Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ratiometric sensing of fluoride ions using Raman spectroscopy†
William J.
Tipping
 a,
Liam T.
Wilson
a,
Liam T.
Wilson
 b,
Sonja K.
Blaseio‡
b,
Sonja K.
Blaseio‡
 a,
Nicholas C. O.
Tomkinson
a,
Nicholas C. O.
Tomkinson
 *b,
Karen
Faulds
*b,
Karen
Faulds
 *a and
Duncan
Graham
*a and
Duncan
Graham
 *a
*a
aCentre for Molecular Nanometrology, WestCHEM, Department of Pure and Applied Chemistry, Technology and Innovation Centre, University of Strathclyde, 99 George Street, Glasgow G1 1RD, UK. E-mail: karen.faulds@strath.ac.uk; duncan.graham@strath.ac.uk
bDepartment of Pure and Applied Chemistry, WestCHEM, University of Strathclyde, 295 Cathedral Street, Glasgow G1 1XL, UK. E-mail: nicholas.tomkinson@strath.ac.uk
First published on 27th October 2020
Abstract
Ratiometric Raman spectroscopy represents a novel sensing approach for the detection of fluoride anions based on alkyne desilylation chemistry. This method enables rapid, anion selective and highly sensitive detection of fluoride in a simple paper-based assay format using a portable Raman spectrometer.
Raman microscopy is a powerful method for chemical analysis and biological imaging because it provides a spectral fingerprint with chemical specificity in a non-destructive manner.1 An emerging approach for chemical sensing is alkyne-tag Raman imaging (ATRI) which has enabled in situ biomolecular detection with high sensitivity, and multiplex analysis using low molecular weight Raman labels and sensor moieties.2 Alkynes have discrete vibrational modes within the cellular-silent region of the Raman spectrum (1800–2800 cm−1) which greatly facilitates their detection in biological samples.3 Early examples of ATRI reported metabolic labelling of intracellular biomolecules with alkyne-labelled precursors for the detection of nascent DNA,4 glycans5 and proteins6 amongst others. Since then, functionalisation of small molecules with alkyne labels has enabled the intracellular visualisation of drugs,7 natural products8,9 and lipids10 using Raman and stimulated Raman scattering (SRS) microscopy.
An unexplored application of ATRI is the development of small molecule sensors for the detection and quantification of endogenous chemicals, ions and biomolecules. Two isolated examples make use of oligoyne scaffolds for intracellular hydrogen sulphide sensing11 and for pH quantification12 (Fig. 1). In each of these reports, the ratiometric Raman sensor relies on analyte-induced changes of the sensing group resulting in a change in the Raman shift and/or scattering properties of the alkyne moiety. As such, ratiometric sensing approaches are advantageous because there is an effective internal referencing that increases sensitivity and improves quantification.13,14
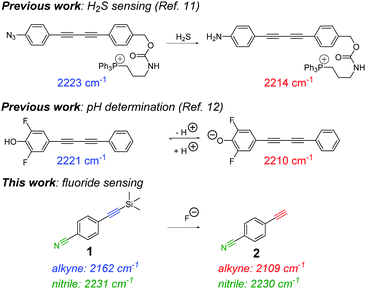 | ||
| Fig. 1 Previous reports of sensing and quantification using alkyne-tag Raman imaging. (i) A previous example of an irreversible sensor for hydrogen sulfide (H2S) detection11 and (ii) a reversible sensor for quantifying intracellular pH.12 This work reports an extremely low molecular weight Raman sensor (1) for ratiometric detection of fluoride ions. | ||
Fluoride is a biologically important anion that is readily absorbed by the body but is excreted slowly, and as such, overexposure to fluoride may result in gastric and kidney problems, bone diseases and fluorosis.15 There has been much interest in the development of fluorescence-based sensors for fluoride detection (Fig. S1, ESI†),16 notably, using the fluoride-mediated desilylation of Si–O groups17–19 and Si–C (alkyne) groups20–26 as prominent examples for military and cellular applications.
Despite 20 years of developing fluorescent fluoride sensors based on Si–F chemistry, significant challenges have been identified.27 First, the structural complexity of fluorescent sensors increases the synthetic effort and expense required to prepare the sensors, restricting translation to low-cost, in-field applications. Second, the use of fluorophores that are not compatible with NIR excitation, which is essential for biological compatibility due to reduced photodamage and enhanced imaging depth penetration. Third, the requirement of a surfactant (e.g. cetyltrimethylammonium bromide, CTAB) to enable detection in organic–aqueous solvent mixtures, once again restricting translation.28 Here we propose the use of Raman spectroscopy as a novel method to address these limitations; an extremely small ratiometric sensor molecule (<200 Da) is used to detect fluoride with NIR excitation in aqueous mixtures without the use of a solubilising agent (Fig. 1). Our method enables rapid monitoring of fluoride ions in solution and in a test-paper assay.
To test the validity of a Raman-based approach to detect fluoride ions, we chose to study the fluoride-mediated desilylation of alkyne groups as a model reaction.29 Raman spectroscopy is well-suited to temporal monitoring of chemical reactions in a non-destructive manner,30 in particular, for reactions of alkynes.31 The cleavage of Si–C alkyne bonds by fluoride is rapid and irreversible owing to the significant differences in the bond dissociation energies (Si–C ∼290 kJ mol−1; Si–F ∼590 kJ mol−1).15 We designed 4-((trimethylsilyl)ethynyl)benzonitrile 1 as an extremely simple molecular Raman sensor, which incorporated two bioorthogonal Raman groups and was readily synthesised in high yield (95%) in a single step from commercially available starting materials (see ESI† for details). The reaction of 1 (50 mM) with tetra-n-butylammonium fluoride (TBAF, 1 equiv.) in THF resulted in complete desilylation of the alkyne group within 1 minute (thus forming compound 2), and resulted in a red-shifting of the alkyne stretching frequency from 2162 cm−1 to 2109 cm−1 (Fig. 2). This observation is consistent with the Raman spectra of the neat compounds (Fig. S2, ESI†).
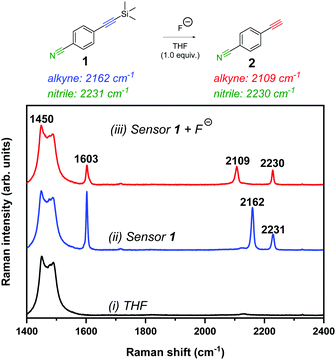 | ||
Fig. 2 Raman spectral analysis of the desilylation reaction of sensor 1. Raman spectrum of (i) THF solvent, (ii) sensor 1 (50 mM) in THF and (iii) sensor 1 (50 mM) + TBAF (50 mM) in THF (spectrum acquired <1 min after TBAF addition). Spectra were acquired using 785 nm laser excitation for 10 s with a 5× objective lens (∼180 mW). The spectra are normalised to the intensity of the peak at 1450 cm−1. The following peak annotations have been included for clarity: 1450 cm−1 (THF CH2 def.);32 1603 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C phenyl); 2109 cm−1 (C C phenyl); 2109 cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C of desilylated compound, 2); 2162 cm−1 (C C of desilylated compound, 2); 2162 cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, sensor 1) and 2230/2231 cm−1 (C C, sensor 1) and 2230/2231 cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N; sensor 1 and compound 2). N; sensor 1 and compound 2). | ||
We proposed that, based on the 53 cm−1 shift in the alkyne frequency, ratiometric sensing could be achieved to confirm the presence of fluoride ions in solution. Furthermore, upon the removal of the silyl group, there was a marginal shift in the nitrile peak (2231 cm−1 → 2230 cm−1) which offers a second motif for ratiometric sensing. In addition, the relative intensity of the phenyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak (∼1600 cm−1) decreased upon desilylation, which is consistent with the relative peak intensities in the Raman spectra of the solid samples. To demonstrate the versatility of this approach, we showed that desilylation of sensor 1 was also successful using inorganic fluoride sources, including NaF and CsF in a THF
C peak (∼1600 cm−1) decreased upon desilylation, which is consistent with the relative peak intensities in the Raman spectra of the solid samples. To demonstrate the versatility of this approach, we showed that desilylation of sensor 1 was also successful using inorganic fluoride sources, including NaF and CsF in a THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) mixture (Fig. S3, ESI†).
1 v/v) mixture (Fig. S3, ESI†).
A series of control reactions were followed by Raman spectroscopy and validated using UV-visible spectrometry. Fig. S4A demonstrates the control reaction of sensor 1 in the absence of fluoride which showed no change in the Raman spectra over 30 min. In addition, no reaction was observed upon addition of a range of different TBA+ and Na+ counter anions (Fig. S4B–D (ESI†) respectively). The sensor is, however, unstable at high pH (>pH 10, Fig. S4E, ESI†) and in the presence of NaI, which is consistent with observations of previous sensors of this type.20 Fig. S5A (ESI†) shows that the detection limit of sensor 1 in solution using Raman spectroscopy is ∼250 μM, enabling sensitive detection of fluoride in solution (Fig. S5B, ESI†), whilst Fig. S5C (ESI†) shows the reaction proceeds in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phosphate buffer (1
phosphate buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) mixture. UV-visible spectrometry showed a reduction of the λmax at ∼280 nm upon addition of fluoride, which is in close agreement with related trimethylsilyl-protected alkyne sensors (Fig. S6, ESI†).20 Together, these data indicated the potential for extremely rapid and sensitive detection of fluoride in organic solution using Raman spectroscopy.
1 v/v) mixture. UV-visible spectrometry showed a reduction of the λmax at ∼280 nm upon addition of fluoride, which is in close agreement with related trimethylsilyl-protected alkyne sensors (Fig. S6, ESI†).20 Together, these data indicated the potential for extremely rapid and sensitive detection of fluoride in organic solution using Raman spectroscopy.
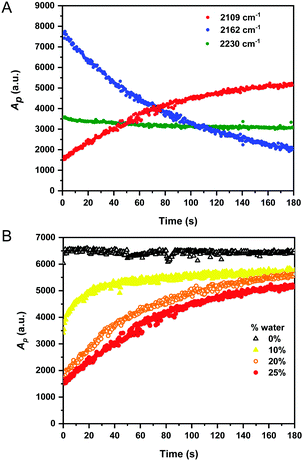
It is desirable to detect fluoride in aqueous solutions for use in analysis of groundwater and drinking water samples, although the majority of fluorescent sensors have only been examined in neat organic medium.28 Water is only weakly Raman active, and therefore, generates minimal interference in Raman analysis.1 Having established the detection of fluoride anions by 1 in organic solvents, we examined our system in aqueous mixtures. We repeated the reaction monitoring experiments with sensor 1 (50 mM) using an increased [TBAF] (75 mM, 1.5 equiv.) to compensate for the high enthalpy of hydration of fluoride in water (ΔH = −504 kJ mol−1).33 Upon addition of excess fluoride to the reaction mixtures, Raman spectra were recorded every 0.5 s for rapid detection. The peak intensities of the starting material (2162 cm−1), the desilylated product (2109 cm−1) and the nitrile band (2230–2231 cm−1) were plotted as a function of time as the water content of the mixture was varied from 0–25% (v/v). Fig. 3A shows a representative example of a reaction conducted in 25% water in THF and indicates the desilylation reaction was extremely rapid, with the formation of desilylated product over the course of the experiment (∼90% completion at 180 s). Similar plots for the other conditions are provided in Fig. S7 (ESI†). Interestingly, the peak area for the nitrile band at ∼2231 cm−1 also varied throughout the reaction time course, confirming that the desilylation of the alkyne group significantly influenced the electronic properties of the benzonitrile group. Fig. 3B presents the integrated peak areas for the desilylated product at 2109 cm−1 as a function of time when the reaction was performed using different initial % water. As the water content of the reaction mixture increased, the rate of product formation decreased. This was likely due to the hydration enthalpy of fluoride in water.33 In spite of this, these results indicate an extremely fast response time (<20 s) for fluoride detection in aqueous–organic mixtures which compares favourably to most fluorescent sensors.
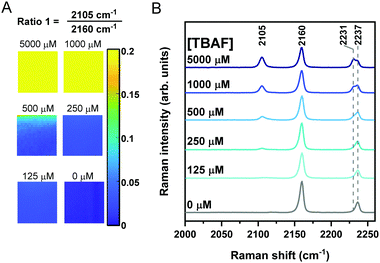
We next sought to assess the application of Raman sensor 1 on different materials to demonstrate the versatility of the approach. Recently, the use of paper-based substrates for fluoride sensing with a fluorescent probe has been reported.34 To demonstrate the application of Raman spectroscopy in this area, we pre-treated filter paper test strips with sensor 1 (10 μL of 100 mM solution in THF) which were air-dried for 5 minutes before a 300 μL solution of TBAF in THF (0.125–5 mM) was applied directly onto the paper. Raman maps (20 μm × 20 μm; 400 individual spectra) were acquired across the sample test strip after drying (∼2 minutes). Representative Raman maps and average Raman spectra from the maps are presented in Fig. 4. As the fluoride concentration increased, so too the ratio of 2105/2160 cm−1 (alkyne 2/alkyne 1) increased, and the ratio 2231/2237 cm−1 (nitrile 2/nitrile 1) arising from the benzonitrile group also increased (ratiometric images provided in Fig. S8 (ESI†) and additional repeat in Fig. S9, ESI†). Interestingly, the Raman shift of the alkyne and nitrile group of the sensor 1 were slightly shifted when analysed on paper (2160 and 2237 cm−1) when compared to solution phase analysis (2162 and 2231 cm−1), potentially due to differences in local intramolecular bonding in solution compared to solid-phase. Using this simple ratiometric approach, fluoride detection at concentrations as low as 125 μM could be achieved (Fig. 4 and Fig. S8, ESI†) which compares favourably to a recently reported colorimetric sensor (5.75 mg L−1, ∼300 μM in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v EtOH
7 v/v EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water)35 and a coordination-based sensor (300 μM in aqueous buffer).36 In addition, we found that our low-cost paper-based sensing system was compatible with handheld, portable Raman detection (Fig. S10, ESI†), which demonstrates a potential translation of this method for in-field fluoride analysis. Together, these results highlight ratiometric Raman spectroscopy as a novel detection method for fluoride ions in solution using a simple paper-based assay format.
water)35 and a coordination-based sensor (300 μM in aqueous buffer).36 In addition, we found that our low-cost paper-based sensing system was compatible with handheld, portable Raman detection (Fig. S10, ESI†), which demonstrates a potential translation of this method for in-field fluoride analysis. Together, these results highlight ratiometric Raman spectroscopy as a novel detection method for fluoride ions in solution using a simple paper-based assay format.
Compound 1 represents an extremely low molecular weight chemical sensor for fluoride detection which works using NIR excitation for ratiometric Raman spectroscopy. Advantages of NIR excitation are biological compatibility and operator safety, particularly given that many fluorescent sensors require cell-damaging UV excitation.15 The narrow resonances of Raman peaks (∼40 cm−1) enables clear, unambiguous and fully resolved detection of the starting material and product in a single Raman spectral acquisition. This represents a significant advantage to the “turn-on” fluorescent probes which are currently reported, as typically only the desilylated product generates a fluorescent signal. Furthermore, the narrow resonances of Raman shifts are advantageous over ratiometric fluorescent sensors, which typically have broad emission profiles (∼1500 cm−1), imposing a limit on the multiplexing capability of the sensing system.37
The detection of the alkyne sensor in solution and on test paper strips highlights the versatility of Raman spectroscopy for small-molecule sensor detection. Our results indicate that fluoride concentrations as low as 125 μM generate a ratiometric output in our simple paper-based assay. This is below the level at which biological toxicity was observed in cells (extracellular [NaF] = 3 mM)38 and is within the maximum contaminant level defined by the U.S. Environmental Protection Agency (4.0 mg L−1, 211 μM).36 We anticipate that the limit of detection of our sensing method could be improved by increasing the Raman scattering intensity of the alkyne sensor through conjugation to form a poly-yne,2 whilst different silicon capping groups could be investigated as alternative sensing motifs for improved pH stability in alkaline solutions.20
We thank the University of Strathclyde, the EPSRC and GlaxoSmithKline for financial support. The research data associated with this paper will become available from the University of Strathclyde at the following link: https://doi.org/10.15129/46582865-502a-43be-907f-bdc41032be1e.
Conflicts of interest
There are no conflicts to declare.Notes and references
- W. J. Tipping, M. Lee, A. Serrels, V. G. Brunton and A. N. Hulme, Chem. Soc. Rev., 2016, 45, 2075–2089 RSC.
- H. Yamakoshi, K. Dodo, A. Palonpon, J. Ando, K. Fujita, S. Kawata and M. Sodeoka, J. Am. Chem. Soc., 2012, 134, 20681–20689 CrossRef CAS.
- A. F. Palonpon, M. Sodeoka and K. Fujita, Curr. Opin. Chem. Biol., 2013, 17, 708–715 CrossRef CAS.
- H. Yamakoshi, K. Dodo, M. Okada, J. Ando, A. Palonpon, K. Fujita, S. Kawata and M. Sodeoka, J. Am. Chem. Soc., 2011, 133, 6102–6105 CrossRef CAS.
- S. Hong, T. Chen, Y. Zhu, A. Li, Y. Huang and X. Chen, Angew. Chem., Int. Ed., 2014, 53, 5827–5831 CrossRef CAS.
- L. Wei, F. Hu, Y. Shen, Z. Chen, Y. Yu, C.-C. Lin, M. C. Wang and W. Min, Nat. Methods, 2014, 11, 410–412 CrossRef CAS.
- M. M. Gaschler, F. Hu, H. Feng, A. Linkermann, W. Min and B. R. Stockwell, ACS Chem. Biol., 2018, 13, 1013–1020 CrossRef CAS.
- W. J. Tipping, M. Lee, A. Serrels, V. G. Brunton and A. N. Hulme, Chem. Sci., 2017, 8, 5606–5615 RSC.
- J. Seidel, Y. Miao, W. Porterfield, W. Cai, X. Zhu, S.-J. Kim, F. Hu, S. Bhattarai-Kline, W. Min and W. Zhang, Chem. Commun., 2019, 55, 9379–9382 RSC.
- L. E. Jamieson, J. Greaves, J. A. McLellan, K. R. Munro, N. C. O. Tomkinson, L. H. Chamberlain, K. Faulds and D. Graham, Spectrochim. Acta, Part A, 2018, 197, 30–36 CrossRef CAS.
- C. Zeng, F. Hu, R. Long and W. Min, Analyst, 2018, 143, 4844–4848 RSC.
- L. T. Wilson, W. J. Tipping, L. E. Jamieson, C. Wetherill, Z. Henley, K. Faulds, D. Graham, S. P. Mackay and N. C. O. Tomkinson, Analyst, 2020, 145, 5289–5298 RSC.
- X. Huang, J. Song, B. C. Yung, X. Huang, Y. Xiong and X. Chen, Chem. Soc. Rev., 2018, 47, 2873–2920 RSC.
- L. E. Jamieson, C. Wetherill, K. Faulds and D. Graham, Chem. Sci., 2018, 9, 6935–6943 RSC.
- Y. Zhou, J. F. Zhang and J. Yoon, Chem. Rev., 2014, 114, 5511–5571 CrossRef CAS.
- Y. Jiao, B. Zhu, J. Chen and X. Duan, Theranostics, 2015, 5, 173–187 CrossRef CAS.
- S. Y. Kim, J. Park, M. Koh, S. B. Park and J.-I. Hong, Chem. Commun., 2009, 4735–4737 RSC.
- D. Kim, S. Singha, T. Wang, E. Seo, J. H. Lee, S.-J. Lee, K. H. Kim and K. H. Ahn, Chem. Commun., 2012, 48, 10243–10245 RSC.
- A. Roy, D. Kand, T. Saha and P. Talukdar, Chem. Commun., 2014, 50, 5510–5513 RSC.
- M. Jo, J. Lim and O. Š. Miljanić, Org. Lett., 2013, 15, 3518–3521 CrossRef CAS.
- L. Fu, F.-L. Jiang, D. Fortin, P. D. Harvey and Y. Liu, Chem. Commun., 2011, 47, 5503–5505 RSC.
- H. Lu, Q. Wang, Z. Li, G. Lai, J. Jiang and Z. Shen, Org. Biomol. Chem., 2011, 9, 4558–4562 RSC.
- D. Buckland, S. V. Bhosale and S. J. Langford, Tetrahedron Lett., 2011, 52, 1990–1992 CrossRef CAS.
- M. R. Rao, S. M. Mobin and M. Ravikanth, Tetrahedron, 2010, 66, 1728–1734 CrossRef CAS.
- L. Fu, F.-F. Tian, L. Lai, Y. Liu, P. D. Harvey and F.-L. Jiang, Sens. Actuators, B, 2014, 193, 701–707 CrossRef CAS.
- M. Kaur, M. J. Cho and D. H. Choi, Dyes Pigm., 2014, 103, 154–160 CrossRef CAS.
- P. Chen, W. Bai and Y. Bao, J. Mater. Chem. C, 2019, 7, 11731–11746 RSC.
- L. Gai, J. Mack, H. Lu, T. Nyokong, Z. Li, N. Kobayashi and Z. Shen, Coord. Chem. Rev., 2015, 285, 24–51 CrossRef CAS.
- G. L. Larson, Synthesis, 2018, 2433–2462 CAS.
- N. E. Leadbeater and J. R. Schmink, Nat. Protoc., 2007, 3, 1 Search PubMed.
- W. J. Tipping, M. Lee, V. G. Brunton, G. C. Lloyd-Jones and A. N. Hulme, Faraday Discuss., 2019, 220, 71–85 RSC.
- H. F. Shurvell and M. C. Southby, Vib. Spectrosc., 1997, 15, 137–146 CrossRef CAS.
- L. Trembleau, T. A. D. Smith and M. H. Abdelrahman, Chem. Commun., 2013, 49, 5850–5852 RSC.
- X. Wu, H. Wang, S. Yang, H. Tian, Y. Liu and B. Sun, ACS Omega, 2019, 4, 4918–4926 CrossRef CAS.
- L. C. Murfin, K. Chiang, G. T. Williams, C. L. Lyall, A. T. A. Jenkins, J. Wenk, T. D. James and S. E. Lewis, Front. Chem., 2020, 8, 10 CrossRef CAS.
- S. Rochat and K. Severin, Chem. Commun., 2011, 47, 4391–4393 RSC.
- L. Wei, Z. Chen, L. Shi, R. Long, A. V. Anzalone, L. Zhang, F. Hu, R. Yuste, V. W. Cornish and W. Min, Nature, 2017, 544, 465 CrossRef CAS.
- H. Matsui, M. Morimoto, K. Horimoto and Y. Nishimura, Toxicol. In Vitro, 2007, 21, 1113–1120 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1–S10 (ESI). See DOI: 10.1039/d0cc05939k |
| ‡ Present address: Technische Universität Braunschweig, Institut für Technische Chemie, Franz-Liszt-Str. 35a, 38106 Braunschweig, Germany. |
| This journal is © The Royal Society of Chemistry 2020 |