Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Site-to-site peptide transport on a molecular platform using a small-molecule robotic arm†
Salma
Kassem
,
Alan T. L.
Lee
,
David A.
Leigh
 *,
Augustinas
Markevicius
,
Daniel J.
Tetlow
*,
Augustinas
Markevicius
,
Daniel J.
Tetlow
 and
Naoyuki
Toriumi
and
Naoyuki
Toriumi

Department of Chemistry, University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: david.leigh@manchester.ac.uk
First published on 10th December 2020
Abstract
Peptides attached to a cysteine hydrazide ‘transporter module’ are transported selectively in either direction between two chemically similar sites on a molecular platform, enabled by the discovery of new operating methods for a molecular transporter that functions through ratcheting. Substrate repositioning is achieved using a small-molecule robotic arm controlled by a protonation-mediated rotary switch and attachment/release dynamic covalent chemistry. A polar solvent mixtures were found to favour Z to E isomerization of the doubly-protonated switch, transporting cargo in one direction (arbitrarily defined as ‘forward’) in up to 85% yield, while polar solvent mixtures were unexpectedly found to favour E to Z isomerization enabling transport in the reverse (‘backward’) direction in >98% yield. Transport of the substrates proceeded in a matter of hours (compared to 6 days even for simple cargoes with the original system) without the peptides at any time dissociating from the machine nor exchanging with others in the bulk. Under the new operating conditions, key intermediates of the switch are sufficiently stabilized within the macrocycle formed between switch, arm, substrate and platform that they can be identified and structurally characterized by 1H NMR. The size of the peptide cargo has no significant effect on the rate or efficiency of transport in either direction. The new operating conditions allow detailed physical organic chemistry of the ratcheted transport mechanism to be uncovered, improve efficiency, and enable the transport of more complex cargoes than was previously possible.
Introduction
The manipulation of matter with molecular machines has intrigued scientists since it was proposed by Feynman over 60 years ago.1 The capability of one molecule to mechanically position another has some precedence in biology: fatty acid synthase2 and polyketide synthase3 both pass tethered molecules between domains located in different parts of enzyme superstructures. In doing so the substrate is prevented from exchanging with others in the bulk enabling the controlled synthesis of complex natural products.4–6 Such physical manipulation is reminiscent of the way robots position and process parts on factory assembly lines. A key feature of assembly line robots, shared by some biological molecular machines, is the ability to deliver different outcomes in response to different programed commands (for example, to build different protein sequences in the case of the ribosome).A step towards the long-term vision of using small-molecule robots to perform useful tasks by passing building blocks and other cargoes between machines and along polymer chains and across surfaces, is to develop molecules that can pick up, reposition and put down substrates at specific sites. Programable DNA devices7–16 include examples that combine building blocks in a defined sequence11–15 or move gold nanoparticles.16 Small-molecule artificial molecular machines17–23 have been developed that can transport macroscopic droplets along a surface24 and perform other mechanical tasks,25–29 and that can carry out sequence-specific30–33 and programable34–40 chemical synthesis. Here we demonstrate that a molecular ‘robotic arm’40–42 can be programed to transport cysteine-containing peptide derivatives, rapidly and with good efficiency in either direction, between two chemically similar sites 2 nm apart on a molecular platform.
Molecular robotic arm and platform design
A programable artificial molecular machine has been developed that is able to pick up, reposition, and set down 3-mercaptopropanehydrazide at either one of two different sites through judicious control of a robotic arm and ‘gripper’.41 The gripper picks up/releases the cargo by formation/breakage of a disulfide bond, while dynamic hydrazone chemistry modulates binding of the cargo to the platform.43 Transport is achieved by selectively inducing configurational (E-to-Z rotation about a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond) and conformational (rotation about a C–N single bond) changes of an embedded bidirectional hydrazone-based rotary switch44,45 that steers the arm. In a three-stage operation, 3-mercaptopropanehydrazide could be transported in either (chosen) direction between the two platform sites. The machine-induced transport is processive: the 3-mercaptopropanehydrazide does not at any time fully dissociate from the machine nor exchange with other molecules in the bulk, ensuring that substrate transport arises solely from the change in position of the robotic arm.
N double bond) and conformational (rotation about a C–N single bond) changes of an embedded bidirectional hydrazone-based rotary switch44,45 that steers the arm. In a three-stage operation, 3-mercaptopropanehydrazide could be transported in either (chosen) direction between the two platform sites. The machine-induced transport is processive: the 3-mercaptopropanehydrazide does not at any time fully dissociate from the machine nor exchange with other molecules in the bulk, ensuring that substrate transport arises solely from the change in position of the robotic arm.
Limitations of these demonstrations included: (i) experiments were only carried out with a single, simple, molecular cargo; (ii) transport in one direction was slow, as the acid-catalyzed hydrazone exchange takes up to six days to equilibrate at the low concentrations of acid necessary for the mechanism used; and (iii) some steps in the transport process proceed in modest yield. We decided to investigate alternative operation mechanisms for the prototypical molecular robotic system while exploring its application for the site-to-site platform transport of more complex molecular substrates, in particular peptides.
Results and discussion
E-to-Z (backward transport) experiments with molecular machine 1 were originally carried out using 5 equivalents of CF3CO2H.41 Selective protonation of the pyridine nitrogen of the hydrazone switch induced transport of 3-mercaptopropanehydrazide from the green site (attachment site 2; E-1) to the blue site (attachment site 1; Z-1). Although transport was achieved in good yield (91%), the process took six days to achieve this level of conversion. Consideration of the intermediates in the transportation pathway suggested the rate-limiting step to be acyl hydrazone exchange of the cargo between the two platform sites, presumably the rate limited by the low concentration of acid (5 equivalents) used. However, higher acid concentrations favour a different switch configuration, preventing transport in the desired direction.In an effort to improve the efficacy of substrate transport by the molecular machine, we studied the effect of acid concentration on the process. We treated, independently, E-1 and Z-1 in C2D2Cl4 (2.5 mM) with increasing amounts of CF3CO2H (10–100 equivalents) and determined the E-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z-1 ratio by 1H NMR spectroscopy once a steady state had been reached (see ESI, Section S3†).
Z-1 ratio by 1H NMR spectroscopy once a steady state had been reached (see ESI, Section S3†).
High acid concentrations had a detrimental effect on E-1 to Z-1 conversion, decreasing from 90% Z-1 with 5 equivalents of CF3CO2H to 30% Z-1 with 70 equivalents. Increasing the concentration of CF3CO2H further did not vary the E-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z-1 ratio from 70
Z-1 ratio from 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. Similarly, when starting from Z-1 the same steady state ratio of 70
30. Similarly, when starting from Z-1 the same steady state ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 E-1
30 E-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z-1 was reached with 70 (or more) equivalents of CF3CO2H.
Z-1 was reached with 70 (or more) equivalents of CF3CO2H.
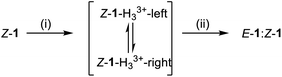
These results are consistent with the equilibrium between Z-1-H33+-right and Z-1-H33+-left (Fig. 1; for identification and characterization of the intermediates, see ESI, Section S4.2†). The conversion between these intermediates occurs via rotation about the C–N (quinoline) bond of the transient acyl hydrazides. This type of conformational change was originally described by Aprahamian,44 in which a large excess of CF3CO2H leads to protonation of both the pyridine and quinoline nitrogens resulting in the doubly-protonated Z-isomer of the rotary switch (Z-1-H33+-right; Fig. 1). The 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 E
30 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio of the intermediates in C2D2Cl4 limits the extent of substrate transport possible. However, by using the results shown in Table 1 for C2D2Cl4
Z ratio of the intermediates in C2D2Cl4 limits the extent of substrate transport possible. However, by using the results shown in Table 1 for C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6D5CD3 (1
C6D5CD3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), forward transport could be achieved with an improved 83% conversion by treating Z-1 with 80 equivalents of CF3CO2H to generate 83
2), forward transport could be achieved with an improved 83% conversion by treating Z-1 with 80 equivalents of CF3CO2H to generate 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 Z-1-H33+-right : Z-1-H33+-left (Table 1; entry 10).41
17 Z-1-H33+-right : Z-1-H33+-left (Table 1; entry 10).41
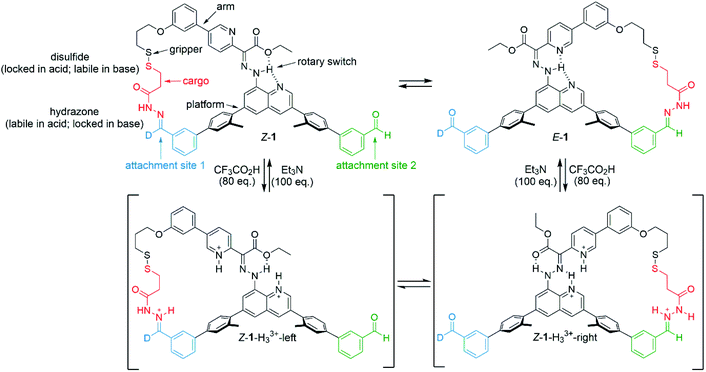 | ||
| Fig. 1 Operation of a platform-bound molecular robotic arm. Mechanism of exchange between E-1 and Z-1 using (i) CF3CO2H (80 equiv.), which causes protonation-induced configurational changes in the switch whilst simultaneously permiting hydrazide exchange at the platform sites, followed by (ii) Et3N (100 equiv.) which neutralizes the acid and switches off hydrazide exchange dynamics, locking the substrate in place on the platform. Z-1-H33+-left and Z-1-H33+-right are intermediates in the reaction mixture that could be identified and characterized by 1H NMR (see ESI†). Other conformations and hydrogen bond patterns may also be present. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z-1a
Z-1a
| Entry | Solvent |
E-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z-1 Z-1 |
ε 46 |
|---|---|---|---|
| a Reagents and conditions: (i) CF3CO2H (80 equiv.), solvent (2.5 mM), r.t. (ii) Et3N (100 equiv.). Conversions determined by 1H NMR. ε is solvent polarity.46,47 | |||
| 1 | C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (CD3)2SO (2 (CD3)2SO (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
>2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98 98 |
|
| 2 | CD3CN | >2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98 98 |
37.5 |
| 3 | C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN (2 CD3CN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
>2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98 98 |
|
| 4 | C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD (2 CD3OD (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
>2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98 98 |
|
| 5 | CD2Cl2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
8.93 |
| 6 | CF3CO2D | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
8.55 |
| 7 | C2D2Cl4 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
8.42 |
| 8 | CDCl3 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
4.81 |
| 9 | C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6D5CD3 (1 C6D5CD3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
4.15 |
| 10 | C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6D5CD3 (1 C6D5CD3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
3.59 |
| 11 | C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6D5CD3 (1 C6D5CD3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
3.11 |
| 12 | CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6D5CD3 (1 C6D5CD3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
2.38 |
| 13 | C6D5CD3 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
2.38 |
Exploring the unanticipated E/Z solvent effect: forward transport (Z-1 to E-1)
The conversion of Z-1 to E-1 was carried out in various solvents to fully explore the effect of the reaction medium on the equilibrium mixture. Z-1 was treated with 80 equivalents of CF3CO2H and the system allowed to reach a steady state. The E-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z-1 ratios after neutralization and workup are shown in Table 1. In polar solvents and solvent mixtures containing (CD3)2SO, CD3CN and CD3OD, little or no conversion to E-1 was observed (Table 1, entries 1–4), indicating that the equilibrium favours Z-1-H33+-left (hydrazone exchange is still dynamic under these reaction conditions). In CD2Cl2, 10% E-1 was formed (Table 1, entry 5). In less polar solvents such as C2D2Cl4 and CDCl3, a substantial increase in the amount of E-1 (corresponding to forward transport of the substrate) was observed (70% and 75%, respectively; Table 1, entries 7 and 8). Even lower polarity solvents, such as C6D5CD3, increased the amount of E-1 formed still further: 85% in 1
Z-1 ratios after neutralization and workup are shown in Table 1. In polar solvents and solvent mixtures containing (CD3)2SO, CD3CN and CD3OD, little or no conversion to E-1 was observed (Table 1, entries 1–4), indicating that the equilibrium favours Z-1-H33+-left (hydrazone exchange is still dynamic under these reaction conditions). In CD2Cl2, 10% E-1 was formed (Table 1, entry 5). In less polar solvents such as C2D2Cl4 and CDCl3, a substantial increase in the amount of E-1 (corresponding to forward transport of the substrate) was observed (70% and 75%, respectively; Table 1, entries 7 and 8). Even lower polarity solvents, such as C6D5CD3, increased the amount of E-1 formed still further: 85% in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 C2D2Cl4
4 C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6D5CD3 and neat C6D5CD3 (Table 1, entries 11 and 13).
C6D5CD3 and neat C6D5CD3 (Table 1, entries 11 and 13).
There is a broad overall trend between solvent dielectric constant and the resulting steady state E-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z-1 ratio (Table 1). Isomer Z-1-H33+-left is favoured in high polarity solvents; Z-1-H33+-right in low polarity media.46–48 It may be that polar, hydrogen-bond-disrupting, solvents solvate Z-1-H33+-left more effectively than Z-1-H33+-right, shifting the equilibrium position to favour the former despite unfavorable interactions between the hydrazone NH and the quinolinium NH proton. In apolar, non-hydrogen-bond-disrupting, solvents, Z-1-H33+-right is favoured because of the more extensive intramolecular hydrogen bonding network. Even in neat CF3CO2D (Table 1, entry 6), a 81
Z-1 ratio (Table 1). Isomer Z-1-H33+-left is favoured in high polarity solvents; Z-1-H33+-right in low polarity media.46–48 It may be that polar, hydrogen-bond-disrupting, solvents solvate Z-1-H33+-left more effectively than Z-1-H33+-right, shifting the equilibrium position to favour the former despite unfavorable interactions between the hydrazone NH and the quinolinium NH proton. In apolar, non-hydrogen-bond-disrupting, solvents, Z-1-H33+-right is favoured because of the more extensive intramolecular hydrogen bonding network. Even in neat CF3CO2D (Table 1, entry 6), a 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 E-1
19 E-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z-1 ratio is produced broadly in line with the general trend regarding media polarity, despite the large excess of acid present.
Z-1 ratio is produced broadly in line with the general trend regarding media polarity, despite the large excess of acid present.
Backward transport (E-1 to Z-1)
We next turned our attention to improving E-1 to Z-1 conversion (backward transport of the substrate). We hypothesized that faster and higher conversions of E-1 to Z-1 might be possible using 80 equivalents of CF3CO2H in polar solvents that favour intermediate Z-1-H33+-left. The molecular machine operation was followed by 1H NMR (see ESI Section S5†). Addition of 80 equivalents of CF3CO2H to E-1 in C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN (2
CD3CN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) led to Z-1-H33+-right, which converts to Z-1-H33+-left (identified by 1H NMR) over an 8 hour period. The 1H NMR spectrum of the product after workup showed >98% conversion to Z-1. This is a significant improvement in speed and efficiency of operation (>98%, 8 h) compared to the previously reported system (91%, 6 days). The findings demonstrate that the direction of cargo transport can be controlled solely by adjusting the polarity of the solvent while keeping other factors (e.g. acid concentration) unchanged. Polar solvent mixtures favour E-1 to Z-1 conversion; apolar solvent mixtures favour Z-1 to E-1 conversion.
1) led to Z-1-H33+-right, which converts to Z-1-H33+-left (identified by 1H NMR) over an 8 hour period. The 1H NMR spectrum of the product after workup showed >98% conversion to Z-1. This is a significant improvement in speed and efficiency of operation (>98%, 8 h) compared to the previously reported system (91%, 6 days). The findings demonstrate that the direction of cargo transport can be controlled solely by adjusting the polarity of the solvent while keeping other factors (e.g. acid concentration) unchanged. Polar solvent mixtures favour E-1 to Z-1 conversion; apolar solvent mixtures favour Z-1 to E-1 conversion.
A significant aspect of the architecture of molecular machine 1 is that binding of the substrate simultaneously to both the gripper of the machine arm and to the platform, forms a macrocycle. The results in Fig. 1 show that incorporating the isomeric forms of the hydrazone rotary switch into such a macrocycle has a profound effect on the chemistry of the switch. It permits the observation of otherwise transient intermediates in the switching process, which was used to modify the machine operation protocols resulting in significantly improved transport efficiency and dynamics.
Site-to-site transport of peptide derivatives
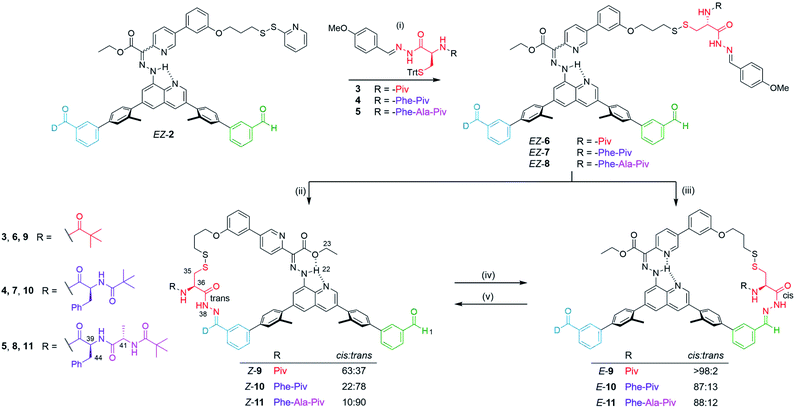
A potential useful feature of molecular machine 1 is that the structure of the 3-mercaptopropanehydrazide cargo can be elaborated upon without changing its interaction points with the platform and the arm. This could allow this unit to be used as a ‘transporter module’ for other small-molecules attached to it. 3-Mercaptopropanehydrazide is the hydrazide derivative of the amino acid cysteine, missing the amino group. Accordingly, we explored the site-to-site transport of a series of peptidic cysteine derivatives in both forward and backward directions to investigate whether the modified cargo would have an effect on the transportation process.A series of peptides (3–5, Fig. 2, see ESI Sections 2.1,† 2.2 and 2.3 for synthesis and characterization) were prepared bearing a cysteine hydrazide at the C-terminus. The thiol was trityl-protected to facilitate loading of the substrate onto the molecular machine arm gripper via silver-mediated disulfide formation.49,50 Deprotection of the trityl group was achieved with silver nitrate, generating the silver thiolate intermediate which reacts with the 2-pyridyl disulfide moiety of E/Z-2 to afford E/Z-6–8 in 63–79% yield (Fig. 2, step (i)). The C-terminus acyl hydrazide was capped as the corresponding p-methoxyphenyl acyl hydrazone in order to facilitate cyclization onto the platform via hydrazone exchange. Subsequent macrocyclization to the corresponding Z and E macrocycles was promoted by CF3CO2H (3 and 70 equivalents respectively, Fig. 2, steps (ii) and (iii)).
1H NMR spectroscopy (e.g.Fig. 3) shows that the machine-substrate-platform macrocycles exist as a mixture of acyl hydrazone rotamers (restricted rotation about the NH–CO bond of the cargo). Generally, acyl hydrazones prefer to adopt a cis conformation.45 However, substituents can destabilize the cis rotamer at the expense of the trans conformer.51 For the single amino acid derivative, Z-9 (R = Piv, Fig. 2), the cis conformer is favoured (63%), whereas with the tri-peptide, Z-11 (R = Phe-Ala-Piv), the trans isomer dominates (90%). The larger E-9–11 macrocycles strongly favour the cis rotamer of the acyl hydrazone irrespective of the size of the peptide cargo: the trans rotamer is not observed in E-9 and only a small amount is present in E-10 and E-11 (13 and 12%, respectively).
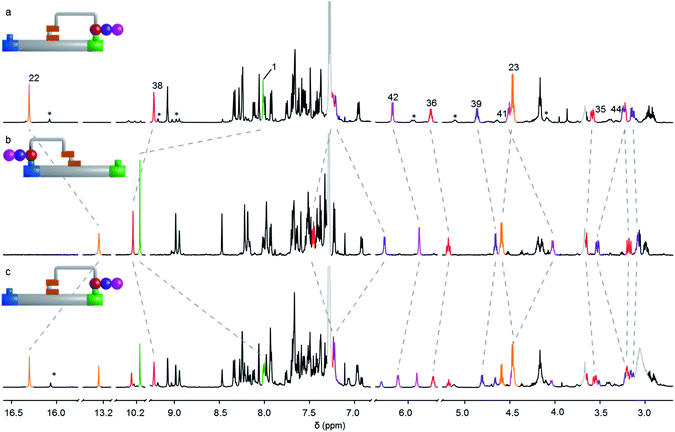 | ||
Fig. 3 Partial 1H NMR (600 MHz, 295 K, CDCl3, 2.5 mM) spectra of the interconversion of E-11 and Z-11. (a) E-11 (tripeptide on the platform green site); (b) backward transport of the tripeptide from the green site to the blue site, i.e. conversion of E-11 to Z-11 (CF3CO2H, 80 equiv., C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN (2 CD3CN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), >98%), (c) forward transport of the tripeptide from the blue site to the green site, i.e. conversion of Z-11 to E-11 (CF3CO2H, 80 equiv., C2D2Cl4 1), >98%), (c) forward transport of the tripeptide from the blue site to the green site, i.e. conversion of Z-11 to E-11 (CF3CO2H, 80 equiv., C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6D5CD3 (1 C6D5CD3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 63%). Proton assignments correspond to the labelling in Fig. 2. Signals marked with an asterisk (*) correspond to the minor rotamer of the acyl hydrazone. Signals due to traces of residual solvents and organic salts are shown in grey. 2), 63%). Proton assignments correspond to the labelling in Fig. 2. Signals marked with an asterisk (*) correspond to the minor rotamer of the acyl hydrazone. Signals due to traces of residual solvents and organic salts are shown in grey. | ||
Site-to-site transport of the peptides was carried out in the backward direction (E to Z) using CF3CO2H (80 equiv.) in C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN (2
CD3CN (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Conversion of E-9 to Z-9 (transport of a single amino acid) was achieved in >98% conversion in line with the results obtained with 3-mercaptopropanehydrazide (Table 1, entry 3). Pleasingly, the same behavior was observed in the backward transport of the di- and tri-peptides, both proceeding in >98% yield. The 1H NMR spectra of the operations to form E-11 and Z-11 are shown in Fig. 3 where successful right-to-left transport of the tripeptide (E-11 to Z-11) is evident from the characteristic shift of the imine proton H1 into the aldehyde region (from 7.94 ppm to 10.16 ppm). The upfield shift of the hydrazone NH proton H22 from 16.30 ppm to 13.26 ppm, and the downfield shift of the hydrazone ester group indicate E to Z isomerization of the rotary switch (Fig. 3a and b). Forward transport (Z-11 to E-11) was carried out with CF3CO2H (80 equiv.) in C2D2Cl4
1). Conversion of E-9 to Z-9 (transport of a single amino acid) was achieved in >98% conversion in line with the results obtained with 3-mercaptopropanehydrazide (Table 1, entry 3). Pleasingly, the same behavior was observed in the backward transport of the di- and tri-peptides, both proceeding in >98% yield. The 1H NMR spectra of the operations to form E-11 and Z-11 are shown in Fig. 3 where successful right-to-left transport of the tripeptide (E-11 to Z-11) is evident from the characteristic shift of the imine proton H1 into the aldehyde region (from 7.94 ppm to 10.16 ppm). The upfield shift of the hydrazone NH proton H22 from 16.30 ppm to 13.26 ppm, and the downfield shift of the hydrazone ester group indicate E to Z isomerization of the rotary switch (Fig. 3a and b). Forward transport (Z-11 to E-11) was carried out with CF3CO2H (80 equiv.) in C2D2Cl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6D5CD3 (1
C6D5CD3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Conversion of Z-11 to E-11 proceeded in 63% yield with the outcome of the operation analyzed by 1H NMR spectroscopy in a similar fashion (Fig. 3c). A decrease in forward transport compared to the 3-mercaptopropanehydrazide cargo (Z-1 to E-1, 83%) was found for Z-9, Z-10 and Z-11 (66%, 65% and 63% respectively). Nevertheless, successful site-to-site transport of the series of peptide-derived cargoes indicates that the size of the cargo does not prevent transport, nor does the size of the peptide affect the efficiency of transport.
2). Conversion of Z-11 to E-11 proceeded in 63% yield with the outcome of the operation analyzed by 1H NMR spectroscopy in a similar fashion (Fig. 3c). A decrease in forward transport compared to the 3-mercaptopropanehydrazide cargo (Z-1 to E-1, 83%) was found for Z-9, Z-10 and Z-11 (66%, 65% and 63% respectively). Nevertheless, successful site-to-site transport of the series of peptide-derived cargoes indicates that the size of the cargo does not prevent transport, nor does the size of the peptide affect the efficiency of transport.
Conclusions
The new operating conditions allow detailed physical organic chemistry of the mechanism of a ratcheted molecular transporter to be uncovered, its efficiency improved, and the transport of more complex cargoes with an artificial molecular machine. The findings demonstrate that site-to-site transport of molecular substrates by a small-molecule robotic arm can proceed within hours in either (chosen) direction with >98% conversion in one direction and 63–85% conversion (depending on the substrate) in the other. The direction of transport can be reversed by changing the polarity of the medium rather than control of the protonation level of the switch. The new operation conditions allow key interconvertible intermediates involved in the process to be identified and characterized. Cysteine hydrazide can act as a ‘transporter module’ through which to attach and transport mono, di- and tripeptide cargoes between different sites on a molecular scaffold within reach of the robotic arm and gripper. The size of the peptide does not affect the effectiveness of programable transport in either direction. The ability to pass a substrate selectively in either direction between chemically similar sites through a ratchet mechanism, without the substrate ever being able to exchange with others in the bulk, is a rare feature for a synthetic molecular machine. The use of a cysteine transporter module for programable repositioning is a significant step towards the vision of complex molecular substrates being manipulated with atomic precision between multiple sites by robotic molecular machines.22,40Conflicts of interest
The authors declare no conflicts of interests.Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EP/P027067/1) and the European Research Council (Advanced Grant No. 786630) for funding, and the University of Manchester Mass Spectrometry Service Centre for mass spectrometry. D. A. L. is a Royal Society Research Professor.References
- R. P. Feynman, Eng. Sci., 1960, 23, 22–36 Search PubMed.
- T. Maier, S. Jenni and N. Ban, Science, 2006, 311, 1258–1262 CrossRef CAS.
- S. Dutta, J. R. Whicher, D. A. Hansen, W. A. Hale, J. A. Chemler, G. R. Congdon, A. R. H. Narayan, K. Håkansson, D. H. Sherman, J. L. Smith and G. Skiniotis, Nature, 2014, 510, 512–517 CrossRef CAS.
- T. Maier, M. Leibundgut and N. Ban, Science, 2008, 321, 1315–1322 CrossRef CAS.
- E. J. Brignole, S. Smith and F. J. Asturias, Nat. Struct. Mol. Biol., 2009, 16, 190–197 CrossRef CAS.
- D. I. Chan and H. J. Vogel, Biochem. J., 2010, 430, 1–19 CrossRef CAS.
- B. Ding and N. C. Seeman, Science, 2006, 314, 1583–1585 CrossRef CAS.
- Z. J. Gartner, M. W. Kanan and D. R. Liu, J. Am. Chem. Soc., 2002, 124, 10304–10306 CrossRef CAS.
- Z. J. Gartner, B. N. Tse, R. Grubina, J. B. Doyon, T. M. Snyder and D. R. Liu, Science, 2004, 305, 1601–1605 CrossRef CAS.
- S. Liao and N. C. Seeman, Science, 2004, 306, 2072–2074 CrossRef CAS.
- Y. He and D. R. Liu, J. Am. Chem. Soc., 2011, 133, 9972–9975 CrossRef CAS.
- M. L. McKee, P. J. Milnes, J. Bath, E. Stulz, R. K. O'Reilly and A. J. Turberfield, J. Am. Chem. Soc., 2012, 134, 1446–1449 CrossRef CAS.
- M. L. McKee, P. J. Milnes, J. Bath, E. Stulz, A. J. Turberfield and R. K. O'Reilly, Angew. Chem., Int. Ed., 2010, 49, 7948–7951 CrossRef CAS.
- T. M. Snyder and D. R. Liu, Angew. Chem., Int. Ed., 2005, 44, 7379–7382 CrossRef CAS.
- W. Meng, R. A. Muscat, M. L. McKee, P. J. Milnes, A. H. El-Sagheer, J. Bath, B. G. Davis, T. Brown, R. K. O'Reilly and A. J. Turberfield, Nat. Chem., 2016, 8, 542–548 CrossRef CAS.
- H. Gu, J. Chao, S.-J. Xiao and N. C. Seeman, Nature, 2010, 465, 202–205 CrossRef CAS.
- S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081–10206 CrossRef CAS.
- J. M. Abendroth, O. S. Bushuyev, P. S. Weiss and C. J. Barrett, ACS Nano, 2015, 9, 7746–7768 CrossRef CAS.
- J.-P. Sauvage, Angew. Chem., Int. Ed., 2017, 56, 11080–11093 CrossRef CAS.
- J. F. Stoddart, Angew. Chem., Int. Ed., 2017, 56, 11094–11125 CrossRef CAS.
- B. L. Feringa, Angew. Chem., Int. Ed., 2017, 56, 11060–11078 CrossRef CAS.
- L. Zhang, V. Marcos and D. A. Leigh, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9397–9404 CrossRef CAS.
- A. W. Heard and S. M. Goldup, ACS Cent. Sci., 2019, 6, 117 CrossRef.
- J. Berná, D. A. Leigh, M. Lubomska, S. M. Mendoza, E. M. Pérez, P. Rudolf, G. Teobaldi and F. Zerbetto, Nat. Mater., 2005, 4, 704–710 CrossRef.
- T. J. Huang, B. Brough, C.-M. Ho, Y. Liu, A. H. Flood, P. A. Bonvallet, H.-R. Tseng, J. F. Stoddart, M. Baller and S. Magonov, Appl. Phys. Lett., 2004, 85, 5391–5393 CrossRef CAS.
- R. Eelkema, M. M. Pollard, J. Vicario, N. Katsonis, B. S. Ramon, C. W. M. Bastiaansen, D. J. Broer and B. L. Feringa, Nature, 2006, 440, 163 CrossRef CAS.
- Q. Li, G. Fuks, E. Moulin, M. Maaloum, M. Rawiso, I. Kulic, J. T. Foy and N. Giuseppone, Nat. Nanotechnol., 2015, 10, 161–165 CrossRef CAS.
- S. Chen, Y. Wang, T. Nie, C. Bao, C. Wang, T. Xu, Q. Lin, D.-H. Qu, X. Gong, Y. Yang, L. Zhu and H. Tian, J. Am. Chem. Soc., 2018, 140, 17992–17998 CrossRef CAS.
- A. Saura-Sanmartin, A. Martinez-Cuezva, D. Bautista, M. R. B. Marzari, M. A. P. Martins, M. Alajarin and J. Berna, J. Am. Chem. Soc., 2020, 142, 13442–13449 CrossRef CAS.
- B. Lewandowski, G. De Bo, J. W. Ward, M. Papmeyer, S. Kuschel, M. J. Aldegunde, P. M. E. Gramlich, D. Heckmann, S. M. Goldup, D. M. D'Souza, A. E. Fernandes and D. A. Leigh, Science, 2013, 339, 189–193 CrossRef CAS.
- G. De Bo, S. Kuschel, D. A. Leigh, B. Lewandowski, M. Papmeyer and J. W. Ward, J. Am. Chem. Soc., 2014, 136, 5811–5814 CrossRef CAS.
- G. De Bo, M. A. Y. Gall, M. O. Kitching, S. Kuschel, D. A. Leigh, D. J. Tetlow and J. W. Ward, J. Am. Chem. Soc., 2017, 139, 10875–10879 CrossRef CAS.
- C. T. McTernan, G. De Bo and D. A. Leigh, Chem, 2020, 6, 2964–2973 CAS.
- J. Beswick, V. Blanco, G. De Bo, D. A. Leigh, U. Lewandowska, B. Lewandowski and K. Mishiro, Chem. Sci., 2015, 6, 140–143 RSC.
- P. Waelès, C. Clavel, K. Fournel-Marotte and F. Coutrot, Chem. Sci., 2015, 6, 4828–4836 RSC.
- A. Martinez-Cuezva, A. Saura-Sanmartin, T. Nicolas-Garcia, C. Navarro, R.-A. Orenes, M. Alajarin and J. Berna, Chem. Sci., 2017, 8, 3775–3780 RSC.
- B. Riss-Yaw, C. Clavel, P. Laurent, P. Waelès and F. Coutrot, Chem.–Eur. J., 2018, 24, 13659–13666 CrossRef CAS.
- A. Martinez-Cuezva, M. Marin-Luna, D. A. Alonso, D. Ros-Ñiguez, M. Alajarin and J. Berna, Org. Lett., 2019, 21, 5192–5196 CrossRef CAS.
- M. Dommaschk, J. Echavarren, D. A. Leigh, V. Marcos and T. A. Singleton, Angew. Chem., Int. Ed., 2019, 58, 14955–14958 CrossRef CAS.
- S. Kassem, A. T. L. Lee, D. A. Leigh, V. Marcos, L. I. Palmer and S. Pisano, Nature, 2017, 549, 374–378 CrossRef CAS.
- S. Kassem, A. T. L. Lee, D. A. Leigh, A. Markevicius and J. Solà, Nat. Chem., 2016, 8, 138–143 CrossRef CAS.
- J. Chen, S. J. Wezenberg and B. L. Feringa, Chem. Commun., 2016, 52, 6765–6768 RSC.
- M. von Delius, E. M. Geertsema and D. A. Leigh, Nat. Chem., 2010, 2, 96–101 CrossRef CAS.
- X. Su and I. Aprahamian, Org. Lett., 2011, 13, 30–33 CrossRef CAS.
- I. Aprahamian, Chem. Commun., 2017, 53, 6674–6684 RSC.
- C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 3rd edn, 2003 Search PubMed.
- J. Nath and A. D. Tripathi, J. Chem. Soc., Faraday Trans. 1, 1984, 80, 1517–1524 RSC.
- E. Raamat, K. Kaupmees, G. Ovjannikov, A. Trummal, A. Kütt, J. Saame, I. Koppel, I. Kaljurand, L. Lipping, T. Rodima, V. Pihl, I. A. Koppel and I. Leito, J. Phys. Org. Chem., 2013, 26, 162–170 CrossRef CAS.
- M. L. Hamm and J. A. Piccirilli, J. Org. Chem., 1997, 62, 3415–3420 CrossRef CAS.
- M. L. Hamm and J. A. Piccirilli, J. Org. Chem., 1999, 64, 5700–5704 CrossRef CAS.
- G. Palla, G. Predieri, P. Domiano, C. Vignali and W. Turner, Tetrahedron, 1986, 42, 3649–3654 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc05906d |
| This journal is © The Royal Society of Chemistry 2021 |