Open Access Article
Open Access ArticleSpeciation of copper in human serum using conjoint liquid chromatography on short-bed monolithic disks with UV and post column ID-ICP-MS detection†
Katarina
Marković
 ab,
Maja
Cemazar
cd,
Gregor
Sersa
ce,
Radmila
Milačič
ab,
Maja
Cemazar
cd,
Gregor
Sersa
ce,
Radmila
Milačič
 *ab and
Janez
Ščančar
*ab and
Janez
Ščančar
 *ab
*ab
aDepartment of Environmental Sciences, Jožef Stefan Institute, Ljubljana, Slovenia. E-mail: janez.scancar@ijs.si; radmila.milacic@ijs.si
bJožef Stefan International Postgraduate School, Ljubljana, Slovenia
cInstitute of Oncology Ljubljana, Department of Experimental Oncology, Zaloška cesta 2, 1000 Ljubljana, Slovenia
dFaculty of Health Sciences, University of Primorska, Polje 42, 6310 Izola, Slovenia
eFaculty of Health Sciences, University of Ljubljana, Zdravstvena pot 5, 1000 Ljubljana, Slovenia
First published on 21st June 2022
Abstract
Ceruloplasmin (Cp) is the major copper-carrying (Cu) protein in human plasma. Due to copper's important physiological functions and its role in various diseases, there is a need to quantify the concentration bound to Cp and the exchangeable form of Cu. In the present work, conjoint liquid chromatography (CLC) on short-bed convective interaction media (CIM) monolithic disks was used to separate the Cu bound to low molecular mass (LMM) species, and the Cu bound to Cp and albumin (HSA) in human serum. Two immunoaffinity CIMmic albumin depletion (α-HSA) disks and one CIMmic weak anion-exchange diethylaminoethyl (DEAE) disk were assembled in a single housing, forming a CLC monolithic column. By applying isocratic elution with a 50 mmol L−1 MOPS buffer (pH 7.4) in the first 3 min, followed by gradient elution with 1 mol L−1 NH4Cl (pH 7.4) in the next 9 min, HSA was retained by the α-HSA disk, allowing subsequent separation of the LMM-Cu from the Cu bound to the Cp on the DEAE disk. Further elution with 0.5 mol L−1 acetic acid in the next 4 min rinsed the HSA from the α-HSA disk. The separated Cu species were quantified by post-column isotope dilution inductively coupled plasma mass spectrometry (ID-ICP-MS), while the elution profile of the proteins was followed by UV detection at 278 nm. Quantitative column recoveries were obtained. Good repeatability of the measurement was achieved for Cu-Cp (±1%), while for Cu-HSA and Cu-LMM species the repeatability of the measurements was slightly worse, due to the much lower Cu concentrations (±6% and ±9%, respectively). The developed method required only 20 μL of a 15-times diluted sample. Low limits of detection for the Cu-Cp, Cu-HSA and Cu-LMM species (6.1, 5.3 and 3.3 ng mL−1 Cu, respectively) were obtained. The technique was successfully applied in the determination of Cu-Cp, Cu-HSA and a fraction that most probably corresponds to the Cu-LMM species in the human serum of healthy individuals, kidney transplant patients and cancer patients.
1. Introduction
Copper (Cu) is an essential trace element for living organisms. It is, like iron (Fe) and zinc (Zn), abundant in healthy diets. It is predominantly required as a cofactor for many enzymes such as ceruloplasmin (Cp), superoxide dismutase, cytochrome c oxidase, and dopamine β-hydroxylase. It is found in all body tissues and plays an important role in the formation of red blood cells and in the maintenance of nerve cells and the immune system.1 In human plasma, the total Cu concentration varies between 500 and 1000 ng mL−1.2 Most of the Cu is strongly bound to Cp, while the rest is exchangeable Cu, bound to albumin (HSA) and amino acids.3 Cp is mainly synthesized in the liver and is the major Cu-carrying protein.4 In the human body, Cp has several vital physiological functions. It regulates Cu and Fe homeostasis5,6 and is a ferroxidase that oxidizes Fe2+ to Fe3+. This is crucially important for the transport and metabolism of Fe and the inhibition of the formation of reactive oxygen species.7,8 According to an X-ray structural study, there are six non-exchangeable Cu binding sites, oriented toward the protein interior, and two additional, so-called ‘labile’, Cu binding sites.9 Metabolic disorders of Cp are reported for several neurodegenerative diseases10,11 such as Wilson's,5,12 Alzheimer's,13 Parkinson's14 and Menkes disease.15 Moreover, Cp is involved in rheumatoid arthritis16 and inflammatory processes.17 Similar associations were reported for various neoplastic diseases.18 They serve as a prognostic biomarker in breast,19 bladder and bile duct cancers.20,21 Cp is also a candidate marker for ovarian clear cell carcinoma.22Cu toxicity in humans is related to its metabolic disorders. In Wilson's disease, the dysfunctional excretion of Cu from the liver, the decreased concentration of Cu-Cp and the increased concentration of exchangeable Cu (mainly Cu associated with HSA) in plasma lead to a Cu overload in the human body.5,12 On the other hand, Cp levels in plasma are high in breast, ovarian, lung, and stomach cancers18 and rheumatoid arthritis.16 In a healthy individual, about 95% of the plasma Cu is bound to Cp.23 Due to important physiological functions and the role of Cu in various diseases, it is necessary to quantify the concentration bound to Cp and the exchangeable fraction.
In clinical practice, Cp in serum or plasma is routinely determined by nephelometry or turbidimetry.24 The main disadvantage is their non-specificity, as both forms of Cp are measured: holoCp (Cu bound to Cp) and apoCp, the form of Cp without Cu atoms. As a biomarker for Wilson's disease, a measurement of the relative exchangeable Cu, which represents the ratio of the exchangeable to the total serum Cu, was proposed. This method can help in the early diagnosis of Wilson's disease and also has a diagnostic value for other Cu related diseases.12,25 Quarles Jr et al.25 developed a rapid method for determining the bound and extractable Cu in serum samples using an automated sample preparation, liquid chromatography inductively coupled plasma mass spectrometry (LC-ICP-MS) instrumental set-up. The Cu species were separated in an Elemental Scientific CF-Cu-02 column, packed with resin bearing negatively charged functional groups, which enabled strong binding of the exchangeable Cu to the resin. As an eluent, 10% HNO3 was used. The bound Cu, which had no interaction with the column, was eluted with a solvent front, while the exchangeable Cu was separated after about 2.8 min. del Castillo Busto et al.26 used a strong anion-exchange fast protein liquid chromatography column MonoQ for the selective separation of Cu-HSA and Cu-Cp in human serum. The separated Cu species were determined by species-specific isotope dilution (ID)-ICP-MS. Based on Cu-HSA concentrations, the developed method made it possible to discriminate between healthy individuals and patients with Wilson's disease. Neselioglu et al.27 reported a simple, automated spectrophotometric method for determining the ferroxidase activity of serum Cp. The method is based on the oxidation of Fe2+ to Fe3+ through the catalytic activity of the ferroxidase enzyme and the colorimetric determination of the concentration of Fe3+ ions. Solovyev et al.28 used strong anion-exchange chromatography coupled to ICP-MS for the speciation of Cu in relation to Wilson's disease. Bernevic et al.29 developed a selective and sensitive method for the determination of Cp in human serum, using an anti-Cp affinity column and an on-line determination of Cu by ICP-MS. A good correlation with an off-line enzyme-linked immunosorbent assay (ELISA) for Cp determination was obtained. Lopez-Avila30 used a method based on off-line immunoaffinity in combination with size-exclusion chromatography (SEC). To remove human serum albumin (HSA), immunoglobulin G (IgG), immunoglobulin A (IgA), transferrin (Tf), haptoglobin and antitrypsin, which may overlap with Cp on an SEC column, the serum sample was first injected into the immunoaffinity column. The fraction that passed through the immunoaffinity column was then injected into an SEC column coupled to an inductively coupled plasma mass spectrometer (ICP-MS) and Cu associated with Cp was determined by monitoring the Cu ions at m/z 63 and 65.
As an alternative to off-line two-dimensional (2D) chromatography, conjoint liquid chromatography (CLC) on monolithic disks allows rapid 2D separation using two chromatographic modes to be carried out in a single chromatographic run. To this end, in our group, the speciation of Pt- and Ru-based chemotherapeutics in human serum and the serum of cancer patients was performed using a set-up that comprises convective interaction media (CIM) affinity (protein G) and weak anion-exchange (diethylaminoethyl DEAE) disks assembled into a single housing, forming a CLC monolithic column.31–34 The development of the albumin-depletion (α-HSA) monolithic columns with immobilized antibodies35,36 provides the possibility to selectively retain HSA prior to the separation of Cp and other human serum proteins on an anion-exchange column.
The main aim of our work was to develop a new analytical method for the speciation of Cu in human serum that is based on the rapid 2D chromatographic separation of Cu-LMM, Cu bound to Cp and Cu bound to HSA. For this purpose, the following objectives were set: (i) to develop the 2D chromatographic separation of Cu species using a CLC monolithic column, which consists of short-bed immunoaffinity CIMmic α-HSA and CIMmic DEAE disks assembled in a single housing, in a single chromatographic run; (ii) to observe the separated serum proteins by UV detection and to quantify the separated Cu species by post-column isotope dilution (ID)-ICP-MS; (iii) to apply the developed method for speciation of Cu in the human serum of healthy individuals, kidney transplant patients and cancer patients.
2. Experimental
2.1. Instrumentation
An Agilent (Tokyo, Japan) series 1200 HPLC system with a quaternary pump was used for the chromatographic separations. It was equipped with an Agilent 1260 Bio-inert Manual Injector valve, fitted with a high-density polyethylene (HDPE) 20 μL injection loop.For the separation of the Cu species in the serum, two 0.1 mL convective interaction media (CIM) CIMmic™ immunoaffinity α-HSA disks and one 0.1 mL CIMmic weak anion-exchange diethylaminoethyl (DEAE) disk from BIA Separations d.o.o. (Ajdovščina, Slovenia) were assembled in a single housing, to form a conjoint liquid chromatography (CLC) monolithic column.
A UV-Vis Agilent 1200 series with a multiple-wavelength (MDW) detector was used to follow the protein elution at 278 nm. The outlet of the detector was coupled online to a single quadrupole ICP-MS, model 7700x from Agilent Technologies (Tokyo, Japan). ICP-MS was also used to determine the total Cu content in human serum samples. The ICP-MS operating parameters were optimized for plasma robustness and for introducing the minimum amounts of salts used in the chromatographic separation procedure. To eliminate polyatomic interferences37 arising from the sample matrix and plasma constituents at m/z 63 and m/z 65, the high energy collision mode (HECM) was applied, using helium as a collision gas. The ICP-MS operating parameters are provided in Table 1S (ESI†).
A CEM Corporation (Matthews, NC, USA) CEM MARS 6 microwave accelerated reaction system was used to digest the serum samples.
A Mettler Toledo MS104 (Zürich, Switzerland) analytical balance was used for weighing.
A WTW (Weilheim, Germany) 330 pH meter was employed to determine the pH.
2.2. Reagents and materials
Ultrapure water (18.2 MΩ cm) was obtained from a Direct-Q 5 Ultrapure water system (Millipore, Watertown, MA, USA). All chemicals were of analytical reagent grade. Human ceruloplasmin (Cp) and human serum albumin (HSA) were purchased from Sigma-Aldrich (Steinheim, Germany).All reagents used for the separation and cleaning of the chromatographic supports were from Merck (Darmstadt, Germany).
Buffer A was composed of 50 mmol L−1 MOPS, pH 7.4. Buffer B contained buffer A + 2 mol L−1 of ammonium chloride (NH4Cl), pH 7.4, eluent C was 0.5 mol L−1 acetic acid, pH 2.45 and buffer D consisted of 2 mol L−1 MOPS, 2 g L−1 EDTA and 1% Tween 20, pH 7.4.
Nitric acid (HNO3) s.p. purchased from Carlo Erba (Milan, Italy) and hydrogen peroxide (H2O2) s.p. (Sigma-Aldrich) were used to digest the samples. Citric acid p.a., was purchased from Merck.
Merck stock Cu solution (1000 mg Cu L−1 in 3% HNO3) was used to prepare the calibration standard solutions.
Copper oxide powder enriched with mass 65 (65CuO) was obtained from Nakima Ltd (Yehud-Monosson, Israel). The declared composition of the enriched 65CuO powder is 0.33 ± 0.03% and 99.67 ± 0.03% for the 63 and 65 isotopes, respectively.
The Seronorm™ trace element serum L-1 quality control material was purchased from SERO AS (Billingstad, Norway).
2.3. Sample preparation
Standard serum proteins were reconstructed in buffer A and were used for the method development. Prior to the chromatographic separations, they were appropriately diluted so as not to exceed the binding capacity of the monolithic disks.Blood samples were obtained with a venous puncture from six healthy individuals (ages 24 to 65), four kidney transplant patients (ages 30 to 42) and six lung cancer patients (ages 39 to 76). 5 mL of blood from the healthy individuals was taken after informed consent. In the kidney transplant patients, about 300 mL of blood was taken during venous puncture. In clinical practice, this blood is discarded. In the present study, it was used for research purposes after informed consent was obtained. Blood samples from the lung cancer patients, treated with Pt-based chemotherapeutics, were obtained about 3 weeks after receiving the chemotherapy, with the approval of the ethical committee (Republic of Slovenia, Ministry for Health, document no. 0120-696/2017/4, 22.01.2018) and conducted according to the rules of Good Clinical Practice (Declaration of Helsinki), and informed consent from the patients.
The blood from the healthy individuals and the cancer patients was collected in Becton–Dickinson vacutainers without additives, while the blood of the kidney transplant patients was gathered in a glass flask. The clot was removed by centrifugation (10 min, 855 g) and the serum in the supernatant was carefully collected using a polyethylene pipette, transferred to 2 mL polyethylene tubes and stored in a freezer at −20 °C.
Before the analysis all the samples were thawed and equilibrated at room temperature (T).
Prior to the speciation analysis, the serum samples were diluted 15-times with buffer A.
For the determination of the total Cu concentration, 0.25 mL of serum was transferred into Teflon vessels. 0.75 mL of MilliQ water, 0.5 mL of H2O2 and 0.5 mL of HNO3 were added and the sample was subjected to closed-vessel microwave digestion at a maxim power of 1200 W, ramped to 120 °C for 15 min, held at 120 °C for 60 min and cooled in the next 20 min. The clear solution was quantitatively transferred into a polyethylene graduated tube and made up to 30 mL with MilliQ water. The same procedure (acids without samples) was used to prepare a blank sample. The Cu concentrations in the digested serum were determined by ICP-MS using matrix-matched standards for the calibration.
2.4. Speciation of Cu on the CLC monolithic column
20 μL of the 15-times diluted serum samples or standard serum proteins were injected into the column. The high dilution of the serum samples was necessary to ensure quantitative sample loading and not to exceed the capacity of the α-HSA disks. To retain Cu-HSA on the α-HSA disks, isocratic elution with 100% buffer A was applied in the first 3 min at a flow rate of 0.3 mL min−1, while the Cu-Cp and Cu-LMM species passed through the α-HSA disks and were retained on the DEAE disk. A linear gradient elution from 100% buffer A to 50% buffer B was then followed for 9 min at a flow rate of 0.6 mL min−1 to separate Cu-Cp from the other serum proteins and the LMM-Cu species retained on the DEAE disk. From 12 to 13.5 min a gradual switching of the eluents from 50% A and 50% B to 100% C was applied at the same flow rate, to ensure optimal mixing of the mobile phase for the quantitative elution of Cu-HSA. Then, Cu-HSA was eluted from the column by isocratic elution with 100% eluent C for 4 min at a flow rate of 1 mL min−1. During the separation step (16 min), the eluate from the CLC column was passed through a UV detector (278 nm) for protein monitoring and was further introduced into the ICP-MS. Quantification of the separated Cu species was based on the peak area using the post-column ID-ICP-MS technique. Regeneration and equilibration followed at a flow rate of 1.5 min, according to the procedure given in Table 2S (ESI†). The eluate from the regeneration and equilibration steps was directed to waste through a software-controlled six-port valve. After approximately 25 injections of serum samples, the α-HSA disks were replaced, while the DEAE disk was subjected to rigorous cleaning. The CLC column was dismantled and cleaning was performed for the DEAE disk at a flow rate of 5 mL min−1. First, it was rinsed with 5 mL of water, followed by rinsing with 5 mL of 1 mol L−1 NaOH, 5 mL of 2 mol L−1 NaCl and finally with 5 mL of buffer A. After cleaning, the α-HSA and DEAE disks were re-assembled into the same CIM housing and the CLC column was ready for further use. The experimental data confirmed the high robustness of the CLC columns. There were no different performances from column to column observed.2.5. Preparation of enriched isotopic solution of 65Cu
12 mg of 65CuO was weighed in a Teflon vessel. 0.5 mL of concentrated HNO3 and 1.5 mL of MilliQ water were added and the samples were subjected to closed-vessel microwave digestion at a maximum power of 1200 W, ramped to T 90 °C for 15 min, held at 90 °C for 5 min, ramped to T 140 °C for 10 min, held at 140 °C for 15 min, and cooled in the next 30 min. The obtained clear solution was quantitatively transferred into polyethylene graduated tubes and made up to 30 mL with MilliQ water. The Cu concentration in the enriched isotopic standard was determined by reverse ID-ICP-MS.2.6. Quantification of the separated Cu species by post-column isotope dilution ICP-MS
The separated Cu species on the CLC column were quantified using the post-column ID-ICP-MS, monitoring Cu isotopes at m/z 63 and 65. The isotopically enriched 65Cu (5.139 ng mL−1) was delivered with a peristaltic pump via a T-piece after the separation of Cu species. The mass flow of Cu was plotted versus time during the chromatographic separation and the concentrations of Cu species were calculated by means of equations for the post-column species-unspecific ID-ICP-MS analysis.38 The analyte-to-spike isotope ratio (Cu present in the sample and 65Cu in the spike solution) was optimized so that the 63Cu-to-65Cu isotopic ratio for the two main Cu species was kept within the optimum (typically 0.1 to 10).38 In the samples analysed, this ratio was about 0.5 for Cu-Cp and 0.1 for Cu-HSA, while it was 0.03 for Cu-LMM (due to very low concentrations of Cu-LMM species).If not stated otherwise, all the analyses were conducted in duplicate.
2.7. Data evaluation
Data were extracted using the Agilent 5.1 MassHunter software and further processed with OriginPro 2015 (Northampton, MA, USA) and Microsoft Excel 2019 MSO (Redmond, WA, USA).A simple statistical analysis was carried out to check for significant differences in Cu-Cp, Cu-HSA and total Cu concentrations between the populations of cancer patients and healthy individuals, and the kidney transplant patients and healthy individuals. For this purpose, the Student's t-test at a 0.05 level of significance was applied.
3. Results and discussion
3.1. Development of an analytical method for Cu speciation in human serum
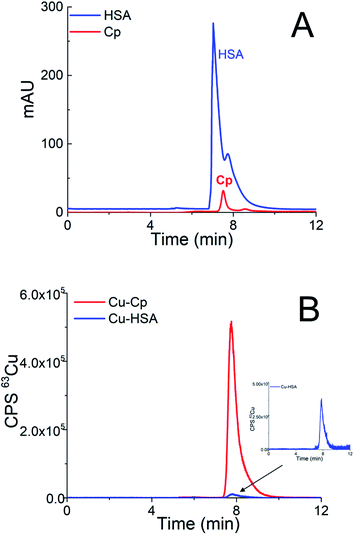
In Cu speciation, the choice of buffer is of great importance. The widely used Tris buffer strongly interacts with Cu2+, forming at neutral pH, dimeric complexes.39 However, this fact was often ignored in previous publications. Therefore, among the different possible buffers, MOPS was selected for the Cu speciation, since it is one of the zwitterionic aminosulfonic (good) buffers that do not interfere with the Cu2+ ions.40First, a weak anion-exchange DEAE disk was used to separate two Cu-binding proteins in human serum, i.e., Cp (molecular weight 132 kDa) from HSA (molecular weight 66.5 kDa). A study was performed with standard serum proteins, so that their final concentrations after dilution with buffer A were high enough to allow UV and ICP-MS detection. Standard serum proteins used in the present study were isolated from human serum. Thus, the serum proteins Cp and HSA naturally contained Cu. HSA (25 g L−1) was diluted 5-times and 20 μL of the sample was injected into the DEAE disk. The injection of 20 μL of 5-times diluted standard serum Cp (1 g L−1) followed. The eluate from the disk first passed through the UV detector and was further transferred to the ICP-MS. To separate the serum proteins, isocratic elution with buffer A was applied for 3 min at a flow rate of 0.3 mL min−1, followed by gradient elution from buffer A to 1 mol L−1 NH4Cl in buffer A in the next 9 min at a flow rate of 0.6 mL min−1. Overlays of two chromatographic separations are presented in Fig. 1.
As can be seen from Fig. 1A, HSA and Cp are co-eluted. The co-elution of Cu-Cp and Cu-HSA is also evident from Fig. 1B, where ICP-MS detection was applied. As expected, the Cu-Cp peak is much higher than that of the Cu-HSA.
To demonstrate that the greater dilution of the sample does not compromise the integrity of the Cu species, the standard serum protein HSA (25 g L−1) was diluted 5- and 15-times and the separation was performed on the DEAE disk. The chromatograms monitored by UV (278 nm) and ICP-MS detection at m/z 63 are provided in Fig. 1SA and 1SB (ESI†). The data from Fig. 1SA and 1SB† indicate that the HSA protein (UV detection) is eluted at the same retention time as the Cu-HSA (ICP-MS detection).
To quantify the separated Cu-HSA species by ICP-MS, the ID technique was applied using equations for the post-column ID-ICP-MS analysis.38 The mass flow of Cu, based on measurements of the isotopic ratios m/z 63 and 65, was plotted versus time during the chromatographic run (Fig. 1SC in ESI†).
The concentration of Cu calculated by post-column ID-ICP-MS was 146 ng mL−1, in both the 5-times and 15-times diluted samples. Therefore, the 15-times dilution of the sample does not compromise the integrity of the Cu species.
In further steps of the method development, a CLC monolithic column was constructed. The chromatographic conditions were optimized so that the α-HSA disks retained HSA, thus, enabling the separation of Cu-Cp and Cu low molecular mass (Cu-LMM) species on the DEAE disk. First, one α-HSA disk was placed in front of one DEAE disk, applying a flow rate of 1 mL min−1 and 0.6 mL min−1, but HSA was not retained. Then, the flow rate was lowered to 0.3 mL min−1 and one more α-HSA disk was added. Applying these chromatographic conditions for 3 min, HSA was quantitatively retained by the α-HSA disks. In order not to overload the α-HSA disks (disk capacity of 0.87 mg mL−1 HSA support), 20 μL of the 15-times diluted sample was injected.
It was found experimentally that under the same chromatographic conditions as previously used to separate the serum proteins on the DEAE disk, HSA was retained on the α-HSA disks, while Cp quantitatively passed the α-HSA disks and was separated on the DEAE disk. Then, the procedure was further optimized to elute the HSA retained on the α-HSA disks. Among the various eluents tested, i.e., 0.1, 0.5 and 1 mol L−1 glycine (pH 2.7), 0.1 mol L−1 citric acid (pH 3), 0.15 mol L−1 NH4OH (pH 10.5), 0.5 mol L−1 arginine (pH 4.4) and 0.5 mol L−1 acetic acid, the last of these was selected since it quantitatively eluted Cu-HSA from the CLC column at a flow rate of 1.0 mL min−1. In addition to Cu-Cp and Cu-HSA, a small portion of serum Cu can also be associated with the low molecular mass (LMM) species, e.g., amino acids.3 To verify whether the developed chromatographic procedure also enables the separation of the Cu-LMM species, a synthetic solution of Cu-glycine was prepared (Cu to glycine ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). Glycine was selected since it is among the most abundant amino acids present in human serum.41
100). Glycine was selected since it is among the most abundant amino acids present in human serum.41
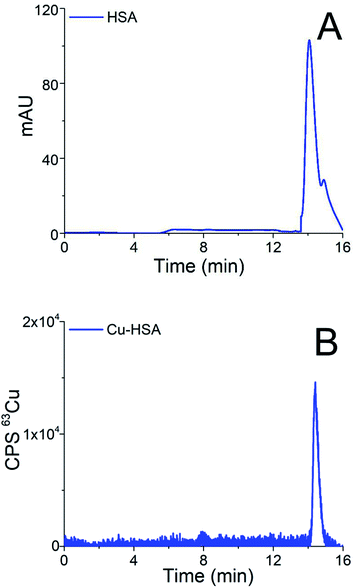
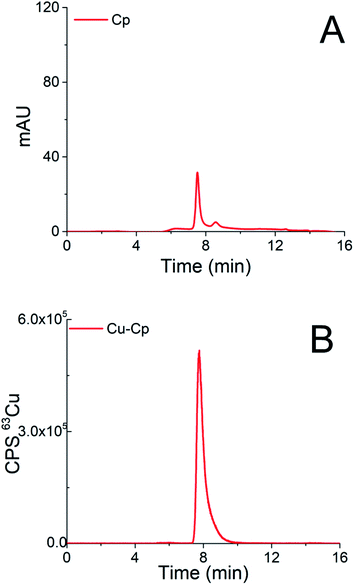
Chromatograms of the separation of the 15-times diluted samples of standard serum proteins, i.e., HSA (25 g L−1) and Cp (3 g L−1) on the CLC monolithic column monitored by UV detection (278 nm), and ICP-MS detection at m/z 63, are presented in Fig. 2 and 3, while the chromatogram of the separation of Cu-glycine (47.0 ng mL−1 Cu) on the CLC monolithic column monitored by ICP-MS detection at m/z 63 is presented in Fig. 4 (the UV signal of glycine is too weak, so it is not shown in Fig. 4).
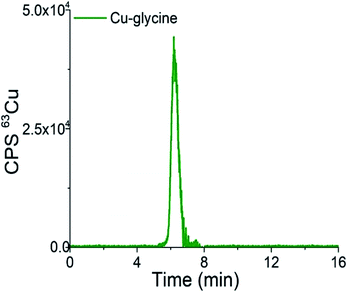 | ||
| Fig. 4 Chromatogram of separation of synthetically prepared Cu-glycine (47.0 ng mL−1 Cu) on the CLC monolithic column monitored by ICP-MS detection at m/z 63. | ||
The data in Fig. 2 show that under optimized chromatographic conditions HSA withstands the gradient elution with NH4Cl and is retained by the α-HSA disks. Finally, HSA is eluted from the α-HSA disks with acetic acid at elution times from 14.0 to 15.2 min. It is further seen that the elution of HSA detected by UV takes place at the same elution time as Cu detected by ICP-MS. To verify whether the developed speciation procedure allows for the quantitative elution of Cu-HSA from the CLC column, the concentration of the eluted Cu was calculated by post-column ID-ICP-MS (the Cu mass flow used for the quantification is shown in Fig. 2S in the ESI†) and compared with the total Cu concentration injected. The Cu concentration eluted was found to be 146.1 ng mL−1, while the total Cu was 152.2 ng mL−1, indicating 96% column recovery. Thus, Cu-HSA was quantitatively eluted from the column.
The data in Fig. 3 demonstrate that Cp passes the α-HSA disks and is separated on the DEAE disk by gradient elution with NH4Cl at elution times from 7.3 to 9.5 min. It can also be seen that it is well separated from Cu-HSA (see Fig. 2). It is evident that the elution of Cp detected by UV takes place at the same elution time as Cu detected by the ICP-MS. To evaluate the efficiency of the Cu-Cp elution from the CLC column, the concentration of eluted Cu (calculated by post-column ID-ICP-MS) was compared with the total Cu concentration injected. The Cu mass flow used for the quantification is shown in Fig. 3S (ESI†). The Cu concentration eluted was found to be 423.5 ng mL−1, while the total Cu was 439.0 ng mL−1, indicating 97% column recovery. Hence, Cu-Cp was quantitatively eluted from the column.
As can be seen from Fig. 4, Cu-glycine is eluted from 5.6 to 6.9 min and is separated from Cu-Cp (elution time from 7.3 to 9.5 min, Fig. 3). From the Cu concentration eluted from the column (47.5 ng mL−1), determined by post-column ID-ICP-MS, and total Cu concentration (47.0 ng mL−1) it is evident that Cu-Cp is quantitatively eluted (column recovery 101%). The Cu mass flow used for the quantification is shown in Fig. 4S (ESI†).
Based on the data presented in Fig. 2–4 and 1S–4S (ESI†), and the overall optimization of the analytical procedure, it can be concluded that the developed method enables the separation of Cu-glycine, Cu-Cp and Cu-HSA, and their quantitative elution from the CLC monolithic column, which extends its use to the determination of the Cu species in human serum.
Finally, the developed CLC-ID-ICP-MS method was applied for Cu speciation in human serum samples. Representative chromatograms of the 15-times diluted serum samples of two healthy individuals (samples H5 and H1) separated on the CLC column and monitored by ICP-MS detection at m/z 63, are presented in Fig. 5. Due to the low Cp concentration in human serum (0.2 to 0.35 g L−1)42 and high sample dilution, it was not possible to follow the Cp elution profile by UV detection.
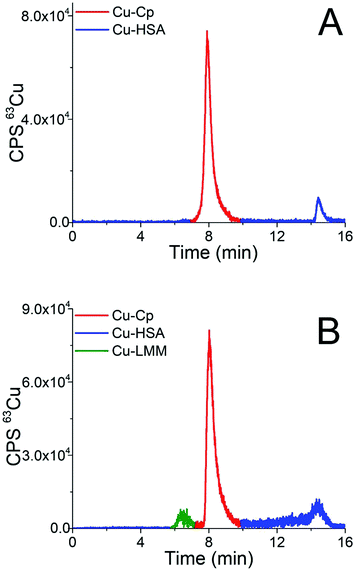 | ||
| Fig. 5 Chromatograms of separation of the 15-times diluted human serum samples (A) H5 and (B) H1 on the CLC monolithic column monitored by ICP-MS detection at m/z 63. | ||
The elution profile of Cu in Fig. 5A (sample H5) shows that Cu-Cp was eluted from 7.3 to 9.5 min and Cu-HSA from 14.0 to 15.2 min, whereas in Fig. 5B (sample H1) the fraction eluted from 5.6 to 6.9 min in front of the Cu-Cp peak is also noticeable. It most probably corresponds to Cu-LMM species, which might also be present in human serum.3 The Cu from the reagent blank contributed less than 1% to the background below the Cu-Cp peak in ICP-MS measurements. The Cu species in human serum were quantified by post-column ID-ICP-MS analysis. A representative chromatogram of the mass flow (sample H1) is shown in Fig. 5 (ESI†).
3.2. Analytical figures of merit
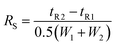 | (1) |
| Human serum sample | Total Cu (ng mL−1) | Cu-LMM (ng mL−1) | Cu-Cp (ng mL−1) | Cu-HSA (ng mL−1) | Sum of Cu species eluted (ng mL−1) | Column recovery (%) |
|---|---|---|---|---|---|---|
| a H – healthy individual; T – kidney transplant patient; C – cancer patient. | ||||||
| H4 | 974 | 58 | 816 | 112 | 986 | 101 |
| 969 | 66 | 804 | 102 | 972 | ||
| T2 | 990 | <3.3 | 864 | 94.0 | 958 | 97 |
| 984 | <3.3 | 877 | 87.0 | 964 | ||
| C3 | 1226 | 41.0 | 1118 | 119 | 1278 | 105 |
| 1205 | 37.0 | 1135 | 109 | 1281 | ||
The data from Table 1 indicate that the separated Cu species in the human serum were quantitatively eluted from the CLC column. The column recoveries ranged between 97 and 105%.
| Cu species | RSD (%) | LOD (ng mL−1 Cu) | LOQ (ng mL−1 Cu) |
|---|---|---|---|
| Cu-LMM | 9.0 | 3.3 | 11 |
| Cu-Cp | 1.0 | 6.3 | 21 |
| Cu-HSA | 6.3 | 5.3 | 18 |
Good repeatability of the measurements was obtained for Cu-Cp. The relative standard deviation (RSD) between consecutive separations for the separated Cu-Cp was ±1%. Slightly worse were the RSDs for the Cu-HSA (±6.3%) and Cu-LMM species (±9.0%) since their concentrations in the serum samples were significantly lower than that of Cu-Cp.
The limits of detection (LODs) and the limits of quantification (LOQs) for the determination of the separated Cu species were calculated as the concentrations that provide signals (peak areas) equal to 3s and 10s of a blank sample (buffer A) in the chromatogram, respectively. The LODs and LOQs were calculated on the basis of six blank samples injected into the CLC monolithic column, considering the same dilution factor (15-times) as in the analysis of the serum samples. The data for the LODs and LOQs for Cu-LMM, Cu-HSA and Cu-Cp are presented in Table 2. The LODs and LOQs for the separated Cu species ranged from 3.3 to 6.3 ng mL−1 Cu and from 11 to 21 ng mL−1 Cu, respectively. These LOQs are low enough to perform Cu speciation analyses in human serum samples.
The accuracy of the determination of the total Cu concentration in serum was tested by analyzing the Seronorm Level 1 quality control material. The determined Cu concentration (1074 ± 9 ng mL−1) agreed well with the certified value (1088 ± 89 ng mL−1), which confirmed the accuracy of the analytical procedure.
The main advantage of the newly developed CLC-ID-ICP-MS method, which combines immunoaffinity and anion-exchange chromatography, over previously reported ones, is its simplicity and ability to perform rapid 2D separation (16 min) and reliable quantification of the Cu species (Cu-Cp, Cu-HSA and Cu-LMM) in human serum in a single chromatographic run. Other researchers reported the determination of Cu-Cp only, applying 2D separation in two chromatographic runs,30 and the determination of Cu-Cp and Cu-HSA on anion-exchange chromatographic columns26,28 or exchangeable Cu in human serum.25
3.3. Speciation of Cu in human serum
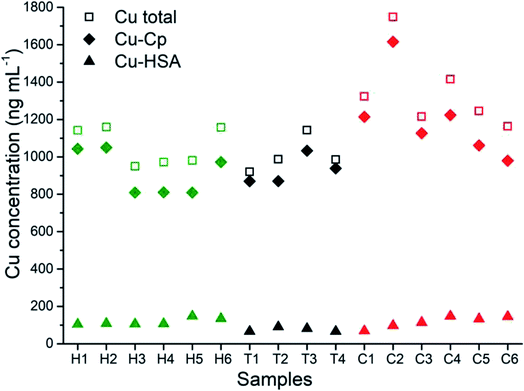
The developed and validated CLC-ID-ICP-MS method for the speciation of Cu in human serum was applied to the analysis of human serum samples from six healthy individuals (samples H1–H6), four kidney transplant patients (samples T1–T4) and six lung cancer patients (samples C1–C6).The distribution of the Cu concentrations in human serum samples between the total Cu concentrations determined by ICP-MS, and Cu-Cp and Cu-HSA species separated on a CLC column and quantified by post-column ID-ICP-MS is shown in Fig. 6. The Cu-LMM species were not considered, as their concentrations generally represented less than 5% of the total Cu content in the serum samples and they were not detected in all the samples analyzed.
The data from Fig. 6 indicate that concentrations of Cu-Cp and those of the total Cu are in general higher in the serum samples of the cancer and kidney transplant patients than in the healthy individuals. Furthermore, it is evident that the Cu-HSA concentrations are generally lower in the kidney transplant patients.
The concentrations for total Cu and Cu species determined in serum samples are provided in Table 3.
| Sample | Total Cu (ng mL−1) | Cu-LMM (ng mL−1) | Cu-Cp (ng mL−1) | Cu-HSA (ng mL−1) | ∑ of Cu species (ng mL−1) |
|---|---|---|---|---|---|
| a H – healthy individual; T – kidney transplant patient; C – cancer patient. | |||||
| H1 | 1147 | 59.0 | 1051 | 109 | 1219 |
| 1138 | 52.0 | 1035 | 98.0 | 1185 | |
| H2 | 1168 | 69.8 | 1055 | 116 | 1241 |
| 1153 | 57.5 | 1044 | 104 | 1206 | |
| H3 | 961 | <3.3 | 816 | 111 | 927 |
| 939 | <3.3 | 804 | 98.0 | 902 | |
| H4 | 974 | 58.0 | 816 | 112 | 986 |
| 969 | 66.0 | 804 | 102 | 972 | |
| H5 | 984 | <3.3 | 815 | 154 | 969 |
| 978 | <3.3 | 802 | 142 | 944 | |
| H6 | 1160 | <3.3 | 979 | 141 | 1120 |
| 1155 | <3.3 | 965 | 130 | 1095 | |
| T1 | 925 | <3.3 | 887 | 69.0 | 956 |
| 916 | <3.3 | 874 | 62.2 | 936 | |
| T2 | 990 | <3.3 | 864 | 94.0 | 958 |
| 984 | <3.3 | 877 | 87.0 | 964 | |
| T3 | 1149 | <3.3 | 1039 | 85.0 | 1124 |
| 1137 | <3.3 | 1027 | 77.0 | 1104 | |
| T4 | 992 | <3.3 | 961 | 69.0 | 1030 |
| 979 | <3.3 | 948 | 63.0 | 1011 | |
| C1 | 1329 | <3.3 | 1223 | 73.05 | 1296 |
| 1317 | <3.3 | 1205 | 65.0 | 1270 | |
| C2 | 1760 | <3.3 | 1628 | 103 | 1731 |
| 1736 | <3.3 | 1604 | 92.4 | 1696 | |
| C3 | 1226 | 41.0 | 1118 | 119 | 1278 |
| 1205 | 37.0 | 1135 | 109 | 1281 | |
| C4 | 1419 | 51.0 | 1232 | 155 | 1438 |
| 1411 | 43.0 | 1215 | 138 | 1396 | |
| C5 | 1253 | 65.0 | 1068 | 138 | 1271 |
| 1238 | 53.0 | 1054 | 127 | 1234 | |
| C6 | 1168 | 64.0 | 987 | 151 | 1202 |
| 1157 | 53.0 | 972 | 139 | 1164 | |
Data from Table 3 show that in 7 out of the 16 serum samples analyzed, the Cu-LMM species were also identified.
In assessing the possible statistical significance of the differences between the population of healthy individuals and the population of cancer patients or the population of healthy individuals and the population of renal patients, the H1 and H2 samples were excluded from the calculations in the healthy population due to recent recovery from viral infection. The mean values between Cu-Cp, Cu-HSA and total Cu were compared for individual Cu species by computing the Student's t-test for the population of cancer patients (n = 6) and healthy individuals (n = 4), or kidney transplant patients (n = 4) and healthy individuals (n = 4).
Higher concentrations of Cu-Cp and total Cu were observed in the cancer patient group in comparison to the healthy individuals, while the Cu-HSA concentrations were similar (Fig. 6). Significant differences (p < 0.05) between the two populations were obtained for Cu-Cp and total Cu. Cu-Cp was found as the most distinctive species (p = 0.0093), while the p value for total Cu (p = 0.0117) differed less. There was no statistical difference observed for Cu-HSA between the populations of cancer patients and healthy individuals (p = 0.979). These observations showed the potential of Cu-Cp as a possible biomarker for cancer diagnosis. Cp has already been proposed as a prognostic biomarker in breast, bladder, and bile duct cancers19–21 and a candidate marker for ovarian clear cell carcinoma.22
Lower concentrations of Cu-HSA, and slightly higher concentrations of Cu-Cp and total Cu, were observed in the kidney transplant patient group compared to the healthy individuals. Significant differences between the two populations were found for Cu-HSA (p = 0.0011), while there was no statistical difference observed for Cu-Cp and total Cu (p = 0.1783 and 0.9251, respectively). The lower Cu-HSA concentrations obtained are consistent with hypo-albuminemia, which is common in patients with end-stage renal disease.44
4. Conclusions
A novel analytical method for the speciation of Cu in human serum that is based on CLC monolithic chromatography with post-column ID-ICP-MS detection was developed. 2D separation of the Cu species was achieved in a single chromatographic run on the column that was constructed by assembling two immunoaffinity CIMmic α-HSA disks and one CIMmic weak anion-exchange DEAE disk into a single housing. Separated Cu species were quantified by post-column ID-ICP-MS. During separation, HSA was first retained on the α-HSA disks, enabling the separation of Cu-LMM species from Cu-Cp using NH4Cl (pH 7.4) as an eluent. The elution with acetic acid that followed, allowed further separation of Cu-HSA. The developed method enables quantitative and reliable determinations of Cu-Cp, Cu-HSA and a fraction that most probably corresponds to the Cu-LMM species in human serum. The previously reported analytical procedures are limited to the determination of exchangeable Cu, or enable the determination of Cu-Cp, or Cu-Cp and Cu-HSA, but are not able to quantify the Cu-LMM species. A statistical evaluation of the results (Student's t-test) revealed that the concentrations of total Cu and Cu-Cp were significantly higher in the population of cancer patients than in the healthy individuals, with Cu-Cp being the most differentiating species. This confirms that Cu-Cp is a potential biomarker in cancer diagnosis. The results also showed that Cu-HSA concentrations were significantly lower in kidney transplant patients, which is consistent with the usually lower HSA levels in this patient population. Our research provides an important new analytical tool that can be used to assess Cu metabolic disorders in many other diseases.Author contributions
KM: methodology, speciation analysis, data curation, validation, and writing – original draft; MC and GS: data interpretation and review & editing; RM and JŠ: conceptualization, methodology, validation, visualization, writing, review & editing, and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Slovenian Research Agency (Program groups P1-0143 and P3-0003) and Junior Researcher Grants for Katarina Marković (52052) for funding. The authors would like to thank BIA Separations, d.o.o. Ajdovščina, Slovenia for providing the CLC monolithic columns. We would also like to thank Aleš Štrancar and Urh Černigoj from Bia Separations d.o.o. for their valuable discussions in support of this work. Furthermore, we would like to thank colleagues and the head of the laboratory, Barbara Možina, from the Department of Laboratory Diagnostics, Institute of Oncology Ljubljana, Ljubljana, Slovenia, for providing serum samples from the cancer patients. We would also like to thank Miha Benedik from the Centre for Dialysis, Department of Nephrology of University Medical Centre Ljubljana, Slovenia, and Maja Uštar from the Centre for Kidney Transplantation, Department of Nephrology of University Medical Centre Ljubljana, Slovenia, for providing serum samples from the kidney transplant patients.Notes and references
- A. Taylor, J. S. Tsuji, M. R. Garry, M. E. McArdle, W. L. Goodfellow Jr, W. J. Adams and C. A. Menzie, Critical review of exposure and effects: implications for setting regulatory health criteria for ingested copper, Environ. Manage., 2020, 65, 131–159, DOI:10.1007/s00267-019-01234-y.
- A. Kubala-Kukuś, D. Banaś, J. Braziewicz, U. Majewska, M. Pajek, J. Wudarczyk-Moćko, G. Antczak, B. Borkowska, S. Góźdź and J. Smok-Kalwat, Analysis of copper concentration in human serum by application of total reflection X-ray fluorescence method, Biol. Trace Elem. Res., 2014, 158, 22–28, DOI:10.1007/s12011-013-9884-4.
- T. Kirsipuu, A. Zadorožnaja, J. Smirnova, M. Friedemann, T. Plitz, V. Tõugu and P. Palumaa, Copper(II)-binding equilibria in human blood, Sci. Rep., 2020, 10, 5686, DOI:10.1038/s41598-020-62560-4.
- M. C. Linder, Ceruloplasmin and other copper binding components of blood plasma and their functions: an update. Critical review, Metallomics, 2016, 8, 887–905, 10.1039/c6mt00103c.
- H. Zischka and C. Einer, Mitochondrial copper homeostasis and its derailment in Wilson disease, Int. J. Biochem. Cell Biol., 2018, 102, 71–75, DOI:10.1016/j.biocel.2018.07.001.
- C. Datz, T. K. Felder, D. Niederseer and E. Aigner, Iron homeostasis in the metabolic syndrome, review, Eur. J. Clin. Invest., 2013, 43, 215–224, DOI:10.1111/eci.12032.
- A. V. Sokolov, V. A. Kostevich, E. T. Zakharova, V. R. Samygina, O. M. Panasenko and V. B. Vasilyev, Interaction of ceruloplasmin with eosinophil peroxidase as compared to its interplay with myeloperoxidase: reciprocal effect on enzymatic properties, Free Radical Res., 2015, 49, 800–811, DOI:10.3109/10715762.2015.1005615.
- A. L. P. Chapman, T. J. Mocatta, S. Shiva, A. Seidel, B. Chen, I. Khalilova, M. E. Paumann-Page, G. N. L. Jameson, C. C. Winterbourn and A. J. Kettle, Ceruloplasmin is an endogenous inhibitor of myeloperoxidase, J. Biol. Chem., 2013, 288, 6465–6477, DOI:10.1074/jbc.m112.418970.
- I. Bento, C. Peixoto, V. N. Zaitsev and P. F. Lindley, Ceruloplasmin revisited: structural and functional roles of various metal cation-binding sites, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2007, 63, 240–248, DOI:10.1107/S090744490604947X.
- M. Manto, Abnormal Copper Homeostasis: Mechanisms and Roles in Neurodegeneration, Toxics, 2014, 2, 327–345, DOI:10.3390/toxics2020327.
- B. Wang and X.-P. Wang, Does ceruloplasmin defend against neurodegenerative diseases? Review, Curr. Neuropharmacol., 2018, 16 DOI:10.2174/1570159x1666618050811.
- F. Woimant, N. Djebrani-Oussedik and A. Poujois, New tools for Wilson's disease diagnosis: exchangeable copper fraction, Ann. Transl. Med., 2019, 7, S70, DOI:10.21037/atm.2019.03.02.
- F. Amtage, D. Birnbaum, T. Reinhard, W.-D. Niesen, C. Weiller, I. Mader, P. T. Meyer and M. Rijntjes, Estrogen intake and copper depositions: implications for Alzheimer's disease, Case Reports in Neurology, 2014, 6, 181–187, DOI:10.1159/000363688.
- M. Barbariga, F. Curnis, A. Andolfo, A. Zanardi, M. Lazzaro, A. Conti, G. Magnani, M. A. Volontè, L. Ferrari, G. Comi, A. Corti and M. Alessio, Ceruloplasmin functional changes in Parkinson's disease-cerebrospinal fluid, Mol. Neurodegener., 2015, 10(1) DOI:10.1186/s13024-015-0055-2.
- R. Ojha and A. N. Prasad, Menkes disease: what a multidisciplinary approach can do. Review, Journal of Multidisciplinary Healthcare, 2016, 9, 371–385, DOI:10.2147/JMDH.S93454.
- D. Spasovski, Lipid peroxidation levels in untreated rheumatoid arthritis and the effect of acute phase reactant, JOJ Urology & Nephrology, 2018, 4, 555643, DOI:10.19080/JOJUN.2018.04.555643.
- B. Bakhautdin, M. Febbraio, E. Goksoy, C. A. de la Motte, M. F. Gulen, E. P. Childers, S. L. Hazen, X. Xiaoxia Li and P. L. Fox, Protective role of macrophage-derived ceruloplasmin in inflammatory bowel disease, Gut, 2012, 62, 209–219, DOI:10.1136/gutjnl-2011-300694.
- K. Michalczyk and A. Cymbaluk-Płoska, The role of zinc and copper in gynecological malignancies. Review, Nutrients, 2020, 12, 3732, DOI:10.3390/nu12123732.
- F. Chen, B. Han, Y. Meng, Y. Han, B. Liu, B. Zhang, Y. Chang, P. Cao, Y. Fan and K. Tan, Ceruloplasmin correlates with immune infiltration and serves as a prognostic biomarker in breast cancer, Aging, 2021, 13, 20438–20467 CrossRef CAS PubMed.
- Y. Mukae, H. Ito, Y. Miyata, Y. Araki, T. Matsuda, N. Aibara, Y. Nakamura, T. Matsuo, H. Sakai and K. Ohyama, Ceruloplasmin levels in cancer tissues and urine are significant biomarkers of pathological features and outcome in bladder cancer, Anticancer Res., 2021, 41, 3815–3823, DOI:10.21873/anticanres.15174.
- I. W. Han, J.-Y. Jang, W. Kwon, T. Park, Y. Kim, K. B. Lee and S.-W. Kim, Ceruloplasmin as a prognostic marker in patients with bile duct cancer, Oncotarget, 2017, 8, 29028–29037 CrossRef PubMed.
- M. Sogabe, S. Kojima, T. Kaya, A. Tomioka, H. Kaji, T. Sato, Y. Chiba, A. Shimizu, N. Tanaka, N. Suzuki, I. Hayashi, M. Mikami, A. Togayachi and H. Narimatsu, Sensitive new assay system for serum wisteria floribunda agglutinin-reactive ceruloplasmin that distinguishes ovarian clear cell carcinoma from endometrioma, Anal. Chem., 2022 DOI:10.1021/acs.analchem.1c04302.
- V. R. Samygina, A. V. Sokolov, M. O. Pulina, H. D. Bartunik and V. B. Vasil'ev, X-ray diffraction study of highly purified human ceruloplasmin, Crystallogr. Rep., 2008, 53, 655–662, DOI:10.1134/s1063774508040172.
- I. Infusino, C. Valente, A. Dolci and M. Panteghini, Standardization of ceruloplasmin measurements is still an issue despite the availability of a common reference material, Anal. Bioanal. Chem., 2009, 397, 521–525, DOI:10.1007/s00216-009-3248-0.
- C. D. Quarles Jr, M. Macke, B. Michalke, H. Zischka, U. Karst, P. Sullivana and M. P. Field, LC-ICP-MS method for the determination of “extractable copper” in serum, Metallomics, 2020, 12, 1348–1355, 10.1039/d0mt00132e.
- M. E. del Castillo Busto, S. Cuello-Nunez, C. Ward-Deitrich, T. Morley and H. Goenaga-Infante, A fit-for-purpose copper speciation method for the determination of exchangeable copper relevant to Wilson's disease, Anal. Bioanal. Chem., 2022, 414, 561–573, DOI:10.1007/s00216-021-03517-y.
- S. Neselioglu, M. Ergin and O. Erel, A new kinetic, automated assay to determine the ferroxidase activity of ceruloplasmin, Anal. Sci., 2017, 33, 1339–1344, DOI:10.2116/analsci.33.1339.
- N. Solovyev, A. Ala, M. Schilsky, C. Mills, K. Willis and C. F. Harrington, Biomedical copper speciation in relation to Wilson's disease using strong anion exchange chromatography coupled to triple quadrupole inductively coupled plasma mass spectrometry, Anal. Chim. Acta, 2020, 1098, 27–36, DOI:10.1016/j.aca.2019.11.033.
- B. Bernevic, A. H. El-Khatib, N. Jakubowski and M. G. Weller, Online immunocapture ICP-MS for the determination of the metalloprotein ceruloplasmin in human serum, BMC Res. Notes, 2018, 11 DOI:10.1186/s13104-018-3324-7.
- V. Lopez-Avila, Determination of ceruloplasmin in human serum by immunoaffinity chromatography and size-exclusion chromatography-ICP-MS, Agilent Technologies, Inc., 2017, 5989-5304EN Search PubMed.
- A. Martinčič, M. Čemažar, G. Serša, V. Kovač, R. Milačič and J. Ščančar, A novel method for speciation of Pt in human serum incubated with cisplatin, oxaliplatin and carboplatin by conjoint liquid chromatography on monolithic disks with UV and ICP-MS detection, Talanta, 2013, 116, 141–148, DOI:10.1016/j.talanta.2013.05.016.
- A. Martinčič, R. Milačič, J. Vidmar, I. Turel, B. K. Keppler and J. Ščančar, New method for the speciation of Ru-based chemotherapeutics in human serum by conjoint liquid chromatography on affinity and anion-exchange monolithic disks, J. Chromatogr. A, 2014, 1371, 168–176, DOI:10.1016/j.chroma.2014.10.054.
- K. Marković, R. Milačič, J. Vidmar, S. Marković, K. Uršič, M. Nikšić Žakelj, M. Čemažar, G. Serša, M. Unk and J. Ščančar, Monolithic chromatography on conjoint liquid chromatography columns for speciation of platinum-based chemotherapeutics in serum of cancer patients, J. Trace Elem. Med. Biol., 2020, 57, 28–39, DOI:10.1016/j.jtemb.2019.09.011.
- K. Marković, R. Milačič, S. Marković, J. Kladnik, I. Turel and J. Ščančar, Binding kinetics of ruthenium pyrithione chemotherapeutic candidates to human serum proteins studied by HPLC-ICP-MS, Molecules, 2020, 25, 1512, DOI:10.3390/molecules25071512.
- I. A. Tarasova, A. A. Lobas, U. Černigoj, E. M. Solovyeva, B. Mahlberg, M. V. Ivanov, T. Panić-Janković, Z. Nagy, M. L. Pridatchenko, A. Pungor, B. Nemec, U. Vidic, J. Gasperšič, N. Lendero Krajnc, J. Vidič, M. V. Gorshkov and G. Mitulović, Depletion of human serum albumin in embryo culture media for in vitro fertilization using monolithic columns with immobilized antibodies, Electrophoresis, 2016, 37, 2322–2327, DOI:10.1002/elps.201500489.
- S. Brudar, U. Černigoj, H. Podgornik, M. Kržan and I. Prislan, Use of differential scanning calorimetry and immunoaffinity chromatography to identify disease induced changes in human blood plasma proteome, Acta Chim. Slov., 2017, 64, 564–570, DOI:10.17344/acsi.2016.2970.
- T. W. May and R. H. Wiedmeyer, A table of polyatomic interferences in ICP-MS, At. Spectrosc., 1998, 19, 150–155 CAS.
- P. Rodríguez-González, J. M. Marchante-Gayón, J. I. García Alonso and A. Sanz-Medel, Isotope dilution analysis for elemental speciation: a tutorial review, Spectrochim. Acta, Part B, 2005, 60, 151–207, DOI:10.1016/j.sab.2005.01.005.
- J. Nagaj, K. Stokowa-Sołtys, E. Kurowska, T. Frączyk, M. Jeżowska-Bojczuk and W. Bal, Revised coordination model and stability constants of Cu(II) complexes of Tris buffer, Inorg. Chem., 2013, 52, 13927–13933, DOI:10.1021/ic401451s.
- H. E. Mash and Y.-P. Chin, Complexation of copper by zwitterionic aminosulfonic (Good) buffers, Anal. Chem., 2003, 75, 671–677, DOI:10.1021/ac0261101.
- J. A. Schmidt, S. Rinaldi, A. Scalbert, P. Ferrari, D. Achaintre, M. J. Gunter, P. N. Appleby, T. J. Key and R. C. Travis, Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: a cross-sectional analysis in the EPIC-Oxford cohort, Eur. J. Clin. Nutr., 2016, 70, 306–312, DOI:10.1038/ejcn.2015.144.
- Health Encyclopedia, University of Rochester, Medical Center, Ceruloplasmin (Blood), 2022, last assessed 23.4.2022, https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=167%26contentid=ceruloplasmin_blood#:%7E:text=The%20normal%20range%20for%20a,your%20body%20to%20absorb%20copper.
- P. Ravisankar, S. Anusha, K. Supriya and U. A. Kumar, Fundamental chromatographic parameters, Int. J. Pharm. Sci. Rev. Res., 2019, 55, 46–50 CAS , https://www.globalresearchonline.net.
- S. Dasdelen and S.-O. Grebe, Infections after renal transplantation, J. Lab. Med., 2017 DOI:10.1515/labmed-2017-0094.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ja00161f |
| This journal is © The Royal Society of Chemistry 2022 |