A general method for endowing hydrophobic nanoparticles with water dispersion abilities†
Han
Wang
 a,
Zi
Fu
a,
Xiuru
Ji
a,
Min
Lu
a,
Lianfu
Deng
a,
Zhuang
Liu
*b,
Bo
Yin
*c and
Dalong
Ni
a,
Zi
Fu
a,
Xiuru
Ji
a,
Min
Lu
a,
Lianfu
Deng
a,
Zhuang
Liu
*b,
Bo
Yin
*c and
Dalong
Ni
 *a
*a
aDepartment of Orthopaedics, Shanghai Key Laboratory for Prevention and Treatment of Bone and Joint Diseases, Shanghai Institute of Traumatology and Orthopaedics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China. E-mail: ndl12353@rjh.com.cn
bDepartment of Radiology, Fudan University Shanghai Cancer Center, Shanghai 200032, China. E-mail: liuzhuang201010@126.com
cDepartment of Radiology, Huashan Hospital, Fudan University, Shanghai 200040, China. E-mail: yinbo@fudan.edu.cn
First published on 8th August 2023
Abstract
Inorganic nanoparticles with long-chain ligands are usually hydrophobic. However, simple and practical methods for converting hydrophobic nanoparticles to hydrophilic nanoparticles are still lacking. Herein, we developed a general method involving using dimercaptosuccinic acid (DMSA) for endowing hydrophobic nanoparticles with water dispersion abilities. By mixing a tetrahydrofuran solution of DMSA with a cyclohexane solution of hydrophobic nanoparticles, the long-chain ligands were replaced with DMSA, with the replacement due to the strong and broad-spectrum coordination abilities of sulphydryls and carboxyls. Four representative kinds of hydrophobic nanoparticles, namely Ag, NaGdF4, TiO2, and ZnS nanoparticles, were selected for verifying the performance of this DMSA-based modification method. Meanwhile, this method can also widen the applications of hydrophobic nanoparticles and facilitate their being subjected to further graft modifications. We hope that our research will increase the chances for applications of nanomaterials to be made.
Introduction
Pyrolysis methods are widely used for the synthesis of inorganic nanomaterials, and could produce monodispersed, uniform and well-crystallized nanomaterials more easily than other methods. Pyrolysis has been used to synthesize many kinds of nanomaterials including rare earth (Re) materials (NaReF4, Re2O3, etc.), magnetic nanoparticles (Fe3O4, etc.), metal nanoparticles (Pd, Fe, Ag, etc.) and metal chalcogenides (ZnS, FeS2, Cu2−xSe, etc.).1–10 Generally, long-chain ligands including oleic acid (OA), oleylamine (OAm), and octadecylamine (OctAm) need to be included when carrying out pyrolysis synthesis, because these ligands can act as reaction solvents, assist to form the precursor, and control the growth of a certain crystal face.10–12 However, the generated nanomaterials with long-chain ligands are hydrophobic, with this feature not suitable for biological applications.In the past decade, various methods have been developed to endow hydrophobic nanoparticles with water dispersion abilities for broader applications in medicine.13–20 Generally, to show water dispersion abilities, the long-chain ligands should be replaced with water-soluble ligands. For example, nitrosonium tetrafluoroborate (NOBF4) has been reported to replace OA and OAm ligands reversibly.14 Polyethylene glycol (PEG)-derivatized phosphine oxide (PO-PEG) has been reported to replace the OA ligands of metal oxide nanoparticles, while the Lemieux-von Rudloff reagent (i.e., KMnO4 and NaIO4) was used for oxidizing the carbon–carbon double bond of OA to obtain azelaic acid ligands.15,16 However, some issues remain with these methods: NOBF4 is unstable at room temperature; PO-PEG is not a commercial product and the process of ligand exchange requires high temperature; and the Lemieux-von Rudloff reagent is not suitable for ligands without a carbon–carbon double bond (such as OctAm) and the high amount of reaction solution is not conducive for the separation of nanomaterials. Hence, it is necessary to develop a new approach that can overcome the limitations of the existing methods.
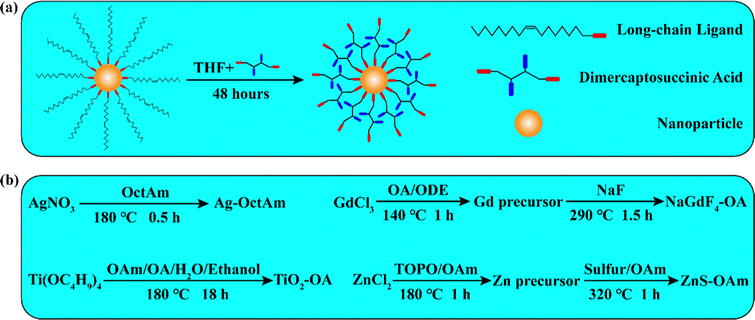
Dimercaptosuccinic acid (DMSA) contains two sulphydryl (–SH) groups and two carboxyl (–COOH) groups per molecule, and has been used as a heavy metal antidote in the clinic. According to the hard and soft acids and bases (HSAB) principle, –SH is a soft base and –COOH is a hard base.21,22 As decreased radius leads to increased base strength, the –COOH in DMSA is a stronger base than OctAm, OA and OAm, which means that DMSA can replace these long-chain ligands. Moreover, with the assistance of –SH (soft base) and –COOH (hard base), DMSA can simultaneously coordinate soft acids (such as elemental Ag, Au, Pt), hard acids (such as Re3+, Ti4+) and borderline acids (such as Cu2+, Zn2+). Herein, we report a generally applicable method involving using DMSA to endow hydrophobic nanoparticles with water dispersion abilities. As shown in Fig. 1a, a tetrahydrofuran (THF) solution of DMSA was mixed with a cyclohexane solution of hydrophobic nanoparticles. After 48 hours, the nanoparticles were modified with DMSA. This method showed several advantages: firstly, the ability of DMSA to strongly coordinate with most metal elements, and the ability of many inorganic nanomaterials produced using pyrolysis to be modified by DMSA; secondly, the process of ligand exchange being carried out at room temperature, with the nanomaterials being easily separated by carrying out distillation at reduced pressure and subsequent methanol/ethanol rinsing; thirdly, both DMSA and THF being low-cost commercial products; and finally, the surface of DMSA-modified nanomaterials containing abundant –SH and –COOH groups, a benefit to the further grafting. We believe that our study will increase the chances for applications of nanomaterials to be made and will provide a method with increased convenience for researchers.
 | ||
| Fig. 1 Illustrations of (a) the DMSA-based modification procedure and (b) the procedures used to synthesize Ag-OctAm, NaGdF4-OA, TiO2-OA and ZnS-OAm. | ||
Results and discussion
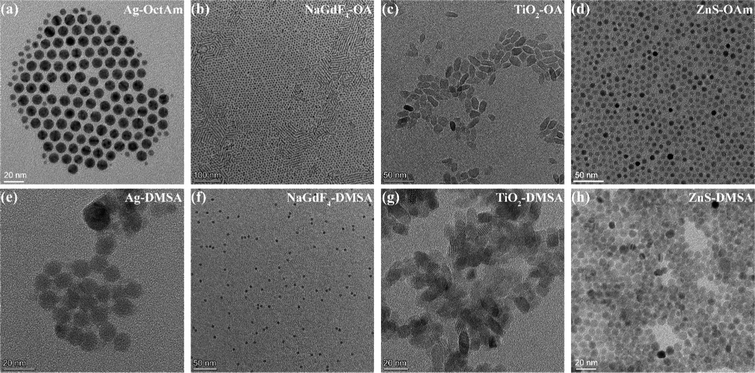
Four representative kinds of hydrophobic nanoparticles, namely Ag, NaGdF4, TiO2, and ZnS, were applied for verifying the performance of this DMSA-based modification method. The procedure used to synthesize these nanomaterials is shown in Fig. 1b. For the synthesis of Ag-OctAm, we poured AgNO3 powder directly into a solution of OctAm at 180 °C, and Ag-OctAm nanoparticles were obtained after 0.5 h.10 For the synthesis of NaGdF4-OA, first GdCl3, OA and octadecene (ODE) were mixed at 140 °C to obtain Gd precursor, and then this Gd precursor was reacted with sodium fluoride (NaF) powder at 290 °C to obtain NaGdF4-OA. For the synthesis of TiO2-OA, we mixed and reacted tetrabutyl titanate (Ti(OC4H9)4), OAm, OA, water and ethanol at 180 °C for 18 h to obtain TiO2-OA.11,23 For the synthesis of ZnS-OAm, first ZnCl2, trioctylphosphine oxide (TOPO) and OAm were mixed at 180 °C to obtain Zn precursor, and then this Zn precursor was reacted with sulfur solution in OAm at 320 °C to obtain ZnS-OAm.5 More experimental details can be found in the Methods section.To clarify the performance of the DMSA-based method, we carried out a series of characterizations of the hydrophobic nanoparticles and DMSA-modified nanoparticles. A photograph of hydrophobic nanoparticles in cyclohexane is shown in Fig. S1a (ESI†) and that of DMSA-modified nanomaterials in water is presented in Fig. S1b (ESI†). All the solutions were clear and transparent liquids. Then, transmission electron microscopy (TEM) was carried out. Both the Ag-OctAm and Ag-DMSA particles were observed to be spheres with dimensions of ∼8 nm (Fig. 2a and e), and both the NaGdF4-OA and NaGdF4-DMSA particles were observed to be spheres with dimensions of ∼5 nm (Fig. 2b and f). In contrast, TiO2-OA and TiO2-DMSA both formed spindles with dimensions of ∼15 nm (Fig. 2e and g). And both the ZnS-OAm and ZnS-DMSA particles were observed to be spheres with dimensions of ∼5 nm (Fig. 2d and h). Hydrodynamic radii were also measured using dynamic light scattering (DLS). The hydrodynamic radii of Ag-OctAm (Fig. S2a, ESI†), NaGdF4-OA (Fig. S2b, ESI†), TiO2-OA (Fig. S2c, ESI†) and ZnS-OAm (Fig. S2d, ESI†) particles were measured to be 9.09 nm, 6.83 nm, 19.87 nm and 6.43 nm, respectively, in accordance with the TEM image data. X-ray diffraction (XRD) patterns showed the crystal structures of Ag-OctAm (Fig. 3a), NaGdF4-OA (Fig. 3b), TiO2-OA (Fig. 3c), ZnS-OAm (Fig. 3d), Ag-DMSA (Fig. 3e), NaGdF4-DMSA (Fig. 3f), TiO2-DMSA (Fig. 3g) and ZnS-DMSA (Fig. 3h) to be in accordance with standard samples, with only a few differences between the XRD patterns of the hydrophobic nanoparticles and those of the DMSA-modified nanoparticles. Hence, neither the morphology nor the crystal structure of any of the nanoparticles changed upon the modification being performed.
 | ||
| Fig. 2 TEM images of (a) Ag-OctAm, (b) NaGdF4-OA, (c) TiO2-OA, (d) ZnS-OAm, (e) Ag-DMSA, (f) NaGdF4-DMSA, (g) TiO2-DMSA and (h) ZnS-DMSA. | ||
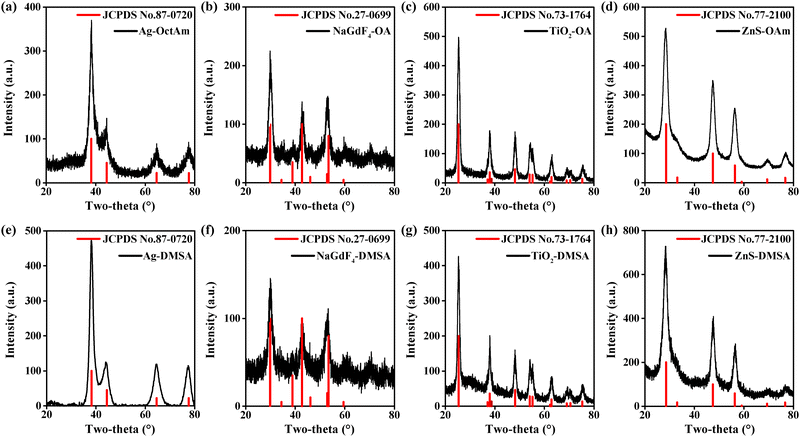 | ||
| Fig. 3 XRD patterns of (a) Ag-OctAm, (b) NaGdF4-OA, (c) TiO2-OA, (d) ZnS-OAm, (e) Ag-DMSA, (f) NaGdF4-DMSA, (g) TiO2-DMSA and (h) ZnS-DMSA. | ||
Fourier-transform infrared spectroscopy (FTIR) data were collected and showed differences between the hydrophobic nanoparticles and the DMSA-modified nanoparticles. As shown in Fig. 4a–d, the four kinds of hydrophobic nanoparticles all showed strong absorption for methylene asymmetric carbon-hydrogen bond (C–H) stretching and methylene symmetric C–H stretching, attributed to the presence of many methylene C–H groups, specifically 28, 30 and 34 of them, per OA molecule, OAm molecule and OctAm molecule, respectively.24 After ligand exchange, the methylene C–H absorption almost disappeared (Fig. 4e–h), attributed to the DMSA molecule having no methylene C–H. Ultraviolet-visible (UV-Vis) spectra of all related nanomaterials were also acquired (Fig. S3a–S3h, ESI†). The differences observed may have been due to the coordination between DMSA and the metal ions.
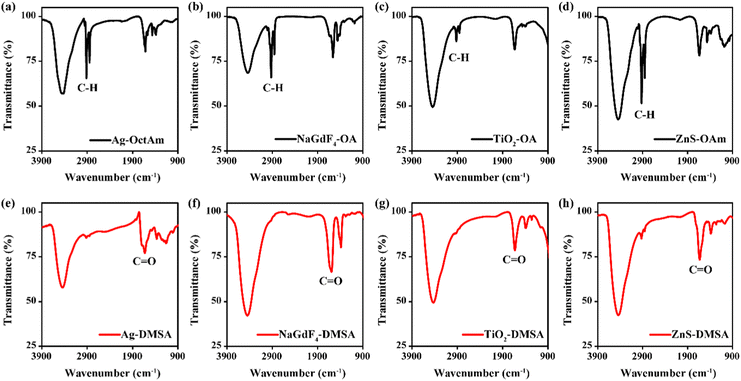 | ||
| Fig. 4 FTIR spectra of (a) Ag-OctAm, (b) NaGdF4-OA, (c) TiO2-OA, (d) ZnS-OAm, (e) Ag-DMSA, (f) NaGdF4-DMSA, (g) TiO2-DMSA and (h) ZnS-DMSA. | ||
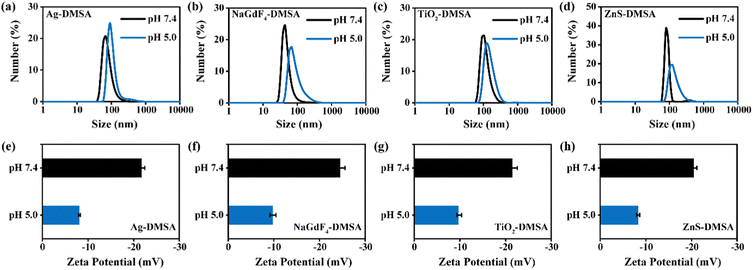
After the DMSA modification, we measured the hydrodynamic radii of the nanoparticles each at different pH values. The abundant –COOH groups on the surfaces of nanoparticles were expected to dissociate into H+ and –COO− reversibly in aqueous solutions. Generally, higher surface charges enhance electrostatic repulsion between nanoparticles. Hence, a higher pH in the solution was expected to promote the dissociation of –COOH and improve surface charge density, leading to better dispersity of DMSA-modified nanoparticles. As shown in Fig. 5a–d, the particles were determined to be smaller at pH 7.4 than at pH 5.0. Additionally, the absolute value of the zeta potential at pH 7.4 was much greater than that under pH 5.0 (Fig. 5e–h). We also characterized the stability levels of the modified nanomaterials at pH 7.4. As shown in Fig. S4a–S4d (ESI†), no significant difference in hydrodynamic size was observed for Ag-DMSA, NaGdF4-DMSA, TiO2-DMSA, and ZnS-DMSA between 1-, 7- and 14-day periods. Hence, the modified nanomaterials could remain stable at pH 7.4. In addition, we measured the leakage of Zn2+ from the ZnS-DMSA surface at pH 5.0. As shown in Fig. S5 (ESI†), only 2% of the Zn2+ was released in 6 days, indicating the stability of ZnS-DMSA in an acid microenvironment. These data confirmed our analysis about the dissociation of –COOH and concurrently indicated that the modification with DMSA occurred.
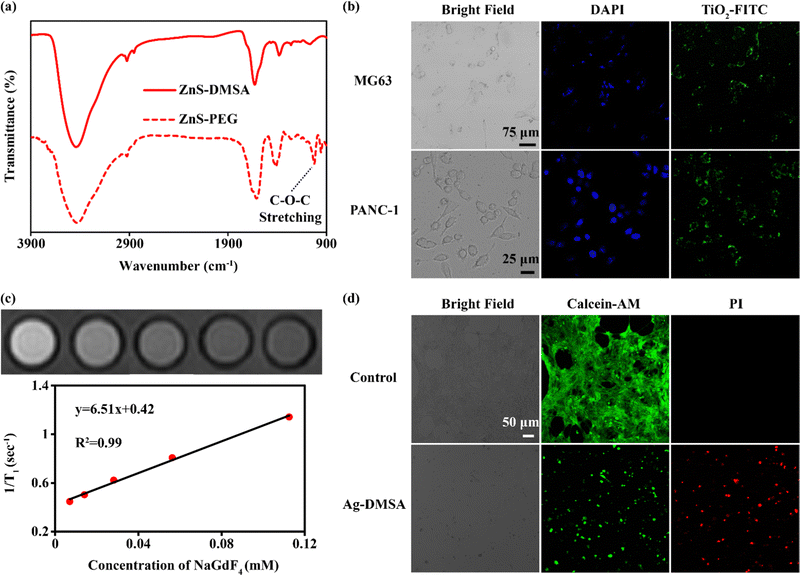
Finally, we verified that there could be biological applications of DMSA-modified nanoparticles. Due to DMSA bringing –SH and –COOH groups to the surfaces of the nanoparticles, further grafting was expected to be feasible. The aminated polyethylene glycol 2000 (PEG2000-NH2) was selected to be reacted with ZnS-DMSA using an amide reaction. C–O–C stretching was not present in the FTIR spectrum of ZnS-DMSA, but appeared in the spectrum of ZnS-PEG (Fig. 6a), indicating the successful modification of PEG.24 Aminated fluorescein isothiocyanate-polyethylene glycol 2000 (FITC-PEG2000-NH2) was also selected for further modification of TiO2-DMSA.25,26 The obtained TiO2-FITC was applied for the fluorescence imaging of cytophagy. As shown in Fig. 6b, after 4 hours of co-culture, MG63 cells (human osteosarcoma cells) and PANC-1 cells (human pancreatic cancer cells) both showed the green fluorescence of TiO2-FITC as measured using a confocal laser scanning microscope (CLSM). In addition, NaGdF4-DMSA was applied for magnetic resonance imaging (MRI). As shown in Fig. 6c, the relaxation rate of NaGdF4-DMSA was 6.51 mM−1 s−1 at 3.0 T, higher than those of commercial MRI contrast agents such as Magnevist (4.1 mM−1 s−1) and Omniscan (3.3 mM−1 s−1).27 Finally, the Ag-DMSA was applied for treating cancer due to the widely reported anticancer activity of Ag nanoparticles.28–30 As shown in Fig. 6d, Ag-DMSA (20 ppm) can effectively kill 4T1 cells (mouse breast cancer cells) as measured using calcein-AM and propidium iodide (PI) stainings. We also used human umbilicus vein endothelial cells (HUVECs) and 4T1 cells to test the cytotoxicity of Ag-DMSA. As shown in Fig. S6 (ESI†), Ag-DMSA exhibited little toxicity to HUVECs, but were obviously lethal for 4T1 cells. Hence, according to these results, Ag-DMSA showed favorable biocompatibility with normal cells and obvious toxicity to cancer cells, in accordance with previous research.31,32 In a word, the DMSA-based modification can widen the applications of hydrophobic nanoparticles.
Conclusions
In summary, we have developed a generally applicable and easy method for endowing hydrophobic nanoparticles with water dispersion abilities. By mixing a THF solution of DMSA with a cyclohexane solution of hydrophobic nanoparticles, the long-chain ligands are replaced with DMSA, due to the strong coordination abilities of –SH and –COOH groups. In principle, this method can be applied at a large scale. DMSA modification widens the applications of hydrophobic nanoparticles to include MRI and cancer treatment. Additionally, further grafting based on –SH and –COOH groups can be achieved. We hope this method will increase the chances for investigating the applications of nanomaterials.Author contributions
Wang H., Lu M., Deng L. and Ni D. conceived the project. Wang H., Fu Z., Ji X., Liu Z. and Yin B. performed the experiments and analyzed the results. Wang H. and Ni D. wrote the manuscript. All the authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by National Natural Science Foundation of China (82102190), the Foundation of National Facility for Translational Medicine (Shanghai) (TMSK-2021-122), China Postdoctoral Science Foundation (Grant No. 2020M681326), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01) and ZJ Lab, Shanghai Center for Brain-Inspired Technology.Notes and references
- D. L. Ni, J. W. Zhang, W. B. Bu, H. Y. Xing, F. Han, Q. F. Xiao, Z. W. Yao, F. Chen, Q. J. He, J. N. Liu, S. J. Zhang, W. P. Fan, L. P. Zhou, W. J. Peng and J. L. Shi, ACS Nano, 2014, 8, 1231–1242 CrossRef CAS PubMed.
- H. Wang, B. Lv, Z. M. Tang, M. Zhang, W. Q. Ge, Y. Y. Liu, X. H. He, K. L. Zhao, X. P. Zheng, M. Y. He and W. B. Bu, Nano Lett., 2018, 18, 5768–5774 CrossRef CAS PubMed.
- Y. L. Hou, H. Kondoh, T. Kogure and T. Ohta, Chem. Mater., 2004, 16, 5149–5152 CrossRef CAS.
- Y. Chong, X. Dai, G. Fang, R. Wu, L. Zhao, X. Ma, X. Tian, S. Lee, C. Zhang, C. Chen, Z. Chai, C. Ge and R. Zhou, Nat. Commun., 2018, 9, 4861 CrossRef PubMed.
- J. Joo, H. B. Na, T. Yu, J. H. Yu, Y. W. Kim, F. Wu, J. Z. Zhang and T. Hyeon, J. Am. Chem. Soc., 2003, 125, 11100–11105 CrossRef CAS PubMed.
- Y. Liu, D. Yao, L. Shen, H. Zhang, X. Zhang and B. Yang, J. Am. Chem. Soc., 2012, 134, 7207–7210 CrossRef CAS PubMed.
- H. Liang, X. Wu, G. Zhao, K. Feng, K. Ni and X. Sun, J. Am. Chem. Soc., 2021, 143, 15812–15823 CrossRef CAS PubMed.
- Y. Lu, Y. J. Xu, G. B. Zhang, D. Ling, M. Q. Wang, Y. Zhou, Y. D. Wu, T. Wu, M. J. Hackett, B. Hyo Kim, H. Chang, J. Kim, X. T. Hu, L. Dong, N. Lee, F. Li, J. C. He, L. Zhang, H. Q. Wen, B. Yang, S. Hong Choi, T. Hyeon and D. H. Zou, Nat. Biomed. Engine, 2017, 1, 637–643 CrossRef CAS PubMed.
- D. Wang, Z. Wang, P. Zhao, W. Zheng, Q. Peng, L. Liu, X. Chen and Y. Li, Chem. – Asian J., 2010, 5, 925–931 CrossRef CAS PubMed.
- D. Wang, T. Xie, Q. Peng and Y. Li, J. Am. Chem. Soc., 2008, 130, 4016–4022 CrossRef CAS PubMed.
- C. T. Dinh, T. D. Nguyen, F. Kleitz and T. O. Do, ACS Nano, 2009, 3, 3737–3743 CrossRef CAS PubMed.
- X. Wang, J. Zhuang, Q. Peng and Y. Li, Nature, 2005, 437, 121–124 CrossRef CAS PubMed.
- A. Nag, M. V. Kovalenko, J. S. Lee, W. Liu, B. Spokoyny and D. V. Talapin, J. Am. Chem. Soc., 2011, 133, 10612–10620 CrossRef CAS PubMed.
- A. Dong, X. Ye, J. Chen, Y. Kang, T. Gordon, J. M. Kikkawa and C. B. Murray, J. Am. Chem. Soc., 2011, 133, 998–1006 CrossRef CAS PubMed.
- H. B. Na, I. S. Lee, H. Seo, Y. I. Park, J. H. Lee, S. W. Kim and T. Hyeon, Chem. Commun., 2007, 5167–5169 RSC.
- Z. G. Chen, H. L. Chen, H. Hu, M. X. Yu, F. Y. Li, Q. Zhang, Z. G. Zhou, T. Yi and C. H. Huang, J. Am. Chem. Soc., 2008, 130, 3023–3029 CrossRef CAS PubMed.
- V. Muhr, S. Wilhelm, T. Hirsch and O. S. Wolfbeis, Acc. Chem. Res., 2014, 47, 3481–3493 CrossRef CAS PubMed.
- G. Tang, J. He, J. Liu, X. Yan and K. Fan, Exploration, 2021, 1, 75–89 CrossRef PubMed.
- Y. Zhao, Z. Zhang, Z. Pan and Y. Liu, Exploration, 2021, 1, 20210089 CrossRef PubMed.
- J. Ouyang, A. Xie, J. Zhou, R. Liu, L. Wang, H. Liu, N. Kong and W. Tao, Chem. Soc. Rev., 2022, 51, 4996–5041 RSC.
- R. G. Pearson, J. Chem. Educ., 1968, 45, 581–587 CrossRef CAS.
- R. G. Pearson, J. Chem. Educ., 1968, 45, 643–648 CrossRef CAS.
- W. W. Su, H. Wang, T. Wang, X. Li, Z. M. Tang, S. Zhao, M. Zhang, D. N. Li, X. W. Jiang, T. Gong, W. Yang, C. J. Zuo, Y. L. Wu and W. B. Bu, Adv. Sci., 2020, 7, 8 Search PubMed.
- B. H. Stuart, Infrared Spectroscopy: Fundamentals and Applications, 2004 Search PubMed.
- X. Huang, C. Liu, N. Kong, Y. Xiao, A. Yurdagul, Jr., I. Tabas and W. Tao, Nat. Protoc., 2022, 17, 748–780 CrossRef CAS PubMed.
- N. Kong, R. Zhang, G. Wu, X. Sui, J. Wang, N. Y. Kim, S. Blake, D. De, T. Xie, Y. Cao and W. Tao, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2112696119 CrossRef CAS PubMed.
- Z. Liu, M. Zhao, H. Wang, Z. Fu, H. Gao, W. Peng, D. Ni, W. Tang and Y. Gu, J. Nanobiotechnol., 2022, 20, 170 CrossRef CAS PubMed.
- V. Porto, E. Borrajo, D. Buceta, C. Carneiro, S. Huseyinova, B. Dominguez, K. J. E. Borgman, M. Lakadamyali, M. F. Garcia-Parajo, J. Neissa, T. Garcia-Caballero, G. Barone, M. C. Blanco, N. Busto, B. Garcia, J. M. Leal, J. Blanco, J. Rivas, M. A. Lopez-Quintela and F. Dominguez, Adv. Mater., 2018, e1801317 CrossRef PubMed.
- R. Foldbjerg, D. A. Dang and H. Autrup, Arch. Toxicol., 2011, 85, 743–750 CrossRef CAS PubMed.
- X. F. Zhang, Z. G. Liu, W. Shen and S. Gurunathan, Int. J. Mol. Sci., 2016, 17, 1534 CrossRef PubMed.
- D. Trachootham, J. Alexandre and P. Huang, Nat. Rev. Drug Discovery, 2009, 8, 579–591 CrossRef CAS PubMed.
- P. V. AshaRani, G. Low Kah Mun, M. P. Hande and S. Valiyaveettil, ACS Nano, 2009, 3, 279–290 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supplementary data. See DOI: https://doi.org/10.1039/d2tb01038k |
| This journal is © The Royal Society of Chemistry 2023 |