Open Access Article
Open Access ArticleA facile and scalable synthetic method for covalent organic nanosheets: ultrasonic polycondensation and photocatalytic degradation of organic pollutants†
Shi-Xian
Gan
,
Chao
Jia
,
Qiao-Yan
Qi
and
Xin
Zhao
 *
*
Key Laboratory of Synthetic and Self-Assembly Chemistry for Organic Functional Molecules, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: xzhao@sioc.ac.cn
First published on 16th December 2021
Abstract
Covalent organic framework nanosheets (COF NSs or CONs), as compared to their bulk counterparts two-dimensional (2D) covalent organic frameworks (COFs), exhibit superior performance in many aspects due to their fully accessible active sites benefiting from their ultrathin porous 2D structures. The development of a scalable synthetic methodology for CONs is crucial to further exploration of their unique properties and practical applications. Herein, we report an efficient strategy to fabricate ultrathin CONs through direct polycondensation of monomers under ultrasonic treatment and mild conditions. This method is facile and scalable, which is demonstrated by gram-scale synthesis of two ultrathin 2D CONs in several hours. Moreover, the as-prepared ultrathin CONs show excellent heterogeneous photocatalytic performance for the degradation of organic pollutants (dyes as representatives), remarkably superior to the bulk COFs prepared from the corresponding monomers under solvothermal conditions. This research provides a new roadmap for the scalable and facile synthesis of ultrathin CONs, which is of paramount importance for fully exploring the tremendous potential of this emerging type of 2D material.
Introduction
Covalent organic frameworks (COFs),1,2 as a burgeoning class of crystalline organic polymers with permanent porosity, have garnered wide attention over the past decade due to their versatile applications in many fields ranging from catalysis,3–6 sensing,7–11 gas storage,12–16 separation,17–20 drug delivery,21–24 nanomedicine,25,26 to energy storage.27–31 These applications have been enabled by the unique structural features of COFs such as ordered internal structures, well-defined nanochannels, large specific surface areas and conjugated skeletons, as well as their predesignable, tailorable, and functionalizable skeletons. COFs are usually prepared via solvothermal synthesis, which gives rise to insoluble polycrystalline powders (bulk COFs). While some applications, for example adsorption, are well implemented with bulk COF materials through the encapsulation of guest molecules in their extended channels, bulk COFs cannot fully exert their functions in many other application scenarios due to poor processibility and inaccessible active sites deeply buried inside the channels. In this context, COF nanosheets, also known as covalent organic nanosheets (CONs),32 have been increasingly developing as a new type of two-dimensional (2D) material to overcome the shortcomings. Compared with bulk 2D COF materials, 2D CONs are few-layer or even single-layer materials, and thus have an extremely large aspect ratio, ultrathin thickness, and fully accessible active sites. These advantages endow them with great application potential in many aspects, such as separation,33,34 catalysis,35–37 energy storage,38,39 drug delivery40 and chemosensing.41Due to the unparalleled advantages of ultrathin CONs over bulk COFs in many application scenarios, a variety of synthetic methods have been developed for them, which can be divided into two types: top-down and bottom-up.32 The former one is based on the exfoliation of layered bulk 2D COF precursors, including sonication exfoliation,42,43 chemical exfoliation,44–47 self-exfoliation,48–50 and mechanical exfoliation.51,52 Among them, sonication exfoliation in the liquid phase is a widely used approach to delaminate layered materials. However, while it is a general method to fabricate 2D CONs, this procedure suffers from the difficulty of defining the size, thickness and shape of nanosheets. Furthermore, an inescapable problem of sonication exfoliation is the quite low yield of ultrathin 2D CONs, which becomes a bottleneck to the practical application of CONs. On the other hand, COF nanosheets can also be fabricated through a bottom-up strategy, for which condensation reactions of monomers usually occur at interfaces, including gas/liquid interface,53,54 liquid/liquid interface,55 and solid surface,56–58 or are assisted by an external modulator.35 To achieve ultrathin 2D CONs via a “bottom-up” strategy, a low concentration of organic monomers is usually required. As a result, they are typically synthesized in very small amounts, which also significantly limits the large-scale applications of 2D CONs. To break the bottleneck problem, a facile, efficient, and scalable method is highly desired. Herein, we report a novel approach to fabricate ultrathin 2D CONs on a gram-scale in a short time. This method is direct ultrasound-mediated polycondensation of monomers under mild conditions, without the use of any matrices, templates or interfaces. The photocatalytic activity of the as-prepared CONs was investigated, which revealed their excellent performance for the degradation of organic dyes.
Results and discussion
To demonstrate this novel synthetic method, two known COF structures are selected as the research objects (Scheme 1).59 The synthetic procedure is facile and was conducted by ultrasonically treating (100 W and 40 KHz) a mixture of the monomers (gram-scale) in organic solvents in a beaker for several hours in an ice water bath under ambient pressure (see the ESI† for details). The CONs were obtained as ultrathin nanosheets, as revealed by morphological characterization. The CON prepared from the condensation of 1,3,5-benzenetricarbaldehyde (BTCA) and 1,3,5-tris(4-aminophenyl)-benzene (TAPB) was named P-CON, while that from BTCA and 2,4,6-tris(4-aminophenyl)-1,3,5-triazine (TAPT) was termed T-CON. For comparison, their corresponding bulk 2D COFs, P-COF and T-COF, were synthesized as microcrystalline powders under solvothermal conditions by heating BTCA with TAPB or TAPT, respectively, in organic solvents at 120 °C for 7 days in sealed tubes.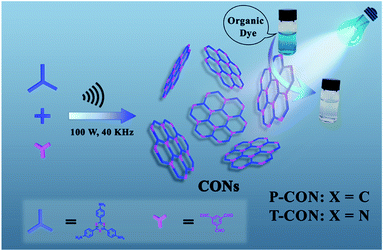 | ||
| Scheme 1 Illustration for the synthesis of the ultrathin 2D CONs and their application as high-performance photocatalysts for dye degradation. | ||
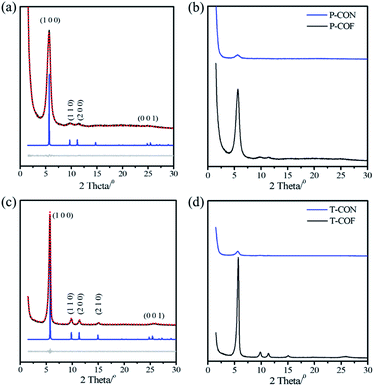
Both the as-prepared 2D CONs (P-CON and T-CON) and the bulk 2D COFs (P-COF and T-COF) were fully characterized by various techniques. As revealed by Fourier-transform infrared (FT-IR) spectroscopy (Fig. S1 and S2†), the CONs have the same chemical compositions as those of the corresponding bulk COFs, which was evidenced by the almost same spectra recorded for the CONs and the COFs. In addition, the crystalline features of the COFs and CONs were indicated by powder X-ray diffraction (PXRD). For P-COF, an intense peak corresponding to the (100) facet appears at 5.66°, along with three weak peaks at 9.67°, 11.17°, and 24.85° attributed to (110), (200), and (001) facets, respectively (Fig. 1a). As to P-CON, the intensity of diffraction peaks was much lower, and only the (100) peak was observed at the same position of the (100) peak of P-COF (Fig. 1b). This result suggests that P-CON holds the same framework structure as that of P-COF, but exists as ultrathin 2D sheets. Owing to the few-layer structures, the interlayer stacking in the CONs is much weaker than that in the bulk COFs, resulting in weaker crystal plane diffraction. On the basis of the PXRD data of P-COF, the unit cell parameters of P-COF were generated through Pawley refinement to be a = b = 18.55 Å, c = 3.60 Å, α = β = 90° and γ = 120°, with Rwp = 4.06% and Rp = 2.89% (Fig. S3†). In the case of T-CON and T-COF, a similar phenomenon was observed (Fig. 1c and d). And the unit cell parameters were calculated to be a = b = 18.32 Å, c = 3.61 Å, α = β = 90° and γ = 120°, with Rwp = 5.01%, Rp = 3.55%, based on the experimental PXRD data of T-COF (Fig. S4†).
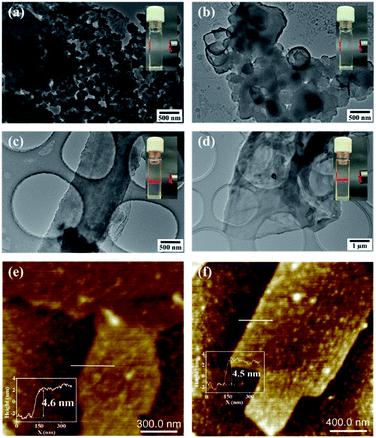
The morphology of the as-prepared CONs and bulk COFs was investigated with transmission electron microscopy (TEM) and atomic force microscopy (AFM). Different from the aggregation morphology of the bulk COFs (Fig. 2a and b), the TEM images of the CONs show a film-like morphology with large 2D dimensions, consistent with the expected structure of nanosheets (Fig. 2c and d). Furthermore, the suspensions of P-CON and T-CON in dimethyl sulfoxide exhibit a typical Tyndall effect (the insets in Fig. 2c and d), while their corresponding bulk COFs do not (the insets in Fig. 2a and b), indicating a colloidal feature of the ultrathin 2D CON nanosheets. Impressively, the CONs exhibit a homogeneous distribution in dimethyl sulfoxide suspension without agglomeration even after two months (Fig. S5†), which is attributed to the weak interlayer stacking between the nanosheets. The thinnest thicknesses of the CONs were measured with AFM to be 4.6 nm for P-CON and 4.5 nm for T-CON (Fig. 2e and f), corresponding to about 13 stacked monolayers, while the maximum thicknesses were ca. 20 nm for P-CON and 19.6 nm for T-CON in a randomly selected micron range (Fig. S6†). By contrast, the bulk COFs prepared by solvothermal condensation exhibit a particle-like morphology with much larger heights (228.5 nm for P-COF and 565.2 nm for T-COF for the selected particles) (Fig. S7†). The above results clearly demonstrate the high efficiency of the ultrasonic synthesis of ultrathin CONs.
The architectural rigidity, Brunauer–Emmett–Teller (BET) surface areas, and permanent porosity of both the CONs and COFs were examined by N2 adsorption–desorption measurements at 77 K. For P-CON, its BET surface area was estimated from the absorption data in the range of P/P0 from 0.003 to 0.05, which afforded a value of 547 m2 g−1. The value is much smaller than that of bulk P-COF (1062 m2 g−1). Such a result is similar to those reported for CONs prepared by the exfoliation of bulk COFs.45,60,61 Similarly, T-CON and T-COF also exhibit the same trend, with BET surface areas of 972 m2 g−1 for T-CONvs. 1323 m2 g−1 for T-COF (Fig. S8–S13†). Meanwhile, a smaller total pore volume of P-CON (0.47 cm3 g−1 at P/P0 = 0.99) than that of P-COF (0.69 cm3 g−1) was found, and a similar phenomenon was also observed for T-CON (0.49 cm3 g−1) and T-COF (0.65 cm3 g−1). Moreover, similar to previous examples,45 the pore size distributions of CONs are consistent with that of COFs (17.5 Å for P-CONvs. 16.7 Å for P-COF, and 17.8 Å for T-CONvs. 17.3 Å for T-COF) (Fig. S14–S17†), agreeing with their theoretical pore sizes (Fig. S18†). This result indicates that the as-prepared ultrathin 2D CONs have uniform microporous frameworks very similar to the layers in their corresponding bulk 2D COFs.
It is well known that ultrathin 2D materials have been revealed to exhibit great potential in heterogeneous photocatalysis associated with their fully accessible active sites and their large surface areas.62 In addition to the large accessible surfaces of CONs, their porous nature also provides a strong guarantee for the adhesion of reactants, making catalytic reactions more adequate. These advantages make CONs very promising to be used as photocatalysts. Such potential was then examined for the as-prepared CONs. To this end, the photocatalytic performance of the two CONs was firstly evaluated by monitoring the degradation of methylene blue (MB), a typical pollutant in dyeing wastewater, under visible light irradiation. For comparison, the photocatalytic performance of their corresponding bulk 2D COFs was also investigated under analogous experimental conditions. Prior to the photocatalytic experiments, we tested the chemical stability of the COFs and CONs. Both the COFs and CONs exhibit good stability in water, as revealed by the maintenance of their crystalline structures after being immersed in water for one week (Fig. S19 and S20†).
To estimate the optical band gaps of the CONs and the COFs, their solid-state UV-Vis diffuse reflectance spectra (DRS) were recorded (Fig. S21 and S22†). These materials exhibit visible light absorption, with the maximum absorption wavelengths of 415 nm for P-COF, 413 nm for P-CON, 411 nm for T-COF, and 407 nm for T-CON. From Tauc's plots, their optical band gaps were calculated to be 2.53, 2.58, 2.47, and 2.52 eV for P-COF, P-CON, T-COF, and T-CON, respectively (Fig. S23†). The energy bands of the CONs are larger than those of their corresponding COFs, suggesting weakened conjugation of the former. It might be attributed to the lower extent of interlayer π–π interactions in the ultrathin nanosheets.60,61 Compared to ZnO (∼3.45 eV), the most commonly used photocatalyst, the as-prepared porous materials have a low optical band gap and thus are more desirable for charge transfer interactions which may lead to the generation of free radicals.63,64 Moreover, COFs have demonstrated great potential in the field of photocatalysis,65,66 which suggests that CONs should also be a new class of promising photocatalysts.
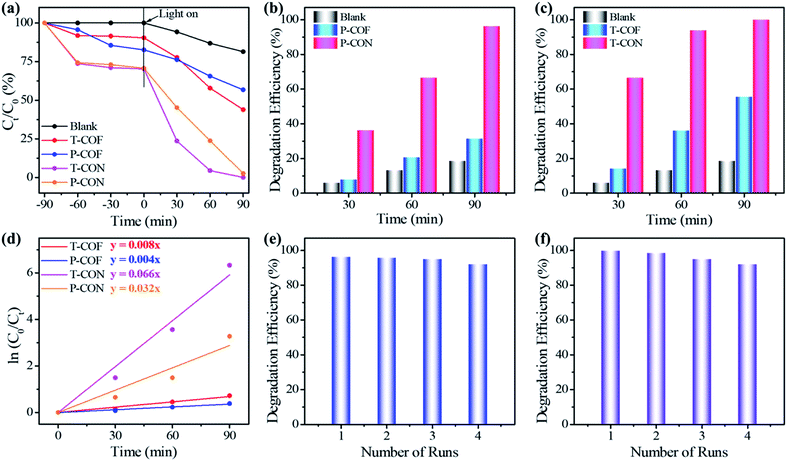
The photo-degradation of MB in water catalyzed by the CONs as well as the bulk COFs was monitored with UV-Vis spectroscopy (Fig. 3 and S24†). The results indicate that the 2D CONs show much higher photocatalytic activity than their corresponding bulk COF materials (Fig. 4a). As illustrated in Fig. 4b, the photo-degradation efficiency of P-CON for MB reaches 96.2% in 90 min with a catalyst dose of 0.5 g L−1, which is more than three times that of P-COF (31.3%). For T-CON, its photo-degradation efficiency for MB is as high as 99.8% in 90 min with the same catalyst dose (Fig. 4c), which further proves that the as-prepared ultrathin 2D CONs have much better photocatalytic performance than the bulk 2D COFs. Moreover, as shown in Fig. 4e and f, after being reused for four cycles, P-CON and T-CON still maintain good photocatalytic activity. The slight decrease in the efficiency after each cycle is attributed to the slight loss of the CONs during sampling in the monitoring reaction and filtering process. PXRD tests after each cycle revealed that the CONs maintained their framework structures during the photocatalytic reactions, indicating their high chemical stability and good recyclability (Fig. S25†). To establish the MB degradation reaction kinetics, the kinetic data of the photocatalytic reaction were fitted using the Langmuir–Hinshelwood kinetics model (ln(C0/Ct) = kt, where C0 and Ct are the concentrations of MB aqueous solution at t = 0 and t minutes of the photocatalytic reaction). As can be seen in Fig. 4d, the model indicates pseudo-first-order reaction kinetics. T-CON shows a high catalytic performance in the degradation of MB with a reaction rate constant of 6.6 × 10−2 min−1, which is 8.25-fold higher than that of T-COF (8.0 × 10−3 min−1). The same situation occurs for P-CON and P-COF, which shows that the reaction rate constant of P-CON (3.2 × 10−2 min−1) is 8-fold larger than that of P-COF (4.0 × 10−3 min−1). This result further confirms that the photocatalytic performance of the ultrathin 2D CONs (P-CON and T-CON) is far superior to that of the bulk 2D COFs (P-COF and T-COF).
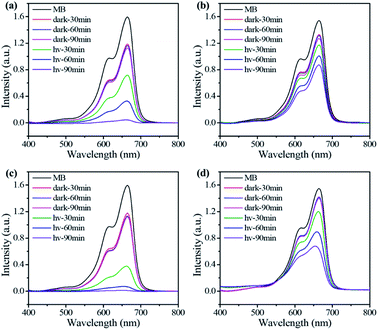 | ||
| Fig. 3 UV-Vis spectra recorded for photocatalytic degradation of MB in water by P-CON (a), P-COF (b), T-CON (c), and T-COF (d) at different times. | ||
To further evaluate the photocatalytic activity of the aforementioned CONs for the removal of organic pollutants, another organic dye, thionine acetate salt (Th), was also selected as the object of photo-degradation. The experimental results indicated that both P-CON and T-CON showed excellent performance to catalyze the photo-degradation of Th, and their efficiencies are also much higher than those of their corresponding bulk COF materials (Fig. S26 and S27†). T-CON showed high catalytic performance with a degradation efficiency of 96.6% in 120 min and a reaction rate constant of 2.8 × 10−2 min−1, much better than T-COF which displayed a degradation efficiency of 65.0% and a reaction rate constant of 8.0 × 10−3 min−1 under the same test conditions (Fig. S28 and S30†). Notably, the degradation efficiency of T-CON for Th was up to 51.2% in 30 min, which was about 3.5 times higher than that of T-COF (14.5%) (Fig. S30†). In the case of P-CON and P-COF, the photocatalytic performance of P-CON (a degradation efficiency of 92.6% in 120 min and a reaction rate constant of 1.6 × 10−2 min−1) was also much higher than that of P-COF (a degradation efficiency of 55.5% in 120 min and a reaction rate constant of 6.0 × 10−3 min−1) (Fig. S28 and S29†). Their photocatalytic activity at the early stage of the degradation reaction was also compared, which indicated that the degradation efficiency of P-CON (22.0%) was 2.27-fold higher than that of P-COF (9.7%) in 30 min (Fig. S29†). Simultaneously, the degradation efficiency of P-CON (89.0%) and T-CON (93.2%) can be well retained after four cycles (Fig. S31 and S32†), with negligible changes of their structures, as revealed by the PXRD study (Fig. S33†). These results again suggest that the ultrathin 2D CONs exhibit superior photocatalytic performance and have a great application prospect in treating environmental pollution.
In order to investigate the photocatalytic mechanism, trapping experiments were carried out. We took the photocatalytic degradation of MB by T-CON as a representative to explore the mechanism of the photocatalysis. It is generally accepted that the superoxide radical (˙O2−), photo-generated hole (h+), and ˙OH are possible reactive species in the photocatalytic degradation of organic pollutants.67,68 To test the role of these reactive species in the photocatalysis, p-benzoquinone (BQ), triethanolamine (TEOA) and isopropylalcohol (IPA) were employed as scavengers for ˙O2−, h+ and ˙OH, respectively. It was found that the photodegradation efficiency of T-CON for MB decreased after adding the three trapping reagents (Fig. S34†), indicating that ˙O2−, h+ and ˙OH were all involved in the degradation process. Moreover, when BQ was added into the reaction system, the degradation efficiency decreased remarkably (with a degradation efficiency of 49.8%), suggesting that ˙O2− plays a crucial role in the degradation of organic pollutants.
To verify the general applicability of this method, BTCA was replaced by 1,3,5-triformylphloroglucinol (Tp) to synthesize β-keto-enamine-based CONs. The CON prepared from the condensation of Tp and TAPB was named Tp-P-CON, while that from Tp and TAPT was termed Tp-T-CON. Their framework structures were confirmed by the experimental PXRD patterns, which showed good agreement with the simulated PXRD patterns of their corresponding bulk 2D COFs (Fig. S35 and S36†). Moreover, thin layers were observed in their TEM images (Fig. S37†), suggesting the formation of nanosheet structures. These experimental results indicated that Tp-P-CON and Tp-T-CON with few layers were synthesized successfully, which demonstrated the general applicability of the method.
Conclusions
In summary, we have developed an efficient, facile, and scalable synthetic method for ultrathin 2D CONs. While in traditional top-down approaches sonication has been used as an external force to assist the delamination of layered 2D COFs to produce CONs, herein we demonstrate that CONs can be directly synthesized on a large scale through ultrasound-mediated polycondensation of monomers. Moreover, the condensation reactions are promoted by the utilization of sonication. The polymerization is completed in just a few hours under mild conditions, by which gram-scale ultrathin CONs could be obtained in one batch. The as-prepared CONs exhibit excellent photocatalytic performance in the degradation of organic pollutants in the aqueous phase, which is much higher than that of their corresponding bulk COF counterparts. The principle of this bottom-up approach is general and thus should be applicable to other COF nanosheets with different structures. With the advantages of facile operation, scalable production, and the scalability of sonication devices, this method offers facile and scalable production of ultrathin CONs, which provides a foundation for the development of practical applications of this emerging class of 2D materials.Data availability
All the data have been included in the ESI.†Author contributions
Conceptualization: X. Zhao and S.-X. Gan; supervision: X. Zhao; synthesis: S.-X. Gan; structural characterization: S.-X. Gan and C. Jia; photocatalytic experiments: S.-X. Gan; structural modeling: Q.-Y. Qi; manuscript writing–revising: X. Zhao and S.-X. Gan; all authors proof-read, provided comments, and approved the final version of this manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We thank the National Natural Science Foundation of China (No. 21632004) and the Science and Technology Commission of Shanghai Municipality (19XD1404900) for financial support.Notes and references
- P. J. Waller, F. Gándara and O. M. Yaghi, Acc. Chem. Res., 2015, 48, 3053–3063 CrossRef CAS PubMed.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Soc. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- S.-Y. Ding, J. Gao, Q. Wang, Y. Zhang, W.-G. Song, C.-Y. Su and W. Wang, J. Am. Chem. Soc., 2011, 133, 19816–19822 CrossRef CAS PubMed.
- J. Hu, H. Mehrabi, Y.-S. Meng, M. Taylor, J.-H. Zhan, Q. Yan, M. Benamara, R. H. Coridan and H. Beyzav, Chem. Sci., 2021, 12, 7930–7936 RSC.
- G.-B. Wang, S. Li, C.-X. Yan, F.-C. Zhu, Q.-Q. Lin, K.-H. Xie, Y. Gen and Y.-B. Dong, J. Mater. Chem. A, 2020, 8, 6957–6983 RSC.
- T. Banerjee, K. Gottschling, G. Savasci, C. Ochsenfeld and B. V. Lotsch, ACS Energy Lett., 2018, 3, 400–409 CrossRef CAS PubMed.
- G. Lin, H. Ding, D. Yuan, B. Wang and C. Wang, J. Am. Chem. Soc., 2016, 138, 3302–3305 CrossRef CAS PubMed.
- Q. Gao, X. Li, G.-H. Ning, K. Leng, B. Tian, C. Liu, W. Tang, H.-S. Xu and K. P. Loh, Chem. Commun., 2018, 54, 2349–2352 RSC.
- L. Ascherl, E. W. Evans, J. Gorman, S. Orsborne, D. Bessinger, T. Bein, R. H. Friend and F. Auras, J. Am. Chem. Soc., 2019, 141, 15693–15699 CrossRef CAS PubMed.
- S.-Y. Ding, M. Dong, Y.-W. Wang, Y.-T. Chen, H.-Z. Wang, C.-Y. Su and W. Wang, J. Am. Chem. Soc., 2016, 138, 3031–3037 CrossRef CAS PubMed.
- D. Yan, Z. Wang, P. Cheng, Y. Chen and Z. Zhang, Angew. Chem., Int. Ed., 2021, 60, 6055–6060 CrossRef CAS PubMed.
- C. J. Doonan, D. J. Tranchemontagne, T. G. Glover, J. R. Hunt and O. M. Yaghi, Nat. Chem., 2010, 2, 235–238 CrossRef CAS PubMed.
- Y. Zeng, R. Zou and Y. Zhao, Adv. Mater., 2016, 28, 2855–2873 CrossRef CAS PubMed.
- Q. Gao, X. Li, G.-H. Ning, H.-S. Xu, C. Liu, B. Tian, W. Tang and K. P. Loh, Chem. Mater., 2018, 30, 1762–1768 CrossRef CAS.
- Y. Ding, Y. Wang, Y. Su, Z. Yang, J. Liu, X. Hua and H. Wei, Chin. Chem. Lett., 2020, 31, 193–196 CrossRef CAS.
- Y. Yang, M. Faheem, L. Wang, Q. Meng, H. Sha, N. Yang, Y. Yuan and G. Zhu, ACS Cent. Sci., 2018, 4, 748–754 CrossRef CAS PubMed.
- H. Yang, L. Yang, H. Wang, Z. Xu, Y. Zhao, Y. Luo, N. Nasir, Y. Song, H. Wu, F. Pan and Z. Jiang, Nat. Commun., 2019, 10, 2101 CrossRef PubMed.
- J. Huang, X. Han, S. Yang, Y. Cao, C. Yuan, Y. Liu, J. Wang and Y. Cui, J. Am. Chem. Soc., 2019, 141, 8996–9003 CrossRef CAS PubMed.
- H. Fan, M. Peng, I. Strauss, A. Mundstock, H. Meng and J. Caro, J. Am. Chem. Soc., 2020, 142, 6872–6877 CrossRef CAS PubMed.
- Z. Jia, Z. Yan, J. Zhang, Y. Zou, Y. Qi, X. Li, Y. LI, X. Guo, C. Yang and L. Ma, ACS Appl. Mater. Interfaces, 2021, 13, 1127–1134 CrossRef CAS PubMed.
- Q. Fang, J. Wang, S. Gu, R. B. Kaspar, Z. Zhuang, J. Zheng, H. Guo, S. Qiu and Y. Yan, J. Am. Chem. Soc., 2015, 137, 8352–8355 CrossRef CAS PubMed.
- V. S. Vyas, M. Vishwakarma, I. Moudrakovski, F. Haase, G. Savasci, C. Ochsenfeld, J. P. Spatz and B. V. Lotsch, Adv. Mater., 2016, 28, 8749–8754 CrossRef CAS PubMed.
- L. Bai, S. Z. F. Phua, W. Q. Lim, A. Jana, Z. Luo, H. P. Tham, L. Zhao, Q. Gao and Y. Zhao, Chem. Commun., 2016, 52, 4128–4131 RSC.
- G. Zhang, X. Li, Q. Liao, Y. Liu, K. Xi, W. Huang and X. Jia, Nat. Commun., 2018, 9, 2785 CrossRef PubMed.
- Q. Guan, L.-L. Zhou, Y.-A. Li, W.-Y. Li, S. Wang, C. Song and Y.-B. Dong, ACS Nano, 2019, 13, 13304–13316 CrossRef CAS PubMed.
- Q. Guan, L.-L. Zhou, F.-H. Lv, W.-Y. Li, Y.-A. Li and Y.-B. Dong, Angew. Chem., Int. Ed., 2020, 59, 18042–18047 CrossRef CAS PubMed.
- X. Zhao, P. Pachfule and A. Thomas, Chem. Soc. Rev., 2021, 50, 6871–6913 RSC.
- J. Li, X. Jing, Q. Li, S. Li, X. Gao, X. Feng and B. Wang, Chem. Soc. Rev., 2020, 49, 3565–3604 RSC.
- F. Xu, H. Xu, X. Chen, D. Wu, Y. Wu, H. Liu, C. Gu, R. Fu and D. Jiang, Angew. Chem., Int. Ed., 2015, 54, 6814–6818 CrossRef CAS PubMed.
- M. Li, J. Liu, Y. Li, G. Xing, X. Yu, C. Peng and L. Chen, CCS Chem., 2020, 2, 696–706 Search PubMed.
- A. K. M, M. Ghosh, V. Vijayakumar, A. Halder, M. Nurhuda, S. Kumar, M. Addicoat, S. Kurungot and R. Banerjee, Chem. Sci., 2019, 10, 8889–8894 RSC.
- D. Rodríguez-San-Miguel, C. Montoro and F. Zamora, Chem. Soc. Rev., 2020, 49, 2291–2302 RSC.
- Y. Ying, M. Tong, S. Ning, S. K. Ravi, S. B. Peh, S. C. Tan, S. J. Pennycook and D. Zhao, J. Am. Chem. Soc., 2020, 142, 4472–4480 CrossRef CAS PubMed.
- J. Yao, C. Liu, X. Liu, J. Guo, S. Zhang, J. Zheng and S. Li, J. Membr. Sci., 2020, 601, 117864 CrossRef.
- W. Liu, X. Li, C. Wang, H. Pan, W. Liu, K. Wang, Q. Zeng, R. Wang and J. Jiang, J. Am. Chem. Soc., 2019, 141, 17431–17440 CrossRef CAS PubMed.
- M. Luo, Q. Yang, W. Yang, J. Wang, F. He, K. Liu, H. Cao and H. Yan, Small, 2020, 16, 2001100–2001108 CrossRef CAS PubMed.
- X. Wang, Z. Fu, L. Zheng, C. Zhao, X. Wang, S. Y. Chong, F. McBride, R. Raval, M. Bilton, L. Liu, X. Wu, L. Chen, R. S. Sprick and A. I. Cooper, Chem. Mater., 2020, 32, 9107–9114 CrossRef CAS.
- J. Sun, A. Klechikov, C. Moise, M. Prodana, M. Enachescu and A. V. Talyzin, Angew. Chem., Int. Ed., 2018, 57, 1034–1038 CrossRef CAS PubMed.
- S. Wang, Q. Wang, P. Shao, Y. Han, X. Gao, L. Ma, S. Yuan, X. Ma, J. Zhou, X. Feng and B. Wang, J. Am. Chem. Soc., 2017, 139, 4258–4261 CrossRef CAS PubMed.
- S. Mitra, H. S. Sasmal, T. Kundu, S. Kandambeth, K. Illath, D. D. Díaz and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 4513–4520 CrossRef CAS PubMed.
- Y. Peng, T. Huang, Y. Zhu, B. Chen, L. Wang, Z. Lai, Z. Zhang, M. Zhao, C. Tan, N. Yang, F. Shao, Y. Han and H. Zhang, J. Am. Chem. Soc., 2017, 139, 8698–8704 CrossRef CAS PubMed.
- I. Berlanga, M. L. Ruiz-González, J. M. González-Calbet, J. L. G. Fierro, R. Mas-Ballesté and F. Zamora, Small, 2011, 7, 1207–1211 CrossRef CAS PubMed.
- G. Das, B. P. Biswal, S. Kandambeth, V. Venkatesh, G. Kaur, M. Addicoat, T. Heine, S. Vermab and R. Banerjee, Chem. Sci., 2015, 6, 3931–3939 RSC.
- X. Chen, Y. Li, L. Wang, Y. Xu, A. Nie, Q. Li, F. Wu, W. Sun, X. Zhang, R. Vajtai, P. M. Ajayan, L. Chen and Y. Wang, Adv. Mater., 2019, 31, 1901640 CrossRef PubMed.
- Y. Yusran, H. Li, X. Guan, D. Li, L. Tang, M. Xue, Z. Zhuang, Y. Yan, V. Valtchev, S. Qiu and Q. Fang, Adv. Mater., 2020, 32, 1907289 CrossRef CAS PubMed.
- M. A. Khayum, S. Kandambeth, S. Mitra, S. B. Nair, A. Das, S. S. Nagane, R. Mukherjee and R. Banerjee, Angew. Chem., Int. Ed., 2016, 55, 15604–15608 CrossRef CAS PubMed.
- D. W. Burke, C. Sun, I. Castano, N. C. Flanders, A. M. Evans, E. Vitaku, D. C. McLeod, R. H. Lambeth, L. X. Chen, N. C. Gianneschi and W. R. Dichtel, Angew. Chem., Int. Ed., 2020, 59, 5165–5171 CrossRef CAS PubMed.
- S. Mitra, S. Kandambeth, B. P. Biswal, A. Khayum, C. K. Choudhury, M. Mehta, G. Kaur, S. Banerjee, A. Prabhune, S. Verma, S. Roy, U. K. Kharul and R. Banerjee, J. Am. Chem. Soc., 2016, 138, 2823–2828 CrossRef CAS PubMed.
- S. Haldar, K. Roy, S. Nandi, D. Chakraborty, D. Puthusseri, Y. Gawli, S. Ogale and R. Vaidhyanathan, Adv. Energy Mater., 2018, 8, 1702170 CrossRef.
- D. N. Bunck and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 14952–14955 CrossRef CAS PubMed.
- S. Chandra, S. Kandambeth, B. P. Biswal, B. Lukose, S. M. Kunjir, M. Chaudhary, R. Babarao, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 17853–17861 CrossRef CAS PubMed.
- H. Liu, Q. Li, Y. Zhu, M. Zhang, R. Liu, X. Li, X. Kang, Z. Li and S. Qiao, J. Mater. Chem. C, 2018, 6, 722–725 RSC.
- W. Dai, F. Shao, J. Szczerbinski, R. McCaffrey, R. Zenobi, Y. Jin, A. D. Schlüter and W. Zhang, Angew. Chem., Int. Ed., 2016, 55, 213–217 CrossRef CAS PubMed.
- K. Liu, H. Qi, R. Dong, R. Shivhare, M. Addicoat, T. Zhang, H. Sahabudeen, T. Heine, S. Mannsfeld, U. Kaiser, Z. Zheng and X. Feng, Nat. Chem., 2019, 11, 994–1000 CrossRef CAS PubMed.
- D. Zhou, X. Tang, H. Wu, L. Tian and M. Li, Angew. Chem., Int. Ed., 2019, 58, 1376–1381 CrossRef CAS PubMed.
- Y.-P. Mo, X.-H. Liu and D. Wang, ACS Nano, 2017, 11, 11694–11700 CrossRef CAS PubMed.
- T. Joshi, C. Chen, H. Li, C. S. Diercks, G. Wang, P. J. Waller, H. Li, J.-L. Bredas, O. M. Yaghi and M. F. Crommie, Adv. Mater., 2019, 31, 1805941 CrossRef PubMed.
- X. Shi, D. Ma, F. Xu, Z. Zhang and Y. Wang, Chem. Sci., 2020, 11, 989–996 RSC.
- J. Dong, Y. Wang, G. Liu, Y. Cheng and D. Zhao, CrystEngComm, 2017, 19, 4899–4904 RSC.
- J. Dong, X. Li, K. Zhang, Y. D. Yuan, Y. Wang, L. Zhai, G. Liu, D. Yuan, J. Jiang and D. Zhao, J. Am. Chem. Soc., 2018, 140, 4035–4046 CrossRef CAS PubMed.
- J. Dong, X. Li, S. B. Peh, Y. D. Yuan, Y. Wang, D. Ji, S. Peng, G. Liu, S. Ying, D. Yuan, J. Jiang, S. Ramakrishna and D. Zhao, Chem. Mater., 2018, 31, 146–160 CrossRef.
- H. Zhang, ACS Nano, 2015, 9, 9451–9469 CrossRef CAS PubMed.
- S. Roy, S. P. Mondal, S. K. Ray and K. Biradha, Angew. Chem., Int. Ed., 2012, 51, 12012–12015 CrossRef CAS PubMed.
- K. Preet, G. Gupta, M. Kotal, S. K. Kansal, D. B. Salunke, H. K. Sharma, S. C. Sahoo, P. V. D. Voort and S. Roy, Cryst. Growth Des., 2019, 19, 2525–2530 CrossRef CAS.
- Y. Qian, D. Li, Y. Han and H.-L. Jiang, J. Am. Chem. Soc., 2020, 142(49), 20763–22077 CrossRef CAS PubMed.
- Y.-N. Gong, W. Zhong, Y. Li, Y. Qiu, L. Zheng, J. Jiang and H.-L. Jiang, J. Am. Chem. Soc., 2020, 142(39), 16723–16731 CrossRef CAS PubMed.
- B. Wang, Z. Xie, Y. Li, Z. Yang and L. Chen, Macromolecules, 2018, 51, 3443–3449 CrossRef CAS.
- Y. Yang, H. Niu, L. Xu, H. Zhang and Y. Cai, Appl. Catal., B, 2020, 269, 118799 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR spectra, PXRD profiles, AFM images, N2 adsorption–desorption isotherms, BET plot, pore size distribution profiles, UV-Vis DRS spectra, UV-Vis spectra and fractional atomic coordinates. See DOI: 10.1039/d1sc05504f |
| This journal is © The Royal Society of Chemistry 2022 |