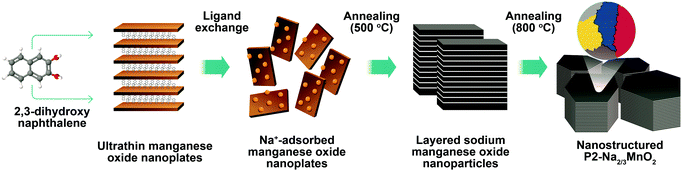
Synthesis of nanostructured P2-Na2/3MnO2 for high performance sodium-ion batteries†
Euiyeon
Jung‡
 ab,
Yuwon
Park‡
ab,
Yuwon
Park‡
 b,
Kunsu
Park‡
ab,
Mi-Sook
Kwon
b,
Mihyun
Park
ab,
Arun Kumar
Sinha
ab,
Byoung-Hoon
Lee
ab,
Jiheon
Kim
b,
Kunsu
Park‡
ab,
Mi-Sook
Kwon
b,
Mihyun
Park
ab,
Arun Kumar
Sinha
ab,
Byoung-Hoon
Lee
ab,
Jiheon
Kim
 ab,
Hyeon Seok
Lee
ab,
Sue In
Chae
ab,
Sung-Pyo
Cho
c,
Kyu Tae
Lee
ab,
Hyeon Seok
Lee
ab,
Sue In
Chae
ab,
Sung-Pyo
Cho
c,
Kyu Tae
Lee
 *b and
Taeghwan
Hyeon
*b and
Taeghwan
Hyeon
 *ab
*ab
aCenter for Nanoparticle Research, Institute for Basic Science (IBS), Seoul 08826, Korea
bSchool of Chemical and Biological Engineering, Seoul National University, Seoul 08826, Korea. E-mail: ktlee@snu.ac.kr; thyeon@snu.ac.kr
cNational Center for Inter-university Research Facilities, Seoul National University, Seoul 08826, Korea
First published on 7th March 2019
Abstract
We report a facile two-step method to synthesize nanostructured P2-Na2/3MnO2via ligand exchange and intercalation of sodium ions into ultrathin manganese oxide nanoplates. Sodium storage performance of the synthesized material shows a high capacity (170 mA h g−1) and an excellent rate performance.
Lithium ion batteries have been the predominant choice of energy storage systems for portable devices and electronic vehicles in the last few decades due to their high energy density and long cycle life.1–3 As a new class of green energy storage and conversion system, sodium ion batteries (SIBs) have received much attention owing to the high natural abundance and low cost of sodium resources.4–6 Sodium has a redox potential (−2.71 V vs. SHE) close to that of lithium (−3.04 V vs. SHE) which makes it an attractive alternative to lithium ion batteries.7 However, there are still challenges remaining for sodium-ion batteries. One critical issue is finding cathode materials with high reversible capacity and good cycle life to meet the performance expectation for electronic devices.8 For this reason, many different types of oxide materials including spinel oxides,9 tunnel-structured oxides,10,11 and layered transition metal oxides, have been explored for the last few years. Among them, layered sodium transition metal oxides (NaxMO2, M = Mn, Ni, Co, Fe, etc., x < 1) are the most studied cathode materials due to their high energy density.12–15
Sodium manganese oxide (NMO) is a promising cathode material because of its high availability and low cost, making it suitable for large-scale batteries. Sodium manganese oxides are known to deliver large reversible capacities. Caballero et al. reported that P2-type layered Na2/3MnO2 (P2-NMO) can deliver a large initial capacity of 160 mA h g−1.16 However, many research groups reported problems with P2-NMO such as poor rate capability and cycle retention.17–19
Nanostructured materials have received a lot of attention as a solution to such problem by enhancing ion kinetics.20–22 Herein, we report a facile and unique synthetic method to produce a nanostructured P2-Na2/3MnO2 cathode material via a two-step method, starting from ultrathin manganese oxide nanoplates. Nanostructured P2-Na2/3MnO2 exhibited remarkable sodium storage performance, such as a high reversible capacity of 170 mA h g−1, excellent rate performance delivering 135 mA h g−1 at 10C rate, and negligible capacity fading over 50 cycles. The improved electrochemical performance is attributable to grain boundaries between the nanostructured domains that are embedded inside the crystals. Our facile synthetic method could be generalized to other sodium metal oxide systems for the design of nanostructured cathodes for high performance SIBs.
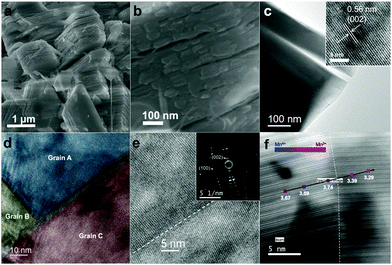
Nanostructured P2-Na2/3MnO2 is synthesized by the two-step method as described in Fig. 1. Firstly, ultrathin manganese oxide nanoplates were synthesized following the previously reported method.23 The as-synthesized brownish nanoplates are 8 nm long and less than 1 nm thick, assembled into a lamellar structure (Fig. 2a and b). For the ligand exchange reaction, the prepared manganese oxide nanoplates were dispersed in tetrahydrofuran (THF) and mixed with 1 M sodium hydroxide (NaOH)–ethylene glycol solution. The mixed solution was stirred at 65 °C for 48 hours and during this ligand exchange reaction, the hydrophobic, 2,3-dihydroxynaphthalene (2,3-DHN) organic surfactant was replaced by the hydroxide ions causing the delamination of the lamellar structure of the nanoplates.23,24 The surface charge was neutralized by the sodium ions on the surface of the nanoplates.25 This process destroyed the π–π interactions between the 2,3-DHN molecules to produce discrete Na+-adsorbed manganese oxide nanoplates. After the heat treatment at 500 °C in air, Na+-adsorbed nanoplates were turned into layered sodium manganese oxide nanoparticles (20–50 nm) (Fig. 2c). From the energy dispersive X-ray spectroscopy (EDS) and high-resolution transmission electron microscopy (HRTEM) images (Fig. 2c and d), we observed sodium manganese oxide nanoparticles (NMO NPs) with a lattice spacing of ∼0.56 nm, similar to the d-spacing of a (002) plane in P2-hexagonal phase. In contrast, we obtained micrometer-sized Mn2O3 particles under the same annealing condition without the NaOH treatment, indicating that the ligand exchange step not only supplies Na+ ions but also restricts the particle growth (Fig. S1, ESI†).26–28 The XRD spectrum of the NMO NPs shows broad peaks (00l), indicating that the particles are stacked in a turbostratic manner without sufficient crystallinity causing poor reversible sodium storage (Fig. 2e and Fig. S2, ESI†). We performed further annealing at 800 °C to acquire a well-crystallized P2-Na2/3MnO2 which is well indexed to a hexagonal structure with a space group of P63/mmc (Na0.7MnO2.05, JCPDS 27-0751) known as the α phase of NMO (Fig. 2e). Additional heat treatment at 800 °C triggered the assembly of the NMO nanoparticles and caused a significant increase in size. The SEM images (Fig. 3a and b) show tens of micrometer-sized plates stacked in an orderly manner and the TEM image (Fig. 3c) demonstrates the highly crystalline nature of the P2-type structure.
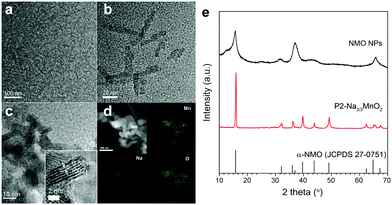 | ||
| Fig. 2 (a) and (b) TEM images of manganese oxide nanoplates, (c) TEM image, (d) STEM image and EDS images of NMO NPs and (e) XRD patterns of NMO NPs and nanostructured P2-Na2/3MnO2. | ||
The cross-sectional specimens of P2-Na2/3MnO2 was obtained using a focused ion beam (FIB) and analyzed by spherical aberration-corrected scanning transmission electron microscopy (Cs-STEM) combined with electron energy loss spectroscopy (EELS). The HRTEM image shows nanoscale domains inside the crystal which are clearly separated by grain boundaries (Fig. 3d). The selective area electron diffraction pattern (SAED) near the grain boundary reveals that all the grains have the P2-type structure despite their different orientations (inset of Fig. 3e). From the EELS line scan across the grain boundary, we calculated the integrated Mn L3/L2 white line ratios (Fig. 3f).29,30 More details on the method of calculating the Mn L3/L2 ratio can be found in Fig. S3 (ESI†). It is well known that the oxidation state of transition metals can be derived from the white line intensity ratio (Fig. S3, ESI†).29–31 The calculated Mn L3/L2 ratios and the corresponding Mn oxidation state values are listed in Table S1 (ESI†). We observed a change in the average Mn oxidation state from 3.74 to 3.29 across the grain boundary, which indicates that the adjacent domains have different oxidation states as indicated in Fig. 3f. There are known to be two polymorphs in Na0.7MnO2: α-Na0.7MnO2+z (0.05 ≤ z ≤ 0.25) with an average Mn oxidation state of 3.8 and β-Na0.7MnO2+z (z < 0.05) with an average Mn oxidation state of 3.3 (Fig. S4, ESI†).32 The characteristics of crystal structures and the average Mn oxidation states depend on the method of cooling during the synthesis. α-Na0.7MnO2+z (0.05 ≤ z ≤ 0.25) and β-Na0.7MnO2+z (z < 0.05) are obtained through a conventional cooling and quenching, respectively. However, we obtained a nanoscale mixture of NMO comprising two different phases through our unique approach of synthesizing nanostructured P2-NMO.
The composite behavior of nanostructured P2-NMO was also supported by the differential capacity plot (dQ/dV) (Fig. 4a). The dQ/dV of nanostructured P2-NMO displays both characteristic peaks of the α-NMO and β-NMO phases. The dQ/dV peaks below 2.27 V indicate redox peaks of α-NMO (Fig. 4a, asterisk signs), whereas the peaks above 2.27 V correspond to those of β-NMO (Fig. 4a, number signs). Redox peaks corresponding to the α phase are much stronger than those corresponding to the β phase. This implies that the majority of grains is in α phase, which is consistent with the XRD pattern of the nanostructured P2-NMO.
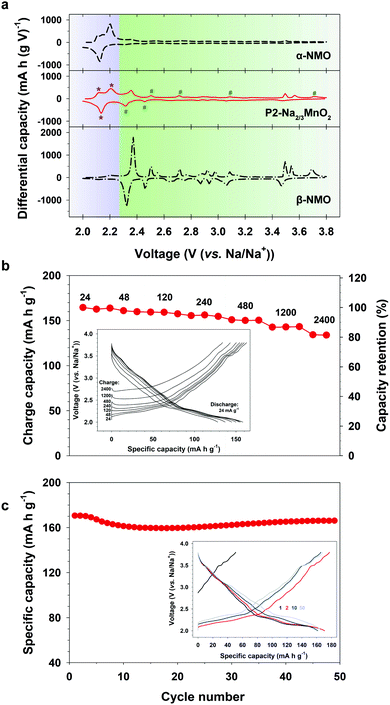 | ||
| Fig. 4 Sodium storage performance of the nanostructured P2-Na2/3MnO2. (a) Differential capacity plots at 12 mA g−1. Data for α-NMO and β-NMO were adapted from ref. 32. (b) Rate performance at various charge current densities. (c) Cycling performance at 12 mA g−1 (inset: corresponding voltage profiles). | ||
Nanostructured P2-Na2/3MnO2 was examined as a cathode material for sodium ion batteries. Firstly, we evaluated rate performance at various charging rates from 24 to 2400 mA g−1 (0.1–10C rate) as shown in Fig. 4b. The cell was discharged at a fixed rate of 24 mA g−1. P2-Na2/3MnO2 showed excellent rate performance, such as 135 mA h g−1 at 10C rate, which corresponds to 80% of the reversible capacity delivered at 0.1C rate. It has been shown that the lithium diffusion coefficient along the grain boundaries is three orders of magnitude higher than that across the grain boundaries due to the much lower activation energy.33,34 Therefore, we suggest that the improved rate performance of nanostructured P2-NMO is due to the nanoscale domains of P2-NMO. Nanostructured grain boundaries (Fig. S5, ESI†) facilitated the solid state diffusion of Na+ ions through a bulk particle, resulting in the excellent rate capability (Fig. 4b). Moreover, the cycle performance and the corresponding charge–discharge profiles were taken at a current density of 12 mA g−1 in the voltage range of 2.0–3.8 V (Fig. 4c). The nanostructured P2-NMO electrode delivered a high initial capacity of 170 mA h g−1 with negligible capacity fading over 50 cycles, outperforming the recently reported sodium manganese oxide electrodes (Table S2, ESI†).
In summary, nanostructured P2-Na2/3MnO2 has been prepared via ligand exchange and crystallization of ultrathin manganese oxide nanoplates. Through this novel scheme of design, we produced well-crystallized P2-NMO bulk particles comprising nanoscale domains. As a cathode for Na-ion batteries, it showed excellent electrochemical performance including a high specific capacity of 170 mA h g−1 and excellent rate performance. Our unique two-step strategy of fabricating nanostructured sodium metal oxide materials will pave a new way for the design and synthesis of electrodes for high-performance rechargeable batteries.
This work was supported by Institute for Basic Science (IBS-R006-D1) and the National Research Foundation of Korea (NRF) Grant (NRF-2018R1A5A1024127).
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. S. Whittingham, Chem. Rev., 2014, 114, 11414 CrossRef CAS.
- J. B. Goodenough, Energy Storage Mater., 2015, 1, 158 CrossRef.
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- W. Luo, F. Shen, C. Bommier, H. Zhu, X. Ji and L. Hu, Acc. Chem. Res., 2016, 49, 231 CrossRef CAS PubMed.
- H. Kim, D. J. Kim, D.-H. Seo, M. S. Yeom, K. Kang, D. K. Kim and Y. Jung, Chem. Mater., 2012, 24, 1205 CrossRef CAS.
- S.-W. Kim, D.-H. Seo, X. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710 CrossRef CAS.
- S. P. Ong, V. L. Chevrier, G. Hautier, A. Jain, C. Moore, S. Kim, X. Ma and G. Ceder, Energy Environ. Sci., 2011, 4, 3680 RSC.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636 CrossRef CAS PubMed.
- X. Liu, X. Wang, A. Iyo, H. Yu, D. Li and H. Zhou, J. Mater. Chem. A, 2014, 2, 14822 RSC.
- Y. Cao, L. Xiao, W. Wang, D. Choi, Z. Nie, J. Yu, L. V. Saraf, Z. Yang and J. Liu, Adv. Mater., 2011, 23, 3155 CrossRef CAS PubMed.
- J.-Y. Li, X.-L. Wu, X.-H. Zhang, H.-Y. Lu, G. Wang, J.-Z. Guo, F. Wan and R.-S. Wang, Chem. Commun., 2015, 51, 14848 RSC.
- M. H. Han, E. Gonzalo, G. Singh and T. Rojo, Energy Environ. Sci., 2015, 8, 81 RSC.
- N. Yabuuchi, M. Kajiyama, J. Iwatate, H. Nishikawa, S. Hitomi, R. Okuyama, R. Usui, Y. Yamada and S. Komaba, Nat. Mater., 2012, 11, 512 CrossRef CAS PubMed.
- D. Yuan, W. He, F. Pei, F. Wu, Y. Wu, J. Qian, Y. Cao, X. Ai and H. Yang, J. Mater. Chem. A, 2013, 1, 3895 RSC.
- D. Kim, S.-H. Kang, M. Slater, S. Rood, J. T. Vaughey, N. Karan, M. Balasubramanian and C. S. Johnson, Adv. Energy Mater., 2011, 1, 333 CrossRef CAS.
- A. Caballero, L. Hernan, J. Morales, L. Sanchez, J. S. Pena and M. A. G. Aranda, J. Mater. Chem., 2002, 12, 1142 RSC.
- N. Bucher, S. Hartung, A. Nagasubramanian, Y. L. Cheah, H. E. Hoster and S. Madhavi, ACS Appl. Mater. Interfaces, 2014, 6, 8059 CrossRef CAS PubMed.
- D. Su, C. Wang, H.-J. Ahn and G. Wang, Chem. – Eur. J., 2013, 19, 10884 CrossRef CAS PubMed.
- T.-R. Chen, Z.-G. Wu, W. Xiang, E.-H. Wang, C.-J. Wu, M.-Z. Chen, X.-D. Guo and B.-H. Zhong, Ceram. Int., 2017, 43, 6303 CrossRef CAS.
- M. F. Oszajca, M. I. Bodnarchuk and M. V. Kovalenko, Chem. Mater., 2014, 26, 5422 CrossRef CAS.
- V. Raju, J. Rains, C. Gates, W. Luo, X. Wang, W. F. Stickle, G. D. Stucky and X. Ji, Nano Lett., 2014, 14, 4119 CrossRef CAS PubMed.
- E. Kang, Y. S. Jung, A. S. Cavanagh, G.-H. Kim, S. M. George, A. C. Dillon, J. K. Kim and J. Lee, Adv. Funct. Mater., 2011, 21, 2430 CrossRef CAS.
- M. Park, N. Lee, S. H. Choi, K. An, S.-H. Yu, J. H. Kim, S.-H. Kwon, D. Kim, H. Kim, S.-I. Baek, T.-Y. Ahn, O. K. Park, J. S. Son, Y.-E. Sung, Y.-W. Kim, Z. Wang, N. Pinna and T. Hyeon, Chem. Mater., 2011, 23, 3318 CrossRef CAS.
- R. Ma, Z. Liu, L. Li, N. Iyi and T. Sasaki, J. Mater. Chem., 2006, 16, 3809 RSC.
- S.-H. Yu, M. Park, H. S. Kim, A. Jin, M. Shokouhimehr, T.-Y. Ahn, Y.-W. Kim, T. Hyeon and Y.-E. Sung, RSC Adv., 2014, 4, 12087 RSC.
- Y. Piao, H. S. Kim, Y.-E. Sung and T. Hyeon, Chem. Commun., 2010, 46, 118 RSC.
- Y. Piao, J. Kim, H. B. Na, D. Kim, J. S. Baek, M. K. Ko, J. H. Lee, M. Shokouhimehr and T. Hyeon, Nat. Mater., 2008, 7, 242 CrossRef CAS PubMed.
- Z. Yang, J. Shen, N. Jayaprakash and L. A. Archer, Energy Environ. Sci., 2012, 5, 7025 RSC.
- D. H. Pearson, B. Fultz and C. C. Ahn, Appl. Phys. Lett., 1988, 53, 1405 CrossRef CAS.
- D. H. Pearson, C. C. Ahn and B. Fultz, Phys. Phys. Rev. B, 1993, 47, 8471 CrossRef CAS.
- H. K. Schmid and W. Mader, Micron, 2006, 37, 426 CrossRef CAS PubMed.
- M.-S. Kwon, S. G. Lim, Y. Park, S.-M. Lee, K. Y. Chung, T. J. Shin and K. T. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 14758 CrossRef CAS PubMed.
- H. Moriwake, A. Kuwabara, C. A. J. Fisher, R. Huang, T. Hitosugi, Y. H. Ikuhara, H. Oki and Y. Ikuhara, Adv. Mater., 2013, 25, 618 CrossRef CAS PubMed.
- N. Balke, S. Jesse, A. N. Morozovska, E. Eliseev, D. W. Chung, Y. Kim, L. Adamczyk, R. E. Garcia, N. Dudney and S. V. Kalinin, Nat. Nanotechnol., 2010, 5, 749 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc01215j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |