Open Access Article
Open Access ArticleRecent advances in carbohydrate-based gelators
Rajdeep
Tyagi
a,
Kavita
Singh
a,
Nitin
Srivastava
*b and
Ram
Sagar
 *a
*a
aGlycochemistry Laboratory, School of Physical Sciences, Jawaharlal Nehru University, New Delhi-110007, India. E-mail: ram.sagar@jnu.ac.in
bDepartment of Chemistry, Amity University Uttar Pradesh Lucknow Campus, 226028, India. E-mail: nitinsriv5@gmail.com
First published on 7th August 2023
Abstract
Carbohydrate-based gelators are the emerging new materials having diversified application fields such as drug delivery, environmental remediation, antibacterial agents, tissue engineering, thixotropy and wound healing. They have been established as biocompatible and renewable materials for sustainable development. Natural monosaccharides have versatile structures that are useful in the synthesis of gelators. In this review, recently synthesized low-molecular weight carbohydrate-based gelators from 2014 to 2023 are meticulously discussed with their diverse application in various fields including biomedical applications. The diverse syntheses and applications of carbohydrate-based gelators establish them as new materials that are cleaner, greener and biocompatible. Therefore, this review may be helpful to the researchers working in the field of biocompatible materials for exploring customized materials and their novel future applications.
1. Introduction
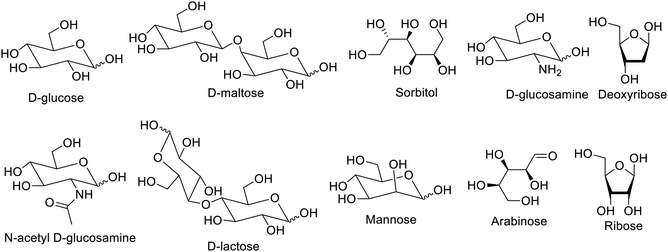
Designing and tailoring smart materials with in-built functionalities in molecules are under keen development in all fields of chemistry. Low-molecular weight gelators (LMGs) are molecular assemblies obtained using versatile small molecules, which have been interesting new materials due to their wide plethora of applications. Such applications include pharmaceuticals, tissue engineering, dye absorption, and drug delivery.1–3 LMGs embedded with stimulus-responsive functional groups have been valuable in research.3–8 Monosaccharides among carbohydrates have been extensively used in the design and synthesis of molecular gelators.9–11 The LMGs yield reversible gels with different solvents and are known as physical gels or supramolecular gels on the basis of their non-covalent bonds such as van der Waals interactions, π–π interactions, hydrophobic interactions, and hydrogen bonding. The gelators give rise to self-assembled, fibrous structures that hold the solvent within their structure, and this property make them unique materials having various applications including biomedical. The hydrogel produced by these gelators shows potential to replace the traditional polymer hydrogel in applications requiring biocompatibility. The formation of such hydrogel is reversible and enhances its applicability in various fields.The formation of supramolecular gels has been extensively studied in diverse fields such as controlled drug delivery,1–3 immobilization of enzyme produced in the body,4 electronics and optics,5–7 and pollution mitigation.9,10 Various metallogels have been employed in the formation of smart materials.11–14 Extensive research for supramolecular gels has been reported for their biomedical applications4–7,9–17 and catalysis.18–21 The diverse applications of LMGs encouraged several research studies to study the nature, design, and synthesis of these LMGs.22–26 There are varieties of organic compounds such as sugars, amino acids, cholesterols, and urea, which have been employed for the design and synthesis of LMGs. Out of these organic compounds, sugars are biocompatible and have internal chirality, which outclass them for biomedical applications such as controlled release systems and segregation of biomolecules. D-Glucose, D-maltose, sorbitol, D-glucosamine, N-acetyl-D-glucosamine, D-lactose and 2-deoxyribose sugars were used to synthesize carbohydrate based LMGs and low-molecular weight hydrogelators (LMHGs), as shown in Fig. 1. Additionally, there are stimulus-responsive gelators that react to light, pH, enzymes, and other factors to stimulate gels. This review will encompass the recently synthesised carbohydrate-based gelators and their biomedical applications.
2. Monosaccharides and their derivatives
Out of the various organic LMGs, carbohydrates are an important class on the basis of their physical and chemical properties and abundant bioavailability. These properties include complex diversified stereochemical structure and unique biocompatibility, making them a suitable novel class of materials.27,282.1. D-Glucose derivatives
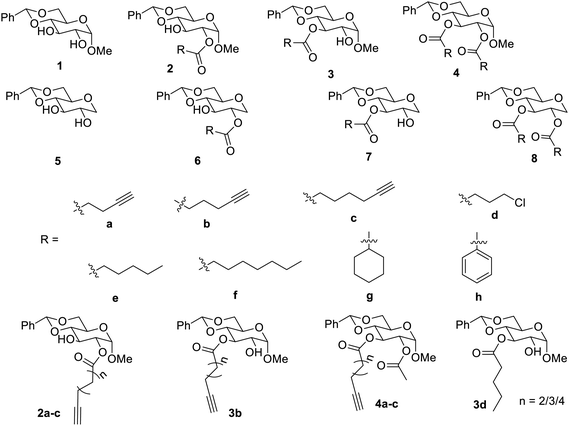
Various monosaccharides and their derivatives have been found to be LMGs and LMHGs29–42 and employed for various applications. A number of LMGs have been synthesised using D-glucose, which is easily accessible, by functionalizing derivatives of α-methyl D-glucose. Diol compounds 1 and their monoesters 2 and 3 and diester derivative 4 were systematically examined for their gelation properties as shown in Fig. 2.36 It was observed that the esters having alkyl chains lacked the properties of good gelators, but the diester 4h was found to yield gels in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H5OH in a volume of 1
C2H5OH in a volume of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 5 mg mL−1, while 3d which is a 3-ester derivative formed hydrogel at 7 mg mL−1.36 The esters with terminal alkynes 2a–2c, 3a–3c and 4a–4c were found to be gelators for many solvents such as water and hexane and their study helped in design and synthesis of more appropriate gelators.
1 at 5 mg mL−1, while 3d which is a 3-ester derivative formed hydrogel at 7 mg mL−1.36 The esters with terminal alkynes 2a–2c, 3a–3c and 4a–4c were found to be gelators for many solvents such as water and hexane and their study helped in design and synthesis of more appropriate gelators.
1-Deoxy glucose diol 5 was synthesized to study the effect of the substituent at the anomeric position on the properties of gels.35 Acyl derivatives, monoesters and diesters 6–8 were also studied for their effect on the properties of gels. It was found that diesters 6a, 6b and 6h showed gelation in various solvents such as ethanol, its aqueous solution and an aqueous solution of DMSO. Such 1-deoxy sugar monoester was reported to be more soluble in organic solvents than 1-methoxy analogues. The researchers found that while long-chain esters were effective gelators, the α-OMe group was crucial in causing this molecule to act as a gelator.35
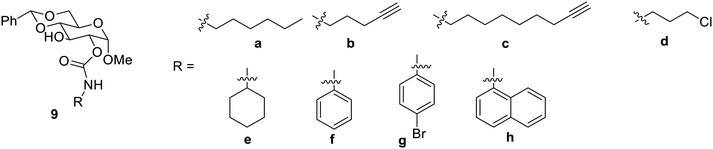
Carbamates on the basis of their hydrogen bond accepting and donating properties have also been found to be the effective functional groups to make carbohydrates behave as gelators. The carbamate's functionalized carbohydrates have been found to develop readily at low concentrations and to be compatible with a wide range of substituents. Many O-linked carbamates 9 were synthesized and studied,38 as shown in Fig. 3.
It was reported here that alkyl derivative 9a and cyclohexyl derivative 9d were most effective as gelators in aqueous DMSO and ethyl alcohol. In aqueous ethanol, 9d showed gelation at 0.91 mg mL−1.38 The carbamates 9b and 9c and their aromatic counterparts 9e–9f exhibited strong gelation in ethyl alcohol and water mixture as well as DMSO and water mixtures. It was also reported that the naphthyl derivative 9g gave gelation only in aqueous DMSO.
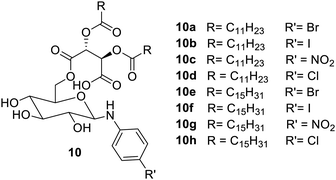
Monosaccharide-based glycolipid derivatives were also reported to exhibit gelation properties in various aliphatic and aromatic solvents43 (Fig. 4). Such glycolipids 10b and 10g were gelators in aliphatic solvents with a critical gelation concentration (CGC) of 0.8% (w/v). The compounds 10c, 10e and 10h showed gelation at a CGC of 1.0% (w/v). It was observed in this study that the R′ group on aniline is primarily responsible for the gelation properties of the compounds. Nitro and iodo groups on the linear chain 10a–d showed good gelation properties. The substituent on the aniline ring was responsible for the self-assembly and stability of gels.
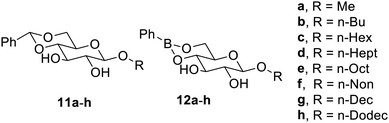
4,6-Benzylidene-protected alkyl glucosides4411a–11h have also been reported to be organogelators, as shown in Fig. 5. Similarly, phenyl boronic-protected alkyl glucosides 12a–12h have also been reported as water-sensitive gelators, which are similar to 4,6-benzylidene-protected alkyl glucosides. Such gelators were effective in isopropyl palmitate, ethyl myritate, cyclohexane and toluene. The boronate gelators are water-sensitive and undergo hydrolysis, which destabilize the gel.44 The sensitivity of the gelators approved their application as smart materials in topical drug delivery.
Similar tartaric acid derivatives of phenylboronic acid were reported to be forming a white metallogel in DMSO with LiOH. When D-(+)-glucose was added, this white gel turned red and exhibited enhanced conduction.45 Therefore, these metallogels may be employed in the development of smart materials in the field of conduction.
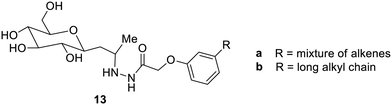
Renewable sophorolipids have been reported to be supramolecular gelators.46 The compounds 13a,b (Fig. 6) have been found to form gels neither with water nor with the organic solvent but with the mixture of methanol, ethanol, DMF, DMSO and water in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Compound 13b undergoes sol–gel transformation at 48 °C. The fibrous structure of these gels was due to π–π interaction and hydrogen bonding.
5. Compound 13b undergoes sol–gel transformation at 48 °C. The fibrous structure of these gels was due to π–π interaction and hydrogen bonding.
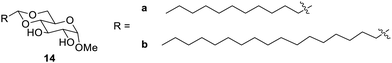
Glucose-based LMGs have been reported as new materials that form gels in both water and organic solvents.47 Such new materials have been found to be methyl α-D-glucopyranosides with an acetal 4,6-protecting group. It was found that the compound 14b (Fig. 7) formed a silicon oil gel, which is an extruded gel and found to retain the viscoelastic behaviour of the gel. The gel formed by 14b was able to restore itself once the shearing stress relieved at room temperature.
2.2. Glucosamine and N-acetyl-D-glucosamine derivatives
D-Glucosamine and N-acetyl-D-glucosamine derivatives have been reported to be used for the synthesis of organogelators and hydrogelators. The amino group here may be employed for the functionalization and synthesis of carbamates, urea, amides, etc.
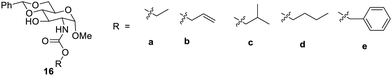
N-linked carbamate derivatives3816 from glucosamines, as shown in Fig. 9, gave a stable gel. Here the synthesized derivatives 16a–e were explored for their gelation properties. The derivative 16a gave a hydrogel at 6.6 mg mL−1. The compound 16c was found to form gels in aqueous ethanol, aqueous DMSO and hexane. It had minimum gelation concentration in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (v
DMSO (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v
v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) but yielded a hydrogel at 0.4%.
1) but yielded a hydrogel at 0.4%.
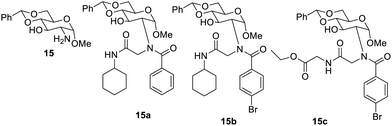
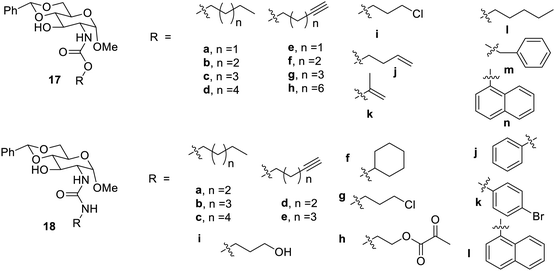
Gelation properties were also observed from urea48,49 and amide derivatives for a larger span of solvents. Urea derivatives act as a good gelator due to hydrogen bonding, which allows the molecule to congregate in the form of a three-dimensional network. Hydrogen bonding was also found to play a vital role in the gelation properties of amide derivatives.50,51 The glucosamine 15 was used for the synthesis37 of amides 17a–n and urea derivatives 18a–l, as shown in Fig. 10. It was found that most of the synthesized compounds formed gel at 33% aqueous solution of DMSO and ethanol and only a few compounds were able to form gels in either organic or aqueous solvents. The compound 17g yielded a very stable gel at a minimum concentration of 0.7 mg mL−1 in aqueous ethyl alcohol. It was found that the compound 17m behaved as a hydrogelator at a concentration 2 mg mL−1. In this research38 it was reported that a very stable gel was formed by heptyl amide 17d in aqueous DMSO, which stayed intact for 6 months.
The alkyl derivatives of synthesized urea (18a–l) responded for the gel formation at lower concentrations than those of the aromatic derivatives. The compound 18i formed a stable glassy gel in aqueous solvents. It was also observed in this research that gels formed by ester and carbamates are less firm than that formed by urea and amides.
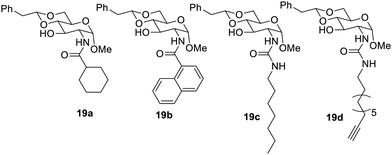
A 4,6-protective phenyl group was explored with the synthesis of a new series,34 in which the methylene group was flanked by a 4,6-protective site and sugar. The compounds of this synthesized series are shown in Fig. 11. It was observed that amide derivatives yielded gels in aqueous solvents, while the urea derivatives formed good gels in organic solvents. During the study of the gelation property in engine and pump oil, the amide derivatives were more prominent in gelation than urea derivatives. The compound 19a was most efficient in forming the gel with six different solvents at lower concentrations. Compound 19b formed a gel in isopropyl alcohol and an aqueous solution of ethyl alcohol and DMSO with a concentration of 3.3 mg mL−1 in aqueous ethyl alcohol. The compounds 19c and 19d made effective gels in ethylene glycol.
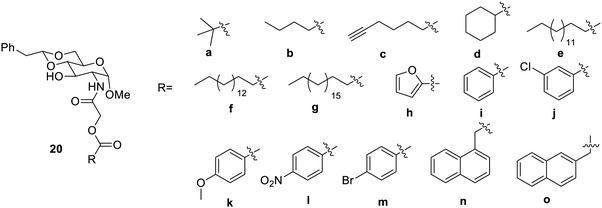
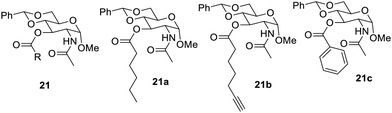
The modification of glucosamines was carried out further by introduction of ester group52 in the amide. Some of the synthesized compounds are shown in Fig. 12. The compound 20b formed an effective gel in aqueous solvents at a concentration of 1.4 mg mL−1. These gelators were found to interact with the enzyme lipase and other basic solutions. Therefore, these gelators were found to be potent new materials for the drug delivery and helpful in dye removal from the environment.
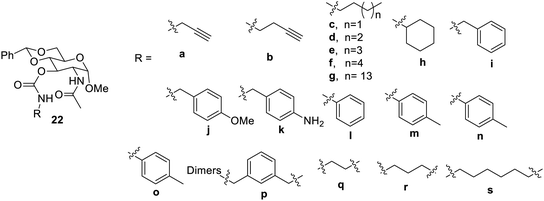
Carbamate derivatives54 of the sugar were also prepared using N-acetyl-D-glucosamines, as shown in Fig. 14. The synthesized compound acted as a gelator in most of the solvents and their binary mixtures. The derivatives 22b–g formed gels with pump oil at a concentration ranging from 5 to 20 mg mL−1. The aromatic substituted compounds showed gelation properties in aqueous DMSO. Compound 22j was found to form gels in the presence of metal ions such as Fe2+, Zn2+, Cu2+, and Pb2+. It was observed that compounds 22j and 22l showed very good gelation properties, which further adds to the new gelation material.
The multicomponent gelator56,57 is yet another type of the recent material to modify or alter the properties of the gelator. The binary system of the gelators is of three types: (i) one component forms gel and another does not, (b) both components forming gel independently and (iii) both components do not form gel but their mixture makes gel easily. The first type of the material is used when no change in the properties of the gel is required. The second one gives rise to the material whose properties depend upon the assembly of the components at the molecular level.57
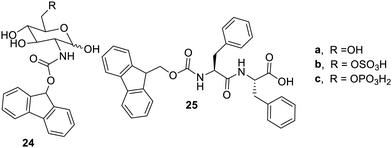
The gelation properties of Fmoc-Glucosamine derivatives58 were studied for the two-component system in cell culture. The compounds 24a and 25 as shown in Fig. 16 were soluble in water with low solubility. The solubility of compound 24a was enhanced by compounds 24b and 24c. The compound 25 co-assembled with 24b and 24c to undergo gelation.59 The advanced research has mentioned that binary component gels have reduced toxicity and may be used as biomimetics. Compound 24c (N-fluorenylmethyloxycarbonylglucosamine-6-phosphate) is a gelator precursor and dissociates alkaline phosphate and yields gelator 24a.60 The tumor cells in breast cancer and osteosarcoma have excess of alkaline phosphate and compounds 24a and 24c due to their sugar moiety binding GLUT1 (glucose transporter 1), and they stop glycolysis in the cell. The cleaved precursor 24cvia ALP, the resulting molecule undergoes self-assembly and disturbs the cell function and promoting cell death to the tumor cells. This biomaterial may become effective for the treatment of cancer.
2.3. D-Mannose, D-arabinose and D-galactose derivatives as LMOGs
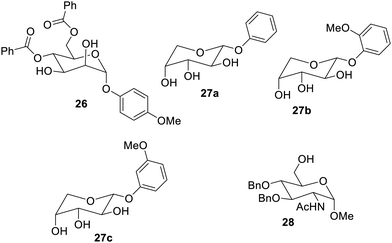
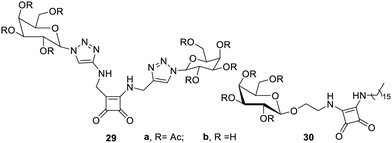
Low-molecular weight organogelators (LMOGs) based on mannose 26 have also been reported which have self-healing and phase selective properties in gelation,61 as shown in Fig. 17. They have been found to gel a large number of organic solvents, oils, fuels, alcohols, aromatic solvents, etc. Arabinose glycosides6227a–c were synthesized and studied for their gelation in aforesaid solvents. Their gelation action in the fuels was reported to be very effective for cleaning oil spills. An important multifunctional gelator 28 based on glucose has also been reported, which has the ability to form organogels and hydrogels. Gelator 28 shows the shortest gelation time (5 s) for N-acetyl-glucosamine-based gelators, and it was also proved to be efficient not only for organic solvents but also for oils such diesel and petrol. Organogels of the same gelator have been proven to be useful for the removal of waterborne synthetic dyes, whereas their hydrogels were explored as growth media for the fabrication of gold nanoparticles63 (Fig. 17).Galactose derivatives have also been reported as new materials on the basis of their gelation. The combination of squaramides and galactosyl moieties was used to synthesize64 low-molecular weight gels, as shown in Fig. 18. It was found that the symmetrical triazole derivatives 29a and 29b were weak gelators, giving gels only in ethyl alcohol and ethyl acetate. However, the amphiphilic derivative 30a showed very good gelation properties in a large number of solvents with lower gelation concentrations. The amphiphilic derivative 30b after amputation of the acetyl group under basic conditions behaved as a supergelator in aqueous ethyl alcohol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with a minimum gelation concentration of 0.1% w/v. This research gave a new material, which shows potential for novel applications.
