Microwave-assisted nucleophilic degradation of organophosphorus pesticides in propylene carbonate†
Daniela
Millán
 *a,
Mabel
Rojas
b,
Ricardo A.
Tapia
c and
Paulina
Pavez
*a,
Mabel
Rojas
b,
Ricardo A.
Tapia
c and
Paulina
Pavez
 c
c
aCentro Integrativo de Biología y Química Aplicada (CIBQA), Universidad Bernardo O'Higgins, General Gana 1702, Santiago, Chile. E-mail: daniela.millan@ubo.cl
bDepartamento de Ingeniería Química y Bioprocesos, Pontificia Universidad Católica de Chile, Avenida Vicuña Mackenna 4860, Macul, Santiago, Chile
cFacultad de Química y de Farmacia, Pontificia Universidad Católica de Chile, Santiago 6094411, Chile
First published on 21st September 2020
Abstract
Propylene carbonate is becoming a suitable green alternative to volatile organic solvents in the study of chemical reactions. In this study, an efficient method for nucleophilic degradation of five organophosphorus pesticides, fenitrothion, malathion, diazinon, parathion, and paraoxon, using propylene carbonate as a solvent is proposed. The effect of changing the nature of the nucleophile and the influence of microwave (MW) heating were investigated. A screening of temperatures (50 °C–120 °C) was performed under microwave heating. The pesticide degradation was followed by 31P NMR, and the extent of conversion (%) was calculated by the integration of phosphorus signals. Keeping in mind that recently it has been reported that some ionic liquids play a nucleophilic role, in this work we report for the first time the degradation of organophosphorus pesticides by using an amino acid-based ionic liquid such as Bmim[Ala] as a nucleophile and a bio-based solvent (propylene carbonate) as a reaction medium in combination with microwave heating.
1. Introduction
In the last few decades, the requirements for sustainable and safe processes have been gaining much attention, not only in academics and the chemical industry but the whole society. This trend towards what has become known as sustainable technologies can be defined as green chemistry which is driven by twelve principles described by Anastas.1 Effort has been made to meet the demands of green chemistry principles mainly in the replacement of volatile organic solvents (VOS) by suitable alternatives such as biodegradables and non-volatile green solvents.2–4 In this context, very recently, bio-based solvents, which are derived from natural resources, such as vegetable and animal raw materials, have emerged as a new class of green solvents.5–8 They have been used as reaction media in some organic processes and have shown to be effective without compromising the safety and the environmental acceptability of the process.9–11 It is interesting to note that propylene carbonate (PC) is nowadays one of the greenest solvents12,13 and it has been used as reaction media in many useful organic transformations.14–20 PC is known as a carbon dioxide neutral solvent and can be obtained from propylene oxide and carbon dioxide21 and also from a microwave-assisted process intensification of lipase-catalyzed transesterification of 1,2 propanediol with dimethyl carbonate.22On the other hand, organophosphorus pesticides (OPP), which are one of the most used pesticides worldwide, have increased the danger of their bioaccumulation in soils and groundwater.23–25 As a result, the degradation of these kinds of pesticides is an important issue to study. In this context, our group has been investigating the degradation of OPP by nucleophilic attack in several kinds of solvents such as aqueous solutions,26 volatile organic solvents (VOS), ionic liquids (ILs) and bio-based solvents.27–29 For example, the nucleophilic degradation of fenitrothion with piperidine in PC at room temperature was very efficient (t1/2 = 9 min), while the degradation of fenitrothion in Bmim[NTF2] was quite similar (t1/2 = 13.2 min) and much more slow in ethyl lactate (t1/2 = 210 min).
It is well known that the efficiency of nucleophilic degradation of organophosphorus pesticides depends strongly on the nucleophile, the nature of the phosphoryl center of the pesticide, and the type of solvent used in the reaction. In this context, we have studied the nucleophilic degradation of paraoxon in a series of ILs containing 1-butyl-3-methylimidazolium (Bmim)+ as a cation and different amino acids (AA) as anions (Bmim[AA]).29 It was observed that the reaction was completed at room temperature in the absence of an external nucleophile in about one hour, and the ionic liquid acted as both, the nucleophile and solvent. This is a relevant result and probably both the solvent (Bmim[AA]) and the imidazolium cation–amino acid–anion interactions increase the nucleophilicity of AA anions.29 Besides solvents, nucleophiles, time and energy play an essential role in organic reactions to meet the requirement of sustainable chemistry. In this context, microwave (MW) heating is becoming a useful tool in sustainable chemistry, because it dramatically diminishes reaction times, increases yields and decreases product impurities by reducing unwanted side reactions compared to conventional heating methods;30 the use of MW heating can be another way to meet the demands of green chemistry principles. Microwaves have been used in combination with ionic liquids for several purposes such as syntheses of inorganic nanomaterials, polymers, carbon-derived composites, and biomass-based composites.31 Abbiati et al. recently reported the microwave-mediated synthesis of 6-substituted 3,4-fused 2-pyranones in an acidic deep eutectic solvent achieving yields of up to 100%.32 Cravotto et al. performed MW-assisted oxidative C–H/C–H cross-coupling reactions in three bio-based solvents, γ-valerolactone, ethyl lactate and ethyl levulinate, achieving yields much higher than reported before.33 Regarding the MW-assisted degradation of OPP, Janeba et al. reported a complete degradation of organophosphates by microwave-assisted hydrolysis in D2O after 10 minutes of irradiation at 160 °C.34
Our group reported a study of MW- and ultrasound-assisted degradation of diazinon in ionic liquids and bio-based solvents, showing that degradation of the pesticide occurs in less than 30 minutes at 50 °C under MW irradiation35 while at room temperature, degradation did not take place. But to the best of our knowledge, the nucleophilic microwave-assisted degradation of organophosphorus pesticides in propylene carbonate has not been studied.
Based on all these facts, this work aims to study the influence of microwave heating on the nucleophilic degradation of five organophosphorus pesticides using an ionic liquid as the reagent and PC as the solvent. Considering the previous results, an ionic liquid containing 1-butyl-3-methylimidazolium (Bmim) as a cation and alanine as an anion (Bmim[Ala]) was chosen to explore its potential for MW-assisted degradation of OPP compared with piperidine.
Therefore, in this study we will investigate the influence of microwave heating on the degradation of OPP with Bmim[Ala] and piperidine as nucleophiles using PC as the solvent. The pesticides malathion (1), fenitrothion (2), paraoxon (3), diazinon (4) and parathion (5) were chosen based on their electrophilic center phosphate (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) or phosphorothioate (P
O) or phosphorothioate (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) with different leaving groups (Scheme 1).
S) with different leaving groups (Scheme 1).
2. Materials and methods
2.1. Materials
Propylene carbonate (PC), piperidine and organophosphorus pesticides were purchased from Aldrich. Piperidine was distilled before use. Bmim[Ala] was prepared as described.29 PC was stored over molecular sieves.2.2. Kinetic measurements
Kinetic measurements were performed by 31P NMR using a 400 MHz spectrometer, following the disappearance of the pesticide signal. The kinetic experiments were carried out in PC as the solvent at 25 °C, and Bmim[Ala] and piperidine as nucleophiles. The spectra were recorded at different reaction times and pseudo-first-order rate coefficients (kobsd) were obtained by integration of the NMR signals for pesticides and plotting log (integration) vs. time. Each measurement was made in triplicate, and the kobsd values reported in Table S1, in the ESI,† correspond to the average of the three measurements. In a typical experiment, a NMR tube containing 500 μL of the PC was thermostatted at 25 °C for 10 min, and then 10 μL of pesticide (0.5 M in ACN) and 20 μL of neat piperidine or 40 μL of Bmim[Ala] were added. A capillary tube of deuterated water was used as a reference solvent for NMR experiments.2.3. Microwave-assisted degradation
Microwave-assisted reactions were carried out in an Anton Paar Monowave 300 Microwave Synthesis Reactor (Anton Paar GmbH, Graz, Austria) in 10 mL sealed vials by adding 500 μL of solvent, 10 μL of pesticide (0.5 M in MeCN) and 20 μL of neat piperidine or 40uL of Bmim[Ala]. Reaction temperatures in the range of 50–120 °C for 10–60 minutes at 500 rpm were selected. To evaluate the percentage of conversion (%) of the pesticide, the reaction mixture was analyzed by 31P NMR.2.4. Gas chromatography–mass spectrometry (GC/MS)
GC/MS analysis was performed using a gas chromatograph fitted with a split–splitless injector and an Elite-5MS column (30 m × 0.25 mm i.d., 0.25 μm d.f.). Helium was used as a carrier gas at a flow rate of 1.0 mL min−1. The injection port was maintained at 25.0 °C, and the split ratio was 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The oven temperature was programmed from 70 °C for 2 min, then ramp 1: 5 °C min−1 to 140 °C hold for 12 min and ramp 2: 10 °C min−1 to 240 °C hold for 12 min. The ionization mode was electron impact ionization and the scanning range was from 50 amu to 400 amu. Mass spectra were obtained at 0.35 s intervals.
1. The oven temperature was programmed from 70 °C for 2 min, then ramp 1: 5 °C min−1 to 140 °C hold for 12 min and ramp 2: 10 °C min−1 to 240 °C hold for 12 min. The ionization mode was electron impact ionization and the scanning range was from 50 amu to 400 amu. Mass spectra were obtained at 0.35 s intervals.
2.5. Electrospray ionization mass spectrometry (ESI-MS)
The identification of the reaction products was performed using an ABSciex Triple Quad 4500 (UHPLC-MS/MS) mass spectrometer equipped with a Turbo Ion Spray (AB Sciex) ion source. A microsyringe pump delivered the reaction mixture of the pesticide with piperidine in propylene carbonate at infinite time dissolved in 10% (v/v) acetonitrile into the ESI source at a flow rate of 10 μL min−1. ESI and QQ (linear trap) mass spectrometry were performed in the negative-ion mode for detecting organophosphate compounds by using the multiple reaction monitoring (MRM) scan type. Main conditions: curtain gas nitrogen flow 10 mL min−1; ion spray voltage −4500 eV; declustering potential −60 eV; entrance potential −10 eV; collision cell exit potential −12 eV; source temperature was set at 300 °C and source gases GS1 and GS2 were set to 12 and 0, respectively. All data were acquired using Analyst 1.6.2 (AB Sciex).3. Results and discussion
It was previously described that Bmim[AA]-type ionic liquids have a dual function, as nucleophiles and solvents, in the degradation of pesticides.29 In this study, we investigated the degradation of pesticides 1–5 (Scheme 1) using propylene carbonate (PC) as the solvent and Bmim[Ala] as the nucleophile (0.4 M) under pseudo-first order conditions. The reactions were followed by 31P NMR experiments, and the degradation of the pesticides was calculated by the integration of the phosphorus signals and expressed as percentage (%) of extent of conversion.First, the reaction of pesticides with Bmim[Ala] in PC was explored at room temperature, and it was found that fenitrothion, malathion and paraoxon were fully degraded, but not diazinon and parathion (Fig. S1–S3 in the ESI†). For comparison purposes, we then tested the reactivity of the five pesticides with a known nucleophile such as piperidine in PC at room temperature. The results showed that fenitrothion (2) and malathion (1) were quickly degraded (Fig. S4 and S5 in the ESI†), but paraoxon, parathion, and diazinon did not react even after 24 hours of reaction. From the plots of log % degradation vs. time, we first calculated the pseudo-first order constants (kobs) and then half-life (t1/2) values which are shown in Table 1.
| Half-life (t1/2) (min) | ||
|---|---|---|
| Bmim[Ala] | Piperidine | |
| — no reaction observed. Values are the mean and standard deviation (±S.D.) of three independent experiments. | ||
| Malathion (1) | 10.7 ± 0.60 | 9.9 ± 0.6 |
| Fenitrothion (2) | 425.0 ± 11.4 | 23.1± 0.6 |
| Paraoxon (3) | 1203 ± 96.1 | — |
| Diazinon (4) | — | — |
| Parathion (5) | — | — |
The best rate of degradation was found for malathion with both nucleophiles, which could be due to the fact that aliphatic pesticides are degraded faster than aromatic pesticides together with a loss of stability in OPP with –OMe groups.36
We can observe that both nucleophiles can degrade fenitrothion, but piperidine is much more efficient. Noteworthily, malathion degradation with both nucleophiles shows similar half-life (t1/2) values, which is very interesting due to the fact that we can use Bmim[Ala] without compromising the rate of the reaction. But the most promising result is that observed for paraoxon degradation when Bmim[Ala] is the nucleophile, since this nucleophile achieves a total degradation of this pesticide at room temperature (t1/2 = 1220 min, Fig. S3 in the ESI†). These results confirm that Bmim[Ala] can be used as a reagent for the degradation of malathion (1) and paraoxon (2) at room temperature using a green and sustainable solvent such as PC.
The reactivity order found is interesting. When piperidine is the nucleophile, malathion reacts faster than fenitrothion; both pesticides have thiophosphoryl moieties, but malathion has a better leaving group than fenitrothion. The lack of nucleophilic attack of piperidine on the electrophilic centers of paraoxon, parathion and diazinon in propylene carbonate as the solvent could be explained by the lower reactivity at the aliphatic carbon by the stereoelectronic effects of the ethoxy substituent.
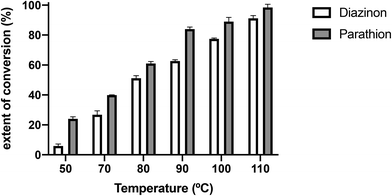
Taking in account that parathion and diazinon did not react at room temperature, the degradation of these pesticides was studied under MW irradiation increasing the temperature in the range of 50–120 °C for 30 minutes using Bmim[Ala] as the nucleophile.
As shown in Fig. 1, at higher temperature, the extent of conversion (%) of both pesticides increases. Parathion's reaction with Bmim[Ala] is faster than with diazinon, and 100% of parathion's degradation occurs around 110 °C while at the same temperature diazinon degradation reaches 90%. Although the structures of both pesticides have certain similarities, such as an electrophilic P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S centre linked to –OEt groups, the aromatic substituents, O-phenyl in parathion and O-pyrimidyl in diazinon, can lead them to react through different reaction pathways (see below).
S centre linked to –OEt groups, the aromatic substituents, O-phenyl in parathion and O-pyrimidyl in diazinon, can lead them to react through different reaction pathways (see below).
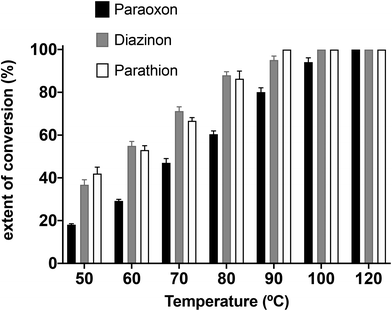
Subsequently, the MW-assisted nucleophilic degradation of paraoxon, parathion and diazinon with piperidine increasing the temperature in the range of 50–120 °C for 30 minutes was analyzed.
As we can see in Fig. 2, complete degradation of paraoxon requires higher temperature (120 °C) compared to parathion (90 °C) and diazinon (100 °C). This result is contrary to what was expected, because it has been described that P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O compounds are more reactive with nucleophiles than their thio-analogues.37 This is an interesting finding and may provide a useful tool for the nucleophilic degradation of thiophosphate pesticides.
O compounds are more reactive with nucleophiles than their thio-analogues.37 This is an interesting finding and may provide a useful tool for the nucleophilic degradation of thiophosphate pesticides.
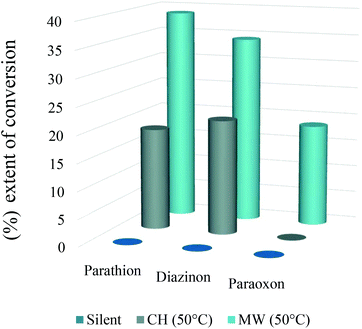
To evaluate the MW effect on the degradation of the pesticides studied, we carried out the same nucleophilic reactions of paraoxon, parathion and diazinon at 50 °C for 30 minutes under conventional heating (oil bath) with controlled stirring and silent conditions (no heating, no stirring). The extent of conversion (%) in all cases was calculated by integration of the 31P NMR signals of each pesticide and is shown in Fig. 3.
As we can see in Fig. 3, there is a great influence of MW irradiation on the rate of the degradation of organophosphate pesticides compared with conventional heating (oil bath). In the case of silent conditions (no heating, no stirring), no degradation was observed after 30 minutes of reaction, which clearly demonstrates the effectiveness of the developed microwave process.
Keeping in mind that the reactivity of organophosphorus pesticides depends on their structure, the nature of the leaving group and nucleophile, and the solvent,27 product analyses of the reactions were performed in order to understand the mechanism of the degradations. Then, the nucleophilic reactions were followed by the 31P NMR technique, and the spectra were recorded at different reaction times as shown in Fig. S1–S5 and S11–S13 in the ESI.†
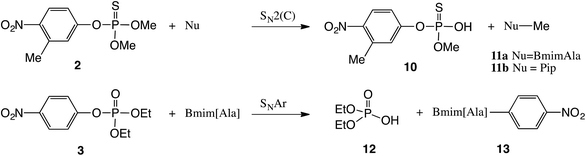
The highest degradation rate was found for the reaction of malathion with both nucleophiles at room temperature. This result is probably related to the very good electrophilic carbon center in malathion which makes it more reactive than the aromatic pesticides studied in this work. This finding is in agreement with a previous work of Lartiges et al. who studied the degradation kinetics of 19 organophosphorus and organonitrogen pesticides in different water types.36 The 31P NMR spectrum for the degradation with piperidine showed two new phosphorus signals (Fig. S2 in the ESI†), one at 118 ppm and another at 67 ppm. The signal at 118 ppm was assigned to O,O-dimethyl dithiophosphate (6) which is produced by an SN2(C–S) pathway (aliphatic carbon bonded to a sulphur atom), identified by ESI-MS (-Q, m/z 156.97, Fig. S6 in the ESI†). In addition, the signal at 67 ppm in Fig. S2† was assigned to compound 8 (Scheme 2), which is formed when piperidine attacks the phosphorus center of malathion SN2(P). The product 8 was identified by comparison with the phosphoric product obtained by the reaction of piperidine and O,O-dimethyl chlorothiophosphate in PC, as shown in Scheme S1 and Fig. S7 in the ESI.† When the reaction of malathion with Bmim[Ala] at room temperature was followed by 31P NMR, only one new phosphorus signal (118.5 ppm) was observed. This was assigned to O,O-dimethyl dithiophosphate (6), produced by an SN2(C–S) pathway.
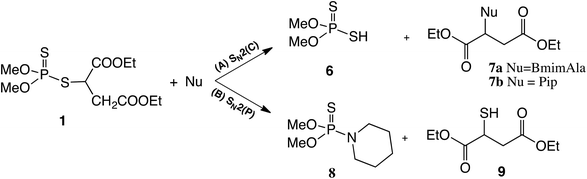 | ||
| Scheme 2 Reaction pathways for the nucleophilic degradation of malathion (1) with both nucleophiles (piperidine and Bmim[Ala]) in propylene carbonate at room temperature. | ||
Regarding the degradation of fenitrothion (2) at room temperature, the 31P NMR spectra showed only one phosphorus signal at 52.3 ppm when Bmim[Ala] was the nucleophile. This signal was attributed to demethylfenitrothion (10) formed when the nucleophile attacks the aliphatic carbon by an SN2(C) pathway (Fig. S1 in the ESI†); see Scheme 3. According to Fig. S4,† the nucleophilic degradation of fenitrothion (2) by piperidine in PC is similar and showed a phosphorus signal at 52 ppm, which was also assigned to demethylfenitrothion (10) as shown in Scheme 3.
On the other hand, the degradation of paraoxon (3) with Bmim[Ala] at room temperature shows the decrease of the signal of 3 (−7.4 ppm) and the increase of a phosphorus signal at −0.30 ppm (Fig. S3 in the ESI†), which was assigned to O,O-diethylphosphate (12); see Scheme 3. Compound 12 is formed when Bmim[Ala] attacks the aromatic carbon of paraoxon (SNAr), involving an aryl-O breakage according to the literature.27 Probably different interactions could explain these results: (i) stabilization of the transition state of the SNAr reaction through a π stacking interaction between the phenyl moiety of paraoxon and the aromatic cation (Bmim) of the nucleophile and (ii) interaction of the imidazolium cation of the IL with the starting material paraoxon which was described by Hawker et al.38 as an entropic effect.
Then, the product analysis of the nucleophilic degradation of the pesticides under MW heating in the range of 50–120 °C for 30 minutes was performed. In the reaction of diazinon with Bmim[Ala], a new signal appears at 49.5 ppm in the 31P NMR spectra (Fig. S8 in the ESI†), assigned to diethyl thiophosphate (14). This is produced when the nucleophile attacks the aromatic carbon of diazinon (SNAr) and it was confirmed by ESI-MS (-Q, m/z 168.9) (Fig. S9 in the ESI†). The degradation of diazinon by piperidine after MW irradiation shows only one phosphoryl product (Fig. S10 in the ESI†), which is the same product 14 described above when Bmim[Ala] was the nucleophile (see Scheme 4).
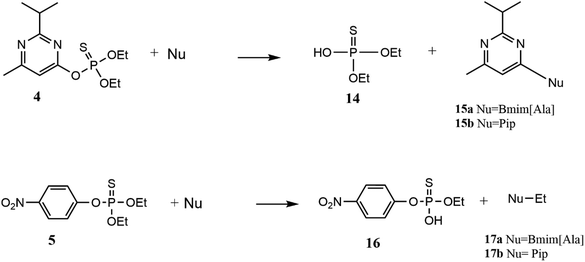 | ||
| Scheme 4 Reaction pathways for the nucleophilic degradation of diazinon (4) and parathion (5) by both nucleophiles (piperidine and Bmim[Ala]) in propylene carbonate under MW heating. | ||
In the case of parathion's degradation with Bmim[Ala], 31P NMR spectra at different times showed the decrease of the substrate signal (62 ppm) and the increase of a new signal at 50.5 ppm which was assigned to the O-ethyl 4-nitrothiophenylphosphate diester (16) (Fig. S11 in the ESI†). This product is formed by the nucleophilic attack of the nucleophile on the aliphatic carbon of parathion, through an SN2(C) pathway as shown in Scheme 4. When the nucleophile is piperidine, it was seen that degradation occurred by the same pathway as described above (Fig. S12 in the ESI†), i.e., via the attack on the aliphatic carbon of the ethyl group. The non-phosphoryl product of this reaction was identified by GC-MS and was found to be N-ethylpiperidine (17b) (r.t. = 17.15 min, m/z 113) (Fig. S13 in the ESI†).
A comparison of parathion and diazinon degradation under MW heating with both nucleophiles, piperidine and Bmim[Ala], shows that in the whole range of studied temperatures, the variation in the extent of conversion (%) is very small. Therefore, we can choose Bmim[Ala] as the best nucleophile to degrade these pesticides under these experimental conditions.
Finally, degradation of paraoxon under MW heating using piperidine as the nucleophile shows only one phosphorus signal at −6 ppm (Fig. S14 in the ESI†), which was assigned to the O-ethyl 4-nitrophenylphosphate diester (18), formed when piperidine attacks the aliphatic carbon of paraoxon.27 Nevertheless, when the reaction of paraoxon with piperidine was carried out using ionic liquids and bio-based solvents, the degradation process was not selective and three reaction pathways were observed: to the aliphatic carbon of O-ethyl, to the aromatic carbon C1 and to the phosphorus centre.28 Interestingly, in this work, the microwave-assisted degradation of paraoxon and parathion in PC is selective and proceeds only through nucleophilic attacks by piperidine on the aliphatic carbon (SN2(C)) of the pesticides resulting in products 16 and 18 (Schemes 4 and 5).
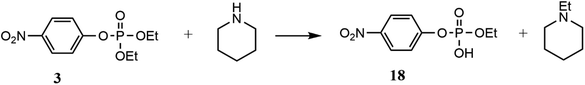 | ||
| Scheme 5 Reaction pathway for the degradation of paraoxon by piperidine as the nucleophile in propylene carbonate under MW heating. | ||
As can be seen, the aromatic OPP studied in this work (2–5) react solely via SN2 at the aliphatic carbon when piperidine is the nucleophile. The preference for this pathway depends on the nucleophile39,40 but also depends on the solvent. It has been described that hard nucleophiles (oximates and −OH) react with the hard P center and soft nucleophiles (butylamine) react with the soft aliphatic carbon center preferentially.39,41 Furthermore, the preference by the SN2(C) in OPP's degradation has been reported before and it was explained as a consequence of the lower basicity of the medium.42,43
Interestingly, the use of propylene carbonate is important to control the selectivity of this reaction. Previously, we have reported the degradation of paraoxon with piperidine in some conventional organic solvents and the SN2(C) pathway is the main degradation route (above 75%) in solvents less polar than DMSO, such as 1,4-dioxane and acetonitrile.27 The kobsd value found for the degradation of paraoxon in PC with Bmim[Ala] as the nucleophile (this study) is similar (9.6 × 10−6 s−1) to those reported for the degradation of paraoxon in conventional ionic liquids such as Bmim[NTF2], B2mim[NTF2] and B2mim[BF4] by the same reaction pathway.27 On the other hand, in a previous study, the degradation of fenitrothion with piperidine was carried out in some bio-based solvents and conventional ionic liquids and the only reaction pathway found was the SN2(C) pathway, propylene carbonate being the best solvent to degrade this pesticide.28 Finally, for malathion degradation, this study showed that the t1/2 value was around 10 minutes with both nucleophiles, which is a very promising result since in previous works the degradation of malathion was studied in aqueous solutions using strong chemical oxidants and UV irradiation. The t1/2 values were higher than 12 hours while the best method to degrade this pesticide was under O3 radiation with a t1/2 value of 2.4 hours.44
These results are interesting from the toxicity point of view since it is known that phosphate monoesters and diesters are much less lipophilic, and therefore less toxic, than their triester precursor, paraoxon or parathion. It is important to mention that even at long reaction times under MW heating, the same products of the reaction were found in all cases.
4. Conclusions
An efficient process for the chemical degradation of organophosphorus pesticides through microwave-assisted degradation in the green solvent “propylene carbonate” was developed.This study helps to understand the mechanism of the reaction of OPP which can be used to create efficient and sustainable degradation methods.
Fenitrothion and malathion do not need MW irradiation to achieve complete degradation with either nucleophile used in this study. Fenitrothion degradation was faster with piperidine as the nucleophile, while malathion degradation was carried out at a similar rate with both nucleophiles (t1/2 values are 9.57 min and 10.9 min with piperidine and Bmim[Ala] respectively). On the other hand, the degradation of paraoxon with Bmim[Ala] was accomplished at room temperature in a few hours, which is an important result because the degradation with piperidine was not successful.
MW heating is a promising technique that accelerates the degradation of the OPP paraoxon, diazinon, and parathion with piperidine which is effective in only 30 minutes at above 90 °C.
The temperature does not affect the reaction pathways of the degradation reactions studied. For the reaction of paraoxon, only one phosphorylic compound was found in contrast to the three phosphorylic compounds observed in the nucleophilic degradation of paraoxon in other solvents.27
This study highlights the efficiency of the microwave-assisted degradation of OPP in propylene carbonate that proceeded at a significantly higher rate compared to conventional heating and silent conditions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Fondecyt, grant 1170976, 11170569 and postdoctoral grant 3150122. The authors would also like to acknowledge the NMR facility in the Unidad Central de Investigación at the Pontificia Universidad Catolica de Chile for NMR support.References
- P. T. Anastas and J. C. Warner, Green chemistry: theory and practice, Oxford University Press, 1998 Search PubMed.
- R. A. Sheldon, Green Chem., 2005, 7, 267–278 Search PubMed.
- J. Sherwood, M. De, A. Constantinou, L. Moity, C. R. Mcelroy, T. J. Farmer, T. Duncan, W. Raverty, A. J. Hunt and J. H. Clark, Chem. Commun., 2014, 50, 9650–9652 Search PubMed.
- P. G. Jessop, Green Chem., 2011, 13, 1391–1398 Search PubMed.
- Y. Gu and F. Jérôme, Chem. Soc. Rev., 2013, 42, 9550–9570 Search PubMed.
- J. H. Clark, D. J. Macquarrie and J. Sherwood, Green Chem., 2012, 14, 90–93 Search PubMed.
- J. R. Sherwood, Bio-based solvents for organic chemistry, University of York, 2013 Search PubMed.
- A. Czompa, B. L. Pásztor, J. A. Sahar, Z. Mucsi, D. Bogdán, K. Ludányi, Z. Varga and I. M. Mándity, RSC Adv., 2019, 9, 37818–37824 Search PubMed.
- G. Paggiola, A. J. Hunt, C. R. McElroy, J. Sherwood and J. H. Clark, Green Chem., 2014, 16, 2107–2110 Search PubMed.
- A. Farrán, C. Cai, M. Sandoval, Y. Xu, J. Liu, M. J. Hernáiz and R. J. Linhardt, Chem. Rev., 2015, 115, 6811–6853 Search PubMed.
- C. S. M. Pereira, V. M. T. M. Silva and A. E. Rodrigues, Green Chem., 2011, 13, 2658–2671 Search PubMed.
- J. Bayardon, J. Holz, B. Schäffner, V. Andrushko, S. Verevkin, A. Preetz and A. Börner, Angew. Chem., Int. Ed., 2007, 46, 5971–5974 Search PubMed.
- C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879–3890 Search PubMed.
- H. L. Parker, J. Sherwood, A. J. Hunt and J. H. Clark, ACS Sustainable Chem. Eng., 2014, 2, 1739–1742 Search PubMed.
- R. J. Cervasio, J. S. Bello Forero, J. A. Hernández Muñoz, J. Jones Jr. and F. M. da Silva, Curr. Org. Synth., 2017, 14, 715–720 Search PubMed.
- K. B. Jr., W. Bergfeld and W. Berndt, J. Am. Coll. Toxicol., 1987, 6, 23–51 Search PubMed.
- S. Mokhatab, W. A. Poe and J. Y. Mak, in Handbook of Natural Gas Transmission and Processing, Elsevier, 2019, pp. 231–269 Search PubMed.
- E. K. Reeves, O. R. Bauman, G. B. Mitchem and S. R. Neufeldt, Isr. J. Chem., 2020, 60, 406–409 Search PubMed.
- P. Gautam, N. J. Tiwari and B. M. Bhanage, ACS Omega, 2019, 4, 1560–1574 Search PubMed.
- H. Lu, L. He, X. Li, W. Zhang, J. Che, X. Liu, Z. Hou, H. Du and Y. Qu, J. Mater. Sci.: Mater. Electron., 2019, 30, 13933–13938 Search PubMed.
- N. Oncel, V. T. Kasumov, E. Sahin and M. Ulusoy, J. Organomet. Chem., 2016, 811, 81–90 Search PubMed.
- G. D. Yadav, M. P. Hude and A. D. Talpade, Chem. Eng. J., 2015, 281, 199–208 Search PubMed.
- B. K. Singh, Nat. Rev. Microbiol., 2009, 7, 156–164 Search PubMed.
- J. E. Casida and G. B. Quistad, Chem. Res. Toxicol., 2004, 17, 983–998 Search PubMed.
- M. A. Matouq, Z. A. Al-Anber, T. Tagawa, S. Aljbour and M. Al-Shannag, Ultrason. Sonochem., 2008, 15, 869–874 Search PubMed.
- R. Aguayo, F. Arias, A. Cañete, C. Zuñiga, E. A. Castro, P. Pavez and J. G. Santos, Int. J. Chem. Kinet., 2013, 45, 202–211 Search PubMed.
- P. Pavez, D. Millán, J. I. Morales, E. A. Castro, A. C. López and J. G. Santos, J. Org. Chem., 2013, 78, 9670–9676 Search PubMed.
- P. Pavez, G. Oliva and D. Millán, ACS Sustainable Chem. Eng., 2016, 4, 7023–7031 Search PubMed.
- J. I. Morales, R. Figueroa, M. Rojas, D. Millán, R. A. Tapia and P. Pavez, Org. Biomol. Chem., 2018, 16, 7446–7453 Search PubMed.
- C. O. Kappe, D. Dallinger and S. S. Murphree, Practical Microwave Synthesis for Organic Chemists: Strategies, Instruments, and Protocols, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2009 Search PubMed.
- Y. Wang, Q. Hou, M. Ju and W. Li, Nanomaterials, 2019, 9, 647 Search PubMed.
- F. Curti, M. Tiecco, V. Pirovano, R. Germani, A. Caselli, E. Rossi and G. Abbiati, Eur. J. Org. Chem., 2019, 2019, 1904–1914 Search PubMed.
- S. Tabasso, E. C. Gaudino, E. Acciardo, M. Manzoli, A. Giacomino and G. Cravotto, Molecules, 2019, 24, 288 Search PubMed.
- P. Jansa, L. Cechova and Z. Janeba, Curr. Microwave Chem., 2016, 3, 219–226 Search PubMed.
- D. Millán, R. A. Tapia and P. Pavez, Front. Chem., 2019, 6, 669 Search PubMed.
- S. B. Lartiges and P. P. Garrigues, Environ. Sci. Technol., 1995, 29, 1246–1254 Search PubMed.
- I. H. Um, Y. H. Shin, S. E. Lee, K. Yang and E. Buncel, J. Org. Chem., 2008, 73, 923–930 Search PubMed.
- K. S. Schaffarczyk McHale, R. R. Hawker and J. B. Harper, New J. Chem., 2016, 40, 7437–7444 Search PubMed.
- N. M. Rougier, R. V. Vico, R. H. de Rossi and E. I. Buján, J. Org. Chem., 2010, 75, 3427–3436 Search PubMed.
- X. Han, V. K. Balakrishnan, G. W. Vanloon and E. Buncel, Langmuir, 2006, 22, 9009–9017 Search PubMed.
- D. Mandal, B. Mondal and A. K. Das, J. Phys. Chem. A, 2012, 116, 2536–2546 Search PubMed.
- V. B. Silva and E. S. Orth, J. Braz. Chem. Soc., 2019, 30, 2114–2124 Search PubMed.
- V. B. Silva, L. L. Q. Nascimento, M. C. Nunes, R. B. Campos, A. R. M. Oliveira and E. S. Orth, Chem. – Eur. J., 2019, 25, 817–822 Search PubMed.
- M. Tony, M. S. El-Geundi, S. M. Hussein and M. Z. Abdelwahab, Sci. Agric., 2017, 20, 174–185 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Stacked 31P NMR plots, GC/MS and ESI-MS chromatogram and mass spectra. See DOI: 10.1039/d0ob01620a |
| This journal is © The Royal Society of Chemistry 2020 |