Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nitrogen-rich porous organic cages with high acetylene storage and separation performance†
Lijuan
Feng
ab,
Yifei
Xie
a,
Wenjing
Wang
 a,
Kongzhao
Su
a,
Kongzhao
Su
 *ac and
Daqiang
Yuan
*ac and
Daqiang
Yuan
 *ac
*ac
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350002, China. E-mail: skz@fjirsm.ac.cn; ydq@fjirsm.ac.cn
bCollege of Chemistry, Fuzhou University, Fuzhou, 350116, China
cUniversity of the Chinese Academy of Sciences, Beijing, 100049, China
First published on 20th September 2023
Abstract
Searching for porous materials that can safely store and efficiently separate acetylene (C2H2), a commonly used petrochemical feedstock with highly explosive properties, is a pressing and significant task. Nitrogen-rich porous materials have garnered substantial attention for their ability to interact strongly with acidic C2H2. Herein, we report two novel nitrogen-rich porous organic cages (POCs), namely CPOC-107 and CPOC-203, constructed from the same bowl-shaped tetraformylcalix[4]resorcinarene but different nitrogen-rich imidazolium-based diamine synthons. X-ray crystallographic analysis reveals that CPOC-107 adopts a [2 + 4] lantern-shaped structure, whereas CPOC-203 takes on a [3 + 6] triangular prism shape. Moreover, the cages exhibit large cavity volumes of up to 787 Å3 and high specific surface areas of up to 1202 m2 g−1. Owing to their high surface areas and high nitrogen content, both cages exhibit impressive C2H2 adsorption capabilities. Specifically, CPOC-107 achieves a remarkable C2H2 uptake value of up to 146 cm3 g−1 at 298 K and 1 atm, the highest among those reported for all porous organic materials to date. Moreover, experimental breakthrough tests have confirmed the effective separation of C2H2/CO2 mixtures using the CPOC-107 adsorbent.
Introduction
Acetylene (C2H2) is a crucial petrochemical material used in the production of industrial products involving polyurethane, polyester plastics, synthetic rubber, and so on.1,2 However, storing C2H2 safely remains challenging due to its flammability and explosive nature when subjected to pressures above 2 atm at room temperature.3 Moreover, C2H2 is usually produced from fractionation and oil cracking, which always involve a small amount of carbon dioxide (CO2). Therefore, the discrimination of CO2 to afford high-purity C2H2 is critical to high-level safety for various industrial applications.4 Traditional methods to separate C2H2/CO2 mixtures, such as solvent extraction and cryogenic distillations, not only suffer from high energy-consuming and environment-unfriendly problems but also possess the risk of introducing secondary by-products. Consequently, it is of great importance and urgency to seek better materials for safer storage of C2H2 and high-efficiency and low-energy technologies to realize C2H2/CO2 separation.In recent years, adsorption and separation technologies based on porous materials have shown promise in addressing C2H2 storage and separation problems.5–7 In this region, porous metal–organic frameworks (MOFs) have rapidly developed.8,9 For instances, FJI-H8 and SNNU-98, reported by Hong and Zhai groups, respectively, have realized gravimetric C2H2 uptake values more than 220 cm3 g−1 under ambient conditions.10,11 Furthermore, SNNU-45, a MOF prepared by the Zhai group, has demonstrated the separation of C2H2/CO2 with breakthrough time up to 80 min g−1.12 In contrast, the development of porous organic materials (POMs) for C2H2 storage and separation is markedly slower than that of MOFs. To the best of our knowledge, POMs' C2H2 uptake values are generally less than 100 m2 g−1 under ambient conditions, and their ability to separate C2H2/CO2 mixtures via actual breakthrough experiments is also underexplored.13 Therefore, it is crucial to increase their C2H2 adsorption capacity and explore their C2H2 purification ability to promote the development of POMs in this field.
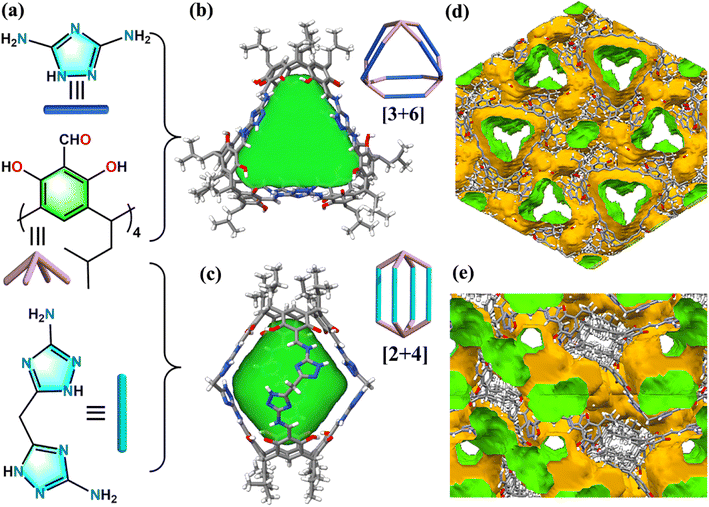
Porous organic cages (POCs) are an emerging class of low-density crystalline POMs, which are held together from discrete (zero-dimensional; 0D) covalent-bonded macromolecules with permanent intrinsic cavities (0D framework) via weak intermolecular interactions.14–16 Since their inception in 2009 from Cooper's group,17 research on the design and synthesis of different linkages, shapes, topologies, sizes, and functions of POCs has attracted much attention from chemists and materials scientists.18–33 Our group focuses on using concave-shaped calix[4]resorcinarene (C4RA) as synthons in construction of novel C4RA-based POCs and their applications.34–40 In this work, we designed and synthesized two robust nitrogen-rich POCs aiming at efficient storage and separation of C2H2, as the basic nitrogen groups have been verified to have strong interaction with acidic C2H2 gas. Both the [2 + 4] lantern-shaped cage (CPOC-107) and [3 + 6] triangular prism-shaped cage (CPOC-203) are constructed from the same bowl-shaped tetraformylcalix[4]resorcinarene (C4RACHO) but different nitrogen-rich imidazolium-based diamine synthons (Fig. 1a–c and S1–S7†). We found that both materials show high C2H2 uptake capacity under ambient conditions (>110 cm3 g−1), and the nitrogen content greatly influences their C2H2 uptake capacity and C2H2/CO2 separation ability.
Results and discussion
A mixture of C4RACHO and 3,5-diamino-1,2,4-triazole (DTA) organic synthons with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in chlorobenzene and methanol mixture at 80 °C for 24 hours affords red block crystals of CPOC-203. Single crystallographic X-ray determination (SCXRD) suggests that CPOC-203 adopts a triangular prism-shaped cage structure (Fig. 1b), with a [3 + 6] assembly mode that is similar to the previously reported CPOC-201, constructed from C4RACHO and m-phenylenediamine synthons. CPOC-203 crystallizes in the triclinic system with the space group P
2 in chlorobenzene and methanol mixture at 80 °C for 24 hours affords red block crystals of CPOC-203. Single crystallographic X-ray determination (SCXRD) suggests that CPOC-203 adopts a triangular prism-shaped cage structure (Fig. 1b), with a [3 + 6] assembly mode that is similar to the previously reported CPOC-201, constructed from C4RACHO and m-phenylenediamine synthons. CPOC-203 crystallizes in the triclinic system with the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with Z = 2, and its asymmetric unit contains one full cage molecule as well as a large number of disordered solvate molecules (∼46.2% of the unit cell volume), which have been removed by the routine SQUEEZE function of PLATON.41 The central triangular prism core of CPOC-203 comprises three C4RACHO faces and six DTA edges. It has a volume of 787 Å3 and a window diameter of 7.04 Å, as calculated using Voidoo and Pywindow, respectively.42–44 The cages pack window-to-window in the solid state with the closest distance of ∼9 Å between neighboring prism faces, forming one-dimensional channels (Fig. 1d).
with Z = 2, and its asymmetric unit contains one full cage molecule as well as a large number of disordered solvate molecules (∼46.2% of the unit cell volume), which have been removed by the routine SQUEEZE function of PLATON.41 The central triangular prism core of CPOC-203 comprises three C4RACHO faces and six DTA edges. It has a volume of 787 Å3 and a window diameter of 7.04 Å, as calculated using Voidoo and Pywindow, respectively.42–44 The cages pack window-to-window in the solid state with the closest distance of ∼9 Å between neighboring prism faces, forming one-dimensional channels (Fig. 1d).
Then we further increase the nitrogen content in imidazolium-based diamine synthons by using bis(5-amino-1,2,4-triazol-3-yl)methane (BATM). Notably, suitable red block single crystals of CPOC-107 can be obtained by an eightfold Schiff base reaction of C4RACHO (1 equiv.) and BATM (2 equiv.) in dimethyl sulfoxide at 100 °C for 72 hours. SCXRD reveals that CPOC-107 crystallizes in a triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with Z = 4, and contains a whole [2 + 4] organic cage in its asymmetric unit, and residual electron density as highly disordered solvent molecules (∼45.3% of the unit cell volume), which were removed by SQUEEZE. The structure of CPOC-107 is s a lantern-shaped structure with four near rhombic windows (Fig. 1c), featuring two C4RACHO as faces and four BATMs as pillars. The height of the cavity is 15.4 Å which was measured from the center of the C4RACHO face, and its windows can be passed by a sphere with a diameter of about 4.47 Å. Remarkably, CPOC-107 has a cavity volume of about 744 Å3, the largest among those of all previously reported lantern-shaped CPOCs, with cavity volumes ranging from 358 to 581 Å3.36 The side length of the rhombic windows is about 4.47 Å, which can be passed by a sphere with a diameter of about 4.47 Å. An examination of the CPOC-107's solid-state packing suggested that cage molecules pack via weak van der Waals interactions, and there is also a one-dimensional channel in window-to-window mode (Fig. 1e).
space group with Z = 4, and contains a whole [2 + 4] organic cage in its asymmetric unit, and residual electron density as highly disordered solvent molecules (∼45.3% of the unit cell volume), which were removed by SQUEEZE. The structure of CPOC-107 is s a lantern-shaped structure with four near rhombic windows (Fig. 1c), featuring two C4RACHO as faces and four BATMs as pillars. The height of the cavity is 15.4 Å which was measured from the center of the C4RACHO face, and its windows can be passed by a sphere with a diameter of about 4.47 Å. Remarkably, CPOC-107 has a cavity volume of about 744 Å3, the largest among those of all previously reported lantern-shaped CPOCs, with cavity volumes ranging from 358 to 581 Å3.36 The side length of the rhombic windows is about 4.47 Å, which can be passed by a sphere with a diameter of about 4.47 Å. An examination of the CPOC-107's solid-state packing suggested that cage molecules pack via weak van der Waals interactions, and there is also a one-dimensional channel in window-to-window mode (Fig. 1e).
The presence of 1D channels in CPOC-107 and CPOC-203 prompts us to study their porosity. Thermal gravimetric analysis measurements showed their stabilities up to about 280 °C (Fig. S7†). Before the gas sorption test, both cages are immersed and exchanged 6 times every 24 hours in MeOH before activating at 100 °C under a high vacuum for 12 hours. Powder X-ray diffraction reveals that after desolvation, no phase change occurs in both materials (Fig. S8 and S9†). N2 gas sorption experiments confirmed their permanent porosity at 77 K (Fig. 2), and both the isotherms of CPOC-107 and CPOC-203 exhibited typical type I adsorption behavior with sharp increases at a pressure below 0.1 P/Po, which reveals their microporous nature. The Brunauer–Emmett–Teller (BET) specific surface areas were 1202 m2 g−1 for CPOC-107 and 1132 m2 g−1 for CPOC-203, respectively (Fig. S10 and S11†). The non-local density functional theory (NLDFT) was used to calculate the pore-size distribution (PSD), and both cages showed microporous cavities with ∼1.08 nm for CPOC-107 and ∼0.98 nm for CPOC-203 (inset in Fig. 2), which are respectively related to the calculated largest pore diameter of 0.94 and 1.02 nm using Zeo++ software.45
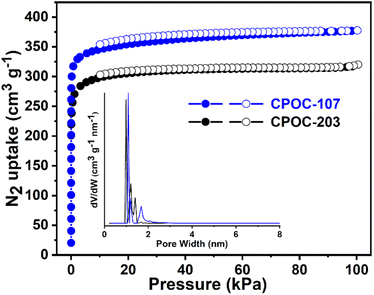 | ||
| Fig. 2 N2 gas sorption isotherms at 77 K for CPOC-107 and CPOC-203, inset: the calculated PSD of CPOC-107 and CPOC-203. | ||
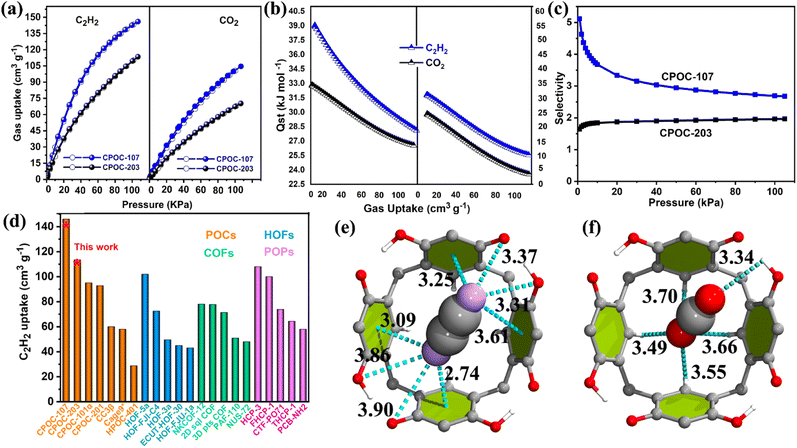
The high surface, together with the presence of a high content of basic nitrogen sites (14.8% for CPOC-203 and 20.1% for CPOC-107) for both cages, further prompts us to use them as solid adsorbents for capturing acidic C2H2 gas and separating C2H2/CO2 mixtures. Single-component gas equilibrium sorption isotherms of CPOC-107 and CPOC-203 were recorded at 298 K and pressure up to 1 bar. As shown in Fig. 3a, the saturated C2H2 adsorption value under the conditions above is 146 cm3 g−1 for CPOC-107, which is higher than that of CPOC-203, with a value of 114 cm3 g−1. Both values are higher than those of our previously reported cages with similar structural assemblies. Specifically, the C2H2 uptakes for the previously reported lantern-shaped [2 + 4] CPOC-101 to CPOC-104 (nitrogen content < 6.2%) are all less than 95 cm3 g−1;38,46 while for the reported similar triangular prism-shaped [3 + 6] CPOC-201 with a nitrogen content of 5.8% the C2H2 uptake is only 91 cm3 g−1.38 The discussions above reveal that increasing the nitrogen content in CPOCs can improve their C2H2 uptake ability. Notably, the C2H2 uptake value (146 cm3 g−1) of CPOC-107 is the highest among those reported for all POMs (Fig. 3d), including POCs,47,48 hydrogen-bonded organic frameworks (HOFs),49–52 covalent organic frameworks (COFs)53–56 and porous organic polymers (POPs).57–59
In contrast, under the same conditions, the CO2 absorbed capacities of CPOC-107 and CPOC-203 are only 100 and 70 cm3 g−1 (Fig. 3a), much lower than their C2H2 uptake capacities under the same conditions. Such a result reveals that the affinity for C2H2 is stronger than that for CO2 in both cages. In order to quantify the affinity (low-coverage heat of adsorption; Qst) between the cage hosts and the guest gas molecules, their C2H2 and CO2 sorption isotherms at 273 K have also been measured (Fig. S12†). The Qst values are calculated using adsorption isotherms at 273 and 298 K and fitted by a virial equation (Fig. S13–S16†). The calculated Qst values for C2H2 were 39.1 and 34.9 kJ mol−1 for CPOC-107 and CPOC-203, respectively, which are higher than those for CO2 with values of 32.8 and 23.2 kJ mol−1, respectively (Fig. 3b). Such calculated results highly indicate the preferential sorption of C2H2 over CO2 in both cages. Moreover, their gas selectivity values have been calculated using the widely studied ideal adsorbed solution theory (IAST), and the equimolar binary C2H2/CO2 for CPOC-107 is 2.7 at 298 K and 100 kPa, which is much higher than 1.9 for CPOC-203. Moreover, the higher C2H2 capacity and C2H2/CO2 selectivity of CPOC-107 suggest that its separation ability for C2H2/CO2 may be better than that of CPOC-203. Therefore, their separation potentials (Δq) are a comprehensive index to evaluate the separation effectiveness of adsorbents based on combining adsorption capacity and selectivity. The calculated Δq value for CPOC-107 is 2.67 mmol g−1, and for CPOC-203 it is 1.30 mmol g−1 (Fig. S17†), consistent with our guess.60
Moreover, the detailed dispersion-corrected density functional theory (DFT-D) calculations for CPOC-107 were also carried out by loading CO2 and C2H2 molecules into its crystal structure for further optimization. The lowest-energy binding configurations of the final gas-loaded structures of CPOC-107 were calculated using DFT-D. In the gas-loaded CPOC-107, both C2H2 and CO2 molecules were observed to preferentially reside around C4RA's cavities via weak Waals interactions (Fig. 3e and f). Specifically, for the C2H2 molecule, both hydrogen atoms were oriented toward the four C4RA's phenyl rings through H⋯π interactions at distances ranging from 2.74 to 3.61 Å. In contrast, their two hydrogen tails were observed to interact with the four C4RA's hydroxy groups through H⋯O interactions at distances ranging from 3.31 to 3.90 Å. For the CO2 molecule, one oxygen atom remained closer to the cage's window through O⋯H interactions at a distance of 3.34 Å. At the same time, the other one was oriented towards the bottom of C4RA through O⋯H interactions at distances ranging from 3.49 to 3.70 Å. The calculated static binding energies of C2H2 and CO2 were −38.9 and −31.1 kJ mol−1, respectively, for CPOC-107. These binding energies and the hydrogen-bond number confirmed the stronger host–guest interactions between C2H2 and the cage compared to that of CO2, which is entirely consistent with our experimental observations.
Lab-scale fixed-bed breakthrough experiments have been performed under ambient conditions to make clear the separation performance of C2H2/CO2 by using CPOC-107 and CPOC-203 as adsorbents. An equimolar CO2/C2H2 gas mixture typically flowed over a packed column of the activated samples with a total flow of 2 mL min−1 at 298 K and 1 bar. As shown in Fig. 4a, CO2 passed through the packed column first to produce an outflow of pure gas containing no detectable C2H2, and then C2H2 eluted following a substantial time-lapse for both CPOC materials. The breakthrough time values of CPOC-107 and CPOC-203 are ∼13 and 5 min g−1, respectively. The longer effective separation time of CPOC-107 than CPOC-203 suggested that the C2H2/CO2 separation ability of CPOC-107 is better than that of CPOC-203, consistent with the abovementioned IAST and Δq results. An ideal separation material should possess recyclability performance to meet practical applications. To evaluate the durability, multiple cycling breakthrough tests for CPOC-107 have been carried out under the same conditions. As displayed in Fig. 4b, the breakthrough time remains unchanged after three recycling experiments, indicating that CPOC-107 is an auspicious C2H2/CO2 separation system.
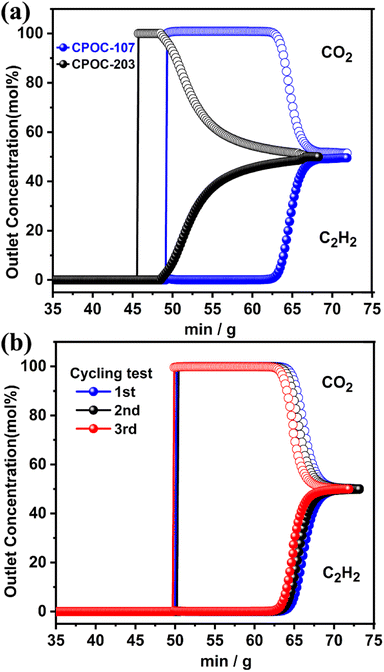 | ||
| Fig. 4 (a) Experimental breakthrough curves for an equimolar mixture of C2H2/CO2 at 298 K and 1 bar; (b) the recyclability of CPOC-107 under multiple mixed gas column breakthrough tests. | ||
Conclusions
In summary, we presented two novel C4RA-based POCs with high nitrogen content (>14%), including [2 + 4] lantern-shaped CPOC-107 and [3 + 6] triangular prism-shaped CPOC-203. Both cages exhibit high surface areas (>1100 m2 g−1), and high C2H2 uptake capacity (>110 cm3 g−1) at 298 K and 1 atm. Notably, the nitrogen content of CPOC-107 is up to 20.1%, and thus its C2H2 uptake capacity under ambient conditions is up to 146 cm3 g−1, which is the highest among those reported for POMs. Moreover, CPOC-107 can also efficiently separate C2H2/CO2 with a breakthrough time of 13 min g−1. This work suggests that nitrogen-rich POCs can be promising materials for safer C2H2 storage, as well as C2H2 purification application. Further studies are focusing on design and synthesis of functionalized POCs by introducing specific group sites for improving their gas storage and separation performances.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (22071244 and 22275191), Youth Innovation Promotion Association CAS (2022305), and the Natural Science Foundation of Fujian Province of China (2022J01503 and 2020J05087).Notes and references
- V. V. Voronin, M. S. Ledovskaya, A. S. Bogachenkov, K. S. Rodygin and V. P. Ananikov, Molecules, 2018, 23, 2442 CrossRef PubMed.
- P. J. Stang and F. Diederich, Modern acetylene chemistry, VCH Weinheim, 1995 Search PubMed.
- R. Matsuda, R. Kitaura, S. Kitagawa, Y. Kubota, R. V. Belosludov, T. C. Kobayashi, H. Sakamoto, T. Chiba, M. Takata, Y. Kawazoe and Y. Mita, Nature, 2005, 436, 238–241 CrossRef CAS PubMed.
- A. Corma, E. Corresa, Y. Mathieu, L. Sauvanaud, S. Al-Bogami, M. S. Al-Ghrami and A. Bourane, Catal. Sci. Technol., 2017, 7, 12–46 RSC.
- H. Li, C. Liu, C. Chen, Z. Di, D. Yuan, J. Pang, W. Wei, M. Wu and M. Hong, Angew. Chem., Int. Ed., 2021, 60, 7547–7552 CrossRef CAS PubMed.
- W. Gong, H. Cui, Y. Xie, Y. Li, X. Tang, Y. Liu, Y. Cui and B. Chen, J. Am. Chem. Soc., 2021, 143, 14869–14876 CrossRef CAS PubMed.
- B. Zhang, Y. Rao, L. Hou, B. Liu and Q. Li, ACS Mater. Lett., 2022, 4, 1774–1779 CrossRef CAS.
- Y. Li and G. Wen, Eur. J. Inorg. Chem., 2020, 2020, 2303–2311 CrossRef CAS.
- G. Verma, J. Ren, S. Kumar and S. Ma, Eur. J. Inorg. Chem., 2021, 2021, 4498–4507 CrossRef CAS.
- J.-W. Wang, S.-C. Fan, H.-P. Li, X. Bu, Y.-Y. Xue and Q.-G. Zhai, Angew. Chem., Int. Ed., 2023, e202217839 CAS.
- J. Pang, F. Jiang, M. Wu, C. Liu, K. Su, W. Lu, D. Yuan and M. Hong, Nat. Commun., 2015, 6, 7575 CrossRef PubMed.
- Y.-P. Li, Y. Wang, Y.-Y. Xue, H.-P. Li, Q.-G. Zhai, S.-N. Li, Y.-C. Jiang, M.-C. Hu and X. Bu, Angew. Chem., Int. Ed., 2019, 58, 13590–13595 CrossRef CAS PubMed.
- W. Wang, L. Wang, F. Du, G.-D. Wang, L. Hou, Z. Zhu, B. Liu and Y.-Y. Wang, Chem. Sci., 2023, 14, 533–539 RSC.
- X. Yang, Z. Ullah, J. F. Stoddart and C. T. Yavuz, Chem. Rev., 2023, 4602–4634 CrossRef CAS PubMed.
- T. Hasell and A. I. Cooper, Nat. Rev. Mater., 2016, 1, 16053 CrossRef CAS.
- M. Mastalerz, Acc. Chem. Res., 2018, 51, 2411–2422 CrossRef CAS PubMed.
- T. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner and A. I. Cooper, Nat. Mater., 2009, 8, 973–978 CrossRef CAS PubMed.
- T. Jiao, H. Qu, L. Tong, X. Cao and H. Li, Angew. Chem., Int. Ed., 2021, 60, 9852–9858 CrossRef CAS PubMed.
- X. Liu, G. Zhu, D. He, L. Gu, P. Shen, G. Cui, S. Wang, Z. Shi, D. Miyajima, S. Wang and S. Zhang, CCS Chem., 2022, 4, 2420–2428 CrossRef CAS.
- S. Huang, Z. Lei, Y. Jin and W. Zhang, Chem. Sci., 2021, 12, 9591–9606 RSC.
- H. Duan, F. Cao, H. Hao, H. Bian and L. Cao, ACS Appl. Mater. Interfaces, 2021, 13, 16837–16845 CrossRef CAS PubMed.
- S. Bera, K. Dey, T. K. Pal, A. Halder, S. Tothadi, S. Karak, M. Addicoat and R. Banerjee, Angew. Chem., Int. Ed., 2019, 58, 4243–4247 CrossRef CAS PubMed.
- M. Hua, S. Wang, Y. Gong, J. Wei, Z. Yang and J.-K. Sun, Angew. Chem., Int. Ed., 2021, 60, 12490–12497 CrossRef CAS PubMed.
- Q.-P. Hu, H. Zhou, T.-Y. Huang, Y.-F. Ao, D.-X. Wang and Q.-Q. Wang, J. Am. Chem. Soc., 2022, 144, 6180–6184 CrossRef CAS PubMed.
- S. Wu, Y. Ni, Y. Han, S. Xin, X. Hou, J. Zhu, Z. Li and J. Wu, J. Am. Chem. Soc., 2022, 144, 23158–23167 CrossRef CAS PubMed.
- G. Montà-González, F. Sancenón, R. Martínez-Máñez and V. J. C. R. Martí-Centelles, Chem. Rev., 2022, 122, 13636–13708 CrossRef PubMed.
- H. Wang, Y. Jin, N. Sun, W. Zhang and J. Jiang, Chem. Soc. Rev., 2021, 50, 8874–8886 RSC.
- F. Qiu, X. Chen, W. Wang, K. Su and D. Yuan, CCS Chem., 2023 DOI:10.31635/ccschem.023.202302903.
- R. D. Mukhopadhyay, Y. Kim, J. Koo and K. Kim, Acc. Chem. Res., 2018, 51, 2730–2738 CrossRef CAS PubMed.
- L. Chen, C. Li, E. Fu, M. Li, Y. Kuboi, Z.-Y. Li, Z. Chen, J. Chen, X. Liu, X. Tang, L. Frederic, F. Maurel, C. Adachi, F. Mathevet and S. Zhang, ACS Mater. Lett., 2023, 5, 1450–1455 CrossRef CAS.
- W.-T. Dou, C.-Y. Yang, L.-R. Hu, B. Song, T. Jin, P.-P. Jia, X. Ji, F. Zheng, H.-B. Yang and L. Xu, ACS Mater. Lett., 2023, 5, 1061–1082 CrossRef CAS.
- M. C. Brand, F. Greenwell, R. Clowes, B. D. Egleston, A. Kai, A. I. Cooper, T. D. Bennett and R. L. Greenaway, J. Mater. Chem. A, 2021, 9, 19807–19816 RSC.
- S. Lee, I. Kevlishvili, H. J. Kulik, H.-T. Kim, Y. G. Chung and D.-Y. Koh, J. Mater. Chem. A, 2022, 10, 24802–24812 RSC.
- F. Gao, C. Luo, X. Wang, C. Zhan, Y. Li, Y. Li, Q. Meng, M. Yang, K. Su, D. Yuan, R. Zhu and Q. Zhao, Adv. Funct. Mater., 2023, 33, 2211900 CrossRef CAS.
- N. Xu, K. Su, E.-S. M. El-Sayed, Z. Ju and D. Yuan, Chem. Sci., 2022, 13, 3582–3588 RSC.
- M. Yang, F. Qiu, E.-S. M. El-Sayed, W. Wang, S. Du, K. Su and D. Yuan, Chem. Sci., 2021, 12, 13307–13315 RSC.
- X. Zhang, K. Su, A. G. A. Mohamed, C. Liu, Q. Sun, D. Yuan, Y. Wang, W. Xue and Y. Wang, Energy Environ. Sci., 2022, 15, 780–785 RSC.
- K. Su, W. Wang, S. Du, C. Ji, M. Zhou and D. Yuan, J. Am. Chem. Soc., 2020, 142, 18060–18072 CrossRef CAS PubMed.
- M. Yang, X. Chen, Y. Xie, E.-S. M. El-Sayed, N. Xu, W. Wang, K. Su and D. Yuan, Sci. China: Chem., 2023, 66, 1763–1770 CrossRef CAS.
- K. Su, W. Wang, S. Du, C. Ji and D. Yuan, Nat. Commun., 2021, 12, 3703 CrossRef CAS PubMed.
- A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 CrossRef CAS PubMed.
- https://xray.bmc.uu.se/usf/ .
- G. J. Kleywegt and T. A. Jones, Acta Crystallogr., Sect. D: Biol. Crystallogr., 1994, 50, 178–185 CrossRef CAS PubMed.
- S. Sanz, K. Ferreira, R. D. McIntosh, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2011, 47, 9042–9044 RSC.
- S. M. Taylor, R. D. McIntosh, C. M. Beavers, S. J. Teat, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2011, 47, 1440–1442 RSC.
- W. Wang, K. Su, E.-S. M. El-Sayed, M. Yang and D. Yuan, ACS Appl. Mater. Interfaces, 2021, 13, 24042–24050 CrossRef CAS PubMed.
- S. M. Elbert, N. I. Regenauer, D. Schindler, W.-S. Zhang, F. Rominger, R. R. Schroeder and M. Mastalerz, Chem.–Eur. J., 2018, 24, 11438–11443 CrossRef CAS PubMed.
- C. D. Charles and E. D. Bloch, Supramol. Chem., 2019, 31, 508–513 CrossRef CAS.
- P. Li, Y. He, Y. Zhao, L. Weng, H. Wang, R. Krishna, H. Wu, W. Zhou, M. O'Keeffe, Y. Han and B. Chen, Angew. Chem., Int. Ed., 2015, 54, 574–577 CrossRef CAS PubMed.
- H. Wang, B. Li, H. Wu, T.-L. Hu, Z. Yao, W. Zhou, S. Xiang and B. Chen, J. Am. Chem. Soc., 2015, 137, 9963–9970 CrossRef CAS PubMed.
- Y. Yang, H. Zhang, Z. Yuan, J.-Q. Wang, F. Xiang, L. Chen, F. Wei, S. Xiang, B. Chen and Z. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202207579 CrossRef CAS PubMed.
- L. Wang, L. Yang, L. Gong, R. Krishna, Z. Gao, Y. Tao, W. Yin, Z. Xu and F. Luo, Chem. Eng. J., 2020, 383, 123117 CrossRef CAS.
- L. Chen, C. Gong, X. Wang, F. Dai, M. Huang, X. Wu, C.-Z. Lu and Y. Peng, J. Am. Chem. Soc., 2021, 143, 10243–10249 CrossRef CAS PubMed.
- L. Jiang, Y. Tian, T. Sun, Y. Zhu, H. Ren, X. Zou, Y. Ma, K. R. Meihaus, J. R. Long and G. Zhu, J. Am. Chem. Soc., 2018, 140, 15724–15730 CrossRef CAS PubMed.
- Z. Zhang, C. Kang, S. B. Peh, D. Shi, F. Yang, Q. Liu and D. Zhao, J. Am. Chem. Soc., 2022, 144, 14992–14996 CrossRef CAS PubMed.
- P. Zhang, Z. Wang, Y. Yang, S. Wang, T. Wang, J. Liu, P. Cheng, Y. Chen and Z. Zhang, Sci. China: Chem., 2022, 65, 1173–1184 CrossRef CAS.
- S. Zhang, M. K. Taylor, L. Jiang, H. Ren and G. Zhu, Chem.–Eur. J., 2020, 26, 3205–3221 CrossRef CAS PubMed.
- Z. Jia, J. Pan and D. Yuan, ChemistryOpen, 2017, 6, 554–561 CrossRef CAS PubMed.
- X. Liu, C. Xu, X. Yang, Y. He, Z. Guo and D. Yan, Microporous Mesoporous Mater., 2019, 275, 95–101 CrossRef CAS.
- Y. Jiang, J. Hu, L. Wang, W. Sun, N. Xu, R. Krishna, S. Duttwyler, X. Cui, H. Xing and Y. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202200947 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2255900 and 2255901. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ta04154a |
| This journal is © The Royal Society of Chemistry 2023 |