1) with a minimum gelation concentration of 0.1% w/v. This research gave a new material, which shows potential for novel applications.
2.4. Glyconamide derivatives by modification at the anomeric position
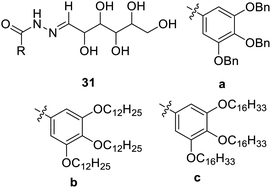
Various linear derivatives have been reported as gelators, synthesized by C-1 anomeric functionalization. The amide and ester derivatives of glyconic acid and glyconolactone have been reported as new gelation materials.Poly alkyl ether and poly aryl ether glucose-cored LMGs have been reported.65 Strong gel formation has been reported by poly aryl ether glucose cored low-molecular weight gelators 31a–c. The fibrous networking of the gel makes it a good novel material for the controlled release of the drug (Fig. 19).
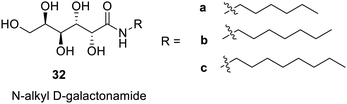
The reaction between galactonolactone and amines such as hexyl, heptyl, and octyl amines yielded galactonamides6632a, which have been reported to be effective gelators in various solvents (Fig. 20). The minimum gelation concentration for these was 1%, 0.45% and 0.5% for hexyl, heptyl and octyl derivatives respectively. These gels have been successfully employed for the growth of neuronal cells. N-Heptyl galactonamide (32b) in DMSO has been reported to form an injectable gel in water bath.67,68
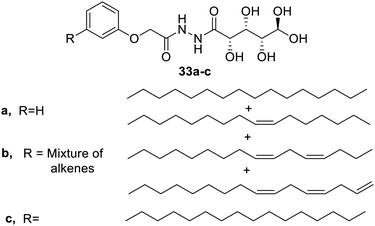
The gluconamide amphiphiles were extracted from cashew nut. The derivatives6933a and 33b were able to form gels easily. The former derivative formed gels in water and the latter in the organic solvent at a concentration of 1% w/v and 0.8% w/v respectively. The derivatives were found to contain a fibrous network characteristic of the supramolecular gel and possess notable antibacterial properties (Fig. 21).
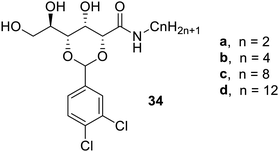
D-Gluconic gelators70 have also been reported, which are similar to gluconamide-based gelators. Such gelators contain 3,4-chlorobenzylidene acetal-protecting groups, and are shown in Fig. 22. Among the synthesized gelator, compound 34c was most prominent and found to form gels in a large number of organic solvents and aqueous mixtures. The gel of compound 34c in n-butyl acetate has exceptional extraction properties for pump oil at room temperature and has good mechanical strength. The gel of compound 34c in chloroform was sensitive to anions. These gelators were found to form moldable clear gels, which may be employed as valuable materials in the optics.
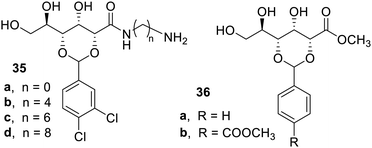
The interaction of the terminal amino group on D-gluconic acetal derivatives with aliphatic acid leading to the formation of two-component gels was also studied.71 It was found in the study that the gelation property was dependent on the aliphatic chain length 35b–d in comparison to 35a. The compound 35c was reported as a super gelator in chlorobenzene and o-xylene with a concentration of 0.1% w/w. The compounds 35b–d in comparison to 35a were able to give a co-gel with o-xylene, dioxane, and chlorobenzene (Fig. 23). The compound 35c formed strong, self-healing co-gels with stearic acid, myristic acid, and capric acid in chlorobenzene. This co-gel was clear and may be used in the optical apparatus. The compounds 36a–b were good gelators in various organic solvents such as nitrobenzene, chlorobenzene, benzene, and toluene at a minimal concentration of 0.06–0.19% w/w.72
2.5. Alditol derivatives from reduced sugar alcohols
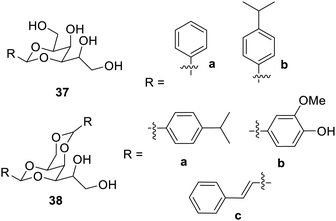
According to a study,73 1,3-dibenzylidene-D-sorbitol and 2,4-dibenzylidene-D-sorbitol (DBS) can produce gels in polyethylene glycol (PEG) that is impacted by silica due to the disruption of the hydrogen connection between DBS and PEG. The inclusion of silica had an impact on the temperature and time of gel formation. A spherulite structure with lamellar packing was discovered through structural analysis. This study brought forward the concept of modification of the self-assembly in solvents by the addition of silica, which led to the development of versatile hybrid materials.Another research developed mono- and di-benzylidene-protected sorbitol low-molecular weight gelators,74 as shown in Fig. 24. Compound 37b was not a hydrogelator but was able to form gels in most of the solvents and in aqueous ethyl alcohol. Compound 38a was the best hydrogelator, while the compounds 38b and 38c did not show any gelation properties. These multicomponent gels may be employed in the tunable soft materials, which have numerous applications.
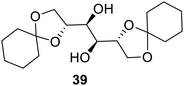
Carbohydrate-based LMGs, as shown in Fig. 25, have been found to form gels in ionic liquids.75 Ammonium phosphonium, pyridinium and imidazolium-based ionic liquid gels were obtained in this research. It was found that a higher amount of the gel was required in the case of bulky cations if the ionic liquid. The gelator 39 was able to form gels with most of the ionic liquids. Ionic liquid gels were found to be thermally more stable than the ionic liquid itself. Reversible ionic liquid gels may play numerous roles in the electrochemistry.
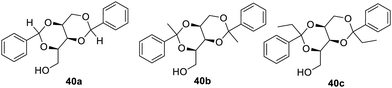
Xylitol-based76 organogelators have also been reported as shown in Fig. 26, which yield gel in crude oil and most of the organic solvents. Compound 40a was found to be a powerful gelator for crude oil and organic solvents. The gelation of the crude oil and refinery effluent was observed to be very fast within minutes. Thus, such gelators proved themselves as new materials, which may be used in the refinery process to minimize the waste.
2.6. Glycosyl triazole derivatives as molecular gelators
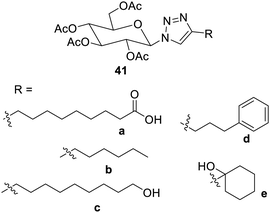
Triazoles and other heterocycles are also used as functional groups in low-molecular weight gelators. The hydrogen bond acceptors and donors of triazoles make it simple to create them by the click reaction, and they also readily establish π–π interactions. Such properties of the triazole make it a valuable low-molecular weight gelator.77,78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The compounds 41c and 41e were also found to be good gelators. The derivatives without polar groups 41b and 41d were able to yield gels in isopropanol and in a mixture of ethyl alcohol and water in a ratio of 1
2. The compounds 41c and 41e were also found to be good gelators. The derivatives without polar groups 41b and 41d were able to yield gels in isopropanol and in a mixture of ethyl alcohol and water in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
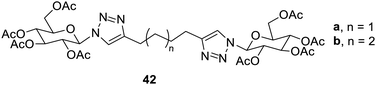
The dimers of these sugar triazoles 42a–b were found to be more promising gelators than the monomers (Fig. 28). The compound 42a formed gels in ethanol and toluene as well as the aqueous mixture of DMSO and ethanol.31,38
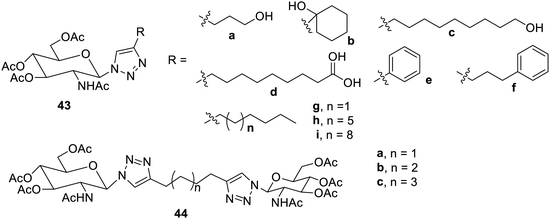
Some effective low-molecular weight peracetylated D-glucosamine triazole gelators were obtained from D-glucosamines40 (Fig. 29). The compounds 43a–d and 43a–b were hydrogelators and yielded gels in aqueous DMSO and alcohol. The derivatives 43a–b were reported to be gelators in toluene. The dimers of these 44b and 44c are still more effective gelators and represent effective hydrogelators.
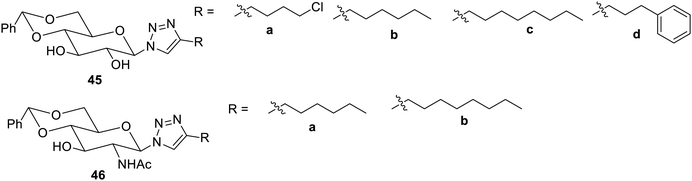
The influence of the functional group on making the molecule an effective gelator was also studied.79 For this protected sugar, triazoles with 4,6-O-benzylidene acetal 45 and 46 were formed from azides. The compounds 45b and 45c were found to possess effective gelation properties for engine oil. The compound 45d yielded gel in aqueous DMSO. The N-acetyl D-glucosamine triazole derivatives 46a and 46b yielded gel in aqueous DMSO and ethanol as shown in Fig. 30.
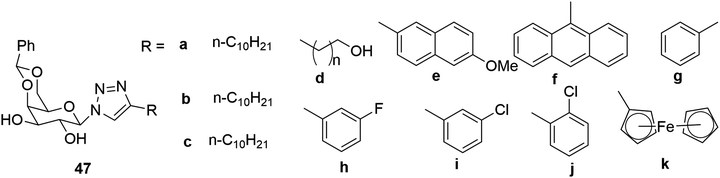
D-Galactose substituted with benzylidene acetal-protected triazoles was found to be an effective gelator,80 as shown in Fig. 31. It was observed that 47a–c were gelators for oils, while the aromatic compounds yielded gels in organic solvents. The gelator containing an anthracene ring was found to be fluorescent, and shows potential for use in medical diagnosis and optical applications.
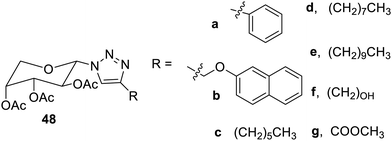
Triazolylarabinosides81 have also been reported as organogelators, as shown in Fig. 32. The derivatives 48a and 48g acted as supergelators in kerosene, diesel and petrol, and hence, they may be employed as new materials for the solidification of crude oil and its various products, which are used for the purification and recovery of oils.
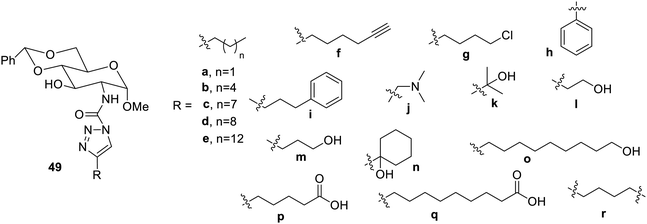
The N-acetyl-glucosamines having triazoles linked at C-6 positions (Fig. 34) have also been synthesized and reported to be good gelators in oils and organic solvents.82 The compounds 50a,b and 50f were found to exhibit phase-selective gelation in crude oil, which became incapable of gelation in powdered form using ethyl acetate as a carrier.
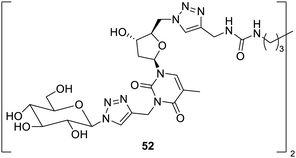
Bolaamphiphiles which were generated with nucleosides were reported to form hydrogels at a lower gelation concentration.84 The compound 52 was found to remain intact for almost less than a month in mouse without any toxicity and immunogenicity. Therefore, this compound is reported to be a new biomaterial, which may find many applications in the live tissues, as shown Fig. 36.
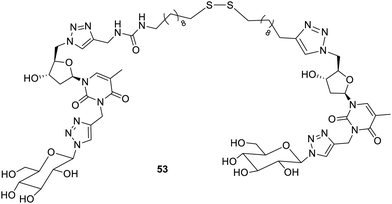
The disulfide linkage in the bolaamphiphiles 5385 was also explored for the sustained release of proteins containing thiols. The thixotropic nature of the resulting gels made them suitable for injecting into the body. The disulfide group enables the thiol-disulfide exchange, so these hydrogels may be employed for their functionalization with thiol-containing proteins for their sustained release in the tissues (Fig. 37).
2.7. Disaccharide derivatives and glycoclusters
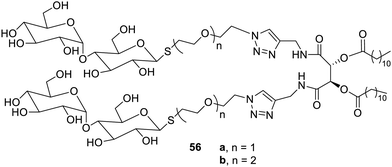
Thiolactose-based amphiphiles have also been reported as low-molecular weight gelators, which yielded colloidal solutions in water, as shown in Fig. 39.86 The compound 56a yielded hydrogels easily at lower concentrations. The compound 56b showed chain inversion. The binding nature of these amphiphiles to the peanut agglutinin lectin lead to the conclusion that these structures may be helpful in designing nanostructures to study sugar-specific ligands.
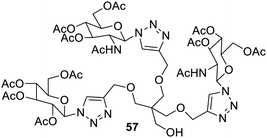
The dendritic glycoclusters have been synthesized88 and the compounds with few branches were appreciable hydrogelators. Hexameric and trimeric gelators were combined to produce co-metallogels, which can be used in supramolecular catalysts that can be recycled and used repeatedly. Such materials may reduce the use of metal ions in the catalysis for the synthetic processes.
2.8. Nucleotide and nucleoside-based gelators
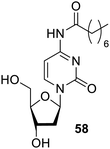
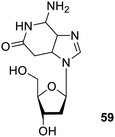
Nucleotide and nucleoside are the self-assembled and may be employed for supramolecular studies. They are basically ribose sugar having pyrimidine or purine forming intermolecular attraction via hydrogen bonding, electrostatic interactions, and π–π interaction.89 Out of the four nucleobases, guanidine is the most desired molecule for such gelator systems, as it has biochemically notable extended two-dimension ribbon with G-4 wires. Supramolecular substances based on nucleic acid are significant for biotechnical and biomedical applications.90Further research has been done on guanosine-based92 gelators. Among these gelators, the 8-aza-20-deoxyisoguanosine derivative 59 (Fig. 42) was discovered to be fluorescent under UV light, and exhibits thixotropy, where the gel form was restored as soon as the radiation was turned off. The gelation process was temperature and pH dependent, which make it a potential molecule in the nanomedicine.
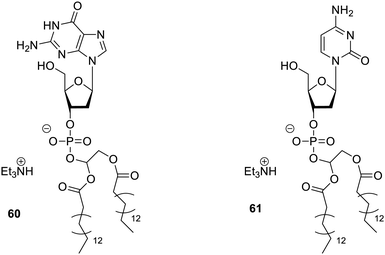
Nucleotide-lipids93 having pyrimidine and purine bases have also been reported, as shown in Fig. 43, which were able to yield hydrogels. Cytosine and guanosine derivatives 60 and 61 are the main reported compounds, as shown in Fig. 44. Among them, cytosine hydrogels were stabilized by NaCl but guanosine hydrogels stabilized even in the absence of NaCl. It was established that the supramolecular structure of such hydrogels was dependent on nucleobase employed along with other conditions. These compounds 60 and 61 were further studied for the slow release of diazepam from the matrix of the gel, and it confirmed the potential of this gelator for controlled delivery of drugs.
Guanosine monophosphate95 crosslinked with Ca2+ and Fe3+ was synthesized, which exhibited pH-dependent zero-order doxorubicin release, which is a well-known chemotherapeutic agent. In the acidic buffer, the release of the drug was at fast rate. This hydrogel was reported to be stable at a pH of 7.4 for three weeks, and there was release of just 3% of the drug. This establishes the metallogel as a potential candidate for drug delivery.
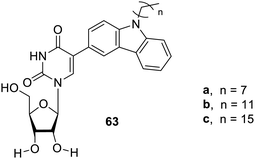
Carbazole97 gelators 63a–c (Fig. 45) have also been reported to possess numerous properties such as electrical conduction, charge carrier, and luminescence. These are presently employed as chemo sensors for fluorescence imaging and several other related biomedical applications. The exceptional self-assembling property of these gelators is due to the hydrogen bonding. These gelators may forecast numerable morphologies in various solvents, which need to be explored for their future applications.
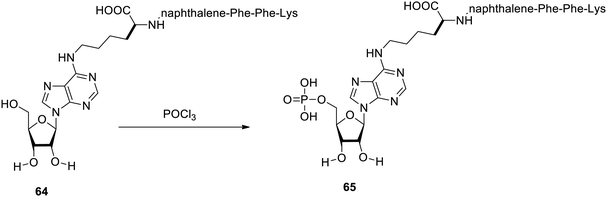
It was reported that the enzymatic self-assembly triggered adenosine-based gelators,98 as shown in Fig. 46. Treatment of a mixture of hydrogelator naphthalene–Phe–Phe–Lys 64 and adenosine with phosphoryl chloride yielded a phosphorylated form of hydrogelator 65. The dephosphorylated form 64 has fibrous structures, while the phosphorylated form does not have any fibrous structure and the self-assembly here was due to the enzyme. This work represents the enzymatic action for the production of hydrogels using adenosine nucleoside.
3. Stimulus-responsive gels
Smart materials are in great demand in the diagnosis and treatment of diseases. One such substance that uses chemicals to stimulate biomolecules is a stimulus-responsive gel.99 The stimulation may be physical, chemical or biological. The enzymes which are biological stimuli have advantages for the peptide bond generation, and the other product is generally water. The physical stimulation is caused by electric field, magnetic field and temperature or radiation. The chemical stimulation includes pH changes and specific ion-presence. The sol–gel transition is reversible and may be employed for the stimulation. Stimulus-responsive hydrogels are biocompatible and are used in the immunotherapy as non-immunogenic vaccines. Many more applications of the stimulus-responsive gels need to be explored for biomedical applications.3.1. pH-Responsive gelators
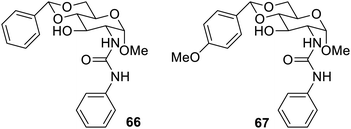
The gelators have been designed to work on change in pH as external stimuli. The pH stimulation can be of two types. The first one are those which work on protonation and deprotonation,100 while the second one are those which work on dissociation of the gelator functional groups on changing the pH of the medium. The first type sol–gel transformation work on the principle of production of positive and negative charges on the system by protonation and deprotonation of the gelator respectively.101 The second type of the gelators have labile pH-dependent functional groups,102 which break away as the pH is changed. Nano particles based on cytidine-5′-monophosphate (5′-CMP)-mediated akaganeite (β-FeOOH) were used to give hydrogels,103 in the pH range of 5.5–8.5 and the temperature range of 30–40 °C. The hydrogel gave a colloidal solution at a lower pH. The porous nature of the hydrogel was reported due to the conformation change from C2′ endo to C3′ endo. This change enhanced the non-covalent forces. The presence of the ribose sugar made this gelator non-cytotoxic, injectable, and self-healing. Therefore, it may be used for the loading and release of the drug at the desired pH in the organic tissues, thereby making it a potential biomedical material.Carbohydrate-based pH-responsive D-glucosamine gelators have also been studied, as given in Fig. 47. The protecting group of benzylidene acetal in 66 was changed to more labile and pH-responsive p-methoxybenzylidene acetal in 67. The gelator 67 dissolved completely in sulfuric acid in 48 hours. This enables the gelator 67 suitable for the drug release in acidic media.
3.2. Photosensitive gelators
Light has also been used to stimulate the gelation action of several gelators. Light or radiation-controlled transformation of gels may enhance the potential of gelators in many biomedical applications.104 Carbohydrate moieties with photo-responsive azobenzene functional groups have been reported as photo-sensitive gelators.105,1063.3. Photo-isomerizable gelators
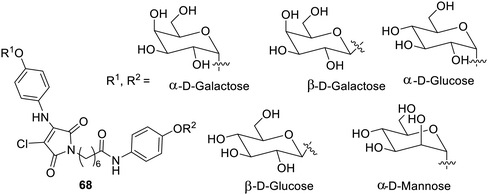
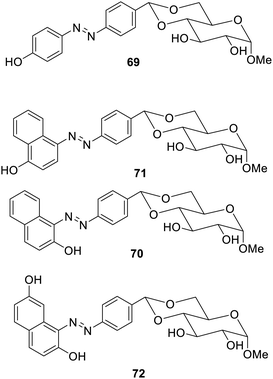
Besides sol–gel transformations, there are several other changes which occur in response to the light falling on the gelators. Bolaamphiphilic glycolipid LMGs 68 having N-alkyl-2-anilino-3-chloromaleimide have shown additional thermochromism,107 as shown in Fig. 48. This material upon increase in temperature became transparent on the alteration of the congregation without affecting the assembly of gels. The bathochromic shift was observed under UV-visible radiation. This research has given an overview of the application of gelators responsible for glycol-related enzymes.Supramolecular gelators responsive to multiple stimuli have also been reported. Glucose-based multi-stimuli-responsive supramolecular gelators10869–72 containing a photosensitive aryl azo moiety and a metal and pH-responsive hydroxyl group have been synthesized, as shown in Fig. 49. Upon exposure to light, the sugar azobenzene derivatives collapsed on treatment with UV radiation for 30 min. The exposure to the visible radiation changed it to the solution in 2–4 days. The gel was found to become a solution at a pH higher than 12.4 and lower than 1.6.
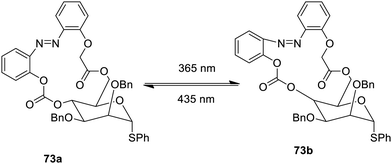
Another azobenzene carbohydrate macrocyclic low-molecular weight gelator has been reported which shows cis–trans isomerism on stimulation with the radiation, as shown in Fig. 50.109 The azo moiety predominates in cis form 73a with radiation at a wavelength 365 nm, while trans form 73b dominates at a wavelength of 435 nm. Half-life of the Z form 73b is 51 days. These gelators may be employed as new photo responsive, biocompatible materials.
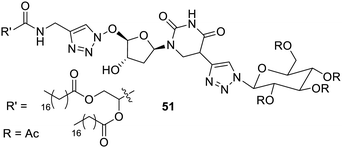
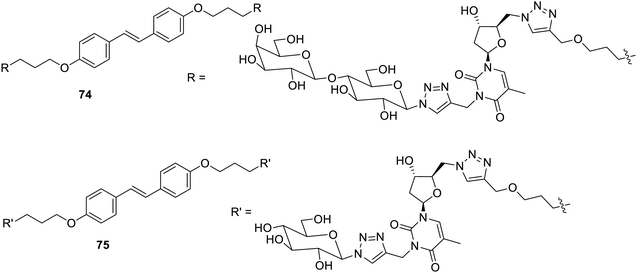
Stilbene has been introduced in the gelator to make it photosensitive. Glyconucleoside bolaamphiphilic low-molecular weight gelators110 have been reported as photo-sensitive gelation. The compounds 74 and 75 were found to form gels in aqueous ethanol (Fig. 51). The E–Z isomerism in stilbene upon UV radiation causes sol–gel transformation. The UV irradiation with a 312 nm deformed supramolecular structure lead to only Z-isomers in dominance. These photo-responsive materials may be considered to replace the polymer-based radiation stimulation employed in drug delivery.
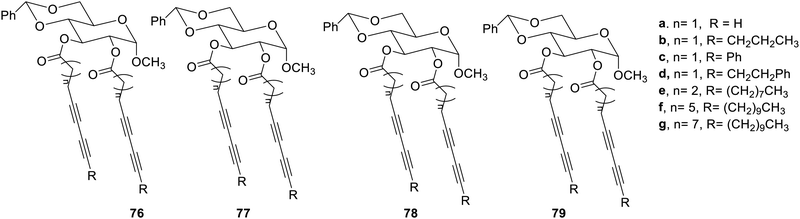
The compounds 79f and 79g polymerized on exposure to the UV-radiation and may be used as new materials for photo-stimulating applications.
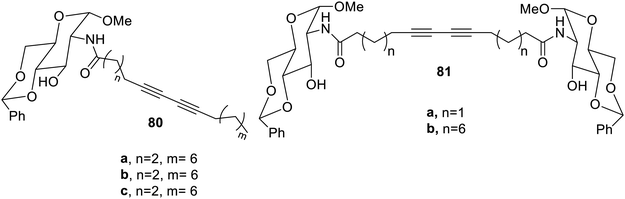
Urea and amide functional groups may also be used for synthesizing the gelators,42 as shown in Fig. 53. These compounds yielded gels in numerous solvents and their aqueous mixtures. Compound 80c gave gel in toluene, ethanol and isopropyl alcohol easily with low gelation concentration followed by compound 81b. These molecules were reported to be sensitive towards UV radiation proposing to be new materials in this field.
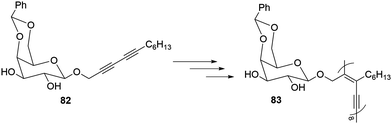
Gelators based on β-D-glucopyranoside diacetylene have been reported as shown in Fig. 54.111 Compound 82 yielded gels at low concentrations in non-polar solvents and gels. The diyne groups existed in close proximity in gels facilitating the polymerization on exposure to the UV radiation (300 nm) for two days. The resulting polymer 83 has exceptional attraction for galactose-binding lectin, which was much more than that of monosaccharides. Such materials pose themselves as biomaterials with exceptional binding with lectin.
3.4. Enzyme-responsive gelators and biomedical applications
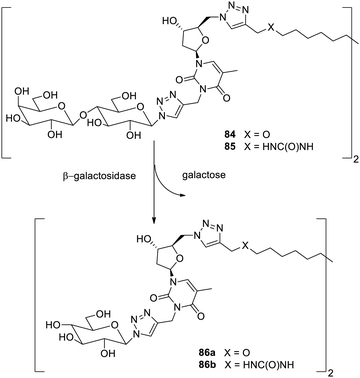
The gelation process has also been induced by the enzymes112 which are catalysts for the synthesis in biochemical processes. The enzymes may be employed for the drug delivery or delivery of the specific biomolecules at the specific site. If the gelator has the enzymatic moiety, then on degradation of the enzyme in a specific environment, the gelator will also degrade leading to the release of desired drugs or the biomolecule.113 Enzymatic stimulation is preferred than other stimulations, as they are harmless, more specific, and selective in their action. Carbohydrate-based materials as gelators whose gelation is induced by the enzymes are lesser in number than those of peptide and other polymer-based gelators.The enzyme β-galactosidase has been exploited frequently for the stimulating supramolecular gelators. Glyconucleo bolaamphiphiles (GNBA) have been employed for the generation of stimuli-responsive materials.114 Gelator precursors 84 and 85 based on the lactose were generated and used to attach nucleotides with carbohydrates (Fig. 55). The compound 84 gave a viscous solution in PBS. While the compound 85 yielded hydrogels. Both the compounds 84 and 85 yielded hydrogels 86a and 86b upon treatment with enzyme β-galactosidase, and it was found that the supramolecules formed after enzymatic reactions had good mechanical strength. This study concluded that materials which are stimulated by enzyme β-galactosidase may be employed to alter the internal cell functioning, which may be utilized in therapeutics.
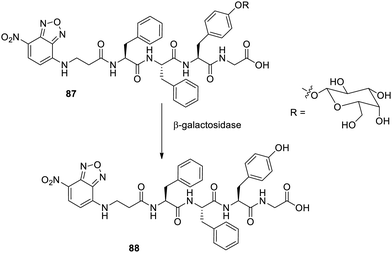
Supramolecular 4-nitro-2,1,3-benzoxadiazole attached with diphenylalanine and tyrosine through β-galactose.115 The solubility of the precursor 87 (Fig. 56) was enhanced by the sugar attached to tyrosine, which made the cleavage by β-galactosidase yielding self-assembly 88. The cleavage of enzyme took place at 37 °C in saline solutions buffered with phosphates. The gelator precursors on incubation with HeLa cancer cells resulted in the self-assembly of the compound, and this material may be employed for detecting the type and extent of these cells. The results of the research also confirmed toxicity on cancer cells, which was due to the self-assembly inside the cell occurring upon cleavage of galactose in the presence of β-galactosidase.
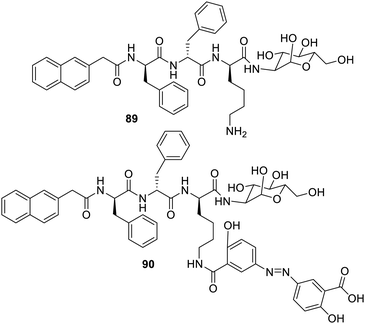
The gelator11689 based on glucosamine peptide yielded hydrogels, which were biocompatible (Fig. 57). The derivatives of such gelators when combined with the drug olsalazine (anti-inflammatory) yielded compound 90. In an acidic medium, the molecule undergoes self-assembly yielding nanofibrous structures, which release the drug mesalazine on treatment with azoreductase. Another material involving glycopeptide was found to have potential applications in the functioning of cells.117
Enzyme stimulation has also been reported involving enzyme histidine triad nucleotide-binding protein 1. Here nucleoside phosphoramidate having bases such as pyrimidine and purine was employed as the gelator and yielded the hydrogel in the presence of enzymes.118
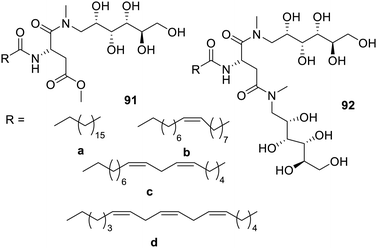
Glycolipids with a N-methyl-D-glucamine group were synthesized119 (Fig. 58). The glycolipid had a polar head with an unsaturated chain. It was found in the research that the gelators 91a and 92b with small unsaturated chains yielded hydrogels, while the gelator with longer unsaturated tails gave organogelation, which was induced by water via agitation. Hydrogel formation was enhanced upon increasing the polar head group size. The gelation of these hydrogels was found to engulf the bioactive compound riboflavin and curcumin, immaterial of their hydrophobic or hydrophilic nature. The engulfing property was further enhanced on increasing the size of the polar headgroup. The release of the bioactive molecule was carried out using trypsin which is an amidase. The bioactive molecule was released on the hydrolysis of glycolipid. It was also found that the hydrogels also exhibited potential for the removal of copper and chromium from the edible oils, which was further enhanced upon increasing the unsaturation of the chain.
Alkaline phosphatase is the most prominent enzyme used for stimulation in carbohydrate-based gels. In one of the reported research studies,120 peptide-containing phosphate was cleaved by alkaline phosphate. The gelator here contained a naphthyl group for the self-assembly and galactose headgroup to interact with the lectin on Pseudomonas aeruginosa to screen for the antibacterial property of the gelator. The dephosphorylation and production of the fibrous structure occurred, which rendered galactose on the outer boundary of the self-assembled fibre, making its interaction with the lectin on the bacterial cell wall. Here the enzyme stimulated the self-assembly and formation of a gel, which was proved to have bactericidal effects.
Similar molecules have a naphthyl group on peptide with a phosphate group and attaching alkaline phosphatase for enzyme stimulation and carbohydrate for binding to the lectin. Phe–Phe–Ser–Tyr(H2PO3) and D-mannose composing gelators based on glycopeptide were found to be bactericidal with healing effects on wounds.121 The mannose here interacted with lectin to cause protein agglutination, which causes adhesion of the bacteria on Escherichia coli. The other tested bacteria were Staphylococcus aureus, but in this case, the protein of the bacterial cell wall does not interact with mannose.
4. New carbohydrate-based gelators
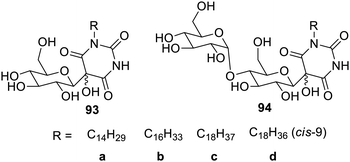
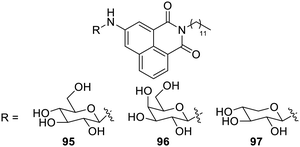
Glucose- and maltose-based Amphiphilic β-C-glycosylbarbiturate hydrogelators 93a–d and 94a–d were synthesized by a H2O2-mediated green method, as shown in Fig. 59. These hydrogelators produce glycol nanostructures that self-assemble due to intermolecular interactions and packing factors. Supramolecular gelation of water at neutral pH can be obtained using the synthesized gelators. It was interesting to note that amyloglucosidase from Aspergillus niger was used to hydrolyze a precursor made of maltose into a hydrogelator made of glucose.122Amphiphilic N-glycosyl naphthalimides123 (glucose, galactose and xylose derived) 95d, 96d, and 97d have also been synthesized by a green method, which furnished the gel in both hydrophilic and hydrophobic solvents due to their supramolecular self-assembly. These gels possess molecular level interactions such as H-bonding, p–p stacking and van der Waals forces. Compound 95d (Fig. 60) shows hydrogelation in DMSO–H2O (40% v/v) with a critical gelation concentration (CGC) of 0.5% (wt/v) and organogelation in CHCl3, cyclohexane and xylene with a CGC of 0.3, 0.8 and 0.6% (wt/v).
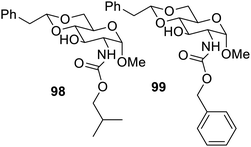
Further, synthesis of C-2 carbamates of para-phenylethylidene acetal-protected D-glucosamine derivatives124 was also reported along with their gelation properties. The study also reported isopropyl carbamate as a highly efficient hydrogelator, which also exhibited the ability to form gel electrolytes. Gelators 98 and 99 possessed ability to form metallogels with various metal ions, and copper and cobalt xerogels showed good catalytic properties, as shown in Fig. 61. Gelator 99 was reported to form a highly stable gel with naproxen sodium and facilitated controlled release.
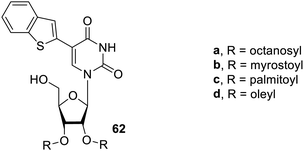
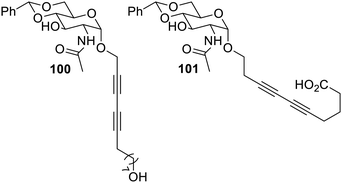
The research group further progressed to study the effect of diacetylene functional groups introduced at the anomeric position by using N-acetyl-D-glucosamine derivatives on gelating properties. The diacetylenic group was attached at the anomeric position using α-glycosidic linkage separated by one, two and three methylene groups from anomeric oxygen. The glycosides formed red and purple coloured gels, which were prone to photopolymerization. The compound 100, a hydroxyl-modified derivative, could form hydrogels at a low concentration of 1 wt%. The derivative 101 which was modified using carboxylic acid formed transparent gels and has the ability to immobilize basic solutions, which encourages its use as a pH-responsive gelator. The synthesized gelators, as shown in Fig. 62, could be used as stimulus-responsive materials, which are highly advantageous in biomedical applications.125
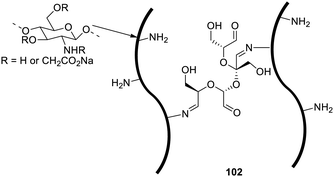
In another work, a highly environment-friendly and biologically safe cross-linker, oxidized sucrose (OS) was used to form carboxymethyl chitosan hydrogel (CMCG) 102 (Fig. 63) of a polysaccharide, carboxymethyl chitosan (CMC).126 The structural characterization revealed that the reaction proceeded smoothly even in the absence of catalysts via cross-linking of aldehyde groups in OS and primary amine groups in CMC. This study focuses on the utilization of oxidised sucrose as a natural cross-linking agent for selective linking of amino sugars to facilitate the formation of polysaccharide gels, which hold enormous potential for biomedical applications.
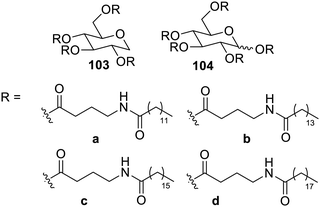
Recent years have also witnessed the synthesis and utilization of gelators for various applications. The ability of N-linear saturated fatty acyl-GABA present in the human brain synthesized using amide-bonded γ-aminobutyric acid (GABA) and linear saturated fatty acids of varying lengths to gelate organic solvents has been reported by Komba et al. Along with this, ester derivatives of GABA127 and 1,5-anhydro-D-glucitol (1,5-AG) 103a–d or D-glucopyranose (Glc) 104a–d (Fig. 64) also delivered gelating properties, which demonstrated high gel hardness and transparency of N-tetradecanoic acyl-GABA bonded to 1,5-AG via ester linkage (C14GABA-AG).
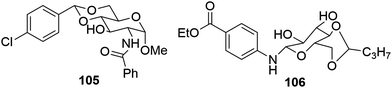
Another interesting study reports the effect of halide substitution on gelation properties by demonstrating the synthesis of para-chlorobenzylidene acetal-protected D-glucosamine amide derivatives. The self-assembling characteristics of these amides were examined in a variety of solvents, organics as well as mixtures, which revealed the property of these derivatives to form gels at low minimum gelation concentrations (MGCs) even below 0.1 wt%. The exploration resulted in the formation of a highly stable gel in water and EtOH/H2O by a gelator128 benzamide 105 (Fig. 65) at an extremely low concentration of 0.04 wt%. The gel was further employed for entrapment as well as controlled release of chloramphenicol and naproxen as well as for dye removal for toluidine blue aqueous solutions.
Alkyl-substituted sugar-aminobenzoate-based organogelator 106 has also been synthesized at low costs by a simple and effective method, as shown in Fig. 65. It can remove water-soluble dyes from their concentrated aqueous solutions. Organogelator 106 shows high gelling ability towards a wide range of solvents along with the exceptional detection ability towards copper metal ions with a state change from gel to solution129 Gel–sol transformation takes place due to high stability of [Cu(G13)] complex formation.
5. Conclusions
This review presented an overview of carbohydrate-based gelators as new materials and their potential applications in various emerging fields such as drug delivery, environmental remediation, antibacterial agents, tissue engineering, thixotropy and wound healing. Carbohydrates are natural materials which are compatible for cells and tissues of living beings, and hence, they are extensively researched and useful for the generation of soft materials. The carbohydrate-based LMGs that fall into this category with numerous potential applications in almost all fields including biomedical applications are discussed in the review. Ribose, arabinose, galactose, mannose, glucosamine, and glucose have been studied for the development of LMGs. The role of the C-1 oxidized and reduced sugar has also been reviewed. The functionalization of carbohydrates for specific stimulation has also been discussed with several examples, which enrich the literature for the development of novel hydro or organogelators for specific requirement in the future. This review compiles stimulus-responsive gelators that are sensitive to acids, bases, enzymes, photoradiation and ions. Thus, the carbohydrate-based gelator as a soft material is an emerging field, which may be studied in collaboration with material scientists to develop new materials for future use.Conflicts of interest
The authors declare that there is no conflict of interest regarding this manuscript.Acknowledgements
Authors are thankful to the Jawaharlal Nehru University (JNU) for providing facilities to write this review. RT is thankful to UGC New Delhi for the Junior/Senior Research Fellowship. KS is thankful to CSIR for Junior/Senior Research Fellowship. RS is grateful to Ultra International and Sanganeria Foundation for supporting lab furniture to Glycochemistry laboratory at JNU.References
- S. Mitchell, R. Qin, N. Zheng and J. Pérez-Ramírez, Nat. Nanotechnol., 2021, 16, 129–139 CrossRef CAS PubMed.
- J. Zhou, J. Li, X. Du and B. Xu, Biomaterials, 2017, 129, 1–27 CrossRef CAS PubMed.
- H. Shigemitsu and I. Hamachi, Acc. Chem. Res., 2017, 50, 740–750 CrossRef CAS PubMed.
- J. Wang, X. Miao, Q. Fengzhao, C. Ren, Z. Yang and L. Wang, RSC Adv., 2013, 3, 16739–16746 RSC.
- T. Kato, Y. Hirai, S. Nakaso and M. Moriyama, Chem. Soc. Rev., 2007, 36, 1857–1867 RSC.
- S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973–2129 CrossRef CAS PubMed.
- A. Ajayaghosh, V. K. Praveen and C. Vijayakumar, Chem. Soc. Rev., 2008, 37, 109–122 RSC.
- J. Morris, J. Bietsch, K. Bashaw and G. Wang, Gels, 2021, 7, 1–61 CrossRef PubMed.
- B. O. Okesola and D. K. Smith, Chem. Soc. Rev., 2016, 45, 4226–4251 RSC.
- A. M. Vibhute and K. M. Sureshan, ChemSusChem, 2020, 13, 5343–5360 CrossRef CAS PubMed.
- J. Zhang and C. Y. Su, Coord. Chem. Rev., 2013, 257, 1373–1408 CrossRef CAS.
- A. Y. Y. Tam and V. W. W. Yam, Chem. Soc. Rev., 2013, 42, 1540–1567 RSC.
- Y. Zheng, G. Li and Y. Zhang, ChemNanoMat, 2016, 2, 364–375 CrossRef CAS.
- M. Häring and D. D. Díaz, Chem. Commun., 2016, 52, 13068–13081 RSC.
- F. Versluis, J. H. van Esch and R. Eelkema, Adv. Mater., 2016, 28, 4576–4592 CrossRef CAS PubMed.
- R. Tian, J. Chen and R. Niu, Nanoscale, 2014, 6, 3474–3482 RSC.
- R. Dong, Y. Pang, Y. Su and X. Zhu, Biomater. Sci., 2015, 3, 937–954 RSC.
- B. Escuder, F. Rodríguez-Llansola and J. F. Miravet, New J. Chem., 2010, 34, 1044–1054 RSC.
- F. Rodríguez-Llansola, B. Escuder and J. F. Miravet, J. Am. Chem. Soc., 2009, 131, 11478–11484 CrossRef PubMed.
- A. Döring, W. Birnbaum and D. Kuckling, Chem. Soc. Rev., 2013, 42, 7391–7420 RSC.
- N. Singh, K. Zhang, C. A. Angulo-Pachón, E. Mendes, J. H. Van Esch and B. Escuder, Chem. Sci., 2016, 7, 5568–5572 RSC.
- N. Basu, A. Chakraborty and R. Ghosh, Gels, 2018, 4(2), 52 CrossRef PubMed.
- R. Kubota, S. Liu, H. Shigemitsu, K. Nakamura, W. Tanaka, M. Ikeda and I. Hamachi, Bioconjugate Chem., 2018, 29, 2058–2067 CrossRef CAS PubMed.
- M. Liu, G. Ouyang, D. Niu and Y. Sang, Org. Chem. Front., 2018, 5, 2885–2900 RSC.
- J. Hoque, N. Sangaj and S. Varghese, Macromol. Biosci., 2019, 19, 1–16 CrossRef.
- H. Wang, Z. Feng and B. Xu, Theranostics, 2019, 9, 3213–3222 CrossRef CAS PubMed.
- S. Datta and S. Bhattacharya, Chem. Soc. Rev., 2015, 44, 5596–5637 RSC.
- K. Soundarajan, R. Periyasamy and T. Mohan Das, RSC Adv., 2016, 6, 81838–81846 RSC.
- G. Wang, S. Cheuk, K. Williams, V. Sharma, L. Dakessian and Z. Thorton, Carbohydr. Res., 2006, 341, 705–716 CrossRef CAS PubMed.
- X. Nie and G. Wang, J. Org. Chem., 2006, 71, 4734–4741 CrossRef CAS PubMed.
- H. P. R. Mangunuru, J. R. Yerabolu, D. Liu and G. Wang, Tetrahedron Lett., 2015, 56, 82–85 CrossRef CAS.
- G. Wang, A. Chen, H. P. R. Mangunuru and J. R. Yerabolu, RSC Adv., 2017, 7, 40887–40895 RSC.
- I. S. Okafor and G. Wang, Carbohydr. Res., 2017, 451, 81–94 CrossRef CAS PubMed.
- A. Chen, S. B. Adhikari, K. Mays and G. Wang, Langmuir, 2017, 33, 8076–8089 CrossRef CAS PubMed.
- G. Wang, H. Yang, S. Cheuk and S. Coleman, Beilstein J. Org. Chem., 2011, 7, 234–242 CrossRef CAS PubMed.
- S. Cheuk, E. D. Stevens and G. Wang, Carbohydr. Res., 2009, 344, 417–425 CrossRef CAS PubMed.
- N. Goyal, S. Cheuk and G. Wang, Tetrahedron, 2010, 66, 5962–5971 CrossRef CAS.
- G. Wang, S. Cheuk, H. Yang, N. Goyal, P. V. Narasimha Reddy and B. Hopkinson, Langmuir, 2009, 25, 8696–8705 CrossRef CAS PubMed.
- N. Goyal, H. P. R. Mangunuru, B. Parikh, S. Shrestha and G. Wang, Beilstein J. Org. Chem., 2014, 10, 3111–3121 CrossRef PubMed.
- H. P. R. Mangunuru, J. R. Yerabolu and G. Wang, Tetrahedron Lett., 2015, 56, 3361–3364 CrossRef CAS.
- H. P. R. Mangunuru, H. Yang and G. Wang, Chem. Commun., 2013, 49, 4489–4491 RSC.
- G. Wang, N. Goyal, H. P. R. Mangunuru, H. Yang, S. Cheuk and P. V. N. Reddy, J. Org. Chem., 2015, 80, 733–743 CrossRef CAS PubMed.
- K. Soundarajan, M. Rajasekar and T. M. Das, Mater. Sci. Eng., C, 2018, 93, 776–781 CrossRef CAS.
- A. D. Ludwig, A. Saint-Jalmes, C. Mériadec, F. Artzner, O. Tasseau, F. Berrée and L. Lemiègre, Chem. – Eur. J., 2020, 26, 13927–13934 CrossRef CAS.
- C. Mahendar, M. K. Dixit, Y. Kumar and M. Dubey, J. Mater. Chem. C, 2020, 8, 11008–11012 RSC.
- K. Lalitha, K. Gayathri, Y. S. Prasad, R. Saritha, A. Thamizhanban, C. U. Maheswari, V. Sridharan and S. Nagarajan, Gels, 2018, 4(1), 1 CrossRef PubMed.
- F. Ono, K. Ichimaru, O. Hirata, S. Shinkai and H. Watanabe, Chem. Lett., 2020, 49, 156–159 CrossRef CAS.
- J. Brinksma, B. L. Feringa, R. M. Kellogg, R. Vreeker and J. Van Esch, Langmuir, 2000, 16, 9249–9255 CrossRef CAS.
- N. N. Adarsh, D. K. Kumar and P. Dastidar, Tetrahedron, 2007, 63, 7386–7396 CrossRef CAS.
- M. Suzuki, M. Yumoto, H. Shirai and K. Hanabusa, Tetrahedron, 2008, 64, 10395–10400 CrossRef CAS.
- A. Pal, Y. K. Ghosh and S. Bhattacharya, Tetrahedron, 2007, 63, 7334–7348 CrossRef CAS.
- J. Morris, P. Kozlowski and G. Wang, Langmuir, 2019, 35, 14639–14650 CrossRef CAS PubMed.
- A. Chen, L. P. Samankumara, C. Garcia, K. Bashaw and G. Wang, New J. Chem., 2019, 43, 7950–7961 RSC.
- D. Wang, A. Chen, J. Morris and G. Wang, RSC Adv., 2020, 10, 40068–40083 RSC.
- T. Xiong, X. Li, Y. Zhou, Q. Song, R. Zhang, L. Lei and X. Li, Acta Biomater., 2018, 73, 275–284 CrossRef CAS PubMed.
- E. R. Draper and D. J. Adams, Chem, 2017, 3, 390–410 CAS.
- L. E. Buerkle and S. J. Rowan, Chem. Soc. Rev., 2012, 41, 6089–6102 RSC.
- L. S. Birchall, S. Roy, V. Jayawarna, M. Hughes, E. Irvine, G. T. Okorogheye, N. Saudi, E. de Santis, T. Tuttle, A. A. Edwards and R. V. Ulijn, Chem. Sci., 2011, 2, 1349–1355 RSC.
- A. Brito, Y. M. Abul-Haija, D. S. Da Costa, R. Novoa-Carballal, R. L. Reis, R. V. Ulijn, R. A. Pires and I. Pashkuleva, Chem. Sci., 2019, 10, 2385–2390 RSC.
- A. Brito, P. M. R. Pereira, R. L. Reis, R. V. Ulijn, J. S. Lewis, R. A. Pires and I. Pashkuleva, Nanoscale, 2020, 12, 19088–19092 RSC.
- K. B. Pal and B. Mukhopadhyay, ChemistrySelect, 2017, 2, 967–974 CrossRef CAS.
- N. P. Pathak, Rajkamal and S. Yadav, Chem. Commun., 2020, 56, 2999–3002 RSC.
- C. Narayana, R. K. Upadhyay, R. Chaturvedi and R. Sagar, New J. Chem., 2017, 41, 2261–2267 RSC.
- J. Ramos, S. Arufe, H. Martin, D. Rooney, R. B. P. Elmes, A. Erxleben, R. Moreira and T. Velasco-Torrijos, Soft Matter, 2020, 16, 7916–7926 RSC.
- R. Kannan, V. Muthuvijayan and E. Prasad, New J. Chem., 2017, 41, 7453–7462 RSC.
- A. Chalard, L. Vaysse, P. Joseph, L. Malaquin, S. Souleille, B. Lonetti, J. C. Sol, I. Loubinoux and J. Fitremann, ACS Appl. Mater. Interfaces, 2018, 10, 17004–17017 CrossRef CAS PubMed.
- A. Chalard, P. Joseph, S. Souleille, B. Lonetti, N. Saffon-Merceron, I. Loubinoux, L. Vaysse, L. Malaquin and J. Fitremann, Nanoscale, 2019, 11, 15043–15056 RSC.
- A. Chalard, M. Mauduit, S. Souleille, P. Joseph, L. Malaquin and J. Fitremann, Addit. Manuf., 2020, 33, 101162 CAS.
- Y. Siva Prasad, S. Manikandan, K. Lalitha, M. Sandeep, R. Vara Prasad, R. Arun Kumar, C. S. Srinandan, C. Uma Maheswari, V. Sridharan and S. Nagarajan, Nano Sel., 2020, 1, 510–524 CrossRef.
- X. Guan, K. Fan, T. Gao, A. Ma, B. Zhang and J. Song, Chem. Commun., 2016, 52, 962–965 RSC.
- J. Liu, J. Li, P. Lin, N. Zhang, X. Han, B. Zhang and J. Song, Chem. Commun., 2016, 52, 13975–13978 RSC.
- K. Fan, X. Wang, X. Wang, H. Yang, G. Han, L. Zhou and S. Fang, RSC Adv., 2020, 10, 37080–37085 RSC.
- W. C. Lai and P. H. Huang, Soft Matter, 2017, 13, 3107–3115 RSC.
- G. C. Dizon, G. Atkinson, S. P. Argent, L. T. Santu and D. B. Amabilino, Soft Matter, 2020, 16, 4640–4654 RSC.
- P. McNeice, Y. Zhao, J. Wang, G. F. Donnelly and P. C. Marr, Green Chem., 2017, 19, 4690–4697 RSC.
- C. S. Kesava Raju, B. Pramanik, R. Ravishankar, P. V. Chalapathi Rao and G. Sriganesh, RSC Adv., 2017, 7, 37175–37180 RSC.
- J. E. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249–1262 RSC.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS PubMed.
- A. Chen, I. S. Okafor, C. Garcia and G. Wang, Carbohydr. Res., 2018, 461, 60–75 CrossRef CAS PubMed.
- B. P. Krishnan and K. M. Sureshan, Chem. – Asian J., 2018, 13, 187–193 CrossRef CAS PubMed.
- Rajkamal, N. P. Pathak, D. Chatterjee, A. Paul and S. Yadav, RSC Adv., 2016, 6, 92225–92234 RSC.
- C. Narayana, P. Kumari, G. Tiwari and R. Sagar, Langmuir, 2019, 35, 16803–16812 CrossRef CAS PubMed.
- M. A. Ramin, J. Baillet, S. Benizri, L. Latxague and P. Barthélémy, New J. Chem., 2016, 40, 9903–9906 RSC.
- M. A. Ramin, L. Latxague, K. R. Sindhu, O. Chassande and P. Barthélémy, Biomaterials, 2017, 145, 72–80 CrossRef CAS PubMed.
- N. D. Bansode, K. R. Sindhu, C. Morel, M. Rémy, J. Verget, C. Boiziau and P. Barthélémy, Biomater. Sci., 2020, 8, 3186–3192 RSC.
- M. E. Cano, P. H. Di Chenna, D. Lesur, A. Wolosiuk, J. Kovensky and M. L. Uhrig, New J. Chem., 2017, 41, 14754–14765 RSC.
- A. Chen, D. Wang, J. Bietsch and G. Wang, Org. Biomol. Chem., 2019, 17, 6043–6056 RSC.
- G. Wang, D. Wang, J. Bietsch, A. Chen and P. Sharma, J. Org. Chem., 2020, 85, 16136–16156 CrossRef CAS PubMed.
- G. M. Peters and J. T. Davis, Chem. Soc. Rev., 2016, 45, 3188–3206 RSC.
- J. Baillet, V. Desvergnes, A. Hamoud, L. Latxague and P. Barthélémy, Adv. Mater., 2018, 30, 1–24 CrossRef PubMed.
- M. G. F. Angelerou, P. W. J. M. Frederix, M. Wallace, B. Yang, A. Rodger, D. J. Adams, M. Marlow and M. Zelzer, Langmuir, 2018, 34, 6912–6921 CrossRef CAS PubMed.
- H. Zhao, D. Jiang, A. H. Schäfer and F. Seela, ChemPlusChem, 2017, 82, 778–784 CrossRef CAS PubMed.
- B. Alies, M. A. Ouelhazi, A. Patwa, J. Verget, L. Navailles, V. Desvergnes and P. Barthélémy, Org. Biomol. Chem., 2018, 16, 4888–4894 RSC.
- J. Li, H. Wei, Y. Peng, L. Geng, L. Zhu, X. Y. Cao, C. Sen Liu and H. Pang, Chem. Commun., 2019, 55, 7922–7925 RSC.
- N. Thakur, B. Sharma, S. Bishnoi, S. Jain, D. Nayak and T. K. Sarma, ACS Appl. Bio Mater., 2019, 2, 3300–3311 CrossRef CAS PubMed.
- A. Nuthanakanti and S. G. Srivatsan, Nanoscale, 2016, 8, 3607–3619 RSC.
- X. Jia, J. Zhao, S. Xu, F. Zhang, J. Sun and R. Lu, Eur. J. Org. Chem., 2018, 1910–1915 CrossRef CAS.
- X. Du, J. Li, Y. Gao, Y. Kuang and B. Xu, Chem. Commun., 2012, 48, 2098–2100 RSC.
- M. Ikeda, Polym. J., 2019, 51, 371–380 CrossRef CAS.
- Y. Qiu and K. Park, Adv. Drug Delivery Rev., 2012, 64, 49–60 CrossRef.
- A. G. Cheetham, R. W. Chakroun, W. Ma and H. Cui, Chem. Soc. Rev., 2017, 46, 6638–6663 RSC.
- W. Gao, J. M. Chan and O. C. Farokhzad, Mol. Pharm., 2010, 7, 1913–1920 CrossRef CAS PubMed.
- A. Kumar and Priyanka, New J. Chem., 2019, 43, 14997–15013 RSC.
- E. R. Draper and D. J. Adams, Chem. Commun., 2016, 52, 8196–8206 RSC.
- M. J. Clemente, R. M. Tejedor, P. Romero, J. Fitremann and L. Oriol, RSC Adv., 2012, 2, 11419–11431 RSC.
- R. Rajaganesh, A. Gopal, T. Mohan Das and A. Ajayaghosh, Org. Lett., 2012, 14, 748–751 CrossRef CAS PubMed.
- R. Oosumi, M. Ikeda, A. Ito, M. Izumi and R. Ochi, Soft Matter, 2020, 16, 7274–7278 RSC.
- Z. Khayat and H. Zali-Boeini, Dyes Pigm., 2018, 159, 337–344 CrossRef CAS.
- C. Lin, S. Maisonneuve, R. Métivier and J. Xie, Chem. – Eur. J., 2017, 23, 14996–15001 CrossRef CAS PubMed.
- J. Baillet, A. Gaubert, D. M. Bassani, J. Verget, L. Latxague and P. Barthélémy, Chem. Commun., 2020, 56, 3397–3400 RSC.
- B. P. Krishnan, S. Raghu, S. Mukherjee and K. M. Sureshan, Chem. Commun., 2016, 52, 14089–14092 RSC.
- J. Gao, J. Zhan and Z. Yang, Adv. Mater., 2020, 32, 1–13 Search PubMed.
- J. Mu, J. Lin, P. Huang and X. Chen, Chem. Soc. Rev., 2018, 47, 5554–5573 RSC.
- J. Baillet, A. Gaubert, J. Verget, L. Latxague and P. Barthélémy, Soft Matter, 2020, 16, 7648–7651 RSC.
- T. Xu, Y. Cai, X. Zhong, L. Zhang, D. Zheng, Z. Gao, X. Pan, F. Wang, M. Chen and Z. Yang, Chem. Commun., 2019, 55, 7175–7178 RSC.
- J. Zhou, M. O’Keeffe, G. Liao, F. Zhao, C. Terhorst and B. Xu, Tetrahedron, 2016, 72, 6078–6083 CrossRef CAS PubMed.
- J. Zhou, X. Du, X. Chen and B. Xu, Biochemistry, 2018, 57, 4867–4879 CrossRef CAS PubMed.
- H. T. West, C. M. Csizmar and C. R. Wagner, Biomacromolecules, 2018, 19, 2650–2656 CrossRef CAS PubMed.
- K. P. C. Sekhar, D. K. Swain, S. A. Holey, S. Bojja and R. R. Nayak, Langmuir, 2020, 36, 3080–3088 CrossRef CAS PubMed.
- S. Liu, H. Li, J. Zhang, X. Tian and X. Li, RSC Adv., 2020, 10, 33642–33650 RSC.
- J. Li, S. Liang, Y. Yan, X. Tian and X. Li, Macromol. Biosci., 2019, 19, 1–10 CrossRef.
- S. Yao, R. Brahmi, A. Bouschon, J. Chen and S. Halila, Green Chem., 2023, 25, 330–335 RSC.
- A. K. Rachamalla, V. P. Rebaka, T. Banoo, R. Pawar, M. Faizan, K. Lalitha and S. Nagarajan, Green Chem., 2022, 24, 2451–2463 RSC.
- P. Sharma and G. Wang, Gels, 2022, 8, 191, DOI:10.3390/gels8030191.
- G. Wang, D. Wang, A. Chen, I. S. Okafor and L. P. Samankumara, ACS Omega, 2022, 7, 11330–11342 CrossRef CAS PubMed.
- H. Kono, J. Noda and H. Wakamori, Molecules, 2022, 27, 6137, DOI:10.3390/molecules27186137.
- S. Komba and R. Iwaura, ACS Omega, 2021, 6, 20912–20923 CrossRef CAS PubMed.
- J. Bietsch, M. Olson and G. Wang, Gels, 2021, 7, 21–23 CrossRef PubMed.
- P. V. Bhavya, K. Soundarajan, J. G. Malecki and T. Mohan Das, ACS Omega, 2022, 7, 39310–39324 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |