblies in cell culture, drug delivery, antibacterial and tissue engineering. Finally, we present the major challenges and perspectives of supramolecular microgel assemblies to indicate future research directions (Fig. 1).
2. Fabrication of microgels
A variety of fabrication techniques of microgels have been developed,10 including mechanical crushing, emulsion polymerization, microfluidic/microchannels and templating47 (Fig. 2). In general, these methods involve particle formation and gelation. Particles are prepared by dispersing an aqueous monomer solution droplet in organic solution. Then, the monomers in particles are initiated to polymerize and crosslink by heating or irradiation to form microgels with 100 nm–1 mm diameters.48,49 This section briefly outlines representative microgel preparation methods.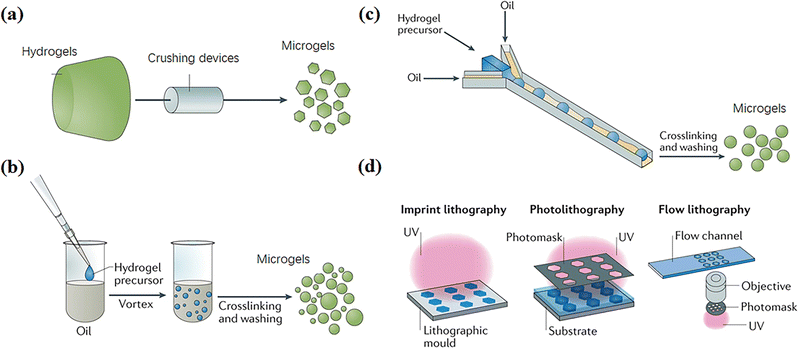 | ||
| Fig. 2 Fabrication techniques of microgels, including (a) mechanical crushing, (b) emulsion polymerization, (c) microfluidic and microchannel and (d) templating methods. Reproduced with permission from ref. 47. Copyright 2019 Springer Nature. | ||
2.1 Mechanical crushing
Microgels can be produced by mechanically breaking a preformed bulk hydrogel into microparticles (Fig. 2a).47 For example, Sinclair et al. produced zwitterionic microgels by extruding bulk gels through progressively fine steel meshes using a stainless-steel piston and cylinder apparatus. The microgels were sieved for a least three times to obtain diameters of 15–30 μm.11 Moreover, Fu et al. extruded crosslinked chitosan methacrylate (CHMA)/polyvinyl alcohol (PVA) hydrogels through nozzles repeatedly with specific internal diameters from 300 to 500 μm to produce a slurry of microparticles with an average diameter of 197 ± 68 μm.12 Hinton et al. used a rotational blender to break a crosslinked gelatin hydrogel into microgels with diameters of 120–300 μm,13 which can be used as ink for 3D printing various structures into a supporting bath. These mechanical crushing methods are high-speed, simple and suitable for various materials; however, the particle sizes are usually polydisperse.2.2 Emulsion polymerization
Many methods based on emulsion polymerization have been reported to prepare microgels, mainly including surfactant-free emulsion polymerization and emulsion polymerization with added surfactant (Fig. 2b).Surfactant-free emulsion polymerization (SFEP) means that no surfactant is added at all or only a small amount of surfactant is added in the reaction process (with a concentration lower than the critical micelle concentration CMC), which usually applies for thermosensitive monomers, typically with a lower critical solution temperature (LCST).50 Such monomers are fully dissolved in water below the LCST to form a homogeneous system, and undergo phase separation to form droplets above the LCST. Therein, polymerization and crosslinking take place to yield microgels with collapsed configurations. Xue et al. prepared different microgels with narrow size distribution by SFEP of six acrylamide-type thermoresponsive monomers through free radical copolymerization.15 However, SFEP is applicable to only a few thermosensitive monomers.
Emulsion polymerization with added surfactant includes conventional emulsion polymerization and inverse emulsion polymerization, which can form oil-in-water or water-in-oil emulsion by high-speed dispersing different monomers in the oil phase or water phase, respectively. Then, polymer microgels are formed by thermal initiation or photoinitiation before purification centrifugal washing. A homogeneous emulsification method, speed and time will affect the size and dispersity of microgels.51 Inverse emulsion polymerization has the advantages of simplicity, rapidity and small particle size, and is suitable for the synthesis of various water-soluble microgels, including poly(2-acrylamido-2-methyl-propanesulfonic acid) (PAMPS), poly-acrylamide (PAAm), poly-acrylic acid (PAAc), poly(N-isopropylacrylamide) (PNIPAm), poly(2-hydroxyethyl methacrylate) (PHEMA), poly(ethylene glycol) (PEG), etc., as well as biocompatible and degradable polysaccharide-based hybrid microgels like gelatin, alginate, chitosan, hyaluronic acid cellulose, etc.16,17
2.3 Microfluidic and microchannels
Microfluidic and microchannel techniques have been widely used to continuously prepare microgels with uniform and controllable particle compositions, dimensions, shapes, and pore sizes by altering flow rates and channel properties like diameters, shapes, polarity, and architecture.52–54 Common microchannel structures are T-shaped and ψ-shaped (Fig. 2c). Generally, the hydrogel precursor solution is injected into the oil phase to form spherical particles through a narrow channel due to interfacial tension, and then the environmental conditions are changed to crosslink the particles into microgels with diameters ranging from around 100 nm to 1000 μm.55 The microgels usually have excellent monodispersity.56 Xu et al. used a flow-focusing V-shaped microfluidic chip with two water phase channels and one oil phase channel manufactured by soft lithography to prepare cross-linked gelatin microgels with enzymes.18 A method to control the spatial distribution of structural proteins in microgels under mild gelation conditions by controlling the mixing of gelatin and physical and enzymatic cross-linking through microfluidics was demonstrated.In addition, the microfluidic droplet generator can be used to generate highly uniform microgel building blocks, manufacture controlled interconnected microporous scaffolds, promote cell proliferation and network, and accelerate wound healing.57–59 For example, Di Carlo et al. described a method for manufacturing highly uniform microgel building blocks in a continuous, scalable, high-throughput manner, so as to form a highly modular microporous environment for cell and tissue growth.19 Hydrogel precursors are injected into the parallel step emulsification microfluidic device at a reduced nonreactive pH to generate uniform spherical microgel templates. Then, a unique pH adjustment was used to induce controllable and uniform crosslinking of microgel building blocks, and the productivity was one to two orders of magnitude higher than that of similar projects.20,21 These controllable and degradable microgels fabricated by microfluidic and microchannel methods have the potential of non-uniform or gradient reactors, which can be used for 3D cell culture scaffolds and as components of complex and natural-inspired robots.
2.4 Templating
It is convenient to use templates to form particles of various sizes for crosslinking to form microgels triggered by environmental stimulation.22–24 Among these, the photolithography process of hydrogel templating in a highly controllable and repeatable manner has become an attractive “top-down” alternative to traditional microgel synthesis technologies,60 because it meets the requirement of preparing microgels with highly controllable size and morphology. Photolithographic patterned microgels can be prepared by any routes commonly used to generate cross-linked polymer networks, including chemical cross-linking, physical association and molecular self-assembly.61 Essentially, the photolithographic synthesis of microgels involves transferring pre-designed template patterns to hydrogel precursors, and then (or accompanying) polymerization and/or cross-linking, separating to form microgels.62 Various emerging lithography technologies for the synthesis of complex microgels can be divided into three categories: imprint lithography, lithography and flow lithography (Fig. 2d). Doyle et al. demonstrated a simple method for preparing non spherical chitosan polyethylene glycol (PEG) microgels based on a photolithographic template method for coupling of high surface density biomolecules.22 Wu et al. used a soft lithography technology to produce alginate microgels with a highly controllable shape and uniform size ranging from several microns to millimeters by changing the corresponding parameters in the soft lithography method.633. Supramolecular assembling of microgels
Supramolecular interactions between microgels, including guest–host recognition, hydrogen bonding, electrostatic attraction, metal-ligand coordination, can work alone or collectively to assemble microgels into aggregates, structured constructs, and even macroporous materials under mild ambient conditions. Supramolecular assembling of microgels is reversible and usually friendly to cells and tissues, which is beneficial to biomedical applications. By introducing supramolecular moieties into microgels, it is convenient to tune the ability to assemble microgels in the desired manner into pre-designed microstructures. Besides, the assemblies exhibit shear thinning and self-healing, which is highly desired for injection, processing, and 3D printing. In this way, microgels with different components and functionalities can be assembled into multifunctional constructs. Specifically, microgels carrying different drugs or cells can assemble into single devices to achieve concerted therapy or regeneration. This section introduces recent progress in representative supramolecular assembling of microgels.3.1 Host–guest recognition
Host–guest recognition usually refers to supramolecular interactions between molecules based on geometric fitting and non-covalent association (Fig. 3a).40 Host molecules with cavities, including crown ethers, cyclodextrins, calixarenes, column aromatics and cucurbitacin, etc., usually offer a tiny amount of room for a guest molecule to fit in. Therein, both the geometry recognition and hydrophobic/hydrophilic/coordination interactions work together to form a stable non-covalent associate. This is known as host–guest supramolecular recognition. The association constant for a host–guest pair is primarily determined by the hydrophilicity, size, polarity, and charge of the guest. Guests with different association constants may compete to occupy the host cavity. When the host or guest moieties are linked to polymer chains, numerous host–guest complexes can form to serve as non-covalent bonding and crosslinking, resulting in non-covalent crosslinks that are responsive to external stimuli (Fig. 3b).44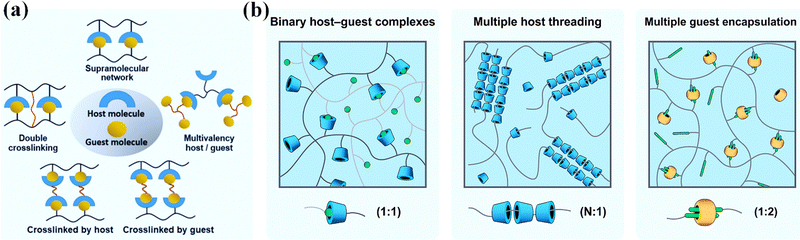 | ||
| Fig. 3 (a) Host–guest recognition. Reproduced with permission from ref. 40. Copyright 2022 John Wiley and Sons. (b) Different kinds of host–guest complexes. Reproduced with permission from ref. 44. Copyright 2021 Elsevier. | ||
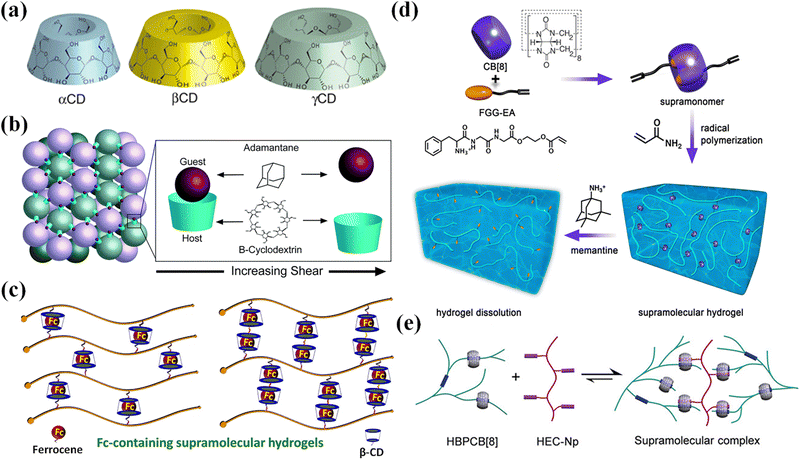 | ||
| Fig. 4 The host–guest recognition for CDs and CB[8]. (a) Schematic structures of α-, β-, and γ-CD. Reproduced with permission from ref. 64. Copyright 2014 American Chemical Society. (b) A reversible host–guest interaction between microgel particles functionalized with Ad and β-CD. Reproduced with permission from ref. 75. Copyright 2021 The Royal Society of Chemistry. (c) Guest molecules (β-CD) and host molecules (Fc) for the construction of Fc-containing hydrogels. Reproduced with permission from ref. 76. Copyright 2020 Elsevier. (d) Supramolecular hydrogel fabrication through the host–guest interactions between the FGG-EA and CB[8]. Reproduced with permission from ref. 77. Copyright 2017 American Chemical Society. (e) Supramolecular assembly between HBPCB[8] and HEC-Np. Reproduced with permission from ref. 31. Copyright 2018 John Wiley and Sons. | ||
Hydrogels with host and/or guest moieties can form assemblies through supramolecular recognition at the interface between dangling host or guest groups. Of particular interest is the supramolecular assembly of microgels as multifunctional building blocks into macroscopic constructs with biomimetic structures and functionalities for drug release, cell encapsulation, and tissue engineering. In order to synthesize microgels functionalized with host and/or guest groups, four-armed PEG is functionalized on the ends by using thiol click chemistry to bond either β-cyclodextrin or adamantane. Microgels separately synthesized from β-CD- or Ad-functionalized four-arm PEGs self-assemble through reversible guest–host interactions between microgel particles when mixed, generating an interlinked network (Fig. 4b).75 Burdick et al. used a PDMS microfluidic device78 to manufacture cross-linked hyaluronic acid (HA) microgels with covalent cross-linking in modular particles. The microgels modified with cyclodextrin and adamantane formed hydrogels through interparticle guest–host linking.20,79 The two-component microgels show shear-thinning and self-healing, which makes it easy to inject into dynamic environments. The multiple material components and high porosity make the microgels suitable for cell delivery. Besides, methacrylate modified β-cyclodextrin (host) and vinyl ferrocene (guest) formed redox cleavable crosslinkers through host–guest inclusion for the synthesis of microgels with variable content of redox cleavable crosslinkers through precipitation polymerization. The microgel assemblies were used for effective loading and release of anticancer drugs.80
Host–guest supramolecular assemblies are usually responsive to external stimuli, including light, temperature, and redox reactions,76,81–83 primarily depending on the responsive properties of the guests. Such responsiveness is usually reversible and can be utilized for injection, advanced manufacturing, drug delivery, cell encapsulation and delivery, etc.
Self-assembly of soft colloids with light-switchable supramolecular interactions can be triggered to dissociate upon exposure to light. Azobenzene (Azo) functionalized nanogels (the guest colloid) and α-cyclodextrin functionalized microgels (the host colloid) can form stable colloid clusters through supramolecular recognition between the Azo and α-CD moieties dangling on the surfaces of the nanogels.81 When exposed to UV light, the Azo groups experience a transition from trans-conformation to cis-conformation. Since the cis-Azo has a much lower association constant with α-CD than that for trans-Azo/α-CD,84,85 the interface recognition becomes weaker, thus the colloidal clusters disassemble under UV light. Subsequently, when the solution is exposed to visible light, the colloids re-assemble into clusters. The reversible colloidal self-assembly can be controlled by the interplay between the supramolecular and covalent crosslinking, and can also be adjusted by the addition of competitive host molecules. Han et al. incorporate supramolecular assembling and electrostatic interactions between microgels, and investigate the delicate balance between colloidal-range electrostatic repulsion and supramolecular-range host/guest driven attraction in α-CD/azobenzene modified host/guest co-assembling microgel systems.86 By labeling microgels with fluorescent moieties, the authors identified three different states of assembly (disassembled, dispersed co-assembled clusters, co-assembled flocculates). The kinetic balances between the multivalent and cooperative binding of the host/guest complexes and the electrostatic repulsion leads to trapped metastable configurations and reconfiguration into string-like assemblies.
Redox reactions can change the chemical structure and hydrophilicity of guest molecules, which trigger disassembly of host–guest microgels.76 Ferrocene (Fc) is a famous guest molecule that complexes with β-CD through supramolecular recognition (Fig. 4c). Once ferrocene is oxidized into Fc ions, the complex decomposes. Microgels copolymerized from acrylic acid, ferrocene-modified N-(3-aminopropyl) methacryl-amide and N,N′-methylenebisacrylamide (MBAA) are responsive to electrochemical oxidation of the ferrocene groups.82 Such electrochemical responsive Fc-containing microgels may find applications as particle stabilizers for potential-stimulated Pickering emulsions. Microgels are synthesized from cyclodextrin functionalized 8-arm poly(ethylene glycol) (8A PEG-CD) and ferrocene modified counterparts (8A PEG-Fc) via CD/Fc host–guest chemistry to form Pickering emulsions. Taking advantage of the redox reaction of Fc, the formation and deformation of the microgels and corresponding Pickering emulsions can be reversibly triggered by external potential.83 Poly(ionic liquid) (PIL) microgels modified with ferrocene (Fc) are electrochemically active and can respond reversibly to redox stimulus. Fc-Anchored PIL microgels are used as the building blocks for supramolecular self-assembly with a β-cyclodextrin (β-CD) dimer through host–guest inclusion complexation. The assembled PIL microgels could undergo reversible association and dissociation as triggered by electrochemical redox reaction.87 Such supramolecular crosslinking on the basis of Fc-β-CD host–guest interactions is cleavable.80
Supramonomers assembled from reactive host and guest molecules have been developed for the synthesis of supramolecular microgels. Zhang et al.77 devised and assembled tripeptide Phe–Gly–Gly/cucurbit[8]uril (CB[8]) host–guest supra-monomers with one acrylate moiety on each end (Fig. 4d). Then the supramonomers were copolymerized with N-isopropylacrylamide (NIPAm) in aqueous solution to synthesize thermo-responsive supramolecular microgels. Moreover, the microgels exhibited stimuli-responsive and degradable properties benefitting from the dynamic nature of supramonomers.92 Yu et al.31 encapsulated two kinds of guests (viologen and naphthyl) in the cavity of macrocyclic host molecule CB[8] through molecular recognition to form a solid hetero-ternary host–guest supramolecular copolymer to prepare microgels (Fig. 4e).93,94 The interaction between molecules is reversible, so the scaffolds formed by microgels are dynamically stable and self-healing. Inclusion complexes of alkyl pyridinium-containing acrylamide monomers with CB[8] facilitate supramolecular crosslinking in a series of hydrogels formed through in situ polymerization. The resulting hydrogels show greatly enhanced mechanical properties over a purely acrylamide-based gel. Moreover, by selecting fluorescent pyridine moieties, the materials exhibit fluorescence properties for sensing applications.95
3.2 Hydrogen bonding
Hydrogen bonding is prevalent between functional groups with electron donating and accepting capabilities. Upy, for example, is a group that is widely known to establish robust hydrogen bonding through three donor–acceptor pairs to form a dimer. Other functional groups like imidazole, amine, carboxylic acid, and OH groups are recognized as important elements for hydrogen bonding. Although a single hydrogen bond is weak, abundant hydrogen bonds between microgels can provide a high mechanical strength to microgel assemblies.96 DNA base complementation and peptide self-assembly, as ubiquitous hydrogen bond interactions in organisms, have sequence specificity and high programmability, and can precisely regulate hydrogen bond interactions between microgels.97 Qi et al. modified the DNA strands on the specified surfaces of PEGDA microgel cubes to produce an asymmetric glue pattern,33 which induced the assembly of microgel cubes with different structures, such as linear chains, open networks, T-junctions and square structures in aqueous or interface systems (Fig. 5a). Microgels based on complementary nucleobase molecular recognition98 were synthesize by polymerizing a series of bis-thymine end decorated flexible poly(N-isopropyl acrylamide) (T-PNIPAM-T) with a rigid poly[1-(4-vinyl benzyl)] adenine (PSA) (Fig. 5b). The nucleobase complementary pairing resulted in supramolecular cross-linked 3D networks and microgel self-assemblies. Such supramolecular 3D microgels are responsive to thermo- and pH-stimulus.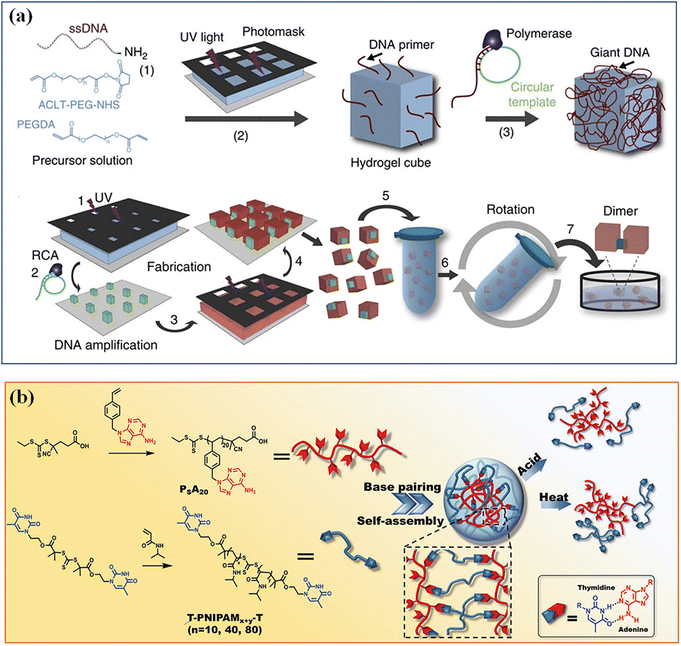 | ||
| Fig. 5 Hydrogen bonding. (a) Hydrogen bond interactions of DNA base complementation on the specified surfaces of PEGDA microgel. Reproduced with permission from ref. 33. Copyright 2013 Springer Nature. (b) Microgels based on complementary nucleobase molecular recognition. Reproduced with permission from ref. 98. Copyright 2022 John Wiley and Sons. | ||
Microgels crosslinked by tannic acid (TA)99 or catechin100 contain abundant hydrogen bonds between the catechol groups and other functional groups. The tannic acid content has a key influence on the microgel properties. The microgels exhibit reversible temperature-dependent swelling/deswelling and undergo pH-triggered degradation in aqueous solutions.101 PNIPAM–TA microgels contain both physical cross-linking via hydrogen bonding between TA and PNIPAM chains and chemical cross-linking via capturing the radicals of propagating polymer chains by catechol and pyrogallol groups of TA (Fig. 6).102 The microgels and hybrids with Fe3O4 nanoparticles are thermoresponsive and degradable dependent on pH values.
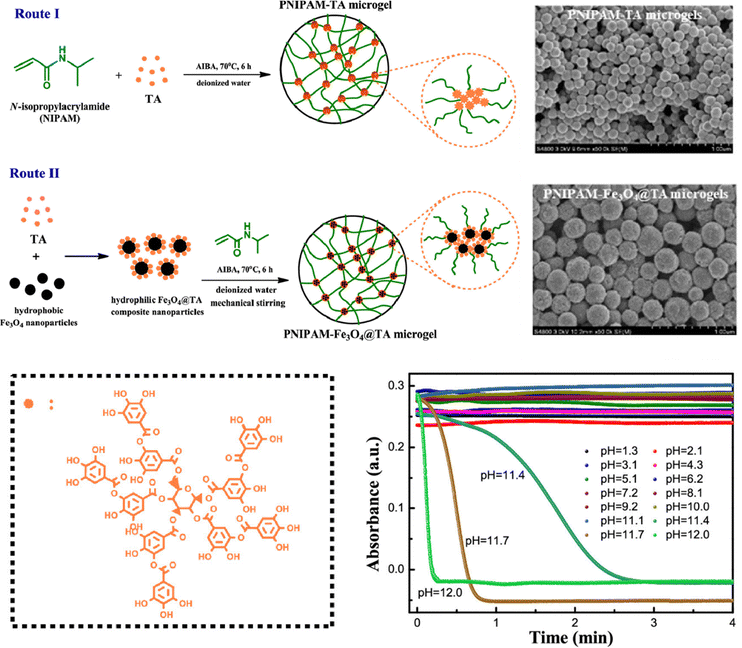 | ||
| Fig. 6 PNIPAM–TA microgels fabricated via hydrogen bonding and the pH-dependent degradation. Reproduced with permission from ref. 102. Copyright 2019 American Chemical Society. | ||
3.3 Metal coordination
Multivalent metal ions with empty orbitals can readily coordinate with ligands with lone pair electrons. Metal coordination has been widely used as non-covalent crosslinking to create or reinforce polymer networks to generate hydrogels with tough, self-healing, and self-recovery properties.103 Most metal/ligand complexes are reversible. Terpyridine (Tpy)-functionalized stimuli-responsive p(NIPAM-co-MAA) microgels can be assembled reversibly with several metal ions (Ni2+, Fe2+, Co2+, or Zn2+) through metal-ligand interactions.104 The assembled Tpy-MG-M2+ showed different rheological properties depending on the metal ion type: the weakly bound ions (Co2+, Zn2+) showed fast dynamics for “inter-particular” exchange, resulting in enhanced storage (G′) and loss (G′′) moduli. By using iron(II)–bis(terpyridine) coordination supramolecular complexes as links between poly(N-isopropylmethacrylamide) (PNiPMAM) chains, cleavable supramolecular microgels are synthesized by dispersion copolymerization.105 Moreover, thermosensitive PNIPAM microgels incorporating terpyridine ligand linkers can serve as a jammed microgel ink for 3D printing that is cured post-printing by iron(II) cations for long-term stabilization. Upon curing, supramolecular iron(II)-bis-terpyridine coordination complexes between neighboring microgels provide sufficient strength and robustness of printed scaffolds to maintain integrity during applications for over two months.106 The metallo-supramolecular assemblies are reversible (Fig. 7a). The [MIITPy2]2+ complexes with metal cation (Fe(II) or Co(II)) as supramolecular links between microgels can break up upon oxidation and thus the connected microgels can disassemble into individual microgel building blocks (Fig. 7b).107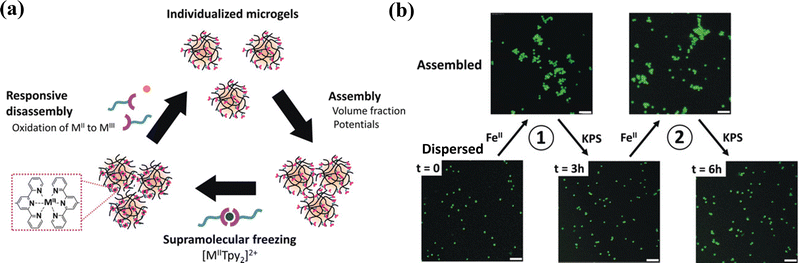 | ||
| Fig. 7 Reversible metallo supramolecular assemblies of the [MIITPy2]2+ complexes with metal cation (Fe(II) or Co(II)). Scale bar: 5 μm. Reproduced with permission from ref. 107. Copyright 2020 John Wiley and Sons. | ||
3.4 Electrostatic interaction
Microgels carrying negative or positive charges can self-assemble into clusters primarily driven by electrostatic attractions at the interface. Microgels can gain either negative charges or positive charges by using anionic or cationic monomers for the synthesis of microgels. Besides, microgels with chargeable functional groups in the network can gain charges in acidic or alkaline conditions. Electrostatic attractions are dynamic, reversible, and responsive to environmental conditions. For example, changes in pH and/or ion strength in solution can significantly strengthen or block the electrostatic interactions between microgels, which induce assembling or disassembling of microgels.Highly anisotropic supramolecular microtubules and soft spherical fluorescent microgels carrying charges self-assembled into various suprastructures. Microgels with positive and negative charges are synthesized by using polystyrene nanoparticles as seeds modified with cationic CTAB and anionic V50 respectively for polymerization with PNIPAM and PNIPMAM. These charged microgels self-assemble with supramolecular bile salt tubules with cationic or anionic charges to form hierarchical self-assembly into various superstructures varying from virus-like assemblies to supracolloidal networks.108
Zwitterionic moieties, comprised of equivalent negative and positive charges linked by a spacer of a few C–C bonds, can form strong ion–dipole and dipole–dipole interactions between polymer chains and microgels. Hydrogels formed from pure zwitterionic polycarboxybetaine (PCB) have been reported to inhibit the foreign body response and resist collagenous encapsulation in vivo,109 and shield proteins from immunogenic responses.110 Moreover, stem cells encapsulated in PCB hydrogels maintain their therapeutic multipotency and avoid nonspecific differentiation.111 Zwitterionic microgels based on carboxybetaine acrylamide monomers are used as self-healing “building blocks” for injectable and malleable PCB hydrogel platform and cell-protective constructs (Fig. 8).112 The microgels are beneficial to evade the foreign body reaction and preserve stem cell multipotency. The non-covalent interactions between microgels enhance the overall self-healing associations (zwitterionic fusion) and increase construct elasticity, with an ideal modulus for cell culture and injection applications.
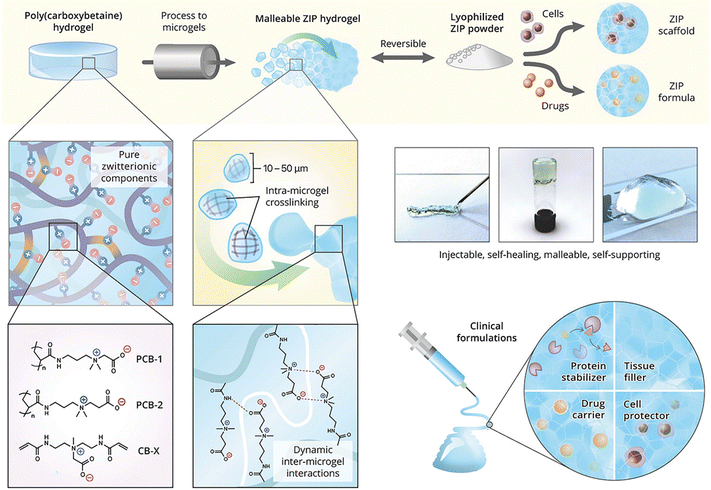 | ||
| Fig. 8 Injectable hydrogels formed by electrostatic and dipole interactions between zwitterionic microgels. Reproduced with permission from ref. 112. Copyright 2018 John Wiley and Sons. | ||
4. 3D printing of supramolecular microgels
As an extremely important additive manufacturing method, 3D printing mainly includes extrusion bioprinting,113,114 digital light processing (DLP) printing115,116 and inkjet printing.117,118 Among these, extrusion 3D bioprinting usually relies on the rheological behaviours, shear thinning, and self-healing capability, and subsequent gelation of ink. Supramolecularly assembled microgels are linked through reversible non-covalent interactions and thus show necessary rheological behaviors for extrusion 3D printing. Such unique rheological properties allow for 3D printing under mild conditions, which are important for fabrication of cell-laden biological constructures. On the other hand, microgel assemblies are used as a supporting bath for 3D printing solutions of biomacromolecules like collagen into engineered scaffolds.4.1 Microgel inks
Microgel assemblies based on supramolecular interactions usually exhibit high viscosity, reversible shear thinning, creep resistance and rapid self-healing behaviors, which are suitable for extrusion-based 3D printing. Microgel assemblies based on interparticle hydrogen bonding or host–guest recognition may break up to flow under shearing/extruding, and then rapidly recover to the gel state once the shearing vanishes after extruding out of the nozzle. Meanwhile, the non-covalent interactions between microgels synergetically provide great resistance against creep, which is important for the fidelity and stability of printed structures.119 Therefore, microgels can be applied as bioinks for 3D printing of constructs with multimodal and functional structure properties. For this purpose, balanced printability, high fidelity, and biocompatibility are highly desired.120 Fu et al. prepared microgels with controllable size by repeatedly extruding and fracturing crosslinked CHMA/PVA hydrogels through nozzles12 (Fig. 8a). The obtained microgels have irregular shapes and abundant hydroxyl groups that can establish numerous hydrogen bonds between hydrogel microparticles, which works with geometric jamming of the microparticles to form extrudable microgel hydrogels. The microparticle hydrogels undergo shear thinning and rapid self-healing into hydrogels after unloading. The microgel hydrogels have good mechanical stability and creep resistance, which provide outstanding printability and fidelity. The microgels have been used as inks to build a variety of bionic structures, which has excellent self-supporting, high aspect ratio and complex structures (Fig. 9b).121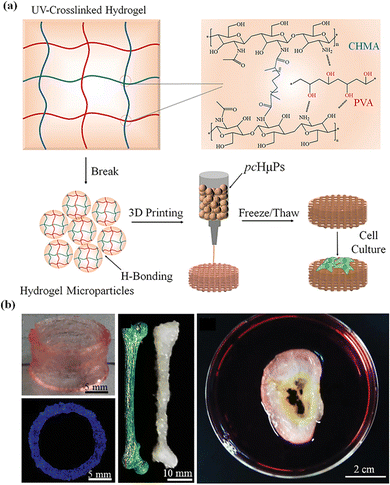 | ||
| Fig. 9 (a) Self-healing pre-cross-linked hydrogel microparticle (pcHμPs) inks; (b) a variety of bionic structures. Reproduced with permission from ref. 12. Copyright 2020 John Wiley and Sons. | ||
Dynamic cross-linking reactions among microgels have been utilized to assemble microgels for extrusion bioprinting with great printability, microporosity, tissue-adhesion, and self-healing.122 Feng et al. proposed a new strategy to prepare dynamic cross-linked microgel assembly (DC-MA) bioink (Fig. 10a). By using a droplet microfluidic device, methacrylate and phenylboronic group modified hyaluronic acid (HAMA-PBA) and methacrylic gelatin (GelMA) were crosslinked to produce microgels, and then the dynamic crosslinker (dopamine modified hyaluronic acid, HA-DA) was assembled into the DC-MA bioink to strengthen interactions between microgels through dynamic covalent bonds while maintaining the relatively low mechanical modulus of microgels in order to achieve high printability, shape-fidelity and cell viability.32 The addition of dynamic crosslinking agent (HA-DA) also improved the microporosity, tissue adhesion and self-healing of DC-MA bioink, which is beneficial for tissue engineering and regenerative medicine applications.
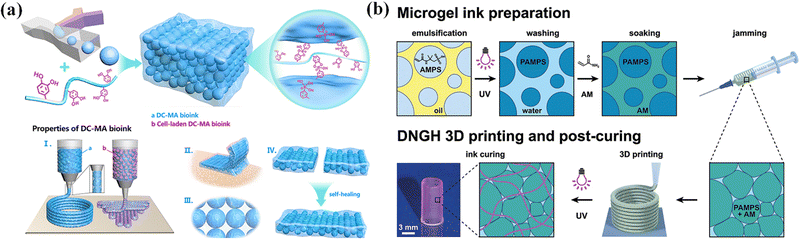 | ||
| Fig. 10 Microgel inks. (a) Dynamic cross-linked microgel assembly (DC-MA) bioink. Reproduced with permission from ref. 32. Copyright 2020 John Wiley and Sons. (b) Double network granular hydrogel (DNGH) inks. Reproduced with permission from ref. 123. Copyright 2022 American Chemical Society. | ||
Microgels can act as sacrificial inks to improve cell infiltration and migration in scaffolds, while maintaining the printability of the microgel inks. Seymour et al. blended UV-crosslinkable gelation methacryloyl (GelMA) microgels with sacrificial gelatin microgels to obtain composite inks high void fraction by optimizing the ratio of GelMA to sacrificial gelatin microgels.27 It was observed that human umbilical vein endothelial cells (HUVEC) seeded onto printed constructs migrated into the microgel inks depending on the void fraction. This strategy has potential for applications in 3D printing and tissue engineering.
Many 3D printing structures by using microgel (JM) bioinks may gradually lose mechanical properties in the long term, in comparison to natural soft tissues,36,124,125 which limits their applications in tissue engineering. To address this problem, microgel particles with a high swelling ratio are dissolved in a second monomer solution to integrate the microgels together to produce microgel-based biphasic (MB) bioinks with both printability and fidelity. Amstad et al. synthesized poly(2-acrylamido-2-methyl-propanesulfonic acid) (PAMPS) microgels by inverse emulsion polymerization, and mixed them with a second monomer solution that is comprised of AAm monomers, initiators, and cross-linkers to create double network granular hydrogels (DNGHs) (Fig. 10b).123 The obtained hydrogels can bear tensile loads up to 1.3 MPa. Zhao et al. prepared various microgels by ball milling, and polymerized the same monomers in the presence of microgels to generate particle-based double-network (P-DN) hydrogels.17 The hydrogels show excellent adhesion and temperature sensitivity. This strategy can be used to print multi-functional hydrogel structures with high mechanical properties and strong adhesion to various materials.
Microgel-based biphasic (MB) bioinks can be combined with different types of cells to form a heterogeneous cell microenvironment in a single printed fiber.126 Zhang et al. swelled sodium alginate microgels or GelMA microgels containing cells into GelMA solution to obtain tightly stacked biological ink127 for printing 3D complex structures including nose, ear, bronchus and brain models with high shape fidelity. In MB bioink, the cell density was increased locally through a space cell pattern, which accelerated cell reorganization and vascularization, and further promoted the functional maturity of printed liver tissue constructs. In general, the MB bioink has mechanical adjustability, hyperelasticity and a heterogeneous microenvironment,128,129 providing new possibilities for 3D bioprinting in biomedical applications such as tissue engineering and soft robots.
4.2 Supporting bath
During conventional extrusion-based 3D printing, the ink is usually layer-by-layer deposited on the substrate and then further cross-linked to improve the shape fidelity and stability of the designed structure, and has therefore limited the selection of ink materials and the construction diversity of complex architectures such as human organs or tissues.130,131 To overcome this shortcoming, bath-supported 3D printing has been applied to the construction of complex structures.132 Different optimized bath materials have been developed to negate the effects of surface tension, gravity, and particle diffusion to support and fix the extruded ink, and firm the printed shapes.133,134 Among these bath materials, the supramolecular assembled microgel bath has a high water content, transparency, and self-healing, and prompts a smooth transition between fluid and solid states, which is an ideal medium for bath-supported 3D printing.135,136 Many studies have shown that there are different yield stress support baths for the embedding bioprinting of hydrogels, including Carbomer, alginate particles, and gelatin microgel, etc.Carbomer is a kind of supramolecular polyacrylic acid (PAA) polymer. The carboxylic groups on the molecular chain can firmly grasp water through hydrogen bonding. It shows a sol to gel transition in aqueous solution as the pH is raised above its pKa (about 5.5).137 Carbomer microgel has been widely used as a 3D printing supporting bath due to its stable, tunable viscoelasticity, and rheological behavior in a biological environment.138,139 Serpooshan et al.140 used Carbomer as the support bath and gelatin-methacryloyl (GelMA) as the 3D printing bioink to create a 3D hydrogel structure with high fidelity and biocompatibility through embedded bioprinting technology, further expanding the application of bioprinting soft tissue construction in various biomedical fields. Huang et al.141 developed a Carbomer microgel supporting material as a bath to support the 3D structure constructed by gelatin-alginate ink, which avoids the instantaneous gelation of each printed layer and nozzle clogging caused by the resultant surface tension. It has been realized as the overall ‘printing-then-gelation’, which is suitable for building a variety of homogeneous soft structures. Angelinl et al.142 generated several complex structures by writing in a Carbomer microgel bath, including a 4 cm-long model of DNA, a thin-shell model and a complex vascular network. It's possible to write and grow living tissue cells in the microgel supporting bath and the jammed microgel provided stability and versatility in a simple framework that can be integrated into a powerful platform in the application of bioprinting soft tissue constructs in various biomedical fields.
The alginate chain is rich in mannuronic acid (M) and glucose uronic acid (G) units. In aqueous solution, the G blocks in different alginate chains can coordinate with multivalent cations (such as Ca2+, Co2+, Cu2+) to form non-covalent crosslinks between alginate chains, thus generating alginate hydrogels.143 As commonly used as a 3D printing supporting bath, alginate microgels show many advantages like cell friendliness, high transparency for realtime monitoring of printing, and can be removed by non-toxic, delicate procedures after printing. Alsberg et al.144 presented a 3D printing strategy in which a bioink rich in stem cells was printed into a support bath comprised of alginate microgels prepared by double crosslinking, oxidation and ionic crosslinking of methacrylate alginate (OMA). The microgel support medium showed similar properties to Bingham plastic fluid, maintaining high-resolution printing of human bone marrow mesenchymal stem cells (hMSCs). Dvir et al.145 used a mixed material comprised of calcium-alginate nanoparticles and xanthan gum as a supporting bath for the printing of extracellular matrix-based biomaterials, allowing high-resolution printing as low as 10 microns to manufacture complex structures and cell structures. It has been proved that the mixed medium can support at least 18 hours of long-time accurate printing. All these characteristics make it a promising supporting medium for 3D printing of tissues and organs. Ozbolat et al.146 studied the feasibility of using human mesenchymal stem cell (hMSC) spheres with an alginate microgel support bath for aspiration-assisted freeform bioprinting (AAfB), which has achieved unprecedented positioning accuracy, and improved the fusion efficiency of bioprinting in alginate microgels with different particle sizes. These studies demonstrated the potential of using alginate microgels as a support bath, which can be used in many different applications, including free bioprinting of spheres, cell loaded hydrogels and unstructured inks, to form viable tissue structures.
Besides, gelatin is a partially hydrolyzed collagen with excellent biocompatibility and degradability, which is widely used in many 3D cell culture, tissue engineering and drug delivery applications. The gelation process of gelatin microgels is thermally reversible.147 Due to the weak interaction between chains, it can automatically transform into a hydrogel at low temperature and reversibly change into a liquid state when heated, which has been widely used in supporting bath materials. For example, Huang et al.148 used the cross-linked composite hydrogel filled with gellan and gelatin microgels with a self-thinning property as the matrix bath to support the fabrication of 2D and 3D fluid network channels to increase the metabolic activity of living cells in the cross-linked matrix. The composite microgel matrix has sufficient mechanical strength to maintain the mechanical integrity of the structure during the removal of sacrificial materials. This method has potential for in vitro and in vivo applications, and may be particularly suitable for chip-based systems. Lee et al.138 proposed a method of 3D biological printing collagen directly by precisely controlling the composition and microstructure (Fig. 11). They used the freeform reversible embedding of suspended hydrogels (FRESH) to design the composition of the human heart at various scales from capillaries to the whole organ in the thermo-reversible gelatin microgel supporting bath, which proved that FRESH v2.0 collagen printing can be used as a platform to build advanced tissue scaffolds for various organ systems.
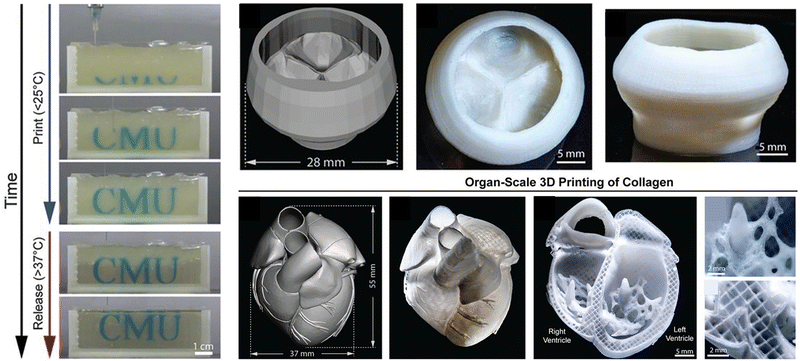 | ||
| Fig. 11 A thermo-reversible gelatin microgel as the supporting bath for FRESH printing. Reproduced with permission from ref. 138. Copyright 2019 The Authors. | ||
5. Applications of supramolecular assemblies of microgels
Microgels have been used as basic units as vehicles to carry drug, factors, and cells, and serve as materials for the fabrication of scaffolds and constructs with biomimetic structures and functionalities. The manufacturing and assembly process of supramolecular microgels is generally fast, biocompatible and non-toxic.149 Due to the dynamic and reversible interactions, supramolecular microgels always show excellent self-healing, injectability, flexibility and biocompatibility, which are crucial for biological and medical applications.40,149 Recent progress in supramolecular microgels for biomedical applications such as cell culture, drug delivery, antibacterial and tissue engineering is summarized in the following sections.5.1 Cell culture
Various strategies have been proposed to culture cells in a 3D environment simulating the microenvironment in vivo, named 3D cell culture, among which the scaffold-based approach has been extensively studied and developed.150 Supramolecular microgel assemblies have been used as scaffolds for 3D cell culture by encapsulating cells inside or inoculating cells on the surface. Microgel assemblies usually have a modulus similar to human tissue. The mechanical and chemical properties can be adjusted as needed to accurately simulate the natural 3D cell microenvironment determined by the ECM with a high water content so as to protect the encapsulated cells from shear force during injection or printing.151,152 In addition, compared with bulk hydrogels, microgels have high porosity and high surface area to mass ratio due to their microscale size, which not only provides enough growth space for cells, but also facilitates rapid nutrient/waste exchange and maintains high cell vitality.19There are many supramolecular biopolymers used to prepare microgels for 3D cell culture. For example, chitosan-based microgels have been applied as microcarriers for cell culture. The water soluble, UV crosslinkable and injectable N-methacryloyl chitosan (N-MAC) was synthesized through a one-step chemically selective N-acylation reaction, which allows for rapid, robust and cost-effective production of patterned cell loaded polysaccharide microgels with unique amino groups, as a building block for cell culture and local and continuous protein delivery.153 PEG-based biocomposite microgels have been widely used in the field of biological applications. The host–guest supramolecular interaction is introduced into the PEG microgel to provide a reversible non-covalent bond and generate a permeable interpenetrating network to form a polyethylene glycol maleimide (PEG-MAL) microgel scaffold.75 The porous structures of the scaffold can enable rapid migration of THP-1 monocytes, which can be used to simulate a natural ECM environment to study the immune cell interaction in the matrix.
Different technologies have been developed to rapidly assemble a heterogeneous 3D cell microenvironment with microgels, including droplet microfluidics, bioprinting, microforming and stop-flow lithography.154 Among them, droplet microfluidics has many advantages, such as accurate diameter control, rapid preparation speed and controllable microsphere structure, which makes the delivery of cells and drugs more flexible, efficient and controllable. It has proved most suitable for continuous high-throughput production of monodisperse spherical microgels.155 For example, Li et al. combined the microdroplet-array-based method with surface-wettability-guided assembly (SWGA) to guide the rapid assembly of heterogeneous 3D cell microenvironment arrays (Fig. 12a).156 This method can accurately control the shape, size, chemical concentration, cell density and 3D spatial distribution of multiple components, which provides a cost-effective solution to meet the growing needs of stem cell research. Besides, the method based on microfluidics was used to encapsulate single cells in the alginate layer of about 6 microns,157 which has increased the proportion of microgels containing cells by 10 times, the encapsulation efficiency exceeding 90%, and helps keep the cells alive in vitro for three days. The intravenous injection of single encapsulated bone marrow stromal cells into mice could maintain the soluble factor provided by the donor in vivo. Single cells encapsulated in tunable microgels can be used in various tissue engineering and regenerative medicine applications (Fig. 12b).
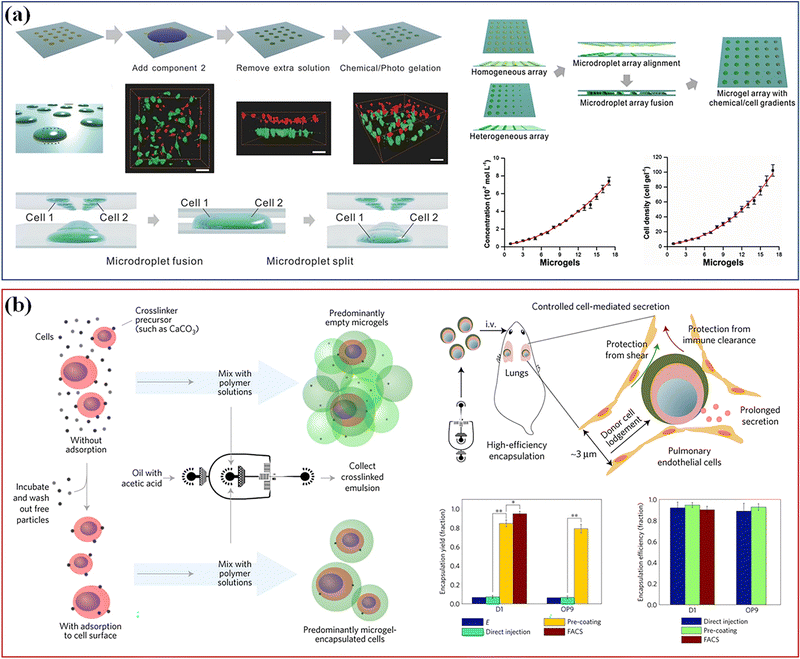 | ||
| Fig. 12 Cell culture. (a) The microdroplet-array-based method with SWGA to guide the assembly of heterogeneous 3D cell microenvironment arrays. Scale bar, 50 μm. Reproduced with permission from ref. 156. Copyright 2016 John Wiley and Sons. (b) A method based on microfluidics used to encapsulate single cells. Reproduced with permission from ref. 157. Copyright 2017 Springer Nature. | ||
5.2 Drug delivery
Compared with chemically crosslinked hydrogels, dynamically crosslinked supramolecular microgels have excellent biocompatibility, biodegradability and a long gelling period. The shear-thinning and self-healing properties of the supramolecular microgel assembly make it injectable and implantable,40 and it can be used as a carrier for local drug delivery in a minimally invasive manner. Moreover, because the microgels have controllable high porosity, when being used to deliver drugs, the drug release rate in vivo can be adjusted by controlling the porosity of microspheres.155 It provides unique advantages for the polymer-based drug delivery system: adjustable size from nanometer to micrometer, large surface area of multivalent biological coupling, and internal network of biological molecule binding.158A wide range of small molecules, proteins and nucleic acid drugs have been encapsulated and released in a controlled manner using supramolecular microgels. Anionic copolymer microgels based on N-isopropylacrylamide and acrylic acid can load a cationic local anesthetic, bupivacaine. The microgel was compounded with an in situ-gelling hydrogel network cross-linked by hydrazide-aldehyde supramolecular chemistry to form hydrogel–microgel soft nanocomposites.159 These nanocomposite hydrogels can provide sustained drug release for up to 60 days, which is significantly longer than the time (<1 week) that can be achieved by using hydrogels or microgels alone. In addition, microgels fabricated via microfluidics are used to encapsulate interleukin-10 (IL-10).8 The degradation and release rate of the microgels can be controlled by the crosslinker. The microgels are injected into a rat model of myocardial infarction (MI), where the released IL-10 reduces macrophage density in 1 week, and improves scar therapy, ejection fraction, cardiac output, and the size of vascular structures in 4 weeks.
The soft nanocomposite hydrogels with long-term local drug delivery have great potential for clinical use. Host–guest supramolecular microgels based on CD have become a promising nanomaterial in biomedical drug delivery applications, benefiting from the complexing ability of CD with a wide range of drugs and hydrophobic molecules.160 Qian et al.161 combined the hydrophobic drug paclitaxel (TAX) and macrocyclic molecule β-CD with the poly-acrylic acid hydrogel skeleton, and then cross-linked it into PAA-β-CD/PAA-TAX nanogels with multiple host–guest contents, which can be used as a new delivery carrier of mucosal adhesive to extend the residence time of TAX in the cervix and reverse the MDR of tumors to improve drug efficiency, and enhance the therapeutic effect of cervical cancer. Si et al.162 designed a supramolecular nanogel for RNase cytoplasmic delivery, and the nanogel was prepared by host–guest interaction between azobenzene (Azo) and β-cyclodextrin (β-CD) conjugated with poly(L-glutamic acid)-graft-poly(ethylene glycol) methyl ether (PLG-g-mPEG). RNase can be loaded into nanogels under mild aqueous conditions. Due to the conformational transformation of azo triggered by UV light, the cross-linking points based on Azo/β-CD recognition were destroyed, which thus ensured the hypoxia sensitive release of nanogels to goods in tumors with NTR overexpression. The hypoxia sensitive supramolecular nanogel provides a universal platform for the delivery of RNase, highlighting its applicability in cancer treatment (Fig. 13a).
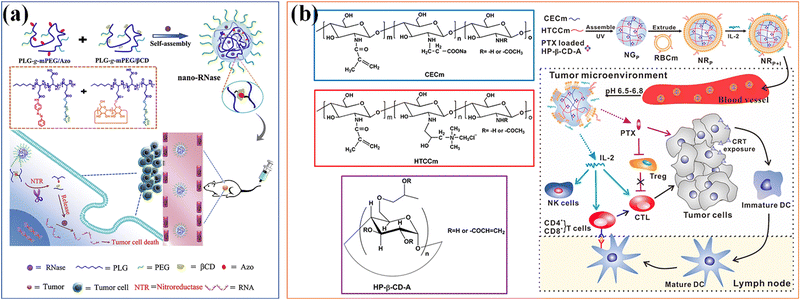 | ||
| Fig. 13 Drug Delivery. (a) A supramolecular nanogel for RNase cytoplasmic delivery. Reproduced with permission from ref. 162. Copyright 2020 Elsevier. (b) A bionic microgel with tumor microenvironment pH response characteristics to achieve controlled release and delivery of paclitaxel (PTX) and IL-2. Reproduced with permission from ref. 163. Copyright 2017 American Chemical Society. | ||
Stimulation responsiveness is another important feature of supramolecular microgel assemblies, which can respond to single or multiple exogenous/endogenous physical or biochemical stimuli (such as: heat, light, magnetic/electric field, mechanical force, pH value, redox potential, etc.).40 Through fine adjustment of the gel–sol phase transition or volume expansion/contraction of microgels, it can be used as intelligent carriers for on-demand drug delivery. According to specific body parts or pathological conditions (such as inflammation), the loaded therapeutic goods can be accurately released in a continuous manner to improve the therapeutic effect. Song and colleagues163 developed a bionic microgel with tumor microenvironment pH response characteristics to achieve co-delivery and controlled release of paclitaxel (PTX) and IL-2 (Fig. 13b). The 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) is compounded with two chitosan derivatives with opposite charges to prepare supramolecular nanocomposite microgels, which are used to embed the anticancer drug paclitaxel, and can precisely control the pH response ability to the weak acidic tumor microenvironment. The RBCm is further coated on the nanogels to enhance the adsorption, protection and delivery of physically and chemically unstable IL-2. The drug released in response can effectively play its role and achieve a promising synergistic anti-tumor effect. Another batch of supramolecular hydrogels used as drug delivery platforms are based on thermal gel polymers with thermal responsiveness.44 Antoniuk et al.164 used electrostatics and host–guest self-assembly to coat positively charged β-cyclodextrin polymers in PNIPAAm microgels to prepare host–guest supramolecular hydrogels through inclusion complexation with adamantane substituted dextran to generate a β-cyclodextrin adamantane (β-CD-Ada) inclusion complex. Due to the synergistic effect between the shrinkage of microgels and the dissociation of β-CD-Ada crosslinks at higher temperatures, the hydrogels exhibit a completely reversible thermally driven gel–sol transition at the temperature range from 37 to 41 °C, opening up attractive prospects for their potential applications in biomedical applications such as stimulation responsive drug delivery.
5.3 Antibacterial
Bacterial infection may lead to serious diseases, including pneumonia, tuberculosis, septicemia, cholera and meningitis, which threaten global human health and are the main causes of death worldwide. Bacterial infection is mainly caused by bacterial biofilm formed by initial bacterial attachment and subsequent bacterial cell proliferation and colonization. In recent years, due to the enhancement of antibiotic resistance, it is urgent to combine antibiotics with other polymers to improve their antibacterial properties or develop new antibacterial drugs to deal with infections in biomedical environments.45,165 Supramolecular microgels can be used as delivery carriers of antibiotics, or directly combined with drugs to form new antibacterial drugs or coatings for application in biological antibacterial products, and have been recognized as a promising method to solve multi drug resistant bacteria.Other than drugs, commonly used antimicrobial agents include quaternary ammonium compounds (QACs),166,167 antimicrobial peptides (AMPs),168–170 and antimicrobial enzymes (AMEs).171,172 Many studies have involved the delivery of antimicrobial agents using supramolecular microgel systems.45,173 Self-assembled polyethylene glycol (PEG) and polyethylene glycol-co-acrylic acid (PEG-AA) microgels can regulate surface cell adhesion on a micron scale comparable to the size of bacteria, and are used for local storage/release of antimicrobial agents to inhibit bacterial colonization on synthetic surfaces.174 Due to electrostatic interactions, the microgels can load cationic antimicrobial peptides (L5). The load in PEG-AA microgels was significantly higher than that in pure PEG microgels. These microgels can be deposited through a nonlinear visual self-assembly process, hinder bacterial colonization, and reduce infection rates associated with biomaterials.
Hydrophilic microgels and nanogels can be utilized to encapsulate and deliver hydrophobic antibacterial agent175 that is urgently needed to tackle antibiotic resistance through nano-injection. Nanogels based on poly(N-isopropylacrylamide-co-N-[3-(dimethylamino) propyl] methacrylamide) and poly(NIPAM-co-DMAPMA), for example, are quaternized with 1-bromododecane to form hydrophobic domains inside the network through self-assembly of aliphatic chains (C12). Triclosan is loaded within the hydrophobic domains serving as containers to load Triclosan inside the nanogels, and the nanogels can adhere to the bacterial cell wall via electrostatic interactions and induce membrane destruction via the insertion of the aliphatic chains into the cell membrane. Thus, Triclosan can be injected into the cell through the destroyed membrane to dramatically increase the effective Triclosan concentration at the bacterial site. As a result, both the minimal inhibitory concentration and minimal bactericidal concentration against the Gram-positive bacteria S. aureus and S. epidermidis are reduced by 3 orders of magnitude, in comparison with free Triclosan. The strategy achieves synergetic physical destruction and active nano-injection, which significantly enhances the antimicrobial efficacy. The designed nanoinjection delivery system shows great promise for combating antimicrobial resistance, and extending applications of hydrophobic drugs delivery for previously challenging applications.
Nanogels with an inherent antibacterial part have been studied.45 The positively charged part causes physical damage to the bacterial cell membrane, which is one of the main antibacterial mechanisms of functional nanogels. The strong charge interaction leads to pore formation and changes in membrane permeability, eventually leading to bacterial cell death.176 Among different cationic compounds for applications to functionalize nanogels, quaternary ammonium salt (QAS) is the most explored. QAS-based PNIPAM (QAS-PNIPAM) microgels177 are synthesized and utilized to modify the surface of a range of materials including metal, plastic, and elastomer. Bacterial culture on QAS-PNIPAM microgel-modified surfaces shows a bactericidal efficiency of nearly 100% (Fig. 14a). The highly efficient bactericidal performance of the QAS-PNIPAM microgel film is attributed to the cationic QAS component. Moreover, the microgel film is robust and cyto-compatible. The cytocompatibility of QAS-PNIPAM microgel thin films can be further improved by modifying with a glycopolymer containing sulfonate groups via attractive electrostatic interactions (Fig. 14b).178 Introduction of the sugar units improves the cytocompatibility of the microgel film without compromising its bactericidal efficacy.
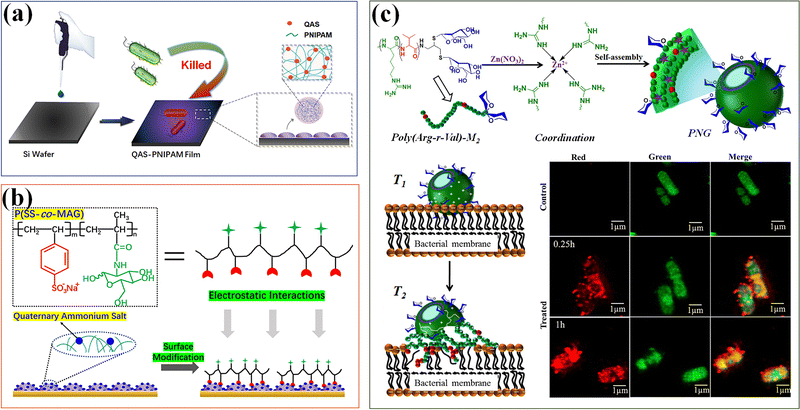 | ||
| Fig. 14 Antibacterial. (a) The antibacterial thin films modified by QAS-based PNIPAM (QAS-PNIPAM) microgels. Reproduced with permission from ref. 177. Copyright 2020 American Chemical Society. (b) Modification of quaternized QPM films with P(SS-co-Mag) via electrostatic interactions has improved the cytocompatibility. Reproduced with permission from ref. 178. Copyright 2020 Elsevier. (c) Polypeptide nanogels (PNGs) with mannose-conjugated AMPs show potential bactericidal effects. Reproduced with permission from ref. 179. Copyright 2019 American Chemical Society. | ||
In addition, AMPs interact with bacteria through non-specific (electrostatic) mechanisms, and have potent antibacterial, anti-inflammatory and other host defense properties.173 A series of polypeptide nanogels (PNGs) have been prepared by using coordination assisted self-assembly of mannose-conjugated AMPs, with poly(arginine)-mannose as the ligand, and Zn2+ ions as a metal ion source, which can minimize the toxicity of the original polypeptide without compromising antimicrobial activity.179 PNG shows potential bactericidal effects on Staphylococcus aureus and Escherichia coli. Compared with the original polypeptide, PNG exhibits higher cell viability (higher than 80%) on mammalian cells (Fig. 14c).
5.4 Tissue engineering
Biotissues and organs are complex systems composed of ECMs, different cells, proteins, and signaling molecules with highly ordered structures. Biofabrication strategies have been developed to engineer 3D tissue models in vitro by mimicking the structure and function of native tissues. The key is to precisely deposit and assemble materials and cells with biomimetic spatiotemporal control.180 Precise control over biophysical and biochemical cues is required to direct cell growth and tissue regeneration.181 Regenerative medicine demands controlled delivery of growth factors and other active substances to promote cell adhesion and guide cell differentiation and tissue formation. Nanogels and microgels could be added to tissue scaffolds for local encapsulation and delivery of bioactive species, modifying their inner architecture, texture and mechanical properties, for regulating cell behavior, and tissue regeneration applications.37 Specifically, nanogels can be fabricated into architectures for regenerating nerve, vessels, cardiac tissue, liver, urothelium and urethral muscle tissues. Multidisciplinary approaches to design nanogels have been employed to meet the diverse needs of regenerative medicine, including active ingredient formulation, controlled release, nanomechanics, tissue engineering and scaffolding with the common purpose of developing clinically relevant tools for the regeneration of complex tissues.Tissue engineering scaffolds play an important role in filling tissue defects, providing mechanical support, promoting cell infiltration, growth and metabolism, achieving the required interactions between cells, releasing encapsulation factors (such as growth factors, chemokines and cytokines) and other functions, so that cells can adhere to biological material scaffolds to form cell-material composites. The compound is implanted into the damaged parts of tissues or organs of the body.182 With gradual degradation and absorption of biomaterials in the body, the implanted cells continue to proliferate and secrete extracellular matrix in vivo, eventually forming the corresponding tissues or organs to achieve the purpose of repairing trauma and rebuilding functions.183 In the past three decades, hydrogel materials have been widely applied in tissue engineering scaffolds for tissue repair and regeneration due to their high-water content, excellent biocompatibility, and biological modulus close to that of tissues and organs, which can be engineered to simulate the natural tissue environment.
Supramolecular microgels are promising for repair and regeneration of different types of tissues and organs due to their highly hydrated nature, tunable microporous structure, ability to encapsulate bioactive factors, and tailorable properties such as stiffness and composition.34 In particular, reversible supramolecular interactions between microgels make the hydrogels injectable and self-healable.184 Supramolecular microgels, as well as their composites with drug, factors, and cells, are ideal candidates for 3D printing ink to fabricate tissue engineering scaffolds with tunable properties to match those of target tissues.36 Microgel-based tissue engineering materials have been recognized for regenerative medicine in the fields of cartilage, cardiac vascular tissue repair, neuron tissue engineering, etc.
Reconstruction of the cartilage defect structure through self-assembly of microgels has a promising application in cartilage tissue engineering and regenerative medicine.185 Microgel assemblies provide a mechanical microenvironment to regulate stem cell chondrogenesis.186 Gelatin/hyaluronic acid hybrid microgels with low, medium and high crosslinking densities are fabricated in microfluidic devices by Michael addition reaction between thiolated gelatin (Gel-SH) and ethylsulfated hyaluronic acid (HA-VS) with different substitution degrees of vinyl sulfone groups. The mouse bone marrow mesenchymal stem cells (BMSC) show a clear trend of differentiating into hyaline cartilage in microgels with a low crosslink density, and fibrocartilage in microgels with medium and high crosslinking densities. Besides, dynamic nanocomposite microgel assemblies are composited with cyclodextrin nanoparticles loaded with kartogenin (KGN) and encapsulate BMSCs via droplet-based microfluidics and photo-crosslinking. The composite hydrogels are bottom-up assembled via dynamic crosslinking between dopamine-modified hyaluronic acid and phenylboronic acid groups on the microgel surface.187 The microgel assemblies can avoid cell endocytosis, ensure high BMSC viability during cell culture, cryopreservation and injection process, and promote chondrogenic differentiation of BMSCs. Injection of the composite hydrogels into articular cartilage defects in animals leads to regeneration of articular cartilage (Fig. 15a). Moreover, injectable hBMSC-laden microgels can be assembled as building blocks into highly ordered tissue-like structures for long-term maintenance and chondrogenesis.188 Cell-laden microgels can be linked by using a 4-arm poly(ethylene glycol)-N-hydroxysuccinimide (NHS) crosslinker into a 3D construct, with the viability and cellular functions of encapsulated hBMSCs well preserved. This assembled microgel construct facilitates upregulation of chondrogenic markers in both gene and glycosaminoglycan (GAG) expression levels, and regeneration of hyaline-like cartilage and high content of type II collagen.
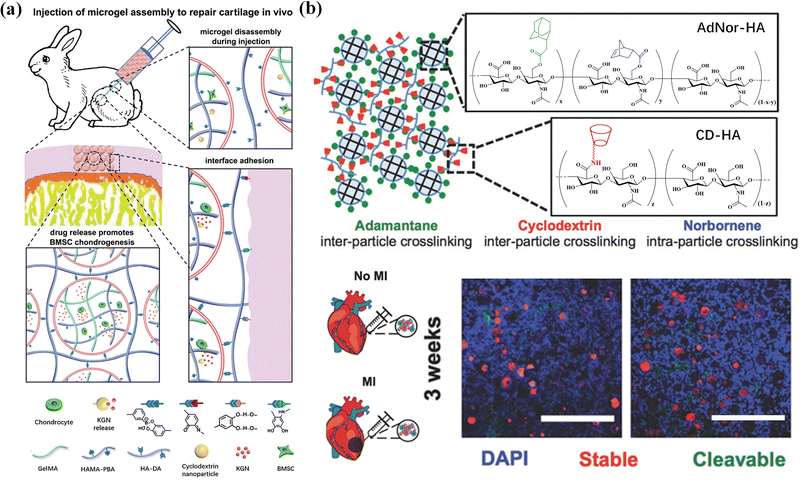 | ||
| Fig. 15 Tissue engineering. (a) BMSC-laden microgel assembly can repair cartilage in vivo. Reproduced with permission from ref. 187. Copyright 2021 John Wiley and Sons. (b) A two-component HA microgel assembly applied in cardiovascular tissue repair. Scale bar = 500 μm. Reproduced with permission from ref. 20. Copyright 2018 John Wiley and Sons. | ||
Cardiac vascular tissue repair aims at cardiac regeneration and vascular repair after injury or myocardial infarction. As a cardiovascular scaffold, microgels can effectively retain and/or deliver cells to affected tissues.189 Mealy et al.20 developed a two-component HA microgel assembly containing protease-cleavable and stable microgels through host–guest interaction, showing shear thinning and self-healing properties, making it easy to inject. More importantly, after injection into the myocardial wall of male Wistar rats with myocardial infarction, the lysable microgels showed significantly accelerated degradation due to the high protease activity under myocardial infarction conditions, while the stable microgels still existed in an undegraded spherical form. The composite properties and high porosity of the two-component granular hydrogel provide unique disease-dependent behavior and high-level cell invasion after injection into myocardial tissue (Fig. 15b). In addition, heparin-modified Pluronic F127 supramolecular nanogels containing basic fibroblast growth factor and VEGF165pDNA introduce proteins and genes into EPCs to promote neovascularization in animal models of limb ischemia,190 effectively promoting the vascularization of female BALB/c mice with femoral artery resection, and form artificial blood vessels.
Nerve degeneration, scar formation and loss of communication between neurons and cells are the main problems of nerve injury. Various neural tissue engineering methods based on supramolecular microgels have been developed to treat nerve injury.191 Hsu et al.192 propose an adaptive microporous hydrogel (AMH), in which high, medium and low concentrations of neuronal growth factor are loaded into negatively charged GelMA microgels, and then mixed with an equal volume of positively charged chitosan methacrylate microgels. The interconnected injectable porous scaffold constructed by electrostatic interaction has suitable micropores to promote cell migration, provides mechanical support, and transports biomolecular clues to manage cell adhesion and growth. Chitosan degradation products have been shown to contribute to peripheral nerve regeneration, inducing a significant bridging effect (axon growth of 4.7 mm) on peripheral nerve defects in SD rats (Fig. 16a). Zhao et al.193 developed a bifunctional microgel to simultaneously reduce the concentration of Ca2+ and glutamate in the extracellular environment and inhibit excessive Ca2+ influx. The microgels are formed by hydrogen bonding between dextran and loaded Ω-conotoxin GVIA (DexGVIA). The DexGVIA significantly accelerates the recovery of motor function in spinal cord injury rats by reducing the injury cavity, protecting neurons and glial cells from injury, improving the continuity and integrity of the spinal cord, and promoting nerve tissue regeneration.
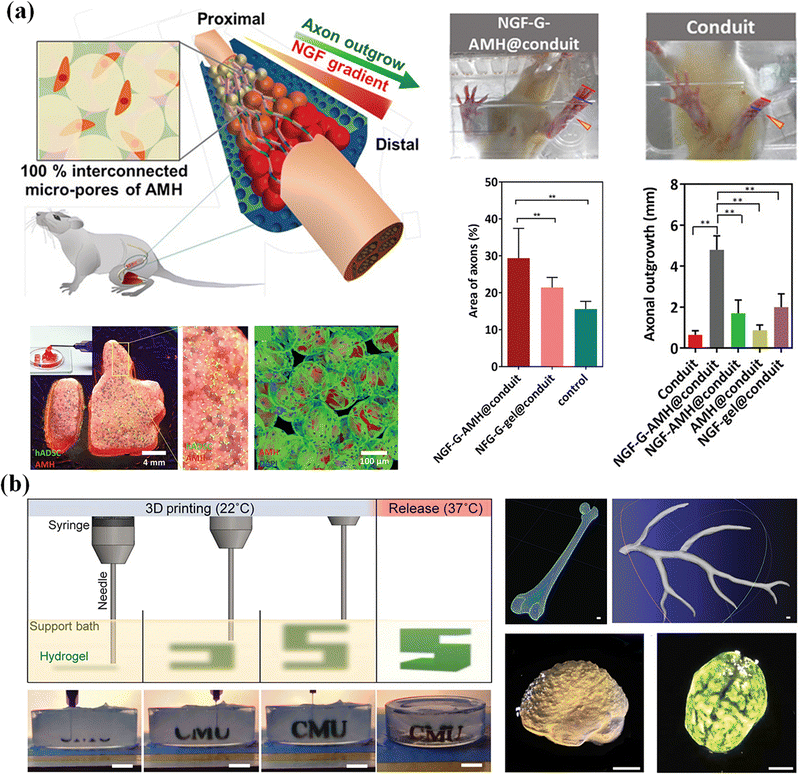 | ||
| Fig. 16 Tissue engineering. (a) An adaptive microporous hydrogel (AMH) has been developed to treat nerve injury and nerve regeneration. Reproduced with permission from ref. 192. Copyright 2019 John Wiley and Sons. (b) The cell-laden OMA microgels are applied in the construction of artificial tissues and organs. Scale bar, 1 cm. Reproduced with permission from ref. 194. Copyright 2019 John Wiley and Sons. | ||
Human organs are composed of complex tissues and have gradient characteristics on various length scales, which is the basis of morphology and function. Microgels have been widely used as building blocks of 3D printed artificial tissues and organs due to their adjustable physical properties, cell adhesion and printability.195,196 So far, many studies have proved the application of cell-laden microgels in the construction of artificial tissues and organs.197 Dual-crosslinkable alginate microgels comprised of oxidized and methacrylated alginate (OMA) are synthesized to encapsulate cells. The cell-laden OMA microgels are directly assembled into well-defined 3D shapes and structures under low-level ultraviolet light. The stem cell-laden OMA microgels can be successfully cryopreserved for long-term storage and on-demand applications (Fig. 16b). The recovered encapsulated cells maintained equivalent viability and functionality to the freshly processed stem cells. The cell-laden microgels can be assembled into complicated 3D tissue structures via freeform reversible embedding of suspended hydrogel (FRESH) 3D bioprinting.194 Then, the second generation of FRESH v2.0 3D bioprinting technology was developed to design the tissue composition of the human heart on multiple length scales.138 Through the use of collagen198 a tri-leaflet heart valve 28 mm in diameter and a human heart chamber of newborn scale were printed,199 and it has proved that FRESH v2.0 collagen printing can be used as a platform to build advanced tissue scaffolds for various organ systems. This bottom-up strategy provides a powerful and highly scalable tool for fabrication of customized and biomimetic 3D tissue constructs.200
6. Conclusions and perspective
In summary, supramolecular microgel assemblies based on non-covalent physical interactions, including host–guest interactions, hydrogen bonding, metal coordination and electrostatic interaction, etc., have great potential for biomedical applications. The alternative integration of bioactive microgels with other functional substances can be applied to drug delivery and release, regenerative medicine, and antibacterial applications, etc. Due to their dynamic self-thinning and self-healing, supramolecular microgels show injection and designability, and can be used as 3D printing bioinks or a supporting bath to fabricate tissue engineering scaffolds and artificial organs. The micron size and macro porosity of microgels are beneficial for cell migration and drug diffusion, which is conducive to cell culture, drug delivery and tissue regeneration, which show huge prospects in the biomedical field.Despite many excellent properties of supramolecular microgel assemblies, there are still many challenges and unmet demands that remain to be addressed. First, massive production of multifunctional microgels is desired to fabricate macro 3D structures by using microgels as building blocks. However, most current manufacturing methods are difficult to simultaneously achieve both high yield and size uniformity of microgels. It is necessary to develop new methods or improve existing technologies to realize high-throughput production, long-term storage and transportation of cell-encapsulated microgels. Second, the degradation rate is preferred to match the regeneration rhythm of tissue regeneration. Besides, the cytotoxicity of degradation by-products may raise problems for applications of supramolecular microgels in cell culture and drug delivery, including possible host immune reactions. It is very important to explore non-toxic, controllable degradation functional microgels for targeted drug delivery through drug matrix affinity supramolecular design.
In addition, supramolecular microgels composed of natural biomaterials are usually cross-linked by non-covalent interactions, and have weak mechanical properties that usually affect the structural integrity of engineering structures. Synthetic biomaterials can provide higher mechanical strength and structural integrity, and become an appropriate choice for load-bearing tissues and nerve conduits. It is necessary to build hybrid microgel tissue engineering scaffolds by combining the advantages of natural and synthetic entities in a single construct. A second network can be incorporated during post-processing to form an interpenetrating network to further stabilize the structures. Moreover, the directional assembly of microgels into arbitrary 3D structures is still in its infancy. Using biomaterials with biocompatibility, heterogeneity and versatility to integrate various biophysical/biochemical clues into the microgels or use signal factors secreted by encapsulated cells or peripheral tissue cells may help promote the spatiotemporal assembly of microgels in vivo. In general, great opportunities and challenges need extensive studies and exploration to broaden the biological applications of supramolecular microgel assemblies for forefront research and clinical applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Natural Science Foundation of China (51873224), and the Ministry of Industry and Information Technology of China (TC190H3ZV/1).References
- J. P. Newsom, K. A. Payne and M. D. Krebs, Acta Biomater., 2019, 88, 32–41 CrossRef CAS PubMed
.
- X. Tong and F. Yang, Adv. Healthcare Mater., 2018, 7, 1701065 CrossRef PubMed
.
- A. Khademhosseini and R. Langer, Nat. Protoc., 2016, 11, 1775–1781 CrossRef CAS PubMed
.
- X. Tong and F. Yang, Adv. Healthcare Mater., 2018, 7, 1701065 CrossRef PubMed
.
- Y. Kittel, A. J. C. Kuehne and L. De Laporte, Adv. Healthcare Mater., 2022, 11, 2101989 CrossRef CAS PubMed
.
- D. Xia, P. Wang, X. Ji, N. M. Khashab, J. L. Sessler and F. Huang, Chem. Rev., 2020, 120, 6070–6123 CrossRef CAS PubMed
.
- C. L. Hedegaard and A. Mata, Biofabrication, 2020, 12, 032002 CrossRef CAS PubMed
.
- M. H. Chen, J. J. Chung, J. E. Mealy, S. Zaman, E. C. Li, M. F. Arisi, P. Atluri and J. A. Burdick, Macromol. Biosci., 2019, 19, 1800248 CrossRef PubMed
.
- A. C. Daly, L. Riley, T. Segura and J. A. Burdick, Nat. Rev. Mater., 2020, 5, 20–43 CrossRef CAS PubMed
.
- T. Farjami and A. Madadlou, Food Hydrocolloids, 2017, 62, 262–272 CrossRef CAS
.
- A. Sinclair, M. B. O'Kelly, T. Bai, H. C. Hung, P. Jain and S. Jiang, Adv. Mater., 2018, 30, 1803087 CrossRef PubMed
.
- H. Zhang, Y. Cong, A. R. Osi, Y. Zhou, F. Huang, R. P. Zaccaria, J. Chen, R. Wang and J. Fu, Adv. Funct. Mater., 2020, 30, 1910573 CrossRef CAS
.
- T. J. Hinton, Q. Jallerat, R. N. Palchesko, J. H. Park, M. S. Grodzicki, M. H. R. H.-J. Shue, A. R. Hudson and A. W. Feinberg, Sci. Adv., 2015, 1, 1–10 Search PubMed
.
- F. A. Plamper and W. Richtering, Acc. Chem. Res., 2017, 50, 131–140 CrossRef CAS PubMed
.
- J. Xue, Z. Zhang, J. Nie and B. Du, Macromolecules, 2017, 50, 5285–5292 CrossRef CAS
.
- A. S. Caldwell, G. T. Campbell, K. M. T. Shekiro and K. S. Anseth, Adv. Healthcare Mater., 2017, 6, 1700254 CrossRef PubMed
.
- D. Zhao, Y. Liu, B. Liu, Z. Chen, G. Nian, S. Qu and W. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 13714–13723 CrossRef CAS PubMed
.
- Y. Xu, R. P. B. Jacquat, Y. Shen, D. Vigolo, D. Morse, S. Zhang and T. P. J. Knowles, Small, 2020, 16, 2000432 CrossRef CAS PubMed
.
- J. M. de Rutte, J. Koh and D. Di Carlo, Adv. Funct. Mater., 2019, 29, 1900071 CrossRef
.
- J. E. Mealy, J. J. Chung, H. H. Jeong, D. Issadore, D. Lee, P. Atluri and J. A. Burdick, Adv. Mater., 2018, 30, 1705912 CrossRef PubMed
.
- L. R. Nih, E. Sideris, S. T. Carmichael and T. Segura, Adv. Mater., 2017, 29, 1606471 CrossRef PubMed
.
- S. Jung and H. Yi, Langmuir, 2012, 28, 17061–17070 CrossRef CAS PubMed
.
- J.-Y. Leong, W.-H. Lam, K.-W. Ho, W.-P. Voo, M.-F. X. Lee, H.-P. Lim, S.-L. Lim, B.-T. Tey, D. Poncelet and E. S. Chan, Particuology, 2016, 24, 44–60 CrossRef CAS
.
- J. E. Meiring, M. J. Schmid, S. M. Grayson, B. M. Rathsack, D. M. Johnson, R. Kirby, R. Kannappan, K. Manthiram, B. Hsia, Z. L. Hogan, A. D. Ellington, M. V. Pishko and C. G. Willson, Chem. Mater., 2004, 16, 5574–5580 CrossRef CAS
.
- K. Song, D. Zhang, J. Yin and Y. Huang, Addit. Manuf., 2021, 41, 101963 CAS
.
- Y. Zhang, S. T. Ellison, S. Duraivel, C. D. Morley, C. R. Taylor and T. E. Angelini, Bioprinting, 2021, 21, 00121 CrossRef
.
- A. J. Seymour, S. Shin and S. C. Heilshorn, Adv. Healthcare Mater., 2021, 10, 2100644 CrossRef CAS PubMed
.
- D. R. Griffin, W. M. Weaver, P. O. Scumpia, D. Di Carlo and T. Segura, Nat. Mater., 2015, 14, 737–744 CrossRef CAS PubMed
.
- H. Li, X. Li, P. Jain, H. Peng, K. Rahimi, S. Singh and A. Pich, Biomacromolecules, 2020, 21, 4933–4944 CrossRef CAS PubMed
.
- K. Song, B. Ren, Y. Zhai, W. Chai and Y. Huang, Biofabrication, 2021, 14, 015014 CrossRef PubMed
.
- Z. Yu, J. Liu, C. S. Y. Tan, O. A. Scherman and C. Abell, Angew. Chem., Int. Ed., 2018, 57, 3079–3083 CrossRef CAS PubMed
.
- F. L. C. Morgan, L. Moroni and M. B. Baker, Adv. Healthcare Mater., 2020, 9, 1901798 CrossRef CAS PubMed
.
- H. Qi, M. Ghodousi, Y. Du, C. Grun, H. Bae, P. Yin and A. Khademhosseini, Nat. Commun., 2013, 4, 2275 CrossRef PubMed
.
- K. Wang, Z. Wang, H. Hu and C. Gao, Supramol. Mater., 2022, 1, 100006 Search PubMed
.
- J. Xu, Q. Feng, S. Lin, W. Yuan, R. Li, J. Li, K. Wei, X. Chen, K. Zhang, Y. Yang, T. Wu, B. Wang, M. Zhu, R. Guo, G. Li and L. Bian, Biomaterials, 2019, 210, 51–61 CrossRef CAS PubMed
.
- Q. Feng, D. Li, Q. Li, X. Cao and H. Dong, Bioact. Mater., 2022, 9, 105–119 CrossRef CAS PubMed
.
- M. A. Grimaudo, A. Concheiro and C. Alvarez-Lorenzo, J. Controlled Release, 2019, 313, 148–160 CrossRef CAS PubMed
.
- M.-C. Tatry, P. Galanopoulo, L. Waldmann, V. Lapeyre, P. Garrigue, V. Schmitt and V. Ravaine, J. Colloid Interface Sci., 2021, 589, 96–109 CrossRef CAS PubMed
.
- K. Marcisz, E. Zabost and M. Karbarz, Appl. Mater. Today, 2022, 29, 101656 CrossRef
.
- S. Wang, P. J. Ong, S. Liu, W. Thitsartarn, M. J. B. H. Tan, A. Suwardi, Q. Zhu and X. J. Loh, Chem. – Asian J., 2022, 17, 202200608 Search PubMed
.
- N. Mitrousis, A. Fokina and M. S. Shoichet, Nat. Rev. Mater., 2018, 3, 441–456 CrossRef CAS
.
- M. Dirksen, C. Dargel, L. Meier, T. Brändel and T. Hellweg, Colloid Polym. Sci., 2020, 298, 505–518 CrossRef CAS
.
- C. A. Dreiss, Curr. Opin. Colloid Interface Sci., 2020, 48, 1–17 CrossRef CAS
.
- S. Bernhard and M. W. Tibbitt, Adv. Drug Delivery Rev., 2021, 171, 240–256 CrossRef CAS PubMed
.
- D. Keskin, G. Zu, A. M. Forson, L. Tromp, J. Sjollema and P. van Rijn, Bioact. Mater., 2021, 6, 3634–3657 CrossRef CAS PubMed
.
- M. Nöth, E. Gau, F. Jung, M. D. Davari, I. El-Awaad, A. Pich and U. Schwaneberg, Green Chem., 2020, 22, 8183–8209 RSC
.
- A. C. Daly, L. Riley, T. Segura and J. A. Burdick, Nat. Rev. Mater., 2020, 5, 20–43 CrossRef CAS PubMed
.
- D. J. McClements, Adv. Colloid Interface Sci., 2017, 240, 31–59 CrossRef CAS PubMed
.
- H. M. Shewan and J. R. Stokes, J. Food Eng., 2013, 119, 781–792 CrossRef CAS
.
- C. D. V. D. Kuckling, H.-J. P. Adler, A. Volkel and H. Colfen, Macromolecules, 2006, 39, 1585–1591 CrossRef
.
- M.-R. Sung, H. Xiao, E. A. Decker and D. J. McClements, J. Food Eng., 2015, 155, 16–21 CrossRef CAS
.
- S. M. Desmarais, H. P. Haagsman and A. E. Barron, Electrophoresis, 2012, 33, 2639–2649 CrossRef CAS PubMed
.
- M. Bjornmalm, Y. Yan and F. Caruso, J. Controlled Release, 2014, 190, 139–149 CrossRef CAS PubMed
.
- G. Prakash, A. Shokr, N. Willemen, S. M. Bashir, S. R. Shin and S. Hassan, Adv. Drug Delivery Rev., 2022, 184, 114197 CrossRef CAS PubMed
.
- S. Bazban-Shotorbani, E. Dashtimoghadam, A. Karkhaneh, M. M. Hasani-Sadrabadi and K. I. Jacob, Langmuir, 2016, 32, 4996–5003 CrossRef CAS PubMed
.
- S. Utech, R. Prodanovic, A. S. Mao, R. Ostafe, D. J. Mooney and D. A. Weitz, Adv. Healthcare Mater., 2015, 4, 1628–1633 CrossRef CAS PubMed
.
- A. G. Hati, T. R. Szymborski, M. Steinacher and E. Amstad, Lab Chip, 2018, 18, 648–654 RSC
.
- J. Koh, D. R. Griffin, M. M. Archang, A. C. Feng, T. Horn, M. Margolis, D. Zalazar, T. Segura, P. O. Scumpia and D. Di Carlo, Small, 2019, 15, 35 Search PubMed
.
- S. Yadavali, H. H. Jeong, D. Lee and D. Issadore, Nat. Commun., 2018, 9, 1222 CrossRef PubMed
.
- T. J. Merkel, K. P. Herlihy, J. Nunes, R. M. Orgel, J. P. Rolland and J. M. DeSimone, Langmuir, 2010, 26, 13086–13096 CrossRef CAS PubMed
.
- H. Z. Stanislav Dubinsky, Z. Nie, I. Gourevich, D. Voicu, M. Deetz and E. Kumacheva, Macromolecules, 2008, 41, 3555–3561 CrossRef
.
- M. E. Helgeson, S. C. Chapin and P. S. Doyle, Curr. Opin. Colloid Interface Sci., 2011, 16, 106–117 CrossRef CAS PubMed
.
- C. Qiu, M. Chen, H. Yan and H. Wu, Adv. Mater., 2007, 19, 1603–1607 CrossRef CAS
.
- A. Harada, Y. Takashima and M. Nakahata, Acc. Chem. Res., 2014, 47, 2128–2140 CrossRef CAS PubMed
.
- E. A. Appel, M. W. Tibbitt, M. J. Webber, B. A. Mattix, O. Veiseh and R. Langer, Nat. Commun., 2015, 6, 6295 CrossRef CAS PubMed
.
- N. G. Hădărugă, G. N. Bandur, I. David and D. I. Hădărugă, Environ. Chem. Lett., 2018, 17, 349–373 CrossRef
.
- D. Harries, D. C. Rau and V. A. Parsegian, J. Am. Chem. Soc., 2005, 127, 2184–2190 CrossRef CAS PubMed
.
- E.-S. Kwak and E. A. Gomez, Chromatographia, 1996, 43, 659–662 CrossRef CAS
.
- W. C. Cromwell, K. Bystróm and M. R. Eftink, J. Phys. Chem., 1985, 89, 326–332 CrossRef CAS
.
- A. Kasprzak, M. Koszytkowska-Stawinska, A. M. Nowicka, W. Buchowicz and M. Poplawska, J. Org. Chem., 2019, 84, 15900–15914 CrossRef CAS PubMed
.
- V. Kolivoška, M. Gál, M. Hromadová, M. Valášek and L. Pospíšil, J. Org. Chem., 2011, 696, 1404–1408 CrossRef
.
- D. Granadero, J. Bordello, M. J. Pérez-Alvite, M. Novo and W. Al-Soufi, Int. J. Mol. Sci., 2010, 11, 173–188 CrossRef CAS PubMed
.
- S. Xian and M. J. Webber, J. Mater. Chem. B, 2020, 8, 9197–9211 RSC
.
- H. Yan, Q. Jiang, J. Wang, S. Cao, Y. Qiu, H. Wang, Y. Liao and X. Xie, Polymer, 2021, 221, 123617 CrossRef CAS
.
- A. E. Widener, M. Bhatta, T. E. Angelini and E. A. Phelps, Biomater. Sci., 2021, 9, 2480–2493 RSC
.
- X. Liu, L. Zhao, F. Liu, D. Astruc and H. Gu, Coord. Chem. Rev., 2020, 419, 213406 CrossRef CAS
.
- W. Xu, Q. Song, J.-F. Xu, M. J. Serpe and X. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 11368–11372 CrossRef CAS PubMed
.
- H. H. Jeong, V. R. Yelleswarapu, S. Yadavali, D. Issadore and D. Lee, Lab Chip, 2015, 15, 4387–4392 RSC
.
- C. B. Rodell, J. W. MacArthur, S. M. Dorsey, R. J. Wade, L. L. Wang, Y. J. Woo and J. A. Burdick, Adv. Funct. Mater., 2015, 25, 636–644 CrossRef CAS PubMed
.
- S.-H. Jung, S. Schneider, F. Plamper and A. Pich, Macromolecules, 2020, 53, 1043–1053 CrossRef CAS
.
- P. Wang, Q. Yang, Z. Ye, C. Zhao and J. Yang, Macromol. Chem. Phys., 2017, 218, 1700280 CrossRef
.
- K. Marcisz, D. Jagleniec, M. Mackiewicz, J. Romanski and M. Karbarz, Mater. Today Chem., 2022, 26, 101151 CrossRef CAS
.
- L. Peng, A. Feng, S. Liu, M. Huo, T. Fang, K. Wang, Y. Wei, X. Wang and J. Yuan, ACS Appl. Mater. Interfaces, 2016, 8, 29203–29207 CrossRef CAS PubMed
.
- D. Taura, S. Li, A. Hashidzume and A. Harada, Macromolecules, 2010, 43, 1706–1713 CrossRef CAS
.
- Y.-L. Lin, S. Zheng, C.-W. Chang, M.-J. Lee, Y.-F. Chen and J.-T. Chen, Macromolecules, 2022, 55, 8940–8949 CrossRef CAS
.
- K. Han, D. Go, D. Hoenders, A. J. C. Kuehne and A. Walther, ACS Macro Lett., 2017, 6, 310–314 CrossRef CAS PubMed
.
- Y. Tang, P. Cao, W. Li, M. He, Z. Dai and Y. Xiong, Polymer, 2021, 220, 123575 CrossRef CAS
.
- Y. Liu, H. Yang, Z. Wang and X. Zhang, Chem. – Asian J., 2013, 8, 1626–1632 CrossRef CAS PubMed
.
- A. C. B. J. Mohanty, W. M. Nau and H. Pal, J. Phys. Chem. B, 2006, 110, 5133 Search PubMed
.
- I. Ghosh and W. M. Nau, Adv. Drug Delivery Rev., 2012, 64, 764–783 CrossRef CAS PubMed
.
- H. Zou, J. Liu, Y. Li, X. Li and X. Wang, Small, 2018, 14, 1802234 CrossRef PubMed
.
- Q. Song, Y. Gao, J. F. Xu, B. Qin, M. J. Serpe and X. Zhang, ACS Macro Lett., 2016, 5, 1084–1088 CrossRef CAS PubMed
.
- Y. Zheng, Z. Yu, R. M. Parker, Y. Wu, C. Abell and O. A. Scherman, Nat. Commun., 2014, 5, 5772 CrossRef CAS PubMed
.
- J. Liu, C. S. Y. Tan, Z. Yu, N. Li, C. Abell and O. A. Scherman, Adv. Mater., 2017, 29, 1605325 CrossRef PubMed
.
- D. J. Whitaker, Z. Huang, B. W. Longbottom, R. L. Sala, G. Wu and O. A. Scherman, Polym. Chem., 2021, 12, 519–525 RSC
.
- H. He, X. Cao, H. Dong, T. Ma and G. F. Payne, Adv. Funct. Mater., 2017, 27, 1605665 CrossRef
.
- A. S. Caldwell, B. A. Aguado and K. S. Anseth, Adv. Funct. Mater., 2020, 30, 1907670 CrossRef CAS PubMed
.
- J. Pan, X. Wen, M. Wang, J. Li, X. Li, A. Feng, L. Zhang and S. H. Thang, Macromol. Rapid Commun., 2022, 43, 2200239 CrossRef CAS PubMed
.
- C. M. López and A. Pich, Macromol. Rapid Commun., 2018, 39, 1700808 CrossRef PubMed
.
- E. Izak-Nau, S. Braun, A. Pich and R. Göstl, Adv. Sci., 2022, 9, 2104004 CrossRef CAS PubMed
.
- C. Molano-López, S. Braun, M. Kather, A. Töpel, G. van Wissen and A. Pich, Macromol. Chem. Phys., 2022, 224, 2200213 CrossRef
.
- J. Xue, W. Ji, S. Dong, Z. Zhang, J. Gao, P. Yang, J. Nie and B. Du, Langmuir, 2019, 35, 16353–16365 CrossRef CAS PubMed
.
- H. Ding, X. Liang, X. N. Zhang, Z. L. Wu, Z. Li and G. Sun, Polymer, 2019, 171, 201–210 CrossRef CAS
.
- J. Lee, E. J. Choi, I. Varga, P. M. Claesson, S. H. Yun and C. Song, Polym. Chem., 2018, 9, 1032–1039 RSC
.
- J. Es Sayed, C. Meyer, N. Sanson and P. Perrin, ACS Macro Lett., 2020, 9, 1040–1045 CrossRef CAS PubMed
.
- J. Es Sayed, M. Khoonkari, M. Oggioni, P. Perrin, N. Sanson, M. Kamperman and M. K. Włodarczyk-Biegun, Adv. Funct. Mater., 2022, 32, 2207816 CrossRef CAS
.
- J. Es Sayed, C. Lorthioir, P. Banet, P. Perrin and N. Sanson, Angew. Chem., Int. Ed., 2020, 59, 7042–7048 CrossRef CAS PubMed
.
- J. Cautela, V. Lattanzi, L. K. Månsson, L. Galantini and J. J. Crassous, Small, 2018, 14, 1803215 CrossRef PubMed
.
- L. Zhang, Z. Cao, T. Bai, L. Carr, J.-R. Ella-Menye, C. Irvin, B. D. Ratner and S. Jiang, Nat. Biotechnol., 2013, 31, 553–556 CrossRef CAS PubMed
.
- P. Zhang, F. Sun, C. Tsao, S. Liu, P. Jain, A. Sinclair, H.-C. Hung, T. Bai, K. Wu and S. Jiang, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 12046–12051 CrossRef CAS PubMed
.
- T. Bai, F. Sun, L. Zhang, A. Sinclair, S. Liu, J.-R. Ella-Menye, Y. Zheng and S. Jiang, Angew. Chem., Int. Ed., 2014, 53, 12729–12734 CrossRef CAS PubMed
.
- A. Sinclair, M. B. O'Kelly, T. Bai, H.-C. Hung, P. Jain and S. Jiang, Adv. Mater., 2018, 30, 1803087 CrossRef PubMed
.
- Z. Fu, L. Ouyang, R. Xu, Y. Yang and W. Sun, Mater. Today, 2022, 52, 112–132 CrossRef CAS
.
- D. Chimene, R. Kaunas and A. K. Gaharwar, Adv. Mater., 2020, 32, 1902026 CrossRef CAS PubMed
.
- M. Dong, Y. Han, X. P. Hao, H. C. Yu, J. Yin, M. Du, Q. Zheng and Z. L. Wu, Adv. Mater., 2022, 34, 2204333 CrossRef CAS PubMed
.
- B. Zhang, H. Li, J. Cheng, H. Ye, A. H. Sakhaei, C. Yuan, P. Rao, Y. F. Zhang, Z. Chen, R. Wang, X. He, J. Liu, R. Xiao, S. Qu and Q. Ge, Adv. Mater., 2021, 33, 2101298 CrossRef CAS PubMed
.
- X. Peng, T. Liu, Q. Zhang, C. Shang, Q.-W. Bai and H. Wang, Adv. Funct. Mater., 2017, 27, 1701962 CrossRef
.
- K. Zub, S. Hoeppener and U. S. Schubert, Adv. Mater., 2022, 34, 2105015 CrossRef CAS PubMed
.
- T. Birman and D. Seliktar, Adv. Funct. Mater., 2021, 31, 2100628 CrossRef CAS
.
- L. Riley, L. Schirmer and T. Segura, Curr. Opin. Biotechnol, 2019, 60, 1–8 CrossRef CAS PubMed
.
- P. S. Gungor-Ozkerim, I. Inci, Y. S. Zhang, A. Khademhosseini and M. R. Dokmeci, Biomater. Sci., 2018, 6, 915–946 RSC
.
- Q. Feng, D. Li, Q. Li, H. Li, Z. Wang, S. Zhu, Z. Lin, X. Cao and H. Dong, ACS Appl. Mater. Interfaces, 2022, 14, 15653–15666 CrossRef CAS PubMed
.
- M. Hirsch, A. Charlet and E. Amstad, Adv. Funct. Mater., 2020, 31, 2005929 CrossRef
.
- C. B. Highley, K. H. Song, A. C. Daly and J. A. Burdick, Adv. Sci., 2019, 6, 1801076 CrossRef PubMed
.
- M. Shin, K. H. Song, J. C. Burrell, D. K. Cullen and J. A. Burdick, Adv. Sci., 2019, 6, 1901229 CrossRef CAS PubMed
.
- Y. Sun, Y. Q. You, W. B. Jiang, B. Wang, Q. Wu and K. Dai, Sci. Adv., 2020, 6, 1–10 Search PubMed
.
- Y. Fang, Y. Guo, M. Ji, B. Li, Y. Guo, J. Zhu, T. Zhang and Z. Xiong, Adv. Funct. Mater., 2021, 32, 2109810 CrossRef
.
- S. P. Pasca, Nature, 2018, 553, 437–445 CrossRef CAS PubMed
.
- A. J. D. Kruger, O. Bakirman, L. P. B. Guerzoni, A. Jans, D. B. Gehlen, D. Rommel, T. Haraszti, A. J. C. Kuehne and L. De Laporte, Adv. Mater., 2019, 31, 1903668 CrossRef PubMed
.
- C. Colosi, S. R. Shin, V. Manoharan, S. Massa, M. Costantini, A. Barbetta, M. R. Dokmeci, M. Dentini and A. Khademhosseini, Adv. Mater., 2016, 28, 677–684 CrossRef CAS PubMed
.
- I. T. Ozbolat and M. Hospodiuk, Biomaterials, 2016, 76, 321–343 CrossRef CAS PubMed
.
- Z.-T. Xie, D.-H. Kang and M. Matsusaki, Soft Matter, 2021, 17, 8769–8785 RSC
.
- A. McCormack, C. B. Highley, N. R. Leslie and F. P. W. Melchels, Trends Biotechnol., 2020, 38, 584–593 CrossRef CAS PubMed
.
- C. B. Highley, K. H. Song, A. C. Daly and J. A. Burdick, Adv. Sci., 2019, 6, 1801076 CrossRef PubMed
.
- D. L. Taylor and M. In Het Panhuis, Adv. Mater., 2016, 28, 9060–9093 CrossRef CAS PubMed
.
- E. M. Ahmed, J. Adv. Res., 2015, 6, 105–121 CrossRef CAS PubMed
.
- H.-R. Lin and K. C. Sung, J. Controlled Release, 2000, 69, 379–388 CrossRef CAS PubMed
.
- A. Lee, A. R. Hudson, D. J. Shiwarski, J. W. Tashman, T. J. Hinton, S. Yerneni, J. M. Bliley, P. G. Campbell and A. W. Feinberg, Science, 2019, 365, 482–487 CrossRef CAS PubMed
.
- J. M. Piau, J. Non-Newtonian Fluid Mech., 2007, 144, 1–29 CrossRef CAS
.
- L. Ning, R. Mehta, C. Cao, A. Theus, M. Tomov, N. Zhu, E. R. Weeks, H. Bauser-Heaton and V. Serpooshan, ACS Appl. Mater. Interfaces, 2020, 12, 44563–44577 CrossRef CAS PubMed
.
- Y. Jin, A. Compaan, T. Bhattacharjee and Y. Huang, Biofabrication, 2016, 8, 025016 CrossRef PubMed
.
- M. Z. Steven, B. Tapomoy, G. R. Kyle, S. Jain, M. N. Ryan, W. G. Sawyer and E. A. Thomas, Sci. Adv., 2015, 1, 1500655 CrossRef PubMed
.
- J. Y. Sun, X. Zhao, W. R. Illeperuma, O. Chaudhuri, K. H. Oh, D. J. Mooney, J. J. Vlassak and Z. Suo, Nature, 2012, 489, 133–136 CrossRef CAS PubMed
.
- O. Jeon, Y. B. Lee, H. Jeong, S. J. Lee, D. Wells and E. Alsberg, Mater. Horiz., 2019, 6, 1625–1631 RSC
.
- A. Shapira, N. Noor, H. Oved and T. Dvir, Biomed. Mater., 2020, 15, 045018 CrossRef CAS PubMed
.
- M. H. Kim, D. Banerjee, N. Celik and I. T. Ozbolat, Biofabrication, 2022, 14, 024103 CrossRef PubMed
.
- S. T. Koshy, R. M. Desai, P. Joly, J. Li, R. K. Bagrodia, S. A. Lewin, N. S. Joshi and D. J. Mooney, Adv. Healthcare Mater., 2016, 5, 541–547 CrossRef CAS PubMed
.
- A. M. Compaan, K. Song, W. Chai and Y. Huang, ACS Appl. Mater. Interfaces, 2020, 12, 7855–7868 CrossRef CAS PubMed
.
- Y. Jiang, J. Chen, C. Deng, E. J. Suuronen and Z. Zhong, Biomaterials, 2014, 35, 4969–4985 CrossRef CAS PubMed
.
- L. Huang, A. M. E. Abdalla, L. Xiao and G. Yang, Int. J. Mol. Sci., 2020, 21, 1895 CrossRef CAS PubMed
.
- F. Li, V. X. Truong, H. Thissen, J. E. Frith and J. S. Forsythe, ACS Appl. Mater. Interfaces, 2017, 9, 8589–8601 CrossRef CAS PubMed
.
- X. Zhao, S. Liu, L. Yildirimer, H. Zhao, R. Ding, H. Wang, W. Cui and D. Weitz, Adv. Funct. Mater., 2016, 26, 2809–2819 CrossRef CAS
.
- B. Li, L. Wang, F. Xu, X. Gang, U. Demirci, D. Wei, Y. Li, Y. Feng, D. Jia and Y. Zhou, Acta Biomater., 2015, 22, 59–69 CrossRef CAS PubMed
.
- S. Selimovic, J. Oh, H. Bae, M. Dokmeci and A. Khademhosseini, Polymers, 2012, 4, 1554 CrossRef CAS PubMed
.
- Z. Zhao, Z. Wang, G. Li, Z. Cai, J. Wu, L. Wang, L. Deng, M. Cai and W. Cui, Adv. Funct. Mater., 2021, 31, 2103339 CrossRef CAS
.
- Y. Li, P. Chen, Y. Wang, S. Yan, X. Feng, W. Du, S. A. Koehler, U. Demirci and B.-F. Liu, Adv. Mater., 2016, 28, 3543–3548 CrossRef CAS PubMed
.
- A. S. Mao, J. W. Shin, S. Utech, H. Wang, O. Uzun, W. Li, M. Cooper, Y. Hu, L. Zhang, D. A. Weitz and D. J. Mooney, Nat. Mater., 2017, 16, 236–243 CrossRef CAS PubMed
.
- J. K. Oh, R. Drumright, D. J. Siegwart and K. Matyjaszewski, Prog. Polym. Sci., 2008, 33, 448–477 CrossRef CAS
.
- D. Sivakumaran, D. Maitland and T. Hoare, Biomacromolecules, 2011, 12, 4112–4120 CrossRef CAS PubMed
.
- F. Topuz and T. Uyar, Carbohydr. Polym., 2022, 297, 120033 CrossRef CAS PubMed
.
- Q. Qian, L. Shi, X. Gao, Y. Ma, J. Yang, Z. Zhang, J. Qian and X. Zhu, Small, 2019, 15, 1903208 CrossRef CAS PubMed
.
- X. Si, S. Ma, Y. Xu, D. Zhang, N. Shen, H. Yu, Y. Zhang, W. Song, Z. Tang and X. Chen, J. Controlled Release, 2020, 320, 83–95 CrossRef CAS PubMed
.
- Q. Song, Y. Yin, L. Shang, T. Wu, D. Zhang, M. Kong, Y. Zhao, Y. He, S. Tan, Y. Guo and Z. Zhang, Nano Lett., 2017, 17, 6366–6375 CrossRef CAS PubMed
.
- I. Antoniuk, D. Kaczmarek, A. Kardos, I. Varga and C. Amiel, Polymers, 2018, 10, 566 CrossRef PubMed
.
- X. Ding, A. Wang, W. Tong and F.-J. Xu, Small, 2019, 15, 1900999 CrossRef PubMed
.
- F. Bures, Top Curr. Chem., 2019, 377, 14 CrossRef PubMed
.
- S. Mohapatra, L. Yutao, S. G. Goh, C. Ng, Y. Luhua, N. H. Tran and K. Y. Gin, J. Hazard. Mater., 2023, 445, 130393 CrossRef CAS PubMed
.
- M. Lei, A. Jayaraman, J. A. Van Deventer and K. Lee, Annu. Rev. Biomed. Eng., 2021, 23, 339–357 CrossRef CAS PubMed
.
- L. J. Zhang and R. L. Gallo, Curr. Biol., 2016, 26, R14–19 CrossRef CAS PubMed
.
- R. Nordstrom and M. Malmsten, Adv. Colloid Interface Sci., 2017, 242, 17–34 CrossRef PubMed
.
- B. Thallinger, E. N. Prasetyo, G. S. Nyanhongo and G. M. Guebitz, Biotechnol. J., 2013, 8, 97–109 CrossRef CAS PubMed
.
- Q. Zhou, Z. Si, K. Wang, K. Li, W. Hong, Y. Zhang and P. Li, J. Controlled Release, 2022, 352, 507–526 CrossRef CAS PubMed
.
- B. C. Borro, R. Nordström and M. Malmsten, Colloids Surf., B, 2020, 187, 110835 CrossRef CAS PubMed
.
- Q. Wang, E. Uzunoglu, Y. Wu and M. Libera, ACS Appl. Mater. Interfaces, 2012, 4, 2498–2506 CrossRef CAS PubMed
.
- G. Zu, M. Steinmüller, D. Keskin, H. C. van der Mei, O. Mergel and P. van Rijn, ACS Appl. Polym. Mater., 2020, 2, 5779–5789 CrossRef CAS PubMed
.
- Y. Wang, Y. Yang, Y. Shi, H. Song and C. Yu, Adv. Mater., 2020, 32, 1904106 CrossRef CAS PubMed
.
- Z. Zhao, X. Ma, R. Chen, H. Xue, J. Lei, H. Du, Z. Zhang and H. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 19268–19276 CrossRef CAS PubMed
.
- H. Xue, Z. Zhao, S. Chen, H. Du, R. Chen, J. L. Brash and H. Chen, Colloid Interface Sci. Commun., 2020, 37, 100268 CrossRef CAS
.
- S. Panja, R. Bharti, G. Dey, N. A. Lynd and S. Chattopadhyay, ACS Appl. Mater. Interfaces, 2019, 11, 33599–33611 CrossRef CAS PubMed
.
- L. Moroni, J. A. Burdick, C. Highley, S. J. Lee, Y. Morimoto, S. Takeuchi and J. J. Yoo, Nat. Rev. Mater., 2018, 3, 21–37 CrossRef CAS PubMed
.
- A. K. Gaharwar, I. Singh and A. Khademhosseini, Nat. Rev. Mater., 2020, 5, 686–705 CrossRef CAS
.
- M. Bhattacharjee, J. Coburn, M. Centola, S. Murab, A. Barbero, D. L. Kaplan, I. Martin and S. Ghosh, Adv. Drug Delivery Rev., 2015, 84, 107–122 CrossRef CAS PubMed
.
- R. D. Pedde, B. Mirani, A. Navaei, T. Styan, S. Wong, M. Mehrali, A. Thakur, N. K. Mohtaram, A. Bayati, A. Dolatshahi-Pirouz, M. Nikkhah, S. M. Willerth and M. Akbari, Adv. Mater., 2017, 29, 1606061 CrossRef PubMed
.
- A. Atala, Chem. Rev., 2020, 120, 10545–10546 CrossRef CAS PubMed
.
- K. Zhang, Z. Jia, B. Yang, Q. Feng, X. Xu, W. Yuan, X. Li, X. Chen, L. Duan, D. Wang and L. Bian, Adv. Sci., 2018, 5, 1800875 CrossRef PubMed
.
- Q. Feng, H. Gao, H. Wen, H. Huang, Q. Li, M. Liang, Y. Liu, H. Dong and X. Cao, Acta Biomater., 2020, 113, 393–406 CrossRef CAS PubMed
.
- Q. Feng, D. Li, Q. Li, S. Li, H. Huang, H. Li, H. Dong and X. Cao, Adv. Healthcare Mater., 2022, 11, 2102395 CrossRef CAS PubMed
.
- F. Li, V. X. Truong, P. Fisch, C. Levinson, V. Glattauer, M. Zenobi-Wong, H. Thissen, J. S. Forsythe and J. E. Frith, Acta Biomater., 2018, 77, 48–62 CrossRef CAS PubMed
.
- R. Madonna, L. W. Van Laake, H. E. Botker, S. M. Davidson, R. De Caterina, F. B. Engel, T. Eschenhagen, F. Fernandez-Aviles, D. J. Hausenloy, J. S. Hulot, S. Lecour, J. Leor, P. Menasche, M. Pesce, C. Perrino, F. Prunier, S. Van Linthout, K. Ytrehus, W. H. Zimmermann, P. Ferdinandy and J. P. G. Sluijter, Cardiovasc. Res., 2019, 115, 488–500 CrossRef CAS PubMed
.
- H. N. Yang, J. H. Choi, J. S. Park, S. Y. Jeon, K. D. Park and K. H. Park, Biomaterials, 2014, 35, 4716–4728 CrossRef CAS PubMed
.
- R. Kumar, K. R. Aadil, S. Ranjan and V. B. Kumar, J. Drug Delivery Sci. Technol., 2020, 57, 101617 CrossRef CAS
.
- R. S. Hsu, P. Y. Chen, J. H. Fang, Y. Y. Chen, C. W. Chang, Y. J. Lu and S. H. Hu, Adv. Sci., 2019, 6, 1900520 CrossRef PubMed
.
- X. Zhao, L. Jin, Z. Zhu, H. Lu, H. Shi, Q. Zhong, J. M. Oliveira, R. L. Reis, C. Gao and Z. Mao, Appl. Mater. Today, 2021, 23, 101064 CrossRef
.
- T. J. Hinton, Q. Jallerat, R. N. Palchesko, J. H. Park, M. S. Grodzicki, H.-J. Shue, M. H. Ramadan, A. R. Hudson and A. W. Feinberg, Sci. Adv., 2015, 1, 1500758 CrossRef PubMed
.
- K. Ali, L. Robert, B. Jeffrey and P. V. Joseph, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 2480–2487 CrossRef PubMed
.
- J. L. Drury and D. J. Mooney, Biomaterials, 2003, 24, 4337–4351 CrossRef CAS PubMed
.
- P. M. Alison and M. V. Sefton, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11461–11466 CrossRef PubMed
.
- C. Frantz, K. M. Stewart and V. M. Weaver, J. Cell Sci., 2010, 123, 4195–4200 CrossRef CAS PubMed
.
- K. Pusch, T. J. Hinton and A. W. Feinberg, HardwareX, 2018, 3, 49–61 CrossRef PubMed
.
- O. Jeon, Y. Bin Lee, T. J. Hinton, A. W. Feinberg and E. Alsberg, Mater. Today Chem., 2019, 12, 61–70 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |