Heterogeneous hydroformylation of long-chain alkenes in IL-in-oil Pickering emulsion†
Lin
Tao
ab,
Mingmei
Zhong
ab,
Jian
Chen
ab,
Sanjeevi
Jayakumar
ab,
Lina
Liu
ab,
He
Li
a and
Qihua
Yang
 *a
*a
aState Key Laboratory of Catalysis, iChEM, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023, China. E-mail: yangqh@dicp.ac.cn; Fax: +86-411-84694447; Tel: +86-411-84379552
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 23rd November 2017
Abstract
An efficient heterogeneous catalytic system for hydroformylation of long-chain alkenes is highly desirable for both academy and industry. In this study, an IL-in-oil Pickering emulsion system was employed for heterogeneous hydroformylation of 1-dodecene with Rh-sulfoxantphos as the catalyst and surface modified dendritic mesoporous silica nanospheres (DMSN) as the stabilizer. The IL-in-oil Pickering emulsion system outperformed IL-oil biphase, water-in-oil Pickering emulsion and IL-oil micelle system under similar reaction conditions to afford n/b ratio of 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, chemoselectivity of 94% and TOF of 413 h−1, among the highest ever reported for IL-oil biphase hydroformylation of long-chain alkenes. The high efficiency of IL-in-oil Pickering emulsion was primarily attributed to the increased interface area and unique properties of ILs. Studies also revealed that solid stabilizers with large and open pore channels could greatly increase the reaction rate of Pickering emulsion systems by accelerating the diffusion rate. The recyclable IL-in-oil Pickering emulsion is promising not only for hydroformylation of long-chain alkenes but also for catalytic reactions with immiscible liquids.
2, chemoselectivity of 94% and TOF of 413 h−1, among the highest ever reported for IL-oil biphase hydroformylation of long-chain alkenes. The high efficiency of IL-in-oil Pickering emulsion was primarily attributed to the increased interface area and unique properties of ILs. Studies also revealed that solid stabilizers with large and open pore channels could greatly increase the reaction rate of Pickering emulsion systems by accelerating the diffusion rate. The recyclable IL-in-oil Pickering emulsion is promising not only for hydroformylation of long-chain alkenes but also for catalytic reactions with immiscible liquids.
Introduction
Aldehydes are important intermediates that can be converted to many final products such as plasticizers, detergents, alcohols, esters and amines.1–4 “Oxo”-hydroformylation reaction developed by Otte Roelen in 1938 has been widely used for production of aldehydes from alkenes in industry.5 The traditional hydroformylation reaction proceeding homogeneously in oil phase confronts difficulties related to recycling of catalysts and separation of products.6 Thus, the oil–water reaction system has been applied in the hydroformylation of C3–C4 olefins industrially with Rh-tris(m-sulfonatophenyl) phosphine (Rh-TPPTS) complexes as catalysts.7 Oil-immiscible catalysts dissolved in the water phase make the recycling of catalysts possible, leading to the reduction of high cost of expensive Rh metal.8 The biphase system is very successful in hydroformylation of light alkenes, but not efficient for hydroformylation of long-chain alkenes due to the mass transfer resistance of lipophilic substrates in the water phase.The emulsion catalytic system is a good choice for reactions facing problems related to diffusion resistance caused by different lipophilicity of reactants, products and catalysts. Pickering emulsions, with solid particles residing at the interface of droplets and bubbles against coalescence or fusion, has been recently used as a platform for the design of efficient catalytic systems.9 Compared with traditional surfactant emulsified microemulsions, the Pickering emulsion system has the advantages of facile emulsion properties tuning by particle surface wettability and easy emulsion breaking by filtration or centrifugation, which are very important for adjusting catalytic performance of the emulsion system, recycling of catalysts and purification of products.10 Recently, several water-in-oil Pickering emulsion systems have been constructed for rhodium-catalysed hydroformylation of long-chain alkenes. The solid particles employed for the abovementioned Pickering emulsion systems include cyclodextrin,11,12 cyclodextrin with PEG as the supramolecular hydrogel,13,14 polymers15 and mesoporous silica nanospheres.16 Obvious increases in both catalytic activity and chemoselectivity are reported in these Pickering emulsion systems.
The reported Pickering emulsions are mainly water-in-oil or oil-in-water emulsion systems.17,18 Though water is regarded as a kind of green solvent, there are still some limitations, such as low solubility for lipophilic substrates, damage to water-sensible compounds, and low boiling point. The extension of Pickering emulsions to other green solvents will be of great significance in but not limited to catalysis. Ionic liquids (ILs) have some environmentally benign properties, for example, extremely low vapor pressure, chemical and thermal stability, high ionic conductivity and good solvent properties towards both ionic and covalent compounds.19,20 In the past decades, there have been many studies on the use of ILs in biphase hydroformylation for increasing the solubility of lipophilic substrates in two-phase reaction systems.21,22 However, the rate acceleration effect is still not very promising, possibly due to the diffusion limitation of reactants in highly viscous ILs. The introduction of Pickering emulsions into ILs will divide bulk ILs into numerous droplets on the micrometer scale, which may increase the interphase area and benefit fast diffusion of reactants and products during the catalytic process. However, the preparation of IL-in-oil/oil-in-IL Pickering emulsions for hydroformylation reactions has been rarely reported.
In the current work, an IL-in-oil Pickering emulsion system for hydroformylation of long-chain alkenes was constructed with Rh-sulfo-xantphos as the catalyst and surface modified dendritic mesoporous silica nanospheres as the solid stabilizers. The overall catalytic performance of the IL-in-oil Pickering emulsion system was superior to that of IL-oil biphase and water-in-oil Pickering emulsion catalytic systems, implying that the combination of the Pickering emulsion with ILs resulted in better outcomes than that of other systems.
Results and discussion
Characterization of interfacially active nanoparticles
Interfacially active nanoparticles are very important for the preparation of Pickering emulsions.9 Silica nanoparticles with tunable surface wettability are generally used as stabilizers for Pickering emulsions.23,24 In this work, the surface wettability of silica nanospheres was modified with C18N (dimethyloctadecyl[3-(trimethoxysilyl)propyl]ammonium chloride) group considering its enrichment properties for negatively charged Rh-sulfo-xantphos through electrostatic interactions. For nonporous silica nanospheres, the interphase area depends mainly on the particle size of nanospheres.25 With mesoporous silica as the stabilizer, the porous structure and pore diameter may affect the diffusion rates of the substrates and products in the Pickering emulsion. Thus, MCM-41 nanospheres and dendritic mesoporous silica nanospheres (DMSN) with similar particle size but different pore structure and pore size were chosen as stabilizers for comparison.All DMSN-C18N-X (X = 0 to 1.2, X refers to mmol of C18N per gram of DMSN used during the grafting process) samples were composed of monodispersed nanospheres with uniform particle size of ∼100 nm based on transmission electron microscopy (TEM) images (Fig. 1a, b and Fig. S1†). Center-radial mesopore arrangement could be clearly observed in the TEM images before and after grafting, revealing the structure robustness of DMSN in the grafting process. Nitrogen adsorption–desorption isotherms of DMSN-C18N-X (X = 0 to 1.2) showed a typical type IV isotherm pattern (Fig. 1c and Fig. S2†), which are characteristic of mesoporous materials. Compared with DMSN, the BET surface area and pore volume of DMSN-C18N-X (X = 0 to 1.2) decreased gradually from 813 m2 g−1 to 339 m2 g−1 as the amount of C18N increased (Table 1 and Fig. 1d). The sharp decrease in pore diameter suggested that the C18N group was mainly grafted in the mesopores of DMSN.
 | ||
| Fig. 1 TEM images of (a) DMSN and (b) DMSN-C18N-0.8, (c) N2 adsorption/desorption isotherms and (d) pore size distribution curves of DMSN and DMSN-C18N-0.8. | ||
| Sample | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) | Content of organic groupsa (%) | Water contact angle (°) |
|---|---|---|---|---|---|
| a Calculated by weight loss in the range of 200 to 800 °C. | |||||
| DMSN | 813 | 1.43 | 6.2 | 0 | 35 |
| DMSN-C18N-0.5 | 387 | 0.78 | 5.4 | 17.1 | 91 |
| DMSN-C18N-0.8 | 345 | 0.68 | 5.1 | 21.3 | 100 |
| DMSN-C18N-1.2 | 339 | 0.93 | 4.4 | 27.9 | 112 |
| MCM-C18N-1.8 | 203 | 0.25 | 2.3 | 33.1 | 101 |
The FT-IR spectrum of DMSN-C18N-0.8 clearly showed characteristic C–H stretching vibration at 2926 cm−1 and 2856 cm−1 and C–H bending vibration at 1468 cm−1, confirming the successful grafting of C18N onto DMSN (Fig. 2a).16 The thermogravimetric (TG) curves of DMSN-C18N-X exhibited three obvious weight loss steps (Fig. 2b). The first one at around 50 to 200 °C was related to physically adsorbed water. The second and third ones in the range of 200 to 700 °C could be attributed to the decomposition of C18N. The TG analysis demonstrated that DMSN-C18N-X samples were stable in air up to 200 °C. The stability was sufficient for hydroformylation of long-chain alkenes, generally performed at temperature below 150 °C. The content of C18N (calculated by weight loss between 200 and 800 °C) increased from 17.1 wt% to 27.9 wt% with increase in initial amount of C18N added, indicating that the content of the organic group on DMSN-C18N-X could be facilely adjusted by the grafting method.
The surface wettability of the nanospheres is one of the key factors to get a stable emulsion.26 Water contact angle experiments were used to test the surface wettability of DMSN-C18N-X (Table 1). DMSN with a water contact angle of 35° has a hydrophilic surface. After modification with C18N, all DMSN-C18N-X materials had a hydrophobic surface with water contact angles larger than 90°. From DMSN-C18N-0.5 to DMSN-C18N-1.2, the water contact angle slightly increased from 91° to 112°, suggesting that grafting is an efficient method for precisely adjusting the surface wettability of silica nanoparticles. A control sample, MCM-C18N-1.8 with water contact angle of 101°, was also prepared using a method similar to that used for DMSN-C18N-X with the exception that MCM-41 nanospheres (particle size: ∼100 nm) were used instead of DMSN (Table 1 and Fig. S1†). MCM-C18N-1.8 with a similar water contact angle as DMSN-C18N-0.8 had much lower BET surface area, pore volume and pore diameter than the latter.
Preparation of IL-in-oil emulsion
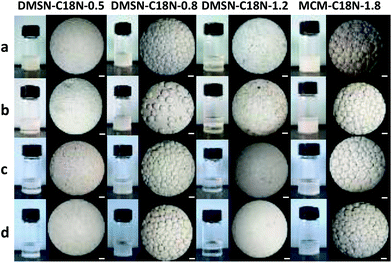
In our previous work, C18N modified silica nanoparticles were used to stabilize oil-in-water Pickering emulsions.16 The IL 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]), possesses different polarity and viscosity compared to water. Therefore, emulsion formation abilities of DMSN-C18N-X (X = 0.5, 0.8, 1.2) were first investigated with 1-dodecene as the oil phase and [BMIM][BF4] as the IL phase in the presence of hydroformylation catalyst Rh-sulfo-xantphos (Fig. 3). The formation of emulsions could be clearly observed with all DMSN-C18N-X samples at room temperature. For identifying the formation of IL-in-oil or oil-in-IL emulsion, confocal laser scanning microscopy (CLSM) experiments were performed with rhodamine 6G, which can dissolve in the IL but not in 1-dodecene. CLSM images of emulsions with DMSN-C18N-0.5, DMSN-C18N-0.8 and DMSN-C18N-1.2 clearly showed the uniform distribution of red colour inside the droplet, suggesting the formation of IL-in-oil emulsions (Fig. 4 and Fig. S3†). The emulsion volume formed under identical conditions increased in the order of DMSN-C18N-1.2 (0.4 mL) < DMSN-C18N-0.5 (0.6 mL) < DMSN-C18N-0.8 (2 mL), suggesting that DMSN-C18N-0.8 had optimized surface wettability for the formation of the IL-in-oil emulsion. For fresh emulsions, the droplet size was strongly related to the wettability of the DMSN-C18N-X samples. Based on the microscopic images, the droplet size of emulsions with DMSN-C18N-0.5 and DMSN-C18N-1.2 was 20–100 μm and 30–50 μm, respectively. Relatively large droplet size of 50–230 μm was observed for the emulsion with DMSN-C18N-0.8 as the stabilizer. | ||
| Fig. 4 CLSM images of IL-in-oil emulsions formed with different amounts of DMSN-C18N-0.8, (a) 40 mg, (b) 60 mg and (c) 80 mg by dying [BMIM][BF4] with rhodamine. Scale bar is 100 μm. | ||
The emulsion with DMSN-C18N-0.8 was very stable and no obvious change in droplet size and emulsion volume could be observed even after 10 days, indicating that DMSN-C18N-0.8 is a good stabilizer for oil-IL emulsions. However, emulsions with DMSN-C18N-0.5 and DMSN-C18N-1.2 separated gradually with time increasing, and broke completely within 10 days. The control sample, MCM-C18N-1.8, also acted as an efficient stabilizer to form a stable emulsion in a manner similar to DMSN-C18N-0.8 (Fig. 3).
The water contact angle of DMSN-C18N-X only varied slightly from 91 to 112° with X varying from 0.5 to 1.2. Nevertheless, the emulsion quality and stability were quite different. In fact, all DMSN-C18N-X samples could form stable water-in-oil emulsions with 1-dodecene as the oil phase and Rh-sulfo-xantphos as the catalyst under identical conditions (Fig. S4†). This reveals that the formation of IL-in-oil emulsions requires more accurate control of surface properties of silica nanospheres.
Hydroformylation of 1-dodecene
Based on the catalytic cycle, two possible isomers, linear and branched aldehydes, can be produced from alkenes with more than two carbon atoms. In industry, linear aldehydes are more desirable.27 Consequently, regioselectivity of hydroformylation should be considered in addition to activity and chemoselectivity. Xantphos based on xanthene backbones with an appropriate “natural” bite angle can improve the n/b ratio of aldehydes via controlling the olefin insertion step.28 Thus, sulfonated xantphos ligand (sulfo-xantphos) was chosen as the IL/water-soluble ligand in this work.To make a comparison of the catalytic performance of the IL-oil biphase system, IL-oil micelle system and IL-in-oil Pickering emulsion system, the hydroformylation of 1-dodecene was performed under identical conditions (Table 2). Taking stability into consideration, the IL-in-oil emulsion system with DMSN-C18N-0.8 as the stabilizer was selected to study the catalytic performance of the Pickering emulsion. Because of the high regioselectivity of sulfonated xantphos ligand, 1-dodecene could be converted to the corresponding aldehyde with an n/b ratio varying in the range of 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 98
4 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in all the abovementioned catalytic systems, but big differences in activity and chemoselectivity were observed. The IL-oil biphase system could only afford 47% conversion with aldehyde selectivity of 89%. The by-products were isomeric olefins and dodecane generated from isomerization and hydrogenation of 1-dodecene. The reason for low conversion was possibly due to the high mass transfer resistance. In the IL-in-oil Pickering emulsion system, the conversion and aldehyde selectivity were improved dramatically to 93% and 94%, respectively. Under similar reaction conditions, the TOF of the IL-in-oil Pickering emulsion system was up to 413 h−1, two times that of the biphase system. This suggests that the Pickering emulsion could efficiently improve both the reaction rate and chemoselectivity.
2 in all the abovementioned catalytic systems, but big differences in activity and chemoselectivity were observed. The IL-oil biphase system could only afford 47% conversion with aldehyde selectivity of 89%. The by-products were isomeric olefins and dodecane generated from isomerization and hydrogenation of 1-dodecene. The reason for low conversion was possibly due to the high mass transfer resistance. In the IL-in-oil Pickering emulsion system, the conversion and aldehyde selectivity were improved dramatically to 93% and 94%, respectively. Under similar reaction conditions, the TOF of the IL-in-oil Pickering emulsion system was up to 413 h−1, two times that of the biphase system. This suggests that the Pickering emulsion could efficiently improve both the reaction rate and chemoselectivity.
| Solid material | P/Rh ratio | Conv. (%) | Sel.b (%) |
n/b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
TOFd (h−1) |
|---|---|---|---|---|---|
a Reaction conditions: Rh 4.5 × 10−3 mmol, 60 mg of solid materials or 30 mg of CTAB, 1.0 mL of [BMIM][BF4], 1.0 mL of 1-dodecene (tetradecane as the internal standard), S/C = 1000, 110 °C, 20 bar CO/H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 1000 rpm, 12 h, 25 mL reactor.
b Selectivity to aldehyde.
c Normal/branched.
d TOF (mmol 1-dodecene per mmol Rh) was calculated with conversion less than 30%.
e 40 mg of DMSN-C18N-0.8.
f 80 mg of DMSN-C18N-0.8.
g 1-Decene was used as the substrate. 1), 1000 rpm, 12 h, 25 mL reactor.
b Selectivity to aldehyde.
c Normal/branched.
d TOF (mmol 1-dodecene per mmol Rh) was calculated with conversion less than 30%.
e 40 mg of DMSN-C18N-0.8.
f 80 mg of DMSN-C18N-0.8.
g 1-Decene was used as the substrate.
|
|||||
| No | 15 | 47 | 89 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
45 |
| CTAB | 15 | 91 | 90 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
315 |
| DMSN-C18N-0.8 | 15 | 93 | 94 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
413 |
| DMSN-C18N-0.8e | 15 | 54 | 77 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
49 |
| DMSN-C18N-0.8f | 15 | 81 | 79 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
144 |
| DMSN-C18N-0.8 | 5 | 95 | 87 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
260 |
| DMSN-C18N-0.8 | 25 | 46 | 73 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
127 |
| DMSN-C18N-0.8g | 15 | 92 | 68 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
250 |
| MCM-C18N-1.8 | 15 | 62 | 81 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
243 |
Surfactants were often used as the phase transfer reagent in reaction systems involving water and oil.29 Thus, CTAB with a functional group similar to C18N was chosen as the phase transfer reagent in hydroformylation of olefins. In the presence of CTAB, the conversion and chemoselectivity were similar to those obtained for the Pickering emulsion system. But the TOF of Pickering emulsion system is much higher than that of the IL-oil-CTAB micelle system. Notably, it was very difficult to separate and recycle the catalyst for the IL-oil-CTAB micelle system. The Pickering emulsion system could not only give better catalytic performance than the biphasic or micelle system under the same conditions, but also be separated and recycled facilely by centrifugation (described later).
