Carbon quantum dots enhanced the activity for the hydrogen evolution reaction in ruthenium-based electrocatalysts†
Weidong
Li‡
a,
Zhihong
Wei‡
a,
Boyang
Wang
a,
Yuan
Liu
ab,
Haoqiang
Song
a,
Zhiyong
Tang
 cd,
Bai
Yang
cd,
Bai
Yang
 e and
Siyu
Lu
e and
Siyu
Lu
 *ac
*ac
aCollege of Chemistry, Zhengzhou University, Zhengzhou, 450001, China. E-mail: sylu2013@zzu.edu.cn
bCollege of Materials Science and Engineering, Zhengzhou University, Zhengzhou, 450001, China
cHenan Institute of Advanced Technology, Zhengzhou University, Zhengzhou, 450001, China
dCAS Key Laboratory of Nanosystem and Hierarchical Fabrication, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing, 100190, China
eState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, 130012, China
First published on 2nd December 2019
Abstract
The development of effective and inexpensive hydrogen evolution reaction (HER) electrocatalysts for future renewable energy systems is highly desired. Carbon-based metal hybrid materials with unique edge sites, good chemical stability, high electrical conductivity, and synergetic effects exhibit great potential. However, there are few studies on the effect of the carbon-based support on catalytic performance. In this study, we explored the synergy between the carrier and metal (Ru), and studied the effect of the carrier and metal crystallinity on the catalytic performance of HER. The performance of Ru nanoparticles (Ru NPs) catalyst supported on carbon quantum dots (Ru@CQDs) was superior to that on other carbon supports. In addition, the HER performance of the Ru@CQDs catalyst was highly dependent on the crystallinity and confinement effects. Experimental results indicate that the Ru NPs were successfully anchored by strong coordination interactions between the d orbitals of Ru and the surface functional groups of CQDs, which is beneficial to the confinement of Ru NPs around the CQDs and to prevent their aggregation. The results will guide the design of inexpensive Ru-based HER electrocatalysts.
Introduction
Hydrogen is the cleanest energy resource in the energy economy. Electrocatalytic water splitting is considered an efficient and green process for sustainable hydrogen generation.1–5 Until now, platinum (Pt) has been the element of choice for catalysing the hydrogen evolution reaction (HER) because of its high current density and low overpotential.6,7 However, the expensive cost and limited reserves of Pt have hindered the technological scalability of this option.8,9 Consequently, alternatives to Pt electrodes are needed. Pt-based alloys10 or earth-abundant non-Pt catalysts such as Fe-,11,12 Ni-,13–16 Co-,17,18 Cu-19 and Mo-based20 materials have been widely studied. Unfortunately, their catalytic performance remains far from satisfactory compared with that of Pt. In addition, after long electrochemical processes, metal particles easily agglomerate or are oxidised, leading to attenuation of the catalytic activity.Benefiting from excellent tuneable surface physical chemical properties, thermal stability and corrosion resistance against acid and alkali, carbon nanomaterials have been widely applied in constructing carbon composite electrocatalysts.21–23 Due to the strong metal–support interactions, support materials usually play a vital role in optimising the local electronic and geometric structures of metal sites.24,25 In the development of support materials, the tuning and stabilising effect over the catalytic sites should be considered. However, there are few studies on the effect of the carbon-based support on catalytic performance. Therefore, it is necessary to improve our understanding of the physical and chemical characteristics of the carbon-based support and metal. In recent decades, a variety of carbon materials such as hollow porous carbon spheres,26,27 graphene,28,29 carbon nanotubes (CNTs),30 carbon nanofibers31,32 and carbon cloth33 have been widely investigated and designed as electrocatalysts support for the HER. However, the preparation process is complicated, and the reduction of metal precursors normally requires excess strong reducing reagents. Moreover, loading the metal nanoparticles (NPs) on the surface of the support leads to insufficient affinity of the NPs to the substrate. In addition, the relatively large particle size and poor distribution of NPs on the carriers result in reduced catalytic performance.34 Therefore, it is desirable to explore an efficient and facile route to fabricate homogeneously dispersed metal NPs firmly confined in a nanocarbon composite via an economic route.
Carbon quantum dots (CQDs) are emerging nanomaterials that show great promise for bioimaging,35 optical sensing,36 catalysis37 and photovoltaic applications.38–41 CQDs with high stability, easy surface functionalisation, and high electrical conductivity are attractive materials for the design and fabrication of photo-electro-catalyst.42–44 The variety of functional groups (–COOH, –NH2, –OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O etc.) on the surfaces of the CQDs can coordinate with metal ions/semiconductors with empty d orbitals to form a relatively stable CQDs–metal ion/CQDs–semiconductor coordination composite.45–47 The metal NPs are restricted between the CQDs to form super-fine nanocrystals with stable structures, which effectively prevents the agglomeration and growth of the metal NPs during the reaction. Therefore, CQDs may be an ideal carrier for the preparation of metal HER electrocatalysts.48,49
O etc.) on the surfaces of the CQDs can coordinate with metal ions/semiconductors with empty d orbitals to form a relatively stable CQDs–metal ion/CQDs–semiconductor coordination composite.45–47 The metal NPs are restricted between the CQDs to form super-fine nanocrystals with stable structures, which effectively prevents the agglomeration and growth of the metal NPs during the reaction. Therefore, CQDs may be an ideal carrier for the preparation of metal HER electrocatalysts.48,49
In this work, we explored the synergy between the carrier and metal (Ru), and investigated the effect of the carrier and metal crystallinity on the HER catalytic performance. The hybrid materials were prepared by a facile pyrolysis method using three typical carbon supports: biomass Porphyra (PC), Porphyra-CQDs, and Porphyra activated carbon (AC), denoted as Ru@PC, Ru@CQDs and Ru@P-AC (Scheme 1). The Porphyra-CQDs-supported Ru NPs catalyst (Ru@CQDs) exhibited the best dispersion and highest catalytic activity among the as-prepared samples. Furthermore, the HER performance of Ru@CQDs was shown to be greatly dependent on the crystallinity of Ru. The fully crystallised Ru exhibited the best HER performance with high electrocatalytic activity and long-term stability in alkaline media.
Results and discussion
The high-crystallinity CQDs were prepared by a simple solvothermal method (200 °C for 4 h). Transmission electron microscopy (TEM) analysis was used to directly observe the morphology of the CQDs nanostructures as well as their particle size distribution. As shown in Fig. 1a and Fig. S1 in the ESI,† the CQDs were uniformly dispersed with an average particle size of 2.13 nm. The high-resolution TEM (HRTEM) in Fig. 1b shows the lattice fringe clearly, with a lattice spacing of 0.21 nm, which corresponded to the (100) plane of graphitic carbon.37,38 The X-ray diffraction pattern (XRD) showed the peak at 22.40° (Fig. 1c), which was consistent with the TEM results. The Fourier transform-infrared (FT-IR) spectrum was used to evaluate the functional groups and chemical composition of the as-synthesised CQDs (Fig. 1d). The stretching vibration at 3334 cm−1 was assigned to N–H. The bands at 1667, 1455, and 1114 cm−1 were assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, and C–O stretching, respectively.35
C, C–N, and C–O stretching, respectively.35
The CQDs aqueous solution emits strong blue-green light under UV irradiation of 365 nm (Fig. 1e and f). From the Fig. 1e, the position and intensity of the emission peak changes as the excitation wavelength changes, which demonstrated the obvious excitation-dependent PL behaviour. When excited with 340 nm, the CQDs show the strongest emission at 450 nm. The peak in UV-vis absorption spectrum at about 280 nm assigned to the π → π* transition of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (Fig. 1f).37,46 The X-ray photoelectron spectroscopy (XPS) was used to characterise the surface chemical component of the CQDs. The survey spectra showed in Fig. S2 (ESI†) contain typical peaks of C 1s (285.1 eV), O 1s (530.9 eV), and N 1s (400.2 eV), confirming the presence of carbon, oxygen, and nitrogen elements, respectively.
C bond (Fig. 1f).37,46 The X-ray photoelectron spectroscopy (XPS) was used to characterise the surface chemical component of the CQDs. The survey spectra showed in Fig. S2 (ESI†) contain typical peaks of C 1s (285.1 eV), O 1s (530.9 eV), and N 1s (400.2 eV), confirming the presence of carbon, oxygen, and nitrogen elements, respectively.
The high-resolution XPS scans in Fig. 1g–i provide further details. Fig. 1g shows that C has four combinations, which correspond to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.6 eV), C–O/C–N (285.9 eV), C
C (284.6 eV), C–O/C–N (285.9 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.7 eV) and –COOH (290.1 eV). The two peaks (Fig. 1h) in 399.9 and 401.4 eV of the N 1s band attribute to pyrrolic N and graphitic N, respectively. The peaks of the O 1s (Fig. 1i) demonstrated there are two kinds of oxygen bond C
O (288.7 eV) and –COOH (290.1 eV). The two peaks (Fig. 1h) in 399.9 and 401.4 eV of the N 1s band attribute to pyrrolic N and graphitic N, respectively. The peaks of the O 1s (Fig. 1i) demonstrated there are two kinds of oxygen bond C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O located in 531.9 and 533.3 eV, respectively. The XPS data showed satisfactory agreement with the FT-IR spectra, which suggested the surface of the as-prepared CQDs contains oxygen- and nitrogen-containing functional groups.
O and C–O located in 531.9 and 533.3 eV, respectively. The XPS data showed satisfactory agreement with the FT-IR spectra, which suggested the surface of the as-prepared CQDs contains oxygen- and nitrogen-containing functional groups.
Ru@CQDs hybrids were synthesised by mixing the CQDs aqueous solution with RuCl3via a hydrothermal process. The products were then carbonised at 800 °C under an argon atmosphere and were denoted as Ru@CQDs800. During the pyrolysis process, the CQDs self-crosslinked to form an N-rich graphene skin surface owing to hydrogen bonding between the amino (–NH2), carboxyl (–COOH), and hydroxyl (–OH) groups of the CQDs and van der Waals bonding induced between adjacent CQDs.46,48 The TEM images clearly reveal the morphology of the Ru@CQDs800. As observed in Fig. 2a, the Ru NPs were uniformly dispersed in the CQDs matrix with an average size of ∼2.21 nm. The HRTEM image in Fig. 2b clearly shows the crystal lattice fringes at 0.205 nm correspond to the spacing of (101) planes of Ru, indicated the Ru@CQDs800 exhibited features of high crystallinity.46 The HAADF-STEM (Fig. 2c) and energy-dispersive X-ray spectroscopy (EDX) elemental mapping images (Fig. 2d–f) also evidenced the uniform homogeneous distributions of carbon, nitrogen, and ruthenium. Besides, the inductively coupled plasma–atom emission spectrometry (ICP–AES) analysis also demonstrated a total Ru content of only 1.24 wt%.
 | ||
| Fig. 2 (a) TEM image; (b) HRTEM image of Ru@CQDs800; (c) HAADF-STEM image corresponding EDX maps of Ru@CQDs for C (d), N (e), Ru (f), respectively. | ||
To explore the crystallisation process, Ru@CQDs samples were prepared at different annealing temperatures of 600 °C, 700 °C, 800 °C, and 900 °C (denoted as Ru@CQDs600–Ru@CQDs900) while keeping all other conditions unchanged. As shown in Fig. 3a–c, the Ru@CQDs600 was first appeared at 600 °C, as indicated by the clear lattice fringe of carbon (Fig. 3a); however, none of the Ru NPs with a mean size of ∼1.12 nm displayed a clear lattice fringe. At 700 °C, Ru NPs were clearly observed, and a few particles began to appear clear lattice fringes with an average size of ∼1.63 nm (Fig. 3d–f). The number of Ru NPs with clear lattice fringes slightly increased at 800 °C (Fig. 3g–i), with a maximum at 900 °C (Fig. S3, ESI†). The Ru NPs clearly grew and aggregated at 900 °C, thereby reducing the effective contact area of the catalyst and hindering the catalytic reaction. These findings indicate that with increasing calcination temperature, the degree of Ru crystallinity and the particle size of the Ru NPs increased; in addition, excessively high temperatures were not conducive to the dispersion of Ru NPs.
The morphologies of various carbon-based carrier-supported Ru nanoparticle catalysts were analysed. Ru@PC and Ru@P-AC were prepared at an annealing temperature of 800 °C. As shown in Fig. 4a, the obtained Ru@CQDs displayed a membrane structure, and the N-doped graphene sheets were thin and transparent, which is favourable for clear observation of the Ru NPs (Fig. 4b). The obtained Ru@PC (Fig. 4d) and Ru@P-AC (Fig. 4g) displayed an amorphous block structure, and the Ru NPs were agglomerated and accumulated. It was difficult to see the crystallised Ru NPs (Fig. 4e and h). We consider that Ru3+ tends to adhere to the surface of the carrier, which can result in an uneven distribution of Ru3+. Upon further carbonisation, the three-dimensional carbon structure may occur collapse, resulting in the aggregation of Ru NPs. The abundant surface groups on the surface of the CQDs could be used as building blocks to form a membrane structure, which could protect the Ru NPs from agglomeration.48
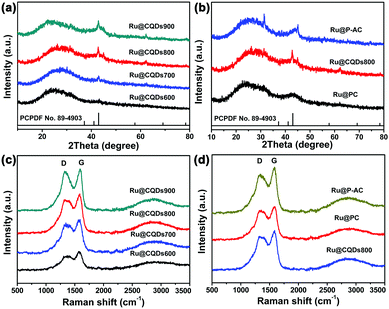
The XRD and Raman measurements were employed to characterise the structures of the Ru@CQDs annealing at different temperature. The peak at 22.40° in Fig. 5a and b can be attributed to the (100) planes of carbon, and the diffraction peaks at 38.4°, 42.2°, 44.0°, 58.3°, 69.4°, and 78.4° can be attributed to the (100), (002), (101), (102), (110), and (103) planes of hexagonal Ru (JCPDS No. 89-4903), respectively.46 With the temperature increasing, the diffraction peak intensity of Ru gradually increases. But the diffraction peak of Ru can hardly be observed in Ru@CQDs600, indicating that Ru did not crystallise at 600 °C. Upon increasing the calcination temperature to 700 °C, the Ru(101) peak became significant, and an additional weak Ru(100) peak appeared, suggesting that the Ru NPs started to crystallise. Upon further increasing the temperature to 900 °C, no clear differences in the diffraction peaks were observed, indicating that the Ru nanocrystals were completely crystallised.15 In addition, the diffraction peaks for Ru@PC and Ru@P-AC were the same as those for Ru@CQDs, indicating that their compositions were the same.
The degree of graphitisation of Ru@CQDs, Ru@PC, and Ru@P-AC were further examined using Raman spectroscopy. As shown in Fig. 5c and d, the broad D bands at 1336 cm−1 and G bands at 1584 cm−1 were clearly observed. The D bands is related to the defected carbons, and the G bands results from the graphitic in-plane vibrations of ideal sp2 carbons. The ratio of the relative intensity of the D bands and the G bands (ID/IG) is widely used to indicate the disorder degree or graphitisation of porous carbon materials.36 With increasing temperature, the ID/IG values of the Ru@CQDs were 0.85, 0.94, 0.95, and 0.94, respectively, indicating that the porous carbons possessed graphitic and disordered structures. No changes in the positions of the D and G peaks were observed for different carriers, and the ID/IG values of Ru@PC and Ru@P-AC were 0.88 and 0.90, respectively. The ID/IG value for Ru@CQDs800 was the largest, indicating that the porous carbon of Ru@CQDs contained more structural defects.
The XPS spectra was employed to identify the chemical component of different elements in the Ru@CQDs. Fig. 6a presents the survey XPS spectrum of Ru@CQDs800, revealing the presence of carbon (∼285 eV), nitrogen (∼400 eV), ruthenium (∼464 eV), and oxygen (∼531.8 eV). As observed in Fig. 6b, the C 1s spectrum of the Ru@CQDs can be deconvoluted into three peaks, which are attributed to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.7 eV), C–N (285.7 eV) and C
C (284.7 eV), C–N (285.7 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.8 eV) respectively. The other peaks at 280.8 and 284.1 eV can be assigned to Ru (3d5/2) and Ru (3d3/2). The N 1s spectrum in Fig. 6c contains two peaks were assigned to pyrrolic-N (399.4 eV) and graphitic-N (401.2 eV).46 The high resolution XPS scan of Ru (Fig. 6d) contains a dual peak at 462.0 and 484.3 eV, corresponding to metallic Ru 3p3/2 and Ru 3p1/2, respectively. These results further indicate that a Ru@CQDs hybrid was obtained.
O (288.8 eV) respectively. The other peaks at 280.8 and 284.1 eV can be assigned to Ru (3d5/2) and Ru (3d3/2). The N 1s spectrum in Fig. 6c contains two peaks were assigned to pyrrolic-N (399.4 eV) and graphitic-N (401.2 eV).46 The high resolution XPS scan of Ru (Fig. 6d) contains a dual peak at 462.0 and 484.3 eV, corresponding to metallic Ru 3p3/2 and Ru 3p1/2, respectively. These results further indicate that a Ru@CQDs hybrid was obtained.
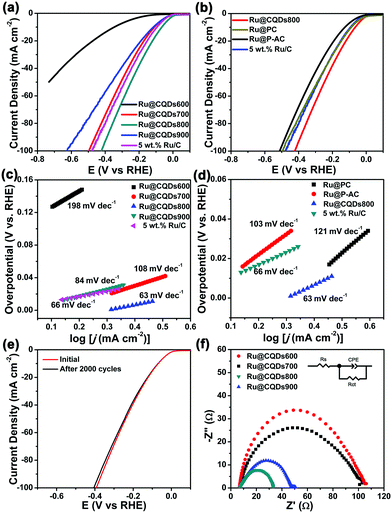
The catalytic performance for the HER of the various samples was evaluated in 1 M KOH solution using a standard three-electrode system (detailed information is provided in the ESI†). All the measurements were performed without correction for iR losses. The HER polarisation curve of commercial Ru/C (5 wt%) was also recorded for comparison. We firstly explored the effect of the crystallisation of the Ru NPs on the catalytic performance of Ru@CQDs. The crystallinity of metal particles is known to increase with increasing temperature; therefore, we tested samples prepared at different temperatures. As observed in Fig. 7a, the Ru@CQDs800 exhibited the highest HER activity of the several catalysts synthesised, requiring an overpotential of ∼65 mV to yield a current density of 10 mA cm−2, which was lower than that of 5 wt% Ru/C (94 mV). The overpotentials followed the trend of Ru@CQDs800 (65 mV) < Ru@CQDs700 (110 mV) < Ru@CQDs600 (346 mV), indicating that the catalytic activity increases with annealing temperature increases; however, when the annealing temperature was increased to 900 °C, the overpotential decreased to 117 mV. Thus, the optimal crystallisation of the Ru@CQDs hybrids was achieved with annealing at 800 °C. This phenomenon may be attributed to the low crystallinity decreased the interfacial interaction between the Ru NPs and CQDs, and higher crystallinity resulting in aggregation of the Ru NPs, which would restrain electron transport.
Next, to study the effect of different carbon-based carriers, we evaluated the catalytic activity of biomass- and biomass-activated-carbon-loaded Ru NPs (annealed at 800 °C). As observed in Fig. 7b, the Ru@CQDs800 exhibited enhanced HER catalytic activity (63 mV) compared with Ru@PC (89 mV) and Ru@P-AC (127 mV). To explore the reaction kinetics during the HER process, the Tafel slopes were determined by fitting the linear portions of the Tafel plots. As shown in Fig. 7c and d, the Tafel slope is ∼63 mV decade−1 for Ru@CQDs800, which is smaller than that of 5 wt% commercial Ru/C (66 mV decade−1), Ru@CQDs600 (198 mV decade−1), Ru@CQDs700 (108 mV decade−1), Ru@CQDs900 (84 mV decade−1), Ru@PC (121 mV decade−1), and Ru@P-AC (103 mV decade−1). The small Tafel slope of the Ru@CQDs suggests that the electrochemical desorption of Hads and H3O+ to generate H2 is the rate-limiting step, which indicates that the HER on Ru@CQDs may occur via the Volmer–Heyrovsky mechanism. The stability of Ru@CQDs800 was measured through a long-term cyclic voltammetry (CV) cycles. Fig. 7e presents the polarisation curves of Ru@CQDs800 before and after 2000 CV cycles. After 2000 CV cycles, the catalytic activity was 4 mV increase, which exhibited an insignificant loss, indicating its superior durability in 1 M KOH. The electrochemical impedance spectroscopy images in Fig. 7f showed the Ru@CQDs800 catalyst has a smallest semicircle radius, indicating that the Ru@CQDs800 displayed a lowest charge-transfer resistance (Rct) than that of the other catalysts (Table S1, ESI†). The small Rct value indicated the Ru@CQDs800 has the good electron-transfer ability. The excellent improvement in charge transport may be attributed to the super electronic transmission capacity between the Ru NPs and CQDs. The CQDs have been shown to be perfect electron donors and acceptors, promoting more efficient electron transport, thereby reducing the interface resistance by combining CQDs and electroactive Ru NPs. In summary, the excellent electrocatalytic performance of Ru@CQDs for the HER is owing to the synergetic effects between CQDs and Ru: an appropriate level of crystallinity is beneficial for electron transfer between the metal and carrier. The CQDs in the hybrid can prevents the agglomeration of Ru NPs, which effectively improves catalytic performance and stability.
Conclusions
In summary, we employed three kinds of carbon-based materials as carriers for carbon-based metal hybrid materials and assessed the relationship between the support and the catalytic performance. Three carbon-based materials (biomass Porphyra, Porphyra-CQDs, and Porphyra activated carbon) were selected to prepare highly dispersed Ru NPs on each support. The Ru NPs catalyst supported on Porphyra-CQDs (Ru@CQDs) exhibited the highest activity with a low overpotential of 65 mV in 1.0 M KOH at 10 mA cm−2. Compared with biomass Porphyra and Porphyra activated carbon, the catalysts with CQDs as the carrier exhibited better dispersibility and higher activity owing to the strong coordination interactions between the d orbitals of Ru and the surface functional groups of the CQDs. Owing to their high degree of crystallinity, the Ru@CQDs prepared at 800 °C exhibited the highest HER activity. Our findings highlight the importance and significance of the rational design of metal–support interactions for carbon-based metal hybrid catalysts. The results provide new insight into the active site of Ru nanocrystals, which will be beneficial for the development of novel highly efficient HER electrocatalysts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21905253, 51973200) and the China Postdoctoral Science Foundation (2018M640681, 2019T120632).Notes and references
- S. Tee, K. Win, W. Teo, L. Koh, S. Liu, C. Teng and M. Han, Recent Progress in Energy-Driven Water Splitting, Adv. Sci., 2017, 4, 1600337 CrossRef PubMed.
- T. Reier, H. Nong, D. Teschner, R. Schlögl and P. Strasser, Electrocatalytic Oxygen Evolution Reaction in Acidic Environments – Reaction Mechanisms and Catalysts, Adv. Energy Mater., 2017, 7, 1601275 CrossRef.
- Y. Jiao, Y. Zheng, M. Jaroniec and S. Qiao, Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions, Chem. Soc. Rev., 2015, 44, 2060–2086 RSC.
- Z. Seh, J. Kibsgaard, C. Dickens, I. Chorkendorff, J. Norskov and T. Jaramillo, Combining theory and experiment in electrocatalysis: Insights into materials design, Science, 2017, 355, eaad4998 CrossRef PubMed.
- B. You, N. Jiang, M. Sheng, W. Drisdell, J. Yano and Y. Sun, Bimetal-Organic Framework Self-Adjusted Synthesis of Support-Free Nonprecious Electrocatalysts for Efficient Oxygen Reduction, ACS Catal., 2015, 5, 7068–7076 CrossRef CAS.
- Y. Xie, J. Cai, Y. Wu, Y. Zang, X. Zheng, J. Ye, P. Cui, S. Niu, Y. Liu, J. Zhu, X. Liu, G. Wang and Y. Qian, Boosting Water Dissociation Kinetics on Pt–Ni Nanowires by N-Induced Orbital Tuning, Adv. Mater., 2019, 31, 1807780 CrossRef PubMed.
- Z. Zhang, G. Liu, X. Cui, B. Chen, Y. Zhu, Y. Gong, F. Saleem, S. Xi, Y. Du, A. Borgna, Z. Lai, Q. Zhang, B. Li, Y. Zong, Y. Han, L. Gu and H. Zhang, Crystal Phase and Architecture Engineering of Lotus-Thalamus-Shaped Pt-Ni Anisotropic Superstructures for Highly Efficient Electrochemical Hydrogen Evolution, Adv. Mater., 2018, 30, 1801741 CrossRef PubMed.
- S. Ye, F. Luo, Q. Zhang, P. Zhang, T. Xu, Q. Wang, D. He, L. Guo, Y. Zhang, C. He, X. Ouyang, M. Gu, J. Liu and X. Sun, Highly stable single Pt atomic sites anchored on aniline-stacked graphene for hydrogen evolution reaction, Energy Environ. Sci., 2019, 12, 1000–1007 RSC.
- L. Li, S. Wang, L. Xiong, B. Wang, G. Yang and S. Yang, Surface-engineered mesoporous Pt nanodendrites with Ni dopant for highly enhanced catalytic performance in hydrogen evolution reaction, J. Mater. Chem. A, 2019, 7, 12800–12807 RSC.
- A. Yang, T. Li, S. Jiang, X. Wang, X. Qiu, W. Lei and Y. Tang, High density growth of ultrafine PdIr nanowires on graphene: reducing the graphene wrinkles and serving as efficient bifunctional electrocatalysts for water splitting, Nanoscale, 2019, 11, 14561–14568 RSC.
- C. Lin, Z. Gao, J. Yang, B. Liu and J. Jin, Porous superstructures constructed from ultrafine FeP nanoparticles for highly active and exceptionally stable hydrogen evolution reaction, J. Mater. Chem. A, 2018, 6, 6387–6392 RSC.
- J. Chen, Y. Yang, J. Su, P. Jiang, G. Xia and Q. Chen, Enhanced Activity for Hydrogen Evolution Reaction over CoFe Catalysts by Alloying with Small Amount of Pt, ACS Appl. Mater. Interfaces, 2017, 9, 3596–3601 CrossRef CAS PubMed.
- A. Meena, M. Ha, S. Chandrasekaran, S. Sultan, P. Thangavel, A. Harzandi, B. Singh, J. Tiwari and K. Kim, Pt-like hydrogen evolution on a V2O5/Ni(OH)2 Electrocatalyst, J. Mater. Chem. A, 2019, 7, 15794–15800 RSC.
- P. Liu, L. Zhang, L. Zheng and H. Yang, Surface engineering of nickel selenide for an enhanced intrinsic overall water splitting ability, Mater. Chem. Front., 2018, 2, 1725–1731 RSC.
- Y. Jin, S. Huang, X. Yue, H. Du and P. Shen, Mo- and Fe-Modified Ni(OH)2/NiOOH Nanosheets as Highly Active and Stable Electrocatalysts for Oxygen Evolution Reaction, ACS Catal., 2018, 8, 2359–2363 CrossRef CAS.
- S. Jeoung, B. Seo, J. Hwang, S. Joo and H. Moon, Direct conversion of coordination compounds into Ni2P nanoparticles entrapped in 3D mesoporous graphene for an efficient hydrogen evolution reaction, Mater. Chem. Front., 2017, 1, 973–978 RSC.
- R. Zhang, X. Wang, S. Yu, T. Wen, X. Zhu, F. Yang, X. Sun, X. Wang and W. Hu, Ternary NiCo2Px Nanowires as pH-Universal Electrocatalysts for Highly Efficient Hydrogen Evolution Reaction, Adv. Mater., 2017, 29, 1605502 CrossRef PubMed.
- X. Yang, A. Lu, Y. Zhu, M. Hedhili, S. Min, K. Huang, Y. Han and L. Li, CoP nanosheet assembly grown on carbon cloth: A highly efficient electrocatalyst for hydrogen generation, Nano Energy, 2015, 15, 634–641 CrossRef CAS.
- J. Feng, J. Wu, Y. Tong and G. Li, Efficient Hydrogen Evolution on Cu Nanodots-Decorated Ni3S2 Nanotubes by Optimizing Atomic Hydrogen Adsorption and Desorption, J. Am. Chem. Soc., 2018, 140, 610–617 CrossRef CAS PubMed.
- X. Xie, M. Song, L. Wang, M. Engelhard, L. Luo, A. Miller, Y. Zhang, L. Du, H. Pan, Z. Nie, Y. Chu, L. Estevez, Z. Wei, H. Liu, C. Wang, D. Li and Y. Shao, Electrocatalytic Hydrogen Evolution in Neutral pH Solutions: Dual-Phase Synergy, ACS Catal., 2019, 9, 8712–8718 CrossRef CAS.
- R. Gao, Q. Dai, F. Du, D. Yan and L. Dai, C60-Adsorbed Single-Walled Carbon Nanotubes as Metal-Free, pH-Universal, and Multifunctional Catalysts for Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution, J. Am. Chem. Soc., 2019, 141, 11658–11666 CrossRef CAS PubMed.
- Y. Tong, X. Yu, H. Wang, B. Yao, C. Li and G. Shi, Trace Level Co–N Doped Graphite Foams as High-Performance Self-Standing Electrocatalytic Electrodes for Hydrogen and Oxygen Evolution, ACS Catal., 2018, 8, 4637–4644 CrossRef CAS.
- Z. Chen, J. Lu, Y. Ai, Y. Ji, T. Adschiri and L. Wan, Ruthenium/Graphene-like Layered Carbon Composite as an Efficient Hydrogen Evolution Reaction Electrocatalyst, ACS Appl. Mater. Interfaces, 2016, 8, 35132–35137 CrossRef CAS PubMed.
- M. Wang, C. Ye, H. Liu, M. Xu and S. Bao, Nanosized Metal Phosphides Embedded in Nitrogen-Doped Porous Carbon Nanofibers for Enhanced Hydrogen Evolution at All pH Values, Angew. Chem., Int. Ed., 2018, 57, 1963–1967 CrossRef CAS PubMed.
- H. Hou, L. Shao, Y. Zhang, G. Zou, J. Chen and X. Ji, Large-Area Carbon Nanosheets Doped with Phosphorus: A High-Performance Anode Material for Sodium-Ion Batteries, Adv. Sci., 2017, 4, 1600243 CrossRef PubMed.
- Y. Liu, X. Yong, Z. Liu, Z. Chen, Z. Kang and S. Lu, Unifed Catalyst for Efficient and Stable Hydrogen Production by Both the Electrolysis of Water and the Hydrolysis of Ammonia Borane, Adv. Sustainable Syst., 2019, 3, 1800161 CrossRef.
- Z. Peng, H. Wang, L. Zhou, Y. Wang, J. Gao, G. Liu, S. A. T. Redfern, X. Feng, S. Lu, B. Li and Z. Liu, Hollow carbon shells enhanced by confined ruthenium as cost-efficient and superior catalysts for the alkaline hydrogen evolution reaction, J. Mater. Chem. A, 2019, 7, 6676–6685 RSC.
- J. Su, Y. Yang, G. Xia, J. Chen, P. Jiang and Q. Chen, Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media, Nat. Commun., 2017, 8, 14969 CrossRef PubMed.
- J. Zhang and L. Dai, Nitrogen, Phosphorus, and Fluorine Tri-doped Graphene as a Multifunctional Catalyst for Self-Powered Electrochemical Water Splitting, Angew. Chem., Int. Ed., 2016, 55, 13296–13300 CrossRef CAS PubMed.
- J. Yu, W. Zhou, T. Xiong, A. Wang, S. Chen and B. Chu, Enhanced electrocatalytic activity of Co@N-doped carbon nanotubes by ultrasmall defect-rich TiO2 nanoparticles for hydrogen evolution reaction, Nano Res., 2017, 10, 2599–2609 CrossRef CAS.
- M. Li, Y. Zhu, H. Wang, C. Wang, N. Pinna and X. Lu, Ni Strongly Coupled with Mo2C Encapsulated in Nitrogen-Doped Carbon Nanofibers as Robust Bifunctional Catalyst for Overall Water Splitting, Adv. Energy Mater., 2019, 9, 1803185 CrossRef.
- M. Wang, C. Ye, M. Xu and S. Bao, MoP nanoparticles with a P-rich outermost atomic layer embedded in N-doped porous carbon nanofibers: Self-supported electrodes for efficient hydrogen generation, Nano Res., 2018, 11, 4728–4734 CrossRef CAS.
- Q. Dang, Y. Sun, X. Wang, W. Zhu, Y. Chen, F. Liao, H. Huang and M. Shao, Carbon dots-Pt modified polyaniline nanosheet grown on carbon cloth as stable and high-efficient electrocatalyst for hydrogen evolution in pH-universal electrolyte, Appl. Catal., B, 2019, 257, 117905 CrossRef CAS.
- G. Zhao, K. Rui, S. X. Dou and W. Sun, Heterostructures for Electrochemical Hydrogen Evolution Reaction: A Review, Adv. Funct. Mater., 2018, 28, 1803291 CrossRef.
- S. Lu, L. Sui, J. Liu, S. Zhu, A. Chen, M. Jin and B. Yang, Near-Infrared Photoluminescent Polymer-Carbon Nanodots with Two-Photon Fluorescence, Adv. Mater., 2017, 29, 1603443 CrossRef PubMed.
- S. Lu, L. Sui, Y. Liu, X. Yong, G. Xiao, K. Yuan, Z. Liu, B. Liu, B. Zou and B. Yang, Red-Emissive Carbon Dots for Fluorescent, Photoacoustic, and Thermal Theranostics in Living Mice, Adv. Sci., 2019, 6, 1801470 CrossRef PubMed.
- W. Li, Y. Liu, B. Wang, H. Song, Z. Liu, S. Lu and B. Yang, Kilogram-scale synthesis of carbon quantum dots for hydrogen evolution, sensing and bioimaging, Chin. Chem. Lett., 2019 DOI:10.1016/j.cclet.2019.06.040.
- B. Wang, J. Li, Z. Tang, B. Yang and S. Lu, Near-infrared emissive carbon dots with 33.96% emission in aqueous solution for cellular sensing and light-emitting diodes, Sci. Bull., 2019, 64, 1285–1292 CrossRef CAS.
- W. Meng, X. Bai, B. Wang, Z. Liu, S. Lu and B. Yang, Biomass-Derived Carbon Dots and Their Applications, Energy Environ. Mater., 2019, 2, 172–192 CrossRef CAS.
- Z. Zhou, P. Tian, X. Liu, S. Mei, D. Zhou, D. Li, P. Jing, W. Zhang, R. Guo, S. Qu and A. L. Rogach, Hydrogen Peroxide-Treated Carbon Dot Phosphor with a Bathochromic-Shifted, Aggregation-Enhanced Emission for Light-Emitting Devices and Visible Light Communication, Adv. Sci., 2018, 5, 1800369 CrossRef PubMed.
- S. Lu, L. Sui, Y. Liu, X. Yong, G. Xiao, K. Yuan, Z. Liu, B. Liu, B. Zou and B. Yang, White Photoluminescent Ti3C2 MXene Quantum Dots with Two-Photon Fluorescence, Adv. Sci., 2019, 6, 1801470 CrossRef PubMed.
- H. Yu, R. Shi, Y. Zhao, G. I. N. Waterhouse, L. Z. Wu, C. Tung and T. Zhang, Smart Utilization of Carbon Dots in Semiconductor Photocatalysis, Adv. Mater., 2016, 28, 9454–9477 CrossRef CAS PubMed.
- Z. Li, L. Wang, Y. Li, Y. Feng and W. Feng, Frontiers in carbon dots: design, properties and applications, Mater. Chem. Front., 2019, 3, 2571–2601 RSC.
- D. Qu, J. Liu, X. Miao, M. Han, H. Zhang, Z. Cui, S. Sun, Z. Kang, H. Fan and Z. Sun, Peering into water splitting mechanism of g-C3N4-carbon dots metal-free photocatalyst, Appl. Catal., B, 2018, 227, 418–424 CrossRef CAS.
- Y. Liu, Y. Yang, Z. Peng, Z. Liu, Z. Chen, L. Shang, S. Lu and T. Zhang, Self-crosslinking carbon dots loaded ruthenium dots as an efficient and super-stable hydrogen production electrocatalyst at all pH values, Nano Energy, 2019, 65, 104023 CrossRef.
- W. Li, Y. Liu, M. Wu, X. Feng, S. A. T. Redfern, Y. Shang, X. Yong, T. Feng, K. Wu, Z. Liu, B. Li, Z. Chen, J. S. Tse, S. Lu and B. Yang, Carbon-Quantum-Dots-Loaded Ruthenium Nanoparticles as an Efficient Electrocatalyst for Hydrogen Production in Alkaline Media, Adv. Mater., 2018, 30, 1800676 CrossRef PubMed.
- L. Pan, S. Sun, A. Zhang, K. Jiang, L. Zhang, C. Dong, Q. Huang, A. Wu and H. Lin, Truly Fluorescent Excitation-Dependent Carbon Dots and Their Applications in Multicolor Cellular Imaging and Multidimensional Sensing, Adv. Mater., 2015, 27, 7782–7787 CrossRef CAS PubMed.
- L. Wang, X. Wu, S. Guo, M. Han, Y. Zhou, Y. Sun, H. Huang, Y. Liu and Z. Kang, Mesoporous nitrogen, sulfur co-doped carbon dots/CoS hybrid as an efficient electrocatalyst for hydrogen evolution, J. Mater. Chem. A, 2017, 5, 2717–2723 RSC.
- Z. Wei, Y. Liu, Z. Peng, H. Song, Z. Liu, B. Liu, B. Li, B. Yang and S. Lu, Cobalt-Ruthenium Nanoalloys Parceled in Porous Nitrogen-Doped Graphene as Highly Efficient Difunctional Catalysts for Hydrogen Evolution Reaction and Hydrolysis of Ammonia Borane, ACS Sustainable Chem. Eng., 2019, 7, 7014–7023 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qm00618d |
| ‡ These authors have contributed equally. |
| This journal is © the Partner Organisations 2020 |