Materials for thermally activated delayed fluorescence and/or triplet fusion upconversion
Malika
Jeffries-EL
 *a,
Nobuhiro
Yanai
*a,
Nobuhiro
Yanai
 *b and
Eli
Zysman-Colman
*b and
Eli
Zysman-Colman
 *c
*c
aDepartment of Chemistry and Divison of Materials Science and Engineering, Boston University, USA. E-mail: malikaj@bu.edu; Web: http://www.sites.bu.edu/EL Web: http://www.twitter.com/Chem_Diva
bDepartment of Applied Chemistry, Graduate School of Engineering, Kyushu University, Japan. E-mail: yanai@mail.cstm.kyushu-u.ac.jp; Web: https://sites.google.com/view/nobuhiroyanai/ Web: http://www.twitter.com/NobuhiroYanai
cOrganic Semiconductor Centre, EaStCHEM School of Chemistry, University of St Andrews, UK. E-mail: eli.zysman-colman@st-andrews.ac.uk; Web: http://www.zysman-colman.com/home Web: http://www.twitter.com/chemguy_eli
 Malika Jeffries-EL |
 Nobuhiro Yanai |
 Eli Zysman-Colman |
Two exciton harvesting mechanisms have shown great promise. Both result in delayed fluorescence but the photophysical mechanisms are divergent.
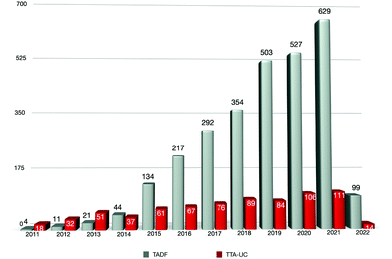
Thermally activated delayed fluorescence (TADF) operates by thermal up-conversion of triplet excitons into singlets by reverse intersystem crossing (RISC), which is possible in materials where the singlet–triplet energy gap, ΔEST, is very small. TADF, originally referred to as E-type fluorescence, was observed by Parker and Hatchard in the organic small molecule eosin in 1961.1 This photophysical phenomenon remained relatively poorly explored until Adachi and his team reported the first example of high-efficiency OLEDs using organic TADF emitters in 2012;2 this is because electroluminescence devices using TADF emitters can attain 100% IQE. Indeed, in the ten years since this seminal discovery, there has been an explosion of interest in the design of materials of this class, particularly as emitters in OLEDs, but also in photocatalysis, sensing and imaging applications. (Fig. 1).
Triplet–triplet annihilation-based photon upconversion (TTA-UC) (aka triplet fusion) materials, by contrast, produce singlet states in a bimolecular fashion when two triplet excitons interact to form a singlet exciton with less than twice the energy of the triplet and a molecule in the ground state. First termed as P-type fluorescence by Parker and Hatchard as it was observed in pyrene, and sensitized upconverted fluorescence was observed in phenanthrene/naphthalene or proflavin hydrochloride/anthracene solutions.3 As with the history of TADF materials, research into TTA-UC materials remained sluggish until the early 2000s when TTA-UC by non-coherent, low-intensity light such as sunlight was first demonstrated.4,5 The ability of TTA-UC materials to convert low-energy photons into higher-energy photons has been exploited in a number of applications beyond OLEDs including photovoltaics, photocatalysis, and optogenetics.
Because of the recent remarkable progress in research related to these exciton harvesting mechanisms, we have organized a themed issue focusing on TADF and TTA-UC materials in Journal of Materials Chemistry C, which covers a wide range of topics from the development of new chromophores to the understanding of the mechanisms and their applications. Since TADF and TTA-UC are closely related topics albeit with different photophysical mechanisms, it is advantageous to combine them in one themed issue. For example, it has been recently reported that TADF materials show excellent performance as triplet sensitizers in TTA-UC because of the small energy loss in the intersystem crossing from the excited singlet state to the triplet state.
Though much chemistry has been recently explored in the development of TADF materials, both organic and metal-based, and the understanding of the underpinning photophysics has greatly improved, evidenced by the more than thirty contributions to this themed issue, much remains to be addressed. For instance, the lifetime and performance of electroluminescence devices are correlated to the RISC rate; compounds that show very fast RISC remain both elusive and highly desired. In this issue, a large range of molecular design strategies, measurement techniques and applications are disclosed, complemented by insight provided by theoretical modeling.
Although numerous TTA-UC materials have been developed in the past 20 years, there are still many issues to be solved for practical applications. In particular, the efficiency of visible-to-ultraviolet TTA-UC and near-infrared-to-visible TTA-UC is still not sufficiently high, requiring the development of better sensitizers and emitters. In this themed issue, the latest TTA-UC materials with excellent performance and a detailed understanding of their exciton dynamics are reported. In addition, the quenching of the excited triplet by oxygen molecules in air is a major problem in the application of TTA-UC in various fields. To solve this problem, rational material designs have been developed in recent years, and excellent examples are presented in this themed issue.
We hope that this themed issue will spark the interest of a wide range of researchers in luminophore design, spectroscopy, theoretical modeling, and device engineering of TADF and TTA-UC materials. We also hope that it will stimulate greater interaction between the TADF and TTA-UC fields and lead to an excellent fusion of research.
References
- C. A. Parker and C. G. Hatchard, Triplet-singlet emission in fluid solutions. Phosphorescence of eosin, Trans. Faraday Soc., 1961, 57, 1894–1904 RSC.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- C. A. Parker and C. G. Hatchard, Sensitised Anti-Stokes Delayed Fluorescence, Proc. R. Soc. London, Ser. A, 1962, 276, 386–387 Search PubMed.
- D. V. Kozlov and F. N. Castellano, Anti-Stokes delayed fluorescence from metal–organic bichromophores, Chem. Commun., 2004, 2860–2861 RSC.
- S. Baluschev, T. Miteva, V. Yakutkin, G. Nelles, A. Yasuda and G. Wegner, Up-Conversion Fluorescence: Noncoherent Excitation by Sunlight, Phys. Rev. Lett., 2006, 97, 143903 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |