How the external solvent in biocompatible reverse micelles can improve the alkaline phosphatase behavior†
Nahir
Dib
*ab,
Valeria R.
Girardi
a,
Juana J.
Silber
ab,
N. Mariano
Correa
 ab and
R. Dario
Falcone
ab and
R. Dario
Falcone
 *ab
*ab
aDepartamento de Química, Universidad Nacional de Rio Cuarto, Agencia Postal 3, C.P. X5804BYA, Ruta Nacional 36, km 601, Rio Cuarto, Córdoba, Argentina
bInstituto de Desarrollo Agroindustrial y de la Salud (IDAS), Universidad Nacional de Río Cuarto, Agencia Postal 3, C.P. X5804BYA, Ruta Nacional 36, km 601, Río Cuarto, Córdoba, Argentina. E-mail: rfalcone@exa.unrc.edu.ar; ndib@exa.unrc.edu.ar
First published on 6th May 2021
Abstract
In the last decade, the nature of the nonpolar solvents that can be part of reverse micelles (RMs) has been the topic of several investigations to improve their applications. In this sense, the hydrolysis of 1-naphthyl phosphate catalyzed by the enzyme alkaline phosphatase (AP) was used as a probe to investigate the effect of the change of the external solvent on RMs formulated with the anionic surfactant sodium diethylhexyl sulfosuccinate (AOT). As external nonpolar solvents, two biocompatible lipophilic esters, isopropyl myristate and methyl laurate, and the traditional nonpolar solvents, n-heptane and benzene, were used. The results were compared among the RMs investigated and with the reaction in homogeneous media. Thus, the effect of the nanoconfinement as well as the impact of the replacement of a conventional external nonpolar solvent by biocompatible solvents were analyzed. The results indicate that the catalytic efficiency in the AOT RMs is larger than that in homogeneous media, denoting a different hydration level over the AP enzyme, which is directly related to the different degrees of nonpolar solvent penetration to the RM interface. Our findings demonstrated that toxic solvents such as n-heptane and benzene can be replaced by nontoxic ones (isopropyl myristate or methyl laurate) in AOT RMs without affecting the performance of micellar systems as nanoreactors, making them a green and promising alternative toward efficient and sustainable chemistry.
1. Introduction
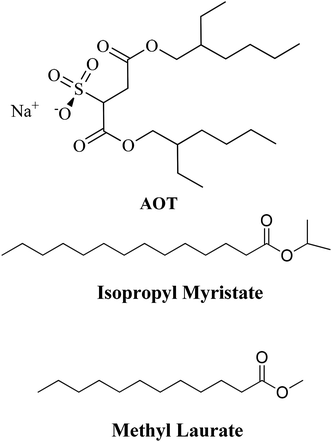
Reverse Micelles (RMs) are a class of organized media with nanometric sizes, composed of at least three components: a polar solvent (generally water), a surfactant, and some nonpolar solvent. The solution formed is optically transparent and thermodynamically stable.1 In the polar core of the RMs, besides water, polar molecules can be dissolved provided that they are non-soluble in the external nonpolar organic solvent.1–5 This nanosized aqueous core is very useful for a wide range of applications such as nanoparticle and polymer synthesis, the enhancement of chemical reaction rates, and even to model water in biological confinement since the water properties are deeply affected when is restrained to a nanoscopic scale.6–11 Thus, properties such as microviscosity, micropolarity, hydrogen bond abilities, and electron donor or acceptor properties are deeply modified.1,12 Consequently, RMs have diverse applications in the field of science and technology.13–20 One of the most important applications is their use as nanoreactors, where a chemical reaction occurs inside the micelle.21,22To prepare RMs different surfactants are used but the majority of the investigations utilize the anionic surfactant sodium diethylhexyl sulfosuccinate (AOT, Scheme 1) since it can form RMs in a wide range of nonpolar solvents. The properties of the AOT RMs can be altered by changing different variables with the most important ones being temperature and nonpolar solvent, and mainly by varying the water content in the solution. Thus, the water content is usually defined as W0 = [H2O]/[surfactant].1
Due to the diverse applications of RMs,1,23 in the last decade, there has been much interest in assessing the nature of nonpolar solvents that can be part of these organized systems.24–26 The traditional RMs cause environmental problems, particularly considering their application to an industrial scale, in food science or drug delivery. The majority of traditional nonpolar solvents used are toxic or at least nonenvironmentally friendly. In this sense, to generate RMs, more biocompatible alternative solvents have been tested.16,23,24,27–38 For example, lipophilic esters such as isopropyl myristate (isopropyl myristate, Scheme 1) have been widely used in biologically resembling systems, pharmaceutics, and drug delivery.16,24,25,32,34,39–42
In particular, in our group, AOT RMs have been formulated with isopropyl myristate and another lipophilic ester (methyl laurate, Scheme 1) with interesting results.24,25,41 Girardi et al.24 showed that methyl laurate and isopropyl myristate can be used as a nonpolar phase in AOT RMs without any cosurfactant. Dynamic light scattering experiments revealed a linear increase of size with the water content as expected for a spherical non-interacting droplet. However, at the same W0, methyl laurate-based RMs are larger than the isopropyl myristate ones. Also, the aggregation number is larger for the methyl laurate RMs. This fact was explained considering that it is possible that isopropyl myristate due to its structural properties can better penetrate the interface leading to smaller RMs. An interesting feature that should be mentioned is the strong similarity observed when comparing droplet sizes, the maximum amount of water solubilized, and the aggregation number values between n-heptane and methyl laurate AOT RMs and benzene and isopropyl myristate AOT RMs. More recently, the micropolarity and the hydrogen-bond ability of the interfaces of these RMs, methyl laurate/AOT/water, and isopropyl myristate/AOT/water, were monitored through the use of the solvatochromism of a molecular probe (1-methyl-8-oxyquinolinium betaine) and Fourier transform infrared spectroscopy (FT-IR).25 The studies confirm that due to the dissimilar penetration of these oils into the interfacial region, the micropolarity and the encapsulated water structure are different. Hence methyl laurate-based RMs have a more polar interface than the isopropyl myristate ones. Moreover, water molecules form stronger hydrogen bond interactions with the polar head of AOT in methyl laurate compared to that in isopropyl myristate. These results evidence the importance of the nonpolar phase penetration in RMs which allows modulating the interface characteristics and could give an alternative when classical toxic solvents need to be replaced by a non-toxic solvent for biological applications.
In this sense, RMs allow the solubilization of hydrophilic biological molecules such as enzymes in organic solvents, so they are also of great interest in enzymatic catalysis.36,43–49 In many cases, it has been found that the application of RMs produces favorable effects compared to that of the homogeneous medium (bulk water), such as superactivity50,51 or greater stability of the enzymes.52,53 Furthermore, RMs are versatile nanoreactors in the study of enzymatic catalysis since they allow controlling the activity and stability of biomolecules through various factors, such as water content or the type of surfactant.43,50
There are numerous reports on enzymatic reactions in RMs. These studies include various enzymes such as α-chymotrypsin,46–48,52,54 trypsin,55 lipase,45,56 peroxidase,57 alcohol dehydrogenase,58 pyruvate kinase,59 cutinase,60 lysozyme,61 and alkaline phosphatase,62–68 among others.
Alkaline phosphatases (APs) are a family of plasma membrane-bound glycoproteins that catalyze the hydrolysis of a wide variety of phosphate esters in an alkaline medium (pH values between 8 and 11).69–73 They are found in many human tissues, including bones, intestines, kidneys, liver, placenta, and white blood cells.71,72 In the first step of the reaction mechanism, the substrate binds to the AP enzyme forming an E–S complex, leading to the phosphorylation of the serine enzyme residue in the active site with the product's release. This is followed by the hydrolysis of the phosphoenzyme, forming an enzyme–phosphate complex. Finally, the dissociation of the phosphate bound to the enzyme occurs. This last step is the limiting one for the reaction in alkaline aqueous solution.74 However, the reaction mechanism is different in RMs, changing the enzymatic hydrolysis's limiting step from phosphate release to phosphorylation or dephosphorylation.63,68
AP has been widely studied, although, compared to other enzymes, such as α-chymotrypsin, its catalytic behavior in RMs has been less explored. Ohshima et al.62 were pioneers in the study of AP in RMs. They used micelles made up of n-heptane and different surfactants AOT, phosphatidylcholine, phosphatidylethanolamine, and phosphatidic acid. The studies revealed that the enzymatic reaction is highly dependent on the water content and the surfactant used. Furthermore, the authors analyzed the effect of the external nonpolar solvent on the enzymatic activity of AP entrapped in AOT RMs. They used four n-alkanes (carbon number: 7–10), finding very similar results in all cases.
More recently, various studies continue to evaluate the enzymatic activity of AP in RMs, and the dependence of their activity on the properties of the system. AP was studied in isooctane/AOT,63,64,66–68n-octane/AOT,75 and n-decane/AOT65 RMs among other systems. In these reports, the effects of pH, water content, temperature, and surfactant concentration have been determined, finding that there are optimal working conditions for each system in which AP presents its maximum activity. Although all the parameters evaluated affect the enzymatic activity, studies have shown that the most relevant parameter in all cases is W0 since AP's kinetic properties in RMs, such as the initial velocity of the reaction, strongly depend on the water content. This indicates that the AP enzyme inside the RM is very sensitive to the amount of water around it and that the optimal W0 depends on several factors.
As mentioned before, there are various reports on the effects of pH, water content, temperature, and surfactant concentration on the catalytic behavior of AP in RMs. However, studies of the effect of the external nonpolar solvent are scarce. Furthermore, most of the AP studies in RMs have been carried out using traditional nonpolar organic solvents, such as isooctane,63,64,66–68n-octane,75 and n-decane.65
In the present work, the study of the hydrolysis of sodium 1-naphthyl phosphate catalyzed by the enzyme AP (Scheme 2) in AOT RMs is reported, using as nonpolar solvents n-heptane, benzene, isopropyl myristate, and methyl laurate. In this way, the effect of the change of the external solvent on the enzyme activity is analyzed. It is important to note that isopropyl myristate and methyl laurate were chosen as they are considered nontoxic lipophilic solvents.24,38,42,76–81 Hence, their use makes them a promising alternative to traditional solvents used in biocatalysis.
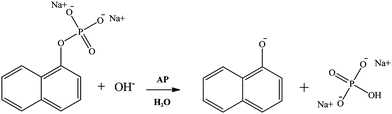 | ||
| Scheme 2 Hydrolysis of sodium 1-naphthyl phosphate catalyzed by the enzyme alkaline phosphatase (AP). | ||
Finally, it is important to note that enzymatic catalysis studies using biocompatible solvents such as isopropyl myristate and methyl laurate as external solvents in RMs are practically not found in the literature. Therefore, our findings provide valuable information on the use of biocompatible organized media as nanoreactors. It is shown that toxic solvents such as n-heptane and benzene can be replaced by isopropyl myristate or methyl laurate in AOT RMs without affecting the performance of micellar systems as nanoreactors, making them a promising green alternative towards efficient and sustainable chemistry. Interestingly, the enzyme seems to work more efficiently because of the confinement effect, as we will show.
2. Results and discussion
To evaluate the effect of the type of external nonpolar solvent in AOT RMs on the enzymatic behavior of AP, first, we monitored the kinetic reaction in homogeneous media (buffer solution), and second, inside the different RMs. Thus, we worked with the RMs formed by isopropyl myristate/AOT/water, methyl laurate/AOT/water, benzene/AOT/water, and n-heptane/AOT/water analyzing the effect of the external solvent's change on the kinetic parameters (kcat and KM) of the reaction catalyzed by AP.2.1. Enzymatic reaction in water
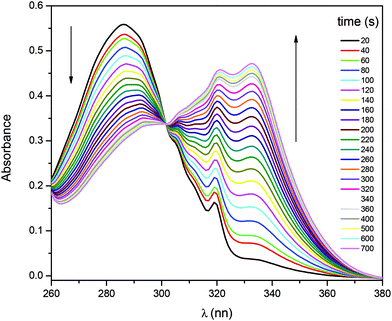
Fig. 1 shows the typical UV-vis absorption spectra of the progress of the hydrolysis reaction of 1-naphthyl phosphate in buffer solution (pH = 10) at different times. A decrease in the intensity of the absorption band of the substrate 1-naphthyl phosphate at 285 nm, an increase in the absorbance of the product (1-naphtholate) at 336 nm with time, and a clear isosbestic point at λ = 301 nm were observed. These facts indicate the formation of the 1-naphtholate without the presence of intermediates and/or product decomposition.To obtain the kinetic parameters of the hydrolysis of 1-naphthyl phosphate catalyzed by AP (kcat and KM), the initial reaction rates were determined at different concentrations of 1-naphthyl phosphate, keeping the enzyme concentration constant (see the ESI† for the detailed procedure). Fig. 2 shows the experimental data evaluated in homogeneous solution and the corresponding fitting according to the Michaelis–Menten mechanism by using the well-known eqn (1).
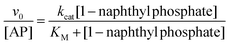 | (1) |
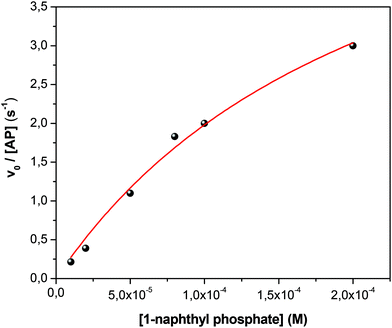 | ||
| Fig. 2 Effect of 1-naphthyl phosphate concentration on the initial reaction rate (v0) of 1-naphthyl phosphate hydrolysis catalyzed by AP in buffer solution at 35 °C. [AP] = 1 × 10−7 M. [buffer] = 0.01 M, pH = 10. The solid line represents the fit according to eqn (1). | ||
From the fitting of Fig. 2, the kcat and KM values were determined and the catalytic efficiency (kcat/KM) was calculated.
As can be observed from Fig. 2, the hydrolysis of 1-naphthyl phosphate catalyzed by AP follows perfectly the reaction model proposed by Michaelis and Menten.82,83 The nonlinear fit of the graph using eqn (1) allowed us to determine values of kcat = 6.5 ± 0.1 s−1 and KM = (23 ± 1) × 10−5 M in homogeneous media. Consequently, the catalytic efficiency (kcat/KM) of AP is 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ± 1400 M−1 s−1.
200 ± 1400 M−1 s−1.
2.2. Enzymatic reaction in AOT RMs
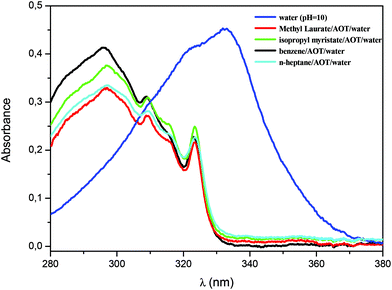
The enzymatic activity of AP was also evaluated in the RM systems formed by isopropyl myristate/AOT/water, methyl laurate/AOT/water, benzene/AOT/water, and n-heptane/AOT/water. The polar solvent used was a 0.01 M Na2CO3/NaHCO3 buffer solution at pH 10. Furthermore, all studies were carried out at W0 = 10 and [AOT] = 0.1 M. The W0 value was chosen equal to 10 to compare exclusively all the systems with the same amount of water. Although the variation in the water content may affect the kinetics parameters, in this case the focus is on the analysis of the effect of the external solvent in RMs.47,48,52,68,84The aqueous AOT RMs show a particular behavior with regard to their pH.85–87 Recently, using an electrochemical technique we suggested that the value of the pH of the AOT RM interface cannot be assumed to be equal to that of the original water solution used to prepare the RMs and, in a large range of water content (W0 = 8–28) it is weakly acidic even when a buffer solution of pH around 12 is used.87 Similarly, previous studies showed that the nonionized form of phenols is the most stable species when entrapped in the AOT RMs; thus the organized media appears to act as a pH buffer system that prevents ionization.86 In this sense, to evaluate what happens with the hydrolysis reaction of 1-naphthyl phosphate catalyzed by AP in AOT RMs even incorporating buffer solution at pH = 10, the appearance of a UV-vis absorption band centered at 323 nm, corresponding to 1-naphthol, was tested. Fig. 3 shows the absorption spectra once the hydrolysis reaction of 1-naphthyl phosphate in isopropyl myristate/AOT/water, methyl laurate/AOT/water, benzene/AOT/water, and n-heptane/AOT/water RMs is completed (time = 15 minutes). Furthermore, the final UV-vis spectrum of the hydrolysis of 1-naphthyl phosphate in the homogeneous solution is shown, where the absorption band corresponds to 1-naphtholate.
As can be observed, in all the AOT RMs the naphtholate species is not detected and only the formation of 1-naphthol is observed. These results reinforce the evidence observed earlier that the AOT RMs act as buffer solution and the interface is acidic.87 In this sense, it is important to remark that the concept of pH in the RMs is not straightforward as in the bulk. Several examples of unusual results have been observed before that support this fact. At the interface, processes such as deprotonation of phenols or protonation of amines are phenomena not detected in AOT RMs. These results were explained as consequences of the different interfacial interactions (mainly strong hydrogen bond interactions with the sulfonate group of the anionic surfactant) present when water is entrapped inside RMs. Thus, solutes show unexpected nucleophilicities, strong electrostatic interactions, inhibition of protonation or deprotonation processes, polarity and viscosity parameters among others, and these are examples of evidence found in RMs.86–89 Interestingly, Fig. 3 also confirms that the enzymatic reaction takes place at the interface and not in the pool. This is because if the reaction is performed in the pool,85 the naphtholate formation should be detected. Moreover, the enzymatic reaction under the same experimental conditions was investigated but with the addition of water without controlling the pH value. The same kinetic parameter values were obtained reinforcing that the reaction takes place at the RM interface.
As in the homogeneous media, the initial velocity values (v0) were determined as a function of the substrate concentration in each micellar system at constant W0 = 10 and [AOT] = 0.1 M. It is important to mention that as both AP and 1-naphthyl phosphate are insoluble in the nonpolar external solvent, no partition process occur and no variation of the surfactant concentration was needed. Additionally, to compare the different RMs and not extend the amount of data collected we decided only to test a single W0. Fig. 4 shows typical data obtained for the isopropyl myristate/AOT/water system. The fit obtained is consistent with the validation of the Michaelis–Menten mechanism in the RMs. Using eqn (1), the values of the catalytic constants kcat = 5.1 ± 0.2 s−1, KM = (11.9 ± 0.6) × 10−5 M and kcat/KM = 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 2000 were obtained. The plots of v0versus substrate concentration for the enzymatic reaction in the systems formed by methyl laurate/AOT/water, benzene/AOT/water, and n-heptane/AOT/water are shown in Fig. S3–S5 in the ESI† and the kinetics parameters are summarized in Table 1.
000 ± 2000 were obtained. The plots of v0versus substrate concentration for the enzymatic reaction in the systems formed by methyl laurate/AOT/water, benzene/AOT/water, and n-heptane/AOT/water are shown in Fig. S3–S5 in the ESI† and the kinetics parameters are summarized in Table 1.
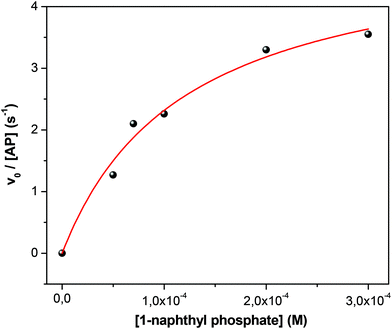 | ||
| Fig. 4 Effect of 1-naphthyl phosphate concentration on the initial reaction rate (v0) of 1-naphthyl phosphate hydrolysis catalyzed by AP in isopropyl myristate/AOT/water, at 35 °C. W0 = 10, [AP] = 1 × 10−7 M. [Na2CO3/NaHCO3] = 0.01 M, pH = 10. The solid line represents the fit according to eqn (1). | ||
| System | k cat (s−1) | K M (M) × 10−5 | k cat/KM (M−1 s−1) |
|---|---|---|---|
| Water | 6.5 ± 0.1 | 23 ± 1 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ± 1400 200 ± 1400 |
| Isopropyl myristate/AOT/water | 5.1 ± 0.2 | 11.9 ± 0.6 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 2000 000 ± 2000 |
| Methyl laurate/AOT/water | 2.4 ± 0.1 | 2.5 ± 0.1 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ± 4800 500 ± 4800 |
| Benzene/AOT/water | 1.3 ± 0.6 | 3.3 ± 0.1 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 092 ± 1800 092 ± 1800 |
| n-Heptane/AOT/water | 15.1 ± 0.7 | 23.7 ± 0.5 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 3800 000 ± 3800 |
The results (Table 1) show that the catalytic efficiency values for the hydrolysis of 1-naphthyl phosphate catalyzed by AP in all the AOT RMs evaluated are higher than those in water. Interestingly, even when the pH is not the appropriated value by AP, the reaction is enhanced in comparison with bulk water. These results are believed to be due to the confinement effect that the enzyme experiences when it is entrapped in AOT RMs. The confinement effect refers to the change in the properties of a compound due to entrapment within a limited space (cavity, chamber, cell, etc.) of nanometric size (1–100 nm).90,91 In this way, the water encapsulated in reverse micellar systems that have dimensions of a few nanometers presents characteristics that are well differentiated from those found for bulk water, mainly due to the interaction of water with the surfactant molecules.92 Confinement severely affects the water structure and dynamics such as dipole orientation, tetrahedral order parameter, and H-bonded populations. Interfacial water molecules were found to be the most severely affected.91,93 Thus, in AOT RMs the water molecules change their properties as a consequence of the strong interactions with the surfactant at the interface, which would produce a suitable interface to solvate the enzyme.
Furthermore, when comparing the RM systems, differences are observed. When analyzing the RMs formed with biocompatible solvents, it is found that, when using methyl laurate as the external compound, kcat/KM is higher than that when using isopropyl myristate. Similarly, when comparing the systems formed by traditional nonpolar solvents, it is observed that in n-heptane/AOT/water, the catalytic efficiency is higher than that in benzene/AOT/water.
At this point, it is important to mention that the change of the external solvent in organized media such as RMs is not trivial and several surprising results were obtained.24,25,32,42,81,94–98 For example, we demonstrated that the polarity and viscosity parameters have a strong impact on the penetration phenomena. Thus, more polar and viscous solvents penetrate deeper the interface in AOT RMs. Moreover, a concept such as the molar volume or the chemical structure does not explain properly the observed results.24,95
The characteristics of AOT RMs formulated in the biocompatible solvents isopropyl myristate and methyl laurate have been previously investigated through different techniques, such as dynamic light scattering and static light scattering, and FT-IR, 1H NMR, and UV-vis absorption spectroscopy using the 1-methyl-8-oxyquinolinium betaine molecular probe.24,42,80,99 The obtained results showed a different degree of penetration of the external solvent into the micellar interface; in particular, isopropyl myristate penetrates more than methyl laurate. The penetration of these external solvents at the interface of the AOT RMs was directly related to the solvent's viscosity and polarity. The more viscous and more polar the external solvent, the greater its ability to penetrate to the micellar interface. Isopropyl myristate is more polar and viscous than methyl laurate, which results in deeper penetration of isopropyl myristate at the interface of micellar systems.24 Considering that the purpose of the present work is to evaluate the external solvent effect using an enzymatic reaction as a probe, we explored a very sensitive reaction to the water properties. Studies using isopropyl myristate and methyl laurate as external solvents in AOT RMs and AP as the enzyme are not found in the literature. Additionally, a comparison of later systems and the traditional RMs created with n-heptane and benzene allows us to understand the effect on the interfacial water when these are employed as reactants in an enzymatic reaction.
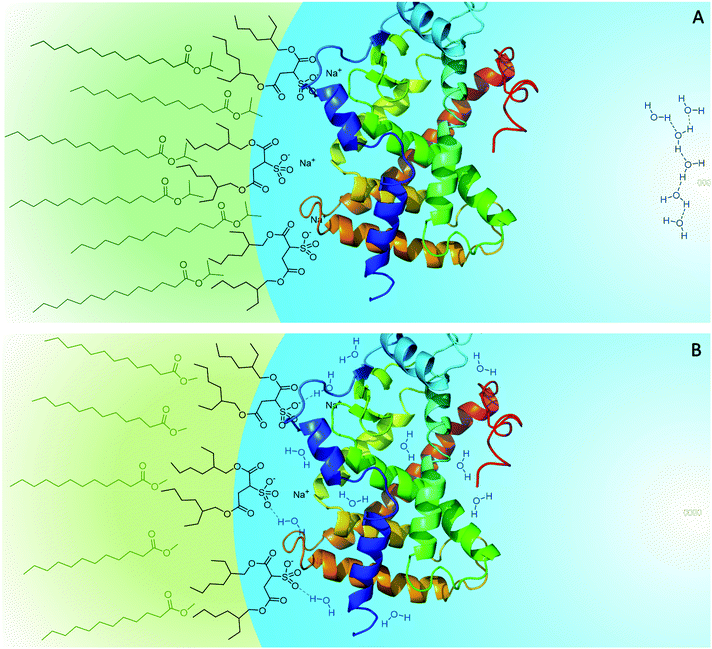
As mentioned above, the different penetration levels of the biocompatible solvents lead to the interfacial properties, such as micropolarity and hydrogen bonding donor capacity, being different for isopropyl myristate/AOT/water and methyl laurate/AOT/water.80 In RMs formed with methyl laurate, the interface is more polar, and there is a higher proportion of water molecules that interact with the micellar interface compared to the isopropyl myristate/AOT system. Then, considering that the 1-naphthyl phosphate hydrolysis reaction occurs at the micellar interface, where the AP enzyme is located, the difference in catalytic efficiency in isopropyl myristate/AOT and methyl laurate/AOT could be explained through the different penetration levels of the external solvent towards the micellar interface, and how this influences the interfacial properties. By increasing the external solvent's penetration in AOT RMs, the proportion of water molecules that interacts with the micellar interface decreases. Consequently, the water molecules around the enzyme's active sites are displaced by the nonpolar solvent, changing the hydration and probably the mobility of the AP, factors essential for its activity; its efficiency decreases. This effect is more pronounced when using isopropyl myristate as an external solvent since, as mentioned before, it penetrates more into the micellar interface (Scheme 3).
On the other hand, in previous studies, it was observed that isopropyl myristate/AOT and methyl laurate/AOT show similar behavior, in terms of solvent penetration and water–surfactant interactions, to the benzene/AOT and n-heptane/AOT systems, respectively. As in the case of biocompatible solvents, when comparing benzene/AOT and n-heptane/AOT, it is found that the aromatic compound penetrates the interface of AOT RMs more than the aliphatic solvent because benzene is more viscous and polar than n-heptane. Thus, the lower catalytic efficiency of AP in benzene/AOT/water compared to that in n-heptane/AOT/water can also be attributed to the lower hydration of the enzyme. In AOT RMs, the aromatic solvent's higher penetration displaces the water molecules from the micellar interface to a greater extent than n-heptane.
It is important to note that Table 1 shows that benzene/AOT/water is the micellar system with lower catalytic efficiency. This is consistent with the fact that aromatic solvents, such as benzene, penetrate the micellar interface more than the aliphatic solvents, such as n-heptane, isopropyl myristate, and methyl laurate.
The results demonstrate the effect of the different penetration levels of the external solvents on the properties of the micellar interface generated, which is reflected in the AP enzyme's catalytic efficiency. Furthermore, they reinforce the idea that, in AOT RMs, isopropyl myristate and methyl laurate have an analogous behavior to benzene and n-heptane, respectively. Just as isopropyl myristate penetrates the micellar interface more than methyl laurate resulting in lower catalytic efficiency, benzene penetrates more than n-heptane obtaining lower catalytic efficiencies in benzene/AOT compared to n-heptane/AOT.
In summary, these results present a promising field as the unique properties of alkane/AOT/water RMs can be obtained using environmentally friendly and nontoxic lipophilic solvents, such as isopropyl myristate and methyl laurate, as an excellent alternative to be used in a sustainable procedure.
3. Conclusions
In this work we evaluate the external component effect using an enzymatic reaction as a probe, exploring a very sensitive reaction to the properties of water. The unknown effect of isopropyl myristate and methyl laurate as external solvents in AOT RMs and AP as the enzyme was unraveled. Additionally, a comparison of these systems and the traditional RMs created with n-heptane and benzene, as well as homogeneous media, allows us to understand the effect on the interfacial water when this is employed as a reactant in an enzymatic reaction. To the best of our knowledge, this is the first report investigating an enzymatic hydrolysis reaction in reverse micellar systems formed in biocompatible organic solvents.The kinetics data of the hydrolysis reaction of 1-naphthyl phosphate inside AOT RMs indicate that the enzyme is perfectly active in all the RMs explored. Interestingly, in the AOT RMs the product is not detected as naphtholate even in the alkaline aqueous solution used to form the RMs. The detection of 1-naphthol is consistent with an acidic interface where the reaction takes place. Even though, the catalytic efficiency in AOT RMs is larger than that in homogeneous media, denoting a different and favorable hydration level over the AP under confinement.
The obtained results reinforced the different penetration levels of the biocompatible external solvents in AOT RMs and the similarities between n-heptane-methyl laurate and benzene-isopropyl myristate. Thus, the use of an enzymatic reaction as a probe is an interesting alternative to explore the interfacial composition and interfacial properties of RMs, since it is very sensitive to the properties of the media.
Finally, our findings provide valuable information on the use of biocompatible organized media as nanoreactors. Most of the antecedents of enzymatic catalysis in reverse micelles involve the use of traditional organic solvents, which represents a problem from the point of view of sustainable chemistry. The present work shows that it is possible to carry out an enzymatic hydrolysis reaction, in particular the hydrolysis of 1-naphthyl phosphate catalyzed by AP, in reverse micellar systems formed in biocompatible organic solvents. Also, the results obtained when using this type of solvent are analogous to those obtained when using traditional solvents. Thus, the toxic solvents such as n-heptane and benzene can be replaced by biocompatible solvents such as isopropyl myristate and methyl laurate without affecting the performance of micellar systems as nanoreactors, making them a green and promising alternative toward efficient and sustainable chemistry.
Abbreviations
| AOT | Sodium diethylhexyl sulfosuccinate |
| AP | Alkaline phosphatase |
| FT-IR | Fourier transform infrared spectroscopy |
| k cat | Catalytic rate constant |
| K M | Michaelis constant |
| RMs | Reverse micelles |
| v 0 | Initial velocity |
| W 0 | [H2O]/[surfactant] |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Consejo Nacional de Investigaciones Científicas y Técnicas (PIP CONICET 112-2015-0100283), Universidad Nacional de Río Cuarto (PPI-UNRC 2016-2019), Agencia Nacional de Promoción Científica y Técnica (PICT 2015-0585, PICT 2018-0508), and Ministerio de Ciencia y Tecnología, Gobierno de la Provincia de Córdoba (PID 2018) is gratefully acknowledged.References
- N. M. Correa, J. J. Silber, R. E. Riter and N. E. Levinger, Chem. Rev., 2012, 112, 4569–4602 CrossRef CAS PubMed.
- D. Langevin, Annu. Rev. Phys. Chem., 1992, 43, 341–369 CrossRef CAS.
- M. P. Pileni, J. Phys. Chem., 1993, 97, 6961–6973 CrossRef CAS.
- J. Eastoe, M. J. Hollamby and L. Hudson, Adv. Colloid Interface Sci., 2006, 128–130, 5–15 CrossRef CAS PubMed.
- N. E. Levinger and L. A. Swafford, Annu. Rev. Phys. Chem., 2009, 60, 385–406 CrossRef CAS PubMed.
- A. Kubacka, U. Caudillo-Flores, I. Barba-Nieto, M. J. Muñoz-Batista and M. Fernández-García, Curr. Opin. Colloid Interface Sci., 2020, 49, 42–59 CrossRef CAS.
- J. A. Gutierrez, R. D. Falcone, M. A. Lopez-Quintela, D. Buceta, J. J. Silber and N. M. Correa, Eur. J. Inorg. Chem., 2014, 2014, 2095–2102 CrossRef CAS.
- J. S. Florez Tabares, N. M. Correa, J. J. Silber, L. E. Sereno and P. G. Molina, Soft Matter, 2015, 11, 2952–2962 RSC.
- J. A. Gutierrez, M. Alejandra Luna, N. Mariano Correa, J. J. Silber and R. Darío Falcone, New J. Chem., 2015, 39, 8887–8895 RSC.
- M. S. Orellano, C. Porporatto, J. J. Silber, R. D. Falcone and N. M. Correa, Carbohydr. Polym., 2017, 171, 85–93 CrossRef CAS PubMed.
- M. S. Orellano, G. S. Longo, C. Porporatto, N. M. Correa and R. D. Falcone, Colloids Surf., A, 2020, 599, 124876 CrossRef CAS.
- J. J. Silber, A. Biasutti, E. Abuin and E. Lissi, Adv. Colloid Interface Sci., 1999, 82, 189–252 CrossRef CAS.
- C. C. Müller-Goymann, Eur. J. Pharm. Biopharm., 2004, 58, 343–356 CrossRef PubMed.
- V. Uskoković and M. Drofenik, Surf. Rev. Lett., 2005, 12, 239–277 CrossRef.
- Y. Liu, X. Dong and Y. Sun, Chin. J. Chem. Eng., 2008, 16, 949–955 CrossRef CAS.
- A. Kogan and N. Garti, Adv. Colloid Interface Sci., 2006, 123–126, 369–385 CrossRef CAS PubMed.
- S. P. Callender, J. A. Mathews, K. Kobernyk and S. D. Wettig, Int. J. Pharm., 2017, 526, 425–442 CrossRef CAS PubMed.
- Y. Singh, J. G. Meher, K. Raval, F. A. Khan, M. Chaurasia, N. K. Jain and M. K. Chourasia, J. Controlled Release, 2017, 252, 28–49 CrossRef CAS PubMed.
- H. Watarai, J. Chromatogr. A, 1997, 780, 93–102 CrossRef CAS.
- B. K. Paul and S. P. Moulik, Curr. Sci., 2001, 80, 990–1001 CAS.
- M. A. López-Quintela, C. Tojo, M. C. Blanco, L. García Rio and J. R. Leis, Curr. Opin. Colloid Interface Sci., 2004, 9, 264–278 CrossRef.
- D. Blach, M. Pessêgo, J. J. Silber, N. M. Correa, L. García-Río and R. D. Falcone, Langmuir, 2014, 30, 12130–12137 CrossRef CAS PubMed.
- X. Sun and N. Bandara, Trends Food Sci. Technol., 2019, 91, 106–115 CrossRef CAS.
- V. R. Girardi, J. J. Silber, N. Mariano Correa and R. Darío Falcone, Colloids Surf., A, 2014, 457, 354–362 CrossRef CAS.
- V. R. Girardi, J. J. Silber, R. D. Falcone and N. M. Correa, ChemPhysChem, 2018, 19, 759–765 CrossRef CAS PubMed.
- M. Kaur, G. Singh, S. Kumar, Navnidhi and T. S. Kang, J. Colloid Interface Sci., 2018, 511, 344–354 CrossRef CAS PubMed.
- K. Kundu and B. K. Paul, J. Surfactants Deterg., 2013, 16, 865–879 CrossRef CAS.
- S. Bardhan, K. Kundu, S. K. Saha and B. K. Paul, J. Colloid Interface Sci., 2013, 411, 152–161 CrossRef CAS PubMed.
- J. X. Liu, X. G. Zhang and H. J. Zhang, J. Chem. Thermodyn., 2014, 72, 1–8 CrossRef CAS.
- B. Mithila and P. Varsha, J. Appl. Pharm. Sci., 2020, 10(08), 008–019 Search PubMed.
- O. P. Lehtinen, R. W. N. Nugroho, T. Lehtimaa, S. Vierros, P. Hiekkataipale, J. Ruokolainen, M. Sammalkorpi and M. Österberg, Colloids Surf., B, 2017, 160, 355–363 CrossRef CAS PubMed.
- N. Dib, J. J. Silber, N. M. Correa and R. D. Falcone, New J. Chem., 2019, 43, 10398–10404 RSC.
- N. Bubic Pajic, I. Nikolic, E. Mitsou, V. Papadimitriou, A. Xenakis, D. Randjelovic, V. Dobricic, A. Smitran, N. Cekic, B. Calija and S. Savic, J. Mol. Liq., 2018, 272, 746–758 CrossRef CAS.
- S. Gupta and S. P. Moulik, J. Pharm. Sci., 2008, 97, 22–45 CrossRef CAS PubMed.
- O. Fadel, L. Girard, D. Gomes Rodrigues, P. Bauduin, X. Le Goff, A. Rossignol-Castera, A. L'Hermitte and O. Diat, Colloids Surf., B, 2017, 154, 279–286 CrossRef CAS PubMed.
- E. Mitsou, A. Xenakis and M. Zoumpanioti, Catalysts, 2017, 7, 52 CrossRef.
- M. Oyarzun, G. Oliva, R. D. Falcone and P. Pavez, ACS Sustainable Chem. Eng., 2020, 8, 5478–5484 CrossRef CAS.
- X. Zhang, Y. Chen, J. Liu, C. Zhao and H. Zhang, J. Phys. Chem. B, 2012, 116, 3723–3734 CrossRef CAS PubMed.
- B. Fubini, M. R. Gasco and M. Gallarate, Int. J. Pharm., 1988, 42, 19–26 CrossRef CAS.
- D. Attwood, C. Mallon and C. J. Taylor, Int. J. Pharm., 1992, 84, R5–R8 CrossRef CAS.
- E. Odella, R. D. Falcone, M. Ceolín, J. J. Silber and N. M. Correa, J. Phys. Chem. B, 2018, 122, 4366–4375 CrossRef CAS PubMed.
- N. Dib, R. D. Falcone, A. Acuña and L. García-Río, J. Mol. Liq., 2020, 304, 112762 CrossRef CAS.
- B. Orlich and R. Schomäcker, Adv. Biochem. Eng. Biotechnol., 2002, 75, 185–208 CrossRef CAS PubMed.
- P. L. Luisi and C. Laane, Trends Biotechnol., 1986, 4, 153–161 CrossRef CAS.
- C. M. L. Carvalho and J. M. S. Cabral, Biochimie, 2000, 82, 1063–1085 CrossRef CAS PubMed.
- F. Moyano, E. Setien, J. J. Silber and N. M. Correa, Langmuir, 2013, 29, 8245–8254 CrossRef CAS PubMed.
- F. Moyano, R. D. Falcone, J. C. Mejuto, J. J. Silber and N. M. Correa, Chem. – Eur. J., 2010, 16, 8887–8893 CrossRef CAS PubMed.
- A. M. Durantini, R. D. Falcone, J. J. Silber and N. M. Correa, ChemPhysChem, 2016, 17, 1678–1685 CrossRef CAS PubMed.
- N. L. Klyachko and A. V. Levashov, Curr. Opin. Colloid Interface Sci., 2003, 8, 179–186 CrossRef CAS.
- E. Ruckenstein and P. Karpe, J. Colloid Interface Sci., 1990, 139, 408–436 CrossRef CAS.
- T. E. Sintra, S. P. M. Ventura and J. A. P. Coutinho, J. Mol. Catal. B: Enzym., 2014, 107, 140–151 CrossRef CAS.
- R. D. Falcone, M. A. Biasutti, N. M. Correa, J. J. Silber, E. Lissi and E. Abuin, Langmuir, 2004, 20, 5732–5737 CrossRef CAS PubMed.
- R. V. Rariy, N. Bec, N. L. Klyachko, A. V. Levashov and C. Balny, Biotechnol. Bioeng., 1998, 57, 552–556 CrossRef CAS.
- E. I. Gomez Rodríguez, R. D. Falcone, P. R. Beassoni, F. Moyano and N. M. Correa, ChemistrySelect, 2019, 4, 7204–7210 CrossRef.
- P. K. Das, G. V. Srilakshmi and A. Chaudhuri, Langmuir, 1999, 15, 981–987 CrossRef CAS.
- P. D. I. Fletcher, B. H. Robinson, R. B. Freedman and C. Oldfield, J. Chem. Soc., Faraday Trans. 1, 1985, 81, 2667 RSC.
- S. Parida, G. R. Parida and A. N. Maitra, Colloids Surf., 1991, 55, 223–229 CrossRef CAS.
- S. Das, S. Mozumdar and A. Maitra, J. Colloid Interface Sci., 2000, 230, 328–333 CrossRef CAS PubMed.
- L. Ramírez-Silva, S. T. Ferreira, T. Nowak, M. Tuena de Gómez-Puyou and A. Gómez-Puyou, Eur. J. Biochem., 2001, 268, 3267–3274 CrossRef PubMed.
- C. M. L. Carvalho, J. M. S. Cabral and M. R. Aires-Barros, J. Mol. Catal. B: Enzym., 1998, 5, 361–365 CrossRef CAS.
- T. Kinugasa, K. Watanabe and H. Takeuchi, Ind. Eng. Chem. Res., 1992, 31, 1827–1829 CrossRef CAS.
- A. Ohshima, H. Narita and M. Kito, J. Biochem., 1983, 93, 1421–1425 CrossRef CAS PubMed.
- G.-G. Chang and S.-L. Shiao, Eur. J. Biochem., 1994, 220, 861–870 CrossRef CAS PubMed.
- M. Gonnelli and G. B. Strambini, J. Phys. Chem., 1988, 92, 2854–2857 CrossRef CAS.
- S. Gupta, L. Mukhopadhyay and S. P. Moulik, Colloids Surf., B, 1994, 3, 191–201 CrossRef CAS.
- H.-C. Hung, T.-M. Huang and G.-G. Chang, J. Chem. Soc., Perkin Trans. 2, 1997, 2757–2760 RSC.
- E. Luchter-Wasylewska and M. Iciek, J. Colloid Interface Sci., 2004, 273, 632–637 CrossRef CAS PubMed.
- T. M. Huang, H. C. Hung, T. C. Chang and G. G. Chang, Biochem. J., 1998, 330, 267–275 CrossRef CAS PubMed.
- J. G. Zalatan, T. D. Fenn and D. Herschlag, J. Mol. Biol., 2008, 384, 1174–1189 CrossRef CAS PubMed.
- U. Sharma, D. Pal and R. Prasad, Indian J. Clin. Biochem., 2014, 29, 269–278 CrossRef CAS PubMed.
- M. M. Kaplan, Gastroenterology, 1972, 62, 452–468 CrossRef CAS.
- J. E. Coleman, Annu. Rev. Biophys. Biomol. Struct., 1992, 21, 441–483 CrossRef CAS PubMed.
- A. K. Cobo Solis, N. M. Correa and P. G. Molina, Langmuir, 2017, 33, 12080–12086 CrossRef CAS PubMed.
- G. G. Chang, T. M. Huang and H. C. Hung, Proc. Natl. Sci. Counc., Repub. China, Part B: Life Sci., 2000, 24, 89–100 CAS.
- A. V. Kabanov, S. N. Nametkin, N. L. Klyachko and A. V. Levashov, FEBS Lett., 1991, 278, 143–146 CrossRef CAS.
- K. Ita, Pharm. Dev. Technol., 2017, 22, 467–475 CrossRef CAS PubMed.
- R. K. Mitra and B. K. Paul, Colloids Surf., A, 2005, 255, 165–180 CrossRef CAS.
- B. K. Paul and R. K. Mitra, Colloids Surf., A, 2006, 273, 129–140 CrossRef CAS.
- R. K. Mitra and B. K. Paul, Colloids Surf., A, 2005, 252, 243–259 CrossRef CAS.
- V. R. Girardi, J. J. Silber, R. D. Falcone and N. M. Correa, ChemPhysChem, 2018, 19, 759–765 CrossRef CAS PubMed.
- N. Dib, J. J. Silber, N. M. Correa and R. D. Falcone, J. Mol. Liq., 2020, 313, 113592 CrossRef CAS.
- R. M. D. Verhaert, R. Hilhorst, M. Vermue, T. J. Schaafsma and C. Veeger, Eur. J. Biochem., 1990, 187, 59–72 CrossRef CAS PubMed.
- E. A. Lissi and E. B. Abuin, Langmuir, 2000, 16, 10084–10086 CrossRef CAS.
- A. Kyriazi, V. Papadimitriou, T. G. Sotiroudis and A. Xenakis, Eur. J. Lipid Sci. Technol., 2013, 115, 601–611 CrossRef CAS.
- B. Baruah, J. M. Roden, M. Sedgwick, N. M. Correa, D. C. Crans and N. E. Levinger, J. Am. Chem. Soc., 2006, 128, 12758–12765 CrossRef CAS PubMed.
- O. F. Silva, M. A. Fernández, J. J. Silber, R. H. De Rossi and N. M. Correa, ChemPhysChem, 2012, 13, 124–130 CrossRef CAS PubMed.
- A. K. Cobo Solís, M. A. Luna, R. D. Falcone, N. M. Correa and P. G. Molina, ChemistrySelect, 2019, 4, 14309–14314 CrossRef.
- O. F. Silva, R. H. De Rossi and N. M. Correa, RSC Adv., 2015, 5, 34878–34884 RSC.
- M. A. Crosio, N. M. Correa, J. J. Silber and R. D. Falcone, Org. Biomol. Chem., 2016, 14, 3170–3177 RSC.
- W. D. Van Horn, M. E. Ogilvie and P. F. Flynn, J. Am. Chem. Soc., 2009, 131, 8030–8039 CrossRef CAS PubMed.
- A. Baksi, P. K. Ghorai and R. Biswas, J. Phys. Chem. B, 2020, 124, 2848–2863 CrossRef CAS PubMed.
- N. E. Levinger, Science, 2002, 298, 1722–1723 CrossRef CAS PubMed.
- A. Baksi and R. Biswas, J. Phys. Chem. B, 2020, 124, 11718–11729 CrossRef CAS PubMed.
- F. M. Agazzi, J. Rodriguez, R. D. Falcone, J. J. Silber and N. M. Correa, Langmuir, 2013, 29, 3556–3566 CrossRef CAS PubMed.
- F. M. Agazzi, R. D. Falcone, J. J. Silber and N. M. Correa, J. Phys. Chem. B, 2011, 115, 12076–12084 CrossRef CAS PubMed.
- F. M. Agazzi, N. M. Correa and J. Rodriguez, Langmuir, 2014, 30, 9643–9653 CrossRef CAS PubMed.
- F. M. Agazzi, R. D. Falcone, J. J. Silber and N. M. Correa, Colloids Surf., A, 2016, 509, 467–473 CrossRef CAS.
- N. Dib, R. D. Falcone and L. García-Río, J. Org. Chem., 2020, 85, 15006–15014 CrossRef CAS PubMed.
- N. Dib, R. D. Falcone, A. Acuña and L. García-Río, Langmuir, 2019, 35, 12744–12753 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fully experimental procedures, the molar absorptivity values (ε) for the hydrolysis product of 1-naphthyl phosphate (1-naphtholate) in the different systems (Table 1). Also contains the following figures: Fig. S1. Changes in absorbance value at 336 nm as a function of time for the hydrolysis of 1-naphthyl phosphate catalyzed by AP in water. Fig. S2. Variation of the concentration of the product 1-naphtholate in buffer solution at different reaction times. Fig. S3–S5. Effect of 1-naphthyl phosphate concentration on the initial rate (v0) of 1-naphthyl phosphate hydrolysis catalyzed by AP in methyl laurate/AOT/water, benzene/AOT/water, and n-heptane/AOT/water RMs. See DOI: 10.1039/d0ob02371j |
| This journal is © The Royal Society of Chemistry 2021 |