The IL-in-oil Pickering emulsion catalytic system was also efficient for hydroformylation of 1-decene for the production of 1-undecanal, an important intermediate in manufacturing of perfumes (Table 2). Up to 92% of conversion with chemoselectivity of 68% and n/b ratio of 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 could be obtained, proving the generality of the IL-in-oil Pickering emulsion catalytic system.
8 could be obtained, proving the generality of the IL-in-oil Pickering emulsion catalytic system.
The droplet size of emulsion determines the interface area of the Pickering emulsion system, which may influence the catalytic performance.30 Therefore, catalytic performances of Pickering emulsion systems with different droplet size were investigated using DMSN-C18N-0.8 as the model stabilizer (Table 2). In order to accurately measure the droplet size of the emulsion, the CLSM technique was used by dying the IL phase with rhodamine 6G as discussed before (Fig. 4). With the amount of DMSN-C18N-0.8 increasing from 40 mg to 60 mg, the droplet size gradually decreased from 150–400 μm to 50–200 μm. Further increasing DMSN-C18N-0.8 content to 80 mg, the droplet size decreased to 30–150 μm.
The n/b ratio of the Pickering emulsion system with different stabilizer amount was very high and only varied slightly from 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 94
3 to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, irrespective of the droplet size (Table 2). However, the conversion and chemoselectivity depended strongly on the droplet size. As the droplet size decreased from 150–400 μm to 50–200 μm, the conversion (from 54% to 93%) and TOF (from 49 h−1 to 413 h−1) increased sharply accompanied by an increase in chemoselectivity from 77% to 94%. Further decreasing the droplet size decreased both activity and chemoselectivity. This is possibly due to the existence of excessive stabilizers, which may hinder the mass transfer during the catalytic process.
6, irrespective of the droplet size (Table 2). However, the conversion and chemoselectivity depended strongly on the droplet size. As the droplet size decreased from 150–400 μm to 50–200 μm, the conversion (from 54% to 93%) and TOF (from 49 h−1 to 413 h−1) increased sharply accompanied by an increase in chemoselectivity from 77% to 94%. Further decreasing the droplet size decreased both activity and chemoselectivity. This is possibly due to the existence of excessive stabilizers, which may hinder the mass transfer during the catalytic process.
In previous research, we found that the Pickering emulsion system formed with MCM-41 nanospheres afforded higher activity than that formed with nonporous silica nanopsheres in the hydroformylation of 1-octene.16 Therefore, the influence of the porous structure of the solid stabilizer on the catalytic performances of the Pickering emulsion was investigated using DMSN with dendritic mesoporous structure and MCM-41 with 2-D hexagonal mesoporous structure as model for comparison (Table 2). DMSN-C18N-0.8 and MCM-C18N-1.8 had similar surface wettability and particle size, but different textural parameters (Table 1). The IL-in-oil emulsion could be efficiently formed with MCM-C18N-1.8 and the droplet size and stability of the emulsion were comparable with emulsion that formed with DMSN-C18N-0.8 (Fig. 3 and Fig. S4†). Pickering emulsion with DMSN-C18N-0.8 as the stabilizer afforded much higher TOF (413 versus 213 h−1), conversion (93% versus 62%) and selectivity to aldehyde (94% versus 81%) than that with MCM-C18N-1.8 as the stabilizer.
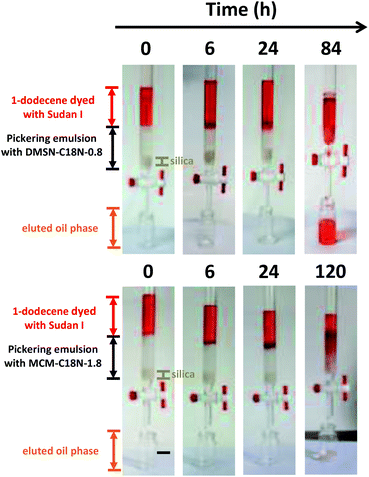
For further understanding the difference in catalytic performance, the diffusion rate of 1-dodecene in the above two Pickering emulsion systems was measured in a column (Fig. 5).31 First, the IL-in-oil Pickering emulsion was transferred in a column. Then, 1-dodecene stained with Sudan I was packed carefully on the top of the Pickering emulsion. For the Pickering emulsion with DMSN-C18N-0.8 as the stabilizer, the stained 1-dodecene gradually passed through the emulsion to elute out and the oil phase in the emulsion was totally replaced by sustained 1-dodecene within 84 h. But for the Pickering emulsion with MCM-C18N-1.8 as the stabilizer, the stained 1-dodecene could not reach the bottom of the column even after 120 h. The diffusion rate of 1-dodecene in the Pickering emulsion with DMSN-C18N-0.8 and MCM-C18N-1.8 as stabilizers was 1.2 mm h−1 and 0.8 mm h−1, respectively. The faster diffusion rate of 1-dodecene could be the main reason for the higher activity and chemoselectivity of the Pickering emulsion with DMSN-C18N-0.8 as the stabilizer. In a simplified model, the interface area was defined as the cross section of nanoparticles floated on the oil-IL interface.32 This is true for nonporous nanoparticles. In case of porous nanoparticles, their nanochannels can also contribute to the interface area. This field is still in its infancy and more theoretical studies are needed.
Another determining factor that could influence both the activity and selectivity of the hydroformylation reaction is the molar ratio of Rh/ligand.33 Hence, the influence of P/Rh ratio on the catalytic performance of the IL-in-oil Pickering emulsion was investigated (Table 2). As the P/Rh ratio increased from 5 to 25, the n/b ratio increased from 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to 98
5 to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The TOF and chemoselectivity reached the highest at P/Rh ratio of 15. Lower activity and chemoselectivity were obtained with P/Rh ratio of 25. The influence of P/Rh ratio on the catalytic performance is possibly due to the variation in the dynamic balance state of the coordination of Rh and ligands.34
2. The TOF and chemoselectivity reached the highest at P/Rh ratio of 15. Lower activity and chemoselectivity were obtained with P/Rh ratio of 25. The influence of P/Rh ratio on the catalytic performance is possibly due to the variation in the dynamic balance state of the coordination of Rh and ligands.34
Under optimized reaction conditions, the IL-in-oil Pickering emulsion showed TOF of 413 h−1, chemoselectivity of 94% and n/b ratio of 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in the hydroformylation of 1-dodecene. Table S1† lists the catalytic performance of biphase systems reported previously (with Rh-sulfo-xantphos as ligand) in hydroformylation of long-chain alkenes. For previously reported oil–water or oil-IL biphase systems, the TOF for the hydroformylation of long-chain alkenes is less than 40 h−1, far less than that of the IL-in-oil Pickering emulsion reported in this work. The catalytic activity of the IL-in-oil Pickering emulsion approaches that of the water-in-oil microemulsion (TOF of 642 h−1, nonionic surfactant as emulsifier), verifying the high efficiency of the current oil-IL Pickering emulsion system.
2 in the hydroformylation of 1-dodecene. Table S1† lists the catalytic performance of biphase systems reported previously (with Rh-sulfo-xantphos as ligand) in hydroformylation of long-chain alkenes. For previously reported oil–water or oil-IL biphase systems, the TOF for the hydroformylation of long-chain alkenes is less than 40 h−1, far less than that of the IL-in-oil Pickering emulsion reported in this work. The catalytic activity of the IL-in-oil Pickering emulsion approaches that of the water-in-oil microemulsion (TOF of 642 h−1, nonionic surfactant as emulsifier), verifying the high efficiency of the current oil-IL Pickering emulsion system.
Hydroformylation of 1-dodecene in water-in-oil and IL-in-oil Pickering emulsion
Catalytic performances of water-in-oil and IL-in-oil Pickering emulsions were compared with that using DMSN-C18N-0.8 as the stabilizer in the hydroformylation of 1-dodecene (Tables 2 and 3). The water-in-oil emulsion exhibited similar conversion and n/b ratio as the IL-in-oil emulsion. But the latter exhibited much higher chemoselectivity (94% versus 69%). Compared with water, ILs have higher solubility towards lipophilic substrates35 and even toward CO.36 The unique properties of ILs prompted the reaction to go through hydroformylation pathways for enhancing chemoselectivity to aldehydes.| Reaction system | Ligand | Conv. (%) | Sel.b (%) | n/bc |
|---|---|---|---|---|
a Reaction conditions: Rh 4.5 × 10−3 mmol, S/C = 1000, P/Rh = 15, 110 °C, 20 bar CO/H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 1000 rpm, 12 h, 25 mL reactor.
b Selectivity to aldehyde.
c Normal/branched.
d TPPTS/ sulfo-xantphos = 13/1.
e Homogeneous system with toluene (2 mL) as solvent. 1), 1000 rpm, 12 h, 25 mL reactor.
b Selectivity to aldehyde.
c Normal/branched.
d TPPTS/ sulfo-xantphos = 13/1.
e Homogeneous system with toluene (2 mL) as solvent.
|
||||
| IL-in-oil Pickering emulsion | TPPTS | 67 | 37 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
| IL-in-oil Pickering emulsiond | TPPTS/sulfo-xantphos | 54 | 97 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| Water-in-oil Pickering emulsiond | TPPTS/sulfo-xantphos | 88 | 43 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| Homogeneous reactione | TPP | >99 | 99 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| Homogeneous reactione | TPP/xantphos | >99 | 99 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| IL-oil biphase reaction | TPPTS | 46 | 35 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| IL-oil biphase reaction | TPPTS/sulfo-xantphos | 21 | 78 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| Water-in-oil Pickering emulsion | TPPTS | 53 | 30 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| Water-in-oil Pickering emulsion | Sulfo-xantphos | 94 | 69 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
It is well known that triphenylphosphine (TPP) and xantphos have different bite angles, which greatly affects the n/b ratio (Scheme 1).37 Xantphos/sulfo-xantphos gave much higher n/b ratio and chemoselectivity than TPP/TPPTS did (Table 3). Unexpectedly, the IL-in-oil emulsion using the mixture of TPPTS and sulfo-xantphos as the ligand (sulfo-xantphos/TPPTS ratio as low as 1/13) gave an n/b ratio and chemoselectivity similar to those obtained using sulfo-xantphos as the ligand, though the conversion was less. However, the n/b ratio (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 versus 74
20 versus 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26) and chemoselectivity (43% versus 30%) of the water-in-oil emulsion using the mixture of TPPTS and sulfo-xantphos as the ligand were only slightly higher than that using TPPTS as the ligand. It revealed that a small amount of sulfo-xantphos in the IL-in-oil emulsion could greatly enhance both the n/b ratio and chemoselectivity. In order to further understand this unique phenomenon, hydroformylation of 1-dodecene was performed in a homogeneous system with toluene as the solvent and in the IL-oil biphase system with different ligands (Table 3). No obvious difference was observed in the conversion and n/b ratio between TPP and the mixture of TPP and xantphos in the homogeneous reaction system, suggesting that a small amount of xantphos has little effect on the n/b and chemoselectivity with toluene as the solvent. The results of the IL-oil biphase system were similar to those of the IL-in-oil Pickering emulsion system in that the n/b ratio and chemoselectivity could be greatly increased with a small amount of sulfo-xantphos. Consequently, the enhancement effect of n/b and chemoselectivity in the presence of a small amount of sulfo-xantphos could be possibly related with ILs. It is well known that ILs and TPPTS/sulfo-xantphos are all composed of cations and anions (Scheme 1), so that anions of TPPTS/sulfo-xantphos could interact with cations of ILs via electrostatic interactions.38 The interaction of the sulfonic group of TPPTS and ILs tended to increase the steric hindrance around the phosphorus atom, which may cause difficulty in the coordination of Rh with TPPTS. It has little effect on the coordination of sulfo-xantphos as the sulfonic group is attached at the 2 and 7 positions of xantphos. Therefore, Rh-sulfo-xantphos complexes are the main active species during the catalytic process of ILs even in the presence of a large amount of TPPTS. For these reasons, a high n/b ratio and chemoselectivity could be obtained. In water or toluene, there are no such strong interactions. As a result, Rh-TPPTS complexes are the main active species and n/b ratio and chemoselectivity could not be improved sharply in the presence of a small amount of sulfo-xantphos.
26) and chemoselectivity (43% versus 30%) of the water-in-oil emulsion using the mixture of TPPTS and sulfo-xantphos as the ligand were only slightly higher than that using TPPTS as the ligand. It revealed that a small amount of sulfo-xantphos in the IL-in-oil emulsion could greatly enhance both the n/b ratio and chemoselectivity. In order to further understand this unique phenomenon, hydroformylation of 1-dodecene was performed in a homogeneous system with toluene as the solvent and in the IL-oil biphase system with different ligands (Table 3). No obvious difference was observed in the conversion and n/b ratio between TPP and the mixture of TPP and xantphos in the homogeneous reaction system, suggesting that a small amount of xantphos has little effect on the n/b and chemoselectivity with toluene as the solvent. The results of the IL-oil biphase system were similar to those of the IL-in-oil Pickering emulsion system in that the n/b ratio and chemoselectivity could be greatly increased with a small amount of sulfo-xantphos. Consequently, the enhancement effect of n/b and chemoselectivity in the presence of a small amount of sulfo-xantphos could be possibly related with ILs. It is well known that ILs and TPPTS/sulfo-xantphos are all composed of cations and anions (Scheme 1), so that anions of TPPTS/sulfo-xantphos could interact with cations of ILs via electrostatic interactions.38 The interaction of the sulfonic group of TPPTS and ILs tended to increase the steric hindrance around the phosphorus atom, which may cause difficulty in the coordination of Rh with TPPTS. It has little effect on the coordination of sulfo-xantphos as the sulfonic group is attached at the 2 and 7 positions of xantphos. Therefore, Rh-sulfo-xantphos complexes are the main active species during the catalytic process of ILs even in the presence of a large amount of TPPTS. For these reasons, a high n/b ratio and chemoselectivity could be obtained. In water or toluene, there are no such strong interactions. As a result, Rh-TPPTS complexes are the main active species and n/b ratio and chemoselectivity could not be improved sharply in the presence of a small amount of sulfo-xantphos.
The recycling stability of water-in-oil and IL-in-oil Pickering emulsion systems was investigated (Fig. 6). In the reaction system, only 1-dodecene was used as the oil phase. After reaction, up to 93% of 1-dodecene was converted to the corresponding aldehyde under optimized conditions. At the end of the reaction, the emulsion was destroyed on account of change in polarity. The influence of the concentration of tridecyl aldehyde on the emulsion stability is shown in Fig. S5.† With increasing aldehyde concentration, the emulsion volume gradually decreased. The existence of the emulsion could still be observed in 1-dodecene conversion less than 80%, suggesting that hydroformylation occurs mainly in the emulsion system. The silica nanospheres were very stable and the emulsions could be easily formed by adding 1-dodecene after removing the oil phase at the end of the reaction (Fig. 6d and Fig. S6†).
 | ||
| Fig. 6 Recycling stability of (a) IL-in-oil Pickering emulsion system and (b) water-in-oil Pickering emulsion system in the hydroformylation of 1-dodecene (with DMSN-C18N-0.8 as the stabilizer, the reaction conditions are the same as those in Table 2), (c) TEM image of the DMSN-C18N-0.8 sample after 6 cycles, (d) photograph and microscopic image of the Pickering emulsion formed with DMSN-C18N-0.8 at the beginning of the fifth cycle (scale bar, 200 μm). | ||
After each cycle, the oil phase was separated after centrifugation, and a fresh oil phase was added again into the mixture of solid and IL or water. The Pickering emulsion regenerated by vigorous shaking was directly used for the next run. For the water-in-oil Pickering emulsion system, the obvious decreases in conversion, aldehyde selectivity and n/b ratio were observed for the third cycle, while for the IL-in-oil Pickering emulsion system, within six cycles, no obvious decreases in conversion, selectivity and n/b ratio could be observed, showing high recycling stability. After each cycle, the Rh content in the oil phase was analyzed by ICP analysis. For six cycles, the total Rh leaching in the oil phase was about 0.33%, indicating that the IL-in-oil Pickering emulsion was an efficient and stable reaction system for hydroformylation of long-chain alkenes. The TEM image of the DMSN-C18N-0.8 sample after six cycles was almost identical to that of fresh one, showing that the stabilizer was stable enough in the hydroformylation process (Fig. 6c).
Conclusions
In this work, an IL-in-oil Pickering emulsion was prepared for catalyzing the hydroformylation of 1-dodecene. The catalytic activity of the IL-in-oil Pickering emulsion was much higher than that of IL-oil biphase and micelle systems, revealing the role of the unique properties of the Pickering emulsion in accelerating reaction rate. The diffusion rate of the hydroformylation of 1-dodecene through the Pickering emulsion with DMSN-C18N-0.8 as the stabilizer was much faster than that with MCM-C18N-1.8 as the stabilizer, which was possibly attributed to the large pore diameter and open porous structure of DMSN. The IL-in-oil Pickering emulsion afforded higher chemoselectivity than the water-in-oil Pickering emulsion owing to the high solubility of ILs towards lipophilic substrates and CO. In ILs, Rh preferred to coordinate with sulfo-xantphos even in the presence of a large amount of TPPTS, probably because of the increased steric hindrance of TPPTS induced by its electrostatic interaction with ILs. The IL-in-oil Pickering emulsion could be recycled at least 5 times without any obvious loss of conversion and selectivity. The stable IL-oil Pickering emulsion may have potential applications in other liquid–liquid biphase reactions involving diffusion problems.Experimental section
Chemicals and reagents
All chemicals were used without further purification. Tetraethyl orthosilicate (TEOS, AR), hexadecyltrimethylammonium bromide (CTAB), toluene, trimethylamine, 1-dodecene and cyclohexane were purchased from Shanghai Chemical Reagent Company of the Chinese Medicine Group. Metal precursor Rh(acac)(CO)2 (acac = acetylacetonato) was purchased from the Energy Chemical Company. Dimethyloctadecyl[3-(trimethoxysilyl)propyl]ammonium chloride (C18N, 60% in MeOH) was purchased from Acros Company. 1-Dodecene, dodecane, undecanal and triphenylphosphine were purchased from TCI Company. 9,9-Dimethyl-4,5-bis(diphenylphosphino)xanthene (xantphos) was purchased from Tianjin Heowns Biochemical Technology Company. Tris(m-sulfonatophenyl) phosphine and tridecanal were purchased from J&K Chemicals. 1-Butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) was purchased from Ark Pharm Company. Rhodamine 6G was purchased from Aladdin Company. 9,9-Dimethyl-2,7-bissulfonato-4,5-bis(diphenylphosphino)xanthene sodium salt (sulfo-xantphos) was prepared according to a reported method.39 MCM-41 nanospheres40 and dendritic mesoporous silica nanospheres41 with a diameter of ∼100 nm were synthesized according to a reported method.Characterization
Transmission electron microscopy (TEM) was performed using an FEI Tecnai G2 Spirit at an acceleration voltage of 120 kV. Thermogravimetric analysis (TGA) was performed under air atmosphere with a heating rate of 5 °C min−1 to 900 °C using a NETZSCHSTA-449F3 thermogravimetric analyzer. FT-IR spectra were collected on a Nicolet Nexus 470 IR spectrometer with KBr pellets. Nitrogen physical adsorption measurements were carried out on Micromeritics ASAP2020 volumetric adsorption analyzer. Before the measurements, the samples were degassed at 393 K for 6 h. The BET surface area was evaluated from the data in the relative pressure range P/P0 of 0.05 to 0.25. The total pore volume was estimated from the amount adsorbed at the P/P0 value of 0.99. The pore size distribution was derived from the adsorption branch using the BJH method. The confocal laser scanning microscopy images of the emulsions were taken on Andor Spinning Disk Evolution WD microscope. The content of rhodium in the organic phase was determined by ICP-MS analysis.Surface modification of DMSN16
After degassing of dendritic mesoporous silica nanospheres (DMSN, 1 g) in a round bottom flask at 120 °C for 3 h, toluene (30 ml) and Et3N (1.2 ml) were added under N2 atmosphere. Then C18N in the MeOH solution (0.76 mL) was added and the mixture was stirred under reflux conditions at 110 °C for 12 h. After cooling down to room temperature, the solid materials were filtered, washed with methanol and toluene, and subsequently dried under vacuum at room temperature. The materials were denoted as DMSN-C18N-X, in which X refers to the mmol amount of C18N per gram of DMSN used during the grafting process. For example, DMSN-C18N-0.8 represents the material synthesized with 1.0 g of DMSN and 0.8 mmol of C18N.Construction of Pickering emulsion system for catalyzing the hydroformylation reaction
IL-in-oil Pickering catalytic emulsion system: a mixture of Rh(acac)(CO)2 (11.67 mg, 4.5 mmol) and desired amount of phosphine ligand were dissolved in 2 mL of degassed methanol for 0.5 h. After addition of 10 ml of [BMIM][BF4], the solution was stirred under N2 atmosphere overnight. The catalyst solution was obtained after removing methanol under vacuum at 40 °C.DMSN-C18N-X, 1-dodecene (1 mL) and tetradecane (0.3 mL as internal standard) were added to a glass vial. After ultrasonication for 1 min, the catalyst solution prepared above (1 mL) was added. Subsequently the mixture was vigorously shaken for about 15 min to form the IL-in-oil emulsion. Then the emulsion was transferred to an autoclave (25 mL). Before the reaction, the autoclave was purged three times with CO/H2 (molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Then, the pressure was raised to 20 bar and the autoclave was stirred in an oil bath at 110 °C for a desired time interval. After the autoclave was cooled down to room temperature, the gas was carefully released. The organic layer was separated by centrifugation, diluted with toluene and analyzed by gas chromatography on a HP-5 capillary column (30 m × 0.32 mm × 0.25 mm).
1). Then, the pressure was raised to 20 bar and the autoclave was stirred in an oil bath at 110 °C for a desired time interval. After the autoclave was cooled down to room temperature, the gas was carefully released. The organic layer was separated by centrifugation, diluted with toluene and analyzed by gas chromatography on a HP-5 capillary column (30 m × 0.32 mm × 0.25 mm).
For the catalyst recycling, the IL phase containing catalyst and solid materials were obtained after centrifugation and washed with toluene 3 times. Then the substrate and internal standard were added for the next catalytic reaction.
Water-in-oil Pickering catalytic emulsion system: the construction of the water-in-oil Pickering emulsion system is similar to that of the IL-in-oil Pickering emulsion system with the exception that water is used instead of [BMIM][BF4].
The IL-oil biphasic catalytic reaction system was prepared in a method similar to that of the IL-in-oil Pickering emulsion system with the exception that no DMSN was used.
General procedure for preparing continuous flow Pickering emulsion31
A glass column with a sand filter and a valve at the bottom was chosen as the column reactor. The inner diameter of the column is 1.05 cm. The desired volume of the emulsion was prepared as mentioned before. After one piece of membrane (oil-permeable but water-impermeable) was spread out on the sand filter, 3 g of silica was added. The prepared Pickering emulsion was poured carefully into this column; after that, 1-dodecene dyed with Sudan I was added on top of the emulsion. The diffusion rate of dyed 1-dodecene in different emulsion systems was calculated briefly with diffusion distant divided by time.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2017YFB0702800), the NSFC (No. 21232008, 21621063) and the Strategic Priority Research Program of the Chinese Academy of Sciences Grant (No. XDB17020200).Notes and references
- R. Franke, D. Selent and A. Borner, Chem. Rev., 2012, 112, 5675 CrossRef CAS PubMed.
- F. Hebrard and P. Kalck, Chem. Rev., 2009, 109, 4272 CrossRef CAS PubMed.
- F. Agbossou, J. F. Carpentier and A. Mortreaux, Chem. Rev., 1995, 95, 2485 CrossRef CAS.
