Open Access Article
Open Access Articles-Block metal ions induce structural transformations between figure-eight and double trefoil knots†‡
Li-Long
Dang
,
Xiang
Gao
,
Yue-Jian
Lin
and
Guo-Xin
Jin
 *
*
Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, State Key Laboratory of Molecular Engineering of Polymers, Department of Chemistry, Fudan University, Shanghai 200438, P. R. China. E-mail: gxjin@fudan.edu.cn
First published on 7th January 2020
Abstract
The formation of two types of heterobimetallic molecular knots, double trefoil and trefoil knots, induced by s-block metal ions, is presented. Coordination of K+, Ca2+, Sr2+ or Ba2+ ions with amide groups plays a crucial role in the formation of these trefoil knots. Remarkably, the reversible topological transformation between a figure-eight knot and double trefoil or trefoil knots can be induced by coordination of s-block metal ions to amide groups. X-ray crystallographic data and NMR experiments support the structural assignments.
Introduction
Molecular knots, a nontrivial form of chemical topology, are ubiquitously found in proteins1,2 and DNA3 and have attracted increasing attention of chemists owing to their fascinating aesthetics and the biomimetic properties of synthetic molecular knots.4–11 A variety of small-molecule knots with different crossings have now been synthesised, either by designed methods or serendipitous discovery.12–28 Recent reports of the synthesis of 819,29 +31 # +31 # +31 composite knots11 and granny knots30 have marked considerable progress in the synthesis of molecular knots. Moreover, a range of structural transformations of molecular knots to other topologically interesting compounds, such as solomon links or monocyclic ring, have also been observed recently.5,9 However, there are very few reports of transformations from one type of molecular knot to another.31 Thus, the adjustment of the spatial arrangements of ligands in order to achieve a reversible transformation between two types of molecular knot remains a formidable challenge.Figure-eight knot, as its name implies, will take on the shape of “8” through the connections of building blocks utilizing coordination and/or covalent bonds, ultimately forming a closed loop, which has four alternating crossings in the reduced representation. Thus, it is also called 41 knot. We recently reported that both figure-eight (41) and trefoil knot species32 could be prepared by treating amide and ester derivatives with [Cp*M]-based (M = Ir, Rh) connecting units under identical reaction conditions. By comparing these knot topologies, we hypothesised that the presence or absence of intramolecular hydrogen bond interactions likely determines the different outcomes of the reactions (Fig. 1).33 Thus, if the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or N–H groups of the amide sites could be shielded, the formation of intramolecular hydrogen bonds would be hampered, which might result in other knot topologies.
O or N–H groups of the amide sites could be shielded, the formation of intramolecular hydrogen bonds would be hampered, which might result in other knot topologies.
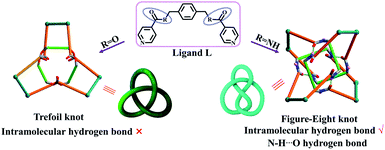 | ||
| Fig. 1 Schematic representation of stick models and topological images of a figure-eight knot and trefoil knot obtained using amide and ester ligands.32 | ||
Pioneering work in this area has proven that amide oxygen atoms are good coordination sites, and a series of interlocked [2]-, [3]catenanes and molecular knots have been formed depending on the coordination between amide groups and metal ions.34,35 A typical example is the fact that three 2,6-pyridinedicarboxamide ligands entwining a lanthanide(III) ion provide a molecular trefoil knot by ring-closing olefin metathesis.36 Thus, this behaviour of amide oxygen atoms coordinated to metal ions may be used to change the self-assembly mechanism of figure-eight knot by fixing the spatial arrangement of an amide ligand L with secondary metal ions in the synthesis of trefoil knot. Intriguingly, when the secondary metal ions are removed, the system could potentially transform into a molecular figure-eight knot.
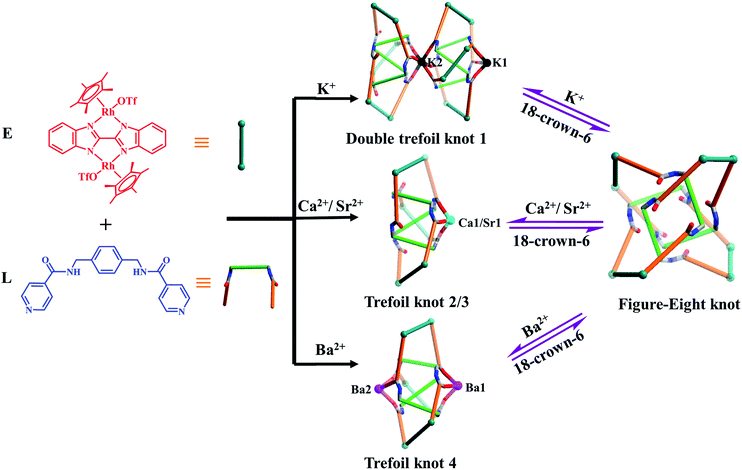
Herein, we present the synthesis of two types of molecular knots, double trefoil and trefoil knots, obtained by introducing secondary metal ions (K+, Ca2+, Sr2+ or Ba2+ ions) into the self-assembly process of amide ligand L with Cp*Rh edge unit E (L + E = figure-eight knot). X-ray crystallographic data show that the central K+ ion bridges trefoil tangles of opposite handedness by coordination with six amide oxygen atoms, leading to the realisation of a novel double trefoil knot 1. In contrast, alkaline earth metal cations induce the formation of a series of trefoil knots due to the coordination with three amide groups, and small molecules or OTf− ions. Of particular note is that all the amide oxygen atoms of trefoil knot 4 were found to be coordinated to two Ba2+ cations, which results in an octanuclear configuration (Fig. 2). In addition, the 1H NMR spectra demonstrate that the addition of secondary metal ions into a solution of figure-eight knot in CD3OD induces the formation of heterometallic trefoil knots. However, removal of K+, Ca2+, Sr2+ and Ba2+ ions from these knots by adding 18-crown-6 promoted transformations to figure-eight knot.
Results and discussion
Self-assembly of a double-trefoil knot (1) based on complete coordination of K+ ions with amide groups
Our initial hypothesis was that the coordination of K+ ions with amide sites could take place in a self-assembly process, thereby hindering the generation of intramolecular N–H⋯O hydrogen bonds and finally resulting in new reaction products. We carried out an experiment involving the addition of K+ ions prior to self-assembly: the yellow solid 1 (yield: 86.4%) was obtained by treating E with ligand L and 2.0 equiv. of KNO3 in methanol after 6 h of stirring under ambient conditions. The structure of 1 was confirmed by NMR spectroscopy, electrospray ionization mass spectrometry (ESI-MS), and single-crystal X-ray diffraction analysis.NMR spectroscopic experiments were carried out to explore the behaviour of 1 in CD3OD. The simpler 1H NMR spectrum obtained (relative to that expected for figure-eight knot) clearly supported the formation of a new topological structure (Fig. S7, ESI‡). The 1H NMR signals show two doublets at δ 8.60, 7.14 ppm and one singlet at δ 1.73 ppm for the protons of pyridinyl and Cp* groups, and the protons of two 2,2′-bisbenzimidazole (BiBzlm) appear as two sets of multiplets at δ 8.15–8.12 and 7.70–7.48 ppm. However, the signals of the phenyl groups show large upfield shifts to 1.0–4.5 ppm, which might be due to the tight π–π stacking of phenyl groups (Fig. S8, ESI‡).30 The 1H DOSY NMR spectrum of 1 shows that the aromatic and Cp* signals are associated with a single diffusion constant (D = 2.6 × 10−10 m−2 s−1), suggesting that only one stoichiometry of assembly is formed (Fig. S10, ESI‡).
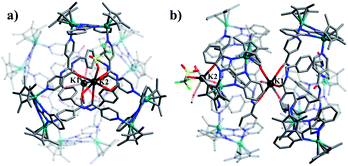
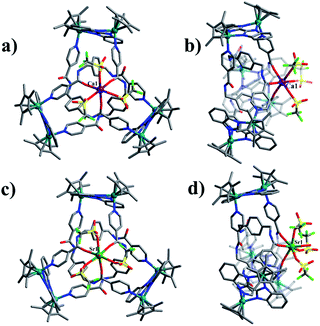
Single crystals of 1 were obtained by slow diffusion of diethyl ether vapor into a solution of 1 in methanol. Its solid-state structure indicated that complex 1 contains twelve Cp*Rh vertexes and two potassium ions, denoted K1+ and K2+ in Fig. 2. The two potassium ions are in different positions in the framework and play specific roles in the formation of 1. The central K1+ ion acts as a bridge to connect two trefoil tangles (+31 and −31) by coordination with six amide oxygen atoms, forming a double trefoil knot. The K2+ ion is bound to another three amide oxygen atoms from the +31 or −31 trefoil tangle, with one OTf− anion and two CH3OH molecules. However, there exist three possible connection forms between two trefoil knots in solution (+31 connecting with +31; −31 connecting with −31; +31 connecting with −31). In the solid state, +31 and −31 trefoil knots being connected can be caused by appropriate stacking of 1 (Fig. 3). Furthermore, ESI-MS data of 1 in methanol showed peaks at m/z 2680.66 assigned to [1 − 2CH3OH − 3OTf−]3+, further confirming the structure of 1 as a double trefoil knot and indicating that complex 1 exhibits remarkable stability in solution (Fig. S31, ESI‡). The formation of double trefoil knot 1 verifies that intramolecular N–H⋯O hydrogen bond interactions play a crucial role in the stabilisation of figure-eight knot. The coordination of the K+ ions with the amide groups is likely the main reason for the synthesis of structure 1 by virtue of hindering the formation of intramolecular N–H⋯O hydrogen bonds. This result provides a new concept for the construction of increasingly complex topological structures and studying the conversion between amide-containing supramolecular compounds.
We next sought to investigate whether NO3− anions have an influence on the formation of double trefoil knot 1. Thereby a self-assembly experiment by using KOTf instead of KNO3 was carried out under the same reaction conditions. Structure 1 was again obtained, thereby demonstrating that NO3− anions had no significant effect on the formation of the complex 1.
The double trefoil knot 1 includes two K+ ions in different chemical environments. However, the 1H NMR spectrum shows the same signal pattern as that of a trefoil knot, most likely due to that the weak coordination interactions between K+ ion and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.37,38
O groups.37,38
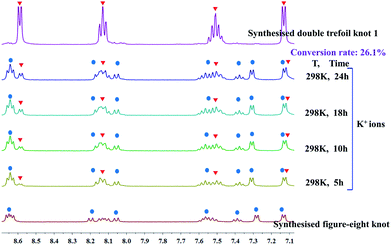
We continued our investigation of the ability of K+ ions to destroy intramolecular N–H⋯O hydrogen bonds by attempting the K+-mediated structural unlinking of figure-eight knot (Fig. 2) to the double trefoil knot 1. Thus, 1H NMR spectroscopic experiments were carried out to explore the behaviour of K+ ions in a methanol solution of figure-eight knot. Upon addition of a slight excess of KNO3 into a CD3OD solution of figure-eight knot in 1.5 mM, a new set of signals appeared that was consistent with the signals of compound 1. However, the conversion was very low and reached only 26.1% after 24 h stirring under ambient conditions. This demonstrates that once the N–H⋯O hydrogen bonds of figure-eight knot are formed, destroying them and forming a double trefoil knot is difficult (Fig. 4).
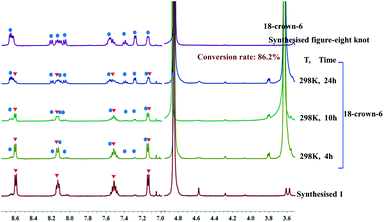
As outlined above, we speculated that the coordination between K+ ions and amide sites hinders the formation of intramolecular N–H⋯O hydrogen bonds in the self-assembly process resulting in the double trefoil knot 1. However, this conjecture might be invalidated if the trefoil monomers of 1 can exist as a stable entity when the K+ ions are removed. In an attempt to obtain trefoil monomers without K+ ions, we sought to remove the K+ ions of the double trefoil knot by using the known K+ scavenger 18-crown-6.39,40 However, attempts to remove the K+ ions by adding 18-crown-6 to a CD3OD solution of 1 while maintaining the trefoil monomer topology proved unsuccessful. We observed that the 1H NMR signals of complex 1 decreased, while those of complex figure-eight knot increased over time, when a slight excess of 18-crown-6 was added to a previously prepared solution of 1 in CD3OD (4.0 mM with respect to Cp*Rh), indicating a gradual structural unraveling of 1 to figure-eight knot (Fig. 5). The conversion reached 86.2% after 24 h of reaction time. This result further proves that in the absence of secondary K+ ions, figure-eight knot has a higher stability than the trefoil knot structure in methanol solution. Furthermore, density functional theory (DFT) calculation also supports this result.41 (Fig. S34 and S35, ESI‡).
Self-assembly of trefoil knots 2, 3, and 4 based on partial coordination of Ca2+, Sr2+ and Ba2+ cations with amide groups
The high charge density, large ionic radii and higher coordination numbers (6–10) of alkaline earth metal ions Ca2+, Sr2+ and Ba2+ has led to their use in the construction of metal–organic frameworks with good thermal stability.42–48 In order to explore the effects of Ca2+, Sr2+ and Ba2+ cations on the self-assembly of E and L in a methanol solution, identical reactions of E and L were performed under ambient conditions with addition of Ca(NO3)2·4H2O, Sr(NO3)2 or Ba(NO3)2 instead of KNO3. Accordingly, complexes 2, 3 and 4 were obtained in yields of 87.3%, 82.3% and 84.0%. The structures of 2, 3 and 4 were confirmed by NMR spectroscopy, electrospray ionization mass spectrometry (ESI-MS), and single-crystal X-ray diffraction analysis.The 1H NMR spectrum of 2 in CD3OD exhibits one sharp singlet at δ = 1.73 ppm corresponding to a single Cp*Rh environment, in marked contrast to the spectrum of figure-eight knot, suggesting a new topology (Fig. S13, ESI‡). The 1H DOSY NMR spectrum of 2 showed that the aromatic and Cp* proton signals were associated with a single diffusion constant, suggesting that only one stoichiometry of assembly was formed (Fig. S15, ESI‡).
Assembly 2 was crystallised by diffusion of diethyl ether into its methanol solution and its trefoil knot structure was elucidated by single-crystal X-ray crystallography. Similarly to figure-eight knot, the unit E and amide ligand L combine with assistance from π–π and CH–π interactions. The single Ca2+ cation was found to be bound to three L amide oxygen atoms and three OTf− ions in a κ6 fashion. The environment around the Ca2+ cation is best described as slightly distorted octahedral. The Ca–Oamide (2.2956(6)–2.2956(11) Å) and Ca–OOTf (2.3323(6)–2.3323(11) Å) distances are comparable to those found in previous work.49 In addition, the remaining three amide groups form stable N–H⋯O intermolecular hydrogen bond interactions with a OTf− anion (2.92 Å) (Fig. 6a and b). ESI-MS data also indicated that the prominent peaks at m/z = 2048.23 ([2 − 2OTf−]2+) is in good agreement with their theoretical distribution (Fig. S32, ESI‡), suggesting that the structure remains intact in solution.
The Sr2+ cation is slightly larger than the Ca2+ cation, thus we speculated that Sr2+ could similarly coordinate with amide oxygen atoms and hinder the formation of intramolecular N–H⋯O hydrogen bonds, ultimately inducing formation of a new topological structure. The 1H NMR and 1H DOSY NMR (D = 2.7 × 10−10 m−2 s−1) spectra of 3 were recorded in CD3OD. As expected, the 1H NMR spectra of 3 show clear differences with those of figure-eight knot (Fig. S19, ESI‡). The 1H DOSY NMR showed a single diffusion coefficient (D = 2.7 × 10−10 m−2 s−1), suggesting a single structure in solution (Fig. S21, ESI‡). Single crystals were obtained by slow diffusion of diethyl ether into a methanol solution and the solid-state structure of 3 was determined to also be a trefoil knot by X-ray diffraction analysis. In addition, two isomers, 3a and 3b, are present in the solid state.
The single-crystal X-ray diffraction analysis of 3a and 3b reveals that both are topological enantiomers with the same P63 point group, with either positive or negative crossings in the unit cells of 3a or 3b, respectively, in the solid state (Fig. 7), consistent with reported structures.50 The Sr2+ cation is coordinated in a κ7 fashion with a single-capped octahedron geometry. The metal ion is coordinated to three amide oxygen atoms, three triflate anions, and one H2O molecule. In addition, another triflate anion forms N–H⋯O intermolecular hydrogen bonds with the remaining three amide groups. The coordination of Ca2+ or Sr2+ cations with amide groups thus effectively hinders the formation of intramolecular N–H⋯O hydrogen bonds and leads to the generation of trefoil knots. Additionally, ESI-MS data of 3 in methanol shows a peak at m/z 2072.21 assigned to ([3 − H2O – 2OTf−]2+), which further confirms the structure of 3 as a trefoil knot and indicates that complex 3 exhibits remarkable stability in solution (Fig. S33, ESI‡).
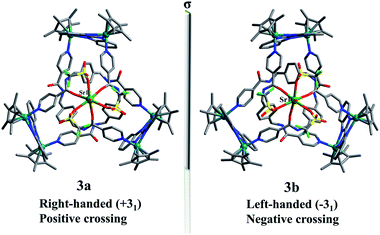 | ||
| Fig. 7 Skeleton representation of the right-handed trefoil knot (+31) of 3a (left); skeleton representation of the left-handed trefoil knot (−31) of 3b (right). | ||
A closer look at the trefoil topologies induced by K+, Ca2+ and Sr2+ ions showed that some amide groups are not involved in the coordination, which form intermolecular N–H⋯O hydrogen bonds with a OTf− ion. We hypothesised that a more strongly-binding metal ion may achieve complete coordination of the six amide groups of a single trefoil knot. The Ba2+ cation was selected to test this hypothesis.51 As expected, the octanuclear trefoil knot 4 was formed by the introduction of Ba(NO3)2, in which the six amide groups coordinate to two Ba2+ cations.
The 1H NMR spectrum of 4 in CD3OD showed all of the expected signals corresponding to the protons of a trefoil knot (Fig. S25, ESI‡), and a single diffusion coefficient (D = 2.4 × 10−10 m−2 s−1) was observed in a 1H DOSY NMR experiment (Fig. S27, ESI‡). These observations clearly demonstrate that trefoil knot 4 is the unique species in solution.
Single crystals of complex 4 suitable for XRD analysis were obtained by diffusion of diethyl ether into a saturated solution of 4 in methanol. Complex 4 crystallises in space group C2/c with the topology of a 31 knot according to the Alexander–Briggs notation. The observation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
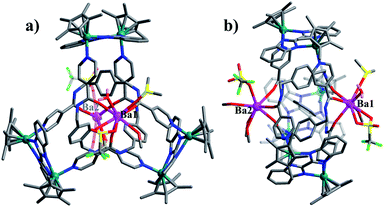
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of both enantiomers (ΛΛΛ and ΔΔΔ) is consistent with reported structure (Fig. S6, ESI‡).52 Close-contact analysis of the structure reveals a trefoil knot arrangement similar to a distorted trigonal bipyramidal configuration composed of two types of metal ion (RhIII and BaII) and two types of organic ligand (L and BiBzlm). The structure is held together by both edge-to-face-type CH–π interactions (3.39 Å) and parallel-displaced π–π interactions (of interlayer distance 3.72 Å) between phenyl moieties and two nearby pyridyl moieties of three L ligands (Fig. 8). This is in marked contrast to figure-eight knot, in which intramolecular N–H⋯O hydrogen bond interactions play a crucial role in the stabilisation of the topological structure. The barium cations (Ba12+ and Ba22+) of trefoil knot 4 are both octacoordinate. Similar to the Ca2+/Sr2+ cations of 2/3, the Ba12+ and Ba22+ cations each bind to three amide oxygen atoms. However, the remaining coordination sites of the two barium cations are occupied by different units. Ba12+ is bound to CH3OH, DMSO and H2O molecules, and one OTf− ion in an O,O′-chelating form. Meanwhile, Ba22+ is bound to three CH3OH molecules, one H2O and one OTf− ion. Again, the interaction of the Ba2+ cations and the amide groups hinders the formation of intramolecular N–H⋯O hydrogen bond interactions and thereby alters the reaction direction.
1 ratio of both enantiomers (ΛΛΛ and ΔΔΔ) is consistent with reported structure (Fig. S6, ESI‡).52 Close-contact analysis of the structure reveals a trefoil knot arrangement similar to a distorted trigonal bipyramidal configuration composed of two types of metal ion (RhIII and BaII) and two types of organic ligand (L and BiBzlm). The structure is held together by both edge-to-face-type CH–π interactions (3.39 Å) and parallel-displaced π–π interactions (of interlayer distance 3.72 Å) between phenyl moieties and two nearby pyridyl moieties of three L ligands (Fig. 8). This is in marked contrast to figure-eight knot, in which intramolecular N–H⋯O hydrogen bond interactions play a crucial role in the stabilisation of the topological structure. The barium cations (Ba12+ and Ba22+) of trefoil knot 4 are both octacoordinate. Similar to the Ca2+/Sr2+ cations of 2/3, the Ba12+ and Ba22+ cations each bind to three amide oxygen atoms. However, the remaining coordination sites of the two barium cations are occupied by different units. Ba12+ is bound to CH3OH, DMSO and H2O molecules, and one OTf− ion in an O,O′-chelating form. Meanwhile, Ba22+ is bound to three CH3OH molecules, one H2O and one OTf− ion. Again, the interaction of the Ba2+ cations and the amide groups hinders the formation of intramolecular N–H⋯O hydrogen bond interactions and thereby alters the reaction direction.
Structural transformations between figure-eight knot and trefoil knots induced by Ca2+, Sr2+ and Ba2+ cations
Trefoil knots being induced by Ca2+, Sr2+ and Ba2+ cations encourages us to explore the effects of these cations on the transformation of figure-eight to trefoil knots in methanol solution. Compared to K+ cation, Ca2+ cation, due to higher valence state and smaller ionic radius, may have greater binding energy and result in higher transformation efficiency of figure-eight to trefoil knots. In addition, previous work53,54 has reported that the binding energies of metal–oxygen increase with decreasing metal ions size (Ba2+ < Sr2+ < Ca2+ ions). Thus, transformation efficiency of figure-eight to trefoil knots may also increase (TE(Ba2+) < TE(Sr2+) < TE(Ca2+)) as the cation radius decreases (r(Ba2+) > r(Sr2+) > r(Ca2+) ions). Three 1H NMR monitoring experiments were performed in methanol solution over a period of 24 h. We observed the slow transformations of figure-eight knot to trefoil knots upon respective addition of calcium, strontium or barium salts to CD3OD solutions of figure-eight knot. After 24 h, the conversion to the corresponding trefoil knot species was 44.7% for Ca2+, 40.1% for Sr2+ and 27.2% for Ba2+ (Fig. S17, S23 and S29, ESI‡). The experimental data are consistent with the expected reactivity based on ionic radii for these alkali earth metal cations, which also indicate that Ca2+, Sr2+, Ba2+ cations have greater capacity to destroy the intramolecular N–H⋯O hydrogen bonds of figure-eight knot to induce their unravelling and formation of trefoil knots than K+ ions (transformation efficiency: 26.1%) (Fig. 9).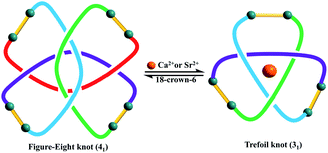 | ||
| Fig. 9 The topological transformation between figure-eight and trefoil knots by adding and removing Ca2+ or Sr2+ ions. | ||
The heavier alkaline-earth cations Ca2+, Sr2+ and Ba2+ are also known to form stable complexes with 18-crown-6.55 Thus, addition of 18-crown-6 to trefoil knots 2, 3 and 4 resulted in their structural unlinking and formation of figure-eight knot. NMR spectroscopic data clearly show the gradual transformation of the trefoil knots to figure-eight knot upon addition of a slight excess of 18-crown-6 to CD3OD solutions of complexes 2–4 (Fig. S18, S24 and S30, ESI‡). After 24 h, all of these transformations had reached conversions above 85.0% by NMR spectroscopy, further indicating that Ca2+, Sr2+ and Ba2+ cations play a critical role in the formation and stabilisation of the respective heterometallic trefoil knots.
Conclusions
In conclusion, s-block metal cations can induce structural transformations between figure-eight knot and double or single trefoil knots by adding or removing K+, Ca2+, Sr2+ or Ba2+ ions. These double or single trefoil knots can also be obtained by employing the corresponding metal salts in the self-assembly process (E + L). Interestingly, in the case of K+, these ions bridge two trefoil monomers of opposite handedness, resulting in the synthesis of a double trefoil knot. In contrast, Ca2+, Sr2+ or Ba2+ cations facilitate the construction of single trefoil knots. Additionally, 18-crown-6 can trigger topological unlinking of double and single trefoil knots to figure-eight knot by removing the s-block metal ions. The destruction and formation of N–H⋯O intramolecular hydrogen bonds is determined to be the main cause of the observed structural transformations between these molecular knots. We believe that a deeper understanding of the topological transformations in amide-containing assemblies induced by the presence or absence of s-block metal ions will be helpful in the future construction of bespoke architectures and responsive supramolecular systems, opening up new vistas in the field of functional molecules and materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation of China (21531002, 21720102004) and the Shanghai Science Technology Committee (13JC1400600).Notes and references
- L. F. Liu, R. E. Depew and J. C. Wang, J. Mol. Biol., 1976, 106, 439–452 CrossRef CAS.
- F. Ziegler, N. C. H. Lim, S. S. Mandal, B. Pelz, W. P. Ng, M. Schlierf, S. E. Jackson and M. Rief, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 7533–7538 CrossRef CAS.
- J. S. Richardson, Nature, 1977, 268, 495–500 CrossRef CAS.
- J. E. Beves, B. A. Blight, C. J. Campbell, D. A. Leigh and R. T. McBurney, Angew. Chem., Int. Ed., 2011, 50, 9260–9327 CrossRef CAS PubMed.
- S. D. P. Fielden, D. A. Leigh and S. L. Woltering, Angew. Chem., Int. Ed., 2017, 56, 11166–11194 CrossRef CAS PubMed.
- H. N. Zhang, W. X. Gao, Y. J. Lin and G. X. Jin, J. Am. Chem. Soc., 2019, 141, 16057–16063 CrossRef CAS PubMed.
- O. Safarowsky, M. Nieger, R. Fröhlich and F. Vögtle, Angew. Chem., Int. Ed., 2000, 39, 1616–1618 CrossRef CAS PubMed.
- J. F. Ayme, G. Gil-Ramírez, D. A. Leigh, J. F. Lemonnier, A. Markevicius, C. A. Muryn and G. Zhang, J. Am. Chem. Soc., 2014, 136, 13142–13145 CrossRef CAS.
- B. B. Guo, Y. J. Lin and G. X. Jin, Chem.–Eur. J., 2019, 25, 9721–9727 CrossRef CAS PubMed.
- V. Marcos, A. J. Stephens, J. Jaramillo-Garcia, A. L. Nussbaumer, S. L. Woltering, A. Valero, J. F. Lemonnier, I. J. Vitorica-Yrezabal and D. A. Leigh, Science, 2016, 352, 1555–1559 CrossRef CAS PubMed.
- L. Zhang, A. J. Stephens, A. L. Nussbaumer, J. F. Lemonnier, P. Jurček, I. J. Vitorica-Yrezabal and D. A. Leigh, Nat. Chem., 2018, 10, 1083–1088 CrossRef CAS PubMed.
- T. Prakasam, et al. , Angew. Chem., Int. Ed., 2013, 52, 9956–9960 CrossRef CAS.
- R. S. Forgan, J.-P. Sauvage and J. F. Stoddart, Chem. Rev., 2011, 111, 5434–5464 CrossRef CAS.
- J. Guo, P. C. Mayers, G. A. Breault and C. A. Hunter, Nat. Chem., 2010, 2, 218–222 CrossRef CAS.
- N. Ponnuswamy, F. B. L. Cougnon, J. M. Clough, G. D. Pantoș and J. K. M. Sanders, Science, 2012, 338, 783–785 CrossRef CAS.
- M. Feigel, R. Ladberg, S. Engels, R. Herbst-Irmer and R. Fröhlich, Angew. Chem., Int. Ed., 2006, 45, 5698–5702 CrossRef CAS.
- J. F. Ayme, J. E. Beves, D. A. Leigh, R. T. McBurney, K. Rissanen and D. Schultz, Nat. Chem., 2012, 4, 15–20 CrossRef CAS.
- L. Zhang, A. J. Stephens, J. F. Lemonnier, L. Pirvu, I. J. Vitorica-Yrezabal, C. J. Robinson and D. A. Leigh, J. Am. Chem. Soc., 2019, 141, 3952–3958 CrossRef CAS.
- V. Marcos, A. J. Stephens, J. Jaramillo-Garcia, A. L. Nussbaumer, S. L. Woltering, A. Valero, J. F. Lemonnier, I. J. Vitorica-Yrezabal and D. A. Leigh, Science, 2016, 352, 1555–1559 CrossRef CAS PubMed.
- C. O. Dietrich-Buchecker and J.-P. Sauvage, Angew. Chem., Int. Ed., 1989, 28, 189–192 CrossRef.
- D. H. Busch, J. Inclusion Phenom. Mol. Recognit. Chem., 1992, 12, 389–395 CrossRef CAS.
- P. E. Barran, H. L. Cole, S. M. Goldup, D. A. Leigh, P. R. McGonigal, M. D. Symes, J. Wu and M. Zengerle, Angew. Chem., Int. Ed., 2011, 50, 12280–12284 CrossRef CAS PubMed.
- D. H. Busch, Science, 1971, 171, 241–248 CrossRef CAS.
- J. F. Ayme, J. E. Beves, C. J. Campbell and D. A. Leigh, Chem. Soc. Rev., 2013, 42, 1700–1712 RSC.
- C. Lincheneau, B. Jean-Denis and T. Gunnlaugsson, Chem. Commun., 2014, 50, 2857–2860 RSC.
- D. M. Engelhard, S. Freye, K. Grohe, M. John and G. H. Clever, Angew. Chem., Int. Ed., 2012, 51, 4747–4750 CrossRef CAS.
- J.-P. Sauvage, Angew. Chem., Int. Ed., 2017, 56, 11080–11093 CrossRef CAS.
- Y. Segawa, M. Kuwayama, Y. Hijikata, M. Fushimi, T. Nishihara, J. Pirillo, J. Shirasaki, N. Kubota and K. Itami, Science, 2019, 365, 272–276 CrossRef CAS.
- J. J. Danon, A. Krüger, D. A. Leigh, J. F. Lemonnier, A. J. Stephens, I. J. Vitorica-Yrezabal and S. L. Woltering, Science, 2017, 355, 159–162 CrossRef CAS.
- J. J. Danon, D. A. Leigh, S. Pisano, A. Valero and I. J. Vitorica-Yrezabal, Angew. Chem., Int. Ed., 2018, 130, 14029–14033 CrossRef.
- N. Ponnuswamy, F. B. L. Cougnon, G. D. Pantoş and J. K. M. Sanders, J. Am. Chem. Soc., 2014, 136, 8243–8251 CrossRef CAS.
- L. L. Dang, Z. B. Sun, W. L. Shan, Y. J. Lin, Z. H. Li and G. X. Jin, Nat. Commun., 2019, 10, 2057 CrossRef.
- E. A. Neal and S. M. Goldup, Chem. Commun., 2014, 50, 5128–5142 RSC.
- F. Zapata, O. A. Blackburn, M. J. Langton, S. Faulkner and P. D. Beer, Chem. Commun., 2013, 49, 8157–8159 RSC.
- M. J. Langton, O. A. Blackburn, T. Lang, S. Faulkner and P. D. Beer, Angew. Chem., Int. Ed., 2014, 53, 11463–11466 CrossRef CAS PubMed.
- G. Zhang, G. Gil-Ramírez, A. Markevicius, C. Browne, I. J. Vitorica-Yrezabal and D. A. Leigh, J. Am. Chem. Soc., 2015, 137, 10437–10442 CrossRef CAS.
- Y. Inokuchi, T. Ebata, T. R. Rizzo and O. V. Boyarkin, J. Am. Chem. Soc., 2014, 136, 1815–1824 CrossRef CAS PubMed.
- K. Tsubaki, D. Tanima, Y. Kuroda, K. Fuji and T. Kawabata, Org. Lett., 2006, 8, 5797–5800 CrossRef CAS.
- T. Dudev and C. Lim, J. Am. Chem. Soc., 2009, 131, 8092–8101 CrossRef CAS PubMed.
- A. Torvisco and K. Ruhlandt-Senge, Inorg. Chem., 2011, 50, 12223–12240 CrossRef CAS PubMed.
- L. L. Gong, D. T. Zhang, C. Y. Lin, L. P. Zhang and Z. H. Xia, Adv. Energy Mater., 2019, 9, 1902625–1902633 CrossRef CAS.
- D. Banerjee and J. B. Parise, Cryst. Growth Des., 2011, 11, 4704–4720 CrossRef CAS.
- S. L. Cai, S. R. Zheng, Z. Z. Wen, J. Fan and W. G. Zhang, Cryst. Growth Des., 2012, 12, 3575–3582 CrossRef CAS.
- M. L. Foo, S. Horike and S. Kitagawa, Inorg. Chem., 2011, 50, 11853–11855 CrossRef CAS.
- J. A. Dolyniuk, H. He, A. S. Ivanov, A. I. Boldyrev, S. Bobev and K. Kovnir, Inorg. Chem., 2015, 54, 8608–8616 CrossRef CAS.
- X. G. Yang, L. F. Ma and D. P. Yan, Chem. Sci., 2019, 10, 4567–4572 RSC.
- Z. Gao, Z. W. Yu, F. Q. Liu, X. F. Feng and F. Luo, Inorg. Chem., 2019, 58, 11500–11507 CrossRef CAS.
- X. G. Yang, X. M. Lu, Z. M. Zhai, Y. Zhao, X. Y. Liu, L. F. Ma and S. Q. Zang, Chem. Commun., 2019, 55, 11099–11102 RSC.
- Y. Sarazin, B. Liu, T. Roisnel, L. Maron and J. F. Carpentier, J. Am. Chem. Soc., 2011, 133, 9069–9087 CrossRef CAS.
- K. E. Horner, M. A. Miller, J. W. Steed and P. M. Sutcliffe, Chem. Soc. Rev., 2016, 45, 6432–6448 RSC.
- D. W. Fu, Y. Zhang, H. L. Cai, H. M. Zhu, X. Y. Chen and R. G. Xiong, J. Mater. Chem., 2012, 22, 17525–17530 RSC.
- L. Zhang, D. P. August, J. K. Zhong, G. F. S. Whitehead, I. J. Vitorica-Yrezabal and D. A. Leigh, J. Am. Chem. Soc., 2018, 140, 4982–4985 CrossRef CAS PubMed.
- S. E. Rodriguez-Cruz, R. A. Jockusch and E. R. Williams, J. Am. Chem. Soc., 1999, 121, 1986–1987 CrossRef CAS PubMed.
- E. D. Glendening and D. Feller, J. Phys. Chem., 1996, 100, 4790–4797 CrossRef CAS.
- Y. Tatematsu, S. Kato, N. Nakata, M. Ebihara, O. Niyomura, K. Sugamata, M. Yukimoto and M. Minoura, Dalton Trans., 2018, 47, 9787–9794 RSC.
Footnotes |
| † Dedicated to Professor Ekkehardt Hahn on the occasion of his 65th birthday. |
| ‡ Electronic supplementary information (ESI) available: CCDC 1965232 (1), 1964477 (2), 1965067 (3a), 1964537 (3b) and 1964536 (4). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc05796j |
| This journal is © The Royal Society of Chemistry 2020 |