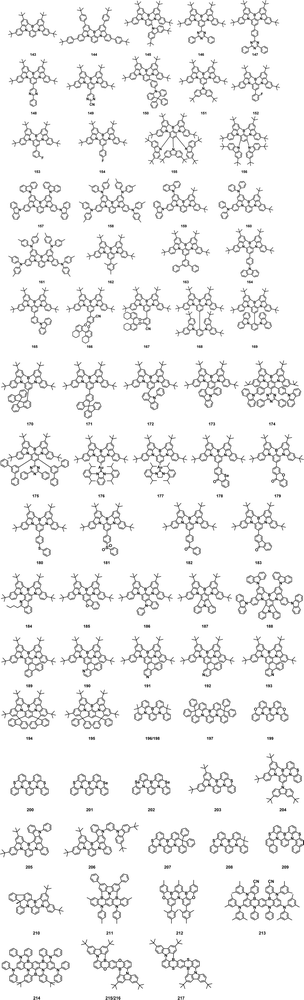
Recent advances and prospects for organoboron-based thermally activated delayed fluorescence emitters
Liang
Wan
,
Zhuang
Cheng
,
Futong
Liu
and
Ping
Lu
 *
*
State Key Laboratory of Supramolecular Structure and Materials, Jilin University, Qianjin Street No. 2699, Changchun, 130012, P. R. China. E-mail: lup@jlu.edu.cn
First published on 7th June 2023
Abstract
The intense interest in organic boron-containing light-emitting materials stems from their attractive potential electron-deficient characteristics and excellent fluorescence efficiency. Delicate molecular design of organic boron-containing emitters and comprehensive studies of the relationship between their chemical structures and photophysical properties are of great significance for developing high-performance emitters. In this review, an overview of the recent studies on organoboron-based emitters for applications in organic light-emitting devices (OLEDs) is presented. First, we give a brief introduction to the basic properties of organoboron materials. And then, the recent research progress of thermally activated delayed fluorescence (TADF) materials containing triaryl/diarylboron, 10H-phenoxaborin, 5,9-dioxa-13b-boranaphtho[3,2,1-de]anthracene (DBNA), and 5,9-diphenyl-5,9-dihydroquinoxalino[3,2,1-de]phenazine (DABAN) is reviewed systematically with a focus on the molecular design, photophysical properties and performance of the corresponding OLEDs. Finally, the future challenges of organoboron-based materials are discussed.
1. Introduction
Boron (B) is the fifth element in the periodic table with an atomic mass of 10.81. It is a non-metallic element with the most electronegativity among elements of Group III. Boron is abundant in nature, mainly distributed in borax (Na2B4O7·10H2O), priceite (Ca2B6O11·5H2O) and magnesite (Mg2B2O5), among which borax was used as medicine in ancient times.1 Boron chemistry has a long history. In 1702, French chemist Holmberg first heated borax and ferrous sulfate together to prepare boric acid.2 In 1808, pure elemental boron was first isolated independently and simultaneously by H. Davy in England, who observed that electric current sent through a solution of borates produced a brown precipitate on one of the electrodes, and J. Gay-Lussac and L. Thenard in France obtained boron by reducing boric acid with iron at high temperature.3 Organoboron chemistry has a profound influence on chemical research and industry, including synthetic chemistry, biochemistry, pharmaceutical chemistry, materials science, etc. In the past decades, it also has witnessed the rapid development of organic optoelectronic functional molecules.4 The electron structure of boron is 1s22s22p1 with four valence orbitals and three valence electrons. Thus, sp2 hybridized three-coordinate boron compounds can be formed with electron-deficient properties. They show high potential in nonlinear optics, chemical sensing, biological imaging, and especially, in the field of organic electroluminescence.5,6In 1987, Tang and VanSlyke first reported the preparation of organic light-emitting diodes (OLEDs).7 Since then, OLEDs have been actively studied owing to their advantages of low power consumption, high brightness, color purity, lightweight, large viewing angle and flexibility and have been commercially applied in some flat-panel display applications such as mobile phones and televisions.8,9 According to spin statics, 25% singlet excitons (S1) and 75% triplet excitons (T1) are generated after hole and electron injection in OLEDs. Thermally activated delayed fluorescence (TADF) emitters emerged as new-generation luminescent materials to fabricate highly efficient OLEDs by enabling 100% IQE through reverse intersystem crossing (RISC) based on a tiny energy gap (ΔEST) between singlet and triplet states.10 To achieve a small ΔEST, the most common way to design TADF molecules is to sufficiently separate the frontier molecular orbitals by appropriate selection of donor (D) and acceptor (A) units with different electron-donating and electron-accepting abilities.11 Organoboron-based groups with an empty p-orbital and intrinsic electron-deficient nature can serve as suitable π-electron acceptors to construct TADF emitters with D–A and D–π–A structures. In such molecules, in order to protect the empty p-orbital from being attacked by nucleophiles and enhance the stability of the compound under ambient conditions, the boron atom is commonly linked with sterically bulky aryl groups, such as 2,4,6-trimethylphenyl and 2,4,6-triisopropylphenyl.12–15 In 2016, Hatakeyama and co-workers proposed a fascinating design strategy for TADF materials based on a polycyclic B/N framework with the multiple resonance (MR) effect.16 On one hand, this heteroaromatic skeleton provides adequate protection for boron atoms; on the other hand, the opposite resonance effect of boron and nitrogen atoms realizes the staggered separation of the HOMO and LUMO, which endows the resulting materials with TADF characteristics. Benefiting from the rigid conjugated framework and short-range intramolecular charge transfer (ICT) characteristics, these materials commonly exhibit emission spectra with narrow full-width at half-maximum (FWHM), high photoluminescence quantum yields (PLQYs) and the maximum external quantum efficiency (EQEmax) of over 20% in OLEDs.
In the light of their unique electronic structure, boron-containing derivatives have aroused wide attention and interest of scientists. By reasonably adjusting the structure, number, and linking fashion of donor and acceptor groups, OLEDs prepared with organic boron-containing functional molecules as the active layers have basically realized the full-visible emission from the blue to red region. We mainly divide the reported boron compounds into three parts, including triaryl/diarylboron-based TADF emitters, boron/oxygen (B/O)-based TADF emitters and boron/nitrogen (B/N)-based TADF emitters. In this paper, the recent research progress and prospect of organoboron-based TADF materials are reviewed systematically with emphasis on the molecular design, photophysical properties and properties of the corresponding OLEDs.
2. Highly efficient TADF emitters based on three-coordinate organoboron compounds
2.1 Triaryl/diarylboron-based TADF emitters
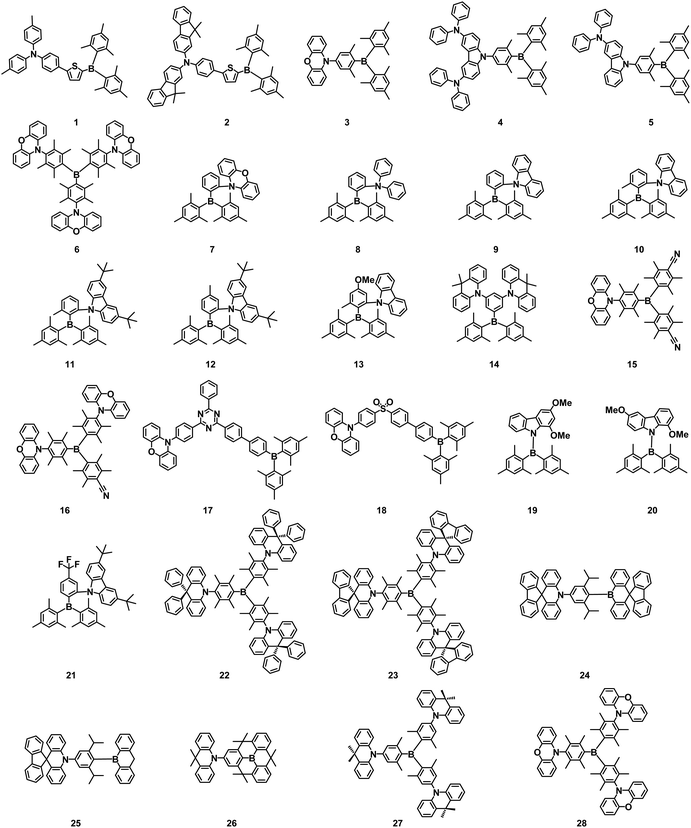
In 2000, Shirota and coworkers reported the first D–A type three-coordinate organoboron compounds, 1 and 2, using diarylboron as the acceptor. In tetrahydrofuran (THF) solution, the emission peaks of 1 and 2 were at 515 nm and 537 nm, respectively. The OLEDs using 1 and 2 as emitters realized blue-green and green emission, respectively, but with a low EQE of only 0.8% and 1.0%. Thermally activated delayed fluorescence (TADF) materials have become the rising star of OLEDs by furnishing electroluminescence (EL) performance comparable with phosphorescent materials, which was initially reported by Adachi et al. in 2011.17 Since then, many highly efficient triarylboron-based TADF molecules have been synthesized and their device performances are also significantly improved.In 2015, Kaji and coworkers successfully prepared TADF emitters 3–5 applying the trimesitylboron (Mes3B) unit as a weak acceptor, and phenoxazine (PXZ), bis(diphenylamino)carbazole (2DAC), and diphenylaminocarbazole (DAC) as respective donors. They displayed sky-blue to green emission, high PLQYs of 87–100% in host matrices, and small ΔEST values of 0.058–0.071 eV, respectively. OLEDs using these emitters as dopants also exhibited EQEmax of 14.0–22.8%, respectively.18 Later, they further reported triarylboron-based TADF emitter 6 by deliberate modulation of the RISC process. 6 possessed a relatively small ΔEST of 0.01 eV and large kRISC of 1.947 × 106 s−1. The solution-processed OLED with 6 as the emitter achieved an EQEmax of 13.9% with an EL peak at 510 nm and CIE coordinates of (0.23, 0.54). The EQE remained at 11.7% at a luminance of 1000 cd m−2 with a slight efficiency roll-off of 16%.19
Lee and coworkers reported a synthetic approach to highly efficient D–A type TADF compounds PXZoB (7), DPAoB (8) and CzoB (9), wherein the acceptor was based on triarylboron and the donor was phenoxazine, diphenylamine, or carbazole. Combined with the ortho D–A connectivity, the bulky nature of the triarylboron endowed the D–A dyads with inherent steric “locking” for a highly twisted arrangement, leading to a small ΔEST and thus exhibiting very efficient TADF with microsecond-range lifetimes. In particular, the pure blue OLEDs based on 9 with a carbazole donor showed a high EQEmax of 22.6% with CIE color coordinates of (0.139, 0.150), well illustrating the validity of the proposed approach. Upon optical optimization, the EQEmax was further improved to 24.1%.20 In 2017, Lu and coworkers also reported two ortho-D–A arranged blue TADF compounds 10 and 11. The significant intramolecular D–A interactions induced a combined charge transfer pathway and thus achieved small ΔEST and high efficiencies. The blue non-doped OLEDs based on 10 prepared from the solution process achieved the EL emission peaking at 463 nm with an EQEmax of 19.1% and CIE coordinates of (0.15, 0.17).21 Next, Lee et al. further developed a series of ortho-carbazole-appended triarylboron compounds by introducing tert-Bu, Me, and OMe to the carbazole donor and/or to the dimesitylphenylboron (PhBMes2) acceptor. Depending on the various substituents on the donor and/or acceptor moieties, the emission color was finely tuned over the entire blue region from sky blue to ultradeep blue. Retention of a twisted D–A structure enabled by the ortho connectivity and bulky triarylboron led to small ΔEST. Among these, OLEDs using 12 as the emitter showed a high EQE of 32.8% with CIE coordinates of (0.135, 0.266). This high device efficiency was caused by a synergistic effect between the high PLQY (93%) and the high horizontal transition dipole ratio of 0.76. Deep blue OLEDs using 13 as the emitter showed an EQEmax of 14.9% with CIE coordinates of (0.151, 0.058).22
Lu and coworkers synthesized three D–A–D borylated compounds with functional acridan derivatives as donors and dimesitylborane as the acceptor in 2018. A small ΔEST and fast intramolecular charge transfer were caused by this crowded structure, which enabled adjacent electron donor and acceptor to form a nearly orthogonal configuration. As the ability of donors increased, the emission spectrum shifted from green to yellow region. The green device based on 14 achieved an EQEmax of 19.3% with CIE coordinates of (0.250, 0.527) and dropped to 18.2% at luminance levels of 1000 cd m−2.23
In 2019, two TADF emitters 15 and 16 by introducing the cyano group into triarylboron/phenoxazine hybrids were prepared by Yang et al.; 15 and 16 acquired PLQYs of 67% and 75%, ΔEST of 0.144 eV and 0.077 eV, respectively. The OLEDs based on 15 and 16 achieved the EQEmax of 9.7% and 11.4%, respectively, with EQEs up to 8.9% and 10.2% at 2000 cd m−2. The small device efficiency roll-offs of 15 and 16 were owing to the short delayed fluorescence lifetimes of 1.6 μs and 1.4 μs, respectively.24
In the same year, a series of D–A–A TADF compounds 17 and 18 were obtained by Wang et al., in which (Mes)2B was combined with triazine and sulfonyl units to construct tandem acceptors. These compounds possessed tiny ΔEST values contributed by the largely expanded LUMO distributions caused by the conjugation between the boron empty p-orbital and the p*-orbital of the other parts of the tandem acceptors. OLEDs based on 18 showed an EQEmax of 24.8% and efficiency roll-off only of 5.6% at a practical luminance of 1000 cd m−2.25
In 2020, Chi et al. designed methoxy-substituted carbazoles, and the corresponding directly N-borylated emitters were prepared with the aim of improving the TADF characteristics of the carbazole-based (Mes)2B emitters. Emitters 19 and 20 showed significantly delayed fluorescence, confirming the potential of methoxy substituents in improving TADF characteristics to harness the electron-donating strength and special orthogonality. Doped devices using 19 and 20 as emitters exhibited EQEmax of 12.5% and 13.3%, respectively.26
A series of ortho-carbazole-appended triarylboron compounds, in which perfluoroalkyl (CF3 and C3F7) or perfluoroaryl (4-CF3C6F4) groups were attached as secondary acceptors, were prepared and characterized by Lee et al. in 2020. In toluene, these compounds revealed emission spectra of light greenish to yellow with high PLQYs up to 100%. The optimized OLEDs based on CF3-substituted emitter 21 realized a high EQE of 29.9% and a low turn-on voltage of 2.35 V.27
Yang et al. constructed TADF materials 22 and 23 with the centrosymmetric 3D-A structures by connecting 9,9-diphenyl-9,10-dihydroacridine-9 (DPAc) and 10H-spiro[acridine-9,9-fluorene] (SpiroAc) with triarylboron groups in 2021. These molecules possessed large dihedral angles between the triarylboron unit and the acridine plane. Because of their large volume and rigid structure, 22 and 23 showed PLQYs of 71.6% and 84.3%, respectively. Solution-processed OLEDs using 22 and 23 as emitters realized EQEmax of 12.8% and 17.3% with EL peaks of 472 nm and 490 nm, respectively.28
In 2021, by combining a weak spiro-donor and a weak spiro-acceptor through a sterically bulky p-spacer, linear D–π–A type TADF molecules with higher molecular rigidity and non-conjugated spiral fragments at both ends of the molecule were constructed by Wang et al. Two deep-blue emitters 24 and 25 were achieved for resolving the lack of highly efficient deep-blue OLEDs matching the display requirements. The weak D and A groups endowed the compounds with deep-blue emission and the helical structure suppressed the concentration quenching simultaneously. These emitters showed high PLQYs (>90%) in toluene. Both doped and non-doped deep-blue OLEDs based on 24 achieved an EQE of over 22% and CIEy coordinates less than 0.1.29
In 2021, Lee and coworkers reported a set of D–A-type blue TADF compounds comprising triply bridged triarylboryl acceptors, the so-called B-heterotriangulenes. Compound 26 exhibited a fast RISC (kRISC ≈ 106 s−1) with short delayed fluorescence (τd ≈ 2 μs), which was found to be promoted by the strong spin–orbit coupling between the local triplet excited state (3LE, T2) and the singlet (S1) state. Devices based on 26 achieved an EQEmax of 28.2% and maintained an EQE of 21.2% at 1000 cd m−2.30
In 2022, two TADF emitters with a D3–A structure, 27 and 28, by the combination of acridine/phenoxazine donors and triarylboron acceptors were obtained by Liu et al. Because of the high steric hindrance and strong intramolecular charge transfer state between the D/A groups, these emitters exhibited well-separated frontier molecular orbitals distributions and obvious TADF characteristics. The high-rigidity “star-shaped” configuration of 27 and 28 endowed both emitters with high PLQYs of up to 94% and 89%, and high ratios of horizontal dipole orientation of 86% and 80%, respectively. Doped OLEDs based on 27 and 28 realized an EQEmax of 38.8% and 29.4%, respectively.31
Triarylboron or diarylboron moieties are the simplest forms of boron acceptors that are generally used in the material design of D–A TADF emitters. The intrinsically reactive boron center is generally embraced by sterically bulky aryl groups to provide enough protection and chemical stability under ambient conditions. Three-coordinate organoboron acceptors are modified with various donors in diversified configurations to achieve small ΔEST and induce delayed fluorescence. This approach is also advantageous for tuning emission wavelength, PLQY in the solid state, and EQE in OLEDs. In this part, we summarize representative TADF emitters using three-coordinate boron derivatives as acceptors including synthetic strategies, photophysical properties and device performance. Blue to green TADF emitters have been illustrated based on a molecular platform employing three-coordinate boron-containing acceptors, and a high EQEmax over 30% in blue and green OLEDs has been realized. However, they suffer from a relatively broad emission spectrum because of the CT nature of S1. Fig. 1 shows the chemical structures of various three-coordinate boron derivatives and the photophysical properties, and device performances for TADF materials discussed above are summarized in Table 1.
| Compound | PLQYa [%] | λ PL [nm] | λ EL [nm] | ΔESTd [eV] | EQEmaxe [%] | CIEf [x, y] | Ref. |
|---|---|---|---|---|---|---|---|
| a Photoluminescence quantum yield. b PL emission maximum. c EL emission maximum. d Singlet–triplet energy gap. e Maximum external EL quantum efficiency. f Maximum external quantum efficiency. | |||||||
| 3 | 92 | 509 | 502 | 0.071 | 22.8 | 0.22, 0.55 | 18 |
| 4 | 100 | 495 | 492 | 0.058 | 21.6 | 0.18, 0.43 | 18 |
| 5 | 87 | 477 | 488 | 0.062 | 14.0 | 0.17, 0.30 | 18 |
| 6 | 65 | 509 | 510 | 0.01 | 13.9 | 0.23, 0.54 | 19 |
| 7 | 49 | 585 | — | 0.01 | 16.3 | 0.460, 0.505 | 20 |
| 8 | 80 | 498 | — | 0.20 | 21.4 | 0.172, 0.488 | 20 |
| 9 | 84 | 463 | — | 0.15 | 22.6 | 0.139, 0.150 | 20 |
| 10 | 61 | 465 | 463 | 0.06 | 8.0 | 0.15, 0.17 | 21 |
| 11 | 94 | 476 | 474 | 0.05 | 19.1 | 0.15, 0.26 | 21 |
| 12 | 93 | 478 | 479 | 0.092 | 32.8 | 0.135, 0.266 | 22 |
| 13 | 63 | 445 | 451 | 0.14 | 17.3 | 0.150, 0.086 | 22 |
| 14 | 46.8 | 505 | 507 | 0.03 | 19.3 | 0.250, 0.527 | 23 |
| 15 | 67 | 533 | 533 | 0.144 | 9.7 | — | 24 |
| 16 | 75 | 517 | 530 | 0.077 | 11.4 | — | 24 |
| 17 | 65 | 546 | 557 | 0.037 | 18.6 | 0.43, 0.54 | 25 |
| 18 | 84 | 533 | 535 | 0.013 | 24.8 | 0.37, 0.55 | 25 |
| 19 | 57 | 501 | 468 | 0.096 | 12.5 | 0.18, 0.24 | 26 |
| 20 | 100 | 452 | 467 | 0.149 | 13.3 | 0.18, 0.19 | 26 |
| 21 | 97 | 513 | 521 | 0.02 | 29.9 | 0.330, 0.598 | 27 |
| 22 | 71.6 | 474 | 472 | 0.12 | 12.8 | 0.15, 0.20 | 28 |
| 23 | 84.3 | 484 | 490 | 0.07 | 17.3 | 0.17, 0.40 | 28 |
| 24 | 95 | 453 | 444 | 0.0005 | 25.4 | 0.151, 0.058 | 29 |
| 25 | 94 | 448 | 437 | 0.0006 | 16.2 | 0.166, 0.066 | 29 |
| 26 | 90 | 462 | 478 | 0 | 28.2 | 0.136, 0.246 | 30 |
| 27 | 94 | 495 | 508 | 0.03 | 38.8 | 0.22, 0.52 | 31 |
| 28 | 89 | 553 | 560 | 0.03 | 29.4 | 0.45, 0.53 | 31 |
2.2 Boron/oxygen (B/O)-based TADF emitters
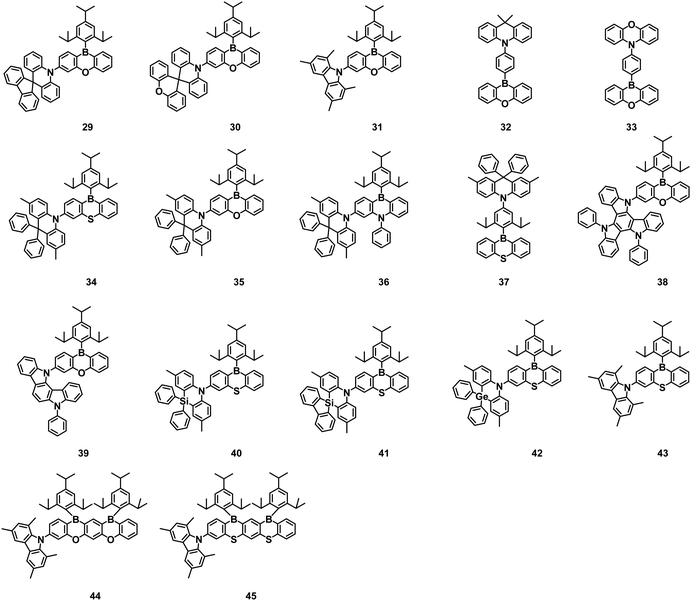
When the boron acceptor structure is modified by oxygen atoms, the acceptor strength will be weakened by the nonbonding electrons of oxygen. These boron/oxygen-based acceptors are suitable for the construction of blue TADF OLEDs. One type is a partially bridged B/O acceptor (10H-phenoxyaborin) by inserting an oxygen atom into the para position of the boron in the acceptor. Another type is the full bridged B/O acceptor (DBNA) consists of two oxygen atoms and one boron atom embedding in three benzene rings.Oi et al. designed and synthesized a series of TADF compounds using the 10H-phenoxaboryl group as the electron acceptor, and carbazole/9,9-dimethylacridine/phenoxazine as the electron donor. Among these, compounds 32 and 33 exhibited high PLQYs of 98% and 99%, and obvious TADF properties because the HOMO and the LUMO were well separated. The devices using 32 and 33 as emitters realized light blue and green emission, with an EQEmax of 15.1% and 22.1%, respectively.33
A new family of TADF molecules (34–37) with a dibenzoheteraborin unit as the acceptor were developed by Yasuda et al. in 2018. By introducing heteroatoms S and N into the 10H-phenoxyaboryl group to obtain dibenzoaborins, all the resulting emitters exhibited PLQY of nearly 100% and narrowband emission. According to the TDDFT calculation, the large spatial repulsive force between the hydrogen atoms around the D and A units limited the structural deformation between the ground and excited states and reduced the recombination energy during the emission process, resulting in narrowband emission in the TADF molecules. The up-conversion process was greatly accelerated owing to the heavy atomic effect of the S atom, improving the RISC rate of over 106 s−1. Both doped and nondoped TADF OLEDs achieved a high EQE of over 20% and negligible efficiency roll-off at actual high luminance.34
Kwon and Lee et al. prepared PXB-DI (38) and PXB-mIC (39) containing oxygen-bridged, symmetric and rigid boron acceptor moieties in 2019. 38 and 39 exhibited deep-blue emission and a small singlet–triplet energy gap of 0.06 eV and 0.11 eV, respectively, in toluene. In addition, they also reported a new high triplet energy and hole transport-type host material, 5-(5-(2,4,6-triiso-propylphenyl)pyridin-2-yl)-5H-benzo[d]benzo[4,5]imidazo[1,2-a]imidazole (PPBI). A device with 39 in the PPBI host exhibited the EQEmax of 12.5% with the CIE coordinates of (0.15, 0.08). The doped device based on 38 using 2,8-bis(diphenylphosphine oxide)dibenzofuran (DBFPO) as the host achieved an EQEmax of 37.4%, and maintained EQE over 30% at 1000 cd m−2 with a small efficiency roll-off.35
As a continuation of the previous article, Yasuda et al. further also developed a series of blue TADF emitters (40–43) by incorporating phenazasiline, phenazagermine, and tetramethylcarbazole as weak D units and phenothiaborin as a weak A unit. The emitters all had narrow bands and allowed systematic modulation of TADF properties by changing the electron strength and rigidity of the donor. In particular, phenazasiline and phenazagermine-based emitters 40 and 42 exhibited blue emissions (464–483 nm), high PLQYs (∼100%), extremely fast spin-converting reverse intersystem crossing rates (>107 s−1), and suppressed concentration quenching. The doped and non-doped OLEDs based on 40 and 42 demonstrated an EQEmax of 27.6% and 20.9% with CIE coordinates of (0.14, 0.26) and (0.14, 0.20), respectively, and suppressed efficiency roll-offs at practically high luminance.36 Later, they also reported excellent blue TADF emitters featuring π-extended ladder-roxaborin and thiaborin acceptors. Steady-state and time-resolved photophysical measurements revealed the advantages of the ladder-oxaborin emitter, including a tiny ΔEST of 10 meV, an ideal PLQY of 100%, and an ultrashort TADF lifetime of 780 ns. 44 and 45 achieved an EQEmax of up to 20.1% and 25.9% with the EL emission at 473 nm and 484 nm, respectively. As the luminance increased to 100 and 1000 cd m−2, the EQE of 44 was still maintained at 19.9% and 17.6%, respectively, corresponding to a 1% and 12% roll-off.37
On account of the good electron-deficient ability of the triarylboron acceptor, the boron-based acceptor structure has been modified in various ways. Introduction of oxygen atoms into the boron-based acceptor structure is a promising way to construct an effective acceptor of 10H-phenoxaborin with the closed ring system inserted by the boron atom. By incorporating boron-based heteroaromatics into the D–A structure, many 10H-phenoxaborin-based compounds have been demonstrated to be effective TADF materials in OLEDs. They mostly exhibit emission in the blue region because of the relatively weak acceptor strength of 10H-phenoxaborin. They also can be applied in combination with other electron-donating heteroatoms, such as oxygen (E = O), nitrogen (N), sulfur (S), and selenium (Se) and offer high PLQY and high RISC rates. However, the device lifetimes of this class have not been covered, and broad spectra issues should also be resolved. The chemical structures of 10H-phenoxaborin-based derivatives are shown in Fig. 2, and the photophysical performances and device performances are summarized in Table 2.
| Compound | PLQYa [%] | λ PL [nm] | λ EL [nm] | ΔESTd [eV] | EQEmaxe [%] | CIEf [x, y] | Ref. |
|---|---|---|---|---|---|---|---|
| a Photoluminescence quantum yield. b PL emission maximum. c EL emission maximum. d Singlet–triplet energy gap. e Maximum external EL quantum efficiency. f Maximum external quantum efficiency. | |||||||
| 29 | 76 | 456 | — | 0.12 | 19.0 | — | 32 |
| 30 | 56 | 451 | 450 | 0.06 | 20.1 | 0.14, 0.16 | 32 |
| 31 | 86 | 443 | — | 0.12 | 13.3 | 0.14, 0.16 | 32 |
| 32 | 98 | 440 | 466 | 0.013 | 15.1 | — | 33 |
| 33 | 99 | 482 | 503 | 0.028 | 22.1 | — | 33 |
| 34 | 96 | 493 | 503 | 0.023 | 25.3 | 0.20, 0.51 | 34 |
| 35 | 100 | 477 | 489 | 0.024 | 24.9 | 0.16, 0.38 | 34 |
| 36 | 50 | 449 | 475 | 0.050 | 16.0 | 0.14, 0.23 | 34 |
| 37 | 91 | 487 | 500 | 0.030 | 16.4 | 0.19, 0.45 | 34 |
| 38 | 97 | 458 | — | 0.09 | 37.4 | 0.16, 0.34 | 35 |
| 39 | 63 | 438 | — | 0.19 | 12.5 | 0.15, 0.08 | 35 |
| 40 | 100 | 479 | 478 | 0.08 | 27.6 | 0.14, 0.26 | 36 |
| 41 | 100 | 483 | 484 | 0.06 | 23.9 | 0.14, 0.32 | 36 |
| 42 | 92 | 468 | 476 | 0.11 | 15.7 | 0.14, 0.22 | 36 |
| 43 | 92 | 476 | 478 | 0.11 | 21.6 | 0.14, 0.26 | 36 |
| 44 | 100 | 476 | 473 | 0.01 | 20.1 | 0.13, 0.20 | 37 |
| 45 | 93 | 483 | 484 | 0.17 | 25.9 | 0.14, 033 | 37 |
Wang and coworkers reported three isomeric boron-containing sky-blue TADF emitters 50–52, which were constructed by incorporating an electron-donor acridine (AC) moiety into meta-, para-, or meta′-positions of an electron-accepting boron-embedded rigid framework in 2019. The experimental results showed that the para-substituted compound 51 exhibited higher decomposition temperature, higher PLQY of 96%, smaller ΔEST of 0.009 eV, shorter delayed fluorescence lifetime as well as a fast RISC rate of over 106 s−1, compared to the meta-isomers 50 and 52. Bright OLEDs with EQEs up to 20.5% and 14.1% were achieved by employing 51 as doped and non-doped emitters in sky-blue OLEDs, respectively.39
In 2019, Kwon et al. prepared two highly efficient deep-blue TADF emitters, 53 and 54, containing oxygen-bridged, symmetric and rigid boron acceptor moieties. The 20 wt%-doped films of 53 and 54 in the DBFPO host showed a high dipole moment and high PLQYs close to 100%. The fabricated 53-based devices using PPBI and DBFPO as hosts exhibited high EQE of 32.23 ± 0.24% and 38.15 ± 0.42%, with CIE coordinates of (0.14, 0.15) and (0.15, 0.28), respectively. The 54-based devices using PPBI as a host realized an EQEmax of 21.50 ± 0.22% with CIE coordinates of (0.15, 0.06) and FWHM of 48 nm.40 To satisfy both high efficiency and long lifetime, the Kwon group further designed and synthesized an efficient and stable blue TADF emitter 55, which exhibited a short-delayed exciton lifetime of 1.25 μs, high PLQY of 95.3%, a small ΔEST of 0.03 eV, high kRISC of 6.21 × 106 s−1 and good electrochemical stability. At the initial luminance of 1000 cd m−2, the fabricated OLEDs displayed a high EQE of 28.1% and a long device lifetime of 329 h. The optimized OLEDs with mixed host exhibited an EQEmax of 26.4% and a two-folded longer lifetime of 540 h than the single host device.41
Choi et al. reported three TADF emitters 56–58 utilizing carbazole derivatives as donors and DBNA as the acceptor in 2020. Compared with the solution state, the PL intensity of these emitters was enhanced by a factor of 11–25 in the aggregated state, confirming the AIE behavior. Non-doped solution-processed OLEDs based on 56–58 exhibited EQEmax and CIE coordinates of 9.90% and (0.17, 0.07), 6.13% and (0.15, 0.08), and 6.04% and (0.18, 0.40), respectively.42
TADF emitters, 59 and 60, connecting boron acceptor with the spiro-type spiro-biacridine (SAB) and spiro-acridine fluorene (SAF), were developed in 2020 by Kim et al. Because of the deep HOMO energy levels of the donors, they exhibited blue and deep-blue emissions. Benefiting from the rigid donor and acceptor, 59 and 60 showed relatively narrow emission spectra with FWHM of less than 65 nm. A high horizontal emitting dipole ratio of over 80% came from the long molecular structure along the transition dipole moment direction. The OLEDs using 59 and 60 as emitters achieved EQEmax of 25.7% and 28.2% and CIE coordinates of (0.144, 0.212) and (0.142, 0.090), respectively.43
Kwon group further reported two deep-blue TADF materials, 61 and 62, by introducing heteroatomic sulfur and oxygen into the heavy rigid donor. As compared with 55 which possessed a similar skeleton, both emitters showed deep-blue emission below 450 nm owing to the enhancement of the optical band gap over 2.8 eV through deeper HOMO level of heteroatom-based donors. The OLEDs based on 61 and 62 demonstrated the EQEmax of 33.2% and 32.8%, respectively. Since they both showed deep blue emissions and high efficiencies, HF-OLEDs were fabricated using ν-DABNA as a fluorescence dopant. 62 as a TADF-sensitized host in HF-OLEDs revealed an outstanding EQE of 38.8% along with narrow FWHM of 19 nm in the bottom emission pure blue OLEDs.44
In 2021, Choi et al. developed three solution-processable A–π-2D-type deep-blue emitters 63–65 by connecting two carbazole analogs as donors and a boron-fused unit as an electron acceptor to the benzene core. The luminescence mechanism of the three emitters changed from fluorescence to TADF as the donor unit was changed from carbazole to indenocarbazole, that is, only 64 and 65 exhibited TADF characteristics. Non-doped solution-processed OLEDs based on 65 showed an EQEmax of 10.11% and CIE coordinates of (0.16, 0.08).45
Yang et al. reported two twisted D–A type TADF materials, 66 and 67, to solve the concentration quenching effects and serious efficiency roll-off of blue TADF materials with high horizontal dipole orientation. Significantly, 66 exhibited excellent TADF properties with a short delayed fluorescence lifetime of only 684 ns and narrow FWHM of 44 nm in the solution state, accompanied by the high PLQYs of over 80% at high doping concentrations, manifesting the alleviated exciton quenching effects. OLEDs based on 66 achieved an EQEmax of 29.3% and a maximum brightness of 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663 cd cm−2. Importantly, an EQE over 20% was realized even at the high brightness of 10
663 cd cm−2. Importantly, an EQE over 20% was realized even at the high brightness of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd cm−2, signifying a small efficiency roll-off.46 In 2021, they further prepared three blue emitters 68–70, by binding boronaphthalene [3,2,1-de] anthracene (BO) acceptor and acridine fluorene donors. The photophysical properties were further adjusted by substituting additional benzene at different locations of the donor to understand the relationship between the structural properties. 68 had a high kRISC of 1.52 × 106 s−1 and 69 exhibited accelerated radiative decay when benzyl was attached to fluorene. The blue OLEDs based on 68–70 achieved EQEmax values of 23.9%, 20.3% and 19.3%, respectively. With the number of phenyl groups increased, CIEy coordinates decreased to 0.197 for 69 and 0.188 for 70, which provided a fine tuning of color purity.47
000 cd cm−2, signifying a small efficiency roll-off.46 In 2021, they further prepared three blue emitters 68–70, by binding boronaphthalene [3,2,1-de] anthracene (BO) acceptor and acridine fluorene donors. The photophysical properties were further adjusted by substituting additional benzene at different locations of the donor to understand the relationship between the structural properties. 68 had a high kRISC of 1.52 × 106 s−1 and 69 exhibited accelerated radiative decay when benzyl was attached to fluorene. The blue OLEDs based on 68–70 achieved EQEmax values of 23.9%, 20.3% and 19.3%, respectively. With the number of phenyl groups increased, CIEy coordinates decreased to 0.197 for 69 and 0.188 for 70, which provided a fine tuning of color purity.47
In 2021, Hong and coworkers obtained efficient deep-blue TADF emitters, 71 and 72 comprising almost perpendicularly linked rigid DBNA electron acceptors and a rigid linear tri-spiral acridine electron donor. The 10 wt% doped films of 71, 72 in a bis[2-(diphenylphosphino)phenyl]ether oxide (DPEPO) host showed efficient TADF emission and PLQYs of 97% and 90%, respectively, because of the rigid and twisted structures and the appropriate singlet and triplet energy levels. The OLEDs based on 71 and 72 exhibited high EQEmax of 31.2% and 28.2%, with CIEy coordinates of 0.092 and 0.061, respectively.48
By the confinement of rigid and planar N- and B-centered donor and acceptor in sandwich-type structures, the Li group obtained two molecules, 73 and 74, which resulted in green TADF emission with up to unity efficiency and improved color purity from through-space charge-transfer excited states. With a relatively short delayed fluorescence lifetime of 11 μs, 73 demonstrated attractive green electroluminescence with an EQEmax of 34.9% and an EQE of 27.4% at 1000 cd m−2.49
Because of the weak electron-accepting ability of boron acceptors, the TADF materials discussed above tend to show excellent performance in blue OLEDs. The red light-emitting materials remain limited. In 2021, Kwon and co-workers designed and synthesized two linear A–D–A orange-red TADF materials, 75 and 76, containing rigid boron acceptors and dihydrophenazine donor moieties. They showed a small ΔEST of 0.05–0.06 eV, PLQYs as high as near unity, and a short delayed exciton lifetime (τd) of less than 2.63 μs in 5 wt% doped film. Doped TADF devices based on 75 and 76 showed EQEmax of 30.3% and 21.8%, and extremely low-efficiency roll-off (3.6% and 3.2%) at 1000 cd m−2, with the EL emission peak at 576 nm and 595 nm, respectively. The devices of 75 and 76 also demonstrated high stability, with operating device lifetime (LT50) of 159 and 193 h at 1000 cd m−2, respectively.50
The DBNA based on three adjacent boron and oxygen atoms shows relatively weak electron-withdrawing ability owing to the nonbonding electrons of oxygen. This polycyclic heteroaromatic framework with big rigidity also provides sufficient structural constraint, which enables the opposite resonance effect of boron and oxygen. As discussed above, the rigid and planar-type boron-based acceptor is effective at improving molecular stability, increasing PLQY and EQE of MR-TADF OLEDs. They show the potential as an acceptor to develop blue to green MR-TADF emitters with narrow FWHM. The above-described MR-TADF emitters containing oxygen-based fully bridged boron acceptors and different donor units are shown in Fig. 3. The photophysical properties and device performances are summarized in Table 3.
| Compound | PLQYa [%] | λ PL [nm] | λ EL [nm] | ΔESTd [eV] | EQEmaxe [%] | CIEf [x, y] | Ref. |
|---|---|---|---|---|---|---|---|
| a Photoluminescence quantum yield. b PL emission maximum. c EL emission maximum. d Singlet–triplet energy gap. e Maximum external EL quantum efficiency. f Maximum external quantum efficiency. | |||||||
| 46 | 65 | 410 | — | 0.21 | — | — | 38 |
| 47 | 60 | 410 | — | 0.31 | — | — | 38 |
| 48 | 57 | 430 | — | 0.14 | — | — | 38 |
| 49 | 92 | 492 | 504 | 0.06 | 13.9 | — | 38 |
| 50 | 89 | 492 | 492 | 0.009 | 17.1 | 0.18, 0.42 | 39 |
| 51 | 96 | 496 | 488 | 0.009 | 20.5 | 0.17, 0.36 | 39 |
| 52 | 87 | 498 | 492 | 0.031 | 14.1 | 0.18, 0.39 | 39 |
| 53 | 99 | 456 | — | 0.11 | 38.15 | 0.15, 0.28 | 40 |
| 54 | 93 | 458 | 448 | 0.06 | 25.71 | 0.14, 0.15 | 40 |
| 55 | 95.3 | 467 | 475 | 0.03 | 28.1 | 0.16, 0.39 | 41 |
| 56 | 99 | 433 | 424 | 0.064 | 9.9 | 0.17, 0.07 | 41 |
| 57 | 93 | 460 | 448 | 0.113 | 6.13 | 0.15, 0.08 | 41 |
| 58 | 90 | 494 | 492 | 0.072 | 6.04 | 0.18, 0.40 | 42 |
| 59 | 90 | — | 456 | 0.11 | 28.2 | 0.142, 0.090 | 43 |
| 60 | 87 | — | 472 | 0.07 | 25.7 | 0.144, 0.212 | 43 |
| 61 | 96.9 | 446 | 476 | 0.17 | 33.2 | 0.15, 0.24 | 44 |
| 62 | 94.8 | 445 | 476 | 0.29 | 32.8 | 0.15, 0.24 | 44 |
| 63 | 61.0 | 414 | 412 | — | 3.44 | 0.18, 0.10 | 45 |
| 64 | 86.4 | 423 | 416 | 0.26 | 6.78 | 0.17, 0.07 | 45 |
| 65 | 75.5 | 431 | 424 | 0.15 | 10.1 | 0.16, 0.08 | 45 |
| 66 | 87 | 448 | 475 | 0.01 | 18.3 | 0.13, 0.21 | 46 |
| 67 | 89 | 450 | 467 | 0.01 | 29.3 | 0.13, 0.15 | 46 |
| 68 | 99 | 477 | 473 | 0.01 | 23.9 | 0.141, 0.215 | 47 |
| 69 | 98 | 472 | 470 | 0.02 | 20.3 | 0.139, 0.197 | 47 |
| 70 | 80 | 475 | 471 | 0.20 | 19.3 | 0.143, 0.188 | 47 |
| 71 | 97 | 452 | 452 | 0.057 | 31.2 | 0.147, 0.092 | 48 |
| 72 | 90 | 446 | 448 | 0.079 | 28.2 | 0.149, 0.061 | 48 |
| 73 | 100 | 502 | 502 | 0.03 | 31.4 | 0.21, 0.50 | 49 |
| 74 | 86 | 518 | 508 | 0.11 | 34.9 | 0.23, 0.54 | 49 |
| 75 | 99.8 | 599 | 576 | 0.06 | 30.3 | 0.49, 0.50 | 50 |
| 76 | 85.4 | 610 | 595 | 0.05 | 21.8 | 0.55, 0.45 | 50 |
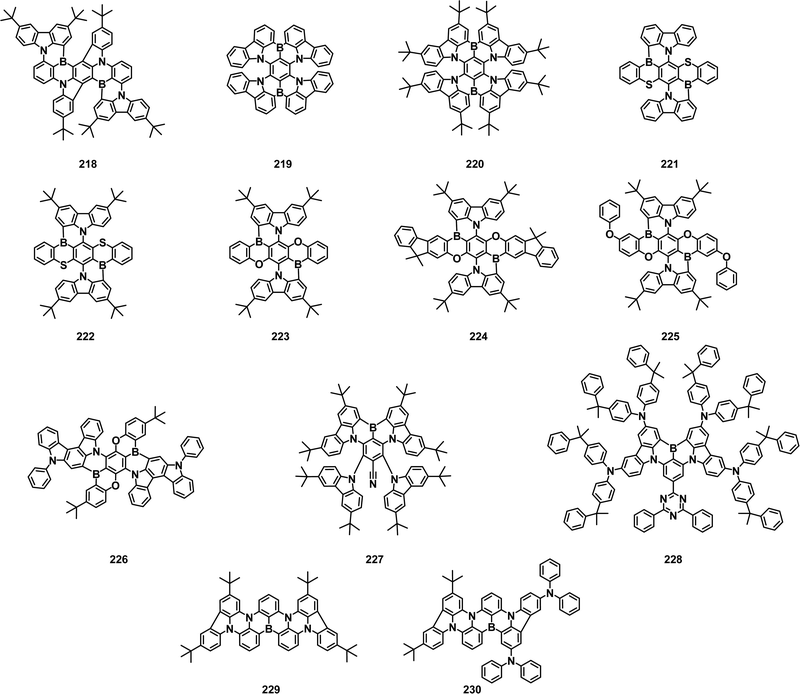
2.3 Boron/nitrogen (B/N)-based TADF emitters
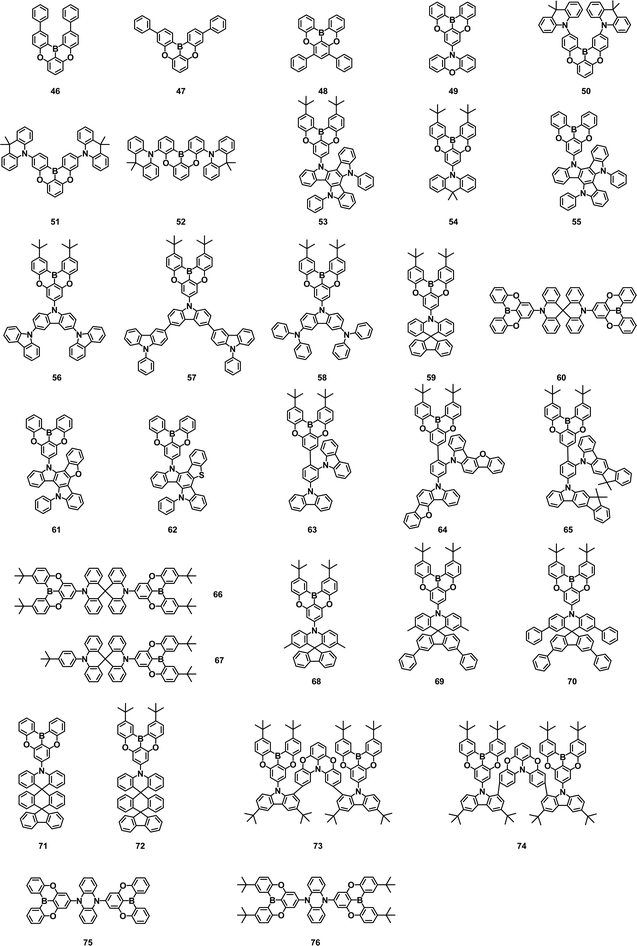
Based on the innovative design strategy of MR-TADF materials, scientists have devoted a lot of effort to obtaining new narrowband B/N compounds. We have summarized the representative design method, hoping to provide instructive guidance for further research of MR-TADF materials. The first method is to use DNBNA as the base skeleton to obtain the blue narrowband materials by directly introducing peripheral modification without changing the B/N core.
In 2018, Huang and coworkers synthesized 79 by introducing a peripheral carbazole group in the para position of the B-substituted phenyl-ring. The introduction of a carbazole unit could significantly boost the resonance effect without compromising the color fidelity. In toluene, 79 had an FWHM of 26 nm and a PLQY of 97.48%. The corresponding OLEDs exhibited excellent performance with an EL emission at 474 nm, an EQEmax of 32.1% and CIE coordinates of (0.12, 0.19).51
Subsequently, Lee and coworkers reported a blue MR-TADF molecule 80. They developed a device structure by using a TADF assistant dopant to enhance fluorescence emission of B/N type blue TADF emitters, named the TATADF device, which underwent a RISC-mediated cascade energy transfer. The combination of 80 and the TATADF device structure enabled a high EQE greater than 30%, pure blue emission color coordinates of (0.13, 0.15), a narrow FWHM of 31 nm and a lifetime improvement by more than 10 times.52 Later, they further reported a deep blue TADF molecule 81, which was resistant to the concentration quenching effect by introducing the di(tert-butylphenyl)amine group at the para position of the boron element. The additional tDPA donor promoted RISC, resulting in a three-times higher kRISC of 81 than that of t-DABNA. OLEDs based on 81 reached an EQEmax of 27.9%, CIE coordinates of (0.13, 0.08) and FWHM of 26 nm.53 As an extension of the previous article, the Lee group also reported a pure blue TADF molecule 82, which possessed an additional di-tert-butylphenyl substituted group on t-DABNA to reduce quenching of the intermolecular interaction. The single-layer device of 82 exhibited FWHM of 22 nm and EQE of 11.4%. The device lifetime was up to LT95–208 hours at 1000 cd m−2 and more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours at 100 cd m−2. The optimized tandem device of 82 achieved an EQEmax of 30.1%, which exhibited long LT95 of over 30
000 hours at 100 cd m−2. The optimized tandem device of 82 achieved an EQEmax of 30.1%, which exhibited long LT95 of over 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/500 h observed at 100/1000 cd m−2.54
000/500 h observed at 100/1000 cd m−2.54
In 2021, Wang's group designed and synthesized three deep blue narrowband MR TADF emitters 83–85 by using the periphery cladding strategy. The intermolecular interactions were suppressed by cladding a large steric hindrance tert-butyl unit at the periphery of the MR emitter, leading to a reduced emission quenching and improved PLQY. The 85-based device with 1,3-di(9H-carbazol-9-yl)benzene (mCP) as host exhibited an EQEmax of 19.3%, CIE coordinates of (0.141, 0.076) and FWHM of 26 nm, respectively.55
B/N-based emitters are usually affected by strong π–π interactions due to their rigid planar structure. In 2022, Kim and coworkers developed blue MR-TADF 86 through a simple synthetic process by introducing meta-xylene and meta-phenyphenyl groups to the core to inhibit the interaction between molecules. As a result, highly efficient pure blue OLEDs with an EQEmax of 24.3%, CIE coordinates of (0.124, 0.140), and FWHM of 28 nm were realized. The corresponding EL emission spectra can be maintained even at a high doping concentration of over 20%.56 In the same year, they reported another isomer 87. Compared with 86, emitter 87 also could effectively suppress the intermolecular interactions deriving from the π–π repulsion between the biphenyl units and the steric hindrance between ortho-xylyl groups. Adding biphenyl moieties to the core body created dense local triplet states in the vicinity of S1 and T1 energetically, letting the emitter harvest excitons efficiently. As a result, kRISC of 87 (6.85 × 104 s−1) was three times higher than that of 86 (1.95 × 104 s−1). The OLED based on 87 showed a high EQE of 23.4%, with an FWHM of 22 nm and CIE coordinates of (0.133, 0.109). By incorporating a conventional TADF sensitizer, the related device achieved deep blue emission with CIE coordinates of (0.133, 0.109) and extremely high EQEmax of 30.1%.57
An alternative strategy to obtain blue MR-TADF emitter involves the modification of the conjugate skeleton of MR-TADF by facilely extending the aromatic rings and expanding the HOMO and LUMO distribution to increase the solubility, enhancing the efficiency and modulating emission color.
In 2020, Chen et al. synthesized two molecules, 88 and 89, by replacing diphenylamine with carbazole groups and introducing tert-butyl groups into the MR skeleton. 88 and 89 exhibited sky-blue and bluish-green emission. It was found that the extension of π-conjugation and the introduction of electron-donating moieties at N-resonance positions to enhance the MR effect could narrow the bandgap and bathochromic-shift the emission spectra for small ΔEST, increased PLQY and good solubility. Unprecedentedly, the first solution-processed MR-TADF OLEDs showed the maximum CEs of 31.1 and 24.3 cd A−1, PEs of 19.5 and 15.9 lm W−1 and EQEmax of 16.3 and 14.7% for 88 and 89-based devices, respectively.58
Adachi et al. reported the utilization of a classic BN skeleton to construct two narrow-emission materials, 90 and 91. The introduction of a carbazole unit in the BN skeleton could fine-tune the energy level and enhance PLQY, while good color purity and fast radiation decay were retained. It was found that the smaller EHOMO difference between the TADF-assisted dopant and the terminal emitter helped to reduce hole capture in the emission layer, resulting in a lower efficiency roll-off and a longer device lifetime. Blue TADF-assisted fluorescence TAF-OLEDs based on 90 and 91 as terminal emitters were fabricated, resulting in a high EQE of up to 21.9%, high color purity, and a high brightness of 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 777 cd m−2 with a small efficiency roll-off (EQE of 21.2% and 19.8% at 100 and 1000 cd m−2, respectively).59
777 cd m−2 with a small efficiency roll-off (EQE of 21.2% and 19.8% at 100 and 1000 cd m−2, respectively).59
Hatakeyama and coworkers synthesized carbazole-based DABNA analogue 92 from triarylamine by regioselective one-shot single and double borylation. The reaction proceeded selectively at the ortho position of the carbazolyl group, where the highest occupied molecular orbital was mainly localized owing to the difference in the electron-donating abilities of the diarylamino and carbazolyl groups. The device using 92 as the emitter showed narrowband sky-blue emission with an EQEmax of 21.8% and CIE coordinates of (0.11, 0.23).60
Yang et al. also developed a series of asymmetric MR-TADF emitters, 93–95, in 2021. In toluene, they all exhibited similar blue emission in the 460 to 469 nm range with FWHM values as low as 20 to 23 nm. By the peripheral decoration design strategy, the diphenylaniline group was introduced into the basic molecule 93 to adjust the photophysical properties, which could maintain PLQY of over 90%, reduce the spectral widening and improve the RISC rate. Among the three OLEDs based on 93–95, the EQEmax was 23.6, 24.0 and 27.7%, and the FWHM was reduced from 34 nm to 28 nm and 24 nm, respectively.61 In 2022, they obtained two MR-TADF compounds 96 and 97 with gradually enlarged ring-fused structures and increased rigidity from the same precursor by lithium-free boration. The extension of the MR skeleton enhanced molecular rigidity, resulting in smaller structural relaxation energy, increased oscillator strength and improved RISC process. OLEDs based on 96 and 97 revealed emission peaks of 457/467nm, FWHM of 28/23 nm, and the EQEmax of 31.2/33.2%.62
In 2022, two blue TADF emitters 98 and 99 using acridan-containing arylamine derivatives as the starting materials of electrophilic C–H borylation reaction were prepared by Wang et al. These compounds had high PLQYs (94.4% and 89.7%) and narrow FWHMs (19 nm and 26 nm). The OLEDs utilizing 98 and 99 as emitters exhibited EQEmax of 21.6% and 22.3% and CIE coordinates of (0.135, 0.094) and (0.116, 0.186), respectively. The electrophilic borylation site of the borylation reaction could be controlled by varying the steric hindrance effect and electron-donating ability of the substrate substituent. The different HOMO distribution originated from the differences in the substrate substituent accounted for different cyclization modes for 98 and 99.63
A “self-host” strategy was developed by Xu et al. to achieve blue MR-TADF emitter 100 by integrating host segments into the MR skeleton without involving additional charge transfer and/or vibrational components to excited states. At a doping concentration of up to 30%, 100 exhibited a blue emission peak at 472 nm, vibrator intensity ≈0.5, radiation rate of up to 2.11 × 108 s−1 and exponentially reduced the non-radiation rate constant. Consequently, at the same time as preserving narrowband blue emission with an FWHM of ≈28 nm at a high doping concentration of 30%, 100 revealed state-of-the-art PLQY of 99% and an EQEmax of 30%, respectively.64
The design strategy of introducing heteroatoms (O/S/Se, etc.) into the MR skeleton is also commonly applied to finely modulate emission colors. For example, in 2021, Yasuda et al. designed a new family of MR-TADF materials that could exhibit narrowband emissions ranging in color from deep blue to yellow. By introducing the electron-withdrawing imine and electron-donating amine moieties into the common boron-embedded MR skeleton (Cz-B), systematic hypsochromic and bathochromic shifts of narrowband TADF emissions could be achieved, allowing effective luminescence color tuning over a wide visible range without degrading the intrinsic high PLQY. Consequently, the corresponding narrowband deep-blue to yellow OLEDs achieved an EQEmax of 19.0–29.2% with desirable EL color purity. The 101-based blue device exhibited an emission peak at 461 nm, a FWHM of 28 nm, and an EQEmax of 19.0%.65
A series of asymmetric blue MR TADF emitters 102–105 were prepared by Lee et al. in 2021. They had an asymmetric molecular structure with one boron, one oxygen, and one nitrogen. The aromatic units linked to the nitrogen were changed into diphenylamine, carbazole, dimethylacridine, and diphenylacridine to manage the light emission properties of the emitters. It was found that they all exhibited emission in the blue region due to the weak electron-donating oxygen atom and the emission wavelength was controlled by the aromatic unit connected to the nitrogen. The 102-based OLEDs possessed an EQEmax of 16.3%, a FWHM of 32 nm, and CIE coordinates of (0.15, 0.05).66
Yang et al. also elaborately constructed three oxygen-containing blue MR materials 106–108, which utilized the opposite MR effect of B and N/O atoms to achieve TADF characteristics. The 106-based deep blue OLEDs achieved an EQEmax of 13.6%, an FWHM of 36 nm with CIE coordinates of (0.14, 0.08). Devices assisted by a sensitizer based on 107 and 108 exhibited an EQE of up to 29.6% with a relatively small efficiency roll-off.67 They further reported a blue MR TADF material 109 with gradual peripheral modification in a boron/nitrogen (B/N) embedded polycyclic skeleton. The ternary TADF-sensitized device based on 109 realized blue emission peaking at 468 nm with an EQEmax of 32.0%.68 In 2022, Shao et al. designed solution-processed MR dendrimers 110–111 by introducing carbazole dendrons in the periphery of the B,O,N-doped polycyclic aromatic skeleton. 110–111 could keep the narrowband emission and suppress aggregation quenching by steric carbazole dendrons. Solution-treated OLEDs based on 110 realized the EL at 488 nm with a FWHM of 39 nm and an EQEmax of 13.4%.69 Wang and co-workers reported two kinds of B, Se, and N-doped polycyclic aromatic hydrocarbons (PAH) narrowband blue emitters (112–113). Benefiting from the heavy atom effect of Se, they exhibited a strong spin–orbit coupling and fast RISC rate (7.5–8.8 × 106 s−1), which was 2 orders of magnitude faster than conventional MR-TADF molecules. The 112-based OLEDs showed blue emission at 481 nm, FWHM of 32 nm, and the EQEmax of 22.3%.70 Subsequently, they also reported two blue emitters 114 and 115 by replacing Se with S. The device with 115 as the emitter exhibited an emission peak at 473 nm, a FWHM of 29 nm and an EQEmax of 22.0%.71
The fourth method is to introduce double or more boron atoms into the MR skeleton by borylation reactions to obtain narrowband blue emitters with even higher performance in OLEDs. In 2017, Hatakeyama and co-workers developed one-shot double, triple, and quadruple borylation reactions of triarylamines through a judicious choice of boron source and Brønsted base. With the aid of borylation reactions, a variety of BN-doped nanographenes 116–118 were synthesized in two steps starting from commercially available materials. OLEDs employing BN-doped nanographene as an emitter exhibited deep pure blue emission at 460 nm, with CIE coordinates of (0.13, 0.11), and an EQEmax of 18.3%.72 In 2019, they succeeded in the synthesis of novel TADF materials with two boron and one nitrogen atom, 119–120, via nucleophilic substitution and electrophilic C–H borylation. The kRISC values of these B–N–B molecules (7.6 × 103 s−1, 9.0 × 103 s−1) were comparable to N–B–N-type DABNA-1 (11.1 × 103 s−1), and the emission wavelength was slightly redshifted without broadening of the emission band. The OLEDs using 120 as the emitter exhibited sky blue emission at 480 nm with an FWHM of 33 nm and a maximum EQE of 21.4%.73 In 2019, Hatakeyama reported a blue emitter, 121, in which the MR core consists of five benzene rings embedded with two boron and four nitrogen atoms, and simultaneously two diphenylamine substitutions on the periphery. MR-TADF with this double meta B–π–B structure had a fully resonant extended π-skeleton, which can inhibit the emission redshift. The MR effects of boron and nitrogen atoms induce significant localization of the HOMO and LUMO on different atoms, minimizing their bonding/antibonding properties and vibration coupling between ground and excited states, leading to an unprecedented ultra-narrow FWHM spectrum of only 14 nm. OLEDs using 121 as the emitter reached the EQEmax of 34.4%, a pure blue emission at 469 nm, CIE coordinates of (0.12, 0.11) and an FWHM of 18 nm. Moreover, the device's efficiency roll-off was greatly suppressed with an efficiency of 32.8% at 100 cd m−2 and 26.0% at 1000 cd m−2, respectively.74 Later, the Hatakeyama group further synthesized an ultrapure blue MR-TADF material 122 based on 121 by introducing an oxygen atom. 122 showed a hypsochromic shift compared to the parent MR-TADF material, because of restricted π-conjugation by oxygen atom incorporation. The OLEDs using 122 as the emitter realized an EL emission peak at 465 nm, an FWHM of 23 nm, CIE coordinates of (0.13, 0.10) and an EQEmax of 29.5%. Compared with the device of 121, the 122-based device provided considerably lower efficiency roll-off and longer device lifetime (LT50 = 314 at 100 cd m−2).75
In 2021, Yasuda et al. reported a series of 121-based MR emitters 123–125 with exquisite combination and interplay of multiple boron, nitrogen, oxygen, and sulfur heteroatoms embedded in a fused polycyclic π-system. Because oxygen and sulfur atoms have weak electron-donating capacity, the emission color could be finely modulated while maintaining a narrow bandwidth. These emitters exhibited ultra-pure narrowband blue emission peaks at 445–463 nm, PLQYs of 64–93%, FWHM of 18–23 nm, and CIEy coordinates of 0.04–0.08. The OLEDs using 124–125 as emitters showed the EQEmax of 26.9% and 26.8% with CIE coordinates of (0.14, 0.06) and (0.13, 0.08), respectively.76 Afterwards, they demonstrated the strategic implementation of electron-accepting tricoordinate boron and electron-donating carbazole subunits into PAHs to produce a family of attractive full-color luminophores with narrowband emission. Among these, the sky-blue emitter 126 showed an EL peak at 471 nm, FWHM of 26 nm and EQEmax of 29.3%. With a similar design strategy, a nanographitic fused-nonacyclic p-system 127, which was strategically embedded with multiple boron, nitrogen, and sulfur atoms, was also developed by them. Narrowband sky-blue emission with a peak at 478 nm, an FWHM of 24 nm, a PLQY of 89% and an EQEmax of 29.3% was obtained in 127-based OLEDs.77,78
Later, Wang et al. found a new reaction that proceeded slowly at room temperature and accelerated at high temperature, paving the way for new, milder procedures for the preparation of B-doped PAHs with high yields and functional group tolerance. Furthermore, the potential of this B-PAH synthesis has been demonstrated by the successful and highly divergent synthesis of a series of B,N,B-doped benzo[4]helicenes 128–131. These ADBNA derivatives showed PL emission peaks at 482–487 nm and PLQYs of 71–88% in CH2Cl2.79 A “one-pot” catalyst-free borylation method was developed by Duan et al. in 2022, which generated deep-blue to pure green MR-TADF emitters by readily peripheral decoration and skeleton fusion of BCzBN. The device based on blue emitter 132 achieved an emission peak at 431 nm with an EQEmax of 13.5% and CIE coordinates of (0.16, 0.05).80
In 2021, Kown et al. developed three deep-blue MR-TADF compounds 133–135 by incorporating methyl groups and fluorine atoms in a diboron-based core. The introduction of methyl groups at para positions to the boron atoms and fluorine atoms at ortho positions to the nitrogen atoms resulted in bandgap enhancement by electron-donating and electron-withdrawing effects. All three emitters exhibit pure blue emissions with high PLQYs around ∼90%, and small ΔEST (≤0.07 eV) values. Among three materials, 134 and 135-based OLEDs demonstrated CIEy coordinates of 0.08 and 0.06, and the EQEmax of 35.8% and 33.7%, respectively.81 Further, they reported two deep blue MR-TADF emitters, 136 and 137, using double boron, three nitrogen and two oxygen atoms. Both materials showed deep blue emissions of ∼450 nm with a high PLQY of ∼90%. These materials showed very small ΔEST less than 0.06 eV and a high rate of reverse intersystem crossing of ∼2.5 × 105 s−1. The TADF devices based on 136 and 137 showed EQEmax of 30.7 and 32.5%, and CIEy coordinates of 0.06 and 0.07, respectively, which satisfy the requirements of blue BT2020.82
Several triple boron-containing MR-TADF compounds with linear structures and V-shaped B–π–B structures have also been developed. In 2020, Zysman-Colman et al. reported a rare example of non-triangulene-based MR-TADF emitter 138, which simultaneously possessed narrow, deep-blue emission with CIE coordinates of (0.17, 0.01). They also synthesized 139 with a similar structure as a derivative of 138. The red-shifted and more intense absorption allowed 139 to pair with a high-performance TADF assistant dopant and achieved an EQEmax of 15%, FWHM of 49 nm at color coordinates of (0.15, 0.10).83,84 In 2022, the Hatakeyama group synthesized MR-TADF molecule 140 with an expanded heterohelicene consisting of three BN2-embedded [4]helicene subunits. Based on the MR effect of three boron and six nitrogen atoms, 140 exhibited a narrowband sky-blue emission with an FWHM of 16 nm. Solution-treated OLEDs with 140 as the emitter showed emission wavelength at 480 nm, FWHM of 27 nm and an EQEmax of 22.9%.85 They also reported two V-shaped MR-TADF materials 141–142. The inductive effect of fluorine atoms reduced the HOMO energy of 141, leading to the blue-shifted emission peak (464 nm) compared with that of 141 (481 nm). 142 demonstrated a small FWHM of 16 nm and a high RISC rate constant (6.5 × 105 s−1). OLEDs using 141–142 as emitters exhibited blue emission at 483 and 468 nm, with FWHMs of 17 and 15 nm and CIE coordinates of (0.09, 0.27) and (0.12, 0.10), respectively. At 1000 cd m−2, both devices achieved high EQE of 26.2% and 26.6%, with tiny efficiency roll-off of 0.9% and 3.2%.86
Blue, green, and red are three fundamental colors for organic EL displays. Thus, organic blue, green, and red luminogens with superhigh color purity are of significant importance for achieving a wide color gamut in OLEDs. Organic light-emitting materials generally showed broad emission spectra and poor color purity, which remains one of the major challenges in OLEDs for many years. The pioneering discovery of blue B/N-containing MR-TADF materials has laid a reliable foundation for the development of organic light-emitting materials with narrowband emission and provided high potential for the manufacture of OLEDs with ultrahigh color purity. In fact, blue TADF OLEDs are of high interest since they are considered as promising alternatives to commercial fluorescent blue materials. In this part, blue MR-TADF materials containing DABNA units that possess maximum EL peaks below 490 nm are summarized in Fig. 4 and Table 4.
| Compound | PLQYa [%] | λ PL [nm] | λ EL [nm] | ΔESTd [eV] | EQEmaxe [%] | CIEf [x, y] | Ref. |
|---|---|---|---|---|---|---|---|
| a Photoluminescence quantum yield. b PL emission maximum. c EL emission maximum. d Singlet–triplet energy gap. e Maximum external EL quantum efficiency. f Maximum external quantum efficiency. | |||||||
| 77 | 88 | 460 | 459 | 0.20 | 13.5 | 0.13, 0.09 | 16 |
| 78 | 89 | 469 | 467 | 0.20 | 20.2 | 0.12, 0.13 | 16 |
| 79 | 97 | 470 | 474 | 0.14 | 32.1 | 0.12, 0.19 | 51 |
| 80 | 88 | — | 466 | 0.17 | 31.4 | 0.13, 0.15 | 52 |
| 81 | 96 | 446 | 459 | 0.10 | 27.9 | 0.13, 0.08 | 53 |
| 82 | 97 | 465 | 471 | 0.19 | 11.4 | 0.11, 0.16 | 54 |
| 83 | 60.8 | 449 | 456 | 0.06 | 14.7 | 0.145, 0.076 | 55 |
| 84 | 67.3 | 456 | 456 | 0.08 | 16.8 | 0.145, 0.076 | 55 |
| 85 | 74.7 | 456 | 460 | 0.10 | 19.3 | 0.141, 0.076 | 55 |
| 86 | 97 | 464 | 468 | 0.12 | 24.3 | 0.124, 0.140 | 56 |
| 87 | 98 | 462 | 464 | 0.176 | 23.4 | 0.132, 0.092 | 57 |
| 88 | 87 | 478 | 480 | 0.12 | 14.7 | — | 58 |
| 89 | 86 | 498 | 490 | 0.11 | 16.3 | — | 58 |
| 91 | 92 | 465 | 471 | 0.18 | 21.9 | 0.160, 0.305 | 59 |
| 92 | 85 | 478 | 477 | 0.14 | 21.8 | 0.11, 0.23 | 60 |
| 93 | 98 | 469 | 475 | 0.11 | 23.6 | 0.14, 0.30 | 61 |
| 94 | 92 | 460 | 469 | 0.12 | 24.0 | 0.13, 0.16 | 61 |
| 95 | 94 | 468 | 472 | 0.13 | 27.7 | 0.12, 0.18 | 61 |
| 96 | 91 | 454 | 457 | 0.20 | 31.2 | 0.14, 0.08 | 62 |
| 97 | 93 | 464 | 467 | 0.16 | 33.2 | 0.13, 0.11 | 62 |
| 98 | 94.4 | 454 | 460 | 0.17 | 21.6 | 0.135, 0.094 | 63 |
| 99 | 89.7 | 468 | 472 | 0.15 | 22.3 | 0.116, 0.186 | 63 |
| 100 | 99 | 466 | 472 | — | 30.8 | 0.14, 0.22 | 64 |
| 101 | 83 | 460 | 461 | 0.12 | 19.0 | 0.13, 0.13 | 65 |
| 102 | 86 | 433 | 443 | 0.18 | 16.3 | 0.15, 0.05 | 66 |
| 103 | 94 | 441 | 481 | 0.15 | 13.4 | 0.13, 0.22 | 66 |
| 104 | 91 | 461 | 475 | 0.11 | 16.2 | 0.12, 0.21 | 66 |
| 105 | 94 | 463 | 473 | 0.06 | 17.0 | 0.12, 0.20 | 66 |
| 106 | 96 | 443 | 454 | 0.21 | 13.6 | 0.14, 0.08 | 67 |
| 107 | 99 | 462 | 472 | 0.23 | 20.4 | 0.13, 0.19 | 67 |
| 108 | 98 | 462 | 468 | 0.19 | 23.0 | 0.13, 0.14 | 67 |
| 109 | 98 | 468 | 476 | 0.17 | 32.0 | 0.15, 0.24 | 68 |
| 110 | 94 | 476 | 488 | 0.14 | 13.4 | 0.13, 0.44 | 69 |
| 111 | 98 | 472 | 487 | 0.10 | 14.9 | 0.15, 0.45 | 69 |
| 112 | 87 | 479 | 490 | 0.15 | 20.3 | 0.13, 0.45 | 70 |
| 113 | 93 | 472 | 481 | 0.14 | 22.3 | 0.11, 0.25 | 70 |
| 114 | 82 | 476 | 482 | 0.13 | 18.9 | 0.11, 0.28 | 71 |
| 115 | 88 | 463 | 473 | 0.12 | 22.0 | 0.11, 0.17 | 71 |
| 116 | 53 | 461 | 460 | 0.19 | 18.3 | 0.13, 0.11 | 72 |
| 117 | 33 | 442 | — | 0.15 | — | — | 72 |
| 118 | 37 | 449 | — | 0.15 | — | — | 72 |
| 119 | 89 | 484 | 481 | 0.18 | 16.2 | 0.10, 0.27 | 73 |
| 120 | 88 | 482 | 480 | 0.18 | 21.4 | 0.11, 0.29 | 73 |
| 121 | 90 | 467 | 469 | 0.017 | 34.4 | 0.12,0.11 | 74 |
| 122 | — | 464 | 465 | 0.029 | 29.5 | 0.13, 0.10 | 75 |
| 123 | 76 | 441 | 445 | 0.15 | 16.6 | 0.15, 0.04 | 76 |
| 124 | 94 | 453 | 455 | 0.159 | 33.1 | 0.14, 0.06 | 76 |
| 125 | 93 | 460 | 463 | 0.144 | 32.2 | 0.13, 0.08 | 76 |
| 126 | 93 | 466 | 469 | 0.15 | 29.3 | 0.12, 0.18 | 77 |
| 127 | 89 | 478 | 478 | 0.14 | 21.0 | 0.11, 0.22 | 78 |
| 128 | 71 | 482 | — | 0.18 | — | — | 79 |
| 129 | 85 | 487 | — | 0.19 | — | — | 79 |
| 130 | 81 | 486 | — | 0.17 | — | — | 79 |
| 131 | 18 | 481 | — | 0.13 | — | — | 79 |
| 132 | 68 | 431 | 431 | 0.31 | 13.5 | 0.16, 0.05 | 80 |
| 133 | 90.5 | 464 | 471 | 0.07 | 36.2 | 0.12, 0.12 | 81 |
| 134 | 90.2 | 457 | 464 | 0.05 | 35.8 | 0.13, 0.08 | 81 |
| 135 | 88.9 | 455 | 461 | 0.07 | 33.7 | 0.13, 0.06 | 81 |
| 136 | 88.1 | 445 | 455 | 0.05 | 30.7 | 0.14, 0.06 | 82 |
| 137 | 90.3 | 451 | 460 | 0.06 | 32.5 | 0.14, 0.07 | 82 |
| 138 | 50 | 390 | — | 0.31 | — | — | 83 |
| 139 | 63 | 442 | 443 | 0.28 | 14.6 | 0.15, 0.10 | 84 |
| 140 | 80 | 484 | 480 | 0.004 | 22.9 | 0.09, 0.21 | 85 |
| 141 | 90 | 481 | 483 | 0.006 | 26.2 | 0.09, 0.27 | 86 |
| 142 | 81 | 464 | 468 | 0.005 | 26.6 | 0.12, 0.10 | 86 |
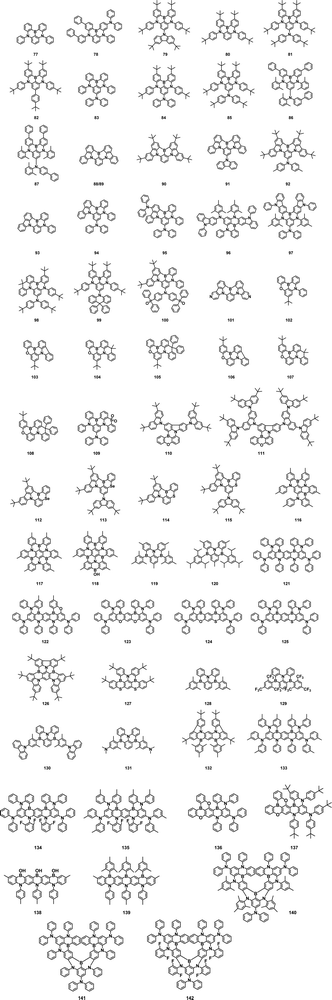
In 2019, by amplifying the influence of the skeleton and the peripheral units, Duan et al. synthesized a series of efficient green MR-TADF materials, 151–154. The electron-accepting groups expanded the distribution of LUMO, enhanced ICT characteristics, and thus narrowed the energy gap. The OLEDs based on 152–154 showed EL peaks at 501, 499, 493 nm, EQEmax of 22.0, 22.7, and 20.9%, and the FWHM of 30–40 nm, respectively.91 In 2020, the Yasuda group also reported two green MR compounds, 155 and 156 by introducing multiple tert-butylcarbazole groups around the periphery. OLEDs of 155 and 156 realized EL emission peak at 515/549 nm, FWHMs of 54/48 nm and EQEmax of 31.8/29.3%.77
For MR systems, it is found that emission regulation can be achieved by introducing the electron-donating units into the carbazole of the MR skeleton. In 2021, Yasuda and co-workers used this design strategy to synthesize two MR compounds 157–158, without compromising narrow spectral features. Because the HOMO extended to the peripheral donors, the energy gap became narrowed, resulting in a redshifted emission wavelength. The OLEDs of 157–158 revealed an EL peak at 515/571 nm, an FWHM of 30/34 nm, and an EQEmax of 29.2/19.6%, respectively.65 Similarly, by manipulating the numbers and electron-donating abilities of the peripheries, 159–161 were obtained by Yang et al.161 realized the first narrowband yellow emitter with emission maxima of 562 nm, an FWHM of 30 nm and an EQEmax of 24.7%.92
In 2021, by introducing neutral or weak donors of the trimethylbenzene unit, m-terphenyl unit, p-phenylcarbazole, and m-phenylcarbazole into the opposite position of the MR framework, four MR TADF emitters 162–165 are obtained by Lu et al. All of them successfully achieved λ values less than 0.12 eV and FWHM of about 0.11 eV (20 nm). The OLEDs based on 163 exhibited the EQEmax of 28.9% and the FWHM of 0.14 eV (28 nm).93
In 2021, Wang's group first combined circular-polarized luminescence (CPL) with MR-TADF to obtain the narrowband green CP-MR-TADF enantiomers, 166 and 167. By grafting chiral (R/S)-octahydro-binaphthol ((R/S)-OBN) derivatives onto DtCzB-Bpin, CPEL signal, narrowband emission and TADF characteristics were obtained simultaneously. The OLEDs based on 166 and 167 showed EQEmax of 29.4% and 24.5% and FWHM of 30 and 33 nm. The EL asymmetry factor (gEL) was +1.43 × 10−3/−1.27 × 10−3 and +4.60 × 10−4/−4.76 × 10−4, respectively.94
Although MR-TADF materials possess outstanding advantages, they suffer from aggregation-induced quenching and efficiency roll-off in OLEDs due to their rigid plane structure and relatively low RISC. To solve these problems, in 2022, Duan et al. sterically wrapped MR dopants with a fluorescent MR core sandwiched by bulk substituents to address the intractable challenges by reducing intermolecular interactions.
The optimal emitter 168 realized remarkably high EQEmax of 36.3–37.2%, identical small FWHMs of 24 nm, and alleviated efficiency roll-offs in OLEDs.95 Similarly, in the same year, by segregating the planar MR-TADF skeleton using two bulky carbazolyl units, a highly emissive molecule 169 with enhanced quenching resistance was reported by Yang et al. The steric effect largely removed the formation of detrimental excimers/aggregates, and boosted the performance of the corresponding device with an EQEmax up to 40.0% and FWHM of 25 nm. Even at a doping ratio of 30 wt%, the EQEmax was retained to be 33.3% with a nearly unchanged emission spectrum.96 In 2022, Jiang et al. proposed a mono-substituted design strategy by introducing spiro-9,9′-bifluorene (SBF) unit with different substituted sites into the MR-TADF system for the first time, and MR-TADF compounds 170 and 171 were thus obtained. As a classic steric group, SBF with a spatial blocking effect could hinder interchromophore interactions without participating in the MR effect. 170 and 171 exhibited high EQEmax of 32.2 and 35.9% and narrow-band emission of ∼27 nm.97 Further, two MR TADF molecules, 172 and 173, were reported by Zheng et al. in 2023 by introducing the sterically hindered unit (N-phenylcarbazole) at the para- and meta-positions of the B/N framework. The face-to-face steric modulation between the non-conjugated benzene ring and B/N skeleton was achieved to separate adjacent MR-TADF skeletons and suppress self-quenching and spectral broadening. Consequently, 172 and 173-based OLEDs showed an EQEmax of up to 33.6% and 32.6% with a FWHM of 26 and 30 nm, respectively.98
The relatively slow kRISC (<105 S−1) of MR-TADF devices commonly leads to the accumulation of triplet excitons in the emitter layer, resulting in serious efficiency roll-off. To address this issue, in 2022, You et al. put forward a “space-confined donor-acceptor (SCDA)” strategy to accelerate the RISC process. The introduction of SCDA units onto the MR-skeleton induced intermediate triplet states, which led to a multichannel RISC process and thus increased kRISC. As illustrated examples, efficient MR-emitters 174 and 175 have been developed with a sub-microsecond delayed lifetime and high kRISC of 2.13 × 10 6 s−1 and 1.55 × 106 s−1. The EQEmax and EQE at 1000 cd m−2 of 174 were up to 32.5% and 22.9%, respectively.99
In 2022, Che et al. described a simple gold(I) coordination strategy to enhance the spin–orbit coupling of green and blue BN(O)-based MR-TADF emitters, which resulted in a notable increase in the rate of the spectroscopically observed ISC process to 3 × 109 s−1 with nearly unitary ISC quantum yield. The vapor-deposited ultrapure-green OLEDs fabricated with 176 emitter delivered a high luminance of up to 2.53 × 105 cd m−2 as well as an EQE up to 30.3% with roll-offs as low as 0.8% and long device lifetimes (LT60) of 1210 h at 1000 cd m−2.100
A new MR-TADF emitter 177 was developed by Yang et al. in 2022 through the coordination of Au with a B/N-embedded polycyclic ligand. Benefitting from the Au perturbation, the RISC rate was dramatically accelerated to 2.3 × 107 s−1, leading to a delayed fluorescence lifetime as short as 4.3 μs. The EQEmax of the 177 device was 35.8% and EQE remained at 32.3% at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2.101 Heavy-atom integration into the TADF molecule could significantly promote the RISC process. Subsequently, they further reported a pure green multi-resonance TADF molecule 178 and 179 by introducing a peripheral heavy atom selenium onto the parent BN-Cz molecule. The OLEDs based on 178 exhibited state-of-the-art performance with an EQEmax of 40.1%, a PE of 176.9 lm W−1, well-suppressed efficiency roll-off and pure green gamut.102
000 cd m−2.101 Heavy-atom integration into the TADF molecule could significantly promote the RISC process. Subsequently, they further reported a pure green multi-resonance TADF molecule 178 and 179 by introducing a peripheral heavy atom selenium onto the parent BN-Cz molecule. The OLEDs based on 178 exhibited state-of-the-art performance with an EQEmax of 40.1%, a PE of 176.9 lm W−1, well-suppressed efficiency roll-off and pure green gamut.102
In 2023, Zhang et al. incorporated the heavy atom effect via facilely hanging heavy atom-containing chains onto an MR framework and thus synthesized two MR emitters (sulfur ether group for 180 and sulfone group for 181). kRISC of 180 was increased to 1.40 × 105 s−1 because of sufficient natural transition orbital (NTO) contributions from the heavy atom, compared to a relatively slow kRISC of 5.6 × 104 s−1 for 181. OLEDs using 180 as the emitter showed an EQEmax of 30.0% and an alleviated efficiency roll-off of 44% at 1000 cd m−2. In contrast, OLEDs based on 181 showed an EQEmax of 33.0% but a more severe efficiency roll-off1000 of 65%.103 In the same year, they also reported two MR-TADF emitters (182 and 183) via managing long- and short-range charge-transfer excitations to study the structure–property relationship. OLEDs based on 182 as the emitter achieved an EQEmax of 35.6% and an FWHM of 35 nm, in which short-range CT excitation was dominant in the S1 excited state. In contrast, OLEDs based on 183 achieved an EQEmax of 27.2% and a broadened FWHM of 56 nm with an overloaded long-range CT excitation in the S1 state.104
Extension of the conjugate skeleton of MR-TADF or introduction of heteroatoms into the MR skeleton is also a generally used method to finely modulate the emission color. In 2020, by fusing the difficult-to-access aza-aromatics onto B (boron)–N (nitrogen) skeleton, a hybridized MR and charge transfer (HMCT) molecule 184 was successfully synthesized by Duan et al. through an effective one-shot multiple cyclization method. 184 showed pure green fluorescence with a PLQY of 99.7%. The corresponding green device exhibited an EQEmax of 28.2% and PE of 121.7 lm W−1, respectively, with FWHM of merely 30 nm and CIEy coordinate of 0.69, representing the purest green bottom-emitting OLEDs.105
In 2022, Zheng et al. achieved two π-extended MR-TADF emitters 185 and 186via fusing conjugated high-triplet-energy units (carbazole, dibenzofuran) into the B/N framework, aiming to increase charge transfer delocalization of the B/N skeleton and minimize ΔEST. This strategy endowed the two emitters with FWHM of 27 and 29 nm, and high PLQYs above 90% in doped films, respectively. The devices of 185 and 186 showed emission peaks at 487 and 500 nm and an EQEmax of 26.1% and 28.0%, respectively.106 They further reported two emitters 187 and 188 based on indolo[3,2,1-jk]carbazole unit and boron–nitrogen skeletons, whose emission peaks were located at 496 and 521 nm with a FWHM of 34 and 29 nm, respectively. Meanwhile, fast rate constants of RISC of above 106 s−1 were obtained due to small ΔEST and large SOC values. Notably, planar molecular structures along the transition dipole moment direction endowed them with high horizontal emitting dipole ratios of up to 94%. Consequently, the corresponding OLEDs of 187 and 188 showed the EQEmax of 31.7% and 32.2%, respectively. Particularly, OLEDs with 188 displayed ultra-pure green emission with CIE coordinates of (0.22, 0.71).107
Wang et al. proposed a novel MR-TADF molecular construction paradigm based on polycyclization of the MR parent core, and constructed a representative MR-PAH based on para-alignment boron and nitrogen atoms into a six-membered ring (p-BNR). The target model molecule 189 showed green fluorescence with an emission peak at 523 nm and a narrow FWHM of 34 nm. The OLED employing BN-TP as an emitter exhibited ultrapure green emission with CIE coordinates of (0.26, 0.70), and an EQEmax of 35.1%.108 Later, based on the unique nitrogen-atom embedding molecular engineering (NEME) strategy, a series of compounds 190–193 have been customized by them. The nitrogen-atom anchored at different positions of the triphenylene hexagonal lattice entailed varying degrees of perturbation to the electronic structure. They demonstrated the precise regulation of emission maxima of MR-TADF emitters to meet the actual industrial demand, and further enormously enriched the MR-TADF molecular reservoir. The device based on 192 showed an FWHM of 33 nm, CIE coordinates of (0.23, 0.71), and an EQEmax of 37.3%.109
In 2022, Zhang et al. linked the outer phenyl groups in MR-type blue-emitting B/N molecules through bonding and spiro-carbon bridges, resulting in rigid green TADF emitters 194 and 195. The MR effect and multiple interlocking strategies greatly suppressed the high-frequency vibrations in the molecules, which emitted green light with an FWHM of 14 nm and a CIEy value of 0.77 in cyclohexane. Doping these emitters into a traditional green-emitting phosphorescence OLEDs endowed the device with a Broadcast Service Television 2020 color-gamut, 50% improved EQE, and an extremely high luminescence of 5.1 × 105 cd m−2, making it the greenest and brightest OLED ever reported.110
In addition to extending the central benzene ring in the B/N skeleton, researchers also modified the carbazole unit of the blue-green molecule DtBuCzB to produce pure green emission of MR TADF derivatives. For example, in 2021, Yang et al. demonstrated 196 and 197 with a simple modification of the B/N framework by insertion of two sp3 carbon atoms, which served as locks to rigidify the molecular backbone and also imposed a significant impact on the corresponding photophysical properties by extending the FMO to the appended phenyl units. 196 and 197 achieved EL peaks above 500 nm. The 197-based device had an EQEmax of 28.2% and CIE coordinates of (0.21, 0.65).111
Kido et al. developed a novel one-pot borylation method that did not require the use of hazardous tert-BuLi in 2021. By inserting carbon and oxygen into the skeleton, two types of green-emitting MR-TADF emitters 198 and 199 were created. OLEDs using 198 and 199 exhibited the EQEmax of 20.3% and 23.3%, respectively, with FWHM values of 49 and 47 nm, respectively.112
In 2022, Yang et al. reported a series of heavy-atom incorporating emitters 200–202, based on a sulfur or selenium-integrated B/N skeleton. 202 possessed a 100% PLQY and a high kRISC of 2.0 × 106 s−1 because of the heavy atom effect of sulfur and selenium atoms. The corresponding green OLEDs exhibited EQEmax up to 36.8% and ultralow efficient roll-off (2.8% and 14.9% at 1000 cd m−2 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2, respectively).123 Lu et al. designed asymmetric MR-TADF emitters 203 and 204. These emitters could facilitate the RISC process because sulfur atoms could effectively enhance SOC through the heavy atom effect. A high PLQY of 96% and a fast kRISC of above 1.0 × 105 s−1 were achieved in 204. The corresponding OLEDs exhibited a pure green emission with an EQEmax of 32.8%, and EQE exceeded 23% at a high brightness of 1000 cd m−2.114 Another two asymmetric molecules, 205 and 206, were realized by suppressing the shoulder peaks in the emission spectra of conventional PAHs by Duan et al. This method simultaneously improved molecular rigidity and reduced vibration frequency. The 205-based device showed an FWHM of 21 nm, an EQEmax of 30.5%, and the CIE coordinate of (0.16, 0.77), which meet the green requirements of BT.2020.115
000 cd m−2, respectively).123 Lu et al. designed asymmetric MR-TADF emitters 203 and 204. These emitters could facilitate the RISC process because sulfur atoms could effectively enhance SOC through the heavy atom effect. A high PLQY of 96% and a fast kRISC of above 1.0 × 105 s−1 were achieved in 204. The corresponding OLEDs exhibited a pure green emission with an EQEmax of 32.8%, and EQE exceeded 23% at a high brightness of 1000 cd m−2.114 Another two asymmetric molecules, 205 and 206, were realized by suppressing the shoulder peaks in the emission spectra of conventional PAHs by Duan et al. This method simultaneously improved molecular rigidity and reduced vibration frequency. The 205-based device showed an FWHM of 21 nm, an EQEmax of 30.5%, and the CIE coordinate of (0.16, 0.77), which meet the green requirements of BT.2020.115
Compared to the peripheral modified chiral unit, several research groups have developed MR-TADF molecules with chiral characteristics. In 2022, Chou et al. successfully designed and synthesized a set of integrated CPMR-TADF molecules, (+)/(−)207 and (+)/(−)208, using a strategy of asymmetric peripheral locking enantiomers. The devices based on (+)/(−)207 and (+)/(−)208 exhibited both TADF and CPL properties with FWHM of 49/50 nm and 48/47 nm, EQEmax of 20.6%/19.0% and 22.0%/26.5%, gEL of +3.7 × 10−3/−3.1 × 10−3 and +1.9 × 10−3/−1.6 × 10−3, respectively.116 Yang et al. also developed a pair of helicene-based enantiomers, (P/M) 209, which merged helical chirality and the B/N/S inserted polycyclic aromatic framework to concurrently feature CPL and narrow TADF characteristics. Green CP-OLEDs based on enantiomers demonstrated an EQEmax of 31.5%/30.7%, an FWHM of 49/50 nm, and gEL of +1.2 × 10−3 and −2.2 × 10−3, respectively.117 They further designed a pair of chiral green emitters, (R)/(S) 210. CPL properties came from the sp3 hybridization of carbon atoms, which also locked the molecular geometry and improved the molecular rigidity. The OLEDs based on (R)/(S) 210 showed an EQEmax of 37.2%/36.1%, and gEL of +2.7 × 10−4/−2.9 × 10−4, respectively.118
Introduction of two or more boron atoms into the MR skeleton also generated excellent narrowband green emitters. These MR TADF molecules are of two types depending on the position of the second B atom, that is, meta B–π–B and para B–π–B system. In 2020, Hatakeyama reported carbazole-based DABNA analogues from one-shot borylation. Under the optimized reaction conditions, the monoborylated and diborylated derivatives were selectively obtained in good yields. This facile and scalable method enabled the preparation of diverse CzDABNAs, which exhibited excellent TADF properties with small FWHMs and tuning of the emission wavelength from deep blue to green region. With the meta B–π–B and N–π–N structure, 211-based OLEDs showed a green emission peak at 497 nm, an FWHM of 29 nm, and an EQEmax of 26.7%.60 They also prepared a solution-processable MR-TADF material 212 with an extended π-skeleton and bulky substituents via a four-step process involving one-shot double borylation. Furthermore, to facilitate charge recombination, two novel semiconducting polymers with similar ionization potentials to that of 212 were synthesized for use as interlayer and emissive layers. These materials were used to fabricate a pure green OLED with CIE coordinates of (0.12, 0.63) and an EQE of 21.8%, representing the first solution-processed OLED featuring high color purity and efficiency.119 Furthermore, they reported two MR-TADF emitters with similar structures, 213 and 214. By introduction of cyano groups into the blue-emitting MR-TADF material (v-DABNA), a remarkable bathochromic shift was induced without a loss of color purity. The OLEDs based on 213 displayed an emission peak at 504 nm, an FWHM of 23 nm, CIE coordinates of (0.13, 0.65), and an EQEmax of 31.9%.120 Later, they demonstrated a sequential multiple borylation reaction that provided new synthetically accessible chemical space. 214, the proof-of-concept material, exhibited narrowband green TADF with an FWHM of 22 nm and a small ΔEST of 13 meV. The OLEDs employing it as an active layer exhibited an EL peak at 512 nm, with CIE coordinates of (0.13, 0.73) and a high EQEmax of 31.1%. Moreover, the device showed minimum efficiency roll-off, with an EQE of 29.4% at 1000 cd m−2.121 Wang et al. reported a ternary B–O–N embedded PAH, 215, by adopting the para boron–π–boron and para oxygen–π–oxygen strategy. 215 presented a vivid green emission with a high PLQY of 96% and an extremely narrow FWHM of 19 nm/0.09 eV, which surpassed all previously reported green TADF emitters to date. In addition, the long molecular structure along the transition dipole moment direction endowed it with a high horizontal emitting dipole ratio of 96%. 215-based OLEDs revealed an emission peak at 504 nm and an FWHM of 24 nm/0.12 eV. The TADF-sensitized device realized an FWHM of 27 nm and an EQEmax of 37.1%.122 Zheng et al. developed a simple strategy to achieve ternary B/N-based polycyclic heteroaromatic emitters from pure blue (463 nm) to yellow (553 nm) via tuning the coordination between B/N and heteroatom, aiming to increase charge transfer delocalization of the polycyclic heteroaromatic emitters and adjust photophysical properties. This strategy endowed the four emitters with FWHMs of 20–28 nm, respectively. Among these, 216–217 achieved FWHM of 20 nm and 28 nm and EQEmax of 26.7% and 21.8% in doped OLEDs, respectively.123
Over the last few years, huge progress has been made in the synthesis of green MR-TADF emitters for OLED applications. Compared to other colors, the definition of FWHM and the maximum emission wavelength is more stringent in green color, which creates a big challenge for ultrapure green emitters. In this section, green B/N-containing emitters possess maximum EL peaks between 490 and 560 nm. Green B/N-based TADF emitters containing DABNA units are summarized in Fig. 5 and Table 5.
| Compound | PLQYa [%] | λ PL [nm] | λ EL [nm] | ΔESTd [eV] | EQEmaxe [%] | CIEf [x, y] | Ref. |
|---|---|---|---|---|---|---|---|
| a Photoluminescence quantum yield. b PL emission maximum. c EL emission maximum. d Singlet–triplet energy gap. e Maximum external EL quantum efficiency. f Maximum external quantum efficiency. | |||||||
| 143 | 91 | 481 | 488 | 0.13 | 21.6 | 0.10, 0.42 | 87 |
| 144 | 97 | 496 | 504 | 0.09 | 23.4 | 0.15, 0.61 | 87 |
| 145 | 97 | 519 | 528 | 0.08 | 31.4 | 0.26, 0.68 | 88 |
| 146 | 94 | 521 | 532 | 0.18 | 24.6 | 0.33, 0.63 | 89 |
| 147 | 97 | 501 | 516 | 0.11 | 29.8 | 0.18, 0.67 | 89 |
| 148 | 96 | 499 | 508 | 0.11 | 28.6 | 0.16, 0.66 | 89 |
| 149 | 93 | 515 | 540 | 0.15 | 25.0 | 0.35, 0.63 | 89 |
| 150 | 97 | 491 | 496 | 0.14 | 42.0 | 0.09, 0.54 | 90 |
| 151 | — | 477 | 474 | — | 18.9 | 0.13, 0.20 | 91 |
| 152 | 88.7 | 494 | 501 | 0.16 | 22.0 | 0.16, 0.60 | 91 |
| 153 | 83.4 | 499 | 499 | 0.08 | 22.7 | 0.20, 0.58 | 91 |
| 154 | 91.4 | 496 | 493 | 0.11 | 20.9 | 0.12, 0.48 | 91 |
| 155 | 90 | 517 | 515 | 0.14 | 31.8 | 0.26, 0.68 | 77 |
| 156 | 85 | 549 | 549 | 0.14 | 29.3 | 0.38, 0.61 | 77 |
| 157 | 89 | 517 | 514 | 0.09 | 29.2 | 0.16, 0.71 | 65 |
| 158 | 87 | 576 | 571 | 0.14 | 19.6 | 0.47, 0.51 | 65 |
| 159 | 99 | 496 | 506 | 0.11 | 24.3 | 0.15, 0.63 | 92 |
| 160 | 98 | 534 | 545 | 0.13 | 24.5 | 0.38, 0.61 | 92 |
| 161 | 98 | 562 | 568 | 0.09 | 24.7 | 0.47, 0.52 | 92 |
| 162 | 92 | 486 | 488 | 0.12 | 27.8 | 0.14, 0.36 | 93 |
| 163 | 94 | 492 | 492 | 0.09 | 28.9 | 0.10, 0.46 | 93 |
| 164 | 95 | 491 | 496 | 0.15 | 27.2 | 0.13, 0.54 | 93 |
| 165 | 88 | 495 | 496 | 0.14 | 25.9 | 0.15, 0.55 | 93 |
| 166 | 99 | 493 | 496 | 0.12 | 29.4 | 0.11, 0.52 | 94 |
| 167 | 96 | 500 | 508 | 0.13 | 24.5 | 0.14, 0.64 | 94 |
| 168 | 98 | 499 | 488 | 0.13 | 37.2 | 0.11, 0.43 | 95 |
| 169 | 93 | 490 | 496 | 0.13 | 40.0 | 0.09, 0.50 | 96 |
| 170 | 93 | 493 | 492 | 0.13 | 35.9 | 0.077, 0.471 | 97 |
| 171 | 90 | 493 | 496 | 0.15 | 32.2 | 0.092, 0.515 | 97 |
| 172 | 92 | 489 | 496 | 0.14 | 33.9 | 0.08, 0.52 | 98 |
| 173 | 95 | 502 | 500 | 0.09 | 32.6 | 0.10, 0.56 | 98 |
| 174 | 93 | 490 | 513 | 0.13 | 32.5 | 0.17, 0.68 | 99 |
| 175 | 95 | 492 | 513 | 0.11 | 31.4 | 0.16, 0.70 | 99 |
| 176 | 91 | 513 | 510 | 0.08 | 30.3 | 0.16, 0.68 | 100 |
| 177 | 95 | 514 | 520 | 0.07 | 35.8 | 0.16, 0.67 | 101 |
| 178 | 96 | 517 | 517 | 0.13 | 40.1 | 0.19, 0.70 | 102 |
| 179 | 93 | 515 | 516 | 0.08 | 37.3 | 0.19, 0.70 | 102 |
| 180 | 97.6 | 488 | 500 | 0.14 | 30.0 | 0.13, 0.60 | 103 |
| 181 | 98.1 | 498 | 512 | 0.14 | 33.0 | 0.17, 0.68 | 103 |
| 182 | 97.6 | 497 | 512 | 0.14 | 35.6 | 0.16, 0.68 | 104 |
| 183 | 75.3 | 516 | 544 | 0.09 | 27.2 | 0.37, 0.61 | 104 |
| 184 | 94 | 522 | 528 | 0.18 | 25.7 | 0.28, 0.69 | 105 |
| 185 | 92 | 457 | 502 | 0.12 | 28.0 | 0.12, 0.62 | 106 |
| 186 | 93 | 500 | 491 | 0.09 | 26.1 | 0.09, 0.41 | 106 |
| 187 | 98 | 496 | 499 | 0.06 | 31.7 | 0.14, 0.56 | 107 |
| 188 | 98 | 521 | 524 | 0.01 | 32.2 | 0.22, 0.71 | 107 |
| 189 | 96 | 524 | 528 | 0.14 | 35.1 | 0.26, 0.70 | 108 |
| 190 | 95 | 534 | 532 | 0.17 | 34.9 | 0.28, 0.69 | 109 |
| 191 | 91 | 535 | 534 | 0.19 | 31.9 | 0.30, 0.67 | 109 |
| 192 | 97 | 526 | 524 | 0.15 | 37.3 | 0.23, 0.71 | 109 |
| 193 | 97 | 530 | 528 | 0.14 | 36.5 | 0.27, 0.70 | 109 |
| 194 | 93 | 531 | 535 | 0.04 | 26.2 | 0.26, 0.72 | 110 |
| 195 | 98 | 523 | 527 | 0.04 | 29.3 | 0.21, 0.75 | 110 |
| 196 | 63 | 485 | 502 | 0.14 | 21.1 | 0.14, 0.54 | 111 |
| 197 | 86 | 490 | 504 | 0.11 | 28.2 | 0.14, 0.56 | 111 |
| 198 | 88 | 4841 | 503 | 0.16 | 20.3 | 0.18, 0.60 | 112 |
| 199 | 90 | 502 | 516 | 0.17 | 23.3 | 0.22, 0.67 | 112 |
| 200 | 68 | 525 | 520 | 0.13 | 34.6 | — | 113 |
| 201 | 86 | 520 | 515 | 0.12 | 35.7 | — | 113 |
| 202 | 95 | 514 | 512 | 0.14 | 36.8 | — | 113 |
| 203 | 91 | 510 | 520 | 0.11 | 27.6 | 0.26, 0.65 | 114 |
| 204 | 96 | 505 | 516 | 0.09 | 32.8 | 0.24, 0.63 | 114 |
| 205 | 99.2 | 521 | 523 | 0.22 | 30.5 | 0.22, 0.74 | 115 |
| 206 | 98.3 | 520 | 523 | 0.18 | 29.8 | 0.22, 0.74 | 115 |
| 207 | 88 | 500 | 510 | 0.14 | 20.6 | 0.186, 0.632 | 116 |
| 208 | 87 | 497 | 506 | 0.14 | 26.5 | 0.167, 0.603 | 116 |
| (P)209 | 98 | 525 | 523 | 0.15 | 31.5 | 0.26, 0.66 | 117 |
| (M)209 | 98 | 525 | 524 | 0.15 | 30.7 | 0.26, 0.66 | 117 |
| (R)210 | 96 | 497 | 504 | 0.11 | 37.2 | 0.12, 0.63 | 118 |
| (S)210 | 96 | 497 | 503 | 0.11 | 36.1 | 0.12, 0.62 | 118 |
| 211 | 87 | 504 | 497 | 0.06 | 26.7 | 0.12, 0.57 | 60 |
| 212 | 71 | 506 | 505 | 0.12 | 21.8 | 0.12, 0.63 | 119 |
| 213 | 86 | 496 | 504 | 0.10 | 31.6 | 0.13, 0.65 | 120 |
| 214 | 87 | 509 | 512 | 0.013 | 31.1 | 0.13, 0.73 | 121 |
| 215 | 96 | 500 | 504 | 0.17 | 37.1 | 0.14, 0.53 | 122 |
| 216 | 98 | 505 | 510 | 0.13 | 26.7 | 0.17, 0.68 | 123 |
| 217 | 98 | 553 | 556 | 0.13 | 21.8 | 0.42, 0.57 | 123 |
The para D–π–D and A–π–A structures could improve the donor and acceptor strength, thus enhancing the intramolecular push-pull electron effect, which contributes to narrowing the energy gap. In 2021, Duan et al. developed two red MR compounds, 219 and 220. The introduction of B-phenyl-B and N-phenyl-N structures enhanced the electronic coupling of those para-positioned atoms, gave rise to restricted p-bonds on the phenyl-core for delocalized excited states and generated a narrow energy gap. 219 and 220 achieved EL peaks at 662 and 692 nm, FWHM of 48 and 49 nm, and EQEmax of 28.1% and 27.6% in a normal planar OLED structure.124
In 2022, MR emitters 221 and 222 were designed and synthesized for narrowband red emission by embedding two pairs of S and N atoms and two B atoms in para-positions of central benzene rings within a tridecacyclic aromatic skeleton. 222 combined high PLQY of 85% and rapid kRISC of 2.2 × 105 s−1. Solution-processed correlated OLEDs realized a maximum emission of 641 nm, an FWHM of 39 nm, and an EQEmax of 7.8%.125
In 2022, Yang et al. reported a series of red MR emitters 223–225 by para-positioning N–π–N, O–π–O, B–π–B pairs onto a benzene ring to construct an MR central core. These emitters could be facilely and modularly synthesized, allowing for easy fine-tuning of emission spectra by peripheral groups. They displayed near-unity PLQY, a fast radiative decay rate up to 7.4 × 107 s−1, and an FWHM of 32 nm. Pure red OLEDs based on 223 sensitized by phosphor realized state-of-the-art device performance with EQE exceeding 36%, ultra-low efficiency roll-off (EQE remains as high as 25.1% at the brightness of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m −2), ultra-high brightness over 130
000 cd m −2), ultra-high brightness over 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2, together with good device lifetime. By roughly assuming a moderate acceleration factor of 1.7, the LT90 at 1000 and 100 cd m−2 was estimated to be as high as 1000 and 52
000 cd m−2, together with good device lifetime. By roughly assuming a moderate acceleration factor of 1.7, the LT90 at 1000 and 100 cd m−2 was estimated to be as high as 1000 and 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h for 223-based devices.126
000 h for 223-based devices.126
In 2023, Duan et al. designed and synthesized a narrowband pure-red MR emitter226 by fusing indolocarbazole segments into a B/O-embedded skeleton. The rigid indolocarbazole segment possessed a strong electron-donating ability due to its para-positioned nitrogen–π–nitrogen backbone and also enlarged the π-extension of the MR skeleton to suppress structural displacement during radiation, achieving concurrently red-shifted and narrowed emission spectrum. An emission maximum at 637 nm with an FWHM of merely 32 nm (0.097 eV) was recorded in toluene. The corresponding device simultaneously exhibited an EQEmax of 34.4% with low roll-off, CIE coordinates of (0.708, 0.292) precisely matching the standard of red BT.2020. An ultra-long lifetime of LT95 of >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h at 1000 cd m−2 was also obtained.127
000 h at 1000 cd m−2 was also obtained.127
In addition to the above design strategy, according to a large number of previous studies, it is found that expanding HOMO/LUMO distribution and improving π-conjugation can make the emission redshift. Alternatively, by introducing a donor or an acceptor into the position of HOMO or LUMO distribution of the MR skeleton, a red-shifted emission spectrum can also be realized. In 2021, an orange-red MR material, 227, was reported by Bin et al. through the attachment of a CN functionality at the lowest unoccupied molecular orbital location of the MR-TADF skeleton which could promote attractive red-shifted emission owing to the exceptional electron-withdrawing capacity of the CN group. With the simultaneous introduction of CN and Cz groups to the blue emitting MR-TADF skeleton (BCz-BN), the emission wavelength was redshifted from 481 nm to 581 nm and the FWHM of 42 nm was maintained. The TADF-sensitized device showed EQE up to 33.7% and CIE coordinates of (0.54, 0.56).128 In 2022, Wang et al. developed a pure red MR TADF molecule, 228, by introducing auxiliary electron donor and acceptor moieties into the HOMO and LUMO distributed positions of the MR skeleton simultaneously. 228 exhibited a narrowband pure-red emission at 624 nm, with a high PLQY of 94% and a narrow bandwidth of 46 nm. Notably, the fabricated solution-processable pure-red OLED exhibited a state-of-the-art EQE over 20% with the CIE coordinates of (0.663, 0.337) and a long operational lifetime (LT50) of 1088 hours at an initial luminance of 1000 cd m−2.129 In 2022, Zhang group reported 229, an orange-red MR molecule through modifying a prototypical MR core of DABNA by fusing carbazoles to the MR framework. 229 maintained the MR-dominated features of DABNA while red-shifting the emission. At the same time, the highly distorted structure could alleviate the aggregation quenching. Its OLED achieved an EQEmax of 39.2% at 588 nm and remained above 30.3% EQE even at high doping concentrations of 30 wt%.130
In 2023, Yang et al. proposed an effective method towards a long-wavelength MR-TADF emitter by integrating a strong electron-donating indolophenazine building block into the B/N-doped polycyclic aromatic hydrocarbons. The investigation of photophysical properties revealed that different electron-donating abilities had significant effects on luminescence features, including emission wavelength and FWHM. The device based on 230 as an emitter exhibited orange-red emission with an EL peak at 584 nm, an FWHM of 0.23 eV and an EQEmax of 32.4%.131
In this section, red-to-NIR emitters are defined as molecules that possess a maximum EL peak larger than 580 nm. Similar to yellow-orange MR-TADF emitters, red, deep-red and near-infrared emitters are still under development as the fact that nonradiative transitions will significantly increase with the decreased energy gap. This effect becomes even stronger for rigid and planar systems, such as in the nonradiative deactivation of PAHs. There still exists a formidable barrier to producing deep-red/near-infrared B/N-containing MR-TADF emitters with simultaneous narrow FWHM and high PLQYs. Red B/N-based TADF emitters containing DABNA units are listed in Fig. 6 and Table 6.
| Compound | PLQYa [%] | λ PL [nm] | λ EL [nm] | ΔESTd [eV] | EQEmaxe [%] | CIEf [x, y] | Ref. |
|---|---|---|---|---|---|---|---|
| a Photoluminescence quantum yield. b PL emission maximum. c EL emission maximum. d Singlet–triplet energy gap. e Maximum external EL quantum efficiency. f Maximum external quantum efficiency. | |||||||
| 218 | 89 | 615 | 616 | 0.19 | 22.0 | 0.67, 0.33 | 77 |
| 219 | 100 | 662 | 664 | 0.18 | 28.1 | 0.719, 0.280 | 124 |
| 220 | 100 | 692 | 686 | 0.16 | 27.6 | 0.721, 0.278 | 124 |
| 221 | 80 | 631 | 613 | 0.20 | 5.8 | 0.63, 0.35 | 125 |
| 222 | 85 | 641 | 616 | 0.19 | 7.8 | 0.65, 0.34 | 125 |
| 223 | 96 | 605 | 610 | 0.25 | 35.7 | 0.64, 0.34 | 126 |
| 224 | 95 | 609 | 618 | 0.27 | 34.4 | 0.65, 0.35 | 126 |
| 225 | 96 | 616 | 625 | 0.26 | 36.1 | 0.66, 0.34 | 126 |
| 226 | 95 | 637 | 643 | 0.09 | 34.4 | 0.708, 0.292 | 127 |
| 227 | 96 | 581 | 583 | 0.18 | 33.7 | 0.54, 0.46 | 128 |
| 228 | 94 | 624 | 617 | 0.11 | 22.0 | 0.654, 0.344 | 129 |
| 229 | 99 | 558 | 588 | 0.12 | 39.2 | 0.54, 0.44 | 130 |
| 230 | 95 | 583 | 584 | 0.06 | 32.4 | 0.54, 0.45 | 131 |
3. Conclusions and outlook
In conclusion, the photophysical properties of the representative boron-containing TADF materials including triaryl/diarylboron, 10H-phenoxaborin, DBNA, DABAN derivatives, and the performance of the corresponding OLEDs are summarized and analyzed systematically. OLEDs based on traditional TADF triaryl/diarylboron compounds have realized comparable, and even surpassed performance compared to state-of-the-art OLEDs adopting organometallic phosphorescent complexes. The broad emission spectra and poor color purity of organic light-emitting materials are one of the major challenges in their practical applications for many years. In this regard, organoboron-based MR-TADF emitters distinguish themselves from traditional D–A TADF materials and provide a potential solution for high-purity OLEDs. The MR-TADF materials with narrowband emission undoubtedly have become one of the most attractive and important projects for future ultrawide-color-gamut OLED displays meeting the B.T.2020 standard. Using a delicate molecular design strategy, the outstanding potential of MR-TADF materials, such as high color purity, color tunability from blue to red region, and excellent EL performance, has been unveiled. In particular, the B/N MR-TADF emitter can efficiently achieve the smallest FWHM reported so far. But many challenges remain in such MR-TADF emitters. Boron-based MR-TADF materials with deep blue and deep red emission are very limited. Further, the synthesis of boron-containing organic functional molecules is difficult, which commonly involves reagents such as butyl lithium and the reaction yields many by-products. Meanwhile, a wider chemical space beyond triangulene-type compounds and a deeper understanding of the design strategy for MR-TADF emitters are required. In addition, the efficiency roll-off and device stability is an issue still to be adequately addressed. Research efforts are needed to be devoted to the TADF system with a short lifetime and high kRISC to minimize triplet-related exciton deactivation processes such as triplet-triplet annihilation or singlet–triplet annihilation. Besides, how to achieve applications of the boron-containing narrowband TADF emitters in laser, biological imaging and other research fields remains unexplored. Although there are various factors ranging from materials to device engineering, it is believed that with the development of organoboron materials science, MR-TADF materials with narrow FWHM and high efficiency will continuously draw interest in both scientific research and industry, and make a significant contribution as a core technology for ultrawide-color-gamut OLED displays.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by the National Natural Science Foundation of China (22075100), the Jilin Provincial Science and Technology Department (No. 20220201082GX) and the Open Fund of the State Key Laboratory of Luminescence and Devices (South China University of Technology, No. 2021-skllmd).Notes and references
-
N. N. Greenwood, Boron, Comprehensive inorganic chemistry, Pergamon Press, Oxford, 1973, pp. 665–993 Search PubMed
.
-
R. E. Krebs, The history and use of our earth's chemical elements: a reference guide, Greenwood Publishing Group, 2006 Search PubMed
.
- B. Albert and H. Hillebrecht, Boron: elementary challenge for experimenters and theoreticians, Angew. Chem., Int. Ed., 2009, 48, 8640 CrossRef CAS PubMed
.
- L. Butterwick, N. Oude and K. Raymond, Safety assessment of boron in aquatic and terrestrial environments, Ecotoxicol. Environ. Saf., 1989, 17, 339 CrossRef CAS PubMed
.
- L. Ji, S. Griesbeck and T. B. Marder, Recent developments in and perspectives on three-coordinate boron materials: a bright future, Chem. Sci., 2017, 8, 846 RSC
.
- Z. M. Hudson and S. Wang, Metal-containing triarylboron compounds for optoelectronic applications, Dalton Trans., 2011, 40, 7805 RSC
.
- C. W. Tang and S. A. VanSlyke, Organic electroluminescent diodes, Appl. Phys. Lett., 1987, 51, 913 CrossRef CAS
.
- S. Reineke, Complementary LED technologies, Nat. Mater., 2015, 14, 459 CrossRef CAS PubMed
.
- B. Zhang, G. Tan, C. S. Lam, B. Yao, C. L. Ho, L. Liu, Z. Xie, W. Y. Wong, J. Ding and L. Wang, High-efficiency single emissive layer white organic light-emitting diodes based on solution-processed dendritic host and new orange-emitting iridium complex, Adv. Mater., 2012, 24, 1873 CrossRef CAS PubMed
.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234 CrossRef CAS PubMed
.
- Y. Tao, K. Yuan, T. Chen, P. Xu, H. Li, R. Chen, C. Zheng, L. Zhang and W. Huang, Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics, Adv. Mater., 2014, 26, 7931 CrossRef CAS PubMed
.
- K. Matsuo and T. Yasuda, Boronate- and borinate-based pi-systems for blue thermally activated delayed fluorescence materials, Chem. Commun., 2019, 55, 2501 RSC
.
- Y. L. Rao and S. Wang, Four-coordinate organoboron compounds with a pi-conjugated chelate ligand for optoelectronic applications, Inorg. Chem., 2011, 50, 12263 CrossRef CAS PubMed
.
- D. Li, H. Zhang and Y. Wang, Four-coordinate organoboron compounds for organic light-emitting diodes (OLEDs), Chem. Soc. Rev., 2013, 42, 8416 RSC
.
- E. von Grotthuss, A. John, T. Kaese and M. Wagner, Doping polycyclic aromatics with boron for superior performance in Materials Science and Catalysis, Am. J. Org. Chem., 2018, 7, 37 CAS
.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO-LUMO separation by the multiple resonance effect, Adv. Mater., 2016, 28, 2777 CrossRef CAS PubMed
.
- A. Endo, K. Sato, K. Yoshimura, T. Kai, A. Kawada, H. Miyazaki and C. Adachi, Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes, Appl. Phys. Lett., 2011, 98, 083302 CrossRef
.
- K. Suzuki, S. Kubo, K. Shizu, T. Fukushima, A. Wakamiya, Y. Murata, C. Adachi and H. Kaji, Triarylboron-based fluorescent organic light-emitting diodes with external quantum efficiencies exceeding 20%, Angew. Chem., Int. Ed., 2015, 54, 15231 CrossRef CAS
.
- Y. Liu, G. Xie, K. Wu, Z. Luo, T. Zhou, X. Zeng, J. Yu, S. Gong and C. Yang, Boosting reverse intersystem crossing by increasing donors in triarylboron/phenoxazine hybrids:
TADF emitters for high-performance solution-processed OLEDs, J. Mater. Chem. C, 2016, 4, 4402 RSC
.
- Y. H. Lee, S. Park, J. Oh, J. W. Shin, J. Jung, S. Yoo and M. H. Lee, Rigidity-induced delayed fluorescence by ortho donor-appended triarylboron compounds: record-high efficiency in pure blue fluorescent organic light-emitting diodes, ACS Appl. Mater. Interfaces, 2017, 9, 24035 CrossRef CAS
.
- X. L. Chen, J. H. Jia, R. Yu, J. Z. Liao, M. X. Yang and C. Z. Lu, Combining charge-transfer pathways to achieve unique thermally activated delayed fluorescence emitters for high-performance solution-processed, non-doped blue OLEDs, Angew. Chem., Int. Ed., 2017, 56, 15006 CrossRef CAS PubMed
.
- Y. H. Lee, S. Park, J. Oh, S.-J. Woo, A. Kumar, J.-J. Kim, J. Jung, S. Yoo and M. H. Lee, High-efficiency sky blue to ultradeep blue thermally activated delayed fluorescent diodes based on ortho-carbazole-appended triarylboron emitters: above 32% external quantum efficiency in blue devices, Adv. Opt. Mater., 2018, 6, 1800385 CrossRef
.
- C.-C. Tsai, W.-C. Huang, H.-Y. Chih, Y.-C. Hsh, C.-W. Liao, C.-H. Lin, Y.-X. Kang, C.-H. Chang, Y. J. Chang and C.-W. Lu, Efficient donor-acceptor-donor borylated compounds with extremely small ΔEST for thermally activated delayed fluorescence OLEDs, Org. Electron., 2018, 63, 166 CrossRef CAS
.
- Y. Liu, H. Huang, T. Zhou, K. Wu, M. Zhu, J. Yu, G. Xie and C. Yang, Boosting photoluminescence quantum yields of triarylboron/phenoxazine hybrids via incorporation of cyano groups and their applications as TADF emitters for high-performance solution-processed OLEDs, J. Mater. Chem. C, 2019, 7, 4778 RSC
.
- C. Qu, G. Xia, Y. Xu, Y. Zhu, J. Liang, H. Zhang, J. Wang, Z. Zhang and Y. Wang, Boron-containing D–A–A type TADF materials with tiny singlet–triplet energy splittings and high photoluminescence quantum yields for highly efficient OLEDs with low efficiency roll-offs, J. Mater. Chem. C, 2020, 8, 3846 RSC
.
- P. Ganesan, D.-G. Chen, W.-C. Chen, P. Gnanasekaran, J.-A. Lin, C.-Y. Huang, M.-C. Chen, C.-S. Lee, P.-T. Chou and Y. Chi, Methoxy substituents activated carbazole-based boron dimesityl TADF emitters, J. Mater. Chem. C, 2020, 8, 4780 RSC
.
- A. Kumar, W. Lee, T. Lee, J. Jung, S. Yoo and M. H. Lee, Triarylboron-based TADF emitters with perfluoro substituents: high-efficiency OLEDs with a power efficiency over 100 lm W−1, J. Mater. Chem. C, 2020, 8, 4253 RSC
.
- Q. Chen, Y. Xiang, X. Yin, K. Hu, Y. Li, X. Cheng, Y. Liu, G. Xie and C. Yang, Highly efficient blue TADF emitters incorporating bulky acridine moieties and their application in solution-processed OLEDs, Dyes Pigm., 2021, 188, 109157 CrossRef CAS
.
- G. Xia, C. Qu, Y. Zhu, J. Ye, K. Ye, Z. Zhang and Y. Wang, A TADF emitter featuring linearly arranged spiro-donor and spiro-acceptor groups: efficient nondoped and doped deep-blue OLEDs with CIE(y) <0.1, Angew. Chem., Int. Ed., 2021, 60, 9598 CrossRef CAS PubMed
.
- Y. H. Lee, W. Lee, T. Lee, D. Lee, J. Jung, S. Yoo and M. H. Lee, Blue TADF emitters based on B-heterotriangulene acceptors for highly efficient OLEDs with reduced efficiency roll-off, ACS Appl. Mater. Interfaces, 2021, 13, 45778 CrossRef CAS PubMed
.
- J. Wang, N. Li, Q. Chen, Y. Xiang, X. Zeng, S. Gong, Y. Zou and Y. Liu, Triarylboron-cored multi-donors TADF emitter with high horizontal dipole orientation ratio achieving high performance OLEDs with near 39% external quantum efficiency and small efficiency Roll-off, Chem. Eng. J., 2022, 450, 137805 CrossRef CAS
.
- M. Numata, T. Yasuda and C. Adachi, High efficiency pure blue thermally activated delayed fluorescence molecules having 10H-phenoxaborin and acridan units, Chem. Commun., 2015, 51, 9443 RSC
.
- Y. Kitamoto, T. Namikawa, D. Ikemizu, Y. Miyata, T. Suzuki, H. Kita, T. Sato and S. Oi, Light blue and green thermally activated delayed fluorescence from 10H-phenoxaborin-derivatives and their application to organic light-emitting diodes, J. Mater. Chem. C, 2015, 3, 9122 RSC
.
- I. S. Park, K. Matsuo, N. Aizawa and T. Yasuda, High-performance dibenzoheteraborin-based thermally activated delayed fluorescence emitters: molecular architectonics for concurrently achieving narrowband emission and efficient triplet-singlet spin conversion, Adv. Funct. Mater., 2018, 28, 1802031 CrossRef
.
- D. H. Ahn, H. Lee, S. W. Kim, D. Karthik, J. Lee, H. Jeong, J. Y. Lee and J. H. Kwon, Highly twisted donor–acceptor boron emitter and high triplet host material for highly efficient blue thermally activated delayed fluorescent device, ACS Appl. Mater. Interfaces, 2019, 11, 14909 CrossRef CAS PubMed
.
- K. Matsuo and T. Yasuda, Blue thermally activated delayed fluorescence emitters incorporating acridan analogues with heavy group 14 elements for high-efficiency doped and non-doped OLEDs, Chem. Sci., 2019, 10, 10687 RSC
.
- T. Agou, K. Matsuo, R. Kawano, I. S. Park, T. Hosoya, H. Fukumoto, T. Kubota, Y. Mizuhata, N. Tokitoh and T. Yasuda, Pentacyclic ladder-heteraborin emitters exhibiting high-efficiency blue thermally activated delayed fluorescence with an ultrashort emission lifetime, ACS Mater. Lett., 2019, 2, 28 CrossRef
.
- H. Hirai, K. Nakajima, S. Nakatsuka, K. Shiren, J. Ni, S. Nomura, T. Ikuta and T. Hatakeyama, One-step borylation of 1,3-diaryloxybenzenes towards efficient materials for organic light-emitting diodes, Angew. Chem., Int. Ed., 2015, 54, 13581 CrossRef CAS PubMed
.
- G. Meng, X. Chen, X. Wang, N. Wang, T. Peng and S. Wang, Isomeric bright sky-blue TADF emitters based on bisacridine decorated DBNA: impact of donor locations on luminescent and electroluminescent properties, Adv. Opt. Mater., 2019, 7, 1900130 CrossRef
.
- D. H. Ahn, S. W. Kim, H. Lee, I. J. Ko, D. Karthik, J. Y. Lee and J. H. Kwon, Highly efficient blue thermally activated delayed fluorescence emitters based on symmetrical and rigid oxygen-bridged boron acceptors, Nat. Photonics, 2019, 13, 540 CrossRef CAS
.
- D. H. Ahn, J. H. Maeng, H. Lee, H. Yoo, R. Lampande, J. Y. Lee and J. H. Kwon, Rigid oxygen-bridged boron-based blue thermally activated delayed fluorescence emitter for organic light-emitting diode: approach towards satisfying high efficiency and long lifetime together, Adv. Opt. Mater., 2020, 8, 2000102 CrossRef CAS
.
- H. J. Kim, M. Godumala, S. K. Kim, J. Yoon, C. Y. Kim, H. Park, J. H. Kwon, M. J. Cho and D. H. Choi, Color-tunable boron-based emitters exhibiting aggregation-induced emission and thermally activated delayed fluorescence for efficient solution-processable nondoped deep-blue to sky-blue OLEDs, Adv. Opt. Mater., 2020, 8, 1902175 CrossRef CAS
.
- H. Lim, H. J. Cheon, S. J. Woo, S. K. Kwon, Y. H. Kim and J. J. Kim, Highly efficient deep-blue OLEDs using a TADF emitter with a narrow emission spectrum and high horizontal emitting dipole ratio, Adv. Mater., 2020, 32, e2004083 CrossRef PubMed
.
- R. Braveenth, H. Lee, J. D. Park, K. J. Yang, S. J. Hwang, K. R. Naveen, R. Lampande and J. H. Kwon, Achieving narrow FWHM and high EQE over 38% in blue OLEDs using rigid heteroatom-based deep blue TADF sensitized host, Adv. Funct. Mater., 2021, 31, 2105805 CrossRef CAS
.
- J. Hwang, H. Kang, J.-E. Jeong, H. Y. Woo, M. J. Cho, S. Park and D. H. Choi, Donor engineered deep-blue emitters for tuning luminescence mechanism in solution-processed OLEDs, Chem. Eng. J., 2021, 416, 129185 CrossRef CAS
.
- T. Hua, Y.-C. Liu, C.-W. Huang, N. Li, C. Zhou, Z. Huang, X. Cao, C.-C. Wu and C. Yang, High-efficiency and low roll-off deep-blue OLEDs enabled by thermally activated delayed fluorescence emitter with preferred horizontal dipole orientation, Chem. Eng. J., 2022, 433, 133598 CrossRef CAS
.
- R. Pei, H. Liu, C. Zhou, J. Miao and C. Yang, Efficient blue thermally activated delayed fluorescent emitters based on a boranaphtho[3,2,1-de]anthracene acceptor, J. Mater. Chem. C, 2021, 9, 17136 RSC
.
- Y. Lee and J. I. Hong, High-efficiency thermally activated delayed fluorescence emitters with high horizontal orientation and narrow deep-blue emission, Adv. Opt. Mater., 2021, 9, 2100406 CrossRef CAS
.
- J. Wang, J. Miao, C. Jiang, S. Luo, C. Yang and K. Li, Engineering intramolecular π-stacking interactions of through-space charge-transfer TADF emitters for highly efficient OLEDs with improved color purity, Adv. Opt. Mater., 2022, 10, 2201071 CrossRef CAS
.
- D. Karthik, Y. H. Jung, H. Lee, S. Hwang, B. M. Seo, J. Y. Kim, C. W. Han and J. H. Kwon, Acceptor-donor-acceptor-type Orange-red thermally activated delayed fluorescence materials realizing external quantum efficiency over 30% with low efficiency roll-off, Adv. Mater., 2021, 33, e2007724 CrossRef PubMed
.
- X. Liang, Z. P. Yan, H. B. Han, Z. G. Wu, Y. X. Zheng, H. Meng, J. L. Zuo and W. Huang, Peripheral amplification of multi-resonance induced thermally activated delayed fluorescence for highly efficient OLEDs, Angew. Chem., Int. Ed., 2018, 57, 11316 CrossRef CAS PubMed
.
- S. H. Han, J. H. Jeong, J. W. Yoo and J. Y. Lee, Ideal blue thermally activated delayed fluorescence emission assisted by a thermally activated delayed fluorescence assistant dopant through a fast reverse intersystem crossing mediated cascade energy transfer process, J. Mater. Chem. C, 2019, 7, 3082 RSC
.
- J. H. Kim, W. J. Chung, J. Kim and J. Y. Lee, Concentration quenching–resistant multiresonance thermally activated delayed fluorescence emitters, Mater. Today Energy, 2021, 21, 100792 CrossRef CAS
.
- J. Park, K. J. Kim, J. Lim, T. Kim and J. Y. Lee, High efficiency of over 25% and long device lifetime of over 500 h at 1000 nit in blue fluorescent organic light-emitting diodes, Adv. Mater., 2022, 34, e2108581 CrossRef PubMed
.
- Y. Wang, Y. Duan, R. Guo, S. Ye, K. Di, W. Zhang, S. Zhuang and L. Wang, A periphery cladding strategy to improve the performance of narrowband emitters, achieving deep-blue OLEDs with CIEy <0.08 and external quantum efficiency approaching 20%, Org. Electron., 2021, 97, 106275 CrossRef CAS
.
- H. J. Cheon, Y. S. Shin, N. H. Park, J. H. Lee and Y. H. Kim, Boron-based multi-resonance TADF emitter with suppressed intermolecular interaction and isomer formation for efficient pure blue OLEDs, Small, 2022, 18, e2107574 CrossRef PubMed
.
- H. J. Cheon, S. J. Woo, S. H. Baek, J. H. Lee and Y. H. Kim, Dense local triplet states and steric shielding of a multi-resonance TADF emitter enable high-performance deep-blue OLEDs, Adv. Mater., 2022, 34, e2207416 CrossRef PubMed
.
- S. Xu, Q. Yang, Y. Zhang, H. Li, Q. Xue, G. Xie, M. Gu, J. Jin, L. Huang and R. Chen, Solution-processed multi-resonance organic light-emitting diodes with high efficiency and narrowband emission, Chin. Chem. Lett., 2021, 32, 1372 CrossRef CAS
.
- Y. T. Lee, C. Y. Chan, M. Tanaka, M. Mamada, U. Balijapalli, Y. Tsuchiya, H. Nakanotani, T. Hatakeyama and C. Adachi, Investigating HOMO energy levels of terminal emitters for realizing high-brightness
and stable TADF-assisted fluorescence organic light-emitting diodes, Adv. Electron. Mater., 2021, 7, 2001090 CrossRef CAS
.
- S. Oda, W. Kumano, T. Hama, R. Kawasumi, K. Yoshiura and T. Hatakeyama, Carbazole-based DABNA analogues as highly efficient thermally activated delayed fluorescence materials for narrowband organic light-emitting diodes, Angew. Chem., Int. Ed., 2021, 60, 2882 CrossRef CAS PubMed
.
- Y. Qiu, H. Xia, J. Miao, Z. Huang, N. Li, X. Cao, J. Han, C. Zhou, C. Zhong and C. Yang, Narrowing the electroluminescence spectra of multiresonance emitters for high-performance blue OLEDs by a peripheral decoration strategy, ACS Appl. Mater. Interfaces, 2021, 13, 59035 CrossRef CAS PubMed
.
- X. Lv, J. Miao, M. Liu, Q. Peng, C. Zhong, Y. Hu, X. Cao, H. Wu, Y. Yang, C. Zhou, J. Ma, Y. Zou and C. Yang, Extending the pi-skeleton of multi-resonance TADF materials towards high-efficiency narrowband deep-blue emission, Angew. Chem., Int. Ed., 2022, 61, e202201588 CAS
.
- Y. Wang, K. Di, Y. Duan, R. Guo, L. Lian, W. Zhang and L. Wang, The selective regulation of borylation site based on one-shot electrophilic C–H borylation reaction, achieving highly efficient narrowband organic light-emitting diodes, Chem. Eng. J., 2022, 431, 133221 CrossRef CAS
.
- J. Bian, S. Chen, L. Qiu, R. Tian, Y. Man, Y. Wang, S. Chen, J. Zhang, C. Duan, C. Han and H. Xu, Ambipolar self-host functionalization accelerates blue multi-resonance thermally activated delayed fluorescence with internal quantum efficiency of 100%, Adv. Mater., 2022, 34, e2110547 CrossRef PubMed
.
- M. Yang, S. Shikita, H. Min, I. S. Park, H. Shibata, N. Amanokura and T. Yasuda, Wide-range color tuning of narrowband emission in multi-resonance organoboron delayed fluorescence materials through rational imine/amine functionalization, Angew. Chem., Int. Ed., 2021, 60, 23142 CrossRef CAS PubMed
.
- J. Park, J. Lim, J. H. Lee, B. Jang, J. H. Han, S. S. Yoon and J. Y. Lee, Asymmetric blue multiresonance TADF emitters with a narrow emission band, ACS Appl. Mater. Interfaces, 2021, 13, 45798 CrossRef CAS PubMed
.
- J. Han, Z. Huang, X. Lv, J. Miao, Y. Qiu, X. Cao and C. Yang, Simple molecular design strategy for multiresonance induced TADF emitter: highly efficient deep blue to blue electroluminescence with high color purity, Adv. Opt. Mater., 2021, 10, 2102092 CrossRef
.
- T. Hua, J. Miao, H. Xia, Z. Huang, X. Cao, N. Li and C. Yang, Sulfone-incorporated multi-resonance TADF emitter for high-performance narrowband blue OLEDs with EQE of 32%, Adv. Funct. Mater., 2022, 32, 2201032 CrossRef CAS
.
- J. Liu, L. Chen, X. Wang, Q. Yang, L. Zhao, C. Tong, S. Wang, S. Shao and L. Wang, Multiple resonance dendrimers containing boron, oxygen, nitrogen-doped polycyclic aromatic emitters for narrowband blue-emitting solution-processed OLEDs, Macromol. Rapid Commun., 2022, 43, e2200079 CrossRef PubMed
.
- Q. Li, Y. Wu, Q. Yang, S. Wang, S. Shao and L. Wang, Selenium-doped polycyclic aromatic hydrocarbon multiresonance emitters with fast reverse intersystem crossing for narrowband blue emission, ACS Appl. Mater. Interfaces, 2022, 14, 49995 CrossRef CAS PubMed
.
- Q. Li, Y. Wu, X. Wang, Q. Yang, J. Hu, R. Zhong, S. Shao and L. Wang, Boron-, sulfur- and nitrogen-doped polycyclic aromatic hydrocarbon multiple resonance emitters for narrow-band blue emission, Chem. – Eur. J., 2022, 28, e202104214 CAS
.
- K. Matsui, S. Oda, K. Yoshiura, K. Nakajima, N. Yasuda and T. Hatakeyama, One-shot multiple borylation toward BN-doped nanographenes, J. Am. Chem. Soc., 2018, 140, 1195 CrossRef CAS PubMed
.
- S. Oda, B. Kawakami, R. Kawasumi, R. Okita and T. Hatakeyama, Multiple resonance effect-induced sky-blue thermally activated delayed fluorescence with a narrow emission band, Org. Lett., 2019, 21, 9311 CrossRef CAS PubMed
.
- Y. Kondo, K. Yoshiura, S. Kitera, H. Nishi, S. Oda, H. Gotoh, Y. Sasada, M. Yanai and T. Hatakeyama, Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter, Nat. Photonics, 2019, 13, 678 CrossRef CAS
.
- H. Tanaka, S. Oda, G. Ricci, H. Gotoh, K. Tabata, R. Kawasumi, D. Beljonne, Y. Olivier and T. Hatakeyama, Hypsochromic shift of multiple-resonance-induced thermally activated delayed fluorescence by oxygen atom incorporation, Angew. Chem., Int. Ed., 2021, 60, 17910 CrossRef CAS PubMed
.
- I. S. Park, M. Yang, H. Shibata, N. Amanokura and T. Yasuda, Achieving ultimate narrowband and ultrapure blue organic light-emitting diodes based on polycyclo-heteraborin multi-resonance delayed-fluorescence emitters, Adv. Mater., 2022, 34, e2107951 CrossRef PubMed
.
- M. Yang, I. S. Park and T. Yasuda, Full-color, narrowband, and high-efficiency electroluminescence from boron and carbazole embedded polycyclic heteroaromatics, J. Am. Chem. Soc., 2020, 142, 19468 CrossRef CAS PubMed
.
- M. Nagata, H. Min, E. Watanabe, H. Fukumoto, Y. Mizuhata, N. Tokitoh, T. Agou and T. Yasuda, Fused-nonacyclic multi-resonance delayed fluorescence emitter based on ladder-thiaborin exhibiting narrowband sky-blue emission with accelerated reverse intersystem crossing, Angew. Chem., Int. Ed., 2021, 60, 20280 CrossRef CAS PubMed
.
- J. A. Knoller, G. Meng, X. Wang, D. Hall, A. Pershin, D. Beljonne, Y. Olivier, S. Laschat, E. Zysman-Colman and S. Wang, Intramolecular borylation via sequential B-Mes bond cleavage for the divergent synthesis of B,N,B-doped benzo[4]helicenes, Angew. Chem., Int. Ed., 2020, 59, 3156 CrossRef PubMed
.
- X. Wang, Y. Zhang, H. Dai, G. Li, M. Liu, G. Meng, X. Zeng, T. Huang, L. Wang, Q. Peng, D. Yang, D. Ma, D. Zhang and L. Duan, Mesityl-functionalized multi-resonance organoboron delayed fluorescent frameworks with wide-range color tunability for narrowband OLEDs, Angew. Chem., Int. Ed., 2022, 61, e202206916 CAS
.
- K. Rayappa Naveen, H. Lee, R. Braveenth, K. Joon Yang, S. Jae Hwang and J. Hyuk Kwon, Deep blue diboron embedded multi-resonance thermally activated delayed fluorescence emitters for narrowband organic light emitting diodes, Chem. – Eur. J., 2022, 432, 134381 CAS
.
- K. R. Naveen, H. Lee, L. H. Seung, Y. H. Jung, C. P. Keshavananda Prabhu, S. Muruganantham and J. H. Kwon, Modular design for constructing narrowband deep-blue multiresonant thermally activated delayed fluorescent emitters for efficient organic light emitting diodes, Chem. – Eur. J., 2023, 451, 138498 CAS
.
- S. M. Suresh, E. Duda, D. Hall, Z. Yao, S. Bagnich, A. M. Z. Slawin, H. Bassler, D. Beljonne, M. Buck, Y. Olivier, A. Kohler and E. Zysman-Colman, A deep blue B,N-doped heptacene emitter that shows both thermally activated delayed fluorescence and delayed fluorescence by triplet-triplet annihilation, J. Am. Chem. Soc., 2020, 142, 6588 CrossRef CAS PubMed
.
- K. Stavrou, S. Madayanad Suresh, D. Hall, A. Danos, N. A. Kukhta, A. M. Z. Slawin, S. Warriner, D. Beljonne, Y. Olivier, A. Monkman and E. Zysman-Colman, Emission and absorption tuning in TADF B,N-doped heptacenes: toward ideal-blue hyperfluorescent OLEDs, Adv. Opt. Mater., 2022, 10, 2200688 CrossRef CAS
.
- S. Oda, B. Kawakami, Y. Yamasaki, R. Matsumoto, M. Yoshioka, D. Fukushima, S. Nakatsuka and T. Hatakeyama, One-shot synthesis of expanded heterohelicene exhibiting narrowband thermally activated delayed fluorescence, J. Am. Chem. Soc., 2022, 144, 106 CrossRef CAS PubMed
.
- S. Oda, B. Kawakami, M. Horiuchi, Y. Yamasaki, R. Kawasumi and T. Hatakeyama, Ultra-narrowband blue multi-resonance thermally activated delayed fluorescence materials, Adv. Sci., 2022, 10, e2205070 CrossRef PubMed
.
- Y. Xu, Z. Cheng, Z. Li, B. Liang, J. Wang, J. Wei, Z. Zhang and Y. Wang, Molecular-structure and device-configuration optimizations toward highly efficient green electroluminescence with narrowband emission and high color purity, Adv. Opt. Mater., 2020, 8, 1902142 CrossRef CAS
.
- Y. Xu, C. Li, Z. Li, Q. Wang, X. Cai, J. Wei and Y. Wang, Constructing charge-transfer excited states based on frontier molecular orbital engineering: narrowband green electroluminescence with high color purity and efficiency, Angew. Chem., Int. Ed., 2020, 59, 17442 CrossRef CAS PubMed
.
- Y. Xu, C. Li, Z. Li, J. Wang, J. Xue, Q. Wang, X. Cai and Y. Wang, Highly efficient electroluminescent materials with high color purity based on strong acceptor attachment onto B–N-containing multiple resonance frameworks, CCS Chem., 2022, 4, 2065 CrossRef CAS
.
- Q. Wang, Y. Xu, T. Yang, J. Xue and Y. Wang, Precise functionalization of a multiple-resonance framework: constructing narrowband organic electroluminescent materials with external quantum efficiency over 40%, Adv. Mater., 2023, 35, e2205166 CrossRef PubMed
.
- Y. Zhang, D. Zhang, J. Wei, Z. Liu, Y. Lu and L. Duan, Multi-resonance induced thermally activated delayed fluorophores for narrowband green OLEDs, Angew. Chem., Int. Ed., 2019, 58, 16912 CrossRef CAS PubMed
.
- Y. Qi, W. Ning, Y. Zou, X. Cao, S. Gong and C. Yang, Peripheral decoration of multi-resonance molecules as a versatile approach for simultaneous long-wavelength and narrowband emission, Adv. Funct. Mater., 2021, 31, 2102017 CrossRef CAS
.
- F. Liu, Z. Cheng, L. Wan, Z. Feng, H. Liu, H. Jin, L. Gao, P. Lu and W. Yang, Highly efficient multi-resonance thermally activated delayed fluorescence material with a narrow full width at half-maximum of 0.14 eV, Small, 2022, 18, e2106462 CrossRef PubMed
.
- Y. Xu, Q. Wang, X. Cai, C. Li and Y. Wang, Highly efficient electroluminescence from narrowband green circularly polarized multiple resonance thermally activated delayed fluorescence enantiomers, Adv. Mater., 2021, 33, e2100652 CrossRef PubMed
.
- Y. Zhang, J. Wei, D. Zhang, C. Yin, G. Li, Z. Liu, X. Jia, J. Qiao and L. Duan, Sterically wrapped multiple resonance fluorophors for suppression of concentration quenching and spectrum broadening, Angew. Chem., Int. Ed., 2022, 61, e202113206 CAS
.
- P. Jiang, J. Miao, X. Cao, H. Xia, K. Pan, T. Hua, X. Lv, Z. Huang, Y. Zou and C. Yang, Quenching-resistant multiresonance TADF emitter realizes 40% external quantum efficiency in narrowband electroluminescence at high doping level, Adv. Mater., 2022, 34, e2106954 CrossRef PubMed
.
- Y. K. Qu, D. Y. Zhou, F. C. Kong, Q. Zheng, X. Tang, Y. H. Zhu, C. C. Huang, Z. Q. Feng, J. Fan, C. Adachi, L. S. Liao and Z. Q. Jiang, Steric modulation of spiro structure for highly efficient multiple resonance emitters, Angew. Chem., Int. Ed., 2022, 61, e202201886 CAS
.
- X. F. Luo, H. X. Ni, X. Liang, D. Yang, D. Ma, Y. X. Zheng and J. L. Zuo, Face-to-face steric modulation to achieve high performance multiple-resonance thermally activated delayed fluorescence emitters with quenching resistant effect, Adv. Opt. Mater., 2023, 11, 2203002 CrossRef CAS
.
- Y. Liu, X. Xiao, Z. Huang, D. Yang, D. Ma, J. Liu, B. Lei, Z. Bin and J. You, Space-confined donor-acceptor strategy enables fast spin-flip of multiple resonance emitters for suppressing efficiency roll-off, Angew. Chem., Int. Ed., 2022, 61, e202210210 CAS
.
- S. Cai, G. S. M. Tong, L. Du, G. K. So, F. F. Hung, T. L. Lam, G. Cheng, H. Xiao, X. Chang, Z. X. Xu and C. M. Che, Gold(I) Multi-resonance thermally activated delayed fluorescent emitters for highly efficient ultrapure-green organic light-emitting diodes, Angew. Chem., Int. Ed., 2022, 61, e202213392 CAS
.
- J. Wang, N. Li, C. Zhong, J. Miao, Z. Huang, M. Yu, Y. X. Hu, S. Luo, Y. Zou, K. Li and C. Yang, Metal-perturbed multiresonance TADF emitter enables high-efficiency and ultralow efficiency roll-off nonsensitized OLEDs with pure green gamut, Adv. Mater., 2023, 35, e2208378 CrossRef PubMed
.
- Y. Hu, J. Miao, C. Zhong, Y. Zeng, S. Gong, X. Cao, X. Zhou, Y. Gu and C. Yang, Peripherally heavy-atom-decorated strategy towards high-performance pure green electroluminescence with external quantum efficiency over 40%, Angew. Chem., Int. Ed., 2023, 62, e2023024 CrossRef PubMed
.
- F. Huang, Y.-C. Cheng, H. Wu, X. Xiong, J. Yu, X.-C. Fan, K. Wang and X.-H. Zhang, Hanging heavy atom-containing chains onto a multiple resonance framework: Influence on the TADF properties and device performances, Chem. Eng. J., 2023, 465, 142900 CrossRef CAS
.
- F. Huang, X. C. Fan, Y. C. Cheng, Y. Xie, S. Luo, T. Zhang, H. Wu, X. Xiong, J. Yu, D. D. Zhang, X. K. Chen, K. Wang and X. H. Zhang, Multiple resonance organoboron OLED emitters with high efficiency and high color purity via managing long- and short-range charge-transfer excitations, Adv. Opt. Mater., 2023, 11, 2202950 CrossRef CAS
.
- Y. Zhang, D. Zhang, J. Wei, X. Hong, Y. Lu, D. Hu, G. Li, Z. Liu, Y. Chen and L. Duan, Achieving pure green electroluminescence with CIEy of 0.69 and EQE of 28.2% from an aza-fused multi-resonance emitter, Angew. Chem., Int. Ed., 2020, 59, 17499 CrossRef CAS PubMed
.
- X. F. Luo, H. X. Ni, H. L. Ma, Z. Z. Qu, J. Wang, Y. X. Zheng and J. L. Zuo, Fused π-extended multiple-resonance induced thermally activated delayed fluorescence materials for high-efficiency and narrowband OLEDs with low efficiency roll-off, Adv. Opt. Mater., 2022, 10, 2102513 CrossRef CAS
.
- X. F. Luo, S. Q. Song, H. X. Ni, H. Ma, D. Yang, D. Ma, Y. X. Zheng and J. L. Zuo, Multiple-resonance-induced thermally activated delayed fluorescence materials based on indolo[3,2,1-jk]carbazole with an efficient narrowband pure-green electroluminescence, Angew. Chem., Int. Ed., 2022, 61, e202209984 CAS
.
- Y. Xu, Q. Wang, J. Wei, X. Peng, J. Xue, Z. Wang, S. J. Su and Y. Wang, Constructing organic electroluminescent material with very high color purity and efficiency based on polycyclization of the multiple resonance parent core, Angew. Chem., Int. Ed., 2022, 61, e202204652 CAS
.
- Q. Wang, Y. Xu, T. Huang, Y. Qu, J. Xue, B. Liang and Y. Wang, Precise regulation of emission maxima and construction of highly efficient electroluminescent materials with high color purity, Angew. Chem., Int. Ed., 2023, 62, e202301930 CrossRef CAS PubMed
.
- J. Liu, Y. Zhu, T. Tsuboi, C. Deng, W. Lou, D. Wang, T. Liu and Q. Zhang, Toward a BT.2020 green emitter through a combined multiple resonance effect and multi-lock strategy, Nat. Commun., 2022, 13, 4876 CrossRef CAS PubMed
.
- P. Jiang, L. Zhan, X. Cao, X. Lv, S. Gong, Z. Chen, C. Zhou, Z. Huang, F. Ni, Y. Zou and C. Yang, Simple acridan-based multi-resonance structures enable highly efficient narrowband green TADF electroluminescence, Adv. Opt. Mater., 2021, 9, 2100825 CrossRef CAS
.
- G. Liu, H. Sasabe, K. Kumada, A. Matsunaga, H. Katagiri and J. Kido, Facile synthesis of multi-resonance ultra-pure-green TADF emitters based on bridged diarylamine derivatives for efficient OLEDs with narrow emission, J. Mater. Chem. C, 2021, 9, 8308 RSC
.
- Y. X. Hu, J. Miao, T. Hua, Z. Huang, Y. Qi, Y. Zou, Y. Qiu, H. Xia, H. Liu, X. Cao and C. Yang, Efficient selenium-integrated TADF OLEDs with reduced roll-off, Nat. Photonics, 2022, 16, 803 CrossRef CAS
.
- F. Liu, Z. Cheng, Y. Jiang, L. Gao, H. Liu, H. Liu, Z. Feng, P. Lu and W. Yang, Highly efficient asymmetric multiple resonance thermally activated delayed fluorescence emitter with EQE of 32.8% and extremely low efficiency roll-off, Angew. Chem., Int. Ed., 2022, 61, e202116927 CAS
.
- Y. Zhang, G. Li, L. Wang, T. Huang, J. Wei, G. Meng, X. Wang, X. Zeng, D. Zhang and L. Duan, Fusion of multi-resonance fragment with conventional polycyclic aromatic hydrocarbon for nearly BT.2020 green emission, Angew. Chem., Int. Ed., 2022, 61, e202202380 CAS
.
- X. Wu, J. W. Huang, B. K. Su, S. Wang, L. Yuan, W. Q. Zheng, H. Zhang, Y. X. Zheng, W. Zhu and P. T. Chou, Fabrication of circularly polarized MR-TADF emitters with asymmetrical peripheral-Lock enhancing helical B/N-doped nanographenes, Adv. Mater., 2022, 34, e2105080 CrossRef PubMed
.
- W. Yang, N. Li, J. Miao, L. Zhan, S. Gong, Z. Huang and C. Yang, Simple double hetero[5]helicenes realize highly efficient and narrowband circularly polarized organic light-emitting diodes, CCS Chem., 2022, 4, 3463 CrossRef CAS
.
- Y. Yang, N. Li, J. Miao, X. Cao, A. Ying, K. Pan, X. Lv, F. Ni, Z. Huang, S. Gong and C. Yang, Chiral multi-resonance TADF emitters exhibiting narrowband circularly polarized electroluminescence with an EQE of 37.2%, Angew. Chem., Int. Ed., 2022, 61, e202202227 CAS
.
- N. Ikeda, S. Oda, R. Matsumoto, M. Yoshioka, D. Fukushima, K. Yoshiura, N. Yasuda and T. Hatakeyama, Solution-processable pure green thermally activated delayed fluorescence emitter based on the multiple resonance effect, Adv. Mater., 2020, 32, e2004072 CrossRef PubMed
.
- S. Oda, T. Sugitani, H. Tanaka, K. Tabata, R. Kawasumi and T. Hatakeyama, Development of pure green thermally activated delayed fluorescence material by cyano substitution, Adv. Mater., 2022, 34, e2201778 CrossRef PubMed
.
- S. Uemura, S. Oda, M. Hayakawa, R. Kawasumi, N. Ikeda, Y. T. Lee, C. Y. Chan, Y. Tsuchiya, C. Adachi and T. Hatakeyama, Sequential multiple borylation toward an ultrapure green thermally activated delayed fluorescence material, J. Am. Chem. Soc., 2023, 145, 1505 CrossRef CAS PubMed
.
- X. Cai, J. Xue, C. Li, B. Liang, A. Ying, Y. Tan, S. Gong and Y. Wang, Achieving 37.1% green electroluminescent efficiency and 0.09 eV full width at half maximum based on a ternary boron-oxygen-nitrogen embedded polycyclic aromatic system, Angew. Chem., Int. Ed., 2022, 61, e202200337 CAS
.
- X. F. Luo, H. X. Ni, A. Q. Lv, X. K. Yao, H. L. Ma and Y. X. Zheng, High-efficiency and narrowband OLEDs from blue to yellow with ternary boron/nitrogen-based polycyclic heteroaromatic emitters, Adv. Opt. Mater., 2022, 10, 2200504 CrossRef CAS
.
- Y. Zhang, D. Zhang, T. Huang, A. J. Gillett, Y. Liu, D. Hu, L. Cui, Z. Bin, G. Li, J. Wei and L. Duan, Multi-resonance deep-red emitters with shallow potential-energy surfaces to surpass energy-gap Law*, Angew. Chem., Int. Ed., 2021, 60, 20498 CrossRef CAS PubMed
.
- Y. Wang, K. Zhang, F. Chen, X. Wang, Q. Yang, S. Wang, S. Shao and L. Wang, Boron-, sulfur- and nitrogen-doped tridecacyclic aromatic emitters with multiple resonance effect for narrowband red emission, Chin. J. Chem., 2022, 40, 2671 CrossRef CAS
.
- Y. Zou, J. Hu, M. Yu, J. Miao, Z. Xie, Y. Qiu, X. Cao and C. Yang, High-performance narrowband pure-red OLEDs with external quantum efficiencies up to 36.1% and ultralow efficiency roll-off, Adv. Mater., 2022, 34, e2201442 CrossRef PubMed
.
- T. Fan, M. Du, X. Jia, L. Wang, Z. Yin, Y. Shu, Y. Zhang, J. Wei, D. Zhang and L. Duan, High-efficiency narrowband multi-resonance emitter fusing indolocarbazole donors for BT. 2020 red electroluminescence and ultra-long operation lifetime, Adv. Mater., 2023, 2301018 CrossRef PubMed
.
- Y. Liu, X. Xiao, Y. Ran, Z. Bin and J. You, Molecular design of thermally activated delayed fluorescent emitters for narrowband orange-red OLEDs boosted by a cyano-functionalization strategy, Chem. Sci., 2021, 12, 9408 RSC
.
- X. Cai, Y. Xu, Y. Pan, L. Li, Y. Pu, X. Zhuang, C. Li and Y. Wang, Solution-processable pure-red multiple resonance-induced thermally activated delayed fluorescence emitter for organic light-emitting diode with external quantum efficiency over 20%, Angew. Chem., Int. Ed., 2023, 62, e202216473 CrossRef CAS PubMed
.
- Y. C. Cheng, X. C. Fan, F. Huang, X. Xiong, J. Yu, K. Wang, C. S. Lee and X. H. Zhang, A highly twisted carbazole-fused DABNA derivative as an orange-red TADF emitter for OLEDs with nearly 40% EQE, Angew. Chem., Int. Ed., 2022, 61, e202212575 CAS
.
- W. Yang, J. Miao, F. Hu, Y. Zou, C. Zhong, S. Gong and C. Yang, An effective approach toward yellow-to-orange multi-resonance TADF emitters by integrating strong electron donor into B/N-based polycyclic architecture: high performance OLEDs with nearly 40% EQE, Adv. Funct. Mater., 2023, 33, 2213056 CrossRef CAS
.
| This journal is © the Partner Organisations 2023 |