Open Access Article
Open Access ArticleRecent developments of a co-immobilized laccase–mediator system: a review
Yaohua Gu a,
Lin Yuana,
Leina Jiaa,
Ping Xue*b and
Huiqin Yao*c
a,
Lin Yuana,
Leina Jiaa,
Ping Xue*b and
Huiqin Yao*c
aKey Laboratory of Environmental Factors and Chronic Disease Control, School of Public Health and Management, Ningxia Medical University, Yinchuan 750004, China
bCollege of Chemistry & Chemical Engineering, Ningxia University, Yinchuan 750021, China. E-mail: ping@nxu.edu.cn
cSchool of Basic Medical Sciences, Ningxia Medical University, Yinchuan 750004, China. E-mail: huiqin_yao@163.com
First published on 2nd September 2021
Abstract
The laccase–mediator is a promising biocatalyst with many possible applications, including bioremediation, chemical synthesis, biobleaching of paper pulp, biosensing, textile finishing and wine stabilization. The immobilization of laccase and the mediator offers several improvements for laccase–mediator system applications because the storage and operational stabilities are frequently enhanced. Moreover, the reusability of the immobilized laccase and mediator represents a great advantage compared with the free laccase and mediator. In this work, we review the methods of co-immobilization of the laccase–mediator system for the first time systematically and comprehensively. In addition, we discuss the different methodologies of laccase and mediator immobilization that have been reported.
1. Laccase
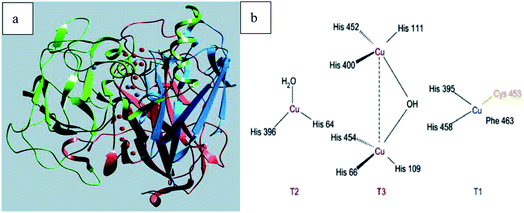
Laccase (p-diphenol: oxygen oxidoreductase, EC 1.10.3.2) is a copper-containing oxidase, and was first discovered in the Japanese lacquer tree in 1883.1 The typical laccase molecular structure (6.5 nm × 5.5 nm × 4.5 nm) generally contains about 500–550 amino acids with molecular weights of about 50–130 kDa (Fig. 1a). Laccase can catalyze the oxidation of a wide variety of substrates, including mono-, di- and polyphenols, aminophenols, methoxyphenols, aromatic amines and ascorbate, with the concomitant four-electron reduction of oxygen to water. These enzymes couple the four single-electron oxidations of the reducing substrate to the four-electron reductive cleavage of the dioxygen bond with four copper atoms. These copper atoms are classified into three groups depending on the characteristics obtained by UV/visible and electron paramagnetic resonance (EPR) spectroscopy. Among them, the T1 copper active site is ubiquitous in all kinds of laccase, which is an important factor to determine the redox potential (0.4–0.8 V) of laccase, and is also the substrate binding site; T2 copper is a mononuclear site with normal EPR parameters, which indicates that there is a tetragonal crystal environment, and only in the UV-vis region does it show weak and unobvious absorption; T3 copper is a coupled ion pair with diamagnetism and a wide absorption band at 330 nm. The two atoms form a binuclear site. The two copper atoms on the site pair antiferromagnetically through the bridging ligand hydroxyl bridge. According to the literature,2–4 the coordination structures of the four copper ions are as follows: T1 copper coordinates with two S of Cys and Phe, and two N of His, forming deformed tetrahedron; T2 copper is a trinuclear cluster, which coordinates with N and O in two His, respectively. The other two Cu atoms of T3 copper coordinate with N in three His and O in Tyr to form oxygen bridge, forming binuclear copper region (Fig. 1b).Laccase is a single electron oxidoreductase, which catalyzes the oxidation of different types of substrates through a single electron reaction to produce free radicals, which is mainly manifested in the formation of free radicals and the synergistic effect of four copper ions in laccase molecule. Laccase could absorb redox substrate through single electron mediated by T1 copper to form free radical cation, which can further carry out laccase catalyzed oxidation. Because the oxidation of single electron substrate is paired with the four electron reduction of oxygen, it could be understood that laccase acts like a battery, storing electrons from each oxidation reaction to reduce oxygen molecules. Therefore, four substrate molecules need to be oxidized to complete the process of oxygen molecules reducing to water; each electron from the four single electron oxidation at the T1 copper site is transferred to the trinuclear copper atom center with the bound O2 molecule, and the oxygen molecule is activated and reduced to water in the T2/T3 trinuclear domain. Typical laccase reaction mechanism is shown in Fig. 2.
 | ||
| Fig. 2 Catalytic mechanism of laccase.5 | ||
The key characteristic of laccase as an oxidoreductase is that the redox potential of its redox center, i.e. T1, T2, T3 copper site, meets the requirements of electrochemical reaction. The research shows that T1 copper site is the initial center for most laccases to receive electrons from the reducing substrate, and the catalytic efficiency of the substrate molecule depends on the redox potential of T1 copper. Christenson6 reported the redox potential values of a large number of different laccase T1 copper sites determined by redox medium potentiometric titration, and pointed out that laccase can be divided into three categories according to the potential of T1 site: low, medium and high potential laccase. The low potential group includes laccase from trees, such as laccase with about 420 mV T1 copper site potential. The intermediate group included laccase from basidiomycetes, such as thermophilic mycelium, basidiomycete C30, Rhizoctonia solani and Coprinopsis cinerea. The T1 potential of these enzymes ranged from 470 mV to 710 mV. The potential of high potential laccase (such as Trametes (Coriolus) laccase, T. versicolor laccase and T. villosa laccase) at T1 site is about 780 mV. Based on this report, the author proposes that the limiting step of reducing substrate in the enzymatic reaction cycle is the single electron transfer of substrate to laccase T1 copper. Yaropolov7 studied the electrochemical behavior of two laccase from different sources on graphite electrode and carbon electrode, and obtained similar conclusion: when the electrode was modified with fungal laccase, the oxygen reduction potential was about 0.35 V higher than that of laccase from lacquer tree modified electrode, and speculated that the main reason might be due to the difference of T1 copper oxidation–reduction potential between the two laccases. Xu8 studied the catalytic oxidation performance of laccase for three N–OH compounds: 1-hydroxybenzotriazole, purpuric acid and N-hydroxyacetanilide, and concluded that the higher the redox potential of laccase, the faster the oxidation rate of substrate molecule, and the rate limiting step of laccase catalytic oxidation depends on the rate of electron transfer from substrate to T1 copper site in laccase.
2. Laccase–mediator system
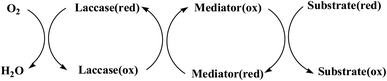
Laccase could catalyze the oxidation of a variety of phenolic substrates, and has potential application value in lignocellulose degradation, environmental pollutant degradation and other fields. It is considered to be a green environmental protection, economic and safe enzyme catalyst.9,10 However, there are still some problems to be solved when laccase is directly used in industrial production. Firstly, a large number of non-phenolic substrates could not directly bind to laccase; secondly, the redox potential of laccase was low (420–780 mV),6 and the low redox potential could not oxidize high potential phenolic compounds and other complex non-phenolic compounds. Studies have shown that the addition of some small molecular mediators can mediate the oxidation between laccase and substrate, and further expand the scope of laccase. The combination of laccase and small molecular mediators is called laccase mediator system (LMS).11 In 1990, Bourbonnais12 found that in the presence of remazol blue and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), laccase could oxidize non-phenolic lignin model compounds, and model compounds isoamyl alcohol and 1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)-propane-1,3-diol were oxidized by laccase mediator obtaining α-carbonyl derivatives. 1-(3,4-Dimethoxyphenyl)-2-phenoxyethane-1,2-diol was cleaved in the presence of laccase and ABTS to produce veratrum aldehyde and benzaldehyde. Based on these observations, Bourbonnais concluded that laccase can oxidize non phenol lignin, but depends on the existence of mediator, so laccase–mediator system is proposed for the first time. Later, other researchers found that laccase could oxidize the non-phenolic structure unit of lignin under the synergetic action of the mediator,13 and could be applied to pulp bleaching.14LMS have led to a dramatic increase in the range of laccase oxidizable compounds. Mediator oxidation by laccase produces a high redox potential intermediate able to oxidize non-phenolic substrates. This intermediate compound is then reduced to restore its initial form and close the redox cycle. The mechanism of LMS acting on substrate is shown in Fig. 3. At present, in addition to ABTS, 1-hydroxybenzotriazole (HBT), 2,2,6,6-tetramethylpiperidine oxide (TEMPO), n-hydroxy-n-acetylaniline (NHA) and phenothiazine (PT) are common synthetic mediators. The molecular structure formula is shown in Table 1.
Compared with the treatment of phenol and non phenol compounds by laccase alone, the addition of these mediators could significantly improve the catalytic oxidation efficiency of laccase to the target substrate. However, most synthetic mediators are toxic, unstable or expensive, and the large amount of use will inevitably cause secondary pollution of the environment. In addition, laccase oxidation mediator could produce a strong oxidation intermediate: the “co-mediator”. In addition to acting as a diffusion electron carrier, the co-mediator also interacts with laccase, resulting in laccase passivation, which greatly reduces the biocatalytic activity of laccase.15 In order to prevent the synthetic mediator from attacking the surface activity sensitive amino acid group of laccase, it is usually used to reduce the concentration of mediator and reaction temperature, which could effectively reduce the effect of mediator on laccase activity.16 In addition, it is reported that the stability of laccase in LMS could be effectively improved by using surfactant trixon.17
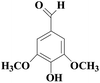
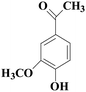
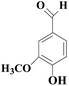
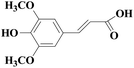
In view of the potential harm of synthetic mediator to laccase and environment, researchers began to look for safer and environment-friendly natural compounds as laccase mediators. Luo18 isolated about 18 kinds of small molecular phenolic compounds from the black liquor of Eucalyptus urophylla kraft pulping. Most of them can be used as natural mediators of laccase and can be used in dye decolorization, oxidation of polycyclic aromatic compounds and degradation of lignin and non lignocellulosic fibers. These phenolic mediators can be rapidly oxidized by laccase because of the existence of power donor in the substituent group of benzene ring, which can reduce the electrochemical potential energy; moreover, it can stabilize the phenoxy group of the mediator and prolong its existence time so that it can be recycled. According to reports,19 not only phenoxy compounds which could exist for a long time can act as mediators, but also their by-products. The phenoxy group of erucic acid is protected by two methoxy groups at the ortho position and can be specifically recognized during oxidation β–β′. The oxidized erucic acid could be rapidly converted into dehydroabietic acid endolipid, and its phenoxy group is still stable, so this phenolic dimer product could act as laccase mediator. At present, researchers have found that a variety of small molecule natural compounds can be used as laccase mediators, such as acetosyringone (As), syringaldehyde (Sa), vanillin (Va), etc. the molecular structure formula is shown in Table 2.
Compared with other synthetic mediators, natural mediators such as acetosyringone (As) and syringaldehyde (Sa) are more conducive to improve the stability of laccase and the ability to treat wastewater. The reason is that the free radical activity of these mediators after oxidation is low, which will greatly reduce the attack probability of laccase surface activity sensitive groups, so that laccase can exist stably for a long time. Camarero20 pointed out that the chemical structure of As and Sa contained two methoxyl substituents on the ortho group of phenol, which could be rapidly oxidized by laccase. The mediator with two methoxyl substituents produced more stable phenoxy (PHO˙) than the mediator with a single substituent. Laccase and natural phenolic mediators As and Sa were used to decolorize azo and heterocyclic dyes, and it was found that the decolorization effect of natural phenolic mediators was similar to that of synthetic mediators; when using As and Sa as mediators to investigate the decolorization performance of laccase for reactive black and sky blue, it was found that the decolorization effect of As and Sa as mediators for reactive black and sky blue was higher than that of synthetic mediator HBT.21 Yang22 found that acetylacetone (AA) can be used as a natural mediator to form laccase AA system, which could induce the graft polymerization of chitosan. The decolorization of malachite green by laccase with AA and HBT was compared. It was found that the degradation effect of malachite green by laccase AA was significantly better than that by laccase HBT. This may be because AA, a natural mediator, can prolong the survival rate and improve the stability of laccase. Fillat23 found a similar phenomenon when using LMS to degrade flax pulp. Compared with using synthesis mediator HBT, natural mediator system such as Sa, As and p-PCA could improve the stability of laccase in the process of degrading flax pulp. Mai24 also found that the natural mediator could be used as the oxidation substrate of laccase, and the natural mediator could effectively improve the stability of laccase by binding with the enzyme active center or the appropriate site in the enzyme protein chain.
3. Immobilization of laccase and mediator
3.1 Immobilization of laccase
Although laccases are excellent biocatalysts for biotechnological and environmental applications because of their high activity, selectivity and specificity, which permit them to perform complex chemical processes under experimental and natural conditions, there are some problems when free laccase is directly used in industrial production. Free laccase has strong water solubility and is not easy to be separated from the substrate products, resulting in the laccase can not be recycled after reaction, and laccase is very sensitive to the environment, easy to denaturation and inactivation. In addition, laccase is expensive, which directly leads to higher production costs,25,26 these problems seriously limit the practical application of laccase. Immobilization serves as an ideal technique for improving the properties and reusability of enzymes as they provide increased operational stability, thermal stability, reusability and tolerance to extreme conditions and chemical reagents. More importantly, the heterogeneity of the immobilized enzyme systems allows an easy recovery of both enzymes and products, multiple re-use of enzymes, continuous operation of enzymatic processes, rapid termination of reactions, and greater variety of bioreactor designs.Conceptually, there are two basic methods for enzyme immobilization, as the enzyme–support link may take place by physical or chemical interactions. These different types of links involve several types of immobilization methodologies. Physical coupling methods include the adsorption or embedding of the enzyme, whereas chemical coupling can occur through covalent binding or cross-linking method. Each of these methods has its advantages and limitations.
The adsorption of laccase onto a support is based on hydrogen bond, water dispersing bond or π-electron affinity of attraction.27 This method has the advantages of environmental protection, mild conditions, simple operation, and little effect on the conformation of laccase. However, there is almost no chemical bond between the carrier and the laccase protein molecules, resulting in weak binding force and low adsorption capacity of the carrier, besides, immobilized laccase is easily affected by the changes of environmental temperature, pH and ionic strength. As a result, the adsorbed laccase molecules are easy to fall off from the surface of the carrier, resulting in the decrease of catalytic activity. Cristóvão28 immobilized commercial laccase on green coconut fiber by physical adsorption method. The effects of immobilization conditions (enzyme concentration, immobilization time and pH value) on the performance of biocatalyst were investigated. It was found that the thermal stability and operational stability of laccase were improved compared with free laccase, however, the activity of immobilized laccase decreased to about 50% after reused twice. Embedding method is a kind of immobilization method that embeds laccase molecules in some material with grid or pore to form immobilized laccase. When the laccase is embedded in the carrier material, the conformation of the laccase will not be changed, so a high recovery of laccase activity can be maintained.29,30 However, the immobilized laccase molecules are easy to fall off from the carrier, resulting in the loss of the laccase, and there is a large mass transfer resistance between the immobilized laccase and the substrate. Zhang31 studied the immobilization of laccase in biomimetic titania. It was found that encapsulation of laccase in water, Tris–HCl buffer, McIlvaine buffer, and phosphate buffer had an efficiency of 80%, 97.6%, 78.5%, and 85.1%, respectively, and a recovery rate of 19.2%, 41.6%, 15.7%, and 25.5%, respectively. The reason for the differences between encapsulated efficiency and recovery of laccase activity was that the embedding method led to mass transfer limitations.
Covalent binding has been the most widely used method for laccase immobilization during the last decade. This method is based on the formation of a covalent bond between the chemical group on support or carrier and the nucleophilic group on the enzyme. Amino, imino, hydroxyl, thiol, guanidyl and imidazole groups present on the carriers are covalently linked to an enzyme. The functional group on the enzyme-like phenol ring of tyrosine, α-carboxyl group at C-terminus of enzyme and α-amino group at N-terminal of enzyme, indole ring of tryptophan is involved in the covalent binding with the support. A wide variety of supports like cellulose, agarose, collagen, inorganic carriers like glass, silica, metallic nanoparticles of gold, silver, cross-linkers like glutaraldehyde are widely used. Kashefi32 believed that covalent immobilization increases the enzyme stability and prevention of enzyme leakage to the reaction mixture and they immobilized laccase on amino-aldehyde modified graphene oxide. The nanobiocatalyst exhibited promising results: laccase loading of 156.5 mg g−1 and immobilization yield of 64.6% at laccase concentration of 0.9 mg mL−1. Skoronski33 immobilized Aspergillus oryzae laccase on graphene nanosheets by physical adsorption and covalent bonding, respectively. Both immobilization methods could improve the pH range of laccase activity, and the thermal stability of the immobilized enzyme was also significantly improved. In terms of operational stability, laccase immobilized by physical adsorption lost its activity quickly after the second reaction cycle, however, the activity of laccase immobilized by covalent method remained about 80% after 6 cycles. Cross-linking immobilized enzyme is a way of covalent immobilization of enzyme, which mostly use bifunctional or multifunctional cross-linking agent to cross-linking between enzyme protein or between enzyme and carrier, so as to form covalent bond with cross-linking agent and obtain cross-linked network structure, and achieve the purpose of enzyme immobilization. Lassouane34 used glutaraldehyde to immobilize the laccase to calcium alginate microspheres. The immobilization efficiency was 30% higher than that of the embedding method, and the leakage rate of laccase was reduced by 7 times. The results showed that the stability of immobilized enzyme by cross-linking covalently was better than that of physical immobilization enzyme. However, some studies have shown that glutaraldehyde as a cross-linking agent can cause partial denaturation of the enzyme molecule, because glutaraldehyde reacts strongly with the amino group of the enzyme, which may cause covalent bonding within and between the molecular structure of the enzyme and inactivate the enzyme. Chen35 studied the effects of glutaraldehyde solution concentration, crosslinking time, crosslinking pH and crosslinking temperature on the activity of galactosidase immobilized on chitosan microspheres. It was found that when the glutaraldehyde solution concentration was higher than 0.3%, the increase of the binding amount between enzyme and active aldehyde might change the space structure of the active center of the enzyme and reduce the activity of the enzyme. Zheng36 also found a similar phenomenon: at a certain concentration, the glutaraldehyde could adequately react with the enzyme to form a Schiff base, so that more enzyme molecules are attached to the support with the glutaraldehyde acting as a bridge. While as the glutaraldehyde concentration increased further, crosslinking reactions often caused conformational changes of enzyme molecules, resulting in damage to the active site, thus inhibiting the immobilization efficiency. Therefore, in the process of immobilization, the appropriate immobilization method should be selected according to the characteristics of the enzyme itself and its application purpose, so as to minimize the loss of enzyme activity.
3.2 Immobilization of mediator
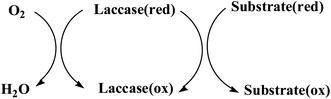
The mediators improve the electron transfer efficiency by indirectly increasing the oxidation potential of laccase and reducing the space barrier between laccase and substrate, thus improving the catalytic efficiency of laccase, expanding the range of laccase substrate and broadening the application field of laccase. However, in the current research and application of LMS, almost all of the mediators play a role in the dissolved state. If a large number of these mediators are used, there will inevitably be the following problems. First, because of the high cost of mediators, it is difficult to recover after use, and with the loss of waste liquid, the cost of laccase mediator system is directly increased; second, the synthetic mediators have certain toxicity, and the direct discharge after use will cause secondary environmental pollution. However, there are few reports on the recovery and utilization of the mediators. Chhabra37,38 used laccase ABTS to degrade basic green 4 and acid violet 17 in continuous membrane reactor, and recycled ABTS. After each degradation experiment, a certain amount of (20%, w/v) ammonium sulfate solution was added to the treated dye solution, and ABTS precipitation was obtained by centrifugation. The recovery rate of ABTS was 70%; although ABTS was recovered by this method, the recovery rate is low, and the introduction of ammonium sulfate may cause secondary pollution to the environment.39The idea of immobilization of biological enzymes gives great inspiration to researchers. If the mediator molecules are immobilized in solid materials, the scientific problem that the mediator can not be applied on a large scale can be overcome. In 1996, Therias40 inserted terephthalic acid (Ta) and ABTS into the interlayer spacing of anionic clay (also known as layered double hydroxides, LDH), and obtained four layered double hydroxides [Zn–Cr–ABTS] and [Zn–Al–ABTS] and [Zn–Cr–Ta] and [Zn–Al–Ta]. The electrochemical behavior of the modified electrodes was investigated, and the cyclic voltammetry response of [Zn–Cr–ABTS] and [Zn–Al–ABTS] modified electrodes was compared with that of ABTS adsorbed on [Zn–Cr–Ta] and [Zn–Al–Ta]. It was found that there was almost no ABTS leaching in the four electrodes under electrolytic conditions, and even if there was leaching phenomenon, it was very slow. The results show that there is a strong interaction between ABTS radical and anionic clay layer. Zebda41 prepared a layered double hydroxide Zn–Cr–ABTS by coprecipitation method, and characterized it by X-ray diffraction and infrared spectroscopy to determine the intercalation of redox anions between inorganic layers. These redox active hybrid materials were used to connect with laccase in biological fuel cell devices. The hybrid layered double hydroxides were co immobilized with enzyme by preparing polypyrrole electropolymerization membrane on porous carbon tube electrode. The structure of layered double hydroxide Zn–Cr–ABTS is shown in Fig. 4. The layered double hydroxides realize the immobilization of mediator molecules, but this immobilization method only has ion exchange effect on anionic substances, such as anthraquinone disulfonate, ferrocene derivatives and ABTS, but can not achieve the immobilization effect on other substances, such as natural molecule acetylsyringone, eugenol, etc.; in addition, the synthesis rate of layered double hydroxides is low, which can only be used in the study of micro modification of electrochemical electrode, and can not be applied in large scale.
 | ||
| Fig. 4 Layered double hydroxide of Zn–Cr–ABTS.41 | ||
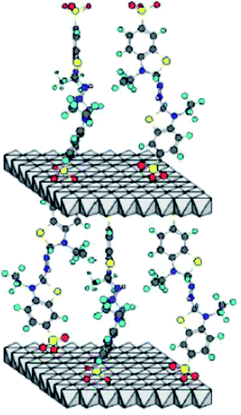
Jahangiri42 freezed the mixture of poly(ethylene glycol) methacrylate, triethylene glycol diacrylate and ABTS at −20 °C, and then irradiate the mixture with 12 kGy radiation dose to make the polymerization react to achieve the effect of cross-linking ABTS. The immobilized ABTS cold gel is used to catalyze the degradation of bisphenol A. Although the immobilization of the mediator was achieved, the immobilization efficiency of the medium was low, because the excessive radiation dose in the preparation process resulted in polymer gel depolymerization and unable to efficiently immobilize the mediator molecule. In addition, the initiation of polymerization reaction needs to be carried out under the condition of high dose radiation, which needs 10 MeV linear accelerator to accelerate the electron beam. The whole process consumes huge energy, which is contrary to the requirements of green and efficient chemical process and is difficult to be applied in practice. Sun43 graft acrylamide onto the surface of chitosan through graft polymerization. Then laccase catalyze the polymerization of acrylamide and acetylacetone (AA), and laccase is embedded in modified chitosan gel by sol–gel method to obtain a co-immobilized laccase mediator biocatalyst as shown in Fig. 5. When the co immobilized laccase mediator catalyst was used for the biodegradation of malachite green, the degradation rate of malachite green reached 95%. However, the way of immobilizing the mediator by the polymerization of the mediator and the polymerizable monomer may lead to the change of the chemical properties of the mediator and the loss of the original redox properties of the mediator. In this process, chitosan is grafted by the graft polymerization, which may lead to low grafting efficiency and poor grafting effect due to the steric hindrance effect, in addition, the final product prepared in this report has no fixed shape and needs to be ground and crushed during use, which is inconvenient to the separation process after reaction.
 | ||
| Fig. 5 Co-immobilized laccase–acetylacetone system.43 | ||
Patra44 prepared MIL-100(Fe) by microwave-assisted hydrothermal synthesis, and ABTS-MIL-100(Fe) was prepared by immersing MIL-100(Fe) in ABTS solution. The modified electrode based on ABTS-MIL-100(Fe) showed good performance in charge transfer kinetics and ionic conductivity, and had stable and repeatable electrochemical response. The results showed that MIL-100(Fe) could provide a suitable and stable microenvironment for the ABTS, and the immobilization of ABTS was realized. Then laccase was immobilized on the surface of ABTS-MIL-100(Fe) by physical adsorption method to achieve the co-immobilization of laccase–ABTS as Fig. 6. However, the immobilized ABTS prepared by impregnation method is very unstable and easy to fall off during continuous operation. The immobilization of laccase also adopts adsorption method, which causes laccase to react directly with the mediator molecules adsorbed on the surface, consumes the redox performance of the mediator, and also has a great loss of laccase activity, which will directly lead to the decrease of reusability of co-immobilized laccase–ABTS catalyst. Liu45 also successfully packed ABTS in the MIL-100(Fe) by a pot hydrothermal synthesis method obtaining ABTS-MIL-100(Fe). The ABTS-MIL-100(Fe) was used as mediator to study the removal energy of indigo Rouge dye. The decolorization rate of indigo rouge is 94% which is not different from that of free ABTS under the same conditions. While the hexagonal window size of MIL-100(Fe) is about 8.6 Å. The molecular size of ABTS is 6.4 × 6.4 × 7.4 Å, therefore, the encapsulation of ABTS molecules is not firm and easy to fall off.
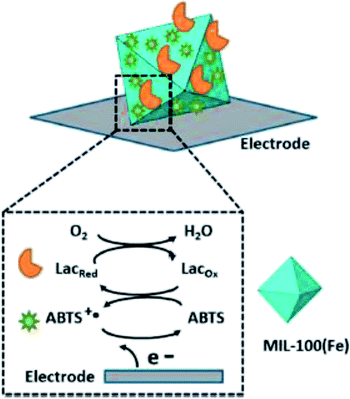 | ||
| Fig. 6 MIL-100(Fe) immobilized laccase–ABTS by impregnated adsorption.44 | ||
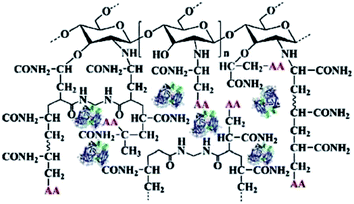
Gao46 prepared amino functionalized magnetic Fe3O4 nanoparticles, and immobilized laccase and mediator 4-amino-TEMPO on the surface of amino functionalized magnetic Fe3O4 nanoparticles through glutaraldehyde crosslinking. The co-immobilized laccase mediator catalyst was used to decolorize acid fuchsin. The decolorization rate of acid fuchsin was up to 77.41%. The results showed that the redox properties of laccase and mediator did not change after immobilization. However, glutaraldehyde as a cross-linking agent will cause partial denaturation of the enzyme molecule, because glutaraldehyde reacts strongly with the amino group of the enzyme, which may cause covalent bond connection within and between the enzyme molecular structure, resulting in the structural rigidity change of the laccase. Our group recently reported a biocatalyst that was prepared through the simultaneous immobilization of laccase and a mediator ABTS into a functionalized calcium alginate and cellulose beads (Fig. 7). This co-immobilized laccase–ABTS was then used for indole and acridine degradation.47,48
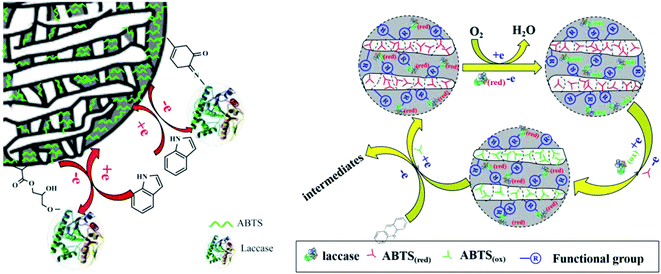 | ||
| Fig. 7 Degradation of indole and acridine by co-immobilized laccase–ABTS.47,48 | ||
4. Conclusion and future outlook
The immobilization of laccase can be performed via many different methods using a large number of supports. However, the methods of immobilization of mediator are not always satisfactory, let alone co-immobilized laccase–mediator system, although improvements are frequently obtained concerning the stability of the laccase–mediator to temperature, pH, organic solvents, storage and operation.Similar to the free laccase and mediator, co-immobilized laccase–mediator can be applied in a huge number of industrial processes, especially in environmental applications and in electrobiochemistry processes. The employment of co-immobilized laccase–mediator for the design of biological fuel cells and biosensors opens up new possibilities, from industrial to healthcare applications. The search for inexpensive supports and the recovery of activity during the immobilization process should be improved to increase the potential application of laccase–mediator co-immobilized systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21663020) and Special talent start-up project of Ningxia Medical University (2001230618).References
- H. Yoshida, Chemistry of lacquer (urushi) part I, J. Chem. Soc., Trans., 1883, 43(1), 472–486 RSC.
- S. Riva, Laccases: blue enzymes for green chemistry, Trends Biotechnol., 2006, 24(5), 219–226 CrossRef CAS PubMed.
- K. Piontek, M. Antorini and T. Choinowski, Crystal structure of a laccase from the fungus Trametes versicolor at 1.90 Å resolution containing a full complement of coppers, J. Biol. Chem., 2002, 277(40), 37663–37669 CrossRef CAS PubMed.
- E. I. Solomon, A. J. Augustine and J. Yoon, O2 reduction to H2O by the multicopper oxidases, Dalton Trans., 2008,(30), 3921–3932 RSC.
- A. M. Mayer and R. C. Staples, Laccase: new functions for an old enzyme, Phytochemistry, 2002, 60(6), 551–565 CrossRef CAS PubMed.
- A. Christenson, N. Dimcheva and E. E. Ferapontova, et al., Direct electron transfer between ligninolytic redox enzymes and electrodes, Electroanalysis, 2004, 16(1314), 1074–1092 CrossRef CAS.
- A. I. Yaropolov, A. N. Kharybin and J. Emnéus, et al., Electrochemical properties of some copper-containing oxidases, Bioelectrochem. Bioenerg., 1996, 40(1), 49–57 CrossRef CAS.
- F. Xu, J. J. Kulys and K. Duke, et al., Redox chemistry in laccase-catalyzed oxidation of N-hydroxy compounds, Appl. Environ. Microbiol., 2000, 66(5), 2052–2056 CrossRef CAS PubMed.
- L. Munk, A. K. Sitarz and D. C. Kalyani, et al., Can laccases catalyze bond cleavage in lignin, Biotechnol. Adv., 2015, 33(1), 13–24 CrossRef CAS PubMed.
- S. K. S. Patel, M. Z. Anwar and A. Kumar, et al., Fe2O3 yolk–shell particle-based laccase biosensor for efficient detection of 2,6-dimethoxyphenol, Biochem. Eng. J., 2018, 132, 1–8 CrossRef CAS.
- O. V. Morozova, G. P. Shumakovich and S. V. Shleev, et al., Laccase-mediator systems and their applications: a review, Prikl. Biokhim. Mikrobiol., 2007, 43(5), 583–597 CAS.
- R. Bourbonnais and M. G. Paice, Oxidation of non-phenolic substrates an expanded role for lactase in lignin biodegradation, FEBS Lett., 1990, 267(1), 99–102 CrossRef CAS PubMed.
- K. Li, R. F. Helm and K. E. L. Eriksson, Mechanistic studies of the oxidation of a non-phenolic lignin model compound by the laccase/1-hydroxybenzotriazole redox system, Biotechnol. Appl. Biochem., 1998, 27(3), 239–243 CAS.
- C. Valls, J. F. Colom and C. Baffert, et al., Comparing the efficiency of the laccase–NHA and laccase–HBT systems in eucalyptus pulp bleaching, Biochem. Eng. J., 2010, 49(3), 401–407 CrossRef CAS.
- C. Johannes, A. Majcherczyk and O. G. D. Univ, Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems, Appl. Environ. Microbiol., 2000, 66(2), 524–528 CrossRef CAS PubMed.
- R. Khlifi-Slama, T. Mechichi and S. Sayadi, et al., Effect of natural mediators on the stability of Trametes trogii laccase during the decolourization of textile wastewaters, J. Microbiol., 2012, 50(2), 226–234 CrossRef CAS PubMed.
- Y. Zhang, Z. Zeng and G. Zeng, et al., Effect of Triton X-100 on the removal of aqueous phenol by laccase analyzed with a combined approach of experiments and molecular docking, Colloids Surf., B, 2012, 97, 7–12 CrossRef CAS PubMed.
- X. Luo, H. Zhan and S. Fu, et al., Separation and GC-MS Analysis of Low-molecular-weight Phenolic Compounds from Black Liquor of Eucalyptus urophylla Kraft Pulpin, Chem. Ind. For. Prod., 2009, 29(4), 53–58 CAS.
- S. Luo, T. Xie and Z. Liu, et al., Laccase-mediator system: a review, Chin. J. Appl. Environ. Biol, 2015, 21(6), 987–995 CAS.
- S. Camarero, D. Ibarra and M. A. J. S. Nez, et al., Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes, Appl. Environ. Microbiol., 2005, 7(4), 1775–1784 CrossRef PubMed.
- S. Camarero, D. Ibarra and Á. T. Martínez, et al., Paper pulp delignification using laccase and natural mediators, Enzyme Microb. Technol., 2007, 40(5), 1264–1271 CrossRef CAS.
- H. Yang, H. Sun and S. Zhang, et al., Potential of acetylacetone as a mediator for Trametes versicolor laccase in enzymatic transformation of organic pollutants, Environ. Sci. Pollut. Res., 2015, 22(14), 10882–10889 CrossRef CAS PubMed.
- A. Fillat, J. F. Colom and T. Vidal, A new approach to the biobleaching of flax pulp with laccase using natural mediators, Bioresour. Technol., 2010, 101(11), 4104–4110 CrossRef CAS PubMed.
- C. Mai, W. Schormann and O. Milstein, et al., Enhanced stability of laccase in the presence of phenolic compounds, Appl. Microbiol. Biotechnol., 2000, 54(4), 510–514 CrossRef CAS PubMed.
- R. A. Sheldon and S. van Pelt, Enzyme immobilisation in biocatalysis: why, what and how, Chem. Soc. Rev., 2013, 42(15), 6223–6235 RSC.
- A. S. Roger, Enzyme immobilization: The quest for optimum performance, Adv. Synth. Catal., 2007, 349, 1289–1307 CrossRef.
- T. Jesionowski, J. Zdarta and B. Krajewska, Enzyme immobilization by adsorption: a review, Adsorption, 2014, 20(5–6), 801–821 CrossRef CAS.
- R. O. Cristóvão, A. P. M. Tavares and A. I. Brígida, et al., Immobilization of commercial laccase onto green coconut fiber by adsorption and its application for reactive textile dyes degradation, J. Mol. Catal. B: Enzym., 2011, 72(1–2), 6–12 CrossRef.
- P. Jian, E. Wu and X. Lou, et al., Anthraquinone removal by a metal–organic framework/polyvinyl alcohol cryogel-immobilized laccase: effect and mechanism exploration, Chem. Eng. J., 2021, 418, 129473–129481 CrossRef.
- K. Sun, S. Li and Y. Si, et al., Advances in laccase-triggered anabolism for biotechnology applications, Crit. Rev. Biotechnol., 2021, 21–25 Search PubMed.
- X. Zhang, M. Wang and L. Lin, et al., Synthesis of novel laccase-biotitania biocatalysts for malachite green decolorization, J. Biosci. Bioeng., 2018, 126(1), 69–77 CrossRef CAS PubMed.
- S. Kashefi, S. M. Borghei and N. M. Mahmoodi, Covalently immobilized Laccase onto graphene oxide nanosheets: Preparation, characterization, and biodegradation of azo dyes in colored wastewater, J. Mol. Liq., 2019, 276, 153–162 CrossRef CAS.
- E. Skoronski, D. H. Souza and C. Ely, et al., Immobilization of laccase from Aspergillus oryzae on graphene nanosheets, Int. J. Biol. Macromol., 2017, 99, 121–127 CrossRef CAS PubMed.
- F. Lassouane, H. Aït-Amar and S. Amrani, et al., A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions, Bioresour. Technol., 2019, 271, 360–367 CrossRef CAS PubMed.
- H. Chen, Q. Zhang and Y. Dang, et al., The effect of glutaraldehyde cross-linking on the enzyme activity of immobilized & beta-galactosidase on chitosan bead, Adv. J. Food Sci. Technol., 2013, 5(7), 932–935 CrossRef CAS.
- F. Zheng, B. Cui and X. Wu, et al., Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes, Int. Biodeterior. Biodegrad., 2016, 110, 69–78 CrossRef CAS.
- M. Chhabra, S. Mishra and T. R. Sreekrishnan, Combination of chemical and enzymatic treatment for efficient decolorization/degradation of textile effluent: High operational stability of the continuous process, Biochem. Eng. J., 2015, 93, 17–24 CrossRef CAS.
- M. Chhabra, S. Mishra and T. R. Sreekrishnan, Laccase/mediator assisted degradation of triarylmethane dyes in a continuous membrane reactor, J. Biotechnol., 2009, 143(1), 69–78 CrossRef CAS PubMed.
- Z. Yang, S. Zhou and Y. Sun, Start-up of simultaneous removal of ammonium and sulfate from an anaerobic ammonium oxidation (anammox) process in an anaerobic up-flow bioreactor, J. Hazard. Mater., 2009, 169(1–3), 113–118 CrossRef CAS PubMed.
- S. Therias, C. Mousty and C. Forano, et al., Electrochemical transfer at anionic clay modified electrodes. Case of 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonate), Langmuir, 1996, 12, 4914–4920 CrossRef CAS.
- A. Zebda, S. Tingry and C. Innocent, et al., Hybrid layered double hydroxides-polypyrrole composites for construction of glucose/O2 biofuel cell, Electrochim. Acta, 2011, 56(28), 10378–10384 CrossRef CAS.
- E. Jahangiri, S. Reichelt and I. Thomas, et al., Electron beam-induced immobilization of laccase on porous supports for waste water treatment applications, Molecules, 2014, 19(8), 11860–11882 CrossRef PubMed.
- H. Sun, W. Huang and H. Yang, et al., Co-immobilization of laccase and mediator through a self-initiated one-pot process for enhanced conversion of malachite green, J. Colloid Interface Sci., 2016, 471, 20–28 CrossRef CAS PubMed.
- S. Patra, S. Sene and C. Mousty, et al., Design of laccase–metal organic framework-based bioelectrodes for biocatalytic oxygen reduction reaction, ACS Appl. Mater. Interfaces, 2016, 8(31), 20012–20022 CrossRef CAS PubMed.
- Y. Liu, Y. Geng and M. Yan, et al., Stable ABTS immobilized in the MIL-100(Fe) metal–organic framework as an efficient mediator for laccase-catalyzed decolorization, Molecules, 2017, 22(6), 920–933 CrossRef PubMed.
- Z. Gao, Y. Yi and J. Zhao, et al., Co-immobilization of laccase and TEMPO onto amino-functionalized magnetic Fe3O4 nanoparticles and its application in acid fuchsin decolorization, Bioresour. Bioprocess., 2018, 5(1), 1–8 CrossRef.
- Y. Gu, P. Xue and F. Jia, et al., Co-immobilization of laccase and ABTS onto novel dual-functionalized cellulose beads for highly improved biodegradation of indole, J. Hazard. Mater., 2019, 365, 118–124 CrossRef PubMed.
- P. Xue, X. Liu and Y. Gu, et al., Laccase-mediator system assembling co-immobilized onto functionalized calcium alginate beads and its high-efficiency catalytic degradation for acridine, Colloids Surf., B, 2020, 196, 111348–111354 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |