Turn-on detection of assorted phosphates by luminescent chemosensors
Pramod
Kumar†
 *,
Sanya
Pachisia
*,
Sanya
Pachisia
 and
Rajeev
Gupta
and
Rajeev
Gupta
 *
*
Department of Chemistry, University of Delhi, Delhi-110007, India. E-mail: rgupta@chemistry.du.ac.in; Tel: +91-11-27666646 Web: http://people.du.ac.in/˜rgupta/
First published on 7th May 2021
Abstract
This review article presents a variety of luminescent chemosensors for the selective detection of assorted biologically relevant phosphates (phosphate ion, pyrophosphate ion and AMP, ADP and ATP) via the “Turn-On” emission mechanism. The focus has been placed to understand the design aspects, chemical structures and luminescence properties including binding constants, detection limits and lifetime parameters of the chemosensors and their emission enhancement based detection mechanism. The article concludes by addressing various future prospects both for the biological and the environmental challenges in front of the scientific community to develop intelligent chemosensors not only for detection purposes but also for remediation processes.
1. Introduction
Phosphate ion and phosphate containing compounds (PPi, AMP, ADP and ATP) play numerous important roles in various biological processes in living organisms.1–3 Phosphate ion and phosphate containing compounds are not only important nutrients for living organisms but also required for the biosynthesis of adenosine phosphates,4 nucleic acids,5,6 phospholipids7 and proteins.3,8 Phosphate containing compounds are also known to participate in various metabolic pathways, including energy transfer,3,9 protein activation10,11 and amino acid metabolism.3,11 For example, while adenosine phosphates (AMP, ADP and ATP) play significant roles in metabolic reactions, such as energy realization,3,9,11 phosphate group-donors12 and as signalling molecules;13 the pyrophosphate ion (PPi) is essential both for DNA sequencing14 and for DNA replication.15In addition to their biological significance, phosphates are also indispensable components of numerous medicinal and pharmaceutical compounds,16 detergents,17 fertilizers,18 personal care products19 and building and construction materials20 while play important roles in the chemical and alloy industries.21 Due to their widespread roles in biology, environment and industry, phosphates are responsible for contaminating the biological and environmental media as well as other habitats including water bodies.22 As a consequence, the WHO has recommended 0.1 mg L−1 as the permissible limit for the phosphate ion in drinking water.23 Therefore, monitoring of phosphate ion and different phosphate containing compounds is very critical and essential.24,25 However, it is very challenging to selectively recognize either a phosphate ion or different phosphate containing compounds.24–26 Such a difficulty is associated with their pH sensitivity and solution dynamics of either a phosphate ion or different phosphate containing compounds.24,26 For example, phosphate ion can exist in PO43−, HPO42−, H2PO4− and of course H3PO4 forms depending on the solution pH, and all such forms are considerably solvated.24,26 Other higher phosphates, such as the pyrophosphate ion (PPi) and adenosine phosphates (AMP, ADP and ATP), are not any exception both in terms of their pH sensitivity and solvation energy.24,26 The abovementioned challenges become more prominent either when the phosphate ion and phosphate containing compounds are present in aqueous media or when dealing with biological samples.26,27
Several analytical techniques have been utilized for the detection of phosphate ions and different phosphate containing compounds.25,26 Such techniques vary from conventional absorption28 and NMR (1H and 31P) spectroscopy29 and electrochemical studies30–32 (cyclic and square-wave voltammetry) to surface-based methods26,33 (electron microscopy including SEM and TEM) to X-ray diffraction methods34 (powder and single crystal) to isothermal titration calorimetry (ITC), which provides quantitative information including enthalpy, entropy and free energy values for a binding event.26,35 However, many such techniques suffer from various disadvantages including detailed instrumentation, sample preparation, only provisions for the solid-sample analysis and the requirement of dried samples that may not correctly represent the actual solution-based species.26 In this context, fluorescence spectroscopy based detection methods are often considered superior due to their high selectivity and sensitivity as well as fast and easy operation.36,37 Moreover, fluorescent spectral techniques are particularly convenient for cell imaging work and when dealing with biological samples.38
Designing a chemosensor for the selective detection of a phosphate ion or various phosphate containing compounds is a challenging task as it requires linking a fluorophore with a receptor with the potential of recognizing an analyte while overcoming pH and solvation related issues.24,26 Despite such challenges, there has been tremendous advancement in the synthesis of assorted chemosensors which utilize various non-covalent interactions including hydrogen bonding, halogen bonding, and anion⋯π and π⋯π interactions for selective binding and therefore sensing of a phosphate ion or various phosphate containing compounds.26,36,37 Consequently, both organic compound based and metal complex based chemosensors have been developed and exploited for the recognition and binding of various phosphates.24,26,36,37 The present review article aims to apprise readers about the chemical diversities of various chemosensors and their mode of interaction that is responsible for a detection event. Efforts have also been made to include crystallographic structures,‡ wherever possible, in order to highlight the mode and/or place of interaction between a chemosensor and an analyte.
2. Scope
This review article presents assorted luminescent chemosensors that have been developed for the “Turn-On” emission based detection of different phosphate ions and related phosphate containing compounds. However, this review article has excluded (i) non-luminescent chemosensors; (ii) emission turn-off chemosensors; and (iii) sequestration based chemosensors for the removal of chelated metal ion(s) by using assorted phosphates and related compounds.The present review article has been divided into two major categories: (i) organic molecule based chemosensors and (ii) metal complex based chemosensors whereas sub-categories have been created based on the nature of the analyte – mono-phosphates, di-phosphates and adenosine phosphates. While this article is aimed to showcase the chemical diversities of assorted chemosensors and their mode of interaction with phosphates, binding information and crystallographic data wherever available have also been included to illustrate the interaction between a chemosensor and an analyte.
3. Organic molecule based chemosensors
In this section, detection of assorted phosphates utilizing organic molecule based chemosensors, showing “Turn-On” emission properties, has been discussed.3.1. Detection of mono-phosphates (PO43−, HPO42−, and H2PO4−)
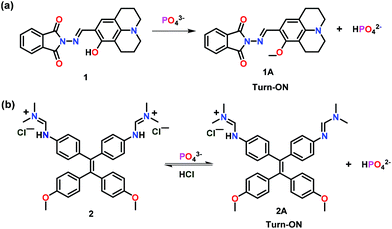
This section has summarized assorted fluorescent chemosensors used for the ‘Turn-On’ detection of mono-phosphates such as PO43−, HPO42− and H2PO4−. Kim and co-workers39 have developed a multifunctional hydroxyjulolidine based chemosensor 1 for the fluorescence detection of PO43− ions (Fig. 1a). Interestingly, the presence of phosphate ions led to a significant emission enhancement in a buffer/DMSO mixture (7/3, v/v, pH = 7.0). In comparison with other competing anions, the presence of PO43− ions manifested a strong fluorescence at 487 nm (λex = 426 nm) to otherwise non-emissive 1. Absorption spectral studies revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between 1 and PO43− ions while emission spectral titration yielded a binding constant of 5.7 × 102 M−1. Enhancement in the emission intensity of chemosensor 1 was related to the deprotonation of the phenolic–OH group (1A), as suggested by the experimental results and theoretical calculations. In addition to phosphate ions, chemosensor 1 was also found to detect Cu2+ and S2− ions.
1 binding stoichiometry between 1 and PO43− ions while emission spectral titration yielded a binding constant of 5.7 × 102 M−1. Enhancement in the emission intensity of chemosensor 1 was related to the deprotonation of the phenolic–OH group (1A), as suggested by the experimental results and theoretical calculations. In addition to phosphate ions, chemosensor 1 was also found to detect Cu2+ and S2− ions.
 | ||
| Fig. 1 Chemical drawings of chemosensors (a) 1 and (b) 2 and their proposed ‘Turn-On’ emission mechanism with PO43− ions. Adapted from ref. 39 and 40. | ||
A cationic tetraphenylethylene dimethylformamidine derivative based chemosensor 2 was developed for the detection of PO43− ions (Fig. 1b).40 The dihydrogen chloride salt of 2 was soluble in water but was non-emissive. Notably, on addition of PO43− ions, non-emissive 2 showed an excellent fluorescence at 502 nm (λex = 368 nm) in aqueous medium. Importantly, other anions as well as alkali metals and alkali earth metals did not interfere in the emission behaviour of 2. UV–Vis spectral titration inferred a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between 2 and PO43− ions with a binding constant of 2.36 × 104 M−1. Authors showed that only one equivalent of PO43− ions transformed non-emissive 2 to its emissive derivative 2A and HPO42− through aggregation-induced emission (AIE).41 Interestingly, 2A and HPO42− ions formed an aggregate due to the low solubility of 2A in water and such a fact resulted in emission enhancement through the AIE phenomenon. The presence of one free dimethyl–formamidine unit in 2A, where imine double bonds and phenyl rings were in conjugation, was responsible to increase the conjugation and thus emission enhancement. However, due to the absence of such a conjugation, chemosensor 2 was non-emissive. The presence of an acid was found to reversibly transform 2A into 2.
1 stoichiometry between 2 and PO43− ions with a binding constant of 2.36 × 104 M−1. Authors showed that only one equivalent of PO43− ions transformed non-emissive 2 to its emissive derivative 2A and HPO42− through aggregation-induced emission (AIE).41 Interestingly, 2A and HPO42− ions formed an aggregate due to the low solubility of 2A in water and such a fact resulted in emission enhancement through the AIE phenomenon. The presence of one free dimethyl–formamidine unit in 2A, where imine double bonds and phenyl rings were in conjugation, was responsible to increase the conjugation and thus emission enhancement. However, due to the absence of such a conjugation, chemosensor 2 was non-emissive. The presence of an acid was found to reversibly transform 2A into 2.
Wu and co-workers42 have reported a tetraphenylethene (TPE) based tripodal tris-urea chemosensor 3 for the selective detection of PO43− ions (Fig. 2a). In the presence of PO43− ions, 3 showed a 20-fold fluorescence enhancement in DMSO. Both experimental and computational studies revealed strong H-bonding between 3 and PO43− ions with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry thus forming a capsular assembly. ‘Turn-On’ behaviour was attributed to strong interaction of PO43− ions with 3 which restricted the rotation of the terminal TPE units. Other monophosphates, such as HPO42− and H2PO4− ions, although inducing significant changes in the NMR spectra of 3 exhibited very weak fluorescence and rather formed 1
1 stoichiometry thus forming a capsular assembly. ‘Turn-On’ behaviour was attributed to strong interaction of PO43− ions with 3 which restricted the rotation of the terminal TPE units. Other monophosphates, such as HPO42− and H2PO4− ions, although inducing significant changes in the NMR spectra of 3 exhibited very weak fluorescence and rather formed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 based half-capsules. The crystal structure of chemosensor 3 displayed a tripodal cavity which exhibited both intramolecular and intermolecular N–H⋯O H-bonds, leading to a 1D assembly. Such a fact was the probable reason for the AIE effect displayed by 3. On the other hand, H-bonding based half-capsular self-assembly between the HPO42− ion and 3via three urea units was confirmed by X-ray crystallography wherein HPO42− ions were bound via seven N–H⋯O H-bonds of the three urea arms present in 3 (Fig. 2b). In contrast, DFT studies of 3·orthophosphate revealed the formation of 12 strong N–H⋯O H-bonds in addition to CH⋯π interactions between the adjacent TPE moieties which restricted their movement and caused emission enhancement. Interestingly, docking studies of 3 with PO43− ions supported the DFT studies in which N–H protons were involved in H-bonding with the phosphate ion (Fig. 2c).
1 based half-capsules. The crystal structure of chemosensor 3 displayed a tripodal cavity which exhibited both intramolecular and intermolecular N–H⋯O H-bonds, leading to a 1D assembly. Such a fact was the probable reason for the AIE effect displayed by 3. On the other hand, H-bonding based half-capsular self-assembly between the HPO42− ion and 3via three urea units was confirmed by X-ray crystallography wherein HPO42− ions were bound via seven N–H⋯O H-bonds of the three urea arms present in 3 (Fig. 2b). In contrast, DFT studies of 3·orthophosphate revealed the formation of 12 strong N–H⋯O H-bonds in addition to CH⋯π interactions between the adjacent TPE moieties which restricted their movement and caused emission enhancement. Interestingly, docking studies of 3 with PO43− ions supported the DFT studies in which N–H protons were involved in H-bonding with the phosphate ion (Fig. 2c).
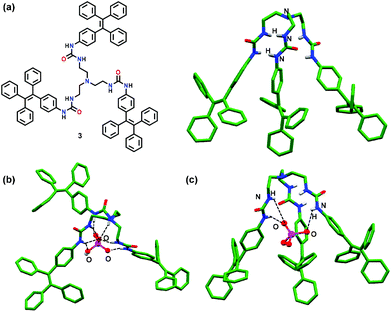 | ||
| Fig. 2 (a) Chemical drawing and crystal structure of chemosensor 3; (b) crystal structure depicting inclusion of the monohydrogen phosphate ion (HPO42−) within the cavity of 3; (c) molecular docking of 3 with the PO43− ion. Hydrogen atoms except for N–H groups have been omitted clarity. Adapted from ref. 42. | ||
Zhang and co-workers43 have reported crystallographically characterized chemosensor 4 for the highly selective sensing of XPO42− ions (X = H+ or Na+) over other phosphates such as PPi and ATP both exogenously and endogenously (Fig. 3a–b). Chemosensor 4 exhibited 91-fold fluorescence enhancement accompanied by a 79 nm red-shift in the presence of XPO42− ions. These spectral changes could be appreciably correlated with a color change from colorless to light yellow. Importantly, the presence of phosphate ions triggered amide bond cleavage in 4 causing the fluorophore to release, thus, inducing a distinct photo-physical change. Theoretical studies including quantum-mechanical calculations and TDFT aided in elucidating the high selectivity of 4 towards phosphate ions over PPi and other higher structural analogues. In hemichannel-closed Sf9 cells, 4 was able to trace both the generation and accumulation of XPO42− ions via the fluorescent spectral changes.
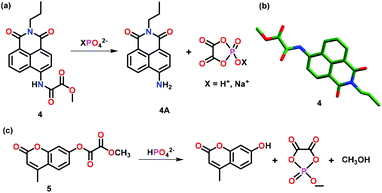 | ||
| Fig. 3 (a) Chemical drawing of chemosensor 4 and its proposed ‘Turn-On’ emission mechanism with XPO42− ion (X = H+ or Na+). (b) Crystal structure of the chemosensor 4. (c) Chemical drawing of chemosensor 5 and its ‘Turn-On’ emission mechanism with HPO42− ions. Adapted from ref. 43 and 44. | ||
Zhou and co-workers44 have reported a colorimetric and fluorescent chemosensor 5 for the detection of HPO42− ions in DMSO–HEPES buffer (v/v = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; pH 7.4) (Fig. 3c). Chemosensor 5 undergoes a unique HPO42− ion induced hydrolytic reaction that produces a colorimetric change associated with a 62 nm red-shift in the UV–Vis spectrum and a 780-fold enhancement in the emission intensity. Theoretical calculations supported the ester bond hydrolysis for the reaction of 5 with HPO42− ions which was further confirmed by the observation of a peak at m/z 151.08 in the mass spectrum corresponding to the cyclic phosphate. Chemosensor 5 was used for localizing exogenous and endogenous phosphate ions in HeLa cells and C. elegans as observed by fluorescence imaging. It was also used to monitor in vivo phosphate ion production due to apyrase catalyzed ATP hydrolysis. The in vitro phosphate ion release was utilized to elucidate the mechanism of Inx3 action in hemichannel-closed Sf9 cells.
1; pH 7.4) (Fig. 3c). Chemosensor 5 undergoes a unique HPO42− ion induced hydrolytic reaction that produces a colorimetric change associated with a 62 nm red-shift in the UV–Vis spectrum and a 780-fold enhancement in the emission intensity. Theoretical calculations supported the ester bond hydrolysis for the reaction of 5 with HPO42− ions which was further confirmed by the observation of a peak at m/z 151.08 in the mass spectrum corresponding to the cyclic phosphate. Chemosensor 5 was used for localizing exogenous and endogenous phosphate ions in HeLa cells and C. elegans as observed by fluorescence imaging. It was also used to monitor in vivo phosphate ion production due to apyrase catalyzed ATP hydrolysis. The in vitro phosphate ion release was utilized to elucidate the mechanism of Inx3 action in hemichannel-closed Sf9 cells.
Rurack and co-workers45 have reported a pyridine-2,6-dicarboxamide based chemosensor 6 containing two styryl chromophores (Fig. 4a). Chemosensor 6 exhibited an emission output between 500 and 600 nm which further increased in the presence of H2PO4− ions due to the formation of intramolecular H-bonds in the resultant host–guest ensemble. The formation of intramolecular H-bonds was confirmed by the crystal structure analysis of 6·DMF and 6·DMSO which assisted in understanding the sensing mechanism and the generation of the actual species responsible for the emission enhancement (Fig. 4b). A solvent molecule was found to create H-bonds through its O-atom with the amidic N–H groups. The chemosensor existed in an unusual conformation where one of its styryl groups was coplanar along with the pyridine-2,6-dicarboxamide unit whereas the second one was at a slightly tilted angle which allowed either a solvent molecule or a guest to reside within the cavity and form both inter- and intra-molecular H-bonds.
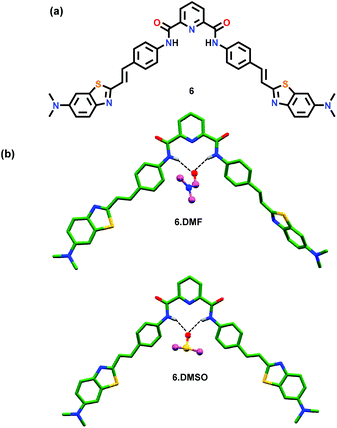 | ||
| Fig. 4 (a) Chemical drawing of chemosensor 6. (b) Crystal structures of 6·DMF and 6·DMSO displaying H-bonds between a solvent molecule (DMF or DMSO) and amidic N–H groups; other hydrogen atoms have been excluded for clarity. Adapted from ref. 45. | ||
Ghosh and co-workers46 have reported a series of anthracene appended bis-amide based chemosensors 7–10 bearing different spacers (Fig. 5). All four chemosensors exhibited significant ‘Turn-On’ fluorescent sensing for the H2PO4− ion in CH3CN. The sensing ability of such chemosensors was highly dependent on the type of spacer between the amide groups. During the binding with the H2PO4− ion, monomer emissions of 7–10 were little perturbed while the excimer emissions at ca. 500 nm were amplified due to the closeness of the anthracene moieties. The excimer emission of 8 was found to increase many-fold as compared to 7. This was presumably due to the fewer conformational changes of 8 containing a picolinamide spacer than that of 7 bearing an isophthalamide spacer. In the case of 9 and 10, glutaroyl and adipoyl spacers introduced greater flexibility and thus repositioning of the anthracenyl groups becomes much easier. Hence, excimer emission was enhanced for chemosensors 9 and 10 in the presence of H2PO4− ions. Interestingly, excimer growth in 10 (with a flexible spacer) was significantly greater than in 7. Due to the significant binding of H2PO4− ions, these chemosensors were used for cell staining and DNA interaction studies. This was also one of the first examples where amide-based receptors exhibited significant interaction with DNA.
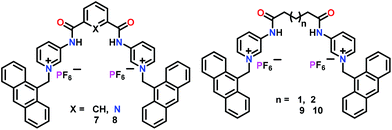 | ||
| Fig. 5 Chemical drawings of chemosensors 7–10. Adapted from ref. 46. | ||
Molina and co-workers47 have developed BINOL-based chiral chemosensors (11–14) bearing triazolium linked ferrocenyl or phenyl groups (Fig. 6). These chemosensors functioned based on the anion⋯anion interactions for the detection of H2PO4− ions. NMR spectral studies indicated selective interaction of these cationic receptors with HP2O73− and H2PO4− ions through non-covalent interactions. However, optical studies confirmed that only H2PO4− ions exhibited changes in absorption and fluorescence spectra in CH3CN. As expected, 14 showed very weak emission due to the presence of bulkier ferrocene and iodide groups and the emission was not restored after the addition of any phosphate. On the other hand, 11 showed little enhancement for monomer emission at 365 nm along with a new excimer emission at 465 nm with H2PO4− ions. 11 also worked as the “naked eye” fluorescent detector for H2PO4− ions in CH3CN. Chemosensor 12 showed weak emission bands at 359, 374, and 390 nm in CH3CN but exhibited significant enhancement with H2PO4− ions. Likewise, 13 exhibited similar sensing behaviour as noted for 12 with 30-fold quantum yield related to the free chemosensor. The crystal structure of 11 with PPi confirmed the existence of H-bonding interactions where C–H units of the triazolyl moiety behaved as the H-bond donor to the H-bond acceptor anion H2P2O72− (Fig. 6b).
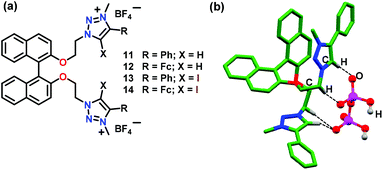 | ||
| Fig. 6 (a) Chemical drawings of chemosensors 11–14. (b) Crystal structure of 11·H2P2O72− depicting H-bonding between the host and H2P2O72− ion. Hydrogen atoms, except for those involved in H-bonding interactions, have been omitted for clarity. Adapted from ref. 47. | ||
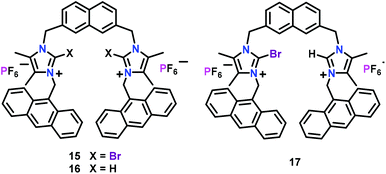
Molina and co-workers48,49 have developed imidazolium-based chemosensors 15–17 containing 2,7-disubstituted naphthalene while containing appended anthracene groups as the fluorophores (Fig. 7). The three chemosensors exhibited characteristic bands at 396, 418 and 442 nm for the anthracene moiety in CH3CN (λex = 370 nm). The Br-substituted 15 worked as a selective chemosensor for the H2PO4− ion and promoted excimer formation of anthracene (at 465 nm) with a concomitant quenching of the monomer emission bands. Chemosensor 15 however showed quenching in the presence of F−, SO42− and HP2O73− anions. Interestingly, addition of HP2O73− ions to 15 showed two different concentration effects; addition of up to 1 equiv. exhibited a weak enhancement of excimer emission and quenching of the monomer emission bands. However, further addition of HP2O73− ions only enhanced the monomer emission bands. Chemosensor 16 also showed quenching of the monomer emission bands in the presence of phosphates, F− and SO42− ions. Similar to 15, 17 showed nearly identical behaviour both towards H2PO4− and HP2O73− ions. In the presence of H2PO4− ions, 17 also showed a remarkable quenching of the monomer emission bands and a progressive formation of the anthracene excimer band. With HP2O73− ions, 17 showed two unlike effects: addition of up to 1.8 equiv. induced an enhancement of the monomer emission bands while further addition of more than 1.8 equiv. promoted a continuous quenching of the monomer emission band. Experiments with HeLa cells proved that 15 showed very good cell membrane permeability and therefore could be used as an efficient imaging agent in living cells.
3.2. Detection of di-phosphates (pyrophosphate ion (PPi) and its derivatives)
In this section, organic molecule based chemosensors have been summarized, which were utilised for the ‘Turn-On’ detection of the pyrophosphate ion (PPi) and its derivatives.Sessler and co-workers50 have developed an excimer disaggregation induced emission (EDIE) method51 for the detection of pyrophosphate and phosphate ions. The dipositively charged macrocycle 18 containing pyridyl-imidazolium groups was found to exhibit a supramolecular polymer in the solid state as established by crystallography (Fig. 8). The presence of pyridine rings, cationic imidazolium and phenylene groups made 18 behave as a potential intermolecular H-bond acceptor as well as donor. Interestingly, 18 showed a concentration-dependent fluorescence at 415 nm (λex = 334 nm) and exhibited emission enhancement up to 0.02 mM concentration in CH3CN. On further increase, concomitant formation of an excimer (ca. 500 nm) was noted due to the self-association led aggregation. Macrocycle 18 showed a 200-fold emission enhancement with HP2O73− ions as compared to other oxo-anions and anions. Turn-on fluorescence was also observed with H2PO4− and HCO3− ions but to a lesser extent. Turn-on detection of HP2O73− ions occurred due to the dis-aggregation of non-fluorescent excimers to generate monomers with strong emission. The formation of a fluorescent H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G complex with HP2O73− (1
G complex with HP2O73− (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and H2PO4− (1
1) and H2PO4− (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) diminished the strong interactions in excimer formation, as confirmed by the crystallographic studies (Fig. 8b). Single crystal analysis of 18 with pyrophosphate ions exhibited a sitting-top anion-complex which minimized the interaction of macrocycles with each other and hence induced an enhancement in the fluorescence intensity due to the dis-aggregation of the excimer. The crystal structure of 18·2H2PO4 exhibited a similar stoichiometry as noted in the solution state (Fig. 8b). Herein, two H2PO4− anions existed together stabilized by H-bonds between them. The structure was further stabilized by the occurrence of both anion⋯π interactions and H-bonds formed between charged (imidazolium rings) and neutral (benzene ring) C–H groups to that of anion–O atoms.
2) diminished the strong interactions in excimer formation, as confirmed by the crystallographic studies (Fig. 8b). Single crystal analysis of 18 with pyrophosphate ions exhibited a sitting-top anion-complex which minimized the interaction of macrocycles with each other and hence induced an enhancement in the fluorescence intensity due to the dis-aggregation of the excimer. The crystal structure of 18·2H2PO4 exhibited a similar stoichiometry as noted in the solution state (Fig. 8b). Herein, two H2PO4− anions existed together stabilized by H-bonds between them. The structure was further stabilized by the occurrence of both anion⋯π interactions and H-bonds formed between charged (imidazolium rings) and neutral (benzene ring) C–H groups to that of anion–O atoms.
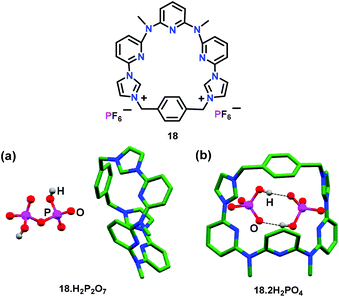 | ||
| Fig. 8 Chemical drawing of chemosensor 18. Crystal structures of (a) 18·H2P2O7 and (b) 18·2H2PO4. Hydrogen atoms except for phosphate anions have been excluded for clarity. Adapted from ref. 50. | ||
Sessler and co-workers52 have reported a calix[4]pyrrole based receptor 19 (Fig. 9a) for the detection of PPi using indicator displacement assay (IDA).53 For this purpose, tetrabutylammonium-2-oxo-4-(trifluoromethyl)-2H-chromen 7-olate (chromenolate ion) was utilized, which formed a non-emissive complex with 19. After the addition of the pyrophosphate ion (PPi) into CH3CN, a non-emissive complex showed an enhanced emission at 500 nm due to the replacement of the chromenolate ion. A similar fluorescence change was observed in aqueous medium. The binding constant (Kb × 106 M−1) for the detection of PPi by 19 was 25.5 in CH3CN and 3.63 in 30% water–CH3CN mixture. A combination of H-bonding, anion⋯π and electrostatic interactions was responsible for the detection of pyrophosphate ions, which was also supported by the crystal structure of free 19 (Fig. 9b).
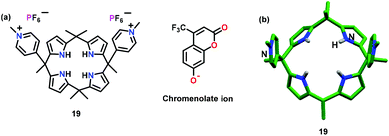 | ||
| Fig. 9 Chemical drawing (a) and crystal structure (b) of chemosensor 19. Adapted from ref. 52. | ||
Molina and co-workers54 have developed a series of dicationic triazolium–pyrene based receptors 20–23 (Fig. 10). In these chemosensors, detection of pyrophosphate ions was triggered by the operation of only H-bonds (20), only halogen-(X)-bonds (21 and 22) and a combination of both H- and X-bonding interactions (23). These chemosensors showed a dramatic fluorescent enhancement of the excimer band at 473 nm (λex = 340 nm) in the presence of only HP2O73− ions as compared to other anions. It was found that only hydrogen pyrophosphate formed X-bonding complexes with these receptors and the association constants were in the order of ≈106 M−1 while detection limits were of order ≈10−7 M. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was calculated via1H NMR spectral titrations of chemosensors 20–23 with HP2O73− ions. NMR spectral titrations and computational studies suggested that in comparison with the H-bonded analogue, X-bonded complexes increased the strength of hydrogen pyrophosphate binding.
1 stoichiometry was calculated via1H NMR spectral titrations of chemosensors 20–23 with HP2O73− ions. NMR spectral titrations and computational studies suggested that in comparison with the H-bonded analogue, X-bonded complexes increased the strength of hydrogen pyrophosphate binding.
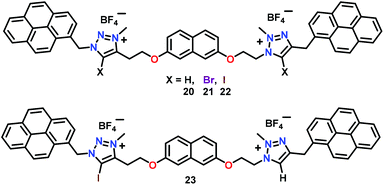 | ||
| Fig. 10 Chemical drawings of chemosensors 20–23. Adapted from ref. 54. | ||
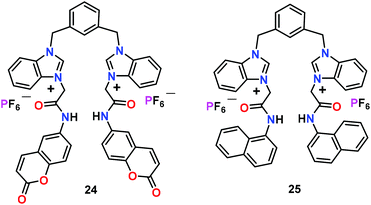
Ghosh and co-workers55 have reported benzimidazolium-based receptors 24 and 25 with identical binding sites, while bearing different fluorophores (Fig. 11). Both receptors displayed different sensing behaviour under identical conditions for different phosphates. At lower concentration of phosphates, emission enhancement was observed for HP2O73− and H2PO4− ions by 24 and 25, respectively. The sensing difference between 24 and 25 was due to the position of the fluorophores which optimized the binding cavity size of a receptor. At higher concentration of anions, 24 revealed selectivity in the sensing of HP2O73− and H2PO4− ions in CH3CN. However, 25 only detected F− ions under identical conditions. It was noted that F− ions exhibited strong affinity with amide and benzimidazolium groups of 24. Interestingly, both chemosensors 24 and 25 formed supramolecular gels with HP2O73− and H2PO4− ions, respectively. The gel formation ratified visual sensing of HP2O73− and H2PO4− ions as other anions were unsuccessful. Authors reasoned that gelation of 24 and 25 took place due to the intermolecular chelation of the benzimidazolium-based receptors to the anion wherein solvent molecules were entrapped. In aqueous CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v), chemosensor 25 showed moderate selectivity for ATP while 24 did not show any selectivity for any phosphate. Experimental results and DFT calculations showed that the different cavity size of 24 and 25, due to different fluorophores around the binding sites as well as the involvement of fluorophores–protons in the interaction with anions, was responsible for anion selectivity.
1; v/v), chemosensor 25 showed moderate selectivity for ATP while 24 did not show any selectivity for any phosphate. Experimental results and DFT calculations showed that the different cavity size of 24 and 25, due to different fluorophores around the binding sites as well as the involvement of fluorophores–protons in the interaction with anions, was responsible for anion selectivity.
 | ||
| Fig. 11 Chemical drawings of chemosensors 24 and 25. Adapted from ref. 55. | ||
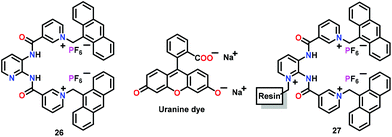
Ghosh and co-workers56 have used a positively charged pyridinium-functionalized chemosensor 26 for the selective detection of HP2O73− ions in both CH3CN and CH3CN/Tris-HCl buffer (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH = 6.5) (Fig. 12). Chemosensor 26 illustrated a new excimer emission peak at 520 nm with HP2O73− ions in CH3CN. However, in aqueous–CH3CN, monomer emission (412 nm) considerably increased without any peak corresponding to the excimer/exciplex. The binding constant for the interaction of 26 with HP2O73− ions in CH3CN/H2O was 9.59 × 104 M−1 while the stoichiometry was 1
1 v/v, pH = 6.5) (Fig. 12). Chemosensor 26 illustrated a new excimer emission peak at 520 nm with HP2O73− ions in CH3CN. However, in aqueous–CH3CN, monomer emission (412 nm) considerably increased without any peak corresponding to the excimer/exciplex. The binding constant for the interaction of 26 with HP2O73− ions in CH3CN/H2O was 9.59 × 104 M−1 while the stoichiometry was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For practical application, they connected receptor 26 with a resin support. The resultant resin-beads (i.e., 27) efficiently sensed HP2O73− ions in CH3CN/H2O (4
1. For practical application, they connected receptor 26 with a resin support. The resultant resin-beads (i.e., 27) efficiently sensed HP2O73− ions in CH3CN/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) and pure aqueous medium at pH 6.5. In addition, both chemosensor 26 and its polymeric version 27 were used in the Indicator Displacement Assay (IDA) technique53 using uranine dye to support naked-eye detection of the HP2O73− ion. A combination of H-bonding, electrostatic interactions and the cleft size controlled the selective recognition of the guest. The blood serum experiments suggested that the resin-bead based 27 can also detect HP2O73− ions in the biological systems.
1 v/v) and pure aqueous medium at pH 6.5. In addition, both chemosensor 26 and its polymeric version 27 were used in the Indicator Displacement Assay (IDA) technique53 using uranine dye to support naked-eye detection of the HP2O73− ion. A combination of H-bonding, electrostatic interactions and the cleft size controlled the selective recognition of the guest. The blood serum experiments suggested that the resin-bead based 27 can also detect HP2O73− ions in the biological systems.
 | ||
| Fig. 12 Chemical drawings of chemosensors 26 and 27. Adapted from ref. 56. | ||
3.3. Detection of adenosine phosphates (AMP, ADP and ATP)
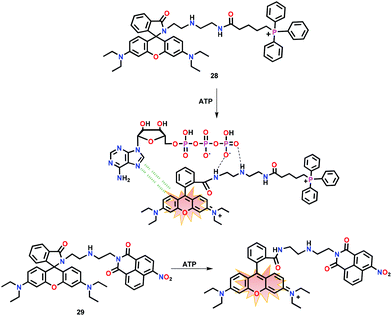
This section has summarized organic molecule based chemosensors for the ‘Turn-On’ detection of various adenosine phosphates such as AMP, ADP and ATP.Tan and co-workers57 have reported a rhodamine based chemosensor 28 containing a diethylenetriamine unit for anion binding and a triphenylphosphonium unit for mitochondrion targeting (Fig. 13). Chemosensor 28 was used for the selective recognition of ATP in mitochondria. In the presence of ATP (PBS, pH = 7.4), a non-emissive spirolactam form of 28 exhibited intense fluorescence at 583 nm (λex = 520 nm), manifesting that the spirolactam ring is in open conformation. The sequential addition of ATP progressively raised the fluorescence of 28 while reaching 81-fold at 10 mM ATP concentration. The spirolactam ring-opening produced a naked-eye colour change from colourless to pink with a detection limit of 0.033 mM. The ATP detection was due to the diethylenetriamine unit which interacted with the phosphate groups of ATP whereas π⋯π stacking between the adenine ring of ATP and xanthene moiety of rhodamine also contributed. The spectral titration showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of 28 with ATP with Kb of 215.6 M−1. The pH dependent fluorescence displayed an appreciable response towards ATP within the pH range of 6.0–8.0 which created the possibility for the detection of ATP in biological media. Notably, 28 was also used for monitoring and the recognition of mitochondrial ATP concentration in HeLa cells.
1 stoichiometry of 28 with ATP with Kb of 215.6 M−1. The pH dependent fluorescence displayed an appreciable response towards ATP within the pH range of 6.0–8.0 which created the possibility for the detection of ATP in biological media. Notably, 28 was also used for monitoring and the recognition of mitochondrial ATP concentration in HeLa cells.
 | ||
| Fig. 13 Chemical drawings of chemosensors 28 and 29 and their proposed binding with ATP through π⋯π interactions with the open conformation of the spirolactum ring. Adapted from ref. 57 and 58. | ||
Ma and co-workers58 have also reported a rhodamine-based chemosensor 29 for the detection of ATP in TRIS buffer at pH 7.4 (Fig. 13). In the presence of ATP, chemosensor 29 showed 110-fold ‘Turn-On’ emission at 580 nm (λex = 540 nm). The emission enhancement was observed as a result of spirolactam ring-opening involving π⋯π stacking and multiple H-bonding interactions. Chemosensor 29 was also used for ATP monitoring in live cells.
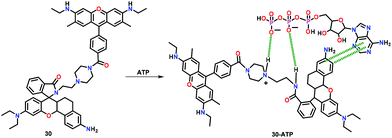
Yuan and co-workers59 have developed a reversible fluorescent chemosensor 30 for the quantitative detection and real-time monitoring of mitochondrial ATP in the living cells (Fig. 14). The chemosensor 30 showed a weak yellow fluorescence at 560 nm and a red fluorescence at 624 nm due to the photo-induced electron transfer (PET) in HEPES buffer (containing 10% EtOH; pH 7.4; λex = 500 nm). Upon addition of ATP, the PET process was blocked while a six-fold enhancement in the intensity ratio (F624/F560) was observed. Chemosensor 30 selectively and reversibly responded to ATP with a dissociation constant of 4.65 mM. Notably, 30 was used for the monitoring of mitochondrial ATP in the normal and the cancer cell lines.
 | ||
| Fig. 14 Chemical drawing of chemosensor 30 and its proposed binding with ATP. Adapted from ref. 59. | ||
Anzenbacher Jr. and co-workers60 have reported an anthracene functionalized chemosensor 31 bearing two imidazolium hydrazone groups for ATP detection in water (Fig. 15). Chemosensor 31 is non-fluorescent in aqueous medium; however, it exhibited a bright green coloured 35-fold ‘Turn-On’ emission at 504 nm in the presence of ATP. In contrast, only 8-fold ‘Turn-On’ emission was perceived in the presence of ADP. Emission spectral titrations yielded binding constants (Kb × 102 M−1) of 13.0 and 1.0 for ATP and ADP, respectively. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry was noted for ATP using Job's plot. The excellent selectivity of 31 towards ATP was the result of combined effects of π⋯π interactions between the adenine ring of ATP and anthracene unit of the chemosensor as well as electrostatic interactions between the oppositely charged phosphates and imidazolium groups of 31.
1 binding stoichiometry was noted for ATP using Job's plot. The excellent selectivity of 31 towards ATP was the result of combined effects of π⋯π interactions between the adenine ring of ATP and anthracene unit of the chemosensor as well as electrostatic interactions between the oppositely charged phosphates and imidazolium groups of 31.
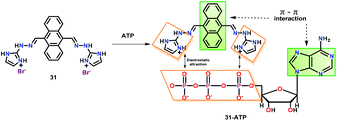 | ||
| Fig. 15 Chemical drawing of chemosensor 31 and its proposed binding with ATP through π⋯π interactions and electrostatic attraction. Adapted from ref. 60. | ||
Cao and co-workers61 have used related tetraphenylethene (TPE) based AIE chemosensors 32–36 for the recognition of ATP in aqueous medium (Fig. 16). In these chemosensors, two TPE moieties were connected to a bis-imidazolium core separated with different methylene spacers. All five chemosensors revealed weak fluorescence in aqueous solution; however, a significant emission enhancement was observed with ATP at 471 nm (λex = 365 nm) in a THF–water mixture due to TPE-based aggregation. Notably, binding affinity of chemosensors 32–36 towards ATP could be tuned with the aid of the spacer length, wherein, binding constants varied in the following order: 32 > 33 > 34 > 35 > 36 (11.9 × 104 to 1.46 × 104 M−1). Detection limits were found to be in the range of 4.15 to 16.8 nm. The dynamic light scattering (DLS) and 1H NMR spectral titrations explained the vital role of two imidazolium units in binding with the anionic triphosphate group of ATP. Such interactions were found to enhance the aggregation of chemosensors by decreasing the solubility and as a result exhibited an AIE-based ‘Turn-On’ emission.41 Importantly, binding affinity towards ATP and the chain length of two charged imidazolium units could be directly correlated. In fact, chemosensors with shorter linkers matched with the size of the phosphate anion of ATP, thus yielding a higher binding towards ATP.
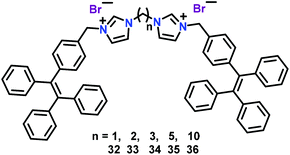 | ||
| Fig. 16 Chemical drawings of chemosensors 32–36. Adapted from ref. 61. | ||
Ma and co-workers62 have reported cationic chemosensors 37–39 containing pyridinium functionalized boronic acid groups for the AIE based41 selective recognition of ATP (Fig. 17). These chemosensors recognized ATP through AIE in a H2O–DMSO mixture at pH = 7. All three chemosensors exhibited emission enhancement with ATP. Among these receptors, the tripodal one (39) showed a better selectivity and sensitivity towards ATP with a detection limit of 0.3 ppm. The strong affinity of 39 with ATP was due to the presence of both electrostatic and covalent bonding interactions involving pyridinium and boronic acid groups, respectively. Authors concluded that the chemosensors with multiple binding sites were capable of detecting ATP efficiently from a pool of other anions. The penetrating power into cells, specifically nucleus, depended on the quantity of positive charges as well as the boronic acid groups in these chemosensors.
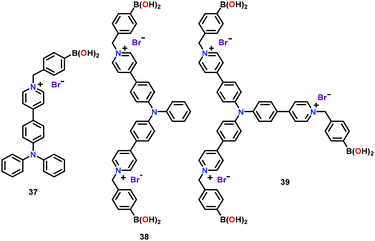 | ||
| Fig. 17 Chemical drawings of chemosensors 37–39. Adapted from ref. 62. | ||
Ghosh and co-workers63 have developed a tripodal receptor 40 containing pyridinium units linked with the appended naphthyl groups (Fig. 18). Chemosensor 40 showed emission at 386 nm (λex = 290 nm) in CH3CN/HEPES buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 6.5). Upon interaction with biologically relevant phosphates, the emission enhancement was observed at 390 nm for ATP over ADP and AMP. The emission peak at 390 nm was due to the exciplex formation between the naphthalene moieties of 40 and adenine unit of ATP. The binding constant and detection limit for ATP were found to be 1.16 × 103 M−1 and 8.44 μM, respectively, with a 1
1, v/v, pH = 6.5). Upon interaction with biologically relevant phosphates, the emission enhancement was observed at 390 nm for ATP over ADP and AMP. The emission peak at 390 nm was due to the exciplex formation between the naphthalene moieties of 40 and adenine unit of ATP. The binding constant and detection limit for ATP were found to be 1.16 × 103 M−1 and 8.44 μM, respectively, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between 40 and ATP. Interestingly, in CH3CN, chemosensor 40 was found to exclusively recognize H2PO4− ions over other anions and formed a significant excimer at 455 nm. The stoichiometry of the 40-H2PO4− complex was found to be 1
1 stoichiometry between 40 and ATP. Interestingly, in CH3CN, chemosensor 40 was found to exclusively recognize H2PO4− ions over other anions and formed a significant excimer at 455 nm. The stoichiometry of the 40-H2PO4− complex was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 while the binding constant and detection limit were 7.06 × 103 M−1 and 1.46 μM, respectively. Chemosensor 40 was also used for intracellular ATP detection through fluorescence imaging.
1 while the binding constant and detection limit were 7.06 × 103 M−1 and 1.46 μM, respectively. Chemosensor 40 was also used for intracellular ATP detection through fluorescence imaging.
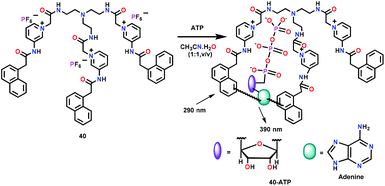 | ||
| Fig. 18 Chemical drawing of chemosensor 40 and its proposed binding with ATP. Adapted from ref. 63. | ||
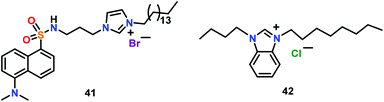
Cao and co-workers64 have reported an amphiphilic receptor 41 for the detection of ATP via emission enhancement through anion-induced self-assembly (Fig. 19). Chemosensor 41 was designed by incorporating a dansyl fluorophore. 41 formed self-assembled micelle (CMC = 7.67 μM) in PBS buffer at pH = 7.4. The micelle structure was highly selective for ATP and was found to increase the emission of 41 accompanied by a 27 nm blue shift while exhibiting a green emission at 508 nm. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, a binding constant of 2.8 × 105 M−1 and a detection limit of 150 nM were calculated for the detection of ATP. Both the significant change in the fluorescence intensity and blue shift were related to the strong interaction of 41 with ATP through imidazolium C–H and amide NH donors. The binding of ATP decreased the electron withdrawing ability of the sulphonyl group and inhibited internal charge transfer (ICT) occurring through the dimethylamino group to the sulfonyl moiety, thus, causing the fluorescent emission to shift towards a lower wavelength. High selectivity of 41 for ATP was utilized for its sensing in the living cells.
1 stoichiometry, a binding constant of 2.8 × 105 M−1 and a detection limit of 150 nM were calculated for the detection of ATP. Both the significant change in the fluorescence intensity and blue shift were related to the strong interaction of 41 with ATP through imidazolium C–H and amide NH donors. The binding of ATP decreased the electron withdrawing ability of the sulphonyl group and inhibited internal charge transfer (ICT) occurring through the dimethylamino group to the sulfonyl moiety, thus, causing the fluorescent emission to shift towards a lower wavelength. High selectivity of 41 for ATP was utilized for its sensing in the living cells.
Kang and co-workers65 have reported a benzimidazolium based ionic liquid 42 for the detection of ADP (Fig. 19). Chemosensor 42 formed micelles in the aqueous medium (pH = 7) and detected ADP over ATP and AMP via ‘Turn-On’ emission. Such an emission enhancement was due to the stabilization of an ADP-micelle ensemble via close proximity of the benzimidazolium moiety and ADP. The binding constant and detection limit for ADP were found to be 1.5 × 108 M−1 and 1.63 nM, respectively.
4. Metal complex based chemosensors
In this section, we have discussed metal complex based chemosensors for the detection of different types of phosphates via a luminescent ‘Turn-On’ mechanism. In general, metal ion(s) in such complexes provided coordination sites for the recognition of the phosphate group(s) whereas the attached ligands were found to participate in other types of interactions.4.1. Detection of mono-phosphates (PO43−, HPO42−, and H2PO4−)
In this section, we have summarized metal complex based chemosensors used for the ‘Turn-On’ detection of mono-phosphates such as PO43−, HPO42− and H2PO4−. Ghosh and co-workers66 have developed several luminescent Ru(II)–polypyridyl complexes for the recognition of different phosphates, mainly H2PO4− and HP2O73− ions. Such Ru–polypyridyl complexes functioned as the excellent phosphorescent chemosensors due to their unique optical properties such as long excited-state lifetime, visible region excitation, large Stokes shift and red-orange emission. The mononuclear Ru(II) complex 43 containing pyridine–triazolium and phenanthroline ligands was used for the sensing of H2PO4− ions and to a lesser extent HP2O73− ion (Fig. 20). Chemosensor 43 exhibited ligand-based π⋯π transitions at 262 nm while the characteristic MLCT bands appeared at 403 nm (Ru-to-phenanthroline) and 445 nm (Ru-to-pyridyl-triazolium). Chemosensor 43 showed a weak emission band at 590 nm on exciting at either λex = 403 or 445 nm. The absorption as well as emission spectra of 43 showed significant changes both with the H2PO4− and HP2O73− ions in CH3CN. The emission intensity of 43 was increased by 6-fold by H2PO4− and 3-fold by the HP2O73− ion, accompanied by a 10 nm red-shift. Upon sequential addition of H2PO4− and HP2O73− ions, absorption spectral features at 403 and 455 nm showed a marginal decrease with an increase at 465 nm. These spectral titrations provided binding constants (Kb × 104 M−1) of 5.28 and 4.67 for H2PO4− and HP2O73− ions, respectively, whereas detection limits were 0.25 and 0.45 μM, respectively. The lifetime of chemosensor 43 (5.45 ns) increased to 28.60 and 12.01 ns in the presence of H2PO4− and HP2O73− ions, respectively. These parameters displayed strong affinity of 43 towards H2PO4− as compared to the HP2O73− ion. 31P NMR spectral studies confirmed the participation of phosphate–O groups in bonding while a proton NMR spectrum showed a gradual downfield shift of the triazole proton. Finally, X-ray structural studies confirmed pyrophosphate ion binding via C–H⋯diphosphate interactions (Fig. 20).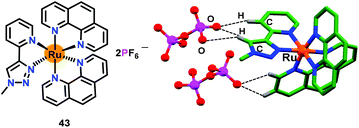 | ||
| Fig. 20 Chemical drawing of chemosensor 43 and the crystal structure of 43·H2P2O72−. Hydrogen atoms, except for those involved in H-bonding interactions, have been omitted for clarity. Adapted from ref. 66. | ||
Encouraged by the remarkable phosphate selectivity of Ru(II)–polypyridyl complex 43via C–H⋯anion interactions, Ghosh and co-workers developed67 a halogen bonding (X-bonding) based anion receptor 44 by substituting the triazole–C–H group with triazole–C–I to enhance the phosphate selectivity (Fig. 21). For comparative studies, an analogous compound 45 was also synthesized. Both Ru(II)–polypyridyl based chemosensors 44 and 45 displayed similar UV-Vis and emission spectra and anion binding properties as noted for 43. Both 44 and 45 detected H2PO4− ions preferably over HP2O73− as compared to other anions in CH3CN. The emission of 44 increased both with H2PO4− (17-fold) and HP2O73− ions (5-fold) in CH3CN. The binding constants (Kb × 104 M−1) were noted as 19.4 and 5.60 for H2PO4− and HP2O73− ions, respectively, while detection limits were 0.018 μM and 0.090 μM, respectively. Similarly, binding constants (Kb × 103 M−1) for the analogous 45 were, respectively, 55.9 and 9.71 for H2PO4− and HP2O73− ions, whereas detection limits were 0.05 μM and 0.25 μM, respectively. Both chemosensors 44 and 45 also worked as the lifetime based detectors where excited-state lifetimes for the free 44 and 45 enhanced both with H2PO4− and HP2O73− ions. Remarkably, chemosensor 44 with an I donor functioned as a superior sensor with higher binding affinities and greater excited-state lifetime enhancement when compared to its H–analogue (45). The crystal structure of 44 with H2PO4− and HP2O73− ions confirmed the presence of C–I⋯anion X-bonding interactions (Fig. 21a and b).
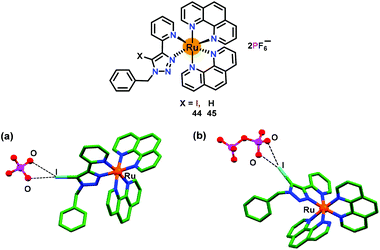 | ||
| Fig. 21 Chemical drawings of chemosensors 44 and 45. Crystal structures of (a) 44·H2PO4− and (b) 45·HP2O73− complexes. Adapted from ref. 67. | ||
After the successful incorporation of the triazole–C–I group into the Ru(II)–polypyridyl complex, Ghosh and co-workers68 integrated an additional anion binding urea arm in the triazole unit (Fig. 22). The chemosensor 46 bearing a triazole unit accompanied by an appended 4-fluorophenyl group was used for the sensing of anions. Chemosensor 46 was crystallographically characterized where a ruthenium ion exhibited a distorted octahedral geometry coordinated by two phenanthroline ligands and a pyridine–triazole unit (Fig. 22a). The binding constants (Kb × 104 M−1) for 46 were found to be 15.0 and 4.1 for H2PO4− and HP2O73− ions, respectively, while detection limits were 0.36 μM and 0.54 μM, respectively. In a similar manner, excited state lifetime of free 46 was enhanced in the presence of H2PO4− and HP2O73− ions. These parameters showed strong affinity of 46 towards H2PO4− as compared to HP2O73− ions. Notably, chemosensor 46 showed better sensing for phosphates in comparison with the non-urea analogue 43.
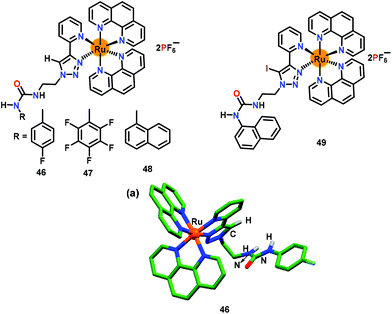 | ||
| Fig. 22 Chemical drawings of chemosensors 46–49. (a) Crystal structure of chemosensor 46; hydrogen atoms, except for the N–H groups, have been omitted for clarity. Adapted from ref. 68–70. | ||
Ghosh and co-workers69 further developed two related chemosensors, 47 and 48, having pentafluorophenylurea and 1-naphthylurea, respectively (Fig. 22). High binding constants (104–105 M−1), detection limits (0.020 and 0.580 μM) and excited state lifetimes were noted both for 47 and 48. Collectively, chemosensor 47 having acidic –NH urea protons revealed higher binding affinities for phosphates when compared to analogous chemosensors 46 and 48 (with urea) and 43 and 45 (non-urea).
Finally, to develop an effective phosphate sensor, Ghosh and co-workers70 synthesised iodotriazole linked naphthyl urea based receptor 49 (Fig. 22). The binding constants (Kb × 105 M−1) for 49 were 12.3 and 8.6 for H2PO4− and HP2O73− ions, respectively, whereas detection limits were 0.012 μM and 0.078 μM, respectively. Chemosensor 49 also showed excited-state lifetime enhancement from 10.7 ns (for free 49) to 206.1 ns and 101.1 ns for H2PO4− and HP2O73− ions, respectively. Chemosensor 49 was superior for phosphate binding in view of its high binding constant, lower detection limit and longer excited state lifetime as compared to other analogues, 43–48.
Similar to 43–49, Khatua and co-workers71 have developed a benzimidazole appended 1,2,3-triazole-pyridyl based chemosensor 50 which was crystallographically characterized (Fig. 23). Chemosensor 50 was used for the exclusive detection of H2PO4− and HP2O73− ions in CH3CN. The emission at 583 nm showed 10-fold enhancement after the addition of HP2O73− and H2PO4− ions. The binding constants (Kb × 103 M−1) were noted as 6.8 and 3.3 whereas detection limits were 5.19 and 5.73 ppb for HP2O73− and H2PO4− ions, respectively. 1H NMR spectral studies displayed downfield shifts for the triazole–CH protons which established its interaction with phosphate ions.
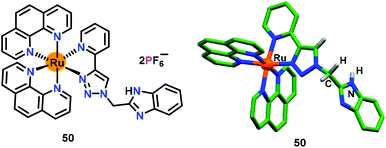 | ||
| Fig. 23 Chemical drawing and crystal structure of chemosensor 50. Hydrogen atoms, except for the N–H and CH2 groups, have been omitted for clarity. Adapted from ref. 71. | ||
Tong and co-workers72 have reported a Eu(III) based chemosensor 51 for the exclusive detection of PO43− ions in aqueous medium (Fig. 24). The detection limit towards the PO43− ion was found to be 4.3 nM which made it an excellent chemosensor for environmental water samples. The authors employed a new strategy by using a FRET sensitizer sodium dodecylbenzenesulfonate (SDBS) surfactant73 for the detection of PO43− ions. The intense blue emission for the free ligand at 415 nm (λex = 330 nm) was quenched to some extent after the formation of 51 due to FRET from the ligand to the Eu(III) centre. Interestingly, this FRET process was not effective to produce the fingerprint bands of the Eu(III) centre at around 615 nm (5D0–7F2). Interestingly, the FRET process was promoted when the carboxylate groups of 51 interacted with SDBS. Consequently, the PO43− ion selectively interacted with the Eu(III) center of 51 and significantly reduced the FRET process which enhanced the emission at 415 nm.
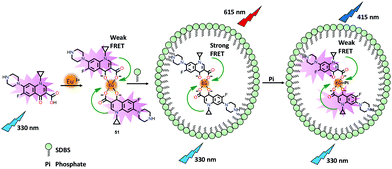 | ||
| Fig. 24 Chemical drawings of ciprofloxacin and its Eu(III) complex 51 that has been used for the detection of PO43− ions based on FRET from ciprofloxacin to Eu(III) ions induced by the surfactant SDBS. Adapted from ref. 72. | ||
4.2. Detection of di-phosphates (pyrophosphate ion (PPi) and its derivatives)
In this section, metal complex based chemosensors have been discussed for the recognition of the pyrophosphate ion (PPi) and its derivative via a ‘Turn-On’ emission mechanism.Gupta and co-workers74 have developed several related chemosensors based on phenol-derivatives while additionally containing an alcohol and an imine group for the recognition of Al3+ and Ga3+ ions. Pro-52 efficiently detected Al3+ and Ga3+ ions via ‘Turn-On’ emission in EtOH (containing 0.5% THF). Notably, the resultant Al3+ and Ga3+ based species 52 and 53 were able to further detect the pyrophosphate ion (PPi) and therefore functioned as the chemosensors (Fig. 25). Pro-52 showed emission enhancement with Al3+ (9-fold) and Ga3+ (3.8-fold) ions, suggesting the formation of 52 and 53. Consequently, addition of PPi further enhanced the emission by 2.5-fold and 4.4-fold for 52 and 53, respectively. Detailed mechanistic studies illustrated that PPi acted as a base and deprotonated the intact phenolic–OH group in 52 and 53. In the 1H NMR spectra, 52 and 53 showed a phenolic–OH signal at δ = 13.7 ppm which was noted to disappear in the presence of the P2O74− ion (PPi). The presence of PPi also produced a visible color change from pale pink to dark green. The pyrophosphate ion mediated deprotonation of 52 and 53 was further supported by the DFT studies.
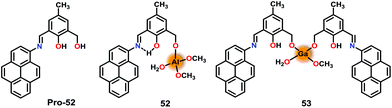 | ||
| Fig. 25 Chemical drawings of Pro-52 and chemosensors 52 and 53. Adapted from ref. 74. | ||
Earlier, Ghosh and co-workers have used Ru(II)–polypyridyl based chemosensors 43–49 for the selective sensing of H2PO4− over HP2O73− ion.66–70 Subsequently, these authors developed75 a trinuclear chemosensor 54 for the detection of phosphates (Fig. 26). Chemosensor 54 revealed similar photophysical properties as noted for 43–49.66–70 Detailed sensing studies revealed that while chemosensors 43–49 were highly selective towards H2PO4− over HP2O73− ion, 54 exhibited selective detection of HP2O73− over H2PO4− ion. Thus, a reverse selectivity was observed for different phosphates on changing a receptor from mononuclear to trinuclear one. Mononuclear receptors 43–49 possessed a single triazole –C–H unit for anion binding which preferred to bind a single negatively charged and smaller phosphate ion, H2PO4−. In contrast, trinuclear yet flexible 54, containing multiple binding sites, preferred to interact with a more negatively charged and bulkier phosphate ion, HP2O73− ion. Emission spectral studies confirmed the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 H
3 H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G complex between 54 and HP2O73−/H2PO4− ions. The overall binding constants (Kb × 1013 M−3) were 6.76 and 91.2 whereas detection limits were noted to be 0.02 and 0.45 mM for HP2O73− and H2PO4− ions, respectively. Interestingly, 54 showed enhancement in the excited state lifetime (4.78 ns) in the presence of H2PO4− (8-times) and HP2O73− (28-times). Furthermore, in different media (10% Tris-HCl buffer–90% CH3CN) 54 showed selectivity for AMP, ADP and ATP over HP2O73− and H2PO4− ions. The presence of multiple C–H⋯anion interactions was confirmed by the single crystal analysis of only chemosensor 54 (Fig. 26a) and its nitrate-complex (Fig. 26b). Notably, various weak C–H⋯O interactions were noted between the acidic C–H protons of the triazole unit and the phenanthroline moiety to that of nitrate ions.
G complex between 54 and HP2O73−/H2PO4− ions. The overall binding constants (Kb × 1013 M−3) were 6.76 and 91.2 whereas detection limits were noted to be 0.02 and 0.45 mM for HP2O73− and H2PO4− ions, respectively. Interestingly, 54 showed enhancement in the excited state lifetime (4.78 ns) in the presence of H2PO4− (8-times) and HP2O73− (28-times). Furthermore, in different media (10% Tris-HCl buffer–90% CH3CN) 54 showed selectivity for AMP, ADP and ATP over HP2O73− and H2PO4− ions. The presence of multiple C–H⋯anion interactions was confirmed by the single crystal analysis of only chemosensor 54 (Fig. 26a) and its nitrate-complex (Fig. 26b). Notably, various weak C–H⋯O interactions were noted between the acidic C–H protons of the triazole unit and the phenanthroline moiety to that of nitrate ions.
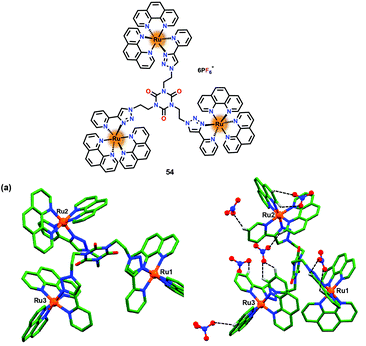 | ||
| Fig. 26 Chemical drawing of chemosensor 54. (a) Crystal structure of chemosensor 54 and (b) its nitrate complex displaying various C–H⋯O interactions between C–H groups and nitrate–O atoms. Hydrogen atoms, except for those involved in weak interactions, have been omitted for clarity. Adapted from ref. 75. | ||
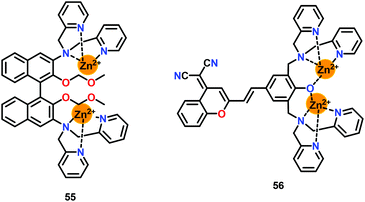
Li and co-workers76 have reported a dinuclear Zn-based chemosensor 55 containing chelating units bridged by a BINOL fluorophore for the detection of HP2O73− ions in HEPES buffer (1% CH3OH) (Fig. 27). The presence of Zn2+ ions induced a ‘Turn-On’ florescence at 383 nm (λex = 316 nm). The addition of HP2O73− ions further enhanced the emission at 383 nm while generating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with 55. The Kb was noted to be 6.7 × 105 M−1 while the detection limit was 95 nM. The ‘Turn-On’ detection of HP2O73− ions was owing to the overall rigidity of the system and the optimal distance between the two Zn2+ ions. Interestingly, chemosensor 55 also showed precipitation-based detection of HP2O73− ions at millimolar concentrations. Chemosensor 55 was successfully used for the detection of HP2O73− ions in the living cells.
1 complex with 55. The Kb was noted to be 6.7 × 105 M−1 while the detection limit was 95 nM. The ‘Turn-On’ detection of HP2O73− ions was owing to the overall rigidity of the system and the optimal distance between the two Zn2+ ions. Interestingly, chemosensor 55 also showed precipitation-based detection of HP2O73− ions at millimolar concentrations. Chemosensor 55 was successfully used for the detection of HP2O73− ions in the living cells.
Feng and co-workers77 have reported a phenol-bridged dinuclear Zn(II) complex 56 appended with a dicyanomethylene–benzopyran group exhibiting near-infrared emission at 639 nm (Fig. 27). Chemosensor 56 selectively sensed HP2O73− ions over other anions in HEPES buffer (pH = 7.4). Interestingly, addition of 0.5 equiv. of HP2O73− ions showed an initial emission quenching with a 14 nm red-shift. Consequently, further addition of HP2O73− ions exhibited emission enhancement at 654 nm. The initial emission quenching at low concentration of HP2O73− ions was due to the PET process from DPA moieties to the fluorophore. However, a stable 56-HP2O73− complex was formed at higher concentration of PPi while the ICT process was responsible for the emission enhancement. The detection of HP2O73− ions by 56 was accompanied by a Stokes shift of 150 nm. Chemosensor 56 also showed an excellent colorimetric detection by exhibiting colour change from orange to pink with PPi in aqueous medium. A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 56-HP2O73− complex was formed between 56 and PPi with a detection limit of 42 nM. Chemosensor 56 was found to operate over a wide pH range (6.05–9.70) and was also used in the cell imaging studies owing to its very low cytotoxicity.
1 56-HP2O73− complex was formed between 56 and PPi with a detection limit of 42 nM. Chemosensor 56 was found to operate over a wide pH range (6.05–9.70) and was also used in the cell imaging studies owing to its very low cytotoxicity.
Yam and co-workers78 have reported a terpyridine-coordinated platinum and DPA-coordinated zinc based chemosensor 57 for the detection of PPi in HEPES buffer (containing 30% CH3CN; pH 7.4) (Fig. 28). The UV–Vis spectral titration of 57 with PPi shows an increase in the 650 nm band assigned to dσ*(Pt⋯Pt) → π*(terpy) and a concomitant decrease in 415 nm band accompanied by an isosbestic point at 347 nm. A colour change from yellow to orange was observed for chemosensor 57 with PPi. The emission spectrum of chemosensor 57 with PPi showed an enhancement at 770 nm with a concomitant decrease in the higher-energy band at 600 nm, signifying the formation of aggregates. The formation of oligomers between 57 and PPi was confirmed by the spectroscopic studies. In 1H NMR spectral titration, aromatic-H signals of 57 were up-field shifted due to the aggregate formation. In electron microscopy studies, 57 was found to be randomly dispersed without any aggregation; however, upon addition of 1.5 equiv. of PPi, 57 formed long fibre-like aggregates. The energy dispersive X-ray (EDX) analysis revealed the presence of Pt, Zn and P elements in the aggregates. PPi formed a (57)2·PPi adduct with 57 through intermolecular Pt⋯Pt interactions (3.4 Å) wherein PPi was held between two zinc centres.
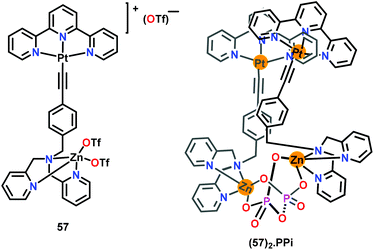 | ||
| Fig. 28 Chemical drawing of chemosensor 57 and its PPi complex (57)2·PPi. Adapted from ref. 78. | ||
4.3. Detection of adenosine phosphates (AMP, ADP and ATP)
In this section, metal complex based chemosensors have been discussed for the detection of adenosine phosphates (AMP, ADP and ATP) via the ‘Turn-On’ emission mechanism.Gupta and co-workers79 have reported hydroxide-bridged dizinc(II) complexes, 58–51, presenting a H-bonding based cavity (Fig. 29). In these complexes, the Zn-bound hydroxide group behaved as an H-bond donor to the heterocyclic rings which functioned as the H-bond acceptors. In contrast, para-anisidine appended rings in 61 were deprived of any H-bonding groups and thus the hydroxide group was not involved in any H-bonding. These chemosensors exhibited emission at ca. 510 nm (λex = 330 nm for 58–60 and 350 nm for 61) in a DMSO/HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v; pH 7.2). Importantly, chemosensors 58 and 59 showed nearly six-fold emission enhancement with ATP whereas negligible changes were observed with other phosphates and other anions. In contrast, 60 and 61 did not exhibit any considerable emission spectral change with any of the phosphates including ATP. The binding stoichiometry between 58 and 59 to that of ATP was found to be 1
1, v/v; pH 7.2). Importantly, chemosensors 58 and 59 showed nearly six-fold emission enhancement with ATP whereas negligible changes were observed with other phosphates and other anions. In contrast, 60 and 61 did not exhibit any considerable emission spectral change with any of the phosphates including ATP. The binding stoichiometry between 58 and 59 to that of ATP was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 while binding constants (Kb × 105 M−1) were 7.5 and 11.1, respectively. The ATP detection limits for 58 and 59 were 59 nM and 10.1 nM, respectively. 31P NMR spectral studies revealed the interaction of ATP with 58 and 59 as the phosphorus nuclei of ATP experienced a downfield shift. The H-bonding based cavity in 58 and 59 played a crucial role in the detection of ATP. In these complexes, synergistic effects involving zinc sites for coordinating the phosphate groups of ATP and H-bonding as well as π⋯π stacking between pyridine ring of a zinc complex and the adenine ring of ATP were together responsible for the unique sensing of ATP. Complex 61, which lacked H-bonding functional groups due to the involvement of para-anisole groups, did not recognize ATP.
1 while binding constants (Kb × 105 M−1) were 7.5 and 11.1, respectively. The ATP detection limits for 58 and 59 were 59 nM and 10.1 nM, respectively. 31P NMR spectral studies revealed the interaction of ATP with 58 and 59 as the phosphorus nuclei of ATP experienced a downfield shift. The H-bonding based cavity in 58 and 59 played a crucial role in the detection of ATP. In these complexes, synergistic effects involving zinc sites for coordinating the phosphate groups of ATP and H-bonding as well as π⋯π stacking between pyridine ring of a zinc complex and the adenine ring of ATP were together responsible for the unique sensing of ATP. Complex 61, which lacked H-bonding functional groups due to the involvement of para-anisole groups, did not recognize ATP.
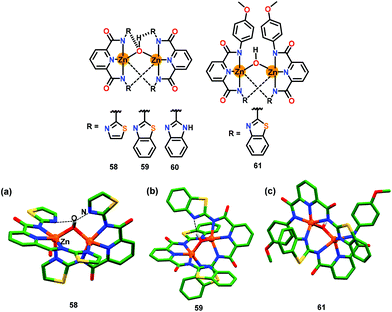 | ||
| Fig. 29 Chemical drawings of chemosensors 58–61. Crystal structures of chemosensors (a) 58, (b) 59 and (c) 61. Hydrogen atoms, except for the bridged hydroxide group, have been omitted for clarity. Adapted from ref. 79. | ||
Das and co-workers80 have developed a mononuclear zinc-based chemosensor 62 for the efficient detection of higher phosphates (Fig. 30). The Zn(II) ion was coordinated to a DPA chelate unit while the appended coumarin moiety acted as the fluorescent probe. Chemosensor 62 showed detection of both ADP and ATP via ‘Turn-On’ emission at 500 nm (λex = 400 nm) in aqueous medium (HEPES, pH = 7.4) by forming 62-ATP and 62-ADP adducts while exhibiting very good detection limits of 8.4 nM (ATP) and 12.5 nM (ADP). 31P NMR spectral studies revealed that both ADP and ATP were found to bind with 62 through their phosphate groups. 1H NMR spectroscopy was used for evaluating interaction of the diphosphate or triphosphate unit of ADP or ATP with the Zn(II) center and for calculating the binding affinities. Chemosensor 62 exhibited a large Stokes shift (ca. 100 nm) while its recognition ability allowed evaluating the ATP concentration change within the mitochondria.
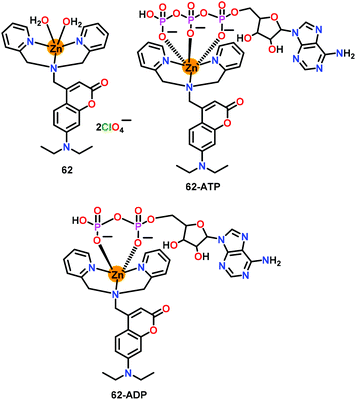 | ||
| Fig. 30 Chemical drawing of chemosensor 62 and its 62·ATP and 62·ADP complexes. Adapted from ref. 80. | ||
Moro and co-workers81 have developed a zinc-based chemosensor 63 bearing naphthalimide linked DPA unit for the detection of ATP (Fig. 31). The DPA based chelating unit coordinated with the Zn(II) ion while the naphthalimide fluorophore was free for the spectroscopic changes. A 2.3-fold emission enhancement at 535 nm was noted upon the introduction of ATP while saturation was noted at 10 mM concentration. The fluorescent quantum yield was enhanced from 0.28 for free 63 to 0.57 for the 63-ATP adduct. The interaction of ATP with Zn–DPA unit of chemosensor 63 decreased the strength of the Zn2+–N bonding interactions which resulted in the emission enhancement. Chemosensor 63 also detected other phosphates such as ADP and PPi but only in the micromolar range while a larger change in the emission was noted only with ATP.
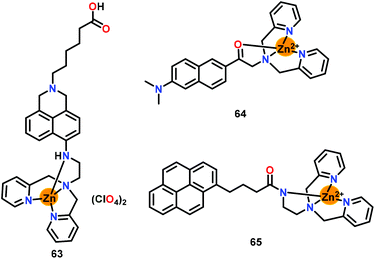 | ||
| Fig. 31 Chemical drawings of chemosensors 63–65. Adapted from ref. 81–83. | ||
Kim and co-workers82 have reported a Zn(II)–DPA based chemosensor 64 containing 6-acetyl-2-(dimethylamino) naphthalene (acedan) as a two-photon fluorophore (Fig. 31). This two-photon chemosensor showed significant absorption/emission spectral changes with ATP over other phosphates (ADP, AMP and HP2O73−) in HEPES buffer (containing 1%-CH3CN; pH = 7.4). In the presence of ATP, chemosensor 64 showed an absorption spectral change at 410 nm accompanied by a 10 nm hypsochromic shift. On the other hand, emission spectral titration against 2 equiv. of ATP showed 8.5-fold enhancement. The quantum yield for the 64-ATP adduct illustrated 5-fold enhancement when compared to free 64. The binding constants (Kb × 105 M−1) from the emission spectral titration were 0.05 (AMP), 3.2 (ADP), 40.0 (PPi) and 62.0 (ATP). The NMR and emission spectral studies suggested the occurrence of π⋯π interactions between the fluorophore and the nucleobases. The ‘Turn-On’ detection was due to the coordination of the carbonyl group to the Zn2+ center. Chemosensor 64 was also used for the effective detection of ATP and ADP in live cells.
Xing and co-workers83 have designed a Zn–DPA based chemosensor 65 containing a pyrene fluorophore for the selective detection of ATP and ADP over other phosphates and anions (Fig. 31). Chemosensor 65 showed a significant emission response and change in the proton NMR spectra with both ATP and ADP. Notably, ATP induced excimer emission at 482 nm while also enhancing the monomer emission at 390 nm. However, ADP comparatively resulted in smaller enhancement of the excimer emission but large enhancement of the monomer emission. The binding constants (Kb × 1010 M−2) were calculated as 3.6 and 0.71 for ATP and ADP, respectively. It was found that both 65-ATP and 65-ADP adducts have similar stabilities in aqueous medium and formed intramolecular sandwich structures. Authors provided spectroscopic evidence that in 65-ATP the adenine ring was outside the pyrene dimer of two molecules of 65 while the phosphate part was coordinated to two zinc centers. However, in 65-ADP, the adenine ring was between pyrene dimers to form a fully intramolecular sandwich structure.
Hamachi and co-workers84 have developed a dinuclear Zn-based chemosensor 66 containing an anthracene bridge as the fluorophore while having appended DPA groups as the chelating units (Fig. 32). The emission of the anthracene unit at 460 nm (λex = 380 nm) showed 3-fold enhancement with ATP over ADP and AMP in HEPES buffer (pH 7.2). A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was noted between 66 and ATP by the emission spectral titration while binding constants (Kb × 104 M−1) were noted to be 220 (ATP), 22 (ADP) and 6.6 (AMP). Binding of 66 with ATP was also confirmed by the downfield shifting of β and γ signals of ATP in the 31P NMR spectra, suggesting that 66 binds only with β and γ phosphates and not with α. The quantity of anionic charges on an analytes played an important role whereas the order of selectivity was found to be ATP > ADP > AMP. Thus, poly-anionic ATP showed highest interaction with the cationic Zn(II) centers of 66 rather than ADP and AMP.
1 stoichiometry was noted between 66 and ATP by the emission spectral titration while binding constants (Kb × 104 M−1) were noted to be 220 (ATP), 22 (ADP) and 6.6 (AMP). Binding of 66 with ATP was also confirmed by the downfield shifting of β and γ signals of ATP in the 31P NMR spectra, suggesting that 66 binds only with β and γ phosphates and not with α. The quantity of anionic charges on an analytes played an important role whereas the order of selectivity was found to be ATP > ADP > AMP. Thus, poly-anionic ATP showed highest interaction with the cationic Zn(II) centers of 66 rather than ADP and AMP.
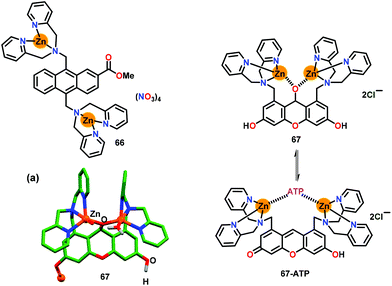 | ||
| Fig. 32 Chemical drawings of chemosensors 66 and 67. (a) Crystal structure of chemosensor 67. Adapted from ref. 84 and 85. | ||
Hamachi and co-workers85 further developed a DPA based dizinc chemosensor 67 containing xanthene as the fluorophore for ATP sensing (Fig. 32). Structurally characterized chemosensor 67 (Fig. 32a) exhibited a weak emission at 522 nm and an absorption spectral band at 509 nm in HEPES buffer (pH 7.4). Chemosensor 67 selectively recognized ATP with a significant emission enhancement, high binding affinity (Kb = 1.3 × 106 M−1), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and high quantum yield (φ = 0.61). The ‘Turn-On’ recognition of ATP was manifested by the formation of a conjugated xanthene form from its non-fluorescent de-conjugated form which was generated by the nucleophilic attack of Zn-bound H2O.
1 stoichiometry and high quantum yield (φ = 0.61). The ‘Turn-On’ recognition of ATP was manifested by the formation of a conjugated xanthene form from its non-fluorescent de-conjugated form which was generated by the nucleophilic attack of Zn-bound H2O.
Chen and co-workers86 have presented two simple terpyridine based Zn(II) complexes 68 and 69 for the detection of ATP (Fig. 33). The crystal structure of chemosensor 69 was substantiated by X-ray crystallography. Both chemosensors 68 and 69 functioned through two-component metallo–hydrogel formation for the detection of ATP. The weak emission of 68 at 617 nm (λex = 448 nm) was enhanced with ATP (44-fold) and ADP (14-fold) while no emission change was found with other anions. The detection limit for ATP was observed as 1.85 μM in aqueous medium. On the other hand, chemosensor 69 displayed a good emission at 450 nm (λex = 284 nm) but showed very small changes in its emission at 450 nm with phosphates and other anions; however, a slight quenching was observed with ATP. The linear geometry of 68 assisted it to form a self-assembly with ATP and that enhanced the emission due to AIE. The geometry of the resultant adduct was strongly influenced by the intramolecular π-stacking involving aromatic ring of 68 and planar nuclear bases of ATP. Notably, the self-assembly process established a new sensing method. Chemosensor 68 exhibited low cytotoxicity and was thus used for monitoring ATP in HeLa cells by using confocal fluorescence microscopy.
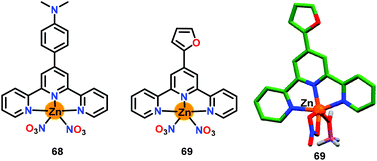 | ||
| Fig. 33 Chemical drawings of chemosensors 68 and 69 and crystal structure of chemosensor 69. Adapted from ref. 86. | ||
Tang and co-workers87 have presented an Eu(III)-based chemosensor 70 for the highly selective recognition of ATP in aqueous medium (Fig. 34). The emission at 615 nm showed enhancement and shifted to 591 nm with ATP. Notably, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was observed between 70 and ATP with a binding constant of 7.85 × 103 M−2 and a detection limit of 8.87 μM. The emission enhancement was largely due to the free terpyridine group which enhanced the coordination interaction as well as π⋯π stacking between ATP and chemosensor 70.
1 stoichiometry was observed between 70 and ATP with a binding constant of 7.85 × 103 M−2 and a detection limit of 8.87 μM. The emission enhancement was largely due to the free terpyridine group which enhanced the coordination interaction as well as π⋯π stacking between ATP and chemosensor 70.
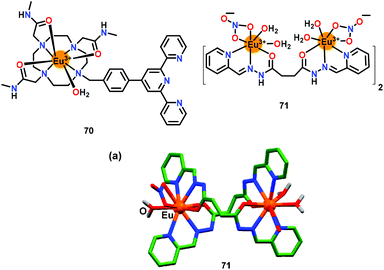 | ||
| Fig. 34 Chemical drawings of chemosensors 70 and 71. (a) Crystal structure of chemosensor 71. Adapted from ref. 87 and 88. | ||
Subramanian and co-workers88 have developed an Eu(III)-based chemosensor 71 for the selective recognition of AMP in HEPES buffer (pH = 4) (Fig. 34). The weak emission of 71 was due to the labile water molecules whereas the presence of AMP led to a 64-fold enhancement in its emission band at 614 nm for the Eu(III) center (λex = 320 nm). Chemosensor 71 formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric adduct with AMP with a Kb value of 6.46 × 103 M−1 and 2 mM detection limit. The crystal structure showed helical arrangement of both ligands and co-ligands around the Eu(III) ions (Fig. 34a) which were found to be easily replaced by the anions. It was noted that AMP bonded both with the Eu(III) centers by replacing the coordinated H2O molecules and co-ligands.
1 stoichiometric adduct with AMP with a Kb value of 6.46 × 103 M−1 and 2 mM detection limit. The crystal structure showed helical arrangement of both ligands and co-ligands around the Eu(III) ions (Fig. 34a) which were found to be easily replaced by the anions. It was noted that AMP bonded both with the Eu(III) centers by replacing the coordinated H2O molecules and co-ligands.
Butler and co-workers89 have presented a series of Eu(III) based chemosensors 72–75 offering different interacting sites (Fig. 35). These chemosensors exhibited significant detection of adenosine phosphates. The absorption spectra of 72 and 74 were very similar and showed a band at 332 nm and 330 nm, respectively. Similarly, both 73 and 75 exhibited absorption maxima at 303 nm and 318 nm, respectively. The emission spectra of 72, 73 and 75 displayed several similarities with respect to ΔJ = 1 (585–605 nm) the ΔJ = 2 (605–630 nm) bands. Chemosensors 72 and 74 showed excellent selectivity between ATP, ADP and other phosphates due to the presence of carboxamide groups in the pendant arms. Addition of ATP to 72 or 74 resulted in a 10-fold emission enhancement of the ΔJ = 2 band (605–630 nm) while addition of ADP produced a 14-fold emission enhancement. Chemosensors 73 and 75, which lacked quinoline–amide groups, only manifested 3-fold emission enhancement (ΔJ = 2) with ATP, ADP and AMP. The quinoline groups of 74 sensitized the Eu(III) center whereas terminal phosphate groups of ADP/ATP coordinated to the Eu(III) centers by replacing the labile H2O molecules. In addition, adjacent phosphate group(s) of ADP/ATP also interacted with the quinoline rings through the H-bonding interactions. Chemosensor 74 formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex both with ATP and ADP with Kb values (×105 M−1) of 6.31 and 5.01, respectively.
1 complex both with ATP and ADP with Kb values (×105 M−1) of 6.31 and 5.01, respectively.
 | ||
| Fig. 35 Chemical drawings of chemosensors 72–75. Adapted from ref. 89. | ||
5. Conclusions and future prospects
The present review article has illustrated several luminescent chemosensors that have been developed for the selective detection of different phosphates through the ‘Turn-On’ emission response. Such chemosensors were summarized in two broad categories; organic molecule based and metal complex based chemosensors whereas their binding affinities were supplemented with the binding constants and detection limits along with the lifetime parameters. Crystal structures of several free chemosensors as well as their adducts and/or complexes with the assorted anions including phosphates provided structural insights about the mode of interactions between a chemosensor and a guest.These examples have highlighted several notable take-home design strategies. In the case of organic molecule based chemosensors, the presence of a suitable fluorophore was an essential requirement. In these chemosensors, such fluorophores were largely responsible for the emission output when excited at an appropriate wavelength. The common sensing mechanisms that were mostly prevalent for the detection-led luminescent output were: (i) acid–base strategy where a phosphate ion acted as a base and removed an acidic proton from a chemosensor thus enhancing the conjugation and therefore emission output; (ii) dissociation strategy where a fluorophore was detached from a chemosensor mostly by a hydrolytic pathway; (iii) displacement strategy where a fluorophore was removed from a [chemosensor–fluorophore] adduct; and (iv) interaction strategy where an analyte was noted to interact with a chemosensor via various weak interactive forces including H-bonding, anion⋯π and π⋯π stacking. On the other hand, the presence of a chelating and/or an interacting site was an essential parameter for the metal complex based chemosensors. In such chemosensors, a phosphate ion was often found to interact with such sites. At the same time, several other non-covalent interactions were clearly observable and cannot be neglected. Several such examples nicely illustrated the significant roles played by the H-bonding, X-bonding, anion⋯π interaction and π⋯π stacking as well as other non-conventional weaker interactions.
The examples contained in the present review article have adequately demonstrated that the challenging analytes such as phosphates and adenosine–phosphates that considerably suffer from pH sensitivity and solution dynamics can be conveniently detected both by using organic molecule based and metal complex based chemosensors. At the same time, involvement of phosphates and various adenosine–phosphates in numerous biological processes certainly pose many more challenges in front of the scientific community, particularly in view of live cell imaging and tracking of adenosine–phosphates as well as phosphate-based drugs.90
From the environmental perspective, the heavy use of phosphates in detergents, fertilizers, pharmaceuticals, medicines, house-hold products and construction materials is known to contaminate both soil and water bodies.16–22 Therefore, design of effective chemosensors for phosphates and particularly those that can remove them from the aqueous media while overcoming pH and solvation energy challenges will be timely and pertinent. In this context, intricately designed materials including gels, metal–organic frameworks, zeolites, modified silica and alumina as well as composite materials offer bright prospects.91–95 Such materials are likely to withstand pH and solvation energy issues prevailing in the aqueous media and the environmental samples. In the quest for next-generation chemosensor-cum-retrievers, it is desirable to integrate the successful design aspects of molecular chemosensors into robust and reusable materials for not only detection of phosphates but also for their effective removal for much-needed biological and environmental remediation applications. Thus, both the biological and the environmental challenges together with the literature precedents and intelligent design strategies are likely to provide fertile ground and create enormous opportunities for the development of next-generation chemosensors.
Abbreviations
| AIE | Aggregation induced emission |
| ADP | Adenosine diphosphate |
| AMP | Adenosine monophosphate |
| ATP | Adenosine triphosphate |
| BINOL | 1,1′-Bi-2-naphthol |
| CMC | Critical micelle concentration |
| DFT | Density functional theory |
| DLS | Dynamic light scattering |
| DMF | Dimethyl formamide |
| DMSO | Dimethyl sulphoxide |
| DPA | 2,2′-Dipicolylamine |
| EDIE | Excimer disaggregation induced emission |
| EDX | Energy dispersive X-ray spectroscopy |
| FRET | Free resonance energy transfer |
| H-bond | Hydrogen bond |
| H-bonding | Hydrogen bonding |
| HEPES | (4-(2-Hydroxyethyl)-1piperazineethanesulfonic acid) |
| ICT | Internal charge transfer |
| ITC | Isothermal titration calorimetry |
| IDA | Indicator displacement assay |
| PBS | Phosphate-buffered saline |
| PET | Photoinduced electron transfer |
| PPi | Pyrophosphate |
| SDBS | Sodium dodecylbenzenesulfonate |
| SEM | Scanning electron microscopy |
| TDFT | Time-dependent density functional theory |
| TEM | Transmission electron microscopy |
| THF | Tetrahydrofuran |
| TPE | Tetraphenylethene |
| TRIS | Trisaminomethane |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
RG gratefully acknowledges financial support from the Science and Engineering Research Board (EMR/2016/ 000888), New Delhi and the Council of Scientific and Industrial Research (01(2841)/16/EMR-II), New Delhi. PK thanks CSIR, New Delhi for the Senior Research Associateship “Scientists’ Pool Scheme” (IA-27577). SP thanks UGC, New Delhi for the SRF fellowship. Reviewers’ comments were very helpful during the revision.References
- A. V. Gourine, E. Llaudet, N. Dale and K. M. Spyer, ATP is a mediator of chemosensory transduction in the central nervous system, Nature, 2005, 436, 108–111 Search PubMed.
- F. Westheimer, Why nature chose phosphates, Science, 1987, 235, 1173–1178 Search PubMed.
- J. K. Heinonen, Biological Role of Inorganic Pyrophosphate, Kluwer Academic Publishers, Norwell, 2001 Search PubMed.
- K. G. Clarke, Bioprocess Engineering: An Introductory Engineering and Life Science Approach, Woodhead Publisher, 2013 Search PubMed.
- R. L. P. Adams, J. T. Knowler and D. P. Leader, The Biochemistry of the Nucleic Acids, Chapman and Hall, 10th edn, 1986 Search PubMed.
- W. Saenger, Principles of Nucleic Acid Structure, Springer-Verlag, 1984 Search PubMed.
- T. S. Moore Jr., Phospholipid biosynthesis, Annu. Rev. Plant Physiol., 1982, 33, 235–259 Search PubMed.
- J. L. Meiera and M. D. Burkart, The chemical biology of modular biosynthetic enzymes, Chem. Soc. Rev., 2009, 38, 2012–2045 Search PubMed.
- A. Shaikh, T. Berndt and R. Kumar, Regulation of phosphate homeostasis by the phosphatonins and other novel mediators, Pediatr. Nephrol., 2008, 23, 1203–1210 Search PubMed.
- J. Gattineni and M. Baum, Genetic disorders of phosphate regulation, Pediatr. Nephrol., 2012, 27, 1477–1487 Search PubMed.
- C. P. Mathews and K. E. van Hold, Biochemistry, The Benjamin/Cummings Publishing Co., Inc., Redwood City, CA, 1990 Search PubMed.
- T. Soderberg, Organic Chemistry with a Biological Emphasis, Chemistry Libre Texts Publisher, 2021 Search PubMed.
- W. G. Junger, Immune cell regulation by autocrine purinergic signalling, Nat. Rev. Immunol., 2011, 11, 201–212 Search PubMed.
- X. Su, C. Zhang, X. Xiao, A. Xu, Z. Xu and M. Zhao, A kinetic method for expeditious detection of pyrophosphate anions at nanomolar concentrations based on a nucleic acid fluorescent sensor, Chem. Commun., 2013, 49, 798–800 Search PubMed.
- L. Stryer, Biochemistry, W. H. Freeman and Company, New York, 4th edn, 1998 Search PubMed.
- C. Rey, in Calcium phosphates in biological and industrial systems, ed. Z. Amjad, Kluwer Academic Publishers, Boston, Dordrecht, London, 1998, pp. 217–251 Search PubMed.
- P. A. Gilbert and A. L. Dejong, The use of phosphate in detergents and possible replacements for phosphate, Ciba Found. Symp., 1977, 57, 253–268 Search PubMed.
- A. N. Sharpley and S. Rekolainen, Phosphorus in agriculture and its environmental implications, in Phosphorus Loss from Soil to Water, ed. H. Tunney, O. T. Carton, P. C. Brookers and A. E. Johnston, CAB International Press, Cambridge, England, 1997, pp. 1–54 Search PubMed.
- F. Nantaba, W.-U. Palm, J. Wasswa, H. Bouwman, H. Kylin and K. Kummerer, Temporal dynamics and ecotoxicological risk assessment of personal care products, phthalate ester plasticizers, and organophosphorus flame retardants in water from Lake Victoria, Uganda, Chemosphere, 2021, 262, 127716–127727 Search PubMed.
- A. M. Rashad, Phosphogypsum as a construction material, J. Cleaner Prod., 2017, 166, 732–743 Search PubMed.
- K. Chandrasekaran, S. N. T. S. Nellaiappan, R. Kulandaivelu and M. H. Lee, Improving the Reactivity and Receptivity of Alloy and Tool Steels for Phosphate Conversion Coatings: Role of Surface Mechanical Attrition Treatment, Ind. Eng. Chem. Res., 2014, 53, 20124–20138 Search PubMed.
- C. F. Mason, Biology of Fresh water Pollution, Longman, New York, 1991 Search PubMed.
- WHO, Guidelines for drinking water quantity, World Health Organization, Geneva, 2nd edn, 1993 Search PubMed.
- E. A. Katayev, Y. A. Ustynyuk and J. L. Sessler, Receptors for tetrahedral oxyanions, Coord. Chem. Rev., 2006, 250, 3004–3037 Search PubMed.
- H. Chen, L. Zhao, F. Yu and Q. Du, Detection of phosphorus species in water: technology and strategies, Analyst, 2019, 144, 7130–7148 Search PubMed.
- S. Pal, T. K. Ghosh, R. Ghosh, S. Mondal and P. Ghosh, Recent advances in recognition, sensing and extraction of phosphates: 2015 onwards, Coord. Chem. Rev., 2020, 405, 213128–213184 Search PubMed.
- P. J. Antony, S. Karthikeyan and C. S. P. Iyer, Ion chromatographic separation and determination of phosphate and arsenate in water and hair, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2002, 767, 363–368 Search PubMed.
- W. Q. Liu, Z. F. Du, Y. Qian and F. A. Li, specific colorimetric probe for phosphate detection based on anti-aggregation of gold nanoparticles, Sens. Actuators, B, 2013, 176, 927–931 Search PubMed.
- E. V. Beletskiy and S. R. Kass, Selective binding and extraction of aqueous dihydrogen phosphate solutions via three-armed thiourea receptors, Org. Biomol. Chem., 2015, 13, 9844–9849 Search PubMed.
- W. L. Cheng, J. W. Sue, W. C. Chen, J. L. Chang and J. M. Zen, Activated nickel platform for electrochemical sensing of phosphate, Anal. Chem., 2010, 82, 1157–1161 Search PubMed.
- C. J. Quintana, L. Idrissi, G. Palleschi, P. Albertano, A. Amine, M. El Rhazi and D. Moscone, Investigation of amperometric detection of phosphate: Application in seawater and cyanobacterial biofilm samples, Talanta, 2004, 63, 567–574 Search PubMed.
- D. Talarico, S. Cinti, F. Arduini, A. Amine, D. Moscone and G. Palleschi, Phosphate detection through a cost-effective carbon black nanoparticle-modified screen-printed electrode embedded in a continuous flow system, Environ. Sci. Technol., 2015, 49, 7934–7939 Search PubMed.
- J. M. Zhu, Y. Shi, X. Q. Zhu, Y. Yang, F. H. Jiang, C. J. Sun, W. H. Zhao and X. T. Han, Optofluidic marine phosphate detection with enhanced absorption using a Fabry-Perot resonator, Lab Chip, 2017, 17, 4025–4030 Search PubMed.
- G. Ji, X. Gao, T. Zheng, W. Guan, H. Liu and Z. Liu, Postsynthetic Metalation Metal–Organic Framework as a Fluorescent Probe for the Ultrasensitive and Reversible Detection of PO43− Ions, Inorg. Chem., 2018, 57, 10525–10532 Search PubMed.
- N. K. Beyeh, I. Diez, S. M. Taimoory, D. Meister, A. I. Feig, J. F. Trant, R. H. S. Ras and K. Rissanen, High-affinity and selective detection of pyrophosphate in water by a resorcinarene salt receptor, Chem. Sci., 2018, 9, 1358–1367 Search PubMed.
- D. A. McNaughton, M. Fares, G. Picci, P. A. Gale and C. Caltagirone, Advances in fluorescent and colorimetric sensors for anionic species, Coord. Chem. Rev., 2021, 427, 213573–213616 Search PubMed.
- S. Lee, K. K. Y. Yuen, K. A. Jolliffe and J. Yoon, Fluorescent and colorimetric chemosensors for pyrophosphate, Chem. Soc. Rev., 2015, 44, 1749–1762 Search PubMed.
- T. Nagano, Development of fluorescent probes for bioimaging applications, Proc. Jpn. Acad., Ser. B, 2010, 86, 837–847 Search PubMed.
- D. H. Joo, J. S. Mok, G. H. Bae, S. E. Oh, J. H. Kang and C. Kim, Colorimetric Detection of Cu2+ and Fluorescent Detection of PO43− and S2− by a Multifunctional Chemosensor, Ind. Eng. Chem. Res., 2017, 56, 8399–8407 Search PubMed.
- Y.-X. Yuan, J.-H. Wang and Y.-S. Zheng, Selective Fluorescence Turn-On Sensing of Phosphate Anion in Water by Tetraphenylethylene Dimethylformamidine, Chem. – Asian J., 2019, 14, 760–764 Search PubMed.
- M. Gao and B. Z. Tang, Fluorescent Sensors Based on Aggregation-Induced Emission: Recent Advances and Perspectives, ACS Sens., 2017, 2, 1382–1399 Search PubMed.
- J. Zhao, D. Yang, Y. Zhao, L. Cao, Z. Zhang, X.-J. Yang and B. Wu, Phosphate-induced fluorescence of a tetraphenylethene-substituted tripodal tris(urea) receptor, Dalton Trans., 2016, 45, 7360–7365 Search PubMed.
- J. F. Zhang, L. E. Guo, T. N. Zang, Y. L. Duan, X. Y. Liu, Z. Yang, P. Verwilst, K. Luo, G. K. Wang, J. F. Kou, Y. Zhou and J. S. Kim, Highly Selective In Vivo Imaging of Endogenous/Exogenous Phosphate Ion over ATP and PPi, Chem. – Asian J., 2015, 10, 1165–1169 Search PubMed.
- L. E. Guo, J. F. Zhang, X. Y. Liu, L. M. Zhang, H. L. Zhang, J. H. Chen, X. G. Xie, Y. Zhou, K. Luo and J. Yoon, Phosphate Ion Targeted Colorimetric and Fluorescent Probe and Its Use to Monitor Endogenous Phosphate Ion in a Hemichannel-Closed Cell, Anal. Chem., 2015, 87, 1196–1201 Search PubMed.
- A. Kovalchuk, J. L. Bricks, G. Reck, K. Rurack, B. Schulz, A. Szumnac and H. Weißhoffd, A charge transfer-type fluorescent molecular sensor that “lights up” in the visible upon hydrogen bond-assisted complexation of anions, Chem. Commun., 2004, 1946–1947 Search PubMed.
- K. Ghosh, A. R. Sarkar, A. Ghorai and U. Ghosh, Design and synthesis of anthracene-based bispyridinium amides: anion binding, cell staining and DNA interaction studies, New J. Chem., 2012, 36, 1231–1245 Search PubMed.
- L. Gonzlez, F. Zapata, A. Caballero, P. Molina, C. R. de Arellano, I. Alkorta and J. Elguero, Chem. – Eur. J., 2016, 22, 7533–7544 Search PubMed.
- P. Sabater, F. Zapata, A. Caballero, N. de la Visitacion, I. Alkorta, J. Elguero and P. Molina, Comparative Study of Charge-Assisted Hydrogen- and Halogen-Bonding Capabilities in Solution of Two-Armed Imidazolium Receptors toward Oxoanions, J. Org. Chem., 2016, 81, 7448–7458 Search PubMed.
- F. Zapata, S. J. Benitez-Benitez, P. Sabater, A. Caballero and P. Molina, Modulation of the Selectivity in Anions Recognition Processes by Combining Hydrogen- and Halogen-Bonding Interactions, Molecules, 2017, 22, 2273–2284 Search PubMed.
- J. Yang, C.-C. Dong, X.-L. Chen, X. Sun, J.-Y. Wei, J.-F. Xiang, J. L. Sessler and H.-Y. Gong, Excimer Disaggregation Enhanced Emission: A Fluorescence “Turn- On” Approach to Oxoanion Recognition, J. Am. Chem. Soc., 2019, 141, 4597–4612 Search PubMed.
- G. Bettistelli, A. Cantelli, G. Guidetti, J. Manzi and M. Monatalti, Ultra–bright and stimuli–responsive fluorescent nanoparticles for bioimaging, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2016, 8, 139–150 Search PubMed.
- P. Sokkalingam, D. S. Kim, H. Hwang, J. L. Sessler and C.-H. Lee, A dicationic calix[4]pyrrole derivative and its use for the selective recognition and displacement-based sensing of pyrophosphate, Chem. Sci., 2012, 3, 1819–1824 Search PubMed.
- A. C. Sedgwick, J. T. Brewster, T. Wu, X. Feng, S. D. Bull, X. Qian, J. L. Sessler, T. D. James, E. V. Anslyn and X. Sun, Indicator displacement assays (IDAs): the past, present and future, Chem. Soc. Rev., 2021, 50, 9–38 Search PubMed.
- F. Zapata, A. Caballero, P. Molina, I. Alkorta and J. Elguero, Open Bis(triazolium) Structural Motifs as a Benchmark To Study Combined Hydrogen- and Halogen-Bonding Interactions in Oxoanion Recognition Processes, J. Org. Chem., 2014, 79, 6959–6969 Search PubMed.
- K. Ghosh, D. Kar, D. Sahub and B. Ganguly, Benzimidazolium-based chemosensors: selective recognition of H2PO4−, HP2O73−, F− and ATP through fluorescence and gelation studies, RSC Adv., 2015, 5, 46608–46616 Search PubMed.
- K. Ghosh, A. R. Sarkar, A. Samadder and A. R. Khuda-Bukhsh, Pyridinium-Based Fluororeceptors As Practical Chemosensors for Hydrogen Pyrophosphate (HP2O7(3−) in Semiaqueous and Aqueous Environments, Org. Lett., 2012, 14, 4314–4317 Search PubMed.
- K.-Y. Tan, C. Li, Y. Li, J. Fei, B. Yang, Y.-J. Fu and F. Li, Real-Time Monitoring ATP in Mitochondrion of Living Cells: a Specific Fluorescent Probe for ATP by Dual Recognition Sites, Anal. Chem., 2017, 89, 1749–1756 Search PubMed.
- Y. Fang, W. Shi, Y. Hu, X. Li and H. Ma, A dual-function fluorescent probe for monitoring the degrees of hypoxia in living cells via the imaging of nitroreductase and adenosine triphosphate, Chem. Commun., 2018, 54, 5454–5457 Search PubMed.
- T.-B. Ren, S.-Y. Wen, L. Wang, P. Lu, B. Xiong, L. Yuan and X.-B. Zhang, Engineering a Reversible Fluorescent Probe for Real-Time Live-Cell Imaging and Quantification of Mitochondrial ATP, Anal. Chem., 2020, 92, 4681–4688 Search PubMed.
- S. Farshbaf and P. Anzenbacher Jr., Fluorimetric sensing of ATP in water by an imidazolium hydrazone based sensor, Chem. Commun., 2019, 55, 1770–1773 Search PubMed.
- H. Tao, L. He, G. Cheng and Q.-Y. Cao, Linear tetraphenylethene-appended bis-imidazolium salts for sensing of ATP, Dyes Pigm., 2019, 166, 233–238 Search PubMed.
- H. Ma, M. Yang, C. Zhang, Y. Ma, Y. Qin, Z. Lei, L. Chang, L. Lei, T. Wang and Y. Yang, Aggregation-induced emission (AIE)-active fluorescent probes with multiple binding sites toward ATP sensing and live cell imaging, J. Mater. Chem. B, 2017, 5, 8525–8531 Search PubMed.
- K. Ghosh, D. Tarafdar, A. Samadder and A. R. Khuda-Bukhsh, Pyridinum-based flexible tripodal cleft: a case of fluorescence sensing of ATP and dihydrogenphosphate under different conditions and cell imaging, RSC Adv., 2015, 5, 35175–35180 Search PubMed.
- J.-H. Zhu, C. Yu, Y. Chen, J. Shin, Q.-Y. Cao and J. S. Kim, A self-assembled amphiphilic imidazolium-based ATP probe, Chem. Commun., 2017, 53, 4342–4345 Search PubMed.
- G. Singh, M. Kaur, B. A. Shiekh and T. S. Kang, Luminescent micellar nano-interfaces of surface active ionic liquid for the selective recognition of ADP in aqueous medium, Chem. Commun., 2018, 54, 7463–7466 Search PubMed.
- B. Chowdhury, S. Khatua, R. Dutta, S. Chakraborty and P. Ghosh, Bis-Heteroleptic Ruthenium(II) Complex of a Triazole Ligand as a Selective Probe for Phosphates, Inorg. Chem., 2014, 53, 8061–8070 Search PubMed.
- B. Chowdhury, S. Sinha and P. Ghosh, Selective Sensing of Phosphates by a New Bis-heteroleptic RuII Complex through Halogen Bonding: A Superior Sensor over Its Hydrogen-Bonding Analogue, Chem. – Eur. J., 2016, 22, 1–10 Search PubMed.
- T. K. Ghosh, S. Chakraborty, B. Chowdhury and P. Ghosh, Bis-Heteroleptic Ruthenium(II) Complex of Pendant Urea Functionalized Pyridyl Triazole and Phenathroline for Recognition, Sensing, and Extraction of Oxyanions, Inorg. Chem., 2017, 56, 5371–5382 Search PubMed.
- T. K. Ghosh and P. Ghosh, Balancing the acidity of the pendant urea arm of bis-heteroleptic ruthenium(II) complex containing pyridyl triazole for improved oxyanion Recognition, Dalton Trans., 2018, 47, 7561–7570 Search PubMed.
- T. K. Ghosh, S. Mondal, S. Bej, M. Nandi and P. Ghosh, An integrated urea and halogen bond donor based receptor for superior and selective sensing of phosphates, Dalton Trans., 2019, 48, 4538–4546 Search PubMed.
- S. K. Sheet, B. Sen, R. Thounaojam, K. Aguan and S. Khatua, Ruthenium(II) Complex-Based Luminescent Bifunctional Probe for Ag+ and Phosphate Ions: Ag+-Assisted Detection and Imaging of rRNA, Inorg. Chem., 2017, 56, 1249–1263 Search PubMed.
- H. Wu and C. Tong, A Specific Turn-On Fluorescent Sensing for Ultrasensitive and Selective Detection of Phosphate in Environmental Samples Based on Antenna Effect-Improved FRET by Surfactant, ACS Sens., 2018, 3, 1539–1545 Search PubMed.
- S. Sandhu, R. Kumar, N. Tripathi, H. Singh, P. Singh and S. Kumar, Lab-on-a-Molecule elaboration for fluorescence based discrimination of commercial surfactants sodium dodecyl sulfate and sodium dodecylbenzenesulfonate, Sens. Actuators, B, 2017, 241, 8–18 Search PubMed.
- V. Kumar, P. Kumar, S. Kumar, D. Singhal and R. Gupta, Turn-On Fluorescent Sensors for the Selective Detection of Al3+ (and Ga3+) and PPi Ions, Inorg. Chem., 2019, 58, 10364–10376 Search PubMed.
- B. Chowdhury, R. Dutta, S. Khatua and P. Ghosh, A Cyanuric Acid Platform Based Tripodal Bis-heteroleptic Ru(II) Complex of Click Generated Ligand for Selective Sensing of Phosphates via C–H⋯Anion Interaction, Inorg. Chem., 2016, 55(1), 259–271 Search PubMed.
- S.-Y. Jiao, K. Li, X. Wang, Z. Huang, L. Pu and X.-Q. Yu, Make PyrophosphateVisible: The First Precipitable and Real-time Fluorescent Sensor for Pyrophosphate in Aqueous Solution, Analyst, 2015, 140, 174–181 Search PubMed.
- S. Yang, W. Feng and G. Feng, Development of a near-infrared fluorescent sensor with a large Stokes shift for sensing pyrophosphate in living cells and animals, Anal. Chim. Acta, 2018, 1034, 119–127 Search PubMed.
- Y.-S. Wong, M. Ng, M. C.-L. Yeung and V. W.-W. Yam, Platinum(II)-Based Host–Guest Coordination-Driven Supramolecular Co-Assembly Assisted by Pt⋯Pt and π–π Stacking Interactions: A Dual-Selective Luminescence Sensor for Cations and Anions, J. Am. Chem. Soc., 2021, 143, 973–982 Search PubMed.
- D. Bansal and R. Gupta, Selective sensing of ATP by hydroxide-bridged dizinc(II) complexes offering a hydrogen bonding cavity, Dalton Trans., 2019, 48, 14737–14747 Search PubMed.
- H. Singh, S. Sreedharan, R. Tiwari, C. Walther, C. Smythe, S. K. Pramanik, J. A. Thomas and A. Das, A Fluorescent Chemodosimeter for Organelle-Specific Imaging of Nucleoside Polyphosphate Dynamics in Living Cells, Cryst. Growth Des., 2018, 18, 7199–7206 Search PubMed.
- A. J. Moro, P. J. Cywinski, S. Korstena and G. J. Mohr, An ATP fluorescent chemosensor based on a Zn(II)-complexed dipicolylamine receptor coupled with a naphthalimide chromophore, Chem. Commun., 2010, 46, 1085–1087 Search PubMed.
- A. S. Rao, D. Kim, H. Nam, H. Jo, K. H. Kim, C. Ban and K. H. Ahn, A turn-on two-photon fluorescent probe for ATP and ADP, Chem. Commun., 2012, 48, 3206–3208 Search PubMed.
- Q.-C. Xu, H.-J. Lv, Z.-Q. Lv, M. Liu, Y.-J. Li, X.-F. Wang, Y. Zhang and G.-W. Xing, A pyrene-functionalized Zinc(II)–BPEA complex: sensing and discrimination of ATP, ADP and AMP, RSC Adv., 2014, 4, 47788–47792 Search PubMed.
- A. Ojida, S.-K. Park, Y. Mito-Oka and I. Hamachi, Efficient fluorescent ATP-sensing based on coordination chemistry under aqueous neutral conditions, Tetrahedron Lett., 2002, 43, 6193–6195 Search PubMed.
- A. Ojida, I. Takashima, T. Kohira, H. Nonaka and I. Hamachi, Turn-On Fluorescence Sensing of Nucleoside Polyphosphates Using a Xanthene-Based Zn(II) Complex Chemosensor, J. Am. Chem. Soc., 2008, 130, 12095–12101 Search PubMed.
- W. Fang, C. Liu, F. Yu, Y. Liu, Z. Li, L. Chen, X. Bao and T. Tu, Macroscopic and Fluorescent Discrimination of Adenosine Triphosphate via Selective Metallo-hydrogel Formation: A Visual, Practical, and Reliable Rehearsal toward Cellular Imaging, ACS Appl. Mater. Interfaces, 2016, 8, 20583–20590 Search PubMed.
- X. Liu, J. Xu, Y. Lv, W. Wu, W. Liu and Y. Tang, An ATP-selective, lanthanide complex luminescent probe, Dalton Trans., 2013, 42, 9840–9846 Search PubMed.
- J. Sahoo, R. Arunachalam, P. S. Subramanian, E. Suresh, A. Valkonen, K. Rissanen and M. Albrecht, Coordinatively Unsaturated Lanthanide(iii) Helicates: Luminescence Sensors for Adenosine Monophosphate in Aqueous Media, Angew. Chem., Int. Ed., 2016, 55, 1–6 Search PubMed.
- R. Mailhot, T. Traviss-Pollard, R. Pal and S. J. Butler, Cationic Europium Complexes for Visualizing Fluctuations in Mitochondrial ATP Levels in Living Cells, Chem. – Eur. J., 2018, 24, 10745–10755 Search PubMed.
- (a) Y. Kurishita, T. Kohira, A. Ojida and I. Hamachi, Organelle-Localizable Fluorescent Chemosensors for Site-Specific Multicolor Imaging of Nucleoside Polyphosphate Dynamics in Living Cells, J. Am. Chem. Soc., 2012, 134, 18779–18789 Search PubMed; (b) L. Wang, L. Yuan, X. Zeng, J. Peng, Y. Ni, J. C. Er, W. Xu, B. K. Agrawalla, D. Su, B. Kim and Y.-T. Chang, A Multisite-Binding Switchable Fluorescent Probe for Monitoring Mitochondrial ATP Level Fluctuation in Live Cells, Angew. Chem., Int. Ed., 2016, 55, 1773–1776 Search PubMed.
- (a) S. Dong, Y. Wang, Y. Zhao, X. Zhou and H. Zheng, La3+/La(OH)3 loaded magnetic cationic hydrogel composites for phosphate removal: Effect of lanthanum species and mechanistic study, Water Res., 2017, 126, 433–441 Search PubMed; (b) E. S. Dragan, D. Humelnicu and M. V. Dinu, Development of Chitosan-Poly(ethyleneimine) Based Double Network Cryogels and Their Application as Superadsorbents for Phosphate, Carbohydr. Polym., 2019, 210, 17–25 Search PubMed.
- (a) M. H. Hassan, R. Stanton, J. Secora, D. J. Trivedi and S. Andreescu, Ultrafast Removal of Phosphate from Eutrophic Waters Using a Cerium-Based Metal–Organic Framework, ACS Appl. Mater. Interfaces, 2020, 12, 52788–52796 Search PubMed; (b) S. Mazloomi, M. Yousefi, H. Nourmoradi and M. Shams, Evaluation of phosphate removal from aqueous solution using metal organic framework; isotherm, kinetic and thermodynamic study, J. Environ. Health Sci. Eng., 2019, 17, 209–218 Search PubMed; (c) Y. Zhang, S. Sheng, S. Mao, X. Wu, Z. Li, W. Tao and I. R. Jenkinson, Highly sensitive and selective fluorescent detection of phosphate in water environment by a functionalized coordination polymer, Water Res., 2019, 163, 114883 Search PubMed; (d) Q. Xie, Y. Li, Z. Lv, H. Zhou, X. Yang, J. Chen and H. Guo, Effective Adsorption and Removal of Phosphate from Aqueous Solutions and Eutrophic Water by Fe-based MOFs of MIL-101, Sci. Rep., 2017, 7, 3316 Search PubMed.
- (a) W. Huang, Y. Zhang and D. Li, Adsorptive removal of phosphate from water using mesoporous materials: A review, J. Environ. Manage., 2017, 193, 470–482 Search PubMed; (b) H. Bacelo, A. M. A. Pintor, S. C. R. Santos, R. A. R. Boaventura and C. M. S. Botelho, Performance and prospects of different adsorbents for phosphorus uptake and recovery from water, Chem. Eng. J., 2020, 381, 122566 Search PubMed.
- (a) S. Morab, K. Chhodena, P. Negia and K. Ravindra, Utilization of Nano-Alumina and Activated Charcoal for Phosphate Removal from Wastewater, Environ. Nanotechnol. Monit. Manage., 2017, 7, 15–23 Search PubMed; (b) D. Yadav, P. Kumar, M. Kapur and M. K. Mondal, Phosphate removal from aqueous solutions by nano–alumina for the effective remediation of eutrophication, Environ. Prog. Sustainable Energy, 2019, 38, S77–S85 Search PubMed.
- (a) B. Wu, J. Wan, Y. Zhang, B. Pan and I. M. C. Lo, Selective Phosphate Removal from Water and Wastewater using Sorption: Process Fundamentals and Removal Mechanisms, Environ. Sci. Technol., 2020, 54, 50–66 Search PubMed; (b) H. H. Nodeh, H. Sereshti, E. Z. Afsharian and N. Nouri, Enhanced removal of phosphate and nitrate ions from aqueous media using nanosized lanthanum hydrous doped on magnetic graphene nanocomposite, J. Environ. Manage., 2017, 197, 265–274 Search PubMed.
Footnotes |
| † Present address: Department of Chemistry, Mahamana Malviya College Khekra (Baghpat), C.C.S. University Meerut, India. E-mail: E-mail: baliyanpk@gmail.com |
| ‡ In the crystal structure drawings, chemosensors are shown in deep green colour whereas counter anions and lattice solvent molecules have been omitted for clarity. The following colour codes have been used: metal atom, orange; nitrogen atom, blue; oxygen atom, red; phosphorus atom, pink; hydrogen atom, grey. |
| This journal is © the Partner Organisations 2021 |