Nine-step total synthesis of (−)-strychnofoline†
Qingzhen
Yu‡
ab,
Pan
Guo‡
ab,
Jie
Jian‡
ab,
Yuye
Chen
abc and
Jing
Xu
 *ab
*ab
aDepartment of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong Province, China. E-mail: xuj@sustc.edu.cn
bSUSTech, Grubbs Institute, Shenzhen, Guangdong Province, China
cState Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau, China
First published on 8th January 2018
Abstract
Strychnofoline is a Strychnos alkaloid that has unique spirooxindole architecture and possesses important anticancer activity. Here, we have, for the first time, reported the enantioselective synthesis of strychnofoline proceeding in only nine steps from commercially available 6-methoxytryptamine. The efficiency of the synthesis derives from the use of two sequential transformation steps in the catalytic asymmetric construction of the spiro[pyrrolidine-3,3′-oxindole] motif in a facile manner. Our route is amenable to the synthesis of other natural and synthetic analogs of bioactive spirooxindole alkaloids to access their therapeutic potential.
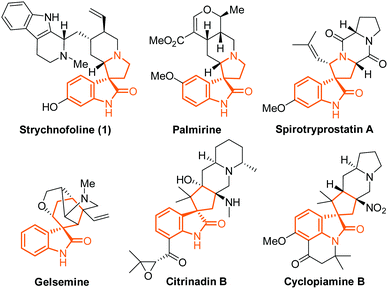
Spirooxindole alkaloids are intriguing and challenging synthetic targets, which have highly complicated architecture combined with promising activity in various therapeutic areas.1 Representative spirooxindole alkaloids include strychnofoline (1), palmirine,2 spirotryprostatins,3 gelsemine,4 citrinadins5 and cyclopiamines6 (Fig. 1). Intensive synthetic studies toward these alkaloids, including many impressive and successful approaches, have been previously reported.7–10
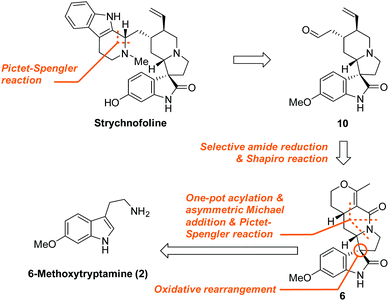
Amongst these fascinating molecules, 1 appears to be an attractive target for chemical synthesis and biological studies. It was isolated from the leaves of Strychnos usambarensis by Angenot et al. in 1978, and has demonstrated very promising antimitotic activity against cultures of mouse melanoma and Ehrlich tumor cells.11 An impressive synthesis of (±)-1 was reported by the Carreira group, in 2002, who used an elegant, highly diastereoselective cyclopropane ring expansion strategy.12 Given the significant physiological functions and complicated chemical skeleton of 1, a simple, asymmetric synthesis is evidently required for accessing its therapeutic potential. The five stereocentres and the unique spiro[pyrrolidine-3,3′-oxindole] motif present a substantial challenge for its synthesis. Here, we describe a concise, catalytic asymmetric synthesis of (−)-strychnofoline in only nine steps from a commercially available starting material.
Various innovative approaches have been developed to efficiently and asymmetrically construct the spirooxindole skeleton.13–15 Inspired by these studies, we devised a synthesis of 1, whose highlights are shown in Fig. 2. We envisioned that the β-carboline skeleton could be constructed via a late stage Pictet–Spengler reaction. A subsequent selective amide reduction and Shapiro tosylhydrazone decomposition would allow access to 10 from intermediate 6, which could arise from the 6-methoxytryptamine 2via sequential acylation/asymmetric Michael addition/Pictet–Spengler reaction/oxidative rearrangement.
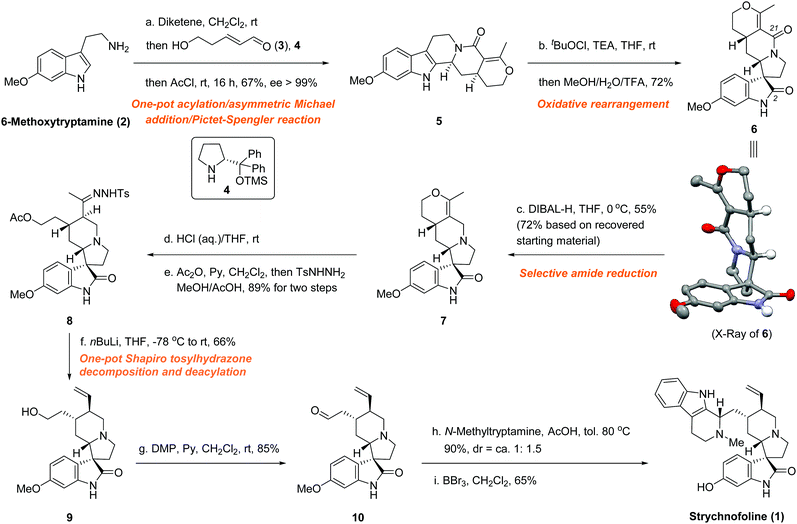
With this in mind, we developed a one-pot, catalytic asymmetric synthesis of the quinolizidine skeleton,14 which, with a subsequent rearrangement, allowed facile access to the spiro[pyrrolidine-3,3′-oxindole] motif in only two steps (Fig. 3). Our synthesis started with commercially available 6-methoxytryptamine (2). By sequentially adding 2; diketene, acrolein derivative 3; organocatalyst 4 (Hayashi–Jørgensen catalyst); and acyl chloride to the reaction mixture, we were able to achieve the quinolizidine derivative 5 in good yield with excellent enantioselectivity (67% yield, ee > 99%).
Subsequently, the seemingly simple transformation from 5 to 7 turned out to be remarkably challenging. Although a reduction using LiAlH4 conditions could easily afford the corresponding tertiary amine in good yield, the various rearrangement conditions7a,b,10,16 tested were unsuccessful, most likely because of the presence of the sensitive tertiary amine. Trials using a Brønsted acid protonated substrate17 also resulted in failure. Therefore, we had to perform the skeleton rearrangement first, hoping that subsequent selective amide reduction would provide access to the pivotal intermediate 7. To this end, a tert-butyl hypochlorite induced rearrangement successfully furnished 6.16a–c,e,h,j The plausible chloroindolenine intermediate was not isolated, but directly transformed into the spiro[pyrrolidine-3,3′-oxindole] intermediate 6 in a one-pot rearrangement using acidic methanol conditions. To our great pleasure, single-crystal X-ray diffraction unambiguously confirmed all the desired stereochemistry.18 The undesired epimer due to the rearrangement was not observed, presumably because of the different thermodynamic stabilities of the two possible epimers. Then, various reductive conditions were intensively screened to selectively reduce the C-21 (based on the carbon numbering of 1) amide functionality over the C-2 amide functionality. Although similar transformations are known from the literature using alane16c,19 or Lawesson's reagent15t,20 followed by certain reducing conditions, the extra unsaturated C–C bond, which is adjacent to the C-21 amide, created a significant challenge for the desired selective reduction. Most tested conditions resulted in either global reduction or disfavoured selectivity. Fortunately, we found that using six equivalents of DIBAL-H successfully differentiated between the two amide functionalities to achieve the desired mono-reduced product 7 (55% yield, 72% brsm).
The final steps of our synthesis began with the acidic hydrolysis of the cyclic enol ether functionality of 7, which easily furnished the corresponding ketone (Fig. 3). Gratifyingly, we found that the acylation of the primary hydroxyl group and the transformation from ketone to tosylhydrazone could be performed in one-pot, thus further improving the overall efficiency, to yield hydrazone 8 (89% overall yield from 7). Shapiro tosylhydrazone decomposition and concomitant deacylation using an excess amount of n-BuLi smoothly produced alkene 9 in 66% yield.14c Subsequently, the Dess–Martin oxidation of the primary hydroxyl group afforded aldehyde 10 in 85% yield. Condensation of aldehyde 10 with N-methyltryptamine produced the corresponding O-methyl strychnofoline in 90% yield. We observed the same diastereoselectivity issue that was encountered by Carreira et al. (dr = ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5).12 Efforts to improve the diastereoselectivity of this Pictet–Spengler reaction were unsuccessful, although a few examples have been reported.21 Nonetheless, this minor botheration was well compensated for by the high overall efficiency of our strategy. Eventually, demethylation of the O-methyl strychnofoline using boron tribromide successfully furnished 1 in 65% yield. To our great pleasure, the synthetic material, thus obtained, exhibited identical spectroscopic and analytical properties to those reported for the natural product.11,12
1.5).12 Efforts to improve the diastereoselectivity of this Pictet–Spengler reaction were unsuccessful, although a few examples have been reported.21 Nonetheless, this minor botheration was well compensated for by the high overall efficiency of our strategy. Eventually, demethylation of the O-methyl strychnofoline using boron tribromide successfully furnished 1 in 65% yield. To our great pleasure, the synthetic material, thus obtained, exhibited identical spectroscopic and analytical properties to those reported for the natural product.11,12
In summary, we have successfully conducted the rapid, enantioselective synthesis of the anti-tumor alkaloid strychnofoline (1) in only nine steps. Our strategy is highlighted by (a) a one-pot, catalytic asymmetric construction of quinolizidine intermediate 5; (b) an efficient, diastereoselective skeleton rearrangement that formed the spiro[pyrrolidine-3,3′-oxindole] motif of 1; and (c) a selective amide reduction that successfully differentiated two similar amide motifs. A convenient preparation of 1 will be of great assistance in addressing its therapeutic promise. Moreover, our work provides an efficient strategy toward the synthesis of various bioactive spirooxindole alkaloids. Encouraged by this promising result, further efforts to synthesize natural and unnatural spirooxindoles and related biological investigations are currently underway in our laboratory and will be reported in due course.
Financial support from the National Natural Science Foundation of China (No. 21402082, No. 21772082 and No. 21702094), SZSTI (Pu20150267, Ji20170314 and Peacock Tech-Innovation 2018), and SZDRC (K16205905) is greatly appreciated. We also thank Professor Bin Tan (SUSTech) for useful comments.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) H. Lin and S. J. Danishefsky, Angew. Chem., Int. Ed., 2003, 42, 36–51 CrossRef CAS; (b) C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209–2219 CrossRef CAS; (c) M. M. C. Lo, C. S. Neumann, S. Nagayama, E. O. Perlstein and S. L. Schreiber, J. Am. Chem. Soc., 2004, 126, 16077–16086 CrossRef CAS PubMed; (d) C. Chen, X. D. Li, C. S. Neumann, M. M. C. Lo and S. L. Schreiber, Angew. Chem., Int. Ed., 2005, 44, 2249–2252 CrossRef CAS PubMed; (e) K. Ding, Y. Lu, Z. Nikolovska-Coleska, S. Qiu, Y. Ding, W. Gao, J. Stuckey, K. Krajewski, P. P. Roller, Y. Tomita, D. A. Parrish, J. R. Deschamps and S. Wang, J. Am. Chem. Soc., 2005, 127, 10130–10131 CrossRef CAS PubMed; (f) A. K. Franz, P. D. Dreyfuss and S. L. Schreiber, J. Am. Chem. Soc., 2007, 129, 1020–1021 CrossRef CAS PubMed; (g) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed; (h) S. Shangary, D. Qin, D. McEachern, M. Liu, R. S. Miller, S. Qiu, Z. Nikolovska-Coleska, K. Ding, G. Wang, J. Chen, D. Bernard, J. Zhang, Y. Lu, Q. Gu, R. B. Shah, K. J. Pienta, X. Ling, S. Kang, M. Guo, Y. Sun, D. Yang and S. Wang, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3933–3938 CrossRef CAS PubMed; (i) M. Rottmann, C. McNamara, B. K. S. Yeung, M. C. S. Lee, B. Zou, B. Russell, P. Seitz, D. M. Plouffe, N. V. Dharia, J. Tan, S. B. Cohen, K. R. Spencer, G. E. Gonzalez-Paéz, S. B. Lakshminarayana, A. Goh, R. Suwanarusk, T. Jegla, E. K. Schmitt, H.-P. Beck, R. Brun, F. Nosten, L. Renia, V. Dartois, T. H. Keller, D. A. Fidock, E. A. Winzeler and T. T. Diagana, Science, 2010, 329, 1175–1180 CrossRef CAS PubMed; (j) B. K. S. Yeung, B. Zou, M. Rottmann, S. B. Lakshminarayana, S. H. Ang, S. Y. Leong, J. Tan, J. Wong, S. Keller-Maerki, C. Fischli, A. Goh, E. K. Schmitt, P. Krastel, E. Francotte, K. Kuhen, D. Plouffe, K. Henson, T. Wagner, E. A. Winzeler, F. Petersen, R. Brun, V. Dartois, T. T. Diagana and T. H. Keller, J. Med. Chem., 2010, 53, 5155–5164 CrossRef CAS PubMed; (k) M. M. M. Santos, Tetrahedron, 2014, 70, 9735–9757 CrossRef CAS; (l) T. L. Pavlovska, R. Gr. Redkin, V. V. Lipson and D. V. Atamanuk, Mol. Diversity, 2016, 20, 299–344 CrossRef CAS PubMed.
- J. Borges, M. T. Manresa, J. L. Martín Ramón, C. Pascual and A. Rumbero, Tetrahedron Lett., 1979, 20, 3197–3200 CrossRef.
- (a) C. B. Cui, H. Kakeya and H. Osada, J. Antibiot., 1996, 49, 832–835 CrossRef CAS PubMed; (b) C. B. Cui, H. Kakeya and H. Osada, Tetrahedron, 1996, 51, 12651–12666 CrossRef.
- (a) F. L. Sonnenschein, Ber. Dtsch. Chem. Ges., 1876, 9, 1182–1186 CrossRef; (b) H. Conroy and J. K. Chakrabarti, Tetrahedron Lett., 1959, 1, 6–13 CrossRef; (c) Y. Schun and G. A. Cordell, J. Nat. Prod., 1985, 48, 969–971 CrossRef CAS PubMed; (d) F. M. Lovell, R. Pepinsky and A. J. C. Wilson, Tetrahedron Lett., 1959, 1, 1–5 CrossRef.
- (a) M. Tsuda, Y. Kasai, K. Komatsu, T. Sone, M. Tanaka, Y. Mikami and J. Kobayashi, Org. Lett., 2004, 6, 3087–3089 CrossRef CAS PubMed; (b) T. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, E. Fukushi, J. Kawabata, M. Watanabe, K. Akao and J. Kobayashi, J. Org. Chem., 2005, 70, 9430–9435 CrossRef CAS PubMed.
- R. F. Bond, J. C. A. Boeyens, C. W. Holzapfel and P. S. Steyn, J. Chem. Soc., Perkin Trans. 1, 1979, 1751–1761 RSC.
- For total syntheses of spirotryprostatins: (a) S. D. Edmonson and S. J. Danishefsky, Angew. Chem., Int. Ed., 1998, 37, 1138–1140 CrossRef; (b) S. D. Edmonson and S. J. Danishefsky, J. Am. Chem. Soc., 1999, 121, 2147–2155 CrossRef; (c) F. von Nussbaum and S. J. Danishefsky, Angew. Chem., Int. Ed., 2000, 39, 2175–2178 CrossRef CAS; (d) L. E. Overman and M. D. Rosen, Angew. Chem., Int. Ed., 2000, 39, 4596–4599 CrossRef CAS; (e) H. Wang and A. Ganesan, J. Org. Chem., 2000, 65, 4685–4693 CrossRef CAS PubMed; (f) P. R. Sebahar and R. M. Williams, J. Am. Chem. Soc., 2000, 122, 5666–5667 CrossRef CAS; (g) P. R. Sebahar, H. Osada, T. Usui and R. M. Williams, Tetrahedron, 2002, 58, 6311–6322 CrossRef CAS; (h) T. D. Bagul, G. Lakshmaiah, T. Kawabata and K. Fuji, Org. Lett., 2002, 4, 249–251 CrossRef CAS PubMed; (i) C. Meyers and E. M. Carreira, Angew. Chem., Int. Ed., 2003, 42, 694–696 CrossRef CAS PubMed; (j) T. Onishi, P. R. Sebahar and R. M. Williams, Org. Lett., 2003, 5, 3135–3137 CrossRef CAS PubMed; (k) T. Onishi, P. R. Sebahar and R. M. Williams, Tetrahedron, 2004, 60, 9503–9515 CrossRef CAS; (l) F. Y. Miyake, K. Yakushijin and D. A. Horne, Angew. Chem., Int. Ed., 2004, 43, 5357–5360 CrossRef CAS PubMed; (m) F. Y. Miyake, K. Yakushijin and D. A. Horne, Org. Lett., 2004, 6, 4249–4251 CrossRef CAS PubMed; (n) C. Marti and E. M. Carreira, J. Am. Chem. Soc., 2005, 127, 11505–11515 CrossRef CAS PubMed; (o) B. M. Trost and D. T. Stiles, Org. Lett., 2007, 9, 2763–2766 CrossRef CAS PubMed; (p) K. Kitahara, J. Shimokawa and T. Fukuyama, Chem. Sci., 2014, 5, 904–907 RSC.
- For total syntheses of gelsemine: (a) Z. Sheikh, R. Steel, A. S. Tasker and A. P. Johnson, J. Chem. Soc., Chem. Commun., 1994, 763–764 RSC; (b) J. K. Dutton, R. W. Steel, A. S. Tasker, V. Popsavin and A. P. Johnson, J. Chem. Soc., Chem. Commun., 1994, 765–766 RSC; (c) N. J. Newcombe, F. Ya, R. J. Vijn, H. Hiemstra and W. N. Speckamp, J. Chem. Soc., Chem. Commun., 1994, 767–768 RSC; (d) T. Fukuyama and G. Liu, J. Am. Chem. Soc., 1996, 118, 7426–7427 CrossRef CAS; (e) S. Atarashi, J.-K. Choi, D.-C. Ha, D. J. Hart, D. Kuzmich, C.-S. Lee, S. Ramesh and S. C. Wu, J. Am. Chem. Soc., 1997, 119, 6226–6241 CrossRef CAS; (f) A. Madin, C. J. O’Donnell, T. Oh, D. W. Old, L. E. Overman and M. J. Sharp, Angew. Chem., Int. Ed., 1999, 38, 2934–2936 CrossRef CAS; (g) S. Yokoshima, H. Tokuyama and T. Fukuyama, Angew. Chem., Int. Ed., 2000, 39, 4073–4075 CrossRef CAS; (h) F. W. Ng, H. Lin and S. J. Danishefsky, J. Am. Chem. Soc., 2002, 124, 9812–9824 CrossRef CAS PubMed; (i) H. Lin, F. W. Ng and S. J. Danishefsky, Tetrahedron Lett., 2002, 43, 549–551 CrossRef CAS; (j) W. G. Earley, J. E. Jacobsen, A. Madin, G. P. Meier, C. J. O'Donnell, T. Oh, D. W. Old, L. E. Overman and M. J. Sharp, J. Am. Chem. Soc., 2005, 127, 18046–18053 CrossRef CAS PubMed; (k) A. Madin, C. J. O'Donnell, T. Oh, D. W. Old, L. E. Overman and M. J. Sharp, J. Am. Chem. Soc., 2005, 127, 18054–18065 CrossRef CAS PubMed; (l) X. Zhou, T. Xiao, Y. Iwama and Y. Qin, Angew. Chem., Int. Ed., 2012, 51, 4909–4912 CrossRef CAS PubMed; (m) X. Chen, S. Duan, C. Tao, H. Zhai and F. G. Qiu, Nat. Commun., 2015, 6, 7204–7210 CrossRef PubMed.
- For total syntheses of citrinadins: (a) Z. Bian, C. C. Marvin and S. F. Martin, J. Am. Chem. Soc., 2013, 135, 10886–10889 CrossRef CAS PubMed; (b) K. Kong, J. A. Enquist Jr., M. E. McCallum, G. M. Smith, T. Matsumaru, E. Menhaji-Klotz and J. L. Wood, J. Am. Chem. Soc., 2013, 135, 10890–10893 CrossRef CAS PubMed; (c) Z. Bian, C. C. Marvin, M. Pettersson and S. F. Martin, J. Am. Chem. Soc., 2014, 136, 14184–14192 CrossRef CAS PubMed.
- For total syntheses of cyclopiamine B: E. V. Mercado-Marin, P. Garcia-Reynaga, S. Romminger, E. F. Pimenta, D. K. Romney, M. W. Lodewyk, D. E. Williams, R. J. Andersen, S. J. Miller, D. J. Tantillo, R. G. S. Berlinck and R. Sarpong, Nature, 2014, 509, 318–324 CrossRef CAS PubMed.
- (a) L. Angenot, Plant. Med. Phytother., 1978, 12, 123–129 CAS; (b) R. Bassleer, M. C. Depauw-Gillet, B. Massart, J.-M. Marnette, P. Wiliquet, M. Caprasse and L. Angenot, Planta Med., 1982, 45, 123–126 CrossRef CAS PubMed.
- (a) A. Lerchner and E. M. Carreira, J. Am. Chem. Soc., 2002, 124, 14826–14827 CrossRef CAS PubMed; (b) A. Lerchner and E. M. Carreira, Chem. – Eur. J., 2006, 12, 8208–8219 CrossRef CAS PubMed.
- For reviews: (a) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381–1407 CrossRef CAS; (b) R. Dalpozzo, G. Bartoli and G. Bencivenni, Chem. Soc. Rev., 2012, 41, 7247–7290 RSC; (c) N. R. Ball-Jones, J. J. Badille and A. K. Franz, Org. Biomol. Chem., 2012, 10, 5165–5181 RSC; (d) L. Hong and R. Wang, Adv. Synth. Catal., 2013, 355, 1023–1052 CrossRef CAS; (e) D. Cheng, Y. Ishihara, B. Tan. and C. F. Barbas, III, ACS Catal., 2014, 4, 743–762 CrossRef CAS; (f) Z.-Y. Cao and J. Zhou, Org. Chem. Front., 2015, 2, 849–858 RSC; (g) R. Dalpozzo, Adv. Synth. Catal., 2017, 359, 1772–1810 CrossRef CAS.
- (a) J. Franzén and A. Fisher, Angew. Chem., Int. Ed., 2009, 48, 787–791 CrossRef PubMed; (b) W. Zhang and J. Franzén, Adv. Synth. Catal., 2010, 352, 499–518 CrossRef CAS; (c) W. Zhang, J. Bah, A. Wohlfarth and J. Franzén, Chem. – Eur. J., 2011, 17, 13814–13824 CrossRef CAS PubMed.
- For related studies, see: (a) X. Y. Wu, X. Y. Dai, L. L. Nie, H. H. Fang, J. Chen, Z. J. Ren, W. G. Cao and G. Zhao, Chem. Commun., 2010, 46, 2733–2735 RSC; (b) H. H. Fang, X. Y. Wu, L. L. Nie, X. Y. Dai, J. Chen, W. G. Cao and G. Zhao, Org. Lett., 2010, 12, 5366–5369 CrossRef CAS PubMed; (c) Z. C. Jin, X. Wang, H. C. Huang, X. M. Liang and J. X. Ye, Org. Lett., 2011, 13, 564–567 CrossRef CAS PubMed; (d) S. L. Zhou, J. L. Li, L. Dong and Y. C. Chen, Org. Lett., 2011, 13, 5874–5877 CrossRef CAS PubMed; (e) X. Y. Wu, X. Y. Dai, H. H. Fang, L. L. Nie, J. Chen, W. G. Cao and G. Zhao, Chem. – Eur. J., 2011, 17, 10510–10514 CrossRef CAS PubMed; (f) X. Sun and D. Ma, Chem. – Asian J., 2011, 6, 2158–2165 CrossRef CAS PubMed; (g) Z. C. Jin, H. C. Huang, W. J. Li, X. Y. Luo, X. M. Liang and J. X. Ye, Adv. Synth. Catal., 2011, 353, 343–348 CrossRef CAS; (h) M. Rueping, C. M. R. Volla, M. Bolte and G. Raabe, Adv. Synth. Catal., 2011, 353, 2853–2859 CrossRef CAS; (i) Z. Jin, F. Yu, X. Wang, H. Huang, X. Luo, X. Liang and J. Ye, Org. Biomol. Chem., 2011, 9, 1809–1816 RSC; (j) M. Rueping and C. M. R. Volla, RSC Adv., 2011, 1, 79–82 RSC; (k) X. Y. Dai, X. Y. Wu, H. H. Fang, L. L. Nie, J. Chen, H. M. Deng, W. G. Cao and G. Zhao, Tetrahedron, 2011, 67, 3034–3040 CrossRef CAS; (l) X. Y. Wu, H. H. Fang, Q. Liu, L. L. Nie, J. Chen, W. G. Cao and G. Zhao, Tetrahedron, 2011, 67, 7251–7257 CrossRef CAS; (m) X. Wu, Y. Zhang, X. Dai, H. Fang, J. Chen, W. Cao and G. Zhao, Synthesis, 2011, 3675–3679 CrossRef CAS; (n) X. Wu, Q. Liu, H. Fang, J. Chen, W. Cao and G. Zhao, Chem. – Eur. J., 2012, 18, 12196–12201 CrossRef CAS PubMed; (o) M. E. Muratore, L. Shi, A. W. Pilling, R. I. Storer and D. J. Dixon, Chem. Commun., 2012, 48, 6351–6353 RSC; (p) S. Z. Lin, L. Deiana, A. Tseggai and A. Córdova, Eur. J. Org. Chem., 2012, 398–408 CrossRef CAS; (q) B.-C. Hong, W.-K. Liao, N. S. Dange and J.-H. Liao, Org. Lett., 2013, 15, 468–471 CrossRef CAS PubMed; (r) I. Aillaud, D. M. Barber, A. L. Thompson and D. J. Dixon, Org. Lett., 2013, 15, 2946–2949 CrossRef CAS PubMed; (s) P. A. Suryavanshi, V. Sridharan and J. C. Menéndez, Chem. – Eur. J., 2013, 19, 13207–13215 CrossRef CAS PubMed; (t) H. Zhang, X. Ma, H. Kang, L. Hong and R. Wang, Chem. – Asian J., 2013, 8, 542–545 CrossRef CAS PubMed; (u) Y. Tan, H.-L. Luan, H. Lin, X.-W. Sun, X.-D. Yang, H.-Q. Dong and G.-Q. Lin, Chem. Commun., 2014, 50, 10027–10030 RSC; (v) H. Du, J. Rodriguez, X. Bugaut and T. Constantieux, Adv. Synth. Catal., 2014, 356, 851–856 CrossRef CAS; (w) L. Li, P. Aibibula, Q. Jia and Y. Jia, Org. Lett., 2017, 19, 2642–2645 CrossRef CAS PubMed.
- (a) N. Finch and W. I. Taylor, J. Am. Chem. Soc., 1962, 84, 1318–1320 CrossRef CAS; (b) J. Shavel and H. Zinnes, J. Am. Chem. Soc., 1962, 84, 1320–1321 CrossRef CAS; (c) S. F. Martin and M. Mortimore, Tetrahedron Lett., 1990, 31, 4557–4560 CrossRef CAS; (d) X. Zhang and C. S. Foote, J. Am. Chem. Soc., 1993, 115, 8867–8868 CrossRef CAS; (e) M. Ito, C. W. Clark, M. Mortimore, J. Betty Goh and S. F. Martin, J. Am. Chem. Soc., 2001, 123, 8003–8010 CrossRef CAS PubMed; (f) X. Z. Wearing and J. M. Cook, Org. Lett., 2002, 4, 4237–4940 CrossRef CAS PubMed; (g) H. Takayama, R. Fujiwara, Y. Kasai, M. Kitajima and N. Aimi, Org. Lett., 2003, 5, 2967–2970 CrossRef CAS PubMed; (h) P. S. Baran and J. M. Richter, J. Am. Chem. Soc., 2005, 127, 15394–15396 CrossRef CAS PubMed; (i) T. J. Greshock and R. M. Williams, Org. Lett., 2007, 9, 4255–4258 CrossRef CAS PubMed; (j) C. Poriel, M. Lachia, C. Wilson, J. R. Davies and C. J. Moody, J. Org. Chem., 2007, 72, 2978–2987 Search PubMed; (k) M. Pettersson, D. Knueppel and S. F. Martin, Org. Lett., 2007, 9, 4623–4626 CrossRef CAS PubMed; (l) J. Yang, X. Z. Wearing, P. W. Le Quesne, J. R. Deschamps and J. M. Cook, J. Nat. Prod., 2008, 71, 1431–1440 CrossRef CAS PubMed; (m) D. A. Mundal and R. Sarpong, Org. Lett., 2013, 15, 4952–4955 CrossRef CAS PubMed.
- C. A. Guerrero and E. J. Sorensen, Org. Lett., 2011, 13, 5164–5167 CrossRef CAS PubMed.
- CCDC 1571159.† Some hydrogen atoms of compound 6 were omitted for clarity.
- M. Amat, C. Ramos, M. Pérez, E. Molins, P. Florindo, M. M. M. Santos and J. Bosch, Chem. Commun., 2013, 49, 1954–1956 RSC.
- J.-Y. Laronzo, B. Guilleteau, D. Cartier, J. Laronze and J. Lévy, Heterocycles, 1989, 29, 2051–2055 CrossRef.
- R. S. Klausen, C. R. Kennedy, A. M. Hyde and E. N. Jacobsen, J. Am. Chem. Soc., 2017, 139, 12299–12309 CrossRef CAS PubMed and references therein.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and crystallographic data of 6. CCDC 1571159. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc08938d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |