Nickel-catalyzed enantioselective electroreductive cross-couplings
Zhijun
Zhou
,
Sheng
Xu
,
Jing
Zhang
* and
Wangqing
Kong
 *
*
The Institute for Advanced Studies (IAS), Wuhan University, Wuhan, Hubei 430072, P. R. China. E-mail: jzhangwhu@whu.edu.cn; wqkong@whu.edu.cn
First published on 3rd September 2020
Abstract
Ni-Catalyzed reductive cross-coupling of two electrophiles has recently evolved into a powerful means for building diverse carbon–carbon bonds in an enantioselective manner. However, this strategy usually requires the use of excessive metal powder as a reducing agent, which poses severe challenges with respect to reproducibility and sustainability. Electrochemical cathode reduction provides new opportunities for reductive cross-coupling reaction in an environmentally benign manner. In this highlight, we summarize the recent progress in Ni-catalyzed enantioselective electroreductive coupling reactions.
Nickel-catalyzed reductive cross-coupling reactions have recently experienced a surge of development and represent a powerful means for the construction of diverse carbon–carbon bonds. This method allows the reaction to be carried out under very mild conditions without the need to prepare sensitive organometallic reagents, therefore showing better functional group compatibility compared with many traditional cross-coupling reactions.1 A very attractive aspect of this transformation is that alkyl electrophiles are effective coupling partners, and enantioselective control of these reactions can be realized by using appropriate chiral ligands.2
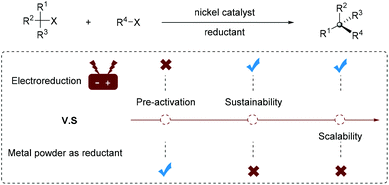
Generally, a superstoichiometric amount of metal powder such as Zn or Mn is essential as a reductant for regenerating an active Ni catalyst. However, the use of excess metal reductant has hindered the development of reductive cross-coupling reactions (Fig. 1): (1) the metal powder requires surface pre-activation, or inorganic salt such as MgCl2, TMSCl or NaI as an activator; (2) capricious stirring often occurs in the process of reaction due to the heterogeneous metal reducing agent; (3) reactions with metal powder from different brands, batches and storage conditions suffer from reproducibility issue; (4) the use of a superstoichiometric metal leads to excess waste generation and complicated post-processing; (5) it is difficult to extend the reaction scale to industrial settings.
Despite being known for many decades, it is not until very recently that organic electrosynthesis has received tremendous attention from organic synthetic chemists.3 In electrochemical processes, the active catalyst can be easily regenerated by precisely modulating the applied potential/current density in the electrolytic cell. The electrochemical continuous flow reaction provides a suitable method for rapid scale-up.4 Therefore, using electrons as green reducing agents instead of metal powder also enables the generation of highly reactive catalytic species. Such a strategy avoids the aforementioned problems associated with the use of superstoichiometric metal reducing agents and represents a green and sustainable synthetic strategy. However, most electrochemically driven nickel-catalyzed cross-coupling reactions are in racemic form and their enantioselective variants remain challenging and underdeveloped.5 The goal of this manuscript is to highlight recent elegant contributions to Ni-catalyzed enantioselective electroreductive coupling reactions.
In 1997, Durandetti, Périchon and Nédélec pioneered the study of enantioselective Ni-catalyzed electroreductive cross-coupling reaction of aryl iodides and activated alkyl electrophiles (Fig. 2).6 The asymmetric induction is achieved through pre-installation of chiral auxiliaries into the alkyl electrophiles. In the experimental setting, the carrier of the chiral auxiliary α-chloropropionic acid derivative 1 must be added constantly to the reaction mixture via a syringe pump to avoid homo-coupling.
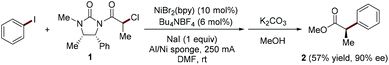 | ||
| Fig. 2 Ni-Catalyzed enantioselective electroreductive cross-coupling between α-chloropropionic acids bearing chiral auxiliaries and aryl halides. | ||
In 2014, Reisman's group developed the nickel-catalyzed enantioselective reductive coupling of alkenyl bromides with secondary benzylic chlorides. A variety of alkenyl products bearing allylic stereogenic centers are obtained in good yields and excellent enantioselectivities employing a combination of 10 mol% of NiCl2(dme), 11 mol% of chiral indanyl substituted bis(oxazoline) ligand L1 and 3 equivalents of Mn0 powder as the stoichiometric reductant (Scheme 1).7 Using the same catalytic system (nickel catalyst and chiral ligand), the same group has recently successfully realized the electrochemical version of this asymmetric transformation. This strategy does not require excessive Mn0 powder as the reducing agent. Instead, reticulated vitreous carbon foam (RVC) is used as the cathode and Zn as a sacrificial anode in an undivided cell.8 Interestingly, both the yields and enantioselectivities obtained from these two different strategies are very similar (Scheme 1). Remarkably, an equimolar ratio of coupling partners was used in the electroreductive cross-coupling process.
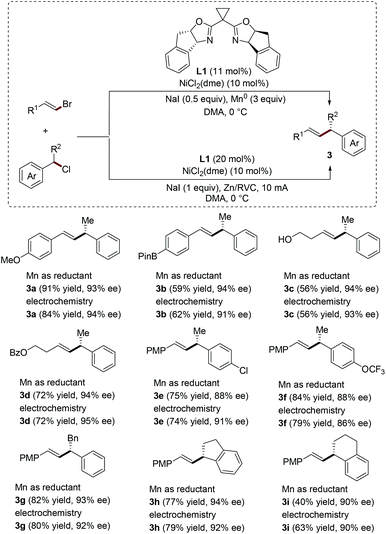 | ||
| Scheme 1 Ni-Catalyzed enantioselective electroreductive cross-coupling between alkenyl bromides and benzyl chlorides. | ||
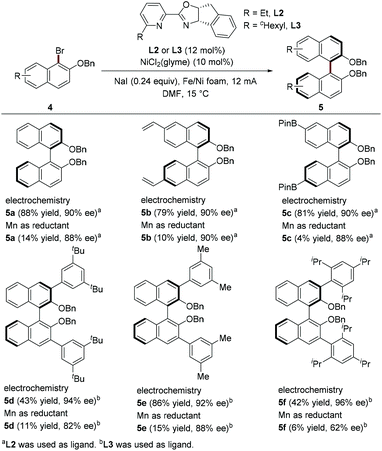
The recent study by Mei et al. constitutes significant progress in the field of Ni-catalyzed enantioselective electroreductive coupling.9 Their work provides an efficient approach to synthesize enantioenriched axially chiral biaryls in good yields with high enantioselectivities through homocoupling of naphthyl bromides using chiral pyridineoxazoline as a ligand in an undivided cell (Scheme 2). In contrast, the use of Mn powder as a reducing agent generally results in low yields, indicating that the electrochemically driven coupling process has superior catalytic efficiency. Attractively, this electroreductive coupling reaction offers a valuable way for the rapid synthesis of 3,3′ bis-arylated BINOL derivatives, which have been widely used in asymmetric organocatalysis.
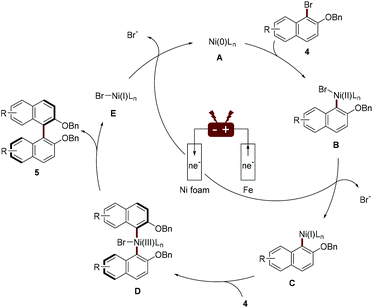
The author proposed a possible mechanism for Ni-catalyzed electroreductive coupling (Scheme 3). Naphthyl-Ni(II) species B, afforded through oxidation addition of naphthyl bromide 4 to Ni(0), is reduced to naphthyl-Ni(I) intermediate C upon cathodic reduction. Further oxidation addition with another molecule of naphthyl bromide 4 and subsequent reductive elimination would deliver the biaryl 5 and Ni(I) species E, which can be reduced to Ni(0) species A by cathodic reduction. A mechanistic study by cyclic voltammetry found that during the electrochemical reduction coupling process, Ni(0) undergoes facile oxidative addition with an aryl halide and Ni(II) catalyst is preferentially reduced.
In conclusion, due to its sustainability, greenness and inherent safety, the direct use of “electrons” as a reducing agent in Ni-catalyzed reductive cross-coupling is on the verge of rising. However, the establishment of enantioselective “electrical” to replace traditional “chemical” organic transformations seems trivial, but it is actually very challenging and still in its infancy. So far, although the electrochemical activation and turnover of the nickel catalyst eliminate the need for a stoichiometric metal powder reducing agent, it still requires the use of active metal electrodes as sacrificial anodes. Therefore, the development of more economical and ecological electrode materials is essential, especially to meet the criteria of future industrial application. We hope that with the reaction development and in-depth mechanistic understanding, more and more successful enantioselective electrochemical reductive coupling reactions will be developed in the near future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledged the financial support from the “1000-Youth Talents Plan”, Wuhan University, National Natural Science Foundation of China (No. 21702149) and Fundamental Research Funds for the Central Universities (2042019kf0008).Notes and references
- For reviews on Ni-catalyzed reductive cross-coupling reactions, see:
(a) C. E. I. Knappke, S. Grupe, D. Gartner, M. Corpet, C. Gosmini and A. Jacobi von Wangelin, Reductive Cross–Coupling Reactions between Two Electrophiles, Chem. – Eur. J., 2014, 20, 6828–6842 Search PubMed
; (b) D. A. Everson and D. J. Weix, Cross-Electrophile Coupling: Principles of Reactivity and Selectivity, J. Org. Chem., 2014, 79, 4793–4798 Search PubMed
; (c) S. Z. Tasker, E. A. Standley and T. F. Jamison, Recent advances in homogeneous nickel catalysis, Nature, 2014, 509, 299–309 Search PubMed
; (d) T. Moragas, A. Correa and R. Martin, Metal-catalyzed reductive coupling reactions of organic halides with carbonyl-type compounds, Chem. – Eur. J., 2014, 20, 8242–8258 Search PubMed
; (e) J. Gu, X. Wang, W. Xue and H. Gong, Nickel-catalyzed reductive coupling of alkyl halides with other electrophiles: concept and mechanistic considerations, Org. Chem. Front., 2015, 2, 1411–1421 Search PubMed
; (f) D. J. Weix, Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles, Acc. Chem. Res., 2015, 48, 1767–1775 Search PubMed
; (g) X. Wang, Y. Dai and H. Gong, Nickel-Catalyzed Reductive Couplings, Top. Curr. Chem., 2016, 374, 43 Search PubMed
.
- For selected reviews on Ni-catalyzed enantioselective reductive cross-coupling reactions, see:
(a) Y. Ping and W. Kong, Ni-Catalyzed Reductive Difunctionalization of Alkenes, Synthesis, 2020, 52, 979–992 Search PubMed
; (b) K. E. Poremba, S. E. Dibrell and S. E. Reisman, Nickel-Catalyzed Enantioselective Reductive Cross-Coupling Reactions, ACS Catal., 2020, 10, 8237–8246 Search PubMed
.
- For recent reviews on electrochemical organic synthesis, see:
(a) R. Francke and R. D. Little, Redox catalysis in organic electrosynthesis: basic principles and recent developments, Chem. Soc. Rev., 2014, 43, 2492–2521 Search PubMed
; (b) E. J. Horn, B. R. Rosen and P. S. Baran, Synthetic organic electrochemistry: an enabling and innately sustainable method, ACS Cent. Sci., 2016, 2, 302–308 Search PubMed
; (c) A. Badalyan and S. S. Stahl, Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators, Nature, 2016, 535, 406–410 Search PubMed
; (d) M. Yan, Y. Kawamata and P. S. Baran, Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance, Chem. Rev., 2017, 117, 13230–13319 Search PubMed
; (e) A. Wiebe, T. Gieshoff, S. Möhle, E. Rodrigo, M. Zirbes and S. R. Waldvogel, Electrifying Organic Synthesis, Angew. Chem., Int. Ed., 2018, 57, 5594–5619 Search PubMed
; (f) S. Tang, Y. Liu and A. Lei, Electrochemical Oxidative Cross-coupling with Hydrogen Evolution: A Green and Sustainable Way for Bond Formation, Chem, 2018, 4, 27–45 Search PubMed
; (g) K. D. Moeller, Using Physical Organic Chemistry To Shape the Course of Electrochemical Reactions, Chem. Rev., 2018, 118(9), 4817–4833 Search PubMed
; (h) S. R. Waldvogel, S. Lips, M. Selt, B. Riehl and C. J. Kampf, Electrochemical Arylation Reaction, Chem. Rev., 2018, 118, 6706–6765 Search PubMed
; (i) K.-J. Jiao, Y.-K. Xing, Q.-L. Yang, H. Qiu and T.-S. Mei, Site-Selective C-H Functionalization via Synergistic Use of Electrochemistry and Transition Metal Catalysis, Acc. Chem. Res., 2020, 53, 300–310 Search PubMed
; (j) J. C. Siu, N. Fu and S. Lin, Catalyzing Electrosynthesis: A Homogeneous Electrocatalytic Approach to Reaction Discovery, Acc. Chem. Res., 2020, 53, 547–560 Search PubMed
; (k) X.-Y. Wang, X.-T. Xu, Z.-H. Wang, P. Fang and T.-S. Mei, Advances in Asymmetric Organotransition Metal-Catalyzed Electrochemistry, Chin. J. Org. Chem. DOI:10.6023/cjoc202003022
.
- H. Li, C. P. Breen, H. Seo, T. F. Jamison, Y.-Q. Fang and M. M. Bio, Ni-Catalyzed Electrochemical Decarboxylative C−C Couplings in Batch and Continuous Flow, Org. Lett., 2018, 20, 1338–1341 Search PubMed
.
- For reviews on asymmetric electrochemical organic synthesis, see:
(a)
O. Onomura, Electrochemical Asymmetric Synthesis, in Encyclopedia of Applied Electrochemistry, ed. G. Kreysa, K. Ota and R. F. Savinell, Springer, New York, NY, 2014 Search PubMed
; (b) M. Ghosh, V. S. Shinde and M. Rueping, A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions, Beilstein J. Org. Chem., 2019, 15, 2710–2746 Search PubMed
; (c) Q. Lin, L. Li and S. Luo, Asymmetric Electrochemical Catalysis, Chem. – Eur. J., 2019, 25, 10033–10044 Search PubMed
; (d) X. Chang, Q. Zhang and C. Guo, Asymmetric Electrochemical Transformations, Angew. Chem., 2020, 59, 12612–12622 Search PubMed
; (e) K. Yamamoto, M. Kuriyama and O. Onomura, Anodic Oxidation for the stereoselective Synthesis of Heterocycles, Acc. Chem. Res., 2020, 53, 105–120 Search PubMed
.
- M. Durandetti, J. Périchon and J. Y. Nédélec, Asymmetric Induction in the Electrochemical Cross-Coupling of Aryl Halides with α-Chloropropionic Acid Derivatives Catalyzed by Nickel Complexes, J. Org. Chem., 1997, 62, 7914–7915 Search PubMed
.
- A. H. Cherney and S. E. Reisman, Nickel-Catalyzed Asymmetric Reductive Cross-Coupling between Vinyl and Benzyl Electrophiles, J. Am. Chem. Soc., 2014, 136, 14365–14368 Search PubMed
.
- T. J. DeLano and S. E. Reisman, Enantioselective Electroreductive Coupling of Alkenyl and Benzyl Halides via Nickel Catalysis, ACS Catal., 2019, 9, 6751–6754 Search PubMed
.
- H. Qiu, B. Shuai, Y.-Z. Wang, D. Liu, Y.-G. Chen, P.-S. Gao, H.-Xi. Ma, S. Chen and T.-S. Mei, Enantioselective Ni-Catalyzed Electrochemical Synthesis of Biaryl Atropisomers, J. Am. Chem. Soc., 2020, 142, 9872–9878 Search PubMed
.
| This journal is © the Partner Organisations 2020 |