Profile and source apportionment of volatile organic compounds from a complex industrial park†
Yuan
Liu
a,
Qing
Xie
a,
Xuehua
Li
 a,
Fulin
Tian
b,
Xianliang
Qiao
a,
Fulin
Tian
b,
Xianliang
Qiao
 *a,
Jingwen
Chen
*a,
Jingwen
Chen
 a and
Wenwen
Ding
a
a and
Wenwen
Ding
a
aKey Laboratory of Industrial Ecology and Environmental Engineering (MOE), School of Environmental Science and Technology, Dalian University of Technology, Dalian 116024, China. E-mail: xlqiao@dlut.edu.cn; Tel: +86-411-84707844
bLiaoning Academy of Analytical Sciences, Shenyang 110015, China
First published on 23rd November 2018
Abstract
Industrial emissions, mainly from industrial parks, are important sources of ambient volatile organic compounds (VOCs). Identification of the major sources of VOCs from industrial parks has practical significance in emission reduction. In this study, the major species of VOCs from a residential area located downwind of a complex industrial park were sampled with Tenax absorption tubes and analyzed by thermal desorption coupled with gas chromatography/mass spectrometry (TD-GC/MS). Receptor models of factor analysis with nonnegative constraints (FA-NNC) and positive matrix factorization (PMF) were employed to recognize the potential emission sources, which suggested an association with the production processes in the nearby industrial park. In order to validate the sources, the profiles of VOC emissions of related workshops under actual manufacturing processes were acquired. It was found that xylenes & amines, phenols and esters were the major species of VOCs for the workshops of foundry, refractory materials and printing, respectively. Similarity analysis indicated that the detected profiles of VOC emissions from the dominant industrial types had good correlations with the identified factors from receptor models. Source contributions to VOCs in the receptor region exhibited that foundry production was the primary contributor (56–64%), followed by refractory material production (22–26%) and printing (14–18%). This study provides a strategy for source apportionment of VOCs from a local complex industrial park, which is helpful in the development of targeted control strategies.
Environmental significanceIn recent decades, thousands of industrial parks have widely been extended in China, and the emission of VOCs from industrial processes is regarded as the main anthropogenic source. Identification of the major sources of VOCs in industrial parks has practical significance for the development of targeted control strategies. However, source apportionment of VOC emissions in industrial parks is generally challenging because of the interactions of different production processes. In this study, the main sources of VOCs in an industrial park were identified by using receptor models and were further validated by in situ sampling in factories under actual manufacturing processes. Our findings provide a strategy for source apportionment of VOC emissions from a local industrial park, which also offer a case study of the VOC issue of thousands of industrial parks in China. |
1. Introduction
Volatile organic compounds (VOCs) play an important role in ambient air quality, which act as precursors of ozone, organic aerosols, etc. through complex photochemical cycles.1,2 VOCs are of high concern because many VOCs have been found to have adverse effects on public health, such as the carcinogenicity of benzene and 1,3-butadiene.3,4 With the rapid development of industrial parks in recent decades, industrial emissions became the dominant sources for VOC emissions in China. Zheng et al. estimated that industrial VOC emissions in China increased from 15.3 Tg in 2011 to 29.4 Tg in 2013 at an annual average growth rate of 38.3%.5 Compared with that of a single enterprise, the issue of VOC emissions for an industrial park is more complex because of the coexistence of different industrial types, which discharge various species of VOCs.6,7 Characterization of emission profiles and identification of source-specific contributions of VOCs are of great significance for the development of targeted and effective control strategies for VOC emissions from industrial parks. Zheng et al. reported that there were four pathways of industrial VOC emissions,5 including (1) production of VOCs, (2) storage and transport, (3) industrial processes using VOCs as raw materials, and (4) use of VOC-containing products. Among them, the production of VOCs accounted for the largest contribution.Characteristic VOC species related to several industrial processes have been identified by previous investigations. Zheng et al. examined sector-based emission profiles of VOCs from printing, shoemaking and coating industries, and they described that ethyl acetate and butyl acetate were the major species from printing.7 Chiu et al. reported that 2-propanol, acetone and benzene were the major chemicals from semiconductor production.8 Wei et al. reported that alkanes, alkenes, and aromatic groups were present in ambient air discharged from a petroleum refinery.9 Pan et al. mentioned that halogenated compounds were the dominant VOCs from a biopharmaceutical company.10 In a complex industrial park, the species of VOCs are supposed to be more diverse because of the contributions from different manufacturing processes,11–13 which give rise of challenges in the clarification of emission sources.
In order to quantitatively apportion the contribution of emissions, source apportionment has been applied in the investigating of air pollution issues associated with VOCs,14 semi-volatile organic compounds (SVOCs),15 particles16 and organic aerosols.17 Generally, three types of models including the dispersion model, inventory model and receptor model are widely employed in source apportionment of air pollutants. Based on source characteristics and meteorological parameters, the time-resolved distribution of VOCs in impacted areas could be estimated by using the dispersion model.18,19 However, the performance of the dispersion model depends strongly on the accuracy of the source characteristics. The local atmospheric circulation or topographic conditions could increase the uncertainty of the modeling output.20 With the database of profiles and emission factors, the inventory model was adopted for the estimation of the total amount of VOC emissions discharged into the atmosphere.21,22 The accuracy of the emission inventories is highly dependent on the comprehensiveness of inventory and emission factors from different industrial processes. Receptor models can be used to investigate the contribution of different sources if source characteristics or emission factors are deficient.23,24
With the advantage of minimum requirement of source characteristics, meteorological conditions and other related information, multivariate receptor models, such as positive matrix factorization (PMF) and factor analysis with nonnegative constraints (FA-NNC), have been applied in source identification of VOCs and SVOCs. By using the PMF model, Yan et al. found that alkanes were the dominant group of VOCs accounting for over 50% of the total concentration in a thermal power station centralized area.25 Wei et al. proposed that catalytic cracking units acted as the most important source of VOCs from a petroleum refinery.26 As one of the advanced receptor models, the FA-NNC model was successfully applied to confirm that coal-fired emission was the main source of PAHs in Dalian.27 To date, source apportionment of VOCs using receptor models in complex industrial parks with various production sectors has rarely been reported.
Besides the typical industries of VOC emissions (e.g. painting industry, petrochemical industry, etc.), several industrial processes that emit VOCs raise further concerns. In recent years, the metal casting industry and refractory materials industry have grown rapidly in China, accounting for 45% and 69% of world production, respectively.28,29 To date, several studies have investigated VOC emissions from the metal casting industry, and the pyrolysis of organic casting materials (e.g., organic binders and additives) during the casting process was regarded as the main source with phenols, benzene, toluene and xylenes being the prominent species.30–33 Organic binders and thermal process are also applied in the refractory materials industry, whereas the corresponding emissions of VOCs have been seldom reported.34 According to previous studies, VOC emissions have been observed to be highly process-specific from the surface coating industry and petrochemical facilities.35,36 Considering the differences in the type of organic binder and heating temperature for the casting industry or refractory materials industry, VOC emissions might vary with the same manufacturing processes and operating conditions. Thus, the investigation of emission profiles of VOCs from actual manufacturing workshops of casting and refractory materials is very meaningful.
A complex industrial park, containing foundry, refractory materials and printing industries, was chosen in this study to investigate the emissions of VOCs. Air samples from an adjacent residential region located in the downwind direction of this park in the summer season were analyzed meanwhile. Two multivariate receptor models (FA-NNC and PMF) were employed to identify VOC sources and to apportion related contributions to the residential region. The contributions from different sources were validated by characterizing the profiles of VOCs emitted from workshops under actual manufacturing processes.
2. Materials and methods
2.1 Sample collection and measurement
Thirty VOC species (J&K Scientific Ltd, Table S1†) were selected in this study based on the data from previous reports30–33 and pre-examination of VOCs in the adjacent residential area of the industrial park. Considering the scale of the workshops, production processes, working periods and other related factors, the preferential sampling regions are shown in Fig. 1 to present the major production types of various workshops. Thirty samples were collected from the residential area and at least five samples were acquired from each workshop to examine the emission profiles of VOCs.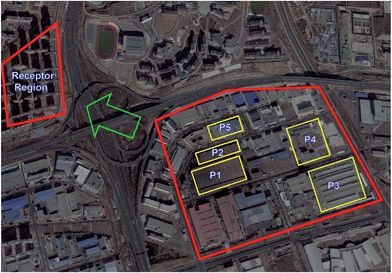 | ||
| Fig. 1 The position of the industrial park and downwind residential region. The wind direction is indicated by the green arrow. | ||
The air samples were collected using absorption tubes filled with Tenax equipped with an air pump at a flow rate of 0.2 L min−1 for 60 min during August to September, 2017. The sampling inlet was set up at 1.5 m above the ground level. All sampling tubes were cleaned in a thermal conditioner at 300 °C for 30 min and sealed with Teflon caps and stored in a Zip-lock bag at 0 °C before use. The Zip-lock bag was filled with silica gel desiccant and activated carbon to avoid passive absorption of water vapor and contaminants.
The sampling tubes were analyzed using a Markes Series 2 Unity thermal desorption unit coupled with an Agilent 7890A gas chromatograph with a 5975C mass spectrometer (TD-GC/MS). In brief, 5 μL of internal standard was added into the sampling tubes, and pre-purged with 100 mL min−1 high purity nitrogen for 1 min. The sampling tubes were firstly desorbed at 280 °C for 10 min. The cold trap was set at −20 °C, and then it was rapidly heated to 310 °C with the secondary desorption maintained for 10 min. The samples were transferred into the GC/MS system through a heated transfer line at 200 °C. A DB-624 GC column (60 m × 0.32 mm × 1.8 μm) was adopted with helium as the carrier at a flow rate of 2 mL min−1. The temperature of the oven was programmed at 40 °C initially, which was held for 5 min and then raised to 250 °C at 10°C min−1 holding for 4 min and later to 260 °C at 10 °C min−1, where it was held for 10 min. The MS was used in electron impact (EI, 70 eV) mode with a source temperature of 230 °C, and the mass fragment scan was from 35 to 400 Da with full scan mode.
The calibration curves were prepared by injecting the standard solutions into Tenax tubes, and the spiked adsorbent tubes were thermally desorbed under the same conditions as the samples.37 A five-point calibration curve was obtained with a linearity >0.98 for each quantified VOC. Following the guidelines of the Compendium Method TO-17 (U.S. EPA),38 the method detection limit (MDL) was calculated as the product of standard deviation (seven replicates of the lowest calibration point) and the Student's t-value at the 99% confidence level (t = 3.14 for the seven replicates). The values of MDL ranged from 1.48 to 24.5 ng m−3 for targeted species based on 12 L sampling volume. The average sampling efficiency for targeted species was 83%, indicating good performance in sampling.37 No major contaminants were detected in the laboratory blank samples. The quantitative parameters of VOCs in TD-GC/MS are shown in Tables S1 and S2.†
2.2 Source apportionment
To accomplish a source-specific analysis, source apportionment studies are conducted using multivariate receptor models based on the mass conservation principle.39–41 The equation can be written using eqn (1),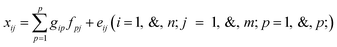 | (1) |
2.3 Uncertainty of the receptor model
Monte Carlo simulation was used to determine the uncertainties of the receptor model output according to eqn (2),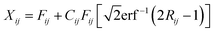 | (2) |
3. Results and discussion
3.1 Characteristics of ambient VOCs in the receptor region
The levels of VOC species in the receptor region are shown in Fig. 2a. The levels of individual species ranged from not detected to 19.8 μg m−3. m/p-Xylene, toluene, butyl acetate, phenol, benzene and ethyl acetate were abundant species, which accounted for 61% of total VOCs (TVOCs). The percentages of different VOC groups varied in the samples from the receptor region (Fig. 2b). On average, aromatics accounted for 46% of TVOCs, followed by oxygen-containing VOCs (OVOCs) (41%), alkanes (10%), and nitrogen-containing VOC (NVOC) (3%).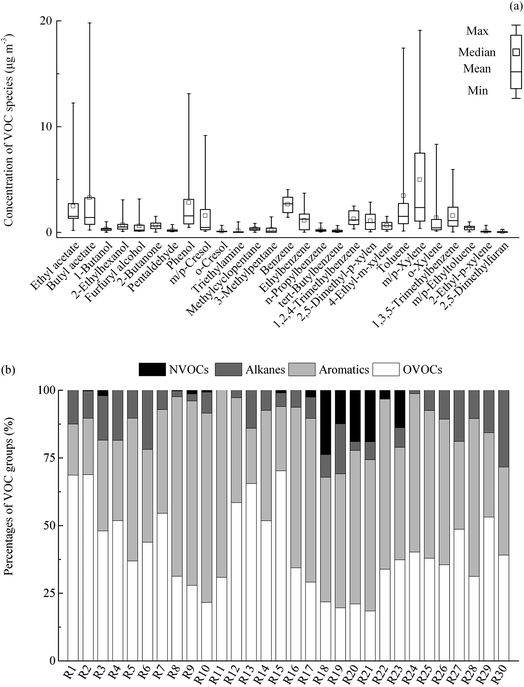 | ||
| Fig. 2 Boxplot of VOC species (a) and percentages of VOC groups (b) in the receptor region. NVOCs as nitrogen-containing VOCs and OVOCs as oxygen-containing VOCs. | ||
The characteristics of ambient VOCs in this study were roughly compared with those in previous studies of receptor regions associated with industrial emission sources. The combustion of fossil fuels and industrial exhaust were described as important contributors to the atmospheric levels of BTEX (benzene, toluene, ethylbenzene, and xylenes).45 Butyl acetate and ethyl acetate as raw materials were usually used in the printing and coating industry with detection rates of 84% and 70%, respectively in related air samples.46 Zhang et al. reported that alkanes were the most abundant VOC species from a petroleum refinery.47 Hsu et al. reported that carbonyls were the largest component of TVOCs from a petrochemical facilities.6 Due to the diverse VOC species examined in this study, relatively complex emission sources were expected, and the contributions of different sources needed to be clarified. According to an in situ survey in enterprises, the composition of VOCs in the receptor region was assumed to be related to the manufacturing processes in the adjacent industrial park.
3.2 Source apportionment of the receptor region
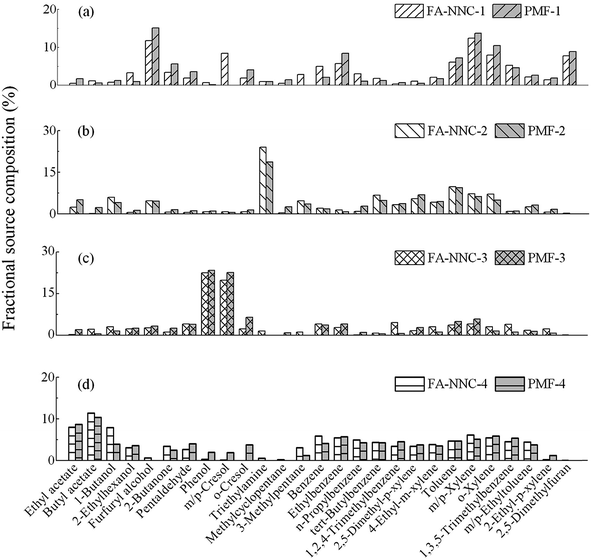
Due to lack of data for source characteristics, meteorological conditions and the complexity of topographic conditions (e.g. blocking by high rise buildings), it was difficult to apply a dispersion model in this study. An inventory model was not applicable either because of the deficiency of emission profiles and factors of VOCs from the potential sources. Receptor models were employed to quantitatively identify the potential emission sources and contributions in this receptor region. Considering the misinterpretation from negative results in the traditional FA model,27,39 the FA-NNC model was applied to identify the potential sources and contributions in this study. Four factors were finally selected in the FA-NNC model based on the performance of coefficient of determination, cumulative percent variance and Exner function (Table S3†). The same number of factors was set for the PMF model to facilitate comparison.In spite of the difference between the FA-NNC model and PMF model, a relatively similar distribution of factor loadings was obtained (Fig. 3), which indicated that there were several distinctive sources for the VOCs in the receptor region. Generally, the predominant species with high loadings on different calculated factors from the receptor model were applied to characterize potential emission sources.39,48 The first factor of the FA-NNC model and PMF model (abbreviated to NNC-1/PMF-1) was characterized with furfuryl alcohol, xylenes and dimethylfuran. Xylenes were reported from residential areas,37,49 whereas furfuryl alcohol and dimethylfuran were seldom reported in related studies. Triethylamine as one kind of NVOC was seldom reported in common VOC emissions, which had obvious loading in NNC-2/PMF-2. Phenol and m/p-cresol was mainly weighted in NNC-3/PMF-3. Ethyl acetate and butyl acetate related to the printing and coating industry had an influence on NNC-4/PMF-4. The calculated contributions of each factors from the FA-NNC model and PMF model were 29–35%, 27–29%, 22–26% and 14–18% respectively (Table 1). Due to the downwind location of the receptor region, we presumed that the identified potential sources were related to the actual manufacturing processes in the complex industrial park. Thus, a further validation of the output of source apportionment with in situ monitor data from actual emission sources was conducted to support local VOC emission control and reduction.
| Emission sources | Model factors of FA-NNC | Model factors of PMF | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 1 | 2 | 4 | 3 | 1 | 2 | 4 | |
| P1 | 0.30 | 0.82 | 0.42 | 0.42 | 0.27 | 0.84 | 0.43 | 0.39 |
| P2 | 0.11 | 0.11 | 0.81 | 0.10 | 0.04 | 0.10 | 0.75 | 0.07 |
| P3 | 0.89 | 0.29 | 0.15 | 0.15 | 0.88 | 0.16 | 0.17 | 0.25 |
| P4 | 0.59 | 0.12 | 0.08 | 0.08 | 0.72 | 0.09 | 0.10 | 0.16 |
| P5 | 0.09 | 0.06 | 0.06 | 0.59 | 0.06 | 0.06 | 0.19 | 0.60 |
| Identified sources | P3/P4 | P1 | P2 | P5 | P3/P4 | P1 | P2 | P5 |
| Source contributions | 22% | 35% | 29% | 14% | 26% | 29% | 27% | 18% |
3.3 Profiles of VOC emissions from the complex industrial park
Generally, the emissions of industrial VOCs could be affected by a number of factors, such as raw materials, manufacturing techniques, product types, etc. According to the investigation of enterprises located in this complex industrial park, the main industrial processes were classified into three types: foundry, refractory materials and printing industry. According to the main procedure of foundry, there were sand and core molding, smelted metal pouring, sand recovery, coating, etc. Several organic binders (e.g. furan resin and phenolic urethanes) and additives (e.g. xylenesulfonic acid and triethylamine) were commonly used as raw materials in the casting industry to cross-link and solidify shaped casting molds. The hot melted metal contacted molds directly after pouring operation, and then the temperature at the metal–mold interface was increased sharply to above 1000 °C, during which VOCs were released simultaneously. Based on the demonstration-scale study by Wang et al.,31,33 pyrolysis of cross-linked casting models was ascribed as the main process for VOC generation.To date, VOC emissions from the production of magnesia-refractory raw materials have been rarely examined, though CO2 emission have been reported in previous studies.34,50 VOC emissions were expected to occur during heat-treatment of bricks or shaped refractory products, since organic binders (e.g. phenolic resin) were applied for consolidation mixed with oxides of aluminium, silicon and magnesium. For example, approximately 200 °C and 1600 °C were required in consolidated processes of unburned and burned bricks, respectively. Printing production as a traditional industry with VOC emissions demonstrated a distinctive profile related to special production processes. Zheng et al. reported that benzene and toluene were the major species in letterpress printing, while ethyl acetate and isopropyl alcohol were exhibited abundantly in offset and gravure printing.7 For various raw material consumption, Wang et al. reported that esters were predominant in water-based paints (WBPs), whereas BTEX was widely present in solvent-based paints.46 Gravure printing processes and WBPs were employed by printing enterprises in this park.
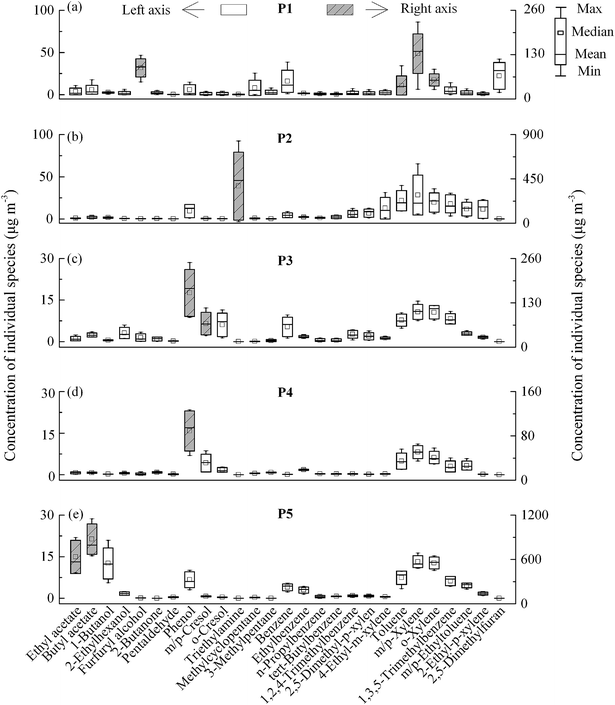
Based on site investigation of actual manufacturing process related VOC discharge, five production processes were labeled P1 (furan no-bake mold system from foundry production with furan resin and xylenesulfonic acid used as organic binders and additives), P2 (cold-box system from foundry production with phenolic urethanes and triethylamine used as binders and additives), P3 (burned bricks with about 1600 °C from refractory material production), P4 (unburned bricks with about 200 °C from refractory material production), and P5 (printing production), respectively (Fig. 1). In order to validate the exact sources, air samples were collected from actual manufacturing workshops of the foundry, refractory materials, and printing industry. The VOC emissions from foundries were mainly generated from pyrolysis of organic casting materials (e.g. organic binders and additives that were used to make sand molds and cores) during the casting processes.31,33 The levels and composition of VOCs from samples collected under actual manufacturing processes are illustrated in Fig. 4. In general, the main groups of VOCs were associated with the employed production system. Aromatics (e.g. xylenes and toluene) were predominant in P1, while triethylamine was the primary species in P2. The results from our study, conducted under the actual production conditions, were largely consistent with the findings by Wang et al. in a lab simulation study.32 They also reported that xylenes predominated in VOC emissions from pyrolysis of the furan binder. The presence of furfuryl alcohol in P1 was associated with the usage of furan resin, which was validated by the analysis of the raw material of furan resin with GC/MS. 2,5-Dimethylfuran was regarded as another indicator of the furan casting process, which was detected in our study and Wang et al.32 Triethylamine was regarded as one of the typical species for the cold-box system employed in P2.
In addition, the levels of generated VOCs (e.g. xylenes and toluene) from the thermal decomposition were obvious different in these two employed systems between P1 and P2 (t-test, p < 0.05). Wang et al. proposed that xylenesulfonic acid was found to be the major source of xylene emissions from pyrolysis of casting materials.32 Zhang et al. reported that aromatic VOC emissions can be considerably decreased if xylenesulfonic acid was replaced by methanesulfonic acid in the pyrolysis of furan no-bake foundry binders.51 In this study, the relatively higher levels of xylenes and toluene from P1 are regarded to be related to xylenesulfonic acid used in the production based on our investigation.
For refractory material production in this park, phenolic resin and calcium lignosulphonate were used as organic binders. The max mold temperatures of 1600 °C and 200 °C were used in P3 and P4, respectively. In general, the distribution of VOC species was roughly similar between these two processes (Fig. 4c and d), whereas a sharp difference in temperature existed. The phenols from phenolic resin represented the largest proportion of TVOCs in refractory material production. Aromatics, which were assumed to be emitted from the pyrolysis of lignosulphonate, acted as the second contributor in TVOCs. In contrast to foundries, the levels of phenol in P3 & P4 were much higher than that in P1 & P2 (t-test, p < 0.05), though phenolic resin was utilized in both of these two factories. To our knowledge, it is the first investigation of VOC profiles from actual production processes of refractory materials. The production technique in P5 was gravure printing with WBPs. The concentrations of VOCs are shown in Fig. 4e, and butyl acetate and ethyl acetate made up a major fraction, which agreed with the results from the gravure printing process.7 In brief, raw materials (e.g. organic binders and additives) consumed within production workshops were the main sources of VOC emissions in the complex industrial park.
3.4 Validation of source apportionment
In order to validate the correlation between VOCs detected in emission sources and identified factors from receptor models, cosine similarity was tested. The calculated factor loadings from receptor models (F1 ∼ F4, Fig. 3) and in situ monitored profiles from this complex industrial park (P1 ∼ P5, Fig. S1†) were considered as multi-dimensional space vectors. The similarity value (cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) φ coefficient) was neatly bounded between 0 and 1, and a value closer to 1 indicated higher similarity.52 The detailed calculations are shown in the ESI.†
φ coefficient) was neatly bounded between 0 and 1, and a value closer to 1 indicated higher similarity.52 The detailed calculations are shown in the ESI.†
Table 1 shows that the actual emission sources from the complex industrial park had obvious similarity with those model results. For example, the emission sources of P1 (Fig. S1a†) had good association with the factor NNC-1/PMF-1 (Fig. 3a). Both of them had evident presentation with characteristic species of xylenes and furfuryl alcohol. P2 had obvious connection with the factor NNC-2/PMF-2 which was enriched with triethylamine (NVOC). The emission sources of P3 and P4 enriched with phenols matched with NNC-3/PMF-3, which could be explained by the similar profiles of the two refractory material industrial types. Ethyl acetate and butyl acetate had primary loadings on P5 and the factor NNC-4/PMF-4. The similarity analysis implicated that the calculated factors from receptor models could represent the profiles of VOC emissions from actual production workshops in this study. Correspondingly, based on the results of FA-NNC and PMF models, foundry production (P1&P2) could be regarded as the primary contributor (56–64%), followed by refractory material production (22–26%) and printing (14–18%) from the complex industrial park to the receptor region.
Source apportionment of VOCs from industrial or urban areas is usually challenging resulting from various interference. In this study, a newly constructed road with low traffic burden is located between the receptor region and the industrial park, which might increase the uncertainty of source apportionment. Previous studies related to the VOC emissions in a traffic-dominated environment of China have observed that alkanes are the most abundant groups (>60%) from TVOCs, and isopentane & propane are regarded as marker species for gasoline evaporation and liquefied petroleum gas, respectively.53,54 The characteristics of VOCs, except BTEX, in this industrial park are clearly different from those related to traffic emissions. Moreover, the levels of BTEX observed from factories in this study, which act as relatively continuous sources because of 24 hour production, are higher than those reported in the roadside environment with a heavy daily traffic frequency.53 The diagnostic ratio of benzene/toluene (1.77–3.22) has been observed as a traffic emission tracer,53,54 which is mainly out of the range from this study (Fig. S2†). Thus, traffic emission is not regarded as an important contributor to VOCs in the receptor region of this study.
Monte Carlo simulation was frequently utilized to test the uncertainty of the receptor model.44 With the aid of Monte Carlo simulation, Rachdawong et al. reported that the profiles of polychlorinated biphenyls predicted by the receptor model from sediments of an estuary were in agreement with the detected profiles in ref. 44. In this study, four in situ monitored emission sources (representing foundry, refractory material production, and printing) from the industrial park were used to generate ten artificial receptor matrices by Monte Carlo simulation. The matrices were further determined with receptor models to calculate the factor loadings. An average uncertainty level of ± 36% for the modeled sources (factor loadings) was acquired in this study (Fig. S3†). Considering the uncertainty of VOCs in analysis and various interference from the adjacent environment, the results of source apportionment in this study were generally reliable. Our findings provide a database for the development of targeted control strategies of VOC emissions from the local industrial park, and provide a case reference of source apportionment related to complex industrial parks widely distributed in China.
4. Conclusions
The distribution of VOCs in a residential area located downwind of a complex industrial park was examined in this study. Four potential VOC sources were identified by applying receptor models of FA-NNC and PMF. By sampling actual manufacturing processes of workshops, the profiles of major VOC emissions from this park were obtained. Xylenes & amine, phenols and esters were the major VOCs for foundry, refractory material and printing production of this industrial park, respectively. In brief, emissions from the pyrolysis of raw materials (e.g. organic binders and additives) consumed within the production workshops were the main sources of VOCs. Factors identified from the receptor models were well validated using the profiles of VOCs emitted from actual industrial processes with similarity analysis. The source contributions of VOCs to the receptor region exhibited that foundry production was the primary contributor (56–64%), followed by refractory material production (22–26%) and printing (14–18%) from the complex industrial park.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study was supported by the National Natural Science Foundation of China (21661142001) and Dalian Municipal Innovation Project “Chemicals Risk Prediction and Risk Reduction Technology” (2015R011).References
- B. C. McDonald, J. A. de Gouw, J. B. Gilman, S. H. Jathar, A. Akherati, C. D. Cappa, J. L. Jimenez, J. Lee-Taylor, P. L. Hayes, S. A. McKeen, Y. Y. Cui, S. W. Kim, D. R. Gentner, G. Isaacman-VanWertz, A. H. Goldstein, R. A. Harley, G. J. Frost, J. M. Roberts, T. B. Ryerson and M. Trainer, Volatile chemical products emerging as largest petrochemical source of urban organic emissions, Science, 2018, 359, 760–764, DOI:10.1126/science.aaq0524.
- R. Li, L. Cui, J. Li, A. Zhao, H. Fu, Y. Wu, L. Zhang, L. Kong and J. Chen, Spatial and temporal variation of particulate matter and gaseous pollutants in China during 2014-2016, Atmos. Environ., 2017, 161, 235–246, DOI:10.1016/j.atmosenv.2017.05.008.
- M. A. Mehlman, Dangerous properties of petroleum-refining products: carcinogenicity of motor fuels (gasoline), Teratog., Carcinog., Mutagen., 1990, 10, 399–408, DOI:10.1002/tcm.1770100505.
- R. Atkinson, Atmospheric chemistry of VOCs and NOx, Atmos. Environ., 2000, 34, 2063–2101, DOI:10.1016/S1352-2310(99)00460-4.
- C. Zheng, J. Shen, Y. Zhang, W. Huang, X. Zhu, X. Wu, L. Chen, X. Gao and K. Cen, Quantitative assessment of industrial VOC emissions in China: historical trend, spatial distribution, uncertainties, and projection, Atmos. Environ., 2017, 150, 116–125, DOI:10.1016/j.atmosenv.2016.11.023.
- C. Hsu, H. Chiang, R. Shie, C. Ku, T. Lin, M. Chen, N. Chen and Y. Chen, Ambient VOCs in residential areas near a large-scale petrochemical complex: spatiotemporal variation, source apportionment and health risk, Environ. Pollut., 2018, 240, 95–104, DOI:10.1016/j.envpol.2018.04.076.
- J. Zheng, Y. Yu, Z. Mo, Z. Zhang, X. Wang, S. Yin, K. Peng, Y. Yang, X. Feng and H. Cai, Industrial sector-based volatile organic compound (VOC) source profiles measured in manufacturing facilities in the Pearl River Delta, China, Sci. Total Environ., 2013, 456–457, 127–136, DOI:10.1016/j.scitotenv.2013.03.055.
- K. Chiu, B. Wu, C. Chang, U. Sree and J. Lo, Distribution of volatile organic compounds over a semiconductor industrial park in Taiwan, Environ. Sci. Technol., 2005, 39, 973–983, DOI:10.1021/es049110m.
- W. Wei, Z. Lv, G. Yang, S. Cheng, Y. Li and L. Wang, VOCs emission rate estimate for complicated industrial area source using an inverse-dispersion calculation method: a case study on a petroleum refinery in Northern China, Environ. Pollut., 2016, 218, 681–688, DOI:10.1016/j.envpol.2016.07.062.
- Y. Pan, Q. Liu, F. Liu, G. Qian and Z. Xu, Regional assessment of ambient volatile organic compounds from biopharmaceutical R&D complex, Sci. Total Environ., 2011, 409, 4289–4296, DOI:10.1016/j.scitotenv.2011.07.014.
- P. Shao, J. An, J. Xin, F. Wu, J. Wang, D. Ji and Y. Wang, Source apportionment of VOCs and the contribution to photochemical ozone formation during summer in the typical industrial area in the Yangtze River Delta, China, Atmos. Res., 2016, 176–177, 64–74, DOI:10.1016/j.atmosres.2016.02.015.
- Y. Xue, S. S. H. Ho, Y. Huang, B. Li, L. Wang, W. Dai, J. Cao and S. Lee, Source apportionment of VOCs and their impacts on surface ozone in an industry city of Baoji, Northwestern China, Sci. Rep., 2017, 7, 9979, DOI:10.1038/s41598-017-10631-4.
- C. Lan, Y. Huang, S. Ho and C. Peng, Volatile organic compound identification and characterization by PCA and mapping at a high-technology science park, Environ. Pollut., 2014, 193, 156–164, DOI:10.1016/j.envpol.2014.06.014.
- F. Wu, Y. Yu, J. Sun, J. Zhang, J. Wang, G. Tang and Y. Wang, Characteristics, source apportionment and reactivity of ambient volatile organic compounds at Dinghu Mountain in Guangdong Province, China, Sci. Total Environ., 2016, 548–549, 347–359, DOI:10.1016/j.scitotenv.2015.11.069.
- F. Wang, T. Lin, J. Feng, H. Fu and Z. Guo, Source apportionment of polycyclic aromatic hydrocarbons in PM 2.5 using positive matrix factorization modeling in Shanghai, China, Environ. Sci.: Processes Impacts, 2015, 17, 197–205, 10.1039/c4em00570h.
- Y. Huang, H. Shen, H. Chen, R. Wang, Y. Zhang, S. Su, Y. Chen, N. Lin, S. Zhuo, Q. Zhong, X. Wang, J. Liu, B. Li, W. Liu and S. Tao, Quantification of global primary emissions of PM 2.5, PM 10, and TSP from combustion and industrial process sources, Environ. Sci. Technol., 2014, 48, 13834–13843, DOI:10.1021/es503696k.
- Y. Li, J. Meng, J. Liu, Y. Xu, D. Guan, W. Tao, Y. Huang and S. Tao, Interprovincial reliance for improving air quality in China: a case study on black carbon aerosol, Environ. Sci. Technol., 2016, 50, 4118–4126, DOI:10.1021/acs.est.5b05989.
- J. H. C. Wang, C. Tsai and C. Chiang, Screening procedure for airborne pollutants emitted from a high-tech industrial complex in Taiwan, Chemosphere, 2015, 139, 268–275, DOI:10.1016/j.chemosphere.2015.06.035.
- L. Chen, F. Jeng, M. Chang and S. Yen, Rationalization of an odor monitoring system:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a case study of Lin-Yuan petrochemical park, Environ. Sci. Technol., 2000, 34, 1166–1173, DOI:10.1021/es990180g.
a case study of Lin-Yuan petrochemical park, Environ. Sci. Technol., 2000, 34, 1166–1173, DOI:10.1021/es990180g. - S. Squizzato, M. Masiol, F. Visin, A. Canal, G. Rampazzo and B. Pavoni, The PM 2.5 chemical composition in an industrial zone included in a large urban settlement: main sources and local background, Environ. Sci.: Processes Impacts, 2014, 16, 1913–1922, 10.1039/c4em00111g.
- Z. Mo, M. Shao and S. Lu, Compilation of a source profile database for hydrocarbon and OVOC emissions in China, Atmos. Environ., 2016, 143, 209–217, DOI:10.1016/j.atmosenv.2016.08.025.
- A. Townsend-Small, J. E. Marrero, D. R. Lyon, I. J. Simpson, S. Meinardi and D. R. Blake, Integrating source apportionment tracers into a bottom-up inventory of methane emissions in the barnett shale hydraulic fracturing region, Environ. Sci. Technol., 2015, 49, 8175–8182, DOI:10.1021/acs.est.5b00057.
- M. A. Bari and W. B. Kindzierski, Concentrations, sources and human health risk of inhalation exposure to air toxics in Edmonton, Canada, Chemosphere, 2017, 173, 160–171, DOI:10.1016/j.chemosphere.2016.12.157.
- S. G. Brown, S. Eberly, P. Paatero and G. A. Norris, Methods for estimating uncertainty in PMF solutions: examples with ambient air and water quality data and guidance on reporting PMF results, Sci. Total Environ., 2015, 518, 626–635, DOI:10.1016/j.scitotenv.2015.01.022.
- Y. Yan, L. Peng, R. Li, Y. Li, L. Li and H. Bai, Concentration, ozone formation potential and source analysis of volatile organic compounds (VOCs) in a thermal power station centralized area: a study in Shuozhou, China, Environ. Pollut., 2017, 223, 295–304, DOI:10.1016/j.envpol.2017.01.026.
- W. Wei, S. Cheng, G. Li, G. Wang and H. Wang, Characteristics of volatile organic compounds (VOCs) emitted from a petroleum refinery in Beijing, China, Atmos. Environ., 2014, 89, 358–366, DOI:10.1016/j.atmosenv.2014.01.038.
- F. Tian, J. Chen, X. Qiao, Z. Wang, P. Yang, D. Wang and L. Ge, Sources and seasonal variation of atmospheric polycyclic aromatic hydrocarbons in Dalian, China: factor analysis with non-negative constraints combined with local source fingerprints, Atmos. Environ., 2009, 43, 2747–2753, DOI:10.1016/j.atmosenv.2009.02.037.
- U.S. Geological Survey, Minerals yearbook: Volume I - metals and minerals (magnesium compounds), 2016, available online at https://minerals.usgs.gov/minerals/pubs/commodity/magnesium/myb1-2015-mgcom.pdf, accessed 31 July 2018.
- A modern casting staff report, Census of world casting production, 2017, available online at http://content.digitalpub.blue-soho.com/web/y5b2/0A1snzj/ModernCastingDec2017/html/index.html?page=26, accessed 31 July 2018.
- Y. Wang, F. S. Cannon, M. Salama, J. Goudzwaard and J. C. Furness, Characterization of hydrocarbon emissions from green sand foundry core binders by analytical pyrolysis, Environ. Sci. Technol., 2007, 41, 7922–7927, DOI:10.1021/es071657o.
- Y. Wang, F. S. Cannon and X. Li, Comparative analysis of hazardous air pollutant emissions of casting materials measured in analytical pyrolysis and conventional metal pouring emission tests, Environ. Sci. Technol., 2011, 45, 8529–8535, DOI:10.1021/es2023048.
- Y. Wang, Y. Zhang, L. Su, X. Li, L. Duan, C. Wang and T. Huang, Hazardous air pollutant formation from pyrolysis of typical Chinese casting materials, Environ. Sci. Technol., 2011, 45, 6539–6544, DOI:10.1021/es200310p.
- Y. Wang, F. S. Cannon, M. Salama, D. A. Fonseca and S. Giese, Characterization of pyrolysis products from a biodiesel phenolic urethane binder, Environ. Sci. Technol., 2009, 43, 1559–1564, DOI:10.1021/es8024929.
- J. An, Y. Li and R. S. Middleton, Reducing energy consumption and carbon emissions of magnesia refractory products: a life-cycle perspective, J. Cleaner Prod., 2018, 182, 363–371, DOI:10.1016/j.jclepro.2018.01.266.
- Z. Zhong, Q. Sha, J. Zheng, Z. Yuan, Z. Gao, J. Ou, Z. Zheng, C. Li and Z. Huang, Sector-based VOCs emission factors and source profiles for the surface coating industry in the Pearl River Delta region of China, Sci. Total Environ., 2017, 583, 19–28, DOI:10.1016/j.scitotenv.2016.12.172.
- Z. Mo, M. Shao, S. Lu, H. Qu, M. Zhou, J. Sun and B. Gou, Process-specific emission characteristics of volatile organic compounds (VOCs) from petrochemical facilities in the Yangtze River Delta, China, Sci. Total Environ., 2015, 533, 422–431, DOI:10.1016/j.scitotenv.2015.06.089.
- J. Zhu, R. Newhook, L. Marro and C. C. Chan, Selected volatile organic compounds in residential air in the city of Ottawa, Canada, Environ. Sci. Technol., 2005, 39, 3964–3971, DOI:10.1021/es050173u.
- U.S. EPA, 1999, Compendium method TO-17 determination of volatile organic compounds in ambient air using active sampling onto sorbent tubes. Environmental Protection Agency: Cincinnati, Ohio, USA, available online at https://www3.epa.gov/ttn/amtic/files/ambient/airtox/to-17r.pdf, accessed 31 July 2018.
- P. K. Hopke, Review of receptor modeling methods for source apportionment, J. Air Waste Manage. Assoc., 2016, 66, 237–259, DOI:10.1080/10962247.2016.1140693.
- Z. Ling, J. Zhao, S. Fan and X. Wang, Sources of formaldehyde and their contributions to photochemical O3 formation at an urban site in the Pearl River Delta, Southern China, Chemosphere, 2017, 168, 1293–1301, DOI:10.1016/j.chemosphere.2016.11.140.
- H. Liao, Y. Yau, C. Huang, N. Chen, J. C. Chow, J. G. Watson, S. Tsai, C. C. K. Chou and C. Wu, Source apportionment of urban air pollutants using constrained receptor models with a priori profile information, Environ. Pollut., 2017, 227, 323–333, DOI:10.1016/j.envpol.2017.04.071.
- G. Norris, R. Duvall, S. Brown and S. Bai, EPA Positive Matrix Factorization (PMF) 5.0 Fundamentals and User Guide, U.S. Environmental Protection Agency, Washington, DC, 2014, available online at https://www.epa.gov/sites/production/files/2015-02/documents/pmf_5.0_user_guide.pdf, accessed 31 July 2018 Search PubMed.
- P. A. Bzdusek, E. R. Christensen, A. Li and Q. Zou, Source apportionment of sediment PAHs in Lake Calumet, Chicago:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) application of factor analysis with nonnegative constraints, Environ. Sci. Technol., 2004, 38, 97–103, DOI:10.1021/es034842k.
application of factor analysis with nonnegative constraints, Environ. Sci. Technol., 2004, 38, 97–103, DOI:10.1021/es034842k. - P. Rachdawong and E. R. Christensen, Determination of PCB sources by a principal component method with nonnegative constraints, Environ. Sci. Technol., 1997, 31, 2686–2691, DOI:10.1021/es970107v.
- A. L. Bolden, C. F. Kwiatkowski and T. Colborn, New look at BTEX: are ambient levels a problem?, Environ. Sci. Technol., 2015, 49, 5261–5276, DOI:10.1021/es505316f.
- D. Wang, L. Nie, X. Shao and H. Yu, Exposure profile of volatile organic compounds receptor associated with paints consumption, Sci. Total Environ., 2017, 603–604, 57–65, DOI:10.1016/j.scitotenv.2017.05.247.
- Z. Zhang, H. Wang, D. Chen, Q. Li, P. Thai, D. Gong, Y. Li, C. Zhang, Y. Gu, L. Zhou, L. Morawska and B. Wang, Emission characteristics of volatile organic compounds and their secondary organic aerosol formation potentials from a petroleum refinery in Pearl River Delta, China, Sci. Total Environ., 2017, 584–585, 1162–1174, DOI:10.1016/j.scitotenv.2017.01.179.
- N. Singh, V. Murari, M. Kumar, S. C. Barman and T. Banerjee, Fine particulates over South Asia: review and meta-analysis of PM 2.5 source apportionment through receptor model, Environ. Pollut., 2017, 223, 121–136, DOI:10.1016/j.envpol.2016.12.071.
- J. Zhu, S. L. Wong and S. Cakmak, Nationally representative levels of selected volatile organic compounds in Canadian residential indoor air: population-based survey, Environ. Sci. Technol., 2013, 47, 13276–13283, DOI:10.1021/es403055e.
- W. Ren, B. Xue, C. Lu, Z. Zhang, Y. Zhang and L. Jiang, Evaluation of GHG emissions from the production of magnesia refractory raw materials in Dashiqiao, China, J. Cleaner Prod., 2016, 135, 214–222, DOI:10.1016/j.jclepro.2016.06.118.
- H. Zhang, H. Zhao, K. Zheng, X. Li, G. Liu and Y. Wang, Diminishing hazardous air pollutant emissions from pyrolysis of furan no-bake binders using methanesulfonic acid as the binder catalyst, J. Therm. Anal. Calorim., 2014, 116, 373–381, DOI:10.1007/s10973-013-3553-x.
- M. Cai, Y. Lin, M. Chen, W. Yang, H. Du, Y. Xu, S. Cheng, F. Xu, J. Hong, M. Chen and H. Ke, Improved source apportionment of PAHs and Pb by integrating Pb stable isotopes and positive matrix factorization application (PAHs): a historical record case study from the Northern South China Sea, Sci. Total Environ., 2017, 609, 577–586, DOI:10.1016/j.scitotenv.2017.07.190.
- B. Li, S. S. H. Ho, Y. Xue, Y. Huang, L. Wang, Y. Cheng, W. Dai, H. Zhong, J. Cao and S. Lee, Characterizations of volatile organic compounds (VOCs) from vehicular emissions at roadside environment: the first comprehensive study in Northwestern China, Atmos. Environ., 2017, 161, 1–12, DOI:10.1016/j.atmosenv.2017.04.029.
- Y. Huang, Z. H. Ling, S. C. Lee, S. S. H. Ho, J. J. Cao, D. R. Blake, Y. Cheng, S. C. Lai, K. F. Ho, Y. Gao, L. Cui and P. K. K. Louie, Characterization of volatile organic compounds at a roadside environment in Hong Kong: an investigation of influences after air pollution control strategies, Atmos. Environ., 2015, 122, 809–818, DOI:10.1016/j.atmosenv.2015.09.036.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8em00363g |
| This journal is © The Royal Society of Chemistry 2019 |