- X. M. Zhang, S. M. Lu, M. M. Zhong, Y. P. Zhao and Q. H. Yang, Chin. J. Catal., 2015, 36, 168 CrossRef CAS.
- H. Adkins and G. Krsek, J. Am. Chem. Soc., 1949, 7, 3051 CrossRef.
- S. M. Lu and H. Alper, J. Am. Chem. Soc., 2003, 125, 13126 CrossRef CAS PubMed.
- P. W. N. M. van Leeuwen and C. Claver, Rhodium Catalysed Hydroformylation, Kluwer, Dordrecht, 2000 Search PubMed.
- C. C. Miyagawa, J. Kupka and A. Schumpe, J. Mol. Catal. A: Chem., 2005, 234, 9 CrossRef CAS.
- T. Ngai and S. A. F. Bon, Particle-stabilized emulsions and colloids: formation and applications, Cambridge, UK, 2015 Search PubMed.
- M. Pera-Titus, L. Leclercq, J. M. Clacens, F. De Campo and V. Nardello-Rataj, Angew. Chem., Int. Ed., 2015, 54, 2006 CrossRef CAS PubMed.
- F. Hapiot, S. Menuel, M. Ferreira, B. Léger, H. Bricout, S. Tilloy and E. Monflier, ACS Sustainable Chem. Eng., 2017, 5, 3598 CrossRef CAS.
- M. Chevry, T. Vanbésien, S. Menuel, E. Monflier and F. Hapiot, Catal. Sci. Technol., 2017, 7, 114 CAS.
- J. Potier, S. Menuel, M. H. Chambrier, L. Burylo, J. F. Blach, P. Woisel, E. Monflier and F. Hapiot, ACS Catal., 2013, 3, 1618 CrossRef CAS.
- J. Potier, S. Menuel, E. Monflier and F. Hapiot, ACS Catal., 2014, 4, 2342 CrossRef CAS.
- K. Kunna, C. Muller, J. Loos and D. Vogt, Angew. Chem., Int. Ed., 2006, 45, 7289 CrossRef CAS PubMed.
- Y. Zhao, X. Zhang, J. Sanjeevi and Q. H. Yang, J. Catal., 2016, 334, 52 CrossRef CAS.
- H. Q. Yang, L. M. Fu, L. J. Wei, J. F. Liang and B. P. Binks, J. Am. Chem. Soc., 2015, 137, 1362 CrossRef CAS PubMed.
- J. P. Huang and H. Q. Yang, Chem. Commun., 2015, 51, 7333 RSC.
- L. Kollar and J. Dupont, Ionic Liquids (ILs) in Organometallic Catalysis, Verlag, Heidelberg, 2015 Search PubMed.
- Q. H. Zhang, S. G. Zhang and Y. Q. Deng, Green Chem., 2011, 13, 2619 RSC.
- S. L. Desset, S. W. Reader and D. J. Cole-Hamilton, Green Chem., 2009, 11, 630 RSC.
- M. Haumann and A. Riisager, Chem. Rev., 2008, 108, 1474 CrossRef CAS PubMed.
- W. J. Zhou, L. Fang, Z. Fan, B. Albela, L. Bonneviot, F. De Campo, M. Pera-Titus and J. M. Clacens, J. Am. Chem. Soc., 2014, 136, 4869 CrossRef CAS PubMed.
- M. Li, R. L. Harbron, J. V. Weaver, B. P. Binks and S. Mann, Nat. Chem., 2013, 5, 529 CrossRef CAS PubMed.
- E. Vignati, R. Piazza and T. P. Lockhart, Langmuir, 2003, 19, 6650 CrossRef CAS.
- L. Davarpanah and F. Vahabzadeh, Starch-Starke, 2012, 64, 898 CrossRef CAS.
- A. Mann and M. Taddei, Hydroformylation for Organic Synthesis, Verlag, Heidelberg, 2013 Search PubMed.
- M. Kumar, R. V. Chaudhari, B. Subramaniam and T. A. Jackson, Organometallics, 2015, 34, 1062 CrossRef CAS.
- G. La Sorella, G. Strukul and A. Scarso, Green Chem., 2015, 17, 644 RSC.
- F. Xue, Y. B. Zhang, F. W. Zhang, X. M. Ren and H. Q. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 8403 CAS.
- M. Zhang, L. Wei, H. Chen, Z. Du, B. P. Binks and H. Q. Yang, J. Am. Chem. Soc., 2016, 138, 10173 CrossRef CAS PubMed.
- B. P. Binks and J. H. Clint, Langmuir, 2002, 18, 1270 CrossRef CAS.
- C. Li and Y. Liu, Bridging Heterogeneous and Homogeneous Catalysis, Wiley-VCH, Weinheim, Germany, 2014 Search PubMed.
- T. Hamerla, A. Rost, Y. Kasaka and R. Schomacker, ChemCatChem, 2013, 5, 1854 CrossRef CAS.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, Germany, 2007 Search PubMed.
- C. A. Ohlin, P. J. Dyson and G. Laurenczy, Chem. Commun., 2004, 1070 RSC.
- M. Kranenburg, Y. E. M. Vanderburgt, P. C. J. Kamer, P. W. N. M. van Leeuwen, K. Goubitz and J. Fraanje, Organometallics, 1995, 14, 3081 CrossRef CAS.
- A. Riisager, R. Fehrmann, M. Haumann, B. S. K. Gorle and P. Wasserscheid, Ind. Eng. Chem. Res., 2005, 44, 9853 CrossRef CAS.
- A. Osichow and S. Mecking, Chem. Commun., 2010, 46, 4980 RSC.
- A. Firouzi, F. Atef, A. G. Oertli, G. D. Stucky and B. F. Chmelka, J. Am. Chem. Soc., 1997, 119, 3596 CrossRef CAS.
- D. Shen, J. Yang, X. Li, L. Zhou, R. Zhang, W. Li, L. Chen, R. Wang, F. Zhang and D. Zhao, Nano Lett., 2014, 14, 923 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The additional figures and tables. See DOI: 10.1039/c7gc02574b |
| This journal is © The Royal Society of Chemistry 2018 |