Open Access Article
Open Access ArticleResearch advances in UV-curable self-healing coatings
Zhen Guo *
*
Industrial Development Promotion Center of Weifang, Sunshine Building, No. 6396, Dongfeng East Street, Weifang, 261061, China. E-mail: 313717234@qq.com
First published on 11th November 2022
Abstract
Self-healing is the ability of a material to recover from physical damage. Because self-healing is crucial to environmental protection and sustainable development, several photochemical approaches, including those involving disulfide group, reversible Diels–Alder reaction, hydrogen bond cleavage, reformation, photo dimerization, and self-filling system, have been used to develop self-healing coating. In this review, the similarities and differences among the approaches to achieving self-healing in synthetic coatings, particularly the approaches related to the preparation, self-healing mechanism, and self-healing efficiency of the coating, are described. Potential future development and application of photochemical approaches to the synthesis of self-healing coating are also discussed.
1. Introduction
Photopolymerization, which uses ultraviolet (UV) light as an energy source to cure photosensitive materials, has many advantages, such as energy efficiency1 and eco friendliness,2 high efficiency,3 speed4 and time-space controllability.5 After decades, with the continuous development of the basic theory and application technology of photopolymerization, this kind of technology has been widely applied in the fields of coating,6 ink,7 and adhesive.8UV-curable coating, which has been widely used as protective and decorative surface coating because of its energy efficiency, eco friendliness, and low cost,9 can be completely cured in as little as a few seconds under the action of UV light. However, the thinness of UV-curable coating makes it vulnerable to damage from external forces, such as bumping and scraping, which can result in micro-cracks forming that are difficult to distinguish with the naked eye.10,11 The gradual accumulation of local damage in UV-curable coating can cause part of the substrate to become exposed, thus causing the coating to lose its protective effect. Inspired by nature, the American military developed the concept of self-healing materials in 198112,13 to reduce material losses caused by the accumulation of micro-cracks and to extend the service life of materials. The American military has applied self-healing materials in fields ranging from biology to materials science, thus opening up new direction for materials research. As the body of research on self-healing expanded, researchers began to explore the integration of UV-curable coatings and self-healing materials.14
Self-healing materials are classified as extrinsic and intrinsic based on their self-healing mechanisms.15 In extrinsic self-healing materials, healing agents (generally liquids) are integrated into the material matrix in the form of microcapsules or micro vascular networks.16 Although extrinsic self-healing materials are the most common, they have some limitations; for example, the self-healing ability of such materials is lost when the supply of healing agents is exhausted. Therefore, researchers have turned to intrinsic strategies to develop coatings that can repeatedly self-heal. Intrinsic self-healing materials involve (1) non covalent bond interactions, including ionic and hydrogen bond formation as well as π–π stacking,17 (2) dynamic covalent bond interactions, such as dynamic trans-esterification, Schiff base and disulfide bond formation, and Diels–Alder (DA) reaction.18,19
Self-healing materials have attracted considerable attention in recent decades owing to their numerous favorable properties, such as their long lifespan and high stability. Herein, advancements in research on intrinsic self-healing coating, specifically those based on disulfide bond exchange, D–A reaction, reverse reaction, hydrogen bond fracture and recombination, photo dimerization reaction, and self-filling coating materials, are briefly summarized. Potential future developments and applications of intrinsic self-healing coating based on photochemical reaction are also discussed.
2 UV-curable self-healing coating
2.1 Disulfide bonds exchange
Disulfide bonds are usually formed by two coupling mercaptan groups, and the energy of such bonds is weaker than that of C–C and C–H bond. The introduction of disulfide bonds into functional material can endow such materials with self-healing properties,20because such bonds are reversible. At certain temperature or in nucleophile system, chain exchange reactions can occur between cross-linked structures containing disulfide bonds, thus initiating self-healing.21 Canadell et al.22 introduced disulfide bonds into molecular chain structure and reported that self-healing can be achieved through disulfide bond exchange. Hideyuki et al.23 prepared new molecular structure with alkyl disulfide as substrate by using a 400 W high-pressure mercury lamp and discovered that disulfide bonds may form between different molecules to create new compound, indicating that disulfide bonds participate not only in heat exchange but also in light exchange.To explore the influence of disulfide bonds content on the self-healing rate of UV-curable coating, Zhao et al.24 designed a series of UV-curable self-healing polyurethane acrylic resins with different disulfide bond contents by introducing polytetramethylene ether glycol, 2,2-dithio diethylene glycol, and isocyanate into the resin molecular structure, as is shown in Fig. 1. The results revealed that the self-healing efficiency of the UV-curable coatings increased significantly with the increase in disulfide bond content. The coating still exhibited favorable self-healing abilities when a UV light source was used instead of high-pressure mercury lamp to eliminate the influence of the thermal effect of the lamp, and the coatings were compatible with commercial acrylic resin, shown in Fig. 2. Li et al.25 synthesized UV-curable water-based polyurethane self-healing coatings with polysulfide prepolymer as the main material through sulfhydryl-ene photopolymerization. Because of the exchange of disulfide bonds in the prepared coating, the coating not only had self-healing properties but also exhibited excellent anticorrosion performance, indicating a new direction for the development of multifunctional anticorrosion coating.
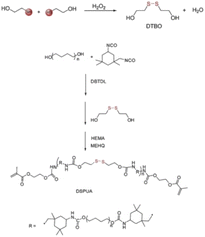 | ||
| Fig. 1 Synthesis of self-healing UV-curable coating containing disulfide bonds. This figure has been adapted from ref. 24 with permission from Elsevier, copyright 2019. | ||
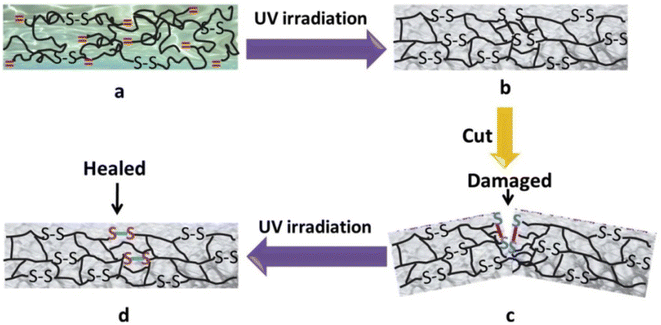 | ||
| Fig. 2 Schematic of preparation and self-healing behavior of UV-curable material based on disulfide bond exchange: (a) before UV irradiation; (b) after UV irradiation; (c) damaged material; (d) self-healed material This figure has been adapted from ref. 24 with permission from Elsevier, copyright 2019. | ||
2.2 D–A reactions
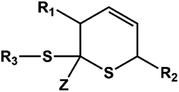
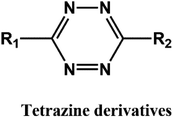
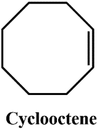
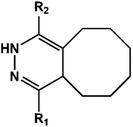
D–A reaction, also known as diene addition, was discovered by German chemists Otto Diels and Kurt Alder in 1928.26 D–A reaction is mainly [4 + 2] Cycloaddition reaction between electron-rich diene, such as cyclopentadiene and furan derivatives, and electron-deficient diene, such as maleimide (MI) derivatives. These reactions produce stable hexatomic ring compound, which has excellent thermal reversibility, mild condition for bond formation and fracture, and few side reactions with no additional catalysts.27 The four most common types of D–A reaction, illustrated in Table 1, occur between the following pairs of compounds (or their derivatives): (1) anthracene and MI;28 (2) furan and MI;29 (3) heteroatom D–A reaction between diene and disulfide ester;30 and (4) tetrazine and cyclooctene with its derivatives.31Self-healing polymers prepared through D–A reaction can realize multiple repairs. The self-healing principle can be summarized as follows.32 When a self-healing coating is damaged by external force, and adequate heat treatment is applied, the film of the damaged coating undergoes D–A reverse reaction, in which the D–A chemical bond is broken. After cooling, another D–A reaction occurs and recombines the chain segments to repair the damaged areas of the coating. Jo et al.33 prepared organic–inorganic hybrid self-healing coating with high hardness by inducing D–A reaction with polysilsesquioxane. They first synthesized polysilsesquioxane with a ladder structure and then introduced diene and dienophile into the side chain to endow the material with self-healing properties. They subsequently introduced acrylic acid or an epoxy group into the polymer network to prepare a series of UV-curable self-healing coatings, shown in Fig. 3. Due to the high crosslinking density significantly improved the mechanical properties of the coating, which had a hardness of up to 6H and an elastic modulus exceeding 9 GPa. The coating also had excellent thermal stability and optical transmission. However, the coating could only fully self-heal when the scratch depth was less than 10 μm. This may be because the thickness of the prepared coating was much lower than that of the coating film, which reduced the interactions among the molecular chains and thus affected the self-healing efficiency of the coating.
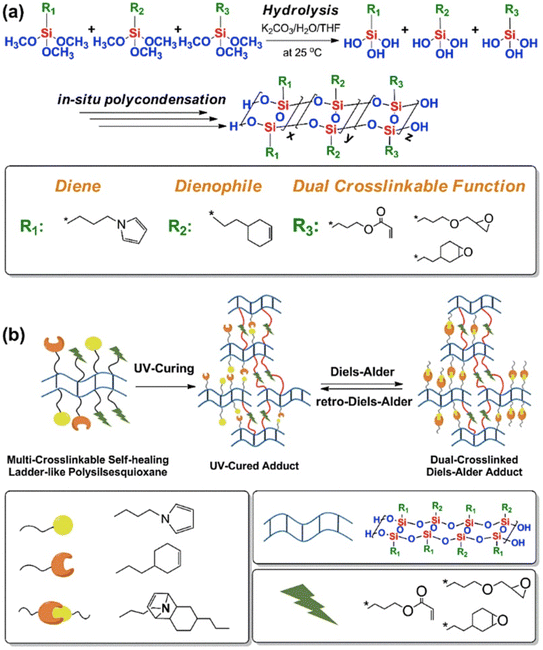 | ||
| Fig. 3 Synthesis and self-healing mechanism of UV-curable polysilsesquioxane-based cross-linked polymers. This figure has been adapted from ref. 33 with permission from Elsevier, copyright 2017. | ||
S. K. Raut34 synthesized polyhedral oligomeric silsesquioxane (POSS) isobutyl maleimide (POSSMI) polymers and induced D–A reaction to endow the polymers with self-healing and ultra-hydrophobic properties. The D–A modified polymers exhibited high hydrophobicity (water contact angle [WCA] > 140°) because of the presence of nano-sized POSS material on the surface. The D–A adduct POSS hybrid elastomer exhibited high thermal stability, surface hardness, and adhesion to metal substrate, illustrated in Fig. 4. The furan maleimide D–A covalent linkages cleaved upon heating at 120 °C, but reformed up on annealing at 60 °C. Consequently, the D–A elastomers exhibited high mechanical and self-healing efficacy.
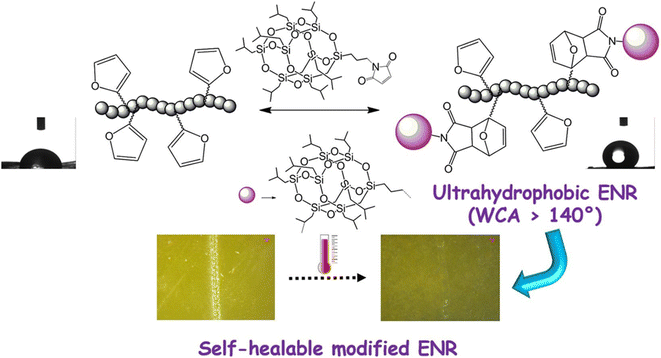 | ||
| Fig. 4 Self-heal ultra-hydrophobic POSS hybrid material. This figure has been adapted from ref. 34 with permission from Elsevier, copyright 2021. | ||
In another study, Ke et al.35 synthesized polyurethane acrylates by using diols containing D–A bond as chain extender and prepared UV-curable coating containing D–A covalent bond through free radical polymerization induced by photoinitiator. The coating exhibited a strong self-healing ability at 120 °C; however, the high temperature required for the coating to self-heal limited its practical application. Chain transfer and chain termination can easily occur during the UV curing process and result in an uneven distribution of D–A covalent bond in the network structure, thus affecting the self-healing ability of the coating.
Wang et al.36 prepared UV-curable, self-healing coating by inducing a click reaction between polyurethane acrylate containing D–A bond and thiol monomer. The free radical inhibitor 2,2,6,6-tetramethylpiperidine-1-oxy (TEMPO) was used in combination with photosensitizer (isopropylthiotonone) and photo-base generator (triazene dicyclododecene tetraphenylborate) to inhibit free radical polymerization during the curing process and to facilitate a polymerization reaction between the double bonds and thiols. The structure of the polyurethane acrylate and diol structures are illustrated in Fig. 5.
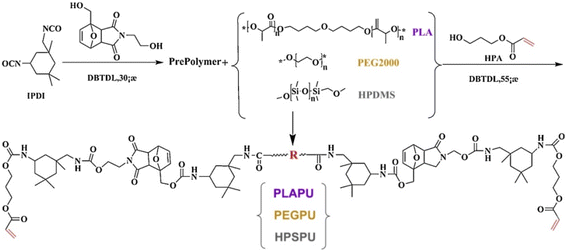 | ||
| Fig. 5 Synthesis of UV-curable polyurethane resins. This figure has been adapted from ref. 36 with permission from Elsevier, copyright 2019. | ||
Compared with free radical polymerization, thiol–ene photo-polymerization is more efficient and produces a more uniform D–A covalent network. This enables the molecular chain to flow more easily during the self-healing process, resulting in rapid self-healing at 90 °C. Thermally induced self-healing is caused by the conversion of thermal energy into internal energy, which breaks D–A bond. Therefore, PU-SH coating has a lower healing temperature and faster healing speed than do polyurethane coating.
2.3 Hydrogen bond
Hydrogen bond is slightly stronger than intermolecular force (e.g., van der Waals forces) and much weaker than covalent and ionic bond. They are less stable than covalent and ionic bond, and their formation is reversible. In addition to the fast response, hydrogen bond is directional, which can enhance the mechanical properties of coating.Hydrogen bond can form when a proton acceptor and donor come within a certain distance of each other. According to their bond energies, hydrogen bond can be divided into three types: strong interaction hydrogen bond, general and weak interaction hydrogen bond, and unconventional hydrogen bond.37,38 They can also be categorized as single, double, triple, quadruple, and multiple hydrogen bonds.
To achieve high self-healing efficiency, researchers often incorporate hydrogen bond into the molecular structural design of self-healing material.39 Han et al.40 prepared various acrylic monomers with imidazole group and subjected them to UV irradiation to synthesize polymers with high conductivity. The hydrogen bond between the imidazole ring and hydroxyl group in the polymer endowed the material produced using the polymer with self-healing property, and the introduction of imidazole ring increased the glass transition temperature of the polymer. Biyani et al.41 developed self-healing nanocomposites based on a telechelic poly(ethylene-co-butene), which was functionalized using tetrahydrogen bonded urea pyrimidine ketone (Upy) and cellulose nanocrystal, shown in Fig. 6. Compared with pure supramolecular polymer, polymer containing tetrahydrogen bonds exhibit more favorable mechanical properties and have faster self-healing rates,42 illustrated in Fig. 7. To explore the effect of hydrogen bond on the self-healing efficiency of coating, Liu et al.43,44 introduced Upy group into polyurethane to produce quadruple hydrogen bonds; the resulting oligomers had favorable self-healing property as well as high hardness, gloss, and adhesion. The other properties of self-healing coating can be adjusted using active diluents. When coating is damaged, increasing the temperature can break the hydrogen bonds in the coating, increasing the fluidity of the polymer chain in the coating. When the temperature cools, the hydrogen bond reform to complete the self-healing process.
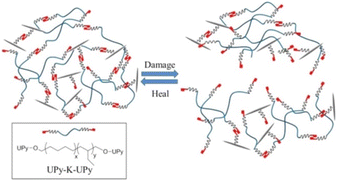 | ||
| Fig. 6 Schematic of formation of supramolecular nanocomposites based on UPy-K-Upy and Upy-decorated CNCs. This figure has been adapted from ref. 41 with permission from American Chemical Society, copyright 2013. | ||
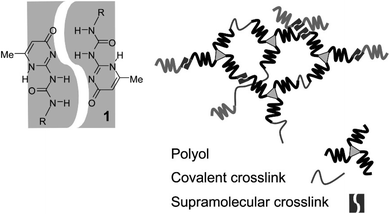 | ||
| Fig. 7 Dimerization of ureidopyrimidinones and schematic of networks with covalent and noncovalent cross-links. This figure has been adapted from ref. 42 with permission from American Chemical Society, copyright 2009. | ||
The fluidity of polymer chain is a crucial factor affecting the self-healing ability of coatings. Most coatings with excellent self-healing property that have been developed have low glass transition temperature and soft coating film. However, self-healing coating with high hardness is required in industrial application and in the field of aerospace engineering. Chen et al.45 were the first to propose the concept of hard/soft micro-phase separation. The soft phase of a material contains reversible hydrogen bond, which can facilitate self-healing, and the hard phase contains rigid ring structure, which can provide strength. Drawing on this concept, Liu et al.46 designed and synthesized UV-curable polyurethane coating with hard cores (consisting of a rigid hexachlorocyclophosphazene group with polyphenyl ring) and multiple soft arm structures (consisting of polyurethane prepared using polycarbonate glycol). The carbamate structure forms hydrogen bonds between the molecular chains in the flexible arm, and the network in the coating can be reconstructed during heating. The coating has excellent self-healing performance and high hardness, indicating that a rigid core can effectively improve the hardness of a coating. Introducing soft/hard phases into the polymer structure of a self-healing coating is an effective approach to improving the coating's hardness.
2.4 Photoreversible interaction
Photosensitive groups, including cinnamoyl,47,48 coumarin,49–51 and anthracene,52 can also endow a material with self-healing property. These photosensitive groups undergo reversible [2 + 2] or [4 + 4] photodimerization and photolysis under UV irradiation, shown in Fig. 8. Researchers often incorporate these groups into the design of self-healing polymers to create cross-linked network structure between the polymer chains, which can facilitate the synthesis of cost-effective and ecofriendly coatings with high self-healing efficiency. When micro-cracks occur, because the chemical bond connecting the dimer is weaker than the other covalent bond, the photosensitive groups are preferentially released. These groups can be separated using long-wavelength UV radiation and re-crosslinked, thus endowing the coatings with self-healing properties.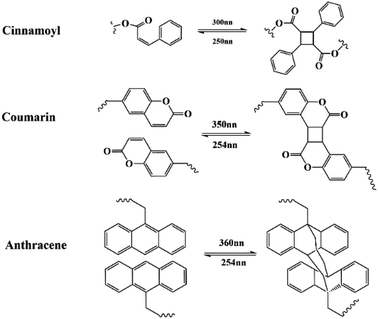 | ||
| Fig. 8 Schematic of photo–reversible reaction of cinnamoyl, coumarin, and anthracene groups at different wavelengths. | ||
Yan et al.53 prepared dynamic photocross-linked polyurethane by introducing cinnamoyl group as a side chain extender during polymerization shown in Fig. 9. Under 300 nm UV light, the strength of the material significantly improved. Because of its reversibility, the dynamic photocrosslinking polyurethane network could rapidly break apart under 250 nm UV irradiation and improve the flow of the thermoplastic polymers. The self-healing of the polymer was influenced by photo-induced crosslinking and de-crosslinking. In the study, a crack was made in the center of a dumbbell-shaped sample with thickness of 1 mm. The scratch depth was half of the thickness. The sample was irradiated with 250 nm UV light for 1 min, then heated at 60 °C for 3 min and irradiated with 300 nm UV light for 24 h. The researchers measured the tensile strength of the sample before and after self-healing and used the ratio of the two measurements as the self-healing efficiency. The self-healing efficiencies immediately after sample preparation and after 72 h of irradiation with 300 nm UV light were 74% and 100%, respectively, shown in Fig. 10. According to the experimental results, this method may also be applicable to other polyurethane systems. Self-healing polyurethane have broad application prospects with respect to developing self-healing coating, electrochemical sensor and device, electronic skin, and other smart materials.
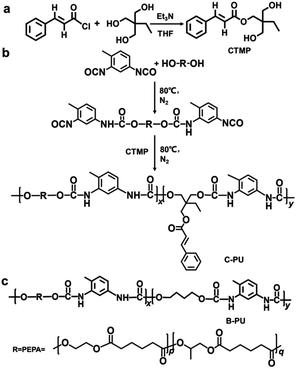 | ||
| Fig. 9 Synthesis of photocrosslinkable polyurethane containing cinnamoyl. This figure has been adapted from ref. 53 with permission from Royal Society of Chemistry, copyright 2019. | ||
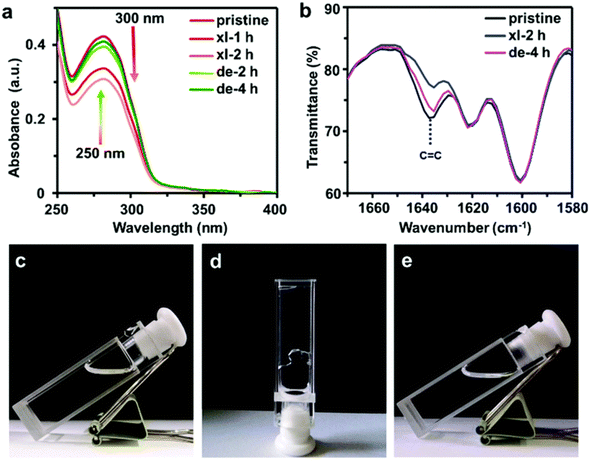 | ||
| Fig. 10 (a) UV absorbance spectra and (b) the Fourier-transform infrared spectroscopy (FT-IR) spectra of C8-PU film after irradiation at 300 nm (xl) and 250 nm (de). Quartz and potassium bromide plates were used for UV-vis and IR measurements, respectively. Photographs of the (c) tetrahydrofuran solution (100 mg mL−1) of C8-PU, (d) gels formed by the solution after 300 nm UV irradiation for 90 h (xl), and (e) reversion of gel to solution after 250 nm UV irradiation for 150 h. This figure has been adapted from ref. 53 with permission from Royal Society of Chemistry, copyright 2019. | ||
2.5 Self-filling
Low surface energy polymers have hydrophobicity and antifouling properties and are widely used in industrial production. When low surface energy additives are introduced into a coating, they can spontaneously migrate to the surface of the coating during surface structure deformation54,55 and thereby achieve self-healing effect. Dikic et al.56 proposed a functional self-healing concept based on self-filling surfaces in which the energy difference between the surface and the inside of the coating is used to drive functional groups upward to fill gaps in the surface shown in Fig. 11. Perfluoroalkyl dangling chains in thermally cross-linked polycaprolactone automatically move to the air-coating interface after damage and thereby automatically repair the surface.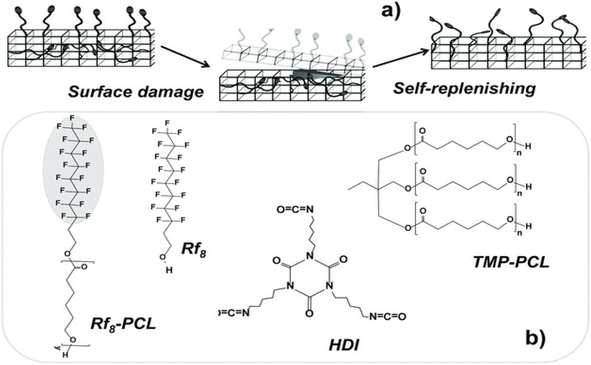 | ||
| Fig. 11 Schematics of (a) polymer self-filling mechanism and (b) chemical structures of components of self-filling coatings. This figure has been adapted from ref. 56 with permission from Wiley, copyright 2012. | ||
Zhang et al.57 prepared UV-curable self-filling polymers by integrating methacrylate-terminated perfluorinitated dangling chains into polyethylene glycol diacrylate-based networks. After multiple consecutive damage, the coating could spontaneously self-heal and restore its surface hydrophobicity. Qiang et al.58 prepared mixed liquids composed of triethyl fluorodecanoate, vinyl-terminated polydimethylsiloxane, and fluoro octavinyl polyhedral oligosilsesquioxane, and used UV curing to synthesize superhydrophobic fabrics with surface microstructures and nanostructures and favorable antifouling property. After 200 cycles of severe wear, the fabric surface could recover their superhydrophobicity by undergoing a short heat treatment and exhibited favorable self-healing property, as is shown in Fig. 12. To contribute to advancements in intelligent textile coating technologies, Chen et al. developed fabric coatings containing pH-responsive polyurethane (pH-PU) and fluoro octavinyl polyhedral oligosilsesquioxane.59 Because the pH-PU protonated and deprotonated with changes in the pH value, the wettability of the fabric coating could be changed between superhydrophobicity and underwater superhydrophobicity through adjustment of the pH, thus achieving intelligent oil/water separation. Additionally, the fluorocarbon chains in the fabric coating could easily migrate to the outer surface of the coating, and the wettability of the coating could be restored using moderate heat. Because the coating exhibited long-lasting, self-cleaning, and oil/water separation abilities, it has potential applications in energy-saving technologies and sustainable development.
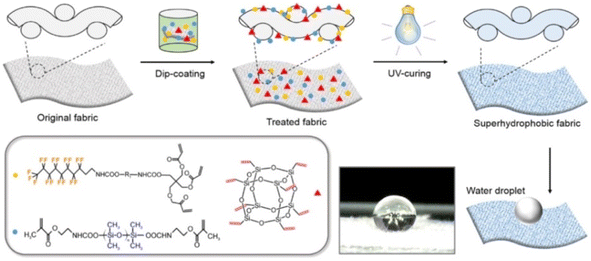 | ||
| Fig. 12 Preparation of UV curable superhydrophobic self-healing textile coating. This figure has been adapted from ref. 58 with permission from Elsevier, copyright 2017. | ||
3. Summary and prospects
To date, UV-curable coatings only account for a small proportion of the entire coating market. However, because of their ecofriendly and energy-saving property and functionality, these coatings can be used to fulfill the strategic needs of the country and are becoming increasingly common each year. Researchers have also been paying an increasing amount of attention to the functionalization of such coatings. Nevertheless, UV-curable self-healing coatings are still being researched, and the main problems that remain to be addressed are as follows: (1) the high cost of the monomers required to synthesize such coatings; (2) the high complexity of the steps involved in the synthesis and the difficulty of the industrial mass production of such coatings; and (3) the relative immaturity of the basic theory of self-healing systems.Self-healing materials can automatically locate and repair cracks within themselves and therefore often have a longer service life and are safer than other materials. The development of self-healing coatings with high hardness and strong adhesion can help expand the application prospects of such coatings in, for example, the manufacturing of automobiles, ships, military technologies, and wood products.
Since the main influence factors of UV curing products include resin, monomer, photo-initiator, UV energy, wavelength, and temperature. So in the actual application process, according to different application fields, comprehensively discuss need to be evaluated, and formulate a comprehensive formula of UV curable self-healing system from the perspective of economy, self-healing effect and customer satisfaction.
Conflicts of interest
There are no conflicts to declare.References
- J. C. Fua, L. Wang, H. J. Yu, F. Haqa, W. L. Shi, B. Wu and L. B. Wang, Research progress of UV-curable polyurethane acrylate-based hardening coatings, Prog. Org. Coat., 2019, 131, 82–99, DOI:10.1016/j.porgcoat.01.061.
- Y. Wang, F. G. Liu and X. X. Xue, Preparation and properties of UV-cured epoxy acrylate/glycidyl POSS coatings, J. Coat. Technol. Res., 2017, 14, 665–672, DOI:10.1007/s11998-016-9886-1.
- J. Fu, H. Yu, L. Wang, C. Zhang and M. Jin, Preparation and properties of UV-curable polyurethane acrylate/SiO2 composite hard coatings, Prog. Org. Coat., 2021, 153, 106–121, DOI:10.1016/j.porgcoat.2020.106121.
- Y. Chen, N. Wang, G. Tong, D. Wu, X. Jin and X. Zhu, Synthesis of multiarm star polymer based on hyperbranched polyester core and poly(ε-caprolactone) arms and its application in UV-Curable coating, ACS Omega, 2018, 3, 13928–13934, DOI:10.1021/acsomega.8b02128.
- K. Wu, S. Xiang, W. Zhi, R. Bian, C. Wang and D. Cai, Preparation and characterization of UV curable waterborne poly(urethaneacrylate)/antimony doped tin oxide thermal insulation coatings by sol-gel process, Prog. Org. Coat., 2017, 113, 39–46, DOI:10.1016/j.porgcoat.2017.08.004.
- K. H. Nama, K. Seo, J. Seo, S. Khan and H. Han, Ultraviolet curable polyurethane acrylate nanocomposite coatings based on surface-modified calcium carbonate, Prog. Org. Coat., 2015, 85, 22–30, DOI:10.1016/j.porgcoat.2014.12.004.
- Z. Liang, X. Wang, L. Feng and X. Wu, Synthesis of UV and thermal dual curing oligomer for solder resist ink using ink-jet printing, J. Appl. Polym. Sci., 2019, 136, 47428, DOI:10.1002/app.47428.
- J. Yang, Z. Wang, Z. Zeng and Y. Chen, Chain-extended polyurethane-acrylate ionomer for UV-curable waterborne coatings, J. Appl. Polym. Sci., 2002, 84, 1818–1831, DOI:10.1002/app.10384.
- T. Liu, X. Pan, Y. Wu, T. Zhang, Z. Zheng, X. Ding and Y. Peng, Synthesis and characterization of UV-curable waterborne polyurethane acrylate possessing perfluorooctanoate side-chains, J. Polym. Res., 2012, 19, 9741, DOI:10.1007/s10965-011-9741-0.
- R.-y. Xing, Q.-y. Zhang and J.-l. Sun, Preparation and properties of self-healing microcapsules containing an UV-curable oligomers of silicone, Polym. Polym. Compos., 2012, 20, 77–82, DOI:10.1177/0967391112020001-215.
- Y.-T. Zhang, H.-C. Yu, M.-C. Shen, Y.-T. Chern and C.-C. Li, Synthesis and application of self-healing microcapsules containing curable glue, Mater. Chem. Phys., 2020, 240, 122161, DOI:10.1016/j.matchemphys.2019.122161.
- S. Ramesh, S. Khan, Y. Park, E. Ford, S. Menegatti and J. Genzer, Self-healing and repair of fabrics: A comprehensive review of the application toolkit, Mater. Today, 2022, 54, 90–109, DOI:10.1016/j.mattod.2021.11.016.
- M. Gharakhloo and M. Karbarz, Autonomous self-healing hydrogels: recent development in fabrication strategies, Eur. Polym. J., 2022, 165(15), 111004, DOI:10.1016/j.eurpolymj.2022.111004.
- N. Wen, T. Song, Z. Ji, D. Jiang, Z. Wu, Y. Wang and Z. Guo, Recent advancements in self-healing materials: mechanicals, performances and features, React. Funct. Polym., 2021, 168, 105041, DOI:10.1016/j.reactfunctpolym.2021.105041.
- E. N. Brown, N. R. Sottos and S. R. White, Fracture testing of a self-healing polymer composite, Exp. Mech., 2002, 42, 372–379, DOI:10.1177/001448502321548193.
- K. S. Toohey, N. R. Sottos, J. A. Lewis, J. S. Moore and S. R. White, Self-healing materials with microvascular networks, Nat. Mater., 2007, 6, 581–585, DOI:10.1038/nmat1934.
- M. Zhu, S. He, Y. Dai, J. Han, L. Gan, J. Liu and M. Long, Long-lasting sustainable self-healing ion gel with triple-network by trigger-free dynamic hydrogen bonds and ion bonds, ACS. Sustainable Chem. Eng., 2018, 6, 17087–17098, DOI:10.1021/acssuschemeng.8b04452.
- D. H. Turkenburg and H. R. Fischer, Diels-Alder based, thermo reversible cross linked epoxies for use in self-healing composites, Polymer, 2015, 79, 187–194, DOI:10.1016/j.polymer.2015.10.031.
- A. Duval, G. Couture, S. Caillol and L. Avérous, Bio-based and aromatic reversible thermoset networks from condensed tannins via the diels-alder reaction, ACS Sustainable Chem. Eng., 2017, 5, 1199–1207, DOI:10.1021/acssuschemeng.6b02596.
- J. Rong, J. Zhong, W. Yan, M. Liu, Y. Zhang, Y. Qiao, C. Fu, F. Gao, L. Shen and H. He, Study on waterborne self-healing polyurethane with dual dynamic units of quadruple hydrogen bonding and disulfide bonds, Polymer, 2021, 221, 123625, DOI:10.1016/j.polymer.2021.123625.
- W. Li, L. Xiao, Y. Wang, J. Chen and A. Nie, Self-healing silicon-containing eugenol-based epoxy resin based on disulfide bond exchange: synthesis and structure-property relationships, Polymer, 2021, 229, 123967, DOI:10.1016/j.polymer.2021.123967.
- J. Canadell, H. Goossens and B. Klumperman, Self-healing materials based on disulfide links, Macromolecules, 2011, 44, 2536–2541, DOI:10.1021/ma2001492.
- O. Hideyuki, N. Shinsuke and K. Yasuharu, A dynamic covalent polymer driven by disulfide metathesis under photoirradiation, Chem. Commun., 2010, 46, 1150–1152, 10.1039/b916128g.
- D. L. Zhao, S. Liu and Y. Wu, Self-healing UV light-curable resins containing disulfide group:synthesis and application in UV coatings, Prog. Org. Coat., 2019, 133, 289–298, DOI:10.1016/j.porgcoat.2019.04.060.
- T. Li, Z. Zhang and M. Rong, Self-healable and thiol-ene UV-curable waterborne polyurethane for anticorrosion coating, J. Appl. Polym. Sci., 2019, 136, 47700, DOI:10.1002/app.47700.
- T. Morofuji and S. N. NaokazuKano, Thermal release of quinoliniums and simple alkenes from their photocycloadducts by a retro-Diels–Alder reaction, Tetrahedron Lett., 2022, 104, 154011, DOI:10.1016/j.tetlet.2022.154011.
- M. Vauthier and C. A. Serra, Controlled reversible aggregation of thermoresponsive polymeric nanoparticles by interfacial Diels-Alder reaction, Colloids Surf., A, 2022, 648, 129321, DOI:10.1016/j.colsurfa.2022.129321.
- N. Alkayal and N. Hadjichristidis, Well-defined polymethylene-based block co/terpolymers by combining anthracene/maleimide Diels-Alder reaction with polyhomologation, Polym. Chem., 2015, 27, 4921–4926, 10.1039/c5py00601e.
- Y. Wei and X. Ma, The self-healing cross-linked polyurethane by Diels-Alder polymerization, Adv. Polym. Technol., 2018, 37, 1987–1993, DOI:10.1002/adv.21857.
- A. H. St. Amant, D. Lemen, S. Florinas, S. Mao, C. Fazenbaker, H. Zhong, H. Wu, C. Gao, J. Christie and J. de Alaniz, Tuning the Diels-Alder reaction for bioconjugation to maleimide drug-Linkers, Org. Biomol. Chem., 2018, 29, 2406–2414, DOI:10.1021/acs.bioconjchem.8b00320.
- L. Xu, M. Raabe, M. M. Zegota, V. Chudasama, S. L. Kuana and T. J. Weil, Site-selective protein modification via disulfide rebridging for fast tetrazine/trans-cyclooctene bioconjugation, Org. Biomol. Chem., 2020, 18, 1140–1147, 10.1039/c9ob02687h.
- N. Wen, T. Song, Z. Ji, D. Jiang, Z. Wu, Y. Wang and Z. Guo, Recent advancements in self-healing materials: Mechanicals, performances and features, React. Funct. Polym., 2021, 168, 105041, DOI:10.1016/j.reactfunctpolym.2021.105041.
- Y. Y. Jo, A. S. Lee, K.-Y. Baek, H. Lee and S. S. Hwang, Multi-crosslinkable self-healing polysilsesquioxanes for the smart recovery of anti-scratch properties, Polymer, 2017, 124, 78–87, DOI:10.1016/j.polymer.2017.06.076.
- S. K. Raut, P. Mondal, B. Parameswaran, S. Sarkar, P. Dey, R. Gilbert, S. Bhadra, S. Nair and N. K. Singh, Self-healable ultra-hydrophobic modified bio-based elastomer using Diels-Alder click chemistry, Eur. Polym. J., 2021, 146, 110204, DOI:10.1016/j.eurpolymj.2020.110204.
- X. Ke, H. Liang and L. Xiong, Synthesis, curing process and thermal reversible mechanism of UV curable polyurethane based on Diels-Alder structure, Prog. Org. Coat., 2016, 100, 63–69, DOI:10.1016/j.porgcoat.2016.03.008.
- Z. Wang, H. Yang and B. D. Fairbanks, Fast self-healing engineered by UV-curable polyurethane contained Diels-Alder structure, Prog. Org. Coat., 2019, 131, 131–136, DOI:10.1016/j.porgcoat.2019.02.021.
- M. Wei, M. Zhan and D. Yu, Novel poly(tetramethylene ether)glycol and poly(ε-caprolactone) based dynamic network via quadruple hydrogen bonding with triple-shape effect and self-healing capacity, ACS Appl. Mater. Interfaces, 2015, 7, 2585–2596, DOI:10.1021/am507575z.
- W. H. Binder and R. Zirbs, Supramolecular polymers and networks with hydrogen bonds in the main and side chain, Adv. Polym. Sci., 2007, 207, 1–78, DOI:10.1007/12.2006.109.
- D. D. Zhu, Q. Ye, X. M. Lu and Q. H. Lu, Self-healing polymers with PEG oligomer side chains based on multiple H-bonding and adhesion properties, Polym. Chem., 2015, 6, 5086–5092, 10.1039/c5py00621j.
- J. W. Han, H. R. Gong, S. L. Jiang, Y. J. Gao, J. Nie and F. Sun, Effect of imidazolium monomer structure on properties of imidazolium functionalized self-healing UV-cured polymers for flexible electronic devices, Macromol. Chem. Phys., 2019, 220, 1900362, DOI:10.1002/macp.201900362.
- M. V. Biyani, E. J. Foster and C. Weder, Light-healable supramolecular nanocomposites based on modified cellulose nanocrystals, ACS Macro Lett., 2013, 2, 236–240, DOI:10.1021/mz400059w.
- J. L. Wietor, A. Dimopoulos and L. E. Govaert, Preemptive healing through supra olecular cross-links, Macromolecules, 2009, 42, 6640–6646, DOI:10.1021/ma901174r.
- R. Liu, X. Yang and Y. Yuan, Synthesis and properties of UV-curable self-healing oligomer, Prog. Org. Coat., 2016, 101, 122–129, DOI:10.1016/j.porgcoat.2016.08.006.
- F. Gao, J. Cao and Q. Wang, Properties of UV-cured self-healing coatings prepared with PCDL-based polyurethane containing multiple H-bonds, Prog. Org. Coat., 2017, 113, 160–167, DOI:10.1016/j.porgcoat.2017.09.011.
- Y. L. Chen, A. M. Kushner, G. A. Williams and Z. B. Guan, Multiphase design of autonomic self-healing thermoplastic elastomers, Nat. Chem., 2012, 4, 467, DOI:10.1038/nchem.1314.
- J. C. Liu, J. C. Cao, Z. Zhou, R. Liu, Y. Yuan and X. Y. Liu, Stiff self-healing coating based on UV-curable polyurethane with a “hard core,flexible arm” structure, ACS Omega, 2018, 3, 11128–11135, DOI:10.1021/acsomega.8b00925.
- J. P. Chesterman, T. C. Hughes and B. G. Amsden, Reversibly photo-crosslinkable aliphatic polycarbonates functionalized with coumarin, Eur. Polym. J., 2018, 105, 186–193, DOI:10.1016/j.eurpolymj.2018.05.038.
- L. Hu, X. Cheng and A. Zhang, A facile method to prepare UV light triggered self-healing polyphosphazenes, J. Mater. Sci., 2015, 50, 2239–2246, DOI:10.1007/s10853-014-8786-y.
- C. S. Wong, N. I. Hassana and M. S. Suait, Photo-activated self-healing bio-based polyurethanes, Ind. Crops Prod., 2019, 140, 111613, DOI:10.1016/j.indcrop.2019.111613.
- B. Kiskan and Y. Yusuf, Self-Healing of poly(propylene oxide) - polybenzoxazine thermosets by photoinduced coumarine dimerization, Polym. Chem., 2014, 52, 2911–2918, DOI:10.1002/pola.27323.
- Y. Wang, Q. Liu and J. Li, UV-triggered self-healing poly-urethane with enhanced stretchability and elasticity, Polymer, 2019, 172, 187–195, DOI:10.1016/j.polymer.2019.03.045.
- H. Xie, M. J. He and X. Y. Deng, Design of poly(l-lactide)- poly(ethylene glycol)copolymer with light-induced shape memory effect triggered by pendant anthracene groups, ACS Appl. Mater. Interfaces, 2016, 8, 9431–9439, DOI:10.1021/acsami.6b00704.
- R. Yan, B. X. Jin, Y. J. Luo and X. Y. Li, Optically-healable polyurethanes with tunable mechanical properties, Polym. Chem., 2019, 1–9, 10.1039/c9py00261h.
- E. K. Sama, D. K. Sama, X. M. Lva, B. T. Liu, X. X. Xiao, S. H. Gong, W. T. Yu, J. Chen and J. Liu, Recent development in the fabrication of self-healing superhydrophobic surfaces, Chem. Eng. J., 2019, 373, 531–546, DOI:10.1016/j.cej.2019.05.077.
- S. Y. Xiang and W. D. Liu, Self-healing superhydrophobic surfaces: healing principles and applications, Adv. Mater. Interfaces, 2021, 12, 2100247, DOI:10.1002/admi.202100247.
- T. Dikic, W. Ming and V. Benthem, Self-replenishing surfaces, Adv. Mater., 2012, 24, 3701–3704, DOI:10.1002/adma.201200807.
- Y. Zhang, C. Rocco, F. Karasu, X. Allonas and C. C. Barghorn, UV-cured self-replenishing hydrophobic polymer films, Polymer, 2015, 9, 384–393, DOI:10.1016/j.polymer.2015.02.036.
- S. Y. Qiang, K. L. Chen, Y. J. Yin and C. X. Wang, Robust UV-cured superhydrophobic cotton fabric surfaces with self-healing ability, Mater. Design, 2017, 15, 395–402, DOI:10.1016/j.matdes.2016.11.099.
- K. Chen, J. Zhou and F. Ge, Smart UV-curable fabric coatings with self-healing ability for durable self-cleaning and intelligent oil/water separation, Colloids Surf., A, 2019, 565, 86–96, DOI:10.1016/j.colsurfa.2019.01.003.
| This journal is © The Royal Society of Chemistry 2022 |