Guiding research in electrochemical CO2 conversion strategies through a systems-level perspective†
Emily
Nishikawa
 a,
Shamiul
Islam
a,
Sylvia
Sleep
a,
Shamiul
Islam
a,
Sylvia
Sleep
 b,
Viola
Birss
b,
Viola
Birss
 c and
Joule
Bergerson
c and
Joule
Bergerson
 *a
*a
aDepartment of Chemical and Petroleum Engineering, University of Calgary, 2500 University Drive NW, Calgary, Alberta T2N 1N4, Canada. E-mail: jbergers@ucalgary.ca
bDepartment of Civil Engineering, University of Calgary, 2500 University Drive NW, Calgary, Alberta T2N 1N4, Canada
cDepartment of Chemistry, University of Calgary, 2500 University Drive NW, Calgary, Alberta T2N 1N4, Canada
First published on 30th November 2022
Abstract
Carbon conversion technologies are gaining interest as a solution to utilize captured CO2 and contribute to efforts to reduce greenhouse gas emissions. This work provides technology developers with a systems-level perspective of the climate impacts of electrochemical CO2 conversion products. Different uses (polymer production, transportation fuels, and power generation) of three CO2-based fuels (methane, methanol, and diesel) are compared considering different combinations of electrolyzers (water or CO2 electrolysis) and thermochemical methods. Additionally, the influence of assumptions and trade-offs between environmental and economic performance are evaluated in sensitivity analyses, using polymer and diesel production as examples. Finally, recommendations are provided based on environmental and economic analyses. The novelty of this work involves the application and communication of LCA methods and insights aimed at helping developers visualize their technology in the full supply chain, providing examples of analyzed systems and a set of recommendations that can be generalized and incorporated into the development of different technologies. Example recommendations include considering that in projects focusing on improving the environmental performance of electrochemical processes, cell degradation and electricity source are major factors. On the other hand, for economic performance, lifetime is more important than cell degradation and electricity source. Electrochemical processes are quite promising from a climate change perspective if the input electricity is from a low-carbon source, if the use phase does not involve combustion, if the product is efficient for the use chosen (e.g., diesel is more efficient for transportation than other fuels), and if the use has a large market size.
1. Introduction
A portfolio of technology solutions will be necessary to keep greenhouse gas (GHG) emissions within limits such as those agreed to in the Paris Agreement.1 Among them, new ways to produce fuels are being developed, such as carbon conversion technologies (CCTs), which aim to use captured CO2 as a raw material for manufacturing valuable products. The production of cement,2 polymers,3 and fuels from CO2,4 as examples, is receiving increasing attention in academic and industrial sectors. Electrolysis is a potentially powerful tool for low-carbon fuel and chemical production. However, aspects such as the supply chain environmental impacts and potential trade-offs between environmental and economic performance have not been holistically assessed to date. This is needed to make better decisions about technology investment and development.Life cycle assessment (LCA) is a tool that is used to evaluate the environmental impacts of a product, process, or service.5 The inputs and outputs (i.e., materials and energy) involved in providing the product, process, or service are identified and linked to potential environmental impacts. LCA guidelines can be found in ISO (International Organization for Standardization) standards ISO 14040 and ISO 14044.6,7 LCA studies have mainly been written and used to inform funding8,9 and policy10 decisions, and environmental labeling,11 rather than to inform technology developers. LCAs tend not to be accessible or directly applicable to the decisions made by researchers as they conceptualize and develop a technology at early stages (e.g., lab-scale experiments). However, at this point, the freedom to make changes (and therefore reduce costs and environmental impacts) is highest.12
One way to make LCA results more relevant and helpful in informing early-stage technology development is to integrate LCA with techno-economic assessments (TEAs) to evaluate potential linkages or trade-offs between environmental impacts and economic performance. There has been some recent literature13–18 exploring this area, but it has not been focused on early-stage technologies and their particular challenges, such as lack of and poor quality data. The technologies considered are mostly at a commercial or mature stage.16 In addition, the life cycle GHG emissions of equipment manufacturing are sometimes neglected, even though in TEA equipment is considered.13 An integrated framework proposed by Thomassen et al.19 was applied to diesel production as a case study to evaluate the system, including environmental and techno-economic aspects. The framework provides a helpful operational example of the integration, but it does not explore the influence of LCA methodological choices (e.g., allocation or how input CO2 is credited) or capital costs, relevant aspects for emerging technologies.
In LCA terminology, the boundaries delimit the system to be analyzed and may influence the results since they determine which phases/processes will be included or excluded from the study.20 For instance, it is possible to study the life cycle of a product from raw material extraction until its end-of-life in a cradle-to-grave study. Alternatively, the use and end-of-life phases are not considered, and the system is drawn until the “gate” of the factory, called a cradle-to-gate study. In the CCTs field, cradle-to-gate studies are most common and are generally sufficient for comparing different production processes of the same product with the same use and end-of-life phases. However, when there are different potential uses and technology developers seeking to understand the role that their technology could play in different markets, this approach becomes inadequate, and information about the full system or supply chain (including the use phase) is then needed. Additionally, only cradle-to-grave analyses allow assessing whether the CCT can result in negative emissions.18 Therefore, this systems-level perspective may offer insights that a cradle-to-gate analysis may not provide.
Different approaches have been developed for CO2 conversion, such as photocatalysis, non-thermal plasma, and electrochemical conversion. Photocatalysis for CO2 conversion uses low-GHG intensity solar energy but suffers from low efficiency and poor selectivity.21 Non-thermal plasma is performed in the plasma state of matter (the fourth state) using electrons with high energy. This approach does not use rare materials and allows rapid system response to fluctuating feed (shut-down/start-up), but energy efficiency and CO2 conversion still cannot be maximized concurrently.21,22 In electrochemical conversion, selectivity and kinetics can be challenges, depending on the type of electrolyzer used and the desired product.21,22 However, this approach can provide electricity storage in the form of fuels and chemicals produced from CO2, especially considering the need for large scale renewable energy storage technologies.
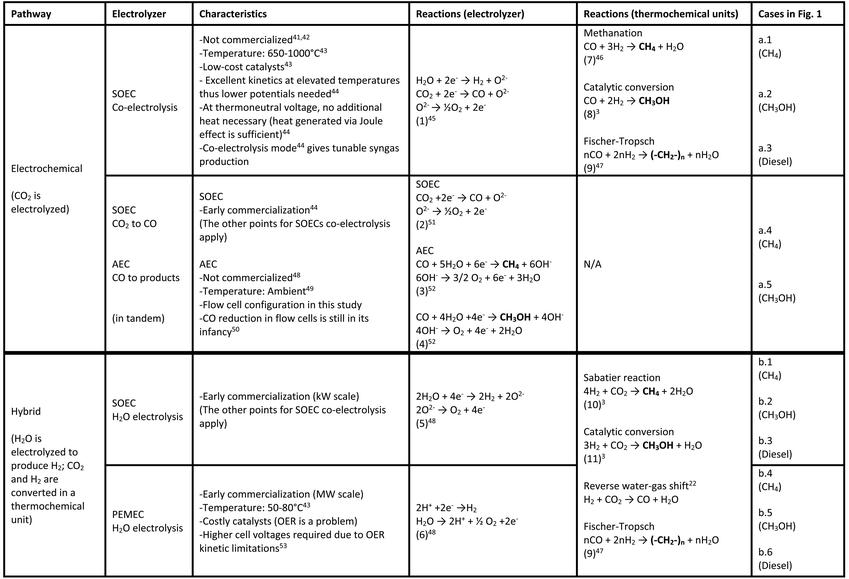
This study provides a bridge between LCA methods and various electrolysis pathways with the aim of informing decisions at early stages of development. For this purpose, this work is focused on Solid Oxide Electrolyzer Cells (SOECs) and Polymer Electrolyte Membrane Electrolyzer Cells (PEMECs) as examples of emerging CCTs, which are considered less mature technologies and therefore offer an opportunity for LCA/TEA to help inform design decisions. In terms of CO2 conversion strategies, two pathways22 are considered in this study: electrochemical (only) and a hybrid pathway of electrochemical and thermochemical units, as illustrated in Table 1 and Fig. 1. In the electrochemical pathway, CO2 is electrochemically converted without the need for H2, such as achieved by the co-electrolysis of CO2 and H2O in SOECs (high-temperature electrolysis), or conversion is carried out in a CO2–CO-product tandem configuration23 (CO2 is converted to CO in a high-temperature SOEC, followed by CO conversion to products in an AEC during low-temperature electrolysis). In the hybrid pathway, H2 from water electrolysis (in an SOEC or PEMEC) is used as an intermediate, followed by a thermochemical process (e.g., Sabatier reaction) for CO2 hydrogenation. Table 1 also shows the cases presented in Fig. 1 related to each strategy.
1.1. Past assessments of electrochemical processes
Several products may be generated using CO2 as a feedstock together with electrochemical and/or thermochemical processes (see Table 1), including methane,3 methanol,3 polyoxymethylene,24 polypropylene,24 and diesel.4,25 The hybrid pathway is the most mature and well-studied in LCA,3,4 with several demonstration projects under development.26,27 Thus, for CCTs, both CO2 electrolysis (electrochemical pathway) and water electrolysis (hybrid pathway) are of interest (reactions (1), (2) and (5), (6), Table 1) and are included as potential pathways.Past LCA studies of fuels production via electrolysis have concluded that the intensity of electricity is the biggest driver of GHG emissions from electrochemical processes.28–30 Wind, hydroelectricity, and solar photovoltaics for electricity generation are attractive sources due to low-GHG intensity.30 Furthermore, coupling electrolysis with surplus renewable electricity may considerably reduce impacts compared to traditional production processes.31,32
In terms of economics, several studies have assessed the costs associated with electrochemical processes. Some of the critical parameters are electricity input and capital costs, and external factors such as local regulations.33 The lifetime of stacks and electricity prices are also relevant.14 However, in terms of GHG emissions, stack lifetime was not an influential factor,14 suggesting that the key factors may differ for environmental and economic performance.
1.2. LCA and R&D (research and development) process
In LCA studies, it is common to define a scenario representing a specific technology configuration, such as an electrolyzer with average or typical performance and specifications. From these “base case” results, further analysis may be performed. For example, a contribution analysis can be conducted to identify the main drivers of impacts. A sensitivity analysis20 that tests the influence of base case assumptions on results is also common. However, LCA studies directed to the R&D process are scarce, and many discuss the product design phase broadly34,35 or explain a theoretical framework with specific application examples.36,37 In these studies, a specific base case is defined, and the conclusions are valid for that system, making it difficult to generalize the insights beyond the base case so that technology developers can apply these insights to their processes.As examples of LCA applied to electrochemical processes, Griffiths et al.,38 Sharma et al.,39 and Nabil et al.40 employed LCA to guide R&D. The synthesis of a material with superior characteristics or with higher conversion efficiency may not result in lower GHG emissions if the synthesis process is more complex, requires more energy, or if more reagents with high embodied GHG emissions (e.g., palladium's upstream GHG emissions due to extraction and processing) are needed.38,39
Nabil et al.40 conducted a cradle-to-gate investigation of strategies for CO2 conversion, including a tandem23 approach: electrolyze CO2 to CO (in a SOEC) followed by the conversion of CO to products in an alkaline electrolyzer flow cell (AEC-flow). This strategy resulted in lower impacts due to lower energy demand (overall lower cell potential and higher selectivity)23,40 compared to other strategies. Separation, especially of liquid products, is an important driver of impacts due to the low concentration of products obtained from the electrolyzer. These studies evaluated electrochemical processes but did not include the use phase (i.e., cradle-to-gate). However, when processes are flexible and different products and uses are possible, cradle-to-gate studies should be expanded to include the use phase (i.e., cradle-to-grave).
Factors not related to the performance of electrochemical processes (e.g., sources of feedstock or energy) may play an essential role in the overall environmental impacts but may not be easily identified. This work aims to study the impacts of upstream and downstream choices in systems that involve electrochemical processes, helping to prioritize research areas.
We aim to answer the following research questions:
(1) Do different uses of products (e.g., methane, syngas) generated from CO2 conversion involving electrolysis (i.e., electrochemical or hybrid pathways) affect the life cycle GHG emissions?
(2) Which factors, if any, drive differences in GHG emissions across the proposed pathways?
(3) What parameters affect the GHG emissions and cost of products? Are there trade-offs?
(4) Are there specific aspects of different strategies (e.g., SOEC co-electrolysis or tandem CO2 to CO to products) that may be an advantage or disadvantage in terms of GHG emissions?
The answers to the above questions are used to generate a set of recommendations that can help technology developers to consider electrochemical processes from a broader perspective.
A cradle-to-grave LCA is conducted for methane, methanol, and diesel production from CO2 when produced using different strategies, which may be classified in two pathways (i.e., electrochemical-only and hybrid). The strategies are summarized in Table 1.
Three potential uses for the chemicals are considered (polymer production, transportation, and power generation), and an integrated LCA and TEA is performed for diesel production. Given the effort to achieve climate targets and the potential role that electrochemical processes may play in this context, this paper focuses on GHG emissions. However, other impacts, such as acidification and water consumption, can and should be included for a more comprehensive investigation of environmental impacts. In this study, other impacts are qualitatively discussed in Section S5 in the ESI.† Caution is advised when analyzing the results since the technologies in this study are at different maturity levels, resulting in different levels of uncertainty, which is beyond the scope of this study.
2. Materials and methods
The goal of this study is to provide technology developers with insights and recommendations using LCA as a tool that can be generalized and applied to the R&D process. As LCA studies are rarely written for technology developers, we also provide clarifications about methodological choices in worked examples to help the audience visualize major aspects that influence a reported GHG emissions estimate. The results of this study are not intended to be used as definitive and assertive identification of the lower impact pathways, which would be difficult given the maturity level of the considered technologies. Instead, the characteristics and differences across strategies (e.g., liquid/gaseous products, tunability) are identified and used to recommend potential improvements for development.The functional units considered are described in Section 2.2. Flows of GHG emissions are the focus of the study (for details, see Sections S1.1–S1.3 in the ESI†), and the characterization factors (i.e., the global warming potential of the various GHGs in relation to the reference CO2) are defined by the IPCC 6th assessment for 100 years.54 We also focus on the operations phase of the life cycle, as the operation is often responsible for most impacts especially in processes that have high energy demands (including non-spontaneous processes such as electrolysis). Therefore, we model material and energy flows for CO2 conversion in more detail, which allows exploring potential associated impacts and effects of CO2 conversion process improvements.
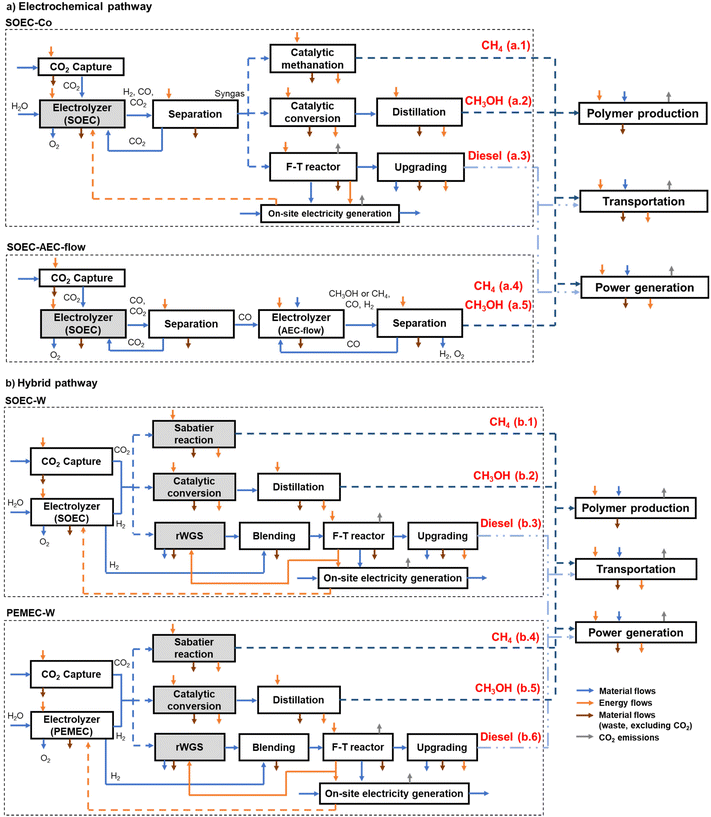
The pathways, electrolyzers, intermediate products, and general characteristics are shown in Table 1. The last column in Table 1 also references the respective cases in Fig. 1, which shows the flow diagram of each case.
Fig. 1 presents the system boundaries of CO2-based fuels production via electrochemical (Fig. 1a) and hybrid (electrochemical + thermochemical) (Fig. 1b) pathways, as well as main flows. Each combination of process and product is labeled between parentheses in front of the respective box, e.g., (a.1) for methane produced from SOEC-Co, where “Co” infers the co-electrolysis of CO2 and water in the same SOEC. Each pathway is explained in detail in Section 2.1.
Three potential uses (polymer production, transportation, and power generation) of the CO2-based fuels are evaluated to put the electrochemical technologies in context and help technology developers envision the impacts of future use.
General assumptions are made to ensure a consistent analysis and are detailed in Section 2.4.
Previous studies that provide a detailed inventory and are preferably based on process simulations were selected to build the model. This requirement is especially relevant for less mature technologies, given that simulations are useful to estimate their performance more accurately than laboratory estimations or use of proxies,9 by including aspects such as heat integration and equipment scale-up. As a note, there is active research in the LCA field regarding scale-up methods and ensuring fair comparisons across technologies at different maturity levels.9
The base case LCA method to handle multi-product systems is system expansion via substitution.55 Allocation methods are tested in the sensitivity analysis (Section 2.3). Some data for the background system (supporting activities) may also employ allocation, noted in Section S1 in the ESI.†
2.1. Description of the system
This section describes the systems, technologies, uses, incumbent processes (conventional way to produce a product or service), assumptions, and metrics.For the electrochemical pathway, SOECs operated in the CO2/H2O co-electrolysis mode are labeled as SOEC-Co, generating syngas that is further processed into fuels. It is also possible to electrochemically convert CO2 in tandem (CO2 to CO in the SOEC and CO to products in an AEC in a flow configuration),40 identified as SOEC–AEC-flow.
The hybrid pathway involves water electrolysis and thermochemical units (Sabatier reaction for methane,3 or reverse water gas shift, rWGS, followed by Fischer–Tropsch, F–T, for diesel).25 In this case, water electrolysis is performed by high-temperature electrolysis in a SOEC (which we identified as SOEC-W) or during low-temperature electrolysis in a PEMEC (PEMEC-W).
In the co-electrolysis processes (SOEC-Co, cases a.1–a.3), H2O and CO2 are fed in the desired ratio and co-electrolyzed to produce syngas. The reference study45 considered a scaled-up process to produce syngas at H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO molar ratio of 2
CO molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 145 (for methane, extra H2 is necessary to adjust the ratio to 3
145 (for methane, extra H2 is necessary to adjust the ratio to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and is assumed to be provided by SOEC water electrolysis). The syngas can be converted to methane, methanol, or F–T diesel in thermochemical units. As in all electrolysis processes discussed here, oxygen is generated as a co-product, and in F–T diesel production, LPG (liquefied petroleum gas), gasoline, wax, and steam are also generated.
3 and is assumed to be provided by SOEC water electrolysis). The syngas can be converted to methane, methanol, or F–T diesel in thermochemical units. As in all electrolysis processes discussed here, oxygen is generated as a co-product, and in F–T diesel production, LPG (liquefied petroleum gas), gasoline, wax, and steam are also generated.
Purification of the gaseous stream is assumed to be done by pressure swing adsorption.40,56 The separation of gaseous streams is a minor contributor to GHG emissions40 (assumption tested in the sensitivity analysis).
It is assumed that the energy demand for methane and methanol production3 from CO and CO2 are equivalent (assumption tested in the sensitivity analysis). In diesel production,57,58 the fuel gas leaving the F–T unit is used for electricity generation,57 which offsets part of the electrolyzer's energy consumption. For details, see Section S1.1 in the ESI.†
Cases a.4 and a.5 (SOEC–AEC-flow) involving low-temperature CO2 conversion in aqueous systems40 consider a combination of a high-temperature SOEC for CO production from CO2 and a low-temperature AEC. The reference study40 assumed optimistic performance, such as 90% selectivity towards methane and methanol (90% faradaic efficiency), and H2 as the only cathodic by-product (along with oxygen from the anode, with oxygen also generated in the SOEC). A range of by-products is expected,59 but the favorable assumptions were selected to verify if electrochemical CO2 conversion, as defined, can be competitive.40 Data about the core processes (electrolysis and separation) is used, and the remaining parts of the system (CO2 capture process, energy, and other utilities production, and use phase) are adjusted to align with the boundaries of our study.
We calculate the net GHG emissions resulting from the use of CO2-based fuels in the uses listed in Table 2. Net GHG emissions are the summation of all GHG sources in the system: CO2 captured, energy consumption during electrolysis and production process, CO2 converted, CO2eq avoided by co-products, and fuel use.
| Use | CO2-based fuels | Metrics |
|---|---|---|
| a POM: polyoxymethylene. b PP: polypropylene. | ||
| Polymer production | Methane (for POMa production) | kg CO2eq per kg POM |
| Methanol (for PPb production) | kg CO2eq per kg PP | |
| Transportation | Methane | kg CO2eq per pkm |
| Methanol | ||
| Diesel | ||
| Power generation | Methane | kg CO2eq per kW h |
| Methanol | ||
| Diesel | ||
Water is electrolyzed in either an SOEC (reaction (5), Table 1; cases b.1–b.3, Fig. 1b) or a PEMEC (reaction (6), Table 1; cases b.4–b.6, Fig. 1b) to produce H2, which reacts with CO2 in a subsequent thermochemical unit, generating methane, methanol, and F–T diesel (reactions (9)–(11), Table 1). For F–T diesel, CO2 is converted to CO using an rWGS reaction to produce CO.4 Additional H2 from the electrolyzer is blended with the product of rWGS to produce syngas with a H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO molar ratio of 2
CO molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 Oxygen is produced during water electrolysis as a co-product, as well as LPG, gasoline, wax, and steam in F–T diesel production.
1.25 Oxygen is produced during water electrolysis as a co-product, as well as LPG, gasoline, wax, and steam in F–T diesel production.
The energy demands associated with using SOEC and PEMEC for water electrolysis are taken as 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48,60 and 54 kW h kg−1 H2,3,48 respectively, and additional information about steam demand and oxygen production is taken from Zhang et al.61 Water electrolysis using the SOEC and PEMEC are referred to as SOEC-W and PEMEC-W, respectively.
48,60 and 54 kW h kg−1 H2,3,48 respectively, and additional information about steam demand and oxygen production is taken from Zhang et al.61 Water electrolysis using the SOEC and PEMEC are referred to as SOEC-W and PEMEC-W, respectively.
The same CO2 conversion units as those described in Section 2.1.1 are employed. For details, see Section S1.2 in the ESI.†
For polymer production, methane is used to produce polyoxymethylene (POM) and methanol is used to produce polypropylene (PP), which are processed by injection molding.24 In the base case, polymers are considered to have a lifetime sufficient to maintain CO2 sequestration. Two alternative end-of-life treatments are considered in the sensitivity analysis (see Section 2.3.1): recycling followed by incineration and direct incineration.24 The incumbent process (conventional way to produce a product or service) for polymer production is the same but uses fossil feedstocks instead of CO2-based chemicals. For POM, additional information is provided in Section S1.3.1 in the ESI.†
For transportation, fuels are assumed to be used in medium-size passenger cars.62,63 The incumbent is assumed to be a gasoline-fueled vehicle complying with Euro 5 standard.62 For power generation, methane and methanol are used as the input to the solid oxide fuel cells (SOFC), generating heat and power.64 Diesel is consumed in a heat and power cogeneration unit,63 and the incumbent is a natural gas combined cycle plant.65
Regarding co-products, gasoline and LPG incumbents are assumed to be from fossil sources, and oxygen from cryogenic air separation. For details, see Sections S1.3 and S1.4 in the ESI.†
Multiple incumbents and competitors may exist. The incumbent may be different in different contexts, and future work should consider this aspect.
We focused on three end uses, but technology developers may also consider other uses for the intermediate products. For instance, methane is an important and potent GHG if released at any point in the supply chain. Methane can also be used for a variety of purposes such as heating29 or substrate for bioproducts.66
2.2. Metrics of evaluation of life cycle GHG emissions and functional units
A functional unit describes the function or service that the process under study intends to fulfill.20 Each use has a different functional unit. For polymer production, the functional unit is one kg of polymer (POM or PP) produced and used. For transportation, the functional unit is one passenger-kilometer (or pkm), which represents the transportation of one passenger for one kilometer.67 For power generation, the functional unit is the generation of 1 kW h of electricity. Table 2 summarizes the CO2-based fuels and their uses, and metrics to support understanding results. Note that polymer production has two units, and diesel is not considered in all end uses.Net GHG emissions, in CO2 equivalents, represent the global warming impact indicator, quantifying the potential impact that a process, product, or service could contribute to increasing global temperatures.
Net GHG emissions are organized into two sets of metrics in this study: the first set is related to the use of CO2-based fuels and is described in the paragraph above.
The second set of metrics focuses on evaluating CCT alternatives consistently, regardless of the use or the product generated, and identifying potential benefits of CCT alternatives compared to incumbent processes. This set of metrics includes kg CO2eq emitted per kg CO2eq converted, avoided emissions in kg CO2eq avoided per kg of polymer (for polymer production, for example), and global emissions reduction potential in Gt CO2eq per year. Avoided emissions compare the GHG emissions from CCTs with incumbent technologies, while the global reduction potential incorporates the market size to understand the potential to reduce emissions according to different uses and markets. For details, see Section S3.4 in the ESI.†
2.3. Sensitivity analyses
In LCA studies, sensitivity analyses are useful tools to evaluate the effect that assumptions or decisions made have on results. They are also helpful for identifying the most influential parameters (e.g., electricity source) on the calculated metrics (e.g., net GHG emissions). Therefore, we employ sensitivity analyses to understand which decisions and parameters are most influential, supporting the generation of a set of recommendations that may be considered during R&D. Two sensitivity analyses are performed: one to evaluate the assumptions (for the CO2-based polymer production) and another to identify the most influential parameters in diesel production. The greater the sensitivity (e.g., the wider the bar in Fig. 5), the more influential that parameter is on the outcome of that analysis.Sensitivity to methanol synthesis energy demand was tested by varying the input by ±50% and evaluating the influence on final impacts. The same procedure was used to evaluate the sensitivity to energy demand for separation in the SOEC-Co case.
2.4. General assumptions
General assumptions are made to harmonize the boundaries of the system, ensuring a consistent analysis across reference studies and data sources. First, the CO2 capture unit is assumed to deliver a CO2 stream with the required purity in all cases. Processes based on absorption by monoethanolamine from flue gases may reach high purity, and CO2 capture via DAC is comparable to the absorption process.3 The sources of CO2 considered in this study were also used in CCT projects in Europe:26,27 coal and natural gas power plants, and DAC. The impact of CO2 source is tested in the sensitivity analysis for polymer production and in the LCA and TEA study of diesel production.The CO2 captured and utilized is assumed to receive full credit, and its use generates a negative emissions credit (−1 kg CO2eq per kg CO2 used), which can be subtracted from the total GHG emissions throughout the life cycle of the CO2-based products. When CO2 is captured from a power plant, for example, there are two product streams, power and CO2, generating the question of who should get credit for the captured CO2 (i.e., the avoided emissions). By assuming that CO2 feedstock can be credited as −1 kg CO2eq per kg CO2 used, we implicitly give full credits of the emissions avoided (in the power plant) to the CO2 product stream. An implication of this assumption is that when negative net GHG emissions are obtained, net CO2 is not necessarily removed from the atmosphere, but that using the systems under study results in lower GHG emissions compared to the incumbent system (e.g., a power plant releasing all CO2 produced to the atmosphere); therefore, we use the term GHG-emissions-reducing18 in this case. In the sensitivity analysis (see Section 2.3.1), we test the extreme high case of this assumption, i.e., the CO2 from the power plant receives no credits for the avoided emissions (with all the credits for avoiding the GHG emissions attributed to the power plant). In this case, instead of subtracting 1 kg CO2eq from the total GHG emissions per each kg CO2 used, no credit is given.
CO2-based fuel transportation and distribution transfer the produced fuel from the supplier to the end user.20 Those phases are assumed to be similar in all cases and small relative to other emissions sources,62 and thus they are not included in the calculations.4
We assume that the SOECs operate at the thermoneutral voltage, where the Joule heating from the electrolysis cell operation maintains the cell temperature;44 therefore, no additional heat is needed once the electrolyzer reaches its operating temperature.
Regarding the deployment context, the natural gas-based scenario assumes that natural gas is the source for utilities. Electricity is assumed to be generated in a natural gas power plant (0.49 kg CO2eq per kW h),70 heat in a natural gas industrial furnace, and H2 from water electrolysis powered by natural gas-based electricity. CO2 is assumed to be obtained by absorption by monoethanolamine from a natural gas power plant. In the low-carbon scenario, electricity is provided by low-carbon sources (0.024 kg CO2eq per kW h),70 and the emission factor used is an average of various sources (hydropower, solar photovoltaics, wind, and nuclear). Low-carbon electricity may vary depending on local contexts, and by assuming a mix of sources, we acknowledge this diversity and the potential intermittent supply. Heat generation is assumed to be from geothermal energy, H2 from water electrolysis powered by low carbon electricity, and the CO2 is captured from ambient air via DAC and powered by low carbon energy. A high carbon scenario was also considered for the LCA and TEA study of diesel production, where electricity is assumed to be generated in a coal power plant, while heat is produced from a combined heat and power plant. The CO2 is assumed to be captured from air via DAC but using natural gas-based electricity and heat. Table S8 (ESI†) presents the emissions factors of each utility in all scenarios.
3. Results and discussion
3.1. Life cycle GHG emissions
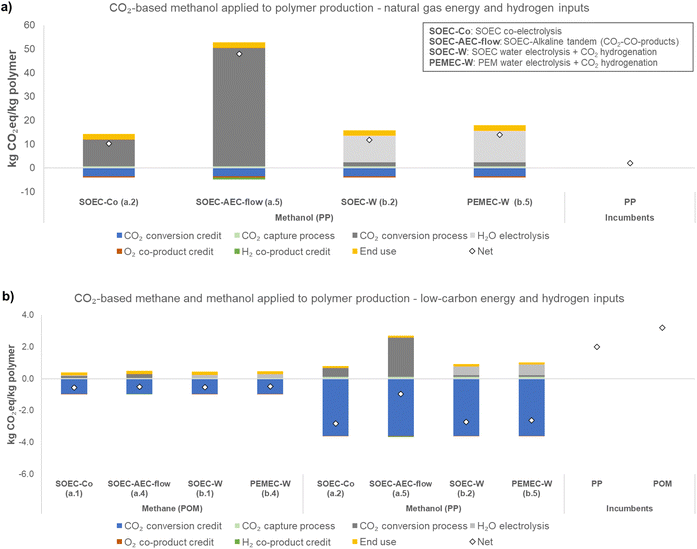
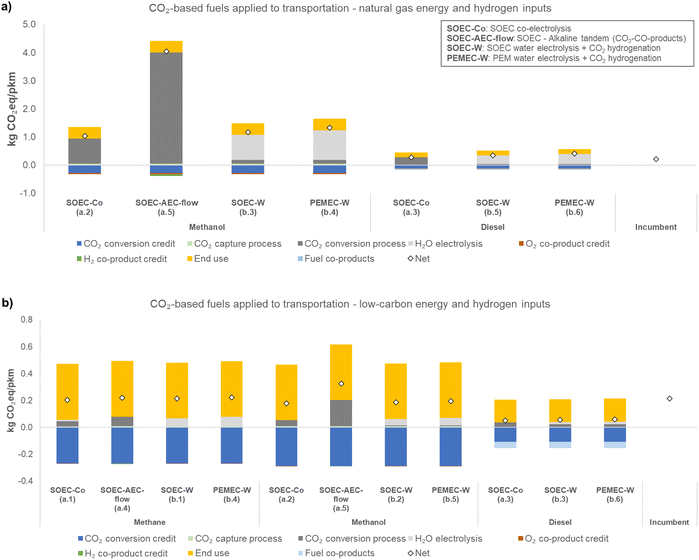
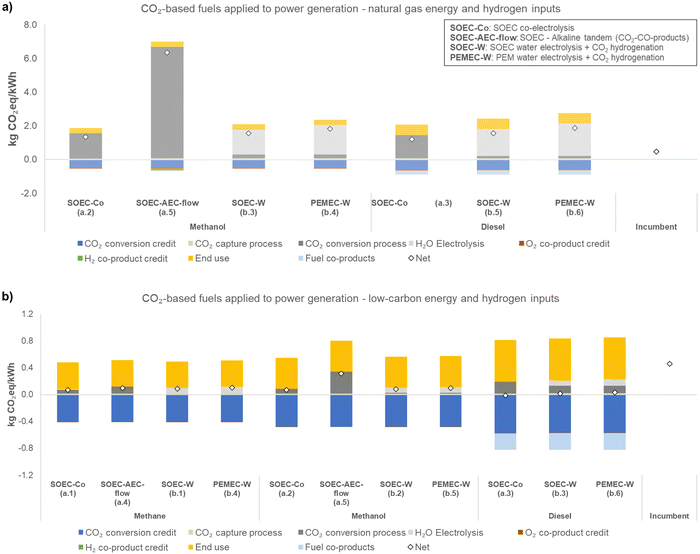
This study estimates the GHG emissions from cradle-to-grave of CO2-based fuels with three different uses. Fig. 2–4 present the life cycle GHG emissions of different uses of each CO2-based fuel, with items (a) referring to natural gas-based inputs and (b) referring to low carbon-based inputs. Methane use in the natural gas-based case was not shown because electricity and CO2 sources are already based on natural gas (main constituent is methane). The natural gas-based case for power generation (Fig. 4) is grayed out because natural gas power plants provide CO2 and electricity (shown to illustrate potential results). Fig. 2 presents the life cycle GHG emissions in kg CO2eq per kg polymer (PP, polypropylene for methanol, and POM, polyoxymethylene for methane), Fig. 3 in kg CO2eq per pkm (passenger-kilometer), and Fig. 4 in kg CO2eq per kW h.A clear general trend is that source of utilities is a major factor influencing the net GHG emissions of electrochemical processes given the difference in net GHG emissions in the low-carbon and natural gas scenarios. The use also influences the net GHG emissions.
In Fig. 2 (polymer production), in the low-carbon case, GHG-emissions-reduction, relative to a baseline in which CO2 is not captured, is possible (−0.53 kg CO2eq per kg POM and −2.7 kg CO2eq per kg PP, SOEC-W) because there is no associated combustion of the fuels, and electricity and CO2 are both obtained from low-carbon sources. The GHG-emissions-reduction is a result of the assumption that the CO2 utilized can be treated as −1 kg CO2eq per kg CO2 used as feedstock (i.e., receives full credit from capture, see Section 2.4), and is a result of the credits given for the co-products, which are also included as negative values. GHG-emissions-reduction means that the GHGs emitted throughout the life cycle is lower than cases when CO2 is not captured (just released from the emitting source, e.g., power plants) and the co-products (e.g., oxygen) are produced by traditional (or incumbent) processes (e.g., cryogenic separation).
In LCA, in systems that generate more than one product, the burdens can be shared among the products by using a partitioning method or a method called system expansion.20 There is no consensus about which method to use, but ISO standards6,7 and Guidelines for CO2 Utilization18 recommend using system expansion whenever possible (i.e., “credits” are given to the production of co-products, avoiding traditional processes). Thus, the impacts of these traditional processes are subtracted from the impacts of our system.
In general, for CO2-based fuels applied to transportation and power generation, further GHG-emissions-reduction is more difficult to achieve due to the combustion step in both cases. Therefore, as one clear message of this work, uses that do not require combustion are more promising to reduce GHG emissions. In transportation (Fig. 3), CO2-based diesel in natural gas-based case presents slightly higher GHG emissions than the incumbent (0.35 kg CO2eq per pkm for SOEC-W diesel, versus 0.21 kg CO2eq per pkm for the gasoline incumbent); however, compared to CO2-based methanol (1.2 kg CO2eq per pkm, SOEC-W) it is considerably lower. Diesel is much more efficient than methanol for this use (diesel energy content is approximately 43 MJ kg−1, versus 20 MJ kg−1 for methanol), and as a result, each kg of methanol can deliver only 4.8 pkm, versus 29 pkm per kg diesel. Therefore, fuel efficiency in the considered use is a factor that should be considered. For power generation, all fuels resulted in similar emissions, except for methanol from SOEC–AEC-flow but it is still lower than natural gas-based power in a low-carbon scenario. In this use, the difference in efficiency between diesel and methanol is not as large as for transportation, with methanol delivering 3 kW h kg−1 and diesel delivering 5 kW h kg−1.
Another important outcome of this analysis is that in terms of the different pathways and technologies involved, electrochemical CO2 conversion via SOEC co-electrolysis (SOEC-Co, cases a.1–a.3) and hybrid pathways (SOEC-W, cases b.1–b.3; and PEMEC-W, cases b.4–b.6) appear to result in lower net GHG emissions than processes involving low-temperature electrochemical CO conversion (SOEC–AEC-flow, cases a.4 and a.5). In these SOEC–AEC-flow cases, the total energy requirements for methane and methanol production were 31 kW h kg−1 methane and 39 kW h kg−1 methanol. In our study, for SOEC-W, the energy requirements are 22 kW h kg−1 methane and 9.0 kW h kg−1 methanol. The large difference between these pathways for methanol production is mainly due to the separation energy needed, which for SOEC–AEC-flow is 27 kW h kg−1 methanol (while the energy for conversion in the electrolyzers is 10 kW h kg−1 methanol).40 In AEC-flow, liquid products are formed in the solution and are recirculated to increase the product concentration (the single pass accumulation is low); however, high concentrations of liquid products in the electrolyte can affect local parameters (e.g., pH),71 impacting the electrolyzer performance40,72 (e.g., instability and increasing cell potential).71 Therefore, the relatively low concentration of liquid products elevates the energy demand for separation.
The hybrid pathway (SOEC-W, case b.2) also requires energy for separation, but this strategy has the advantage of heat integration within the system. The methanol synthesis process is exothermic, which allows the heat generated to be used in the distillation process,73 reducing the external energy input. Regarding the AEC-flow, improvements in the electrochemical process itself (e.g., faster kinetics, improved stability, and higher selectivity)22 and integration with other industrial processes that generate surplus heat that can be reused in liquid products separation may help the process become more competitive in terms of GHG emissions.
It is also seen that SOEC-Co (cases a.1–a.3) tends to result in slightly lower GHG emissions than the hybrid pathway, even for methane (case a.1) that in this study uses additional H2 from water electrolysis to adjust the ratio of H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO (from 2
CO (from 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the scaled-up simulation45 to the 3
1 in the scaled-up simulation45 to the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 needed to produce methane). In the case of tuning the composition from the co-electrolyzer by adjusting the H2O
3 needed to produce methane). In the case of tuning the composition from the co-electrolyzer by adjusting the H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 ratio, we estimate that the GHG emissions may be further decreased between 4% (transportation) to 19% (power generation) compared to the non-tuned case (see Section S1.1 in the ESI†). Regarding the hybrid pathway, it is clear that SOEC-W resulted in lower emissions than PEMEC-W because PEMEC electrolyzers are less efficient and require more electricity to produce the same amount of H2 as SOEC water electrolyzers. The estimates in Fig. 2–4 are uncertain, and while outside the scope of this work, uncertainty should be considered when interpreting the results. The comparisons are an indication of trends, not definitive conclusions. Nonetheless, the results indicate that under certain conditions, CO2 utilization may be more promising than the current incumbent process. For instance, in PP production (Fig. 2), SOEC-Co (a.2) or SOEC-W (b.3) with low-carbon-based energy inputs may result in net GHG emissions almost 3 times lower than the incumbent.
CO2 ratio, we estimate that the GHG emissions may be further decreased between 4% (transportation) to 19% (power generation) compared to the non-tuned case (see Section S1.1 in the ESI†). Regarding the hybrid pathway, it is clear that SOEC-W resulted in lower emissions than PEMEC-W because PEMEC electrolyzers are less efficient and require more electricity to produce the same amount of H2 as SOEC water electrolyzers. The estimates in Fig. 2–4 are uncertain, and while outside the scope of this work, uncertainty should be considered when interpreting the results. The comparisons are an indication of trends, not definitive conclusions. Nonetheless, the results indicate that under certain conditions, CO2 utilization may be more promising than the current incumbent process. For instance, in PP production (Fig. 2), SOEC-Co (a.2) or SOEC-W (b.3) with low-carbon-based energy inputs may result in net GHG emissions almost 3 times lower than the incumbent.
In summary, uses that do not involve combustion may be GHG-emissions-reducing compared to a system that employs incumbent production processes and no CO2 capture. Furthermore, these non-combustion options emit about 2 times less GHG than end uses that result in combustion, on a per kg of CO2 converted basis in the low-carbon scenario (Fig. S4 in the ESI†). When shifting from natural gas-based to a low-carbon scenario, the pathways become similar in terms of GHG emissions. The electrochemical CO2 conversion option that involves low-temperature electrolysis (SOEC–AEC-flow, cases a.4 and a.5) in the low-carbon scenario appears to be competitive with high-temperature electrolysis (SOEC-Co, cases a.1–a.3) and the hybrid pathway for methane production.
Comparing the CO2-based options with the incumbent processes does not capture the GHG mitigation potential that each use presents globally. Thus, including the market sizes of each use in the analysis provides a sense of scale. Fig. S6 and S7 in ESI† presents the avoided emissions and global reduction potential for the base case and low-carbon scenarios. Polymer production appears to be a promising use in a low-carbon scenario according to Fig. 2 compared to the incumbent production process. However, considering the smaller market size compared to transportation and power generation, this use results in a lower potential to reduce emissions globally.
3.2. Sensitivity analyses
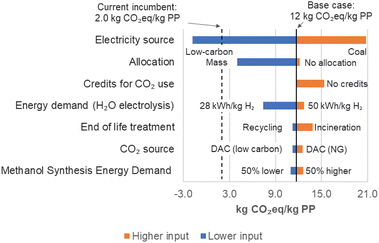
In this section, two sensitivity analyses are discussed: (1) influence of assumptions on the results (CO2-based chemicals in polymer production was selected as this case includes the end-of-life assumption) and (2) exploring the influence and trade-offs of certain parameters on the net GHG emissions and cost of diesel.In this figure, known as a tornado graph, parameters are ranked according to influence. One parameter is varied at each time, and its effect on the net GHG emissions is calculated. The electricity source is the most influential parameter. The use of renewable electricity may decrease net GHG emissions up to 115% compared to the base case, while the use of coal may increase net GHG emissions by 77% from the base case. In this analysis, electricity was evaluated alone, whereas in the former results (Fig. 2), the impact of electricity was combined with the impact of other utilities and feedstocks (e.g., CO2 source). The CO2 source, end-of-life treatment, and energy demand in thermochemical units appears at the bottom of the graph, indicating a weaker influence than the electricity source.
For each parameter, the calculations were repeated with a lower and a higher input compared to the base case. The low and high values are labeled close to their respective bars. For instance, for electricity source, the lower input is low-carbon electricity labeled as “Low-carbon,” and the net GHG emissions calculated is −1.8 kg CO2eq per kg PP, as depicted by the blue bar. The same process was done with the higher input (coal-based electricity, labeled as “Coal”), which resulted in 21 kg CO2eq per kg PP as can be seen in the x-axis.
Methodological choices in LCA, including allocation method and credits for CO2 use, also resulted in appreciable influence. The allocation method chosen to handle the co-products may affect the net emissions, with mass allocation resulting in lower emissions (66% compared to the base case) and no allocation resulting in higher emissions (3% compared to the base case).
The base case employs system expansion, as recommended by ISO standards.6,7 However, other methods are available to handle co-products (see Section S4 in ESI† for an explanation about each method and reasons to consider or discard the methods). The base case assumes all credits for CO2 use are attributed to the CO2 as feedstock, but when no credits are attributed to the CO2 captured and used, net GHG emissions increased 31%.
For the assumption regarding the energy requirement for syngas purification in the SOEC-Co case, the base case input of 0.25 kW h m−3 was varied by ±50%, with the final net GHG emissions for PP production varying by ±0.20%. Thus, this assumption has a low influence on the results.
In Section S2 in ESI,† additional sensitivity analyses are presented, including a breakeven analysis for CO2-based polymers with varying electricity emissions intensity, and a sensitivity analysis for the market penetration assumption to estimate the global emissions reduction potential. For PP production from SOEC-W, electricity emissions intensities below 0.075 kg CO2eq per kW h will likely result in lower net GHG emissions than the current incumbent (2.0 kg CO2eq per kg PP). The electricity emissions intensity in the provinces of Quebec (0.002 kg CO2eq per kW h)74 and Ontario (0.03 kg CO2eq per kW h)74 in Canada, for example, are sufficient to achieve lower net GHG emissions for production and use of CO2-based PP compared to the incumbent. In the province of Quebec, over 94% of the grid is from hydroelectricity and only 0.7% from fuels such as natural gas and diesel.74
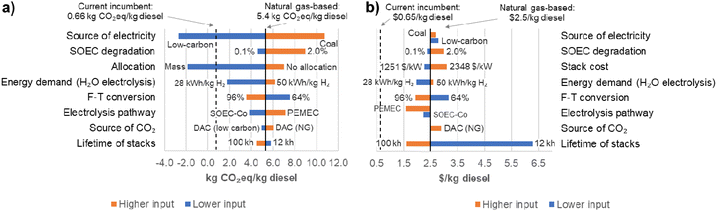
In the LCA sensitivity, net GHG emissions in the base case are 5.4 kg CO2eq per kg diesel, and costs are $2.5 per kg diesel. The most influential LCA parameters appear at the top of the graph; economic parameters are arranged in the same order as the LCA to highlight the trade-offs between the two dimensions. One parameter was varied at a time, and its effect on the net GHG emissions and production costs was estimated. This is not an optimization exercise or a rigorous TEA, but a tool to identify opportunities for improvement and potential trade-offs between environmental and economic performance.
The electricity source, SOEC degradation rate, and allocation method are the most sensitive environmental parameters as changes in any of these factors could result in appreciable variation in the net GHG emissions. The source of electricity is one of the main parameters impacting net GHG emissions, which are higher for coal-based electricity and lower for the low-carbon source. This is due to the higher emission factor for coal-fired electricity (0.80 kg CO2eq per kW h) compared to electricity produced from natural gas (0.49 kg CO2eq per kW h) and low-carbon sources (0.024 kg CO2eq per kW h). A substantial reduction in GHG emissions could be achieved using low-carbon electricity instead of natural gas. However, using renewable electricity may lead to higher costs due to the cost of renewable electricity. The price of renewable electricity in this study was based on the levelized cost, which includes costs for construction and operation, but does not account for intermittency explicitly. In the case of surplus renewable electricity, the electricity is not counted as a cost; however, the operation time (or load factor) depends on when electricity is available, which may lead to higher operational costs per unit produced since production will be lower than a continuous operation with the same capital expense.14 A more detailed discussion about the effect of intermittency on prices may be found in Ganzer and Mac Dowell,76 who concluded that intermittency may account for up to 2/3 of the price of renewable electricity, depending on the location due to additional costs of storage in batteries or in the form of H2. Conversely, coal electricity is being phased out in many developed countries, increasing its cost.77,78 This result may change in the near future since trends in electricity prices show that renewable energy may be cost-competitive in the future.79 Nevertheless, electricity source and price will continue to be crucial for electrolysis performance.
In terms of SOEC degradation, the higher degradation rate (2.0%/1000 h) than the base case has a notable impact on the net emissions (∼65% higher) compared to the base case (SOEC-W, natural gas-based electricity, CO2, heat, and steam, SOEC degradation of 0.5%/1000 h, F–T conversion of 80%, and system expansion via substitution). We assume that the production of diesel would be the same throughout the lifetime of the SOEC (water electrolysis). To compensate for degradation, electricity demand will need to increase with degradation to maintain output. The SOEC would thus consume more electricity than the base case, resulting in higher net GHG emissions with time. However, as the technology matures, emissions are expected to decrease as degradation rates decrease. Promising work is targeting issues that may lead to lowered degradation rates, such as minimizing Ni migration44 and coking,80 and replacement of metal catalysts with more stable metal oxides.81 Cost follows the same trend as the GHG emissions due to the cost of electricity.
Allocation method is another influential parameter for GHG emissions, reinforcing the importance of LCA methodological choices on the results. Mass allocation shares the GHG emissions among the co-products based on their mass, and since the mass of oxygen produced is relevant, a substantial portion of GHG emissions is attributed to oxygen, resulting in lower GHG emissions attributed to diesel (12%). For the no allocation case, 100% of the GHG emissions are attributed to diesel.
Lastly, the lifetime of stacks appeared to be the least relevant factor for the LCA portion, with a lower lifetime of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours (12 kh) only slightly increasing emissions. Infrastructure typically has lower impacts than operational factors, being commonly assumed to have negligible impacts.3 Conversely, stack lifetime is one of the most influential cost drivers. If a research project aims to improve environmental performance, stack lifetime appears to be of minor importance but is relevant if the goal is to reduce costs. In this study, we consider lifetime and degradation separately and having different effects on GHG emissions, even though they are related.14 Degradation is assumed to only increase electricity demand, maintaining the base case lifetime (48
000 hours (12 kh) only slightly increasing emissions. Infrastructure typically has lower impacts than operational factors, being commonly assumed to have negligible impacts.3 Conversely, stack lifetime is one of the most influential cost drivers. If a research project aims to improve environmental performance, stack lifetime appears to be of minor importance but is relevant if the goal is to reduce costs. In this study, we consider lifetime and degradation separately and having different effects on GHG emissions, even though they are related.14 Degradation is assumed to only increase electricity demand, maintaining the base case lifetime (48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours), while the stack lifetime parameter considers the GHG emissions of replacing the electrolyzer in terms of infrastructure (e.g., steel for interconnects and sealings) when the end-of-life voltage of the base case is achieved.
000 hours), while the stack lifetime parameter considers the GHG emissions of replacing the electrolyzer in terms of infrastructure (e.g., steel for interconnects and sealings) when the end-of-life voltage of the base case is achieved.
Most factors in the sensitivity analysis have the same effect on environmental and economic indicators, except for electricity and CO2 source. Renewable sources (with lower GHG emissions) are currently more expensive than fossil options and lead to higher production costs. Thus, these factors are especially important to consider in decision-making processes.
The other tested parameters (energy demand for H2 production, stack cost, electrolysis pathway, the CO2 source, and the F–T conversion) are discussed in Section S3 in the ESI.† The technologies involved in this study are at different maturity levels. For instance, F–T is a mature technology, whereas SOECs are in the early stages of commercialization, and assessment of uncertainty is therefore relevant. However, uncertainty analysis is beyond the scope of this study.
3.3. Recommendations
Based on the analysis and results of this study, we formulated the following recommendations for technology developers of electrochemical CO2 conversion solutions. It is worth noting that our intention is not to predict which technology or strategy is the most preferred but, from an analysis considering a broader scope, to generate insights and provide suggestions about applications and conditions that should be prioritized in research.• Pair with renewables or look for low-carbon grids to decrease GHG emissions, keeping in mind that costs may increase.
• Avoid combustion wherever possible.
• In terms of the magnitude of global GHG emissions reduction, the difference between the alternative (e.g., CO2 conversion) pathway and incumbent is as important as the market size of the use.
• Consider the efficiency of the product-use relation (such as diesel, which is more efficient than the other fuels in transportation).
• In low-temperature electrochemical CO2 conversion, pathways that produce liquid products and need more energy for the separation process than gaseous products, reusing waste heat in the separation unit may help decrease the overall energy requirement.
• Minimize degradation and energy demand, especially if the grid is carbon-intensive or if the electricity price is high.
• Better characterize the long-term behavior of high temperature electrolyzers (especially co-electrolysis) with dynamic loads to understand the degradation pattern, if any, and better assess their lifetime.
• In high temperature dry CO2 or co-electrolysis, issues with carbon deposition or electrode microstructure change that can increase degradation should be minimized, as degradation rate is a relevant parameter for LCA.
• Stack lifetime is more relevant to economic performance than environmental performance. Thus, if the goal is to reduce climate impacts, energy demand is more relevant.
• Where high-temperature co-electrolysis is possible, it may be more promising than low-temperature electrolysis technologies and may even be competitive against thermochemical pathways. Also, tuning the intermediate products composition (e.g., syngas) according to the use may be beneficial.
4. Conclusions
Our study provides a systems-level perspective to technology developers, highlighting the most relevant factors for improvement in the R&D process of electrochemical systems involving CO2 conversion. The results indicate that electricity source and consumption during operation are the main drivers of GHG emissions. Other influential parameters include those related to the processing unit, such as SOEC degradation rates (which are still uncertain), energy demand for H2 production, electrolyzer type, and F–T conversion (diesel case). External parameters also considerably influence the results, including the electricity source, as mentioned above, and the CO2 source, reinforcing the notion that the overall emissions are heavily affected by factors beyond the electrochemical process itself. The LCA methodology may also have an appreciable effect (e.g., the method to handle multiple co-products in diesel production). In terms of costs, stack lifetime, electrochemical process, and thermochemical units (e.g., F–T reactor for diesel) are shown to be relevant factors.Based on the results of each metric and the sensitivity analyses, a list of recommendations was provided with the intent of helping technology developers prioritize research areas of focus, considering environmental and economic performance.
It is worth emphasizing that this study does not aim to compare different technologies or strategies to define which one has the lowest GHG emissions. We use the comparison, which is subject to uncertainty, to investigate characteristics or sources of differences across strategies, and potential improvements that could support decisions during R&D. Additionally, we provide relevant LCA fundamentals to help technology developers identify aspects of LCA studies that influence a reported value, or what assumptions and methodological choices are behind a GHG emissions estimate.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Alberta Innovates through the Strategic Research Projects program and Natural Sciences and Engineering Research Council of Canada through the CREATE ME2 program (Collaborative Research and Training Experience - Materials for Electrochemical Energy Solutions).References
- IPCC, Summary for policymakers, in Global Warming of 1.5 °C, 2018.
- M. H. Youn, K. T. Park, Y. H. Lee, S. P. Kang, S. M. Lee, S. S. Kim, Y. E. Kim, Y. N. Ko, S. K. Jeong and W. Lee, J. CO2 Util., 2019, 34, 325–334 Search PubMed.
- W. Hoppe, N. Thonemann and S. Bringezu, J. Ind. Ecol., 2018, 22, 327–340 CrossRef CAS.
- C. Van Der Giesen, R. Kleijn and G. J. Kramer, Environ. Sci. Technol., 2014, 48, 7111–7121 CrossRef CAS PubMed.
- J. B. Guinée, R. Heijungs, G. Huppes, A. Zamagni, P. Masoni, R. Buonamici, T. Ekvall and T. Rydberg, Environ. Sci. Technol., 2011, 45, 90–96 CrossRef PubMed.
- The International Standards Organisation, Environmental Management—Life Cycle Assessment—Principles and Framework (ISO 14040), 2006.
- The International Standards Organisation, Environmental Management—Life Cycle Assessment—Requirements and Guidelines (ISO 14044), 2006.
- J. A. Bergerson, A. Brandt, J. Cresko, M. Carbajales-Dale, H. L. MacLean, H. S. Matthews, S. McCoy, M. McManus, S. A. Miller, W. R. Morrow, I. D. Posen, T. Seager, T. Skone and S. Sleep, J. Ind. Ecol., 2020, 24, 11–25 Search PubMed.
- S. M. Moni, R. Mahmud, K. High and M. Carbajales-Dale, J. Ind. Ecol., 2020, 24, 52–63 Search PubMed.
- A. Venkatesh, P. Jaramillo, W. M. Griffin and H. S. Matthews, Environ. Sci. Technol., 2011, 45, 8182–8189 CrossRef CAS PubMed.
- B. M. Galindro, S. Welling, N. Bey, S. I. Olsen, S. R. Soares and S. Ryding, J. Ind. Ecol., 2020, 24, 965–975 CrossRef.
- R. Arvidsson, A. M. Tillman, B. A. Sandén, M. Janssen, A. Nordelöf, D. Kushnir and S. Molander, J. Ind. Ecol., 2018, 22, 1286–1294 CrossRef CAS.
- R. E. Davis, D. B. Fishman, E. D. Frank, M. C. Johnson, S. B. Jones, C. M. Kinchin, R. L. Skaggs, E. R. Venteris and M. S. Wigmosta, Environ. Sci. Technol., 2014, 48, 6035–6042 Search PubMed.
- D. Parra, X. Zhang, C. Bauer and M. K. Patel, Appl. Energy, 2017, 193, 440–454 CrossRef.
- C. Fernández-Dacosta, M. van der Spek, C. R. Hung, G. D. Oregionni, R. Skagestad, P. Parihar, D. T. Gokak, A. H. Strømman and A. Ramirez, J. CO2 Util., 2017, 21, 405–422 Search PubMed.
- M. Miotti, J. Hofer and C. Bauer, Int. J. Life Cycle Assess., 2017, 22, 94–110 Search PubMed.
- V. Sick, K. Armstrong, G. Cooney, L. Cremonese, A. Eggleston, G. Faber, G. Hackett, A. Kätelhön, G. Keoleian, J. Marano, J. Marriott, S. McCord, S. A. Miller, M. Mutchek, B. Olfe-Kräutlein, D. Ravikumar, L. K. Roper, J. Schaidle, T. Skone, L. Smith, T. Strunge, P. Styring, L. Tao, S. Völker and A. Zimmermann, Energy Technol., 2020, 8, 1901034 CrossRef CAS.
- A. Zimmermann, L. Müller, Y. Wang, T. Langhorst, J. Wunderlich, A. Marxen, K. Armstrong, G. Buchner, A. Kätelhön, M. Bachmann, A. Sternberg, S. Michailos, S. McCord, A. V. Zaragoza, H. Naims, L. Cremonese, T. Strunge, G. Faber, C. Mangin, B. Olfe-Kräutlein, P. Styring, R. Schomäcker, A. Bardow and V. Sick, Techno-Economic Assessment & Life Cycle Assessment Guidelines for CO2 Utilization (Version 1.1), 2020 Search PubMed.
- G. Thomassen, M. Van Dael and S. Van Passel, Bioresour. Technol., 2018, 267, 271–280 CrossRef CAS PubMed.
- M. Z. Hauschild, Life Cycle Assessment, Springer International Publishing, Cham, 2018 Search PubMed.
- Y. Xing, Z. Ma, W. Su, Q. Wang, X. Wang and H. Zhang, Catalysts, 2020, 10, 1–18 Search PubMed.
- R. G. Grim, Z. Huang, M. T. Guarnieri, J. R. Ferrell, L. Tao and J. A. Schaidle, Energy Environ. Sci., 2020, 13, 472–494 Search PubMed.
- J. Sisler, S. Khan, A. H. Ip, M. W. Schreiber, S. A. Jaffer, E. R. Bobicki, C.-T. Dinh and E. H. Sargent, ACS Energy Lett., 2021, 6, 997–1002 Search PubMed.
- S. Turnau, C. S. Mignot, C. Mostert and S. Bringezu, Green Chem., 2020, 22, 8423–8443 RSC.
- C. M. Liu, N. K. Sandhu, S. T. McCoy and J. A. Bergerson, Sustainable Energy Fuels, 2020, 4, 3129–3142 Search PubMed.
- Helmeth Consortium, Deliverable 5.2: Final LCA Report HELMETH concept system, 2017.
- J. Schaffert, H. Cigarida, D. Coquette, J. Leukefeld, M. Lange, D. Levedag, R. Albus, F. Burmeister and K. Görner, Innovative large-scale energy storage technologies and power-to-gas concepts after optimisation – Report on an EU-wide potential analysis of power-to-gas locations coupled to local CO2 and renewable energy sources, 2020.
- R. Bhandari, C. A. Trudewind and P. Zapp, J. Cleaner Prod., 2014, 85, 151–163 Search PubMed.
- G. Reiter and J. Lindorfer, Int. J. Life Cycle Assess., 2015, 20, 477–489 CrossRef CAS.
- A. Mehmeti, A. Angelis-Dimakis, C. B. Muñoz, M. Graziadio and S. J. McPhail, J. Cleaner Prod., 2018, 199, 723–736 CrossRef CAS.
- V. Uusitalo, S. Väisänen, E. Inkeri and R. Soukka, Energy Convers. Manage., 2017, 134, 125–134 CrossRef CAS.
- D. Yadav and R. Banerjee, Appl. Energy, 2020, 262, 114503 CrossRef CAS.
- W. Hoppe, S. Bringezu and N. Wachter, J. CO2 Util., 2018, 27, 170–178 CrossRef CAS.
- D. Chang, C. K. M. Lee and C. H. Chen, J. Cleaner Prod., 2014, 83, 48–60 CrossRef.
- G. A. Keoleian, J. Cleaner Prod., 1993, 1, 143–149 CrossRef.
- M. Gear, J. Sadhukhan, R. Thorpe, R. Clift, J. Seville and M. Keast, J. Cleaner Prod., 2018, 180, 735–747 CrossRef.
- B. A. Wender, R. W. Foley, V. Prado-Lopez, D. Ravikumar, D. A. Eisenberg, T. A. Hottle, J. Sadowski, W. P. Flanagan, A. Fisher, L. Laurin, M. E. Bates, I. Linkov, T. P. Seager, M. P. Fraser and D. H. Guston, Environ. Sci. Technol., 2014, 48, 10531–10538 CrossRef CAS PubMed.
- O. G. Griffiths, R. E. Owen, J. P. O'Byrne, D. Mattia, M. D. Jones and M. C. McManus, RSC Adv., 2013, 3, 12244–12254 Search PubMed.
- V. Sharma, S. Biswas, B. Sundaram, P. Haldar, B. Dubey and A. Chandra, ACS Sustainable Chem. Eng., 2019, 7, 5385–5392 CrossRef CAS.
- S. Kibria Nabil, S. McCoy and M. G. Kibria, Green Chem., 2021, 23, 867–880 Search PubMed.
- M. Marchese, E. Giglio, M. Santarelli and A. Lanzini, Energy Convers. Manage.: X, 2020, 6, 100041 CAS.
- Y. Zheng, J. Wang, B. Yu, W. Zhang, J. Chen, J. Qiao and J. Zhang, Chem. Soc. Rev., 2017, 46, 1427–1463 RSC.
- O. Schmidt, A. Gambhir, I. Staffell, A. Hawkes, J. Nelson and S. Few, Int. J. Hydrogen Energy, 2017, 42, 30470–30492 CrossRef CAS.
- A. Hauch, R. Küngas, P. Blennow, A. B. Hansen, J. B. Hansen, B. V. Mathiesen and M. B. Mogensen, Science, 2020, 370, eaba6118 Search PubMed.
- Q. Fu, C. Mabilat, M. Zahid, A. Brisse and L. Gautier, Energy Environ. Sci., 2010, 3, 1382–1397 RSC.
- J. Gao, Y. Wang, Y. Ping, D. Hu, G. Xu, F. Gu and F. Su, RSC Adv., 2012, 2, 2358–2368 Search PubMed.
- D. H. König, M. Freiberg, R.-U. Dietrich and A. Wörner, Fuel, 2015, 159, 289–297 Search PubMed.
- IRENA, Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.5 °C Climate Goal, 2020.
- J. J. Kaczur, H. Yang, Z. Liu, S. D. Sajjad and R. I. Masel, Front. Chem., 2018, 6, 1–16 CrossRef PubMed.
- J. He, Y. Li, A. Huang, Q. Liu and C. Li, Electrochem. Energy Rev., 2021, 4, 680–717 CrossRef.
- C. Mittal, C. Hadsbjerg and P. Blennow, Chem. Eng. World, 2017, 52, 44–46 Search PubMed.
- N. S. Romero Cuellar, K. Wiesner-Fleischer, M. Fleischer, A. Rucki and O. Hinrichsen, Electrochim. Acta, 2019, 307, 164–175 Search PubMed.
- R. Bock, H. Karoliussen, F. Seland, B. G. Pollet, M. S. Thomassen, S. Holdcroft and O. S. Burheim, Int. J. Hydrogen Energy, 2020, 45, 1236–1254 CrossRef CAS.
- H. Zhang, F. Wang, S. Y. Zhao and B. Xie, Earth's energy budget, climate feedbacks, and climate sensitivity, 2021, vol. 17 Search PubMed.
- L. J. Müller, A. Kätelhön, S. Bringezu, S. McCoy, S. Suh, R. Edwards, V. Sick, S. Kaiser, R. Cuéllar-Franca, A. El Khamlichi, J. H. Lee, N. von der Assen and A. Bardow, Energy Environ. Sci., 2020, 13, 2979–2992 RSC.
- A. Paturska, M. Repele and G. Bazbauers, Energy Procedia, 2015, 72, 71–78 CrossRef.
- I. J. Okeke, K. Sahoo, N. Kaliyan and S. Mani, J. Cleaner Prod., 2020, 249, 119358 Search PubMed.
- I. J. Okeke and S. Mani, Biofuels, Bioprod. Biorefin., 2017, 11, 472–487 CrossRef CAS.
- D. S. Ripatti, T. R. Veltman and M. W. Kanan, Joule, 2019, 3, 240–256 Search PubMed.
- Sunfire, Sunfire-Hylink, https://www.sunfire.de/en/products-and-technology/sunfire-hylink, accessed 31 March 2020.
- H. Zhang, L. Wang, M. Pérez-Fortes, J. Van herle, F. Maréchal and U. Desideri, Appl. Energy, 2020, 258, 114071 Search PubMed.
- C. A. Trudewind, A. Schreiber and D. Haumann, J. Cleaner Prod., 2014, 70, 38–49 Search PubMed.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 Search PubMed.
- Y. Bicer and F. Khalid, Int. J. Hydrogen Energy, 2020, 45, 3670–3685 Search PubMed.
- D. Ravikumar, G. Keoleian and S. Miller, Appl. Energy, 2020, 279, 115770 Search PubMed.
- A. J. Pieja, M. C. Morse and A. J. Cal, Curr. Opin. Chem. Biol., 2017, 41, 123–131 Search PubMed.
- C. C. Chang, Y. T. Liao and Y. W. Chang, Int. J. Hydrogen Energy, 2019, 44, 18472–18482 CrossRef CAS.
- United State Department of Energy, Status of the Solid Oxide Fuel Cell Program, 2019.
- S. Lee, S. Lee, H.-J. Kim, S. M. Choi, H. An, M. Y. Park, J. Shin, J. H. Park, J. Ahn, D. Kim, H.-I. Ji, H. Kim, J.-W. Son, J.-H. Lee, B.-K. Kim, H.-W. Lee, J. Hong, D. Shin and K. J. Yoon, J. Mater. Chem. A, 2018, 6, 15083–15094 Search PubMed.
- Intergovernmental Panel on Climate Change, Climate Change 2014 Mitigation of Climate Change, Cambridge University Press, Cambridge, 2015, pp. 1329–1356 Search PubMed.
- K. Krause, C. Lee, J. K. Lee, K. F. Fahy, H. W. Shafaque, P. J. Kim, P. Shrestha and A. Bazylak, ACS Sustainable Chem. Eng., 2021, 9, 5570–5579 Search PubMed.
- M. Jouny, W. Luc and F. Jiao, Ind. Eng. Chem. Res., 2018, 57, 2165–2177 Search PubMed.
- L. K. Rihko-Struckmann, A. Peschel, R. Hanke-Rauschenbach and K. Sundmacher, Ind. Eng. Chem. Res., 2010, 49, 11073–11078 CrossRef CAS.
- Environment and Climate Change Canada, National inventory report 1990–2018: Greenhouse gas sources and sinks in Canada, Gatineau, QC, 2020.
- M. Lang, S. Raab, M. S. Lemcke, C. Bohn and M. Pysik, Fuel Cells, 2020, 20, 690–700 Search PubMed.
- C. Ganzer and N. Mac Dowell, Sustainable Energy Fuels, 2020, 4, 3888–3903 RSC.
- P. Breeze, in The Cost of Electricity, ed. P. Breeze, Elsevier, 2021, pp. 117–136 Search PubMed.
- IEA, Projected Costs of Generating Electricity 2020, Paris, 2020.
- EIA, Levelized Cost and Levelized Avoided Cost of New Generation Resources in the Annual Energy Outlook 2016, 2020.
- Y. Wang, W. Li, L. Ma, W. Li and X. Liu, J. Mater. Sci. Technol., 2020, 55, 35–55 Search PubMed.
- H. M. Ansari, A. S. Bass, N. Ahmad and V. I. Birss, J. Mater. Chem. A, 2022, 10, 2280–2294 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc01466a |
| This journal is © The Royal Society of Chemistry 2023 |