Open Access Article
Open Access ArticleFurfurylation protects timber from degradation by marine wood boring crustaceans†
Lucy S.
Martin‡
 a,
Stanislav
Jelavi懧
a,
Stanislav
Jelavi懧
 b,
Simon M.
Cragg
b,
Simon M.
Cragg
 ac and
Lisbeth G.
Thygesen
ac and
Lisbeth G.
Thygesen
 *d
*d
aInstitute of Marine Science, University of Portsmouth, Ferry Road, Portsmouth, PO4 9LY, UK
bCentre for GeoGenetics, GLOBE Institute, University of Copenhagen, Øster Voldgade 5–7, 1350 Copenhagen, Denmark
cCentre for Enzyme Innovation, School of Biological Sciences, University of Portsmouth, King Henry 1st Street, Portsmouth, P01 2DY, UK
dUniversity of Copenhagen, Department of Geosciences and Natural Resource Management, Rolighedsvej 23, 1958 Frederiksberg C, Denmark. E-mail: lgt@ign.ku.dk
First published on 6th September 2021
Abstract
Unmodified timber is susceptible to biodegradation in the marine environment by wood-boring molluscs and crustaceans. Wood is a renewable resource and has a much lower carbon footprint than other alternative materials that are suitable for marine applications, such as concrete and steel. However, biodegradation causes expensive damage to wooden structures and protection by broad spectrum biocides entails environmental risks. Furfurylation offers an effective alternative protection from marine wood-borers. We investigate the changes in feeding rate, behaviour and digestion of the marine wood-boring crustacean, the gribble, on furfurylated wood under laboratory conditions. Pinus radiata was impregnated with furfuryl alcohol in a methanol solvent and polymerised at elevated temperatures. Wood was leached in seawater and then tested in a laboratory setting against the gribble Limnoria quadripunctata, by measuring its feeding rate (faecal pellet production), vitality and mortality. The wood samples were analysed using Attenuated Total Reflectance Infrared Spectroscopy (ATR-IR), while faecal pellets were analysed by Atomic Force Microscopy Infrared Spectroscopy (AFM-IR). A reduction in band intensity ascribed to carbonyl vibration was seen during leaching, possibly due to loss of hemicellulose or acetyl groups therein. Untreated wood faecal pellets showed a decrease in C–O absorbance in the 1100–1000 cm−1 range interpreted as a loss of cellulose and an increase in signal in the 1700–1600 cm−1 range interpreted as increase in lignin modification products. For furfurylated wood similar tendencies were seen, but to a smaller extent. Faecal pellet production was reduced on treated wood and a lack of burrowing behaviour was observed. Mortality began to increase after a month of decreased feeding rates which is comparable to mortality rates of starved gribble. Disruption to enzymatic activity within the gut and/or increased hardness of the wood could be the mechanisms protecting furfurylated wood from biodegradation by gribble. Modification of wood, such as by furfurylation, offers promising levels of protection against such degradation without the reliance on broad spectrum biocides and can reduce costs associated with damaged wooden structures.
Introduction
The marine environment is particularly challenging for the compositional and structural integrity of construction materials. Coastal structures are subject to high UV levels, salt-water-induced corrosion, high diurnal fluctuations in temperature and biodegradation. Furthermore, components of fixed structures located in the intertidal and subtidal zones are difficult to access for repairs. Thus, marine environment specific properties and long maintenance-free service are important features to consider when evaluating general engineering and economic requirements for production of sustainable materials for this application. Steel and concrete are widely used in this context, though their high CO2 emissions are problematic in the drive for carbon neutrality. Timber, on the other hand, is a renewable resource if sourced from suitably managed forests and entails considerably less CO2 emissions during the extraction and production process.1 However, there is a marked biodeterioration hazard for timber in the sea. The main marine organisms causing biodeterioration are shipworm (bivalve molluscs from the family Teredinidae) and gribble (isopod crustaceans, from the family Limnoriidae). Structures of unprotected softwood may fail in a few months in waters where these animals are active.2,3 Shipworm have been found to both host cellulase producing bacteria in the gills and to produce their own cellulases.4Limnoria rely on enzymes in solution within gut fluids and are known to generate carbohydrate polymer degrading enzymes5,6 and phenoloxidase activity.7 Modification might limit access of enzymes to wood cell wall biopolymers. There are also indications that material hardness plays a role for the resistance of wood towards marine biodeterioration.8,9 These two hypotheses do not exclude each other. Other chemical modifications of wood, such as acetylation have demonstrated that hardness is not the sole contributor to borer resistance and it is suggested that enzyme nonrecognition or pore blocking of cell walls may play a part.10 Insight into the chemical composition as well as the amount of the faecal pellets produced by wood degrading animals when feeding on untreated vs. modified wood might indicate which mechanism(s) are most important during different phases of the degradation and thus how wood is best protected by modification.No up-to-date, accurate assessment of the economic cost associated with damage caused by wood boring bivalves and crustaceans to timber structures is available. Distel and Morrell cite decades old estimates ranging from US$200 million to one billion dollars for annual repair costs for US coastal construction, much of which were necessitated by borer attack.11,12 Broad spectrum biocide methods of wood protection established in the last century offered economically viable protection to wood in marine service, but the emissions from these methods such as chromated copper arsenate treated wood have been scrutinized for impacts on non-target organisms.13–15 Similarly, creosote impregnation has come under scrutiny too, particularly for impacts during installation.16 Legislation to restrict the use of these treatments has followed in Europe, North America and Australasia,17,18 accelerating the need for new alternatives for efficient and environmentally acceptable marine wood protection.
In this study, we examine the marine protection offered by a wood modification method, which derives its furan raw materials from plant waste.19 The method is known as furfurylation and comprises vacuum impregnation with furfuryl alcohol, which is subsequently polymerized inside the timber by heat curing. Furfurylation has been known for many years and the process has been industrialized. Furfurylated wood does not pose an environmental hazard.20–23 The treated wood has improved properties such as increased durability when exposed to fungi24 or termites.25,26 However, when it comes to the marine environment, results indicate that long term durability might be linked to the solvent used during furfurylation, as specimens prepared in alcohol showed better performance than those prepared using water as solvent.2 In a separate study it was found that wood specimens furfurylated using isopropanol as solvent had more filled cell lumina than specimens furfurylated using water as solvent, while neither IR spectroscopy or fluorescence spectroscopy revealed any differences in the composition of the final product.27
Here, we tested how gribble (Limnoria) feeding was affected by furfurylation performed using an alcohol as solvent. The feeding trials were performed in a unique laboratory setup, which allows much faster and more flexible examination of results than what is possible with standard marine testing (Fig. 1). Insights into how furfurylation operates by non-biocidal means and possibly interferes with digestive enzyme action are generated by characterising the chemical changes in the wood after passage through the gut of the test organism. We used Attenuated Total Reflectance Infrared Spectroscopy (ATR-IR) to analyse the composition of bulk furfurylated wood and Atomic Force Microscopy Infrared Spectroscopy (AFM-IR) to analyse gribble faecal pellets. Laboratory based ATR-FTIR is commonly used to monitor the composition of wood after various treatments because it is non-destructive, it is relatively simple to use and provides a quick insight into sample composition. However, it is difficult to apply ATR-FTIR for the analysis of small sample sizes and single, micrometre-sized particles such as gribble faecal pellets since the signal is too weak even after prolonged analysis. This is resolved by AFM-IR, which simultaneously exploits the spatial resolution of AFM and chemical analysis by IR spectroscopy28 to permit the analysis of distinct areas within a single faecal pellet. The IR signal in ATR-FTIR originates from within the 0.5–3 μm from the sample surface29 making it essentially an analysis of bulk composition of various wood materials. On the other hand, the composition contained in the AFM-IR spectra originates from ∼0.1 μm within the surface because of the ways the signal is generated and recorded during the analysis.28
This study tests the hypothesis that the furfurylation process hinders wood digestion when performed in alcohol, either because of increased material hardness or by making furfurylated wood a non-viable substrate for Limnoria by interfering with enzyme activity as has been suggested in non-marine contexts.30 In both cases, the treatment acts in a manner that is unlikely to harm non-wood -feeders or the surrounding environment.
Experimental
Wood material
Wood samples were prepared for feeding experiments. Analysis by IR spectroscopy was performed on the wood before and after feeding and on the faecal pellets generated during feeding. The wood used was Pinus radiata (D. Don) from New Zealand plantations. Three treatment batches were used in this study (treatments 1, 2 and 3), generated by experimental furfurylation processes conducted by RISE Sweden at laboratory scale. In all treatments, the impregnation liquid consisted of 35 wt% furfuryl alcohol, 4 wt% water, 59.25 wt% methanol and 1.75% initiators and catalysts that are propriety to Kebony AS. In unrelated studies published earlier, citric acid has successfully been used as catalyst, Laboratory scale impregnation of wood specimens measuring 25 mm × 74 mm × 489 mm (along the grain) was carried out in an autoclave. A vacuum of 0.3 bar was applied, and then the impregnation liquid was added. A pressure of 1.5 bar was then applied using nitrogen. The vacuum-pressure cycle was repeated four times to secure that all air had been pulled out. The samples were then left at 4.5 bar overnight. The next day excess liquid was discharged, and samples were vacuum dried at 0.3 bar until the condensate was no longer colourless, indicating that furfuryl alcohol was being evaporated. The specimens were then subjected to two different drying regimes. For treatment 1 drying took place at 55 °C for two days followed by 80 °C for one day. For treatment 2 drying took place at 70 °C for two days. For treatment 3 preparation was similar to treatment 2, but it was carried out in another laboratory set-up.The average dry densities of the wood used for treatments 1, 2 and 3 were, respectively 450.2, 412.9 and 432.2 kg m−3. The average weight percentage gain relative to original dry weight due to retention of polymerised furfuryl alcohol in treatment 1 samples was 35.2 ± 1.2% (n = 14), while in treatment 2 samples it was 35.3 ± 0.8% (n = 14) and in treatment 3 it was 38% for the plank used to generate the test sticks. After treatment, the wood was darker than the untreated wood, particularly in the case of treatment 2 (Fig. S1†).
Untreated wood was used as control. Gribble feeding trials comparing wood furfurylated in isopropanol with wood furfurylated in water have been performed earlier. They showed that the former was somewhat more efficient in deterring gribble when using a set-up identical to the one used here.31
For the feeding experiments, sticks were cut, measuring 20 mm (along the grain) × 2 mm × 4 mm (Fig. S1†).
Obtaining gribble specimens
Gribble were obtained from a culture maintained in aquaria, replenished at intervals from infested wood from Southsea, Portsmouth, UK and were kept in a flow through tank with water pumped in directly from nearby Langstone Harbour. Fine forceps were used to peel loose wood from the block and expose the burrows. Gribble were collected by gently picking up using a paintbrush. Gribble were then examined under a microscope to identify species and check for suitability for use in the experiment. The species Limnoria quadripunctata was used because of its proven abilities as a test organism.32–34 The underside of the gribble was exposed to check for beating pleopods which is a sign of vitality and an indication that the specimen was not damaged while being extracted from the wood. Only gribble above 1.5 mm in length were chosen. Any individuals that were brooding eggs were removed as gravid animals have a significantly reduced feeding rate.33 Borges et al. 2009 tested different parameters on the effects of gribble feeding in laboratory conditions. They found that light condition and size of individuals (from 1.5 mm–3.1 mm) did not affect the feeding rate of L. quadripunctata. This study found feeding rate optima for salinity between 25 and 35 practical salinity units (≈parts per thousand) and for temperature between 15–25 °C. Borges and colleagues found that moulting markedly reduced feeding rate33 so data from individuals that had moulted were excluded from calculations of feeding rates in our study.Feeding trials
In 14 multi-well plates, with wells sized 20 mm in diameter, one stick of wood, one gribble and 5 ml of seawater were added per well (32–35 ppt). Each plate contained 12 wells and per well, woods of different treatments were added so that each treatment was represented at least once per row and per column (Fig. S2†). This was to avoid confounding from external factors such as light or the position of well plates, which may affect evaporation of water from the plates. Replicate gribbles were used in each treatment. This gave a total of 24 replicates for the untreated control, 72 replicates for treatment 1 wood samples and 72 for treatment 2 wood samples.
Over 28 days, the mortality, vitality, and faecal pellet production of each gribble were measured. Vitality was measured on a scale of 1 to 5. A score of 5 is the highest vitality given where the gribble were within or were creating a burrow in the wood. 4 indicates gribble that were crawling on the surface of the wood but not within burrows. A vitality of 3 was scored when gribble were actively swimming in the water within the well or had rapidly beating legs and pleopods but not on or in the wood. 2 was also given where the gribble was not on the wood but with slowly moving legs and pleopods. A vitality score of 1 was given to dead gribble.
The water in the well plates was changed and collected every 3 to 4 days. This was done by transferring wood sticks and gribble to a duplicate well plate containing 5 ml of seawater per well. A fine paintbrush and forceps were used to pick up and brush any faecal pellets from the wood before transferring, then gribble were carefully picked up using the paintbrush and deposited in the new well. The water and pellets remaining in the original plate were observed under a stereo microscope and pellets were counted manually. Both pellets and water were collected into Eppendorf tubes, using a pipette. Faecal pellets were allowed to settle to the bottom of the tube and the seawater pipetted out. Pellets were then rinsed twice in water purified by reverse osmosis and air dried.
Faecal pellets from individual gribble were counted at each water change every 3 to 4 days and daily faecal pellet production was calculated. Replicates were averaged for each treatment (untreated, n = 24; treatment 1, n = 72; treatment 2, n = 72). As moulting negatively impacts feeding rate,33 counts from individuals were excluded from the average on days when moulting occurred. Dead animals were also excluded from averages as individuals were not replaced after death.
12 multi-well plates were used, again with one stick of wood, one gribble and 5 ml of seawater added per well (32–35 ppt). Replicate gribbles were used, with the addition of twelve starved gribble as a negative control (untreated, n = 12; treatment 1, n = 36; treatment 2, n = 36; starved, n = 12). Starved animals were kept individually within wells containing 5 ml of water but with no access to wood. The experimental period was increased to 57 days, enabling comparisons of vitality and mortality rates from starved gribble to those with a prolonged reduction in feeding rate from treatment 1 and 2 woods. Faecal pellets were counted up to 54 days due to a disruption on the last day of collection from the Covid-19 virus. Water was changed and collected every 3 days. Faecal pellets were counted automatically, using the image processing software Image J (version 1.8.0_172), from images taken under a stereo microscope (Fig. S3†). Daily faecal pellet counts were calculated (faecal pellet count/3) and replicates were averaged for each treatment, again excluding moulting and dead individuals.
SEM
Pellets collected from feeding trial II were rinsed twice in RO water and left to air dry. They were mounted on adhesive carbon tabs and sputter coated with gold/palladium. Images were obtained in secondary electron mode with a Zeiss EVO MA10 SEM with a W-filament, operating at an acceleration voltage of 10 kV and current of 25 pA.ATR-IR spectroscopy
ATR-IR measurements were performed using a Nicolet 6700 FT-IR spectrometer (Thermo Scientific, Waltham, MA, USA) equipped with a Pike Technologies GladiATR diamond ATR (Pike Technologies, Fitchburg, WI 53719, United States). Spectra in the range from 4000 to 400 cm−1 were obtained using 64 scans for samples and 128 scans for the background at a resolution of 4.0 cm−1 and using medium Norton-Bear apodization.35 For all wood samples, three replicate measurements were carried out. For the pooled faecal pellets from feeding trial II there was only enough materiel to obtain a single spectrum for each setup.The spectral range between 2500 and 1800 cm−1 dominated by absorbance by the ATR diamond was excluded from the spectra. Spectra were normalised using Standard Normal Variate.36 To eliminate the risk of information loss before analysis, Principal Component Analysis (PCA) was performed on spectra that were not Standard Normal Variate corrected. We used the PCA to further identify informative and characteristic IR bands and to identify the variation in their intensity as a function of leaching, time or treatment. All data analysis was carried out using Matlab (version R2017b) extended with the PLS toolbox (version 8.02) from Eigenvector Research (Manson, WA, USA).
Spectra were collected from wood samples and faecal pellets collected from feeding trial II, treatments 1 and 2. In addition, for comparison with AFM-IR, wood samples from treatment 3 were analysed.
AFM-IR spectroscopy
We collected localised nanoscale IR spectra of controls and faecal pellets between 900–1800 cm−1 using a nanoIR2 instrument from Nanosys Instruments, Inc, equipped with a pulsed, tunable IR laser. AFM-IR is based on the photothermal effect.37,38 When the wavelength of the incident IR laser corresponds to the maximum absorption of the sample, the material expands to dissipate the absorbed energy. Since the thermal expansion is directly proportional to the absorption coefficient of the excited area,39 by detecting the expansion with the AFM tip and subsequently analysing its resonant amplitudes with Fourier transform, we reconstructed the IR energies adsorbed by the sample, i.e., the IR spectra.We collected and averaged three background IR spectra with the resolution of 4 cm−1 and by averaging 512 scans to account for the variations in power of the IR laser. The wood cuttings (controls) and faecal pellets were fixed with carbon tape to a magnetic puck and we acquired AFM images in contact mode at a scan rate of 1 Hz. Since the pellets have varied topography, we chose relatively flat areas for imaging and IR analysis to minimise damage to the tip during the analysis. Once an area was imaged, we collected multiple spectra from spots within the imaged area to account for surface compositional heterogeneity. The IR signal was reconstructed from the second mode of the AFM tip oscillation, at the frequency centre between 180–220 kHz with a frequency window of 50 kHz to account for the variations in thermoelastic propertied of wood and pellets. We collected the spectra with a resolution of 4 cm−1 and by averaging 256 scans. After the spectral acquisition, we again imaged the analysed area to inspect for possible damage to the samples caused by IR laser. No damage was noticed on wood cuttings or faecal pellets. The AFM-IR spectra were smoothed by taking an average of two neighbouring data points by an algorithm embedded in the Analysis Studio software. The AFM images were flattened to remove the tilt. AFM-IR maps were collected at a scan rate of 0.1 Hz by averaging 8 scans and with a nominal resolution of 10 nm in both x and y.
Spectra were collected from faecal pellets collected at days 7 and 28 from feeding trial I, treatment 2 only due to few pellets being produced for treatment 1. In addition, for comparison with ATR-IR, wood samples from treatment 3 were analysed.
Results
Feeding trials
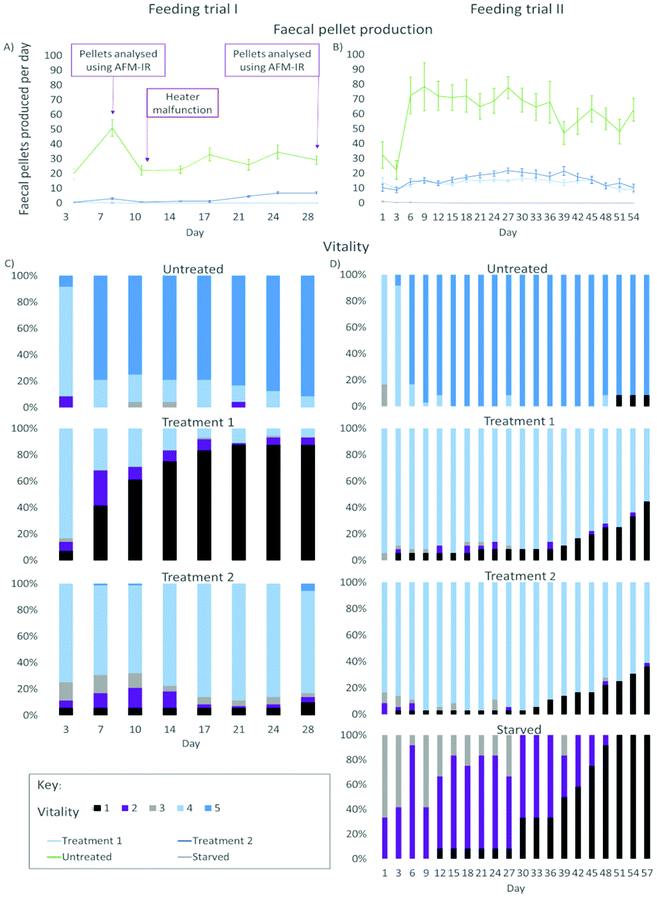 | ||
| Fig. 2 Results from the feeding trials of gribble on two different treatments of furfurylated pine wood and untreated pine wood (control). Left: figures from feeding trial I (28 days). Right: figures from feeding trial II (57 days). (A) Average number of faecal pellets produced by gribble per day (mean ± standard error) from feeding trial I. Indicated at day 7 and 28 is where faecal pellet samples were analysed using AFM-IR (Fig. 6). At day 10, a heater malfunction dropped room temperature to 11 °C, reducing feeding rates. (B) Average number of faecal pellets produced by gribble per day (mean ± standard error) from feeding trial II. (C) Vitality of gribble while feeding on different treatments from feeding trial I. (D) Vitality of gribble while feeding on different treatments from feeding trial II. Starved gribbles were included as a negative control. A vitality of 5 indicates burrowing gribble, a vitality of 4 are gribble found on the wood surface but not burrowed, a vitality of 3 are gribble that were off the wood but active, a vitality of 2 represents gribble that were off the wood but passive and a vitality of 1 shows dead individuals. | ||
A shorter leaching period on the wood, as tested in feeding trial I, demonstrated a clear difference in the mortality rate between treatments 1 and 2. Gribble feeding on treatment 1 had a high mortality rate compared to those feeding on treatment 2 wood and untreated wood. Treatment 1 mortality increased from day 3 (6.94%, n = 5) until day 21 where it plateaued at 87.5% (n = 63). This suggests that, rather than simply restricting the gribble's ability to feed, this treatment may have also caused some biocidal effect, potentially from unpolymerized oligomers due to the lower temperature used for curing. High mortality on this treatment was not seen in feeding trial II, likely due to the increased period of leaching. On treatment 2, there was no increase in mortality from the initial individuals who died on day 3 (5.56%, n = 4) until day 28 (9.72%, n = 7) and no mortality was seen on the untreated control wood.
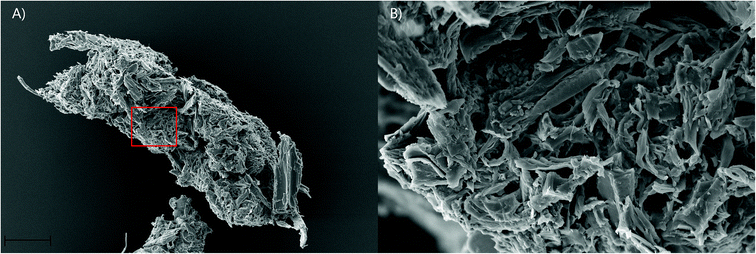
Faecal pellet production is indicative of feeding rate and is used to determine the ability of gribble to feed on different woods. An example of a typical faecal pellet is shown in Fig. 3. The pellet is ~730 μm in length (Fig. 3a). Untreated wood had consistently the highest faecal pellet production between 20 and 51 pellets per day (Fig. 2). A drop in temperature at day 10, to 11 °C, reduced faecal pellet production among all treatments but most notably on untreated wood. Over time, faecal pellet production gradually started to increase again but remained below 40 pellets per day. No faecal pellets were produced on treatment 1 due to the high levels of mortality of gribble. Faecal pellets produced in the feeding trial were analysed using AFM-IR spectroscopy.
On day 3, two individuals died on treatment 1 (5.6%) and one individual on treatment 2 (2.8%) wood. Mortality began to increase on day 21 and day 33 for treatment 1 and treatment 2, respectively. By day 57, 44.4% of gribble had died (n = 16) on treatment 1 and 36.1% (n = 13) on treatment 2.
Starved gribble were included in this trial to compare mortality rates to those feeding on treated woods. As there was no wood given to these animals, no vitality was scored higher than 3. On day 12, one individual had died (8.3%). Mortality began to increase on day 30 (33.3%, n = 4) until all individuals had died by day 51. The addition of a starved control showed that the mortality seen in feeding trial I was most likely a result of reduced leaching time, particularly affecting treatment I, instead of a prolonged reduction in feeding. When mortality in starved gribble increased (between day 30 and day 51), it showed us the maximum amount of time a gribble can survive without feeding. Comparing this to the rise in mortality on treatment 1 and treatment 2 woods indicate whether mortality seen would likely be a result from any residual toxicity from the treatment or from starvation, due to a reduction in feeding and/or digestion.
Faecal pellet production was again highest on untreated control wood and increased between days 3 and 6, where the majority of gribble were creating their burrows (Fig. 2). There was a slight drop in faecal pellet production on day 39 and this corresponds with where the individual who died on day 51 began to show a low vitality and had a daily faecal pellet production below ten, until day 45 where it did not produce any faecal pellets. Treatments 1 and 2 had a similar faecal pellet production rate although both were much lower than untreated, leached wood. Faecal pellet production began to decline from day 39 on treatment 2 wood and from day 45 on treatment 1 wood. Some starved individuals produced between one and three pellets on day 1, from having fed prior to collection, then produced no pellets for the remainder of the experiment. Faecal pellet production per individual showed a slight decline on both treatments towards the end of the feeding trial II, although vitality remained high (excluding dead replicates). The rate of faecal pellet production combined with assessing the gribble's vitality shows how, over time, woods treated using furfurylation impacts their survivability.
IR characterisation of wood and faecal pellets
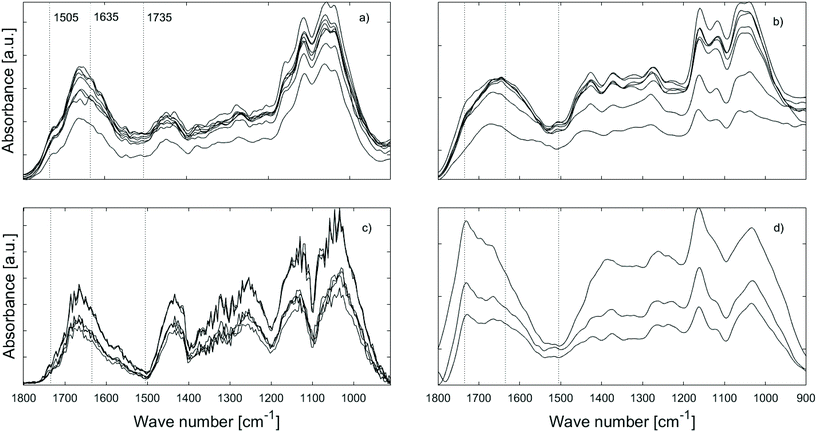
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O range from ∼1700 to 1500 cm−1. Any quantitative comparisons between methods have consequently not been attempted in this study.
O range from ∼1700 to 1500 cm−1. Any quantitative comparisons between methods have consequently not been attempted in this study.
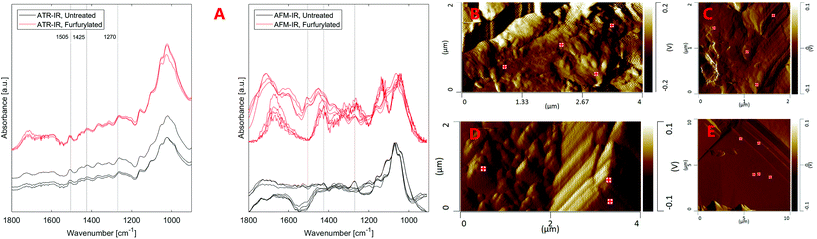
Fig. 4 also shows that the strongest lignin band at ∼1505 cm−1 seems to be missing from AFM-IR spectra or is much weaker than surrounding bands, in contrast to ATR-IR. The replicate spectra seem to fall in two groups based on their overall form, either showing a weak band at ∼1505 cm−1 or no band at this position both for the furfurylated and the untreated wood. Presence of the ∼1505 cm−1 band is not related to any topographic features. This result is in conflict with our previous study40 where lignin was readily observed in cross sections of Larix by AFM-IR. Both the spectra of untreated and furfurylated wood that show a weak band at ∼1505 cm−1 also show a strong band at ∼1270 cm−1, which is likely also a lignin band (1272 cm−1), according to Larsen and Barsberg41 ascribed to ring deformation and C–O stretch combined with aromatic skeletal vibrations and methoxy vibrations at 1268 cm−1.42,43 These spectra also show relatively strong absorbance at ∼1426 cm−1, which has also been ascribed to lignin (1427 cm−1, lignin methoxy deformation, methyl bending and aromatic skeletal vibrations42,43). The spectra that do not show the ∼1505 cm−1 band also lack the ∼1270 and the ∼1426 cm−1 bands, tentatively suggesting that no lignin signal was recorded at these positions. We did observe compositional heterogeneity of wood and pellets on the nanoscale (Fig. S4†), with pellets showing areas with larger heterogeneity than undigested wood. However, we are reluctant to believe that there are areas of about 0.01 μm2 in wood completely devoid of lignin. On the other hand, about half the spectra captured showed this pattern, making it unlikely that it is an artefact. Alternatively, we speculate that either the orientation of the lignin relative to the incident IR laser could modify the signal strength (especially for stretching vibrations44–46), or that halite (NaCl) crystals within the wood or on the surface attenuated signals from aromatic compounds more efficiently than signal from other vibrational groups. Compared to these hypotheses, spots devoid of lignin might after all be the least unlikely speculation.
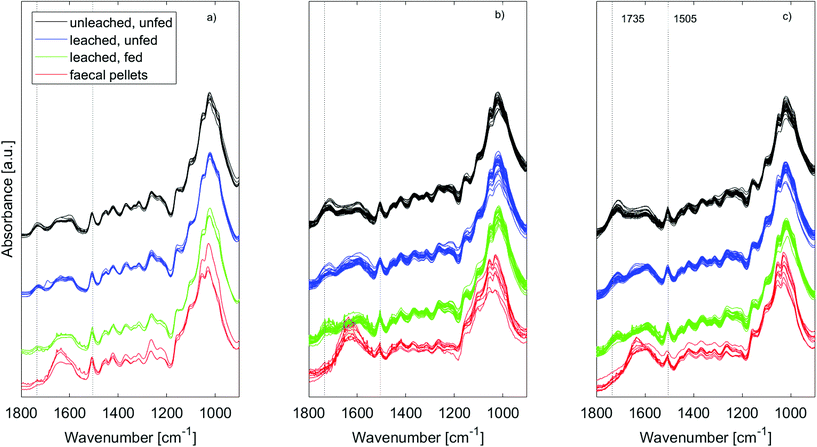
ATR-IR spectra of the wood samples (Fig. 5) showed that leaching affected the composition of both untreated and furfurylated wood, and for both treatment 1 and 2. However, it was not leaching of furfuryl alcohol that was observed, since no differences were seen at band positions corresponding to furfuryl alcohol.47A difference between treatment 1 and 2 was expected, as the feeding trials showed that mortality assumingly due to leaching was much higher for treatment 1 than for treatment 2 (Fig. 2).
In all cases the range between 1800 and 1500 cm−1 was especially affected by leaching. For untreated wood in particular, the carbonyl band at 1735 cm−1 was reduced by leaching, indicating loss of hemicellulose, or of acetyl groups therein48,49 (Fig. 5a – black and blue). This could be due to bacterial degradation of wood or various abiotic processes such as hydrolysis or (photo)oxidation.48 Faecal pellets did not show any signs of the wood having been previously degraded by tunnelling bacteria (Fig. 3). Furfurylation leads to formation of homopolymers of furan within the wood.50 Heteropolymerisation between furan and lignin moieties is also likely,19,51 but binding to hydroxyl groups is thermodynamically unlikely.52 Thus, if accessible, hemicellulose within both furfurylated and untreated wood cell walls might be affected by chemical or biological degradation during leaching, as it is unlikely that its hydroxyl groups copolymerise with furans. Since the 1735 cm−1 band was not completely gone after leaching (Fig. 5 – blue), furfurylation likely sterically hindered degradation of hemicellulose during leaching to some extent.
The increase in signal between 1700 and 1550 cm−1 seen for the furfurylated wood after leaching is more difficult to interpret (Fig. 5b and c – blue). Most bands in this region can be assigned to either C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or C
O or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds (Table 1), the former present as either ketones or aldehydes, the latter in aryl rings. Ketones and aldehydes might arise from modification of lignin.7 Conjugated C
C bonds (Table 1), the former present as either ketones or aldehydes, the latter in aryl rings. Ketones and aldehydes might arise from modification of lignin.7 Conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds are present in furan chains, while C
C bonds are present in furan chains, while C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds could arise from furan ring opening, which can occur if a solvent like water or alcohol is present, as discussed by Falco et al.53 If such ring opening took place during the leaching, it would require that furfuryl alcohol and/or its oligomers were still present in the wood after curing, i.e. it should only be relevant for treatment 1. However, even though feeding trial I strongly suggested that wood from treatment 1 leached unreacted furfuryl alcohol, no spectral changes after leaching suggested loss of unreacted furfuryl alcohol, and no differences in effects of leaching between treatment 1 and 2 spectra were observed. It seems gribble is a more sensitive sensor than IR for these leachates. Use of behavioural assays of sublethal effects of chemicals is now being widely advocated for ecotoxicological studies.54
O bonds could arise from furan ring opening, which can occur if a solvent like water or alcohol is present, as discussed by Falco et al.53 If such ring opening took place during the leaching, it would require that furfuryl alcohol and/or its oligomers were still present in the wood after curing, i.e. it should only be relevant for treatment 1. However, even though feeding trial I strongly suggested that wood from treatment 1 leached unreacted furfuryl alcohol, no spectral changes after leaching suggested loss of unreacted furfuryl alcohol, and no differences in effects of leaching between treatment 1 and 2 spectra were observed. It seems gribble is a more sensitive sensor than IR for these leachates. Use of behavioural assays of sublethal effects of chemicals is now being widely advocated for ecotoxicological studies.54
| Position (cm−1) this work | Position (cm−1) literature | Band assignment | Component | |
|---|---|---|---|---|
| ATR-IR | AFM-IR | |||
| Sh – appears as shoulder; δ – bending vibration; ν – stretching vibration; ρ – rocking (bending) vibration, abc – used as approximations of the unique aromatic ring bonding. | ||||
| 1020–2060 | 1030–1040sh | 1015–1060 | Various C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–H and C–C stretching and bending modes, aromatic and aliphatic O, C–H and C–C stretching and bending modes, aromatic and aliphatic |
Cellulose |
| 1058–1070 | ||||
| 1103 | 1106–1114sh | 1108 |
δC–O–H, δC–H, νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
Cellulose and hemicellulose |
| 1153–1157 | 1158–1162sh | 1125–1162 | ν as C–O–C, aromatic δC–Ha | All |
| 1327–1232 | 1202–1226 | 1221–1235 |
νC–O, νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, νC–C, δC–OH O, νC–C, δC–OH |
Cellulose, lignin |
| 1261–1263 | 1266–1274 | 1266–1270 |
νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, aromatic νC O, aromatic νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ob and νC Ob and νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H H |
Lignin |
| (1275sh) | 1270–1280 | 1277–1280 | δC–H | Cellulose |
| 1313–15 | 1318–1334sh | 1315–1335 | δC–H; δ(ρ)C–H2 | Cellulose |
| 1365–1369 | 1366–1374 | 1335–1375 | δC–H; δC–OH | Cellulose |
| 1421–1423 | 1422–1430 | 1422–1430 | δC–H; δC–H2; aromatic δC–H | Cellulose, lignin |
| 1450–1462 | 1446–1462 | 1460 |
δC–H; νC–H; νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
Hemicellulose, lignin |
| 1502–1510 | 1502–1510 | 1505–1520 | Aromatic νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cc Cc |
Lignin |
| 1593–1595 | 1598–1610 | 1593–1605 | Aromatic νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and νC C and νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
Lignin |
| (1632) | 1626–1646 | 1635–1640 |
νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
All |
| (1659) | 1666–1678 | 1670–1690 |
νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
All |
| 1713–1732 | 1702–1726 | 1738–1709 |
νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
Cellulose, hemicellulose |
The spectra of leached wood samples before and after the feeding trial are similar, indicating that no leaching of unreacted furfuryl alcohol took place during the experiment, but as mentioned above, this may have gone undetected by IR.
The IR spectra of the faecal pellets show a decrease in the signal at 1735 cm−1 compared to the same band in the spectra of both untreated and furfurylated leached wood (Fig. 5 – red). As for the leached wood, this suggests a loss of hemicellulose or its components such as acetyl groups. An increase in the signal ∼1635 cm−1 is also observed for both wood types. This band can be ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of para substituted ketone or aryl aldehydes,40 interpreted as lignin degradation products due to oxidation.7 An increase in the IR signal at this wavenumber is also seen for degraded, waterlogged archaeological wood,48 a material that typically shows depletion in carbohydrates and a relative increase in (possibly modified) lignin content. In the current data, the aromatic skeletal vibrations at ~1505 cm−1 in lignin is still observed in the spectra, slightly weakened for the faecal pellets produced from furfurylated samples when compared to neighbouring bands (Fig. 5).
O stretching of para substituted ketone or aryl aldehydes,40 interpreted as lignin degradation products due to oxidation.7 An increase in the IR signal at this wavenumber is also seen for degraded, waterlogged archaeological wood,48 a material that typically shows depletion in carbohydrates and a relative increase in (possibly modified) lignin content. In the current data, the aromatic skeletal vibrations at ~1505 cm−1 in lignin is still observed in the spectra, slightly weakened for the faecal pellets produced from furfurylated samples when compared to neighbouring bands (Fig. 5).
An overview of the effects of treatment, leaching and feeding on wood composition was obtained by use of principal component analysis (ESI, Fig. S5†). In brief, results confirmed that the largest variations in the spectra occurred when the wood had been digested, and that this change was mostly related to a relative decrease in bands associated with cellulose and a relative increase in bands associated with lignin degradation products.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal between 1700–1600 cm−1 (Fig. 6a and c). The former suggests increasingly more efficient carbohydrate degradation, i.e. carbohydrate utilisation by gribble, while the latter is more difficult to interpret. As mentioned above, the 1700–1600 cm−1 range is ascribed to vibrations of aryl ketones and aldehydes, i.e. possibly lignin oxidation products7 suggesting that lignin is metabolised.
O signal between 1700–1600 cm−1 (Fig. 6a and c). The former suggests increasingly more efficient carbohydrate degradation, i.e. carbohydrate utilisation by gribble, while the latter is more difficult to interpret. As mentioned above, the 1700–1600 cm−1 range is ascribed to vibrations of aryl ketones and aldehydes, i.e. possibly lignin oxidation products7 suggesting that lignin is metabolised.
The variation in spectral features of the furfurylated wood as a function of time (Fig. 6b and d) is different to our observation on the untreated wood. Here, the variations in the 1100–900 cm−1 region are negligible suggesting that there is no difference in the utilisation of carbohydrates over time. The same can be said about the 1700–1600 cm−1 band, indicative of no variation in lignin degradation. Further, it seems that the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band at ∼1735 cm−1 is still present in the pellets even after 28 days, indicating that the acetyl group within hemicellulose was not digested or degraded.
O band at ∼1735 cm−1 is still present in the pellets even after 28 days, indicating that the acetyl group within hemicellulose was not digested or degraded.
Discussion
Lower gribble vitality and reduced feeding rate on treated wood compared to untreated wood in the feeding trials are two aspects of the effect of furan polymer inside the wood structure (Fig. 2). The treatment markedly reduces processing of wood by the borers and furthermore, over time, reduces their vitality and increases mortality. This is likely to prevent establishment of borer colonies within treated wood in marine structures. The inability to form burrows in treated wood, despite being able to feed at a limited extent on it, would be likely to expose the borers to predators, a factor not so far assessed in either lab or field observations. The leaching of the wood material prior to the feeding trials provided a closer approximation to field conditions of rapid dilution, ensuring that readily water-soluble components did not build up in the water used for the test organisms, a factor that has sometimes lead to exaggeration of the effects of wood treatments on non-target biota.14,15 Observations with well-leached wood demonstrate that effects of the treatment were due to the characteristics of the modified wood itself and not to leaching of soluble oligomers of furfuryl alcohol. Higher mortality rates in the first feeding trial, which had a shorter pre-trial leaching period, could be ascribed to release of oligomers of furfuryl alcohol into the seawater containing the gribble and wood. Such leaching effects have been demonstrated with the heartwood of a variety of tropical timbers.32 In the marine environment, adverse effects from leachates are less likely to affect wood borers due to rapid dilution.In the longer-duration second trial (Fig. 2), mortality rates of starved gribbles showed a trend in mortality over time similar to that of animals on treated woods, but with a somewhat earlier onset. The marked reduction in feeding rate, perhaps compounded by less efficient digestion due to hindrance of enzymatic and/or oxidative activity by deposited furan polymer, will greatly reduce nutritional gain. This suggests that the nutrition derived from the reduced food intake was insufficient.
The question is then: was reduced food intake because the furfurylated wood was more difficult to ingest or to digest? In marine exposure studies, furfurylated wood has been found to be long-term resistant to shipworm action, but seemingly only when the furfuryl alcohol impregnation step was performed using alcohol as solvent.2 In the present study, we used methanol. It has been suggested that furfurylation taking place in methanol leads to a higher proportion of earlywood lumina being filled with furan polymer,27 increasing the wood hardness. Such furfurylation-induced increase in hardness hinders shipworm from settling on the wood. However, an increase in hardness at the macroscopic level is also observed if the furfurylation is conducted using water as a solvent,55 so hardness is likely not the only factor affecting shipworm settling. Rather, we speculate that it could be the lumen filling in itself. For gribble, on the other hand, it has been shown that there is an inverse correlation between the hardness of various untreated timbers and the feeding rate.8 In agreement with hardness affecting feeding rate, we observed a decrease in feeding rate on furfurylated wood already during the first days of the trials where the gribble still had a high vitality. They were however unable to make burrows even though they fed and produced a limited amount of faecal pellets (Fig. 2). On the other hand, we also found that AFM-IR spectra of faecal pellets produced by gribble feeding on furfurylated wood were similar over time, while those for untreated wood changed over time (Fig. 6) in a manner indicative of loss of cellulose and increasingly modified lignin. We speculate that for gribble feeding on untreated wood these changes could be due to an increasingly efficient enzyme activity within the gut, i.e. an adjustment to the substrate at hand, a metabolic adjustment not taking place when feeding on the furfurylated wood. These results, advocate for the hypothesis that furfurylation reduces enzymatic efficiency in degradation of wood.
In an earlier study, the biopolymer composition of wood and gribble faecal pellets was compared.7 It was found that only the cellulose content was reduced, while both lignin and hemicellulose content remained largely unchanged, except for a minor reduction in xylan and mannan. In the current study using AFM-IR on single pellets, it was found that the carbonyl band at ∼1735 cm−1 associated with acetyl groups in hemicellulose was reduced compared to the unfed wood for both untreated and furfurylated wood. However, a large part, if not all, of the reduction could be ascribed to degradation processes going on during leaching of the wood prior to the feeding trial.
The finding that ATR-IR and AFM-IR spectra of wood samples were markedly different from each other was surprising, and further investigations into this are needed. Here we are not referring to the result that relative band intensities differed between the methods, but the fact that about half the spectra from both furfurylated and untreated wood and pellets lacked lignin signal. As mentioned in the introduction, the penetration depth of the two methods differ by about a factor of ten, so AFM-IR should be more sensitive to spatial heterogeneity. On the other hand, in our experience, confocal Raman spectra obtained with a spatial resolution of about 0.3 μm normally contain lignin signal in all locations on a cross section of wood. The resolution in depth (z-direction) for these Raman data is however ∼0.7 μm, which is several times larger than for AFM-IR. No doubt AFM-IR is a useful method that offers possibilities for obtaining IR spectra with high spatial resolution, but the lignin-devoid data obtained in the present study needs further confirmation.
Conclusions
Our behavioural assays, measuring faecal pellet production and other categories of behaviour indicative of borer vitality, indicated that furfurylation does reduce wood degradation by gribble. This provides controlled laboratory evidence to complement observations from long-term field trials in which the challenge from gribble is just one of the factors determining treated wood performance. The results indicate that this protection of plantation-grown timber otherwise highly susceptible to borer attack may be partly due to furfurylation increasing wood hardness. However, we also found differences between the IR spectra of faecal pellets produced on untreated and furfurylated wood, indicating that the latter resulted in less efficient cellulose utilization and less modification of lignin during digestion. This result does not disprove the hypothesis that furfurylation interferes with the wood cell wall degradation catalysed by enzyme activity within the gribble's gut. In other words, we cannot dismiss either of the two hypotheses regarding the mechanism responsible for the protection of furfurylated wood against marine borers.We detected interesting effects of seawater immersion on wood and furan composition, which should be examined over an extended time series to assist predictions of long-term performance in the sea. We have no evidence to raise concern regarding environmental impact from commercially treated furfurylated wood, but our findings from wood modified at laboratory scale suggest that ensuring complete polymerisation during polymer curing will minimise any such impact. Thus, it would be desirable to use ecotoxicological tests to evaluate this prediction with respect to wood prepared under the range of operational conditions relevant to commercial curing processes.
Author contributions
The study was conceptualized by SMC and LGT, who also obtained the main part of the funding. Gribble feeding trials and analysis of data therefrom were carried out by LSM and SMC. AFM-IR was carried out by SJ, and ATR-IR by LGT. All IR data were analysed by SJ and LGT. All authors contributed to the first draft of the paper and to subsequent revisions of text and figures. All authors approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The study was carried out with financial support to SMC, LGT and LSM from the Regional Norwegian Research Council for the Oslo Fjord region (Grant No. 268707) for the project Alcohol-based process for wood furfurylation and from Interreg Öresund-Kattegat-Skagerrak for the project MODUWOOD: Modified Durable Wood Products. SJ acknowledges funding from VILLUM FONDEN grant 25352. The authors thank Stig Lande (Kebony) for procuring the wood material and Erika Tönnerfors (RISE) for performing the furfurylation of wood samples. We also thank John McGeehan (University of Portsmouth), Laura Michie (University of Portsmouth) and Andersen & Bach Design Studio for their help with the graphics. LSM acknowledges funding from the University of Portsmouth in support of her doctoral studies.References
- C. A. S. Hill, Front. Built Environ., 2019, 5, 10 CrossRef.
- M. Westin, P. Larson Brelid, T. Nilsson, A. O. Rapp, J. P. Dickerson, S. Lande and S. M. Cragg, presented in part at the 47th International Research Group on Wood Protection Annual Meeting Lisbon, 2016.
- A. Treu, L. Nunes and E. Larnoy, Forests, 2020, 11, 776 CrossRef.
- F. Sabbadin, G. Pesante, L. Elias, K. Besser, Y. Li, C. Steele-King, M. Stark, D. A. Rathbone, A. A. Dowle, R. Bates, J. R. Shipway, S. M. Cragg, N. C. Bruce and S. J. McQueen-Mason, Biotechnol. Biofuels, 2018, 11, 59 CrossRef PubMed.
- A. J. King, S. M. Cragg, Y. Li, J. Dymond, M. J. Guille, D. J. Bowles, N. C. Bruce, I. A. Graham and S. J. McQueen-Mason, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 5345–5350 CrossRef CAS PubMed.
- M. Kern, J. E. McGeehan, S. D. Streeter, R. N. A. Martin, K. Besser, L. Elias, W. Eborall, G. P. Malyon, C. M. Payne, M. E. Himmel, K. Schnorr, G. T. Beckham, S. M. Cragg, N. C. Bruce and S. J. McQueen-Mason, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 10189–10194 CrossRef CAS PubMed.
- K. Besser, G. P. Malyon, W. S. Eborall, G. P. da Cunha, J. G. Filgueiras, A. Dowle, L. C. Garcia, S. J. Page, R. Dupree, M. Kern, L. D. Gomez, Y. Li, L. Elias, F. Sabbadin, S. E. Mohamad, G. Pesante, C. Steele-King, E. R. de Azevedo, I. Polikarpov, P. Dupree, S. M. Cragg, N. C. Bruce and S. J. McQueen-Mason, Nat. Commun., 2018, 9, 5125 CrossRef PubMed.
- S. M. Cragg, C. Danjon and H. Mansfield-Williams, Holzforschung, 2007, 61, 201–206 CAS.
- K. Rosenbusch, L. M. S. Borges, S. M. Cragg, A. O. Rapp and A. J. Pitman, Int. Biodeterior. Biodegrad., 2006, 57, 71–74 CrossRef.
- A. Kluppel, S. M. Cragg, H. Militz and C. Mai, Int. Biodeterior. Biodegrad., 2015, 104, 8–14 CrossRef.
- D. L. Distel, in Wood Deterioration and Preservation, ed. B. S. Goodell, D. D. Nicholas and T. P. Schultz, American Chemical Society Press, Washington, 2003, pp. 253–271 Search PubMed.
- J. J. Morrell, in Handbook of environmental degradation of materials, ed. M. Kutz, Elsevier Science and Technology Books, Oxford, 2018, pp. 343–368 Search PubMed.
- C. J. Brown, R. A. Eaton, S. M. Cragg, P. Goulletquer, A. Nicolaidou, M. J. Bebianno, J. Icely, G. Daniel, T. Nilsson, A. J. Pitman and G. S. Sawyer, Arch. Environ. Contam. Toxicol., 2003, 45, 37–47 CrossRef CAS PubMed.
- S. T. Lebow, D. O. Foster and P. K. Lebow, FPJ, 1999, 49, 80–89 CAS.
- L. Adler-Ivanbrook and V. T. Breslin, Environ. Toxicol. Chem., 1999, 18, 213–221 CrossRef CAS.
- P. T. Smith, Aquat. Toxicol., 2008, 86, 287–298 CrossRef CAS PubMed.
- United States Environment Protection Agency, https://www.epa.gov/ingredients-used-pesticide-products/chromated-arsenicals-cca, 2021, consulted 27 July 2021.
- Official Journal of the European Communities, Commission Directive 2003/2/EC of 6th January 2003, Clause (3).
- S. T. Barsberg and L. G. Thygesen, ChemistrySelect, 2017, 2, 10818–10827 CrossRef CAS.
- S. Lande, M. Eikenes and M. Westin, Scand. J. For. Res., 2004, 19, 14–21 CrossRef.
- A. Pilgard, L. De Vetter, J. Van Acker and M. Westin, Environ. Toxicol. Chem., 2010, 29, 1067–1071 CAS.
- G. Van Eetvelde, S. De Geyter, P. Marchal and M. Stevens, International Research Group on Wood Preservation, 1998, IRG/WP 98-50114 Search PubMed.
- A. Pilgard, A. Treu, A. N. T. van Zeeland, R. J. A. Gosselink and M. Westin, Environ. Toxicol. Chem., 2010, 29, 1918–1924 CAS.
- S. Lande, M. Westin and M. H. Schneider, Manag. Environ. Qual. Int. J., 2004, 15, 529–540 CrossRef.
- Y. S. Hadi, E. N. Herliyana, D. Mulyosari, I. B. Abdillah, R. Pari and S. Hiziroglu, Appl. Sci., 2020, 10, 6101 CrossRef CAS.
- Y. S. Hadi, D. Mulyosari, E. N. Herliyana, G. Pari, W. O. M. Arsyad, I. B. Abdillah and P. Gerardin, Eur. J. Wood Wood Prod., 2021, 79, 1007–1015 CrossRef CAS.
- L. G. Thygesen, G. Ehmcke, S. Barsberg and A. Pilgard, Wood Sci. Technol., 2020, 54, 929–942 CrossRef CAS.
- A. Dazzi and C. B. Prater, Chem. Rev., 2017, 117, 5146–5173 CrossRef CAS PubMed.
- D. T. Djajadi, A. R. Hansen, A. Jensen, L.G Thygesen, M. Pinelo, A.S Meyer and H. Jørgensen, Biotechnol. Biofuels, 2017, 10, 49 CrossRef PubMed.
- D. T. Djajadi, M. M. Jensen, M. Oliveira, A. Jensen, L. G. Thygesen, M. Pinelo, M. Glasius, H. Jorgensen and A. S. Meyer, Biotechnol. Biofuels, 2018, 11, 85 CrossRef PubMed.
- C. R. Slevin, M. Westin, S. Lande and S. M. Cragg, in Proceedings from the Eighth European Conference on Wood Modification, 2015, pp. 464–471 Search PubMed.
- L. M. S. Borges, S. M. Cragg, J. Bergot, J. R. Williams, B. Shayler and G. S. Sawyer, Holzforschung, 2008, 62, 99–111 CAS.
- L. M. S. Borges, S. M. Cragg and S. Busch, Int. Biodeterior. Biodegrad., 2009, 63, 289–296 CrossRef.
- C. C. Delgery, S. M. Cragg, S. Busch and E. A. Morgan, J. Exp. Mar. Biol. Ecol., 2006, 334, 165–173 CrossRef.
- R. H. Norton and R. Beer, J. Opt. Soc. Am. A, 1976, 66, 259–264 CrossRef.
- R. J. Barnes, M. S. Dhanoa and S. J. Lister, Appl. Spectrosc., 1989, 43, 772–777 CrossRef CAS.
- A. Dazzi, R. Prazeres, E. Glotin and J. M. Ortega, Opt. Lett., 2005, 30, 2388–2390 CrossRef CAS PubMed.
- A. Dazzi, F. Glotin and R. Carminati, J. Appl. Phys., 2010, 107, 124519 CrossRef.
- A. Dazzi, C. B. Prater, Q. C. Hu, D. B. Chase, J. F. Rabolt and C. Marcott, Appl. Spectrosc., 2012, 66, 1365–1384 CrossRef CAS PubMed.
- S. Piqueras, S. Fuchtner, R. R. de Oliveira, A. Gomez-Sanchez, S. Jelavic, T. Keplinger, A. de Juan and L. G. Thygesen, Front. Plant Sci., 2020, 10, 1701 CrossRef PubMed.
- K. L. Larsen and S. Barsberg, J. Phys. Chem. B, 2010, 114, 8009–8021 CrossRef CAS PubMed.
- U. P. Agarwal, in Advances in Lignocellulosics Characterization, ed. D. S. Argyropoulos, TAPPI Press, Atlanta GA, 1999, ch. 9, pp. 201–225 Search PubMed.
- U. P. Agarwal, in 16th International Symposium on Wood, Fiber and Pulping Chemistry, China Light Industry Press, Beijing, 2011, pp. 170–173 Search PubMed.
- M. Akerholm and L. Salmen, Polymer, 2001, 42, 963–969 CrossRef CAS.
- L. Salmen, A. M. Olsson, J. S. Stevanic, J. Simonovic and K. Rakotic, BioResources, 2012, 7, 521–532 CAS.
- J. S. Stevanic and L. Salmen, Holzforschung, 2009, 63, 497–503 CAS.
- S. Barsberg and R. W. Berg, J. Phys. Chem. A, 2006, 110, 9500–9504 CrossRef CAS PubMed.
- N. B. Pedersen, N. Gierlinger and L. G. Thygesen, Holzforschung, 2015, 69, 103–112 CAS.
- T. P. Schultz and M. Baranska, Vib. Spectrosc., 2007, 43, 13–25 CrossRef.
- L. G. Thygesen, S. Barsberg and T. M. Venas, Wood Sci. Technol., 2010, 44, 51–65 CrossRef CAS.
- L. Nordstierna, S. Lande, M. Westin, O. Karlsson and I. Furo, Holzforschung, 2008, 62, 709–713 CAS.
- S. Barsberg and L. G. Thygesen, Vib. Spectrosc., 2009, 49, 52–63 CrossRef CAS.
- G. Falco, N. Guigo, L. Vincent and N. Sbirrazzuoli, Polymers, 2018, 10, 529 CrossRef PubMed.
- A. T. Ford, M. Agerstrand, B. W. Brooks, J. Allen, M. G. Bertram, T. Brodin, Z. Dang, S. Duquesne, R. Sahm, F. Hoffmann, H. Hollert, S. Jacob, N. Kluever, J. M. Lazorchak, M. Ledesma, S. D. Melvin, S. Mohr, S. Padilla, W. Kloas, B. B. M. Wong, M. Ziegler and G. Maack, Environ. Sci. Technol., 2021, 55(9), 5620–5628 CrossRef CAS PubMed.
- W. Li, D. Ren, X. Zhang, H. Wang and Y. Yu, BioResources, 2016, 11, 3614–3625 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc01524a |
| ‡ Shared first authors. |
| § Current address: Université Grenoble Alpes, Université Savoie Mont Blanc, CNRS, IRD, Université Gustave Eiffel, ISTerre, F-38000 Grenoble, France. |
| This journal is © The Royal Society of Chemistry 2021 |