Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Indoor particulate matter (PM) from cooking in UK students' studio flats and associated intervention strategies: evaluation of cooking methods, PM concentrations and personal exposures using low-cost sensors†
Ruijie
Tang
a and
Christian
Pfrang
 *ab
*ab
aSchool of Geography, Earth and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham, UK. E-mail: c.pfrang@bham.ac.uk
bDepartment of Meteorology, University of Reading, Whiteknights, Earley Gate, Reading, UK
First published on 20th February 2023
Abstract
Cooking emissions have been identified as a major source of indoor particulate matter (PM), which can contribute to severe health issues, including cardiovascular disease and lung cancer. Both cooking methods and use of extractors were investigated to assess their influence on PM concentrations and emission rates. The impact of PM residence times was also examined in terms of the operator's exposure. PM10 and PM2.5 were monitored by placing carefully validated low-cost sensors, with sufficiently high accuracies of 70.7% and 64.3% on PM10 and PM2.5, respectively, by compared to a reference instrument, in the kitchen areas of five student studio flats. Ranges and medians of mean concentrations of PM10 and PM2.5 of four studied cooking methods ranked (range (median) (μg m−3)) deep-frying (62–236 (151); 6–37 (30)), stir-frying (17–176 (63); 2–38 (7)), boiling (6–41 (17); 2–11 (4)) and steaming (7–23 (12); 2–7 (3)), respectively. The impact of using extractors on PM removal rates ranged between 10.5% and 63.0%. Extractors were shown to accelerate the post-cooking pollutant decay, which therefore resulted in shorter residence times. Personal exposure times also varied with cooking method and operator gender. Indoor PM exposure is associated with PM concentration, cooking duration and decay rate. Based on these results, cooking water-based dishes while operating extractors would improve indoor air quality and reduce PM exposure.
Environmental significanceDomestic cooking plays a critical role in releasing gaseous and particulate pollutants into indoor environments and the outdoor atmosphere. This study tested and deployed low-cost sensors to assess the concentrations, emission rates and decays of particulate matter (PM) from cooking in student studio flats, and examined the results from different cooking methods and the use of extractors. Oil-based methods were found to cause ca. six times the emission rates of water-based cooking; extractors significantly increased removal rates and shortened PM residence times, particularly important in residential settings where kitchen and living/sleeping spaces are closely linked; together with successful deployment of validated low-cost sensors, this work enables decision-makers and the general public to improve indoor air quality experiences. |
Introduction
Personal exposure to indoor air pollution plays one of the most significant roles in threatening public health around the world.1 Various pollutants, such as particulate matter (PM), volatile organic components (VOCs) and polycyclic aromatic hydrocarbons (PAHs) from the indoor air have received much attention. In New Jersey, the concentrations of these contaminants indoor were determined to be higher than those in the outdoor atmosphere.2 In many countries people spend 80–90% of their time indoors and the association between airborne particulate matter and adverse effects on human health has been documented by numerous studies.3,4 Huang et al.5 conducted a study on non-smoking Chinese women, with the results demonstrating that the risk of lung cancer had risen due to cooking. This was due to the emissions of particles, especially ultrafine particles which may cause respiratory ailments and damage to the cardiovascular system, reproductive system, and blood system by inhaling over a long time period.6 In addition, because of indoor exposure to smoke, Abdullahi et al.7 suggested that more than one million people died annually from chronic obstructive pulmonary disease (COPD). Therefore, indoor air quality has become a focus of public attention.8The emissions of indoor air pollution originate from a wide variety of sources. They differ between buildings and depend on the fuels used for cooking and heating, as well as the personal habits of smoking and use of various consumer products.9–11 Emissions from combustion of e.g. mosquito-repellent incense, candles and cooking all play critical roles in the formation of indoor air pollution.12–14 A study by Abt et al.15 characterising sources of indoor particles in houses in Boston, USA, found that the concentrations of indoor particles were impacted significantly by cooking activities, cleaning, and the movement of people. Cooking, known as a considerable source of air pollution and emissions of odour, can emit micro-solid particles, including reduced sulphur compounds (RSCs), aldehydes, organic acids, VOCs and other gases.16,17 Key factors include the choice of cooking oils, duration of cooking, fuel type, food ingredients, cooking temperature, style of cooking and ventilation conditions.18,19
In order to gain a better understanding of the association between cooking and particulate air pollution, the concentrations of particle numbers and size distribution of particles generated during cooking has been assessed in multiple studies. It was found that particles produced by various cooking methods contributed 12–20% to the overall ambient aerosols.20 For instance, an 18 month study conducted by Wallace et al.21 in a house near Washington DC, USA, found that particle numbers from frying reached 1014 after only 15 minutes of cooking with over 90% of the particles in the ultrafine size range. A study conducted by Liu et al.14 suggested that cooking was a significant source of primary organic aerosols (POA) and a potential source to form secondary organic aerosols (SOA); different types of cooking oils were found to release varying amounts of organic aerosols. As a common technique of cooking in numerous cultures, frying with vegetable oils can produce a large quantity of particles and organic gases.7,22,23 Dennekamp et al.6 demonstrated that gas stoves generate high concentrations of particles, while electric rings and grills produce smaller amounts of particles. Noticeably, due to coagulation effects for lower diameter particles, concentration levels of fine particulate (PM2.5) were found to drop with the increasing time of heating, as the concentration of coarse particles (PM10) increased.24 While irritations of upper airways were caused by PM10 and total suspended particulates (TSP), fine particulate matter were osmosing into bronchi where they might ultimately enter the bloodstream.25 The most critical parameter impacting evaporative emission rates was found to be temperature of cooking as it increased particle emission rates.19
A positive correlation between the emission of VOCs, which is key for formation of particulate matter from heated cooking oils, and frying temperature has been found.26 During oil heating, PM2.5 was released with a linkage to emission of heavy metals and organic compounds.27,28 Additionally, in terms of the raw material, food containing a high percentage of fat was likely to produce a much higher amount of PM2.5 compared to vegetables which contain less fat, with a ratio of peak mass concentration to background concentration for fatty food and vegetables at approximately 40 and 8, respectively.29 Lu et al.8 conducted a study of PM2.5 emissions by six types of cooking styles using gas as the fuel. Their results showed that emission rates of PM2.5 decreased in the following order: deep-frying, stir-frying, stewing, quick-frying, boiling and steaming. Also, See and Balasubramanian30 assessed the exposure to indoor aerosols with the association with Chinese cooking methods on gas-phase by controlled experiments in a domestic kitchen. Their results found that the highest particles concentration was produced by deep-frying, with 20 nm of the mode diameter approximately. However, Zhao et al.18 observed that in Chinese cooking, stir-frying and pan-frying caused a significantly higher generation rate of PM2.5 than deep-frying, although it required larger amounts of cooking oil and longer durations of cooking. During the cooking process at a high temperature, it can generate more than 300 reaction products, including fatty acids, alkanes, alkene, aldehydes, ketones, alcohols, esters, aromatic compounds, and heterocyclic compounds.31 In order to manage indoor air pollution, in addition to controlling the source of emission, the removal of the pollutants also plays a significant role.32 The use of an extractor directly influences the level of PM2.5 and an extractor operating at higher air flow rates has a more significant effect on reducing PM2.5 concentrations during cooking.
The majority of previous studies on cooking emissions have been conducted in places such as restaurants, canteens, laboratories and residential kitchens.33,34 Also, a large section of the existing literature focusses on emission rates to the ambient air which differs from the indoor environment investigated in the present study.19 To our knowledge, no previous studies have focused on the cooking emissions within the confined area of student studio accommodation, where young adults are exposed to cooking emissions from its generation through to its decay. In terms of the cooking methods, there are a wide variety of cuisines around the world, associated with geographical environments, cultural traditions, different climates, ethnic customs and other factors. With the increasing number of international students studying at UK universities, students living in university accommodation generally cook for themselves with cuisines and cooking styles showcasing the features from their hometowns. According to a survey conducted by Lu et al.,8 deep-frying, frying, stir-frying, boiling, stewing and steaming are the most favoured cooking styles.
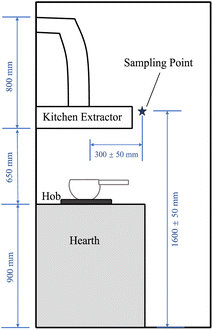
In the present study, measurements of cooking emissions were carried out in five student studio flats. In such studio flats, students from all over the world dwell together with no partitioning between their private living areas and the kitchen; this common set-up may cause a particularly long exposure to the air pollutants produced by cooking activities. A low-cost sensor was used to record these cooking emissions. The concentrations and emission rates of PM, including PM10 and PM2.5, from different cooking methods was assessed as well as the intervention by using the kitchen extractor. The link between the cooking emission concentrations and cooking duration was also examined. The efficiency of the kitchen extractor and residence time of the pollutants was also assessed. Based on the results from the measurements, the personal exposure to the indoor particles from the cooking emissions was determined to estimate potential health impacts.
Experimental
Materials and methods
To confirm the accuracy of the Flow2 measurements for our work, two Flow2 sensors were placed in an office in the Biosciences building at the University of Birmingham with co-location of the reference instrument Fidas® 200 (Palas GmbH), a continuous ambient air quality monitoring system, to assess the inter-Flow2 variation. In line with similar research,37,38 the coefficient of variation (CV), which determines the variation among the sensors, the relative precision errors (RPE), which is averaged to indicate the precision of the sensors, and the accuracy (Acc) by comparisons between Flow2 and Fidas® 200 were calculated and linear regressions (using SPSS; IBM version 28) were chosen to yield the R2 correlation between the two Flow2 sensors and between each Flow2 sensor and the Fidas® 200 reference instrument (see ESI, Text S3, eqn (S1)–(S3) for details†). The results of averaged CV of PM2.5 and PM10 concentrations between the two Flow2 sensors were 12.3% and 15.0%, respectively, and the overall RPE were 17.4% and 21.2%, which demonstrated high precisions between Flow2 sensors. In addition, the overall accuracies of Flow2 sensors by compared to the Fidas® 200 for PM2.5 and PM10 were 70.7% and 64.3%, respectively. The averaged values of R2 for linear regressions between Flow2 sensors and Fidas® 200 for PM2.5 and PM10 were 89.5% and 73.4%, respectively (compare Table 1). The moderately high values of the accuracy and coefficient of determinations illustrate suitable performance in terms of reliability for using the Flow2 sensors for further utilisation on indoor air quality assessment focussing on PM2.5 and PM10 (it should be noted that we have not tested any of the other pollutants Flow2 attempts to measure).
| Flow2Avs. Flow2B | Flow2Avs. Fidas | Flow2Bvs. Fidas | |
|---|---|---|---|
| PM10 | 0.936 (p < 0.001) | 0.771 (p < 0.001) | 0.697 (p < 0.001) |
| PM2.5 | 0.836 (p < 0.001) | 0.873 (p < 0.001) | 0.908 (p < 0.001) |
The Flow2 was set up at least 10 days before the data collection period and synchronised to the apps on personal devices at least once a day for auto-calibration by the AI. During the period for measurements of the PM concentrations, the use of aerosol sprays, laser printers, cleaning and combustion processes such as burning candles and smoking cigarettes, as well as the use of e-cigarettes were prohibited in the kitchens, as these activities may impact on the PM concentration.11 Before cooking, the hobs and the bottom of the pots were well-cleaned to minimise the influences of the residua on the hearth and pot, which could potentially release additional PM and VOCs while cooking. When cooking, the windows were suggested to be closed or only opened at a small acute angle (∼30°) to minimise the direct wind impacts on the air flow in the room. Due to the restrictions in place at the time as a result of the COVID-19 pandemic, we confirm that the distribution and collection of the sensors followed the full guidance from the UK government at the time.
Four groups of typical cooking examples made by the four cooking methods were chosen. In each group, the same dishes were made twice: once with the extractor on and the other with the extractor off. The dishes in each group were cooked with the same food (chicken thighs of the same weight (100 g)), durations and processes at a similar background air pollution level to ensure they were under as similar conditions as possible. The major ingredient was selected to be chicken to minimise the potential impact of differences in fat proportion of the meats. For the deep-frying and stir-frying, which were oil-based, grapeseed oil was used. The chosen dishes were deep-fried chicken, stir-fried chicken, chicken soup and steamed chicken, which represented deep-frying, stir-frying, boiling and steaming, respectively. Detailed recipes of the four cooking examples were provided in S2.†
Emission rate assessment
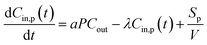 | (1) |
At the start time t0, the particle concentration is determined as Cin,p(t0). This is because the concentration of indoor particles before measurement tended to be at steady-state (i.e. Cin,p(t0) = aPCout/λ) as there would be no other activities before cooking for a period of at least 10 min. Thus, the expression of indoor PM concentration during periods of emissions for eqn (1) corresponds to:
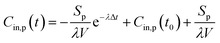 | (2) |
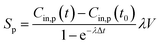 | (3) |
As the kitchen extractors were off, and the windows and doors were closed or opened with only a small angle, it resulted in a small value of air change rate a, which improves the fitting accuracy for emission rate Sp.42 The value of air change rate a was assumed to be 0.075 min−1 in line with a previous study under similar conditions.43 The total removal rate λ was identified by the measurement of particle concentration decay after the cooking finished, which could be briefly described by fitting the time series of total particle concentrations between the peak and the end of each cooking process into a natural exponential decay curve by using:
Ct = Cp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−λt e−λt | (4) |
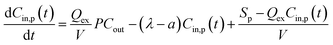 | (5) |
| Shood,p = Sp − QexCin,p(t) | (6) |
The calculated emission rate of PM with kitchen extractors on, Shood,p, is affected by the PM concentration in the exhausted air, which varies over time. To make comparisons between this and the emission rates of the pollutants generated from cooking, Sp, the emission rate of PM with the extractors on, Shood,p, is estimated as a constant from the solution of the concentration of indoor PM for eqn (1):
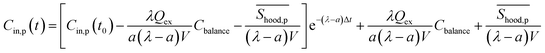 | (7) |
 of PM with kitchen extractors on could be rewritten as:
of PM with kitchen extractors on could be rewritten as: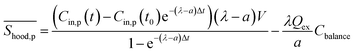 | (8) |
Therefore, the removal efficiency of the kitchen extractors can be expressed as:
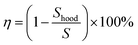 | (9) |
| Caverage = ∑Ci/N | (10) |
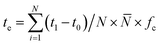 | (11) |
![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) represented the average number of dishes per cooking activity, fc represented the averaged frequency of cooking activities per day.
represented the average number of dishes per cooking activity, fc represented the averaged frequency of cooking activities per day.| Dpot = Caverage × IR × te × EF | (12) |
Results and discussion
PM emissions from different cooking methods
The ranges and medians of mean concentrations of PM10 and PM2.5 generated by the four cooking methods deep-frying, stir-frying, boiling and steaming under conditions of both kitchen extractors on and off in the five study kitchens are displayed in order from high to low emission in Tables 2 and 3. The minimum concentrations for PM10 and PM2.5, i.e., the background pollution levels were 3.0 μg m−3 and 2.0 μg m−3, respectively. The maximum concentrations of PM10 and PM2.5 peaked at 2111.5 μg m−3 and 57.4 μg m−3, respectively, during deep-frying. Statistically significant differences in the mean concentrations of PM10 and PM2.5 between different cooking methods were found (p < 0.001). The average concentrations of PM10 and PM2.5 in the direct respiratory zone during the cooking process were 48.7 and 9.4 μg m−3, respectively. This indicated that the average levels of the cooking emissions were around or below the WHO air quality guideline values for PM10 and PM2.5 for the 24 hour mean (45 and 15 μg m−3, respectively).47 However, the average values of PM included boiling and steaming, which emitted particles at very low levels and lasted longer. The concentrations of PM10 emitted from deep-frying and stir-frying exceeded the PM10 level recommended by the UK Department for Environment Food & Rural Affairs,48 with the standards of annual mean PM10 and PM2.5 at 40 μg m−3 and 25 μg m−3, respectively. Approximately 73% of cooking activities in the studied flats recorded peaks in PM10 concentrations higher than the standard, and 67% of them lasted for more than 10 minutes. Even though the average PM2.5 concentrations did not go beyond the standard, 45% of the surveyed cooking activities recorded peaks above 25 μg m−3, with 28% lasting for more than 10 minutes. Noticeably, the mass concentrations of both PM10 and PM2.5 from deep-frying and stir-frying were greater than those from boiling and steaming. The fact that cooking periods and dishes cooked varied introduced significant uncertainties. Thus, ranges and medians were used to present the concentrations and emission rates of the cooking activities.| C PM10 | C PM2.5 | Duration | |
|---|---|---|---|
| a The column of duration indicated the range of time taken for each dish by each cooking method, so the row of total would not determine any useful value. According to the survey, the average cooking duration per day was 49.4 minutes. | |||
| Deep-frying | 62–236 (151) | 6–37 (30) | 7–49 (16) |
| Stir-frying | 17–176 (63) | 2–38 (7) | 10–61 (23) |
| Boiling | 6–41 (17) | 2–11 (4) | 9–172 (17.5) |
| Steaming | 7–23 (12) | 2–7 (3) | 7–65 (21) |
| Total | 6–236 (30) | 2–38 (5) | —a |
| Extractors off | Extractors on | |||
|---|---|---|---|---|
| C PM10 | C PM2.5 | C PM10 | C PM2.5 | |
| Deep-frying | 91–236 (190) | 22–35 (27) | 62–216 (146) | 10–37 (34) |
| Stir-frying | 52–176 (67) | 5–38 (12) | 17–129 (54) | 2–22 (6) |
| Boiling | 9–41 (17) | 2–9 (4) | 6–35 (12) | 2–11 (4) |
| Steaming | 7–23 (12) | 2–7 (4) | 7–14 (11) | 2–4 (3) |
Several studies have demonstrated that compared to low-fat foods, high-fat foods generate more particulate matter during the process of cooking, and oil-based cooking methods, including deep-frying, stir-frying, pan-frying and grilling emit more particles, especially fine particles, than cooking methods that are water-based, including boiling, stewing and steaming.30,49–51 This is likely due to the enhanced oil and food pyrolysis caused by the high temperature, which results in much higher particle emissions from pan-, deep- and stir-frying than those of boiling and steaming.19 According to To and Yeung,52 by combining the deep-, stir- and pan-frying and the other methods of cooking, the number concentration of PM10 rose to up to 3.9 × 107 cm−3, while PM2.5 concentrations were discovered to be elevated 90 times higher than the background levels during the cooking process of grilling and frying.52 The cooking process of stir-frying was observed to generate the highest concentration of PM10 with a peak at 1300 μg m−3, followed by pan- and deep-frying in domestic kitchens.52 See and Balasubramanian30 conducted experiments by cooking 150 g of tofu via steaming, boiling, stir-frying, pan-frying and deep-frying. The results determined that the largest mass of PM2.5 was generated by deep-frying, followed by stir-frying, boiling and steaming, with average concentrations of PM2.5 at 190, 120, 81 and 66 μg m−3, respectively. This is consistent with the order of the emission rank of PM2.5 in the present study, but the value of PM2.5 concentrations were 8 to 13 times higher in the study by See and Balasubramanian30 than the results from our study. Similar experiments were conducted by Wu et al.53 with 200 g of bean curd cooked with these same cooking methods. It was found that the most ultra-fine particles (UFPs) were emitted by stir-frying, followed by pan-frying, boiling and steaming, and their particle number emission rates were 1483 × 1010, 1044 × 1010, 53 × 1010 and 47 × 10 min−1, respectively, i.e. the emission rate of UFPs from stir-frying was more than 22 times higher than those from boiling and steaming. In the present study, the emission rates of PM2.5 from stir-frying was the highest, followed by deep-frying, boiling and steaming. The average PM2.5 emission rate of stir-frying was approximately three times greater than that of boiling and steaming. The maximum concentrations and emission rates of boiling (0.47 × 106 # cm−3; 0.888 × 1010 # s−1) and steaming (0.36 × 106 # cm−3; 0.783 × 1010 # s−1) were found to be similar, which could be interpreted by a slower growth in the concentrations of UFPs, as the residual oil and food attached to the pot and hob were heated as well, which could undergo pyrolysis.53 The results of PM mass concentrations corresponding to the methods of cooking, heating sources and the use of extractors are displayed in Table 4 also in comparison with other studies.
| Cooking method | Heating source | Range hood mode | Average mass concentration (μg m−3) | Reference | |
|---|---|---|---|---|---|
| PM10 | PM2.5 | ||||
| Boiling | Gas stove | Off | 241.6 | Zhao et al.54 | |
| Pan-fry | 214.1 | ||||
| Stir-fry | 287.5 | ||||
| Boiling | 252.3 | ||||
| Boiling | Gas stove | On | 105.3 | 81.1 | Lee et al.31 |
| Steaming | 33.9 | 28.7 | |||
| Deep-fry | Electric hob | On | 230.9 | Zhang et al.43 | |
| Stir-fry | Gas stove | On | 281 | Wong et al.55 | |
| Stir-fry | Electric hob | 155 | |||
| Mix | Gas stove | 30.3 | 24.5 | Pan et al.56 | |
| Mix | 74.9 | 56.9 | |||
| Mix | Gas stove | On | 75.9 | Wan et al.50 | |
| Mixed | Gas stove | 312.4 | See and Balasubramanian30 | ||
| Pan-fry | Gas stove | On | 1020 | To and Yeung52 | |
| Pan-fry | Electric hob | 520 | |||
| Stir-fry | Gas stove | 1330 | |||
| Stir-fry | Induction hob | 1030 | |||
| Deep-fry | Gas stove | 4720 | |||
| Deep-fry | Induction hob | 3980 | |||
| Boiling (stew) | Electric hob | 117 | 26.1 | Embiale et al.57 | |
| Charcoal | 179 | 50.2 | |||
| Kerosene | 124 | 31.4 | |||
| Deep-fry | Electric stove | On | 154.3 | 21.8 | Current study |
| Stir-fry | 68.7 | 11.5 | |||
| Boiling | 20.5 | 6.2 | |||
| Steaming | 13.9 | 5.0 | |||
Emission rates of PM10 and PM2.5 from different cooking methods with and without the extractors in the five study kitchens on are displayed in Table 5. While we found the same order for the PM10 emission rates in terms of cooking methods as for the concentrations of PM10 (see Table 2), for PM2.5, the highest median emission rate was produced by stir-frying with the median PM2.5 emission rate of deep-frying only being the second highest. The highest emission rates of both PM10 and PM2.5 at a single timepoint were found to be generated by deep-frying with values of 7586 μg min−1 and 1229 μg min−1, respectively. Emission rates of PM from cooking are impacted by the type of appliance used, the condition of cooking, temperatures and the fat content of the ingredients.58 Our results in Table 5 showed that deep-frying and stir-frying had much higher emission rates than boiling and steaming, in line with several previous studies; larger particle production is caused by cooking with oil as opposed to water.30,50 Additionally, the type of ingredients used affects emission rates due to the percentage of fat contained: food with a high proportion of fat tends to result in higher emission rates than ingredients with low fat proportions.29 In terms of the processes of oil heating, the emission rates varied considerably as well. It was reported that the range of emission rates of PM10 was between 0.67 and 2.33 mg s−1, while the range of PM2.5 emission rates varied from 0.06 to 1.46 mg s−1.23,59
| Extractor off | Extractor on | |||
|---|---|---|---|---|
| ERPM10 | ERPM2.5 | ERPM10 | ERPM2.5 | |
| Deep-frying | 636–1015 (921) | 16–83 (50) | 86–913 (535) | 17–221 (58) |
| Stir-frying | 303–1720 (546) | 18–399 (54) | 39–1163 (268) | 4–64 (17) |
| Boiling | 39–420 (124) | 2–54 (16) | 37–139 (119) | 6–28 (16) |
| Steaming | 30–109 (72) | 4–32 (13) | 41–98 (64) | 8–15 (14) |
The effects of air quality intervention using kitchen extractors
According to Khalid and Foulds60 from the UK Energy Research Centre, approximately 60% of the UK residential and commercial kitchens prefer to use natural gas for cooking. Previous research has shown that natural ventilation may not be able to provide a sufficiently high air exchange rate to move indoor aerosol particles outdoors.61 In Table 5, PM emission rates with and without the extractors are displayed. Significant effects (at 99.9% confidence level) from the use of extractors were observed for the emission rates of PM10 from deep-frying and stir-frying. The extractors worked well, with removal efficiencies for PM10 and PM2.5 of 21.9% to 53.5% and 10.5% to 63.0%, respectively (see Fig. S5†). The highest removal efficiencies for both PM10 and PM2.5 were found for stir-frying, followed by deep-frying and steaming. The average removal efficiency of PM10 from oil-based cooking methods in the present work was 51.9%, which was similar to the results from Kang et al.62 at 45.2% (see Table 6). The removal efficiency of PM2.5 from stir-frying in our study was 10.5%, which was much lower than from deep-frying (63.0%), which fitted well with results from Xu and Huang.63 Even though the water-based cooking methods did not contribute large PM emission rates compared with oil-based cooking methods, the extractors still performed with high efficiencies on clearing particles at the average values of 26.4% and 29.1% on PM10 and PM2.5, respectively.| Cooking method | Volume of room (m3) | Range hoods exhaust air volume rate (m3 min−1) | Air exchange rate (min−1) | Total removal rate (min−1) | Removal efficiency | Reference | ||
|---|---|---|---|---|---|---|---|---|
| PM10 | PM2.5 | PM10 | PM2.5 | |||||
| Mixed | Average 256.57 | N/A | 0.077 | 0.0941 | 0.079 | 45.24% | 49.68% | Kang et al.62 |
| Mixed | 10.88 | 15 | 0.36 | 58% | Chen et al.40 | |||
| Stir-fry | N/A | 59.61% | Xu and Huang63 | |||||
| Stir-fry | 14.29% | |||||||
| Deep-fry | Average 43.70 | 7.5 | Average 0.075 | 0.069 | 0.080 | 53.53% | 62.96% | This study |
| Off | 0.066 | 0.079 | ||||||
| Stir-fry | 7.5 | 0.128 | 0.092 | 50.25% | 10.54%% | |||
| Off | 0.123 | 0.079 | ||||||
| Boiling | 7.5 | 0.172 | 0.161 | 21.90% | 14.12% | |||
| Off | 0.151 | 0.143 | ||||||
| Steaming | 7.5 | 0.192 | 0.140 | 30.94% | 44.04% | |||
| Off | 0.180 | 0.068 | ||||||
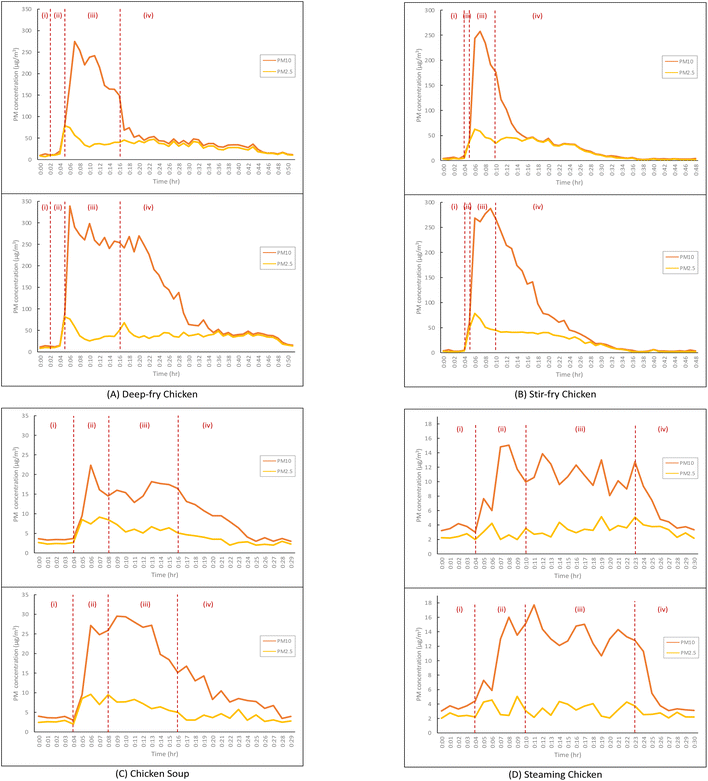
PM emissions of the four typical cooking methods with the extractor on and off are displayed in Fig. 2. Each cooking episode is divided into four stages: (i) is the background testing, (ii) is heating of the pot or pan and the oil or water, (iii) represents the main cooking processes and (iv) is the post-cooking decay after the end of the entire cooking process. The average background concentrations of PM10 and PM2.5 for all examined cooking episodes were 4.6 ± 1.8 μg m−3 and 2.3 ± 1.7 μg m−3, respectively. For deep-frying, with no kitchen extractor, the PM concentrations rose steadily while heating the oil, and PM2.5 (79.0 μg m−3) peaked when the chicken was put in with the oil at a high temperature, while PM10 peaked two minutes later than PM2.5 with the concentration at 274.7 μg m−3 which was 91.5 times above background concentrations. A similar trend was found with the extractor on with higher concentration peaks for PM2.5 (81.5 μg m−3) and PM10 (338.8 μg m−3). When the deep-frying finished, the concentrations of PM10 with extractor fans on and off were 149.4 μg m−3 and 254.5 μg m−3, respectively. In terms of stir-frying, the PM2.5 concentrations under both conditions peaked (extractor on: 62.4 μg m−3; off: 73.4 μg m−3) at the second minute when the chicken was placed into the pan. When the extractor was on, PM10 concentration (257.4 μg m−3) peaked one minute later than the peak of PM2.5. However, with the extractor off, there was an initial PM10 peak (268.4 μg m−3) at the same time as the PM2.5 peak, with the highest PM10 peak (287.7 μg m−3) occurring three minutes later. Under both conditions, PM10 and PM2.5 concentrations waned after their peak. Not all periods of post-cooking decay were recorded until the concentrations fully descended back to background levels. When the kitchen extractors were working, both average and peak concentrations of PM10 and PM2.5 were significantly lower than those with the extractors off.
For PM10, there were second peaks appearing after a first peak with intervals of 1 to 3 minutes. This could be due to coagulation of UFPs and gaseous pollutants such as VOCs forming secondary organic aerosol (SOA). With the constant emissions of primary organic aerosol (POA), these SOA may cause increasing particle numbers, even though the decay and removal occurred simultaneously. After the second peak, the trend of PM concentrations either remained at relatively low levels or started to decrease steadily. When intervening with the extractors, the slopes of the decays were much steeper, which revealed a swifter decay and removal of the particles indoors, even if it was still within the cooking processes. This suggests that it may not be sufficient to rely on uncontrolled natural ventilation for the removal of the particles. A relatively stable supply of air via an open kitchen door, fresh air systems and kitchen extractors could work much more effectively together to reduce the particles in the breathing zone, rather than only opening the kitchen window.23,50 In addition to the volume flow rate of the extractors and air exchange rates, there are other factors, such as the volume of the kitchen and the total removal rates, including the decay rate, that should be taken into account. The summary of comparisons of our work to other studies regarding the efficiencies of extractors operation is presented in Table 6.
PM residence times with extractors on and off. Residence time of the PM generated by cooking was counted from the time of the end of each cooking activity until the concentration of PM returned to the background level. Deep-frying and stir-frying could emit PM10 in high quantities, after which the PM concentrations decreased to below the limit given by the World Health Organization (24 hour mean PM10: 45 μg m−3);47 the time taken for PM concentrations to return to a safe level was also measured.
Table 7 presents the durations of post-cooking decay for each cooking method and the time taken for PM10 to decease to the WHO limit. With the extractor on, the mean duration for PM10 and PM2.5 generated by deep-frying and stir-frying to return to the background level was significantly shorter (PM10: 27.1–38.8%, PM2.5: 26.5–29.3%) than with the extractors off, with confidence levels at 99.9%. For boiling and steaming, the use of extractors also sped up (by 32.0% to 40.5%) the reduction of PM10 significantly at 99% confidence levels. However, no significant effects of extractors on the removal of PM2.5 during post-cooking decay were found, although the average durations were shortened. In terms of the reductions of PM10 to the recommended WHO limit, when the extractors were off, it took approximately a third of the entire duration of PM10 post-cooking decay to reduce PM10 concentrations lower than 50 μg m−3 for both emissions by deep-frying and stir-frying. It was significantly (by 47.1% to 75.5%) faster to reduce PM10 emitted by deep-frying and stir-frying below the WHO level of health concern by using the kitchen extractor at a 99.9% confidence level.
| PM10 | PM2.5 | PM10 (WHO standard) | ||||
|---|---|---|---|---|---|---|
| Extractors off | Extractors on | Extractors off | Extractors on | Extractors off | Extractors on | |
| Deep-fry | 53.0 ± 9.8 | 32.4 ± 2.1 | 20.8 ± 2.8 | 14.7 ± 2.2 | 23.2 ± 3.7 | 6.4 ± 1.5 |
| Stir-fry | 31.6 ± 2.0 | 23.1 ± 2.3 | 17.6 ± 3.8 | 12.9 ± 5.2 | 11.6 ± 2.1 | 6.2 ± 1.4 |
| Boiling | 9.2 ± 2.5 | 5.5 ± 1.6 | 6.2 ± 2.2 | 4.3 ± 1.8 | — | — |
| Steaming | 10.0 ± 1.8 | 6.8 ± 1.9 | 4.1 ± 1.6 | 3.4 ± 1.1 | — | — |
The PM pollutant is presented in either the air compartment or on a surface compartment.64 In the present study, the surface compartment residence time was ignored as it measured the average time a particle remained on a surface before being removed by cleaning or resuspension, while the air compartment residence time examined the average time spent to remove a particle in the indoor air via deposition and exfiltration.64 The post-activity decreasing trends of particles of all sizes were found to be following an exponential decay at the room air exchange rate for this study and in line with previous work.65 It should be noted that the air compartment residence time of PM is impacted by the total removal rate (λ) which includes the air exchange rate and the coagulation and deposition of the particles.
With no mechanical ventilation presented, after a cooking event, the concentrations of nanosized particles larger than 6 nm (ca. 6–1000 nm) (PN>6) started to decay due to the mixing of pollutants throughout the room, or by being removed by ventilation, infiltration and deposition.65,66 In the current study, the air exchange rate was assumed to be 0.075 min−1 (4.5 h−1) based on the studies by Kang et al.62 and Lunden et al.67 Lunden et al.67 conducted two experiments of pan-frying and stir-frying, with the results of the residence time of PN>6 being 19 and 26 minutes, respectively, while their peak concentrations were approximately 104 and 105 # cm−3. Due to its similar conditions of air exchange rates and volume of kitchen, the residence times observed by Lunden et al.67 fit into the range of the current study. However, because of a low air exchange rate, much longer residence times of particles were found by Singer et al.66 who performed repeated experiments investigating cooking emissions of PN>6 and gaseous pollutants. PN>6 were found to deposit indoors at a rate which was quick enough to compete against the air exchange as a removal process, and the average time of decay was approximately 88 minutes with an air exchange rate of 0.0083 min−1.66 Similarly, in the research by Qian et al.,64 the air exchange rates ranged between 0.11–0.58 h−1 (0.0018–0.0097 min−1), which were significantly lower, and the average residence time of PM10 and polystyrene latex (PSL) tracer particles were in 1.2 and 2.6 hours, respectively, in the air compartment.
The average post-cooking PM decays were fitted with regression lines to exponential functions as illustrated in Fig. 3. The averaged PM concentrations after cooking at each time point are displayed together with the regression lines. The regression trend lines generally fit well the decay of PM10 and PM2.5 with and without the kitchen extractors on after cooking activities as demonstrated by high correlation coefficients (all the R2 values are greater than 80%). Although the PM10 concentrations did not quite reach the background levels when the cooking activities finished, with the extractors on, PM10 concentrations dropped 1.43 times faster and the slope of the first three minutes was much steeper than without extractors. The effect of extractors on the post-cooking decay of PM2.5 concentration was not as clear, but a slightly steeper slope of the PM2.5 decay with kitchen extractors on was observed in the first five minutes.
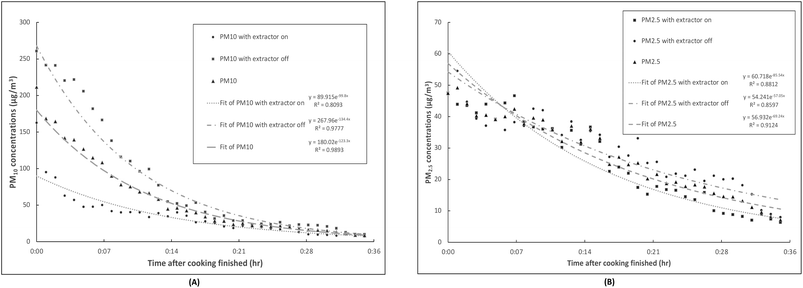 | ||
| Fig. 3 (A) PM10 and (B) PM2.5 concentrations with the extractors on/off during post-cooking decay with exponential regressions. | ||
During the PM decay in the present study, the rates of particle removal were higher for PM10 than for PM2.5 as shown in Fig. 3. This was because, besides the air exchange rate, particle deposition rates significantly contributed to the PM removal rate. This varied broadly across the various conditions, including particle size variation, which was reported to be one of the most critical factors impacting on indoor particle concentrations and removal rates, together with the quantity of interior furnishings.68,69 Thatcher et al.70 conducted experiments to explore the deposition rates of particles by using three different levels of indoor furnishings and four different conditions of air flow in an isolated room with a volume of 14.2 m3. With an increase of the particle diameter from 0.55 to 8.66 μm, the deposition loss rate coefficients increased from 0.10 to 6.79 h−1 (0.0016–0.1132 min−1) in an unfurnished room with a bare, electrically grounded metal floor. For an unfurnished room with a carpeted floor and one that was fully furnished, the deposition loss rate coefficients increased together with the particle diameter (0.55–8.66 μm), varied 0.12–4.92 h−1 (0.002–0.082 min−1) and 0.20–5.54 h−1 (0.0033–0.908 min−1), respectively.70 This indicated that the residence time of the particles with the diameter from 0.55 to 8.66 μm ranged from approximately 10 hours to only 8.83 minutes, and the residence time for particles with a mean diameter at 2.37 μm was about 65 minutes.64 In our present study, the trends of PM10 and PM2.5 decays showed that PM10 had a dramatically faster decay rate in the first 14 minutes compared to that of PM2.5, which indicates that the PM10 deposition occurred much swifter than for PM2.5.
The use of kitchen extractors significantly raised the efficiency of the PM removal and reduced the PM residence time. Quicker depositions were found in the first three minutes after the peak concentrations of PM10 when the extractors were on compared to those with extractors off. From Table 7, it is clear that the residence time of both PM10 and PM2.5 decreased due to the use of extractors. However, the performance of extractors on PM2.5 removal was not as clear as no statistical significance was found between the groups with and without extractors on, which might be because of their relatively lower concentrations at the peak time. Singer et al.66 determined that extractors performed with higher reduction efficiency on PN with a larger diameter than those in small sizes, resulting in approximately 130% faster decay. A similar result was found by Rim et al.71 reporting that removal effectiveness for particles with diameters of 2–6 nm (PN2–6) was lower than for particles larger than 6 nm (PN>6), which led to approximately a 59% shorter time for PN>6 to decay than for PN2–6. In Table 7, it also indicates that an additional five-minute running of the extractors could reduce PM10 to a safe level after deep-frying and stir-frying.
Influences of oil type and temperature
As the oil-based cooking methods contributed higher PM emissions than water-based methods, the type of oil could also play a critical role in the generation of aerosols. It was shown that the composition of oil can influence the temperature at which the oil started to decompose and emit visible smoke fumes.19 When the semi-volatile compounds are released during the heating of oil, they tended to be condensed and form liquid aerosols, which eventually generated particles.29 Thus, the PM emission rates are influenced by the proportions of semi-volatile and volatile species in the oil. According to Gao et al.23 and Torkmahalleh et al.,59 the ranges of concentrations and emission rates of PM10 were 7.4–30 mg m−3 and 0.51–2.33 mg s−1, respectively, while those of PM2.5 were 6.5–18.8 mg m−3 and 0.05–1.46 mg s−1 respectively, due to different oil types. Based on the study by Liu et al.,14 POA ranged from 0.8 to 42.3 μg m−3, with olive oil emitting the highest POA, and SOA ranged from 27.1 to 107.5 μg m−3, with palm oil generating the greatest quantity of SOA. Rapeseed oil, peanut oil and olive oil generated a relatively high particle number, while soybean oil, sunflower oil and blend oil released the lowest concentrations.14,23 In general, oils with higher smoke points tend to generate lower concentrations of particles.19 It was found that when the temperature of oil was between 180 and 210 °C, every five-degree increase could lead to a rise in the concentrations of UFPs of 20–50%.72 The mode diameter of oil droplets was found to grow with increasing temperature, leading to enhanced concentrations of accumulation mode aerosols.72Personal exposure to cooking PM and health effects
![[N with combining macron]](https://www.rsc.org/images/entities/i_char_004e_0304.gif) ) was 1.37, and the averaged frequency of cooking activities (fc) was 1.94 times per day. In Table 3, the exposure time of the four cooking methods is presented. The total exposure time per day (te) for PM10 and PM2.5 was calculated by using eqn (13) resulting in 103.81 and 77.09 minutes, respectively.
) was 1.37, and the averaged frequency of cooking activities (fc) was 1.94 times per day. In Table 3, the exposure time of the four cooking methods is presented. The total exposure time per day (te) for PM10 and PM2.5 was calculated by using eqn (13) resulting in 103.81 and 77.09 minutes, respectively.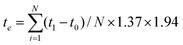 | (13) |
| Average exposure duration per dish (min) | Likelihood of choosing a particular cooking method per day | Average exposure time of each cooking method per day (min) | |||
|---|---|---|---|---|---|
| PM10 | PM2.5 | PM10 | PM2.5 | ||
| Deep-frying | 48.8 | 25.0 | 12% | 16.0 | 8.2 |
| Stir-frying | 40.5 | 26.3 | 33% | 35.5 | 23.1 |
| Boiling | 39.3 | 35.0 | 40% | 42.0 | 37.4 |
| Steaming | 26.9 | 21.8 | 14% | 10.3 | 8.4 |
The average exposure time of each cooking method per day was calculated by multiplying te by the likelihood of choosing a particular cooking method per day, listed in the 4th column of Table 8.
The annual intake for PM10 and PM2.5 are displayed in Table 9. The PM10 annual intake ranked with the same order as the PM10 concentrations, even though deep-frying only contributed 12% to the four cooking methods. Steaming contributed 14% to the cooking activities but its PM10 annual intake was about 18 and 14 times lower than that of deep-frying and stir-frying. Boiling had the largest share in cooking activities, though it donated only ⅓ and ⅖ of deep-frying and stir-frying annual inhalation exposures, respectively. In terms of the annual PM2.5 intake, it was noticed that boiling played the greatest role, followed by stir-frying and deep-frying, with steaming the lowest, which was about 5.7 times lower than that of boiling. The PM10 and PM2.5 intake of male operators in the student flats were approximately 12% higher than those of females, as the average inhalation rates used for calculation were 0.66 m3 h−1 and 0.59 m3 h−1 for males and females, respectively.
| D pot | D pot for male | D pot for female | ||||
|---|---|---|---|---|---|---|
| PM10 | PM2.5 | PM10 | PM2.5 | PM10 | PM2.5 | |
| Deep-fry | 7789 | 564 | 8160 | 591 | 7294 | 528 |
| Stir-fry | 7682 | 836 | 8048 | 876 | 7195 | 783 |
| Boiling | 2713 | 736 | 2842 | 771 | 2540 | 689 |
| Steaming | 452 | 132 | 473 | 138 | 423 | 124 |
| Total | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 636 636 |
2268 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523 523 |
2376 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 452 452 |
2124 |
According to Table 9, the average annual intake to PM10 and PM2.5 from cooking emissions in the studio flats were 18.64 and 2.27 mg per year, respectively. Deep-frying and stir-frying produced high values of particles, even though their cooking durations were the shortest. They also took much longer to decay to the background levels, resulted in longer exposure duration, as shown in Table 7. The exposure by deep-frying contributed the largest fraction of PM10 while it took the second lowest proportion of average cooking time per day due to its relatively short cooking duration and low number of times chosen. Stir-frying and boiling were two of the most popular cooking methods, but the PM10 intake of stir-frying was nearly three times higher than that of boiling, because of its high emission rate. Also, stir-frying contributed the highest PM2.5 intake because of its high frequency and oil-based method. Due to the low emission rate and short cooking duration, steaming provided particularly low intake.
Liao et al.51 studied the PM mass lung/indoor (L/I) ratio and the inhalation exposure dose of major compartments in the human respiratory tract by using a L/I ratio model. It was suggested that the integrated cumulative inhalation dose rates (particles per cm2 per h) in the nasal passage (23.94–24.27), bronchial region (4.92–5.06), bronchiolar region (4.97–10.82) were significantly higher than those in the alveolar interstitial region (0.002–0.02) for the events of cooking. In terms of the L/I ratios of PM mass, the regions of nasal passage and pharynx (0.70–0.83) were found to be higher than those of the bronchial region (0.41–0.62), bronchiolar region (0.12–0.41), alveolar interstitial region (0.02–0.26), where they discovered larger sized particles (diameter >3 μm) with smaller L/I ratios of PM.51 This explained that, due to the deposition during respiration, particles, especially those with bigger size ranges, tended to be no longer airborne, resulting in a lower concentration of PM in deeper lung regions. The greater extent of harmful particles in the lung were those in smaller size ranges (diameter < 3 μm).
Lu et al.8 had conducted experiments on assessing the personal inhalation exposure to PM2.5 during Chinese family cooking. The results showed that the exposure of male and female operators was 346.3 and 309.6 mg per year, respectively, due to physiological differences, which resulting in various amounts of inhalation while breathing. In the present study, the differences were identified as well, but the results were approximately 150 times lower than those from the study by Lu et al.8 According to Ji et al.,73 the annual inhalation exposure of indoor PM2.5 from daily life was 174.84 mg per year, which was approximately 10 times higher than the present study's findings. According to Mohammadyan,74 the concentrations of indoor PM were the best predictors of personal exposure. The measured average concentrations of PM2.5 were the major difference, which were 60 μg m−3 by Ji et al.73 and 599 μg m−3 by Lu et al.8 as they used gas stoves, while the present study result was 9.36 μg m−3 using electric hobs. The experiments by Lu et al.8 counted 3.54 dishes per meal, two times of cooking activities per day, and 365 days of the exposure frequency as it was designed for a family with four people comprising three adults and one child, while the values were 1.37, 1.94 and 300 respectively in the present study for only one adult student. This underlines that the type of stove and duration of cooking plays a critical role in the personal exposure. In addition, the background level of air quality needs to be taken into account. The outdoor concentrations of PM2.5 in these two previous studies ranged from 10 to 220 μg m3; especially the study by Lu et al.8 which took placed in Tianjin, an industrial city in Northern China, during the late winter, was during a period with relatively serious air pollution, resulting in a background PM2.5 range of 41 to 168 μg m−3. In contrast, the data collection period of the present study was during the early summer and a national lockdown was implemented due to the COVID-19 pandemic, which resulted in the PM2.5 concentration ranging from 2.8 to 6.2 μg m−3.
Cooking activities also contribute greatly to people's response to oxidative stress, especially for the vulnerable population.8 The relationship between oxidative stress response and the concentration of PM2.5 was assessed by Kim et al.,81 who demonstrated that the acceptable mean personal exposures were 20.7 ± 12.7 and 80.5 ± 29.9 μg m−3 for young children and the elderly, respectively, due to different bodily functions. Although, in the present study, the mean concentrations of PM2.5 did not exceed these levels, previous studies have reported much higher personal exposures to indoor PM2.5 due to cooking. A recent systematic review on the indoor air pollution impact on the health of children and people with pre-existing lung disease by Maung et al.82 reported that PM2.5 levels vary seasonally with the highest levels reached in winter. From these studies it was strongly suggested that vulnerable people should carefully consider their participation in cooking processes and accessible intervention options.
Limitations of the study
Deploying low-cost sensors in real-world scenarios has the potential to deliver important insights to complement more rigorous, but also more costly as well as time- and location-limited studies with reference instruments. Apart from the limitations in the accuracy and precision of the low-cost sensors themselves (a comparison with reference instruments e.g. through co-location is essential to establish the performance for each individual pollutant of interest), there are a number of further uncertainties that need to be taken into account: air mixing regimes at the chosen sensor locations, variations in building ventilation caused e.g. by local wind conditions, building fabrics, building state and maintenance, as well as influences from external factors such as neighbouring flats as pollution sources and variable outdoor pollutant ingress into the study location are a few examples of such uncertainties. However, the most important uncertainties are likely associated with the behaviours of occupants and the interpretation of pollution measurements largely relies on the accuracy of the activity profiles reported by the volunteers which should ideally not only include the cooking activities, but also any activity that affects the indoor air quality such as opening/closing of windows and doors as well as any other activities that generate or dilute pollutant levels indoors; given the complexity of the activity profiles even for a single occupant, use of increasingly affordable smart technology to reduce the reporting burden on the volunteer could be an important step to reduce the uncertainties associated with this type of study.Conclusion
The present study identified the factors influencing indoor particulate matter levels from cooking emissions, including the cooking methods and the impact of extractors. In addition, the residence time and personal exposure to the cooking-generated PM was measured using a low-cost sensor following validation of the PM data by co-location of the sensor with a reference instrument. Oil-based cooking methods generated much higher PM levels than water-based methods. The use of kitchen extractors provided effective ventilation removing the pollutants, lowering the emission rates and speeding up the pollutant decay. This all resulted in a shorter residence time of the particles indoors. When considering the frequency of each cooking method, the personal exposure varied widely. It is recommended to minimise exposure to PM cooking emissions by choosing to cook water-based dishes, turning on the extractor for a straightforward indoor air quality intervention and leaving the extractor on for an additional five to ten minutes once the cooking process has finished. Although the accuracy of the low-cost sensor has scope for improvement, it successfully determined the trends of PM concentrations from the activities studied. This enables the public to raise their awareness of indoor air pollution caused by cooking activities and seek to apply strategies to maintain an all-round healthier lifestyle. Further research should focus on more effective ways to remove the pollutants generated in the various cooking activities to reduce personal exposure and health impacts.Author contributions
Conceptualisation was jointly by CP & RT; funding acquisition, supervision and project administration was carried out by CP; investigation, visualisation, data curation, formal analysis, validation and writing the original draft of the manuscript was carried out by RT; reviewing and editing of the manuscript was performed jointly by CP & RT.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Ravi Sahu, Siqi Hou and Zongbo Shi for enabling the co-location of low-cost sensors with the reference instrumentation. This work also benefitted from ongoing indoor air quality measurement activities as part of the Met Office/SPF grant “Indoor Air Quality Emissions & Modelling System (IAQ-EMS)” and the NERC grants “Air quality supersite triplets (UK-AQST)” (project reference: NE/V017624/1) and “Air Pollution Solutions for Vulnerable Groups (CleanAir4V)” (project reference: NE/V002414/1). The low-cost sensors deployed in this study were funded by the University of Birmingham's Institute for Global Innovation (IGI)/The Institute of Advanced Studies (IAS) Clean Air Pump Priming Funding via a grant on “Probing the Impact of Air Pollution Exposure in Sandy Urban Playgrounds on Children in Post-Soviet Countries” awarded jointly to Christian Pfrang and Viktor Karamushka, National University of Kyiv-Mohyla Academy (Kyiv, Ukraine). We also would like to thank the volunteers (Xiao Cheng, Chengxu Tong, Xiaoyue Lin, Junyi Liang) for helping with the measurements and surveys, and Roy M. Harrison for RT's personal supervision.References
- D. Norbäck, in Indoor Environmental Quality and Health Risk toward Healthier Environment for All, 2020, ch. 17, pp. 321–333, DOI:10.1007/978-981-32-9182-9_17.
- C. P. Weisel, S. Alimokhtari and P. F. Sanders, Environ. Sci. Technol., 2008, 42, 8231–8238 CrossRef CAS PubMed.
- R. Kamens, C.-t. Lee, R. Wiener and D. Leith, Atmos. Environ., Part A, 1991, 25, 939–948 CrossRef.
- W. G. Kreyling, M. Semmler-Behnke and W. Möller, J. Nanopart. Res., 2006, 8, 543–562 CrossRef CAS.
- Y. Huang, S. S. H. Ho, K. F. Ho, S. C. Lee, J. Z. Yu and P. K. K. Louie, J. Hazard. Mater., 2011, 186, 344–351 CrossRef CAS PubMed.
- M. Dennekamp, Occup. Environ. Med., 2001, 58, 511–516 CrossRef CAS PubMed.
- K. L. Abdullahi, J. M. Delgado-Saborit and R. M. Harrison, Atmos. Environ., 2013, 71, 260–294 CrossRef CAS.
- F. Lu, B. Shen, P. Yuan, S. Li, Y. Sun and X. Mei, Sci. Total Environ., 2019, 654, 671–677 CrossRef CAS PubMed.
- J. A. Adeniran, J. A. Sonibare and L. A. Jimoda, Atmos. Pollut. Res., 2015, 6, 21–28 CrossRef CAS.
- J. A. Adeniran, R. O. Yusuf, S. I. Mustapha and J. A. Sonibare, Environ. Res. Eng. Manag., 2018, 73(4), 21–30 Search PubMed.
- E. Géhin, O. Ramalho and S. Kirchner, Atmos. Environ., 2008, 42, 8341–8352 CrossRef.
- T.-T. Yang, S.-T. Lin, R.-H. Shie, C.-K. Tseng and C.-H. Ku, Aerosol Air Qual. Res., 2016, 16, 2570–2580 CrossRef CAS.
- S. Orecchio, Atmos. Environ., 2011, 45, 1888–1895 CrossRef CAS.
- T. Liu, Z. Wang, D. D. Huang, X. Wang and C. K. Chan, Environ. Sci. Technol. Lett., 2017, 5, 32–37 CrossRef.
- E. Abt, H. H. Suh, G. Allen and P. Koutrakis, Environ. Health Perspect., 2000, 108, 35–44 CrossRef CAS PubMed.
- E. Kabir and K.-H. Kim, J. Hazard. Mater., 2011, 188, 443–454 CrossRef CAS PubMed.
- L. Wang, Z. Xiang, S. Stevanovic, Z. Ristovski, F. Salimi, J. Gao, H. Wang and L. Li, Sci. Total Environ., 2017, 589, 173–181 CrossRef CAS PubMed.
- Y. Zhao, C. Chen and B. Zhao, Atmos. Environ., 2018, 193, 190–197 CrossRef CAS.
- Y. Zhao and B. Zhao, Build. Simul., 2018, 11, 977–995 CrossRef.
- R. M. Healy, J. Sciare, L. Poulain, M. Crippa, A. Wiedensohler, A. S. H. Prévôt, U. Baltensperger, R. Sarda-Estève, M. L. McGuire, C. H. Jeong, E. McGillicuddy, I. P. O'Connor, J. R. Sodeau, G. J. Evans and J. C. Wenger, Atmos. Chem. Phys., 2013, 13, 9479–9496 CrossRef.
- L. A. Wallace, S. J. Emmerich and C. Howard-Reed, Environ. Sci. Technol., 2004, 38, 2304–2311 CrossRef CAS PubMed.
- F. Klein, S. M. Platt, N. J. Farren, A. Detournay, E. A. Bruns, C. Bozzetti, K. R. Daellenbach, D. Kilic, N. K. Kumar, S. M. Pieber, J. G. Slowik, B. Temime-Roussel, N. Marchand, J. F. Hamilton, U. Baltensperger, A. S. H. Prévôt and I. El Haddad, Environ. Sci. Technol., 2016, 50, 1243–1250 CrossRef CAS PubMed.
- J. Gao, C. Cao, L. Wang, T. Song, X. Zhou, J. Yang and X. Zhang, Aerosol Air Qual. Res., 2013, 13, 488–496 CrossRef.
- J. A. Adeniran, R. O. Yusuf, M. O. Abdulkadir, M.-N. O. Yusuf, K. A. Abdulraheem, B. K. Adeoye, J. A. Sonibare and M. Du, Environ. Pollut., 2020, 266(Pt 2), 115169 CrossRef CAS PubMed.
- D. von Bornstädt, A. Kunz and M. Endres, J. Cereb. Blood Flow Metab., 2013, 34, 215–220 CrossRef PubMed.
- W.-T. Tsai, Environments, 2016, 3(4), 23 CrossRef.
- S. Gorjinezhad, A. Kerimray, M. A. Torkmahalleh, M. Keleş, F. Ozturk and P. K. Hopke, Environ. Sci. Pollut. Res., 2017, 24, 9515–9529 CrossRef CAS PubMed.
- J. J. Schauer, M. J. Kleeman, G. R. Cass and B. R. T. Simoneit, Environ. Sci. Technol., 2001, 36, 567–575 CrossRef PubMed.
- G. Buonanno, L. Morawska and L. Stabile, Atmos. Environ., 2009, 43, 3235–3242 CrossRef CAS.
- S. W. See and R. Balasubramanian, Environ. Res., 2006, 102, 197–204 CrossRef CAS PubMed.
- S. C. Lee, W.-M. Li and L. Yin Chan, Sci. Total Environ., 2001, 279, 181–193 CrossRef CAS PubMed.
- Y. Li and X. Li, Int. J. Vent., 2016, 5, 273 Search PubMed.
- Y. Chen, K. F. Ho, S. S. H. Ho, W. K. Ho, S. C. Lee, J. Z. Yu and E. H. L. Sit, J. Environ. Monit., 2007, 9(12), 1402–1409 RSC.
- Y. Zhao, M. Hu, S. Slanina and Y. Zhang, Environ. Sci. Technol., 2006, 41, 99–105 CrossRef PubMed.
- P. Labs, Evaluation of Flow, a Personal Air Quality Sensor, https://blog.plumelabs.com/2019/06/21/how-accurate-is-flow/, accessed 04 Oct, 2022 Search PubMed.
- N. Crnosija, M. Levy Zamora, A. M. Rule and D. Payne-Sturges, Int. J. Environ. Res. Public Health, 2022, 19(12), 7340 CrossRef CAS PubMed.
- F. M. J. Bulot, H. S. Russell, M. Rezaei, M. S. Johnson, S. J. J. Ossont, A. K. R. Morris, P. J. Basford, N. H. C. Easton, G. L. Foster, M. Loxham and S. J. Cox, Sensors, 2020, 20(8), 2219 CrossRef PubMed.
- M. Levy Zamora, F. Xiong, D. Gentner, B. Kerkez, J. Kohrman-Glaser and K. Koehler, Environ. Sci. Technol., 2018, 53, 838–849 CrossRef PubMed.
- D. M. Styne and H. McHenry, Horm. Res., 1993, 39, 3–6 CrossRef PubMed.
- C. Chen, Y. Zhao and B. Zhao, Environ. Sci. Technol., 2018, 52, 1081–1087 CrossRef CAS PubMed.
- S. Bhangar, N. A. Mullen, S. V. Hering, N. M. Kreisberg and W. W. Nazaroff, Indoor Air, 2011, 21, 132–144 CrossRef CAS PubMed.
- J. Thornburg, D. S. Ensor, C. E. Rodes, P. A. Lawless, L. E. Sparks and R. B. Mosley, Aerosol Sci. Technol., 2001, 34, 284–296 CrossRef CAS.
- Q. Zhang, R. H. Gangupomu, D. Ramirez and Y. Zhu, Int. J. Environ. Res. Public Health, 2010, 7, 1744–1759 CrossRef CAS PubMed.
- Q. Zhang, J. Avalos and Y. Zhu, Indoor Air, 2014, 24, 190–198 CrossRef CAS PubMed.
- N. Hodas, M. Loh, H. M. Shin, D. Li, D. Bennett, T. E. McKone, O. Jolliet, C. J. Weschler, M. Jantunen, P. Lioy and P. Fantke, Indoor Air, 2015, 26, 836–856 CrossRef PubMed.
- B. Wang and X. Duan, Epidemiology, 2011, 22, S97 CrossRef.
- W. H. Organization, WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide, World Health Organization, 2021 Search PubMed.
- D. f. E. F. R. Affairs, UK and EU Air Quality Limits, 2021 Search PubMed.
- A. Varlamov, Z. Zhou and Y. Chen, Phys. Economy, 2018, 1/2018, 55–68 Search PubMed.
- M.-P. Wan, C.-L. Wu, G.-N. Sze To, T.-C. Chan and C. Y. H. Chao, Atmos. Environ., 2011, 45, 6141–6148 CrossRef CAS.
- C.-M. Liao, S.-C. Chen, J.-W. Chen and H.-M. Liang, Sci. Total Environ., 2006, 358, 72–84 CrossRef CAS PubMed.
- W. M. To and L. L. Yeung, Indoor Built Environ., 2011, 20, 555–563 CrossRef.
- C. L. Wu, C. Y. H. Chao, G. N. Sze-To, M. P. Wan and T. C. Chan, Indoor Built Environ., 2011, 21, 782–796 CrossRef.
- P. Zhao, K.-P. Yu and C.-C. Lin, Int. Arch. Occup. Environ. Health, 2010, 84, 231–237 CrossRef PubMed.
- T. W. Wong, A. H. S. Wong, F. S. C. Lee and H. Qiu, Occup. Environ. Med., 2011, 68, 746–752 CrossRef CAS PubMed.
- C.-H. Pan, C.-C. Chan and K.-Y. Wu, Cancer Epidemiol., Biomarkers Prev., 2008, 17, 3351–3357 CrossRef CAS PubMed.
- A. Embiale, F. Zewge, B. S. Chandravanshi and E. Sahle-Demessie, Indoor Built Environ., 2019, 28, 1140–1154 CrossRef CAS.
- J. D. McDonald, B. Zielinska, E. M. Fujita, J. C. Sagebiel, J. C. Chow and J. G. Watson, J. Air Waste Manage. Assoc., 2012, 53, 185–194 CrossRef PubMed.
- M. A. Torkmahalleh, I. Goldasteh, Y. Zhao, N. M. Udochu, A. Rossner, P. K. Hopke and A. R. Ferro, Indoor Air, 2012, 22, 483–491 CrossRef CAS PubMed.
- R. Khalid and C. Foulds, The Social Dimensions of Moving Away from Gas Cookers and Hobs: Challenges and Opportunities in Transition to Low-Carbon Cooking, The Department for Business, Energy & Industrial Strategy (BEIS), 2020 Search PubMed.
- T. Hussein, T. Glytsos, J. Ondráček, P. Dohányosová, V. Ždímal, K. Hämeri, M. Lazaridis, J. Smolík and M. Kulmala, Atmos. Environ., 2006, 40, 4285–4307 CrossRef CAS.
- K. Kang, H. Kim, D. D. Kim, Y. G. Lee and T. Kim, Sci. Total Environ., 2019, 668, 56–66 CrossRef CAS PubMed.
- J. Xu and Z. Huang, J. Xi’an Univ. Posts Telecommun., 2014, 19(5), 101–105 Search PubMed.
- J. Qian, A. R. Ferro and K. R. Fowler, J. Air Waste Manage. Assoc., 2012, 58, 502–516 CrossRef PubMed.
- V. Y. Seaman, D. H. Bennett and T. M. Cahill, Atmos. Environ., 2009, 43, 6199–6204 CrossRef CAS.
- B. C. Singer, R. Z. Pass, W. W. Delp, D. M. Lorenzetti and R. L. Maddalena, Build Environ., 2017, 122, 215–229 CrossRef.
- M. M. Lunden, W. W. Delp and B. C. Singer, Indoor Air, 2015, 25, 45–58 CrossRef CAS PubMed.
- R. B. Mosley, D. J. Greenwell, L. E. Sparks, Z. Guo, W. G. Tucker, R. Fortmann and C. Whitfield, Aerosol Sci. Technol., 2001, 34, 127–136 CrossRef CAS.
- T. Thatcher and D. Layton, Atmos. Environ., 1995, 29, 1487–1497 CrossRef CAS.
- T. L. Thatcher, A. C. K. Lai, R. Moreno-Jackson, R. G. Sextro and W. W. Nazaroff, Atmos. Environ., 2002, 36, 1811–1819 CrossRef CAS.
- D. Rim, L. Wallace, S. Nabinger and A. Persily, Sci. Total Environ., 2012, 432, 350–356 CrossRef CAS PubMed.
- L. Tseng and C. Chen, Ann. Occup. Hyg., 2013, 57, 920–933 CAS.
- H. Ji, Y. Fang, T. Song and C. Cao, Environ. Pollut. Control, 2014, 36(9) Search PubMed.
- M. Mohammadyan, Iran. J. Energy Environ., 2012, 3(3), 246–254 Search PubMed.
- L. Wallace, J. Air Waste Manage. Assoc., 1996, 46, 98–126 CrossRef CAS PubMed.
- L. Rojas-Bracho, H. H. Suh, P. J. Catalano and P. Koutrakis, J. Air Waste Manage. Assoc., 2012, 54, 207–217 CrossRef PubMed.
- G. Associates, Exposure Assessment and Risk Characterisation to Inform Recommendations for Updating Ambient Air Quality Standards for PM2.5, PM10, O3, NO2, SO2 Golden Associates, Canberra, 2013 Search PubMed.
- WHO, WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide, World Health Organization, 2015 Search PubMed.
- S. Medina, A. Le Tertre and M. Saklad, Air Qual., Atmos. Health, 2009, 2, 185–198 CrossRef PubMed.
- S. R. Magari, J. Schwartz, P. L. Williams, R. Hauser, T. J. Smith and D. C. Christiani, Epidemiology, 2002, 13, 305–310 CrossRef PubMed.
- K. Kim, E.-Y. Park, K.-H. Lee, J.-D. Park, Y.-D. Kim and Y.-C. Hong, Environ. Health Prev. Med., 2008, 14, 60–66 CrossRef PubMed.
- T. Z. Maung, J. E. Bishop, E. Holt, A. M. Turner and C. Pfrang, Int. J. Environ. Res. Public Health, 2022, 19, 8752 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Further study details (S1), definitions of studied cooking methods (S2), flow 2 accuracy assessment (S3). See DOI: https://doi.org/10.1039/d2ea00171c |
| This journal is © The Royal Society of Chemistry 2023 |