 Open Access Article
Open Access ArticleDevelopment of sustainable bio-adhesives for engineered wood panels – A Review
Venla Hemmilä
*a,
Stergios Adamopoulos
 *a,
Olov Karlsson
b and
Anuj Kumar
*a,
Olov Karlsson
b and
Anuj Kumar
 a
a
aDepartment of Forestry and Wood Technology, Linnaeus University, Lückligs Plats 1, 351 95 Växjö, Sweden. E-mail: venla.hemmila@lnu.se; stergios.adamopoulos@lnu.se; Tel: +46 470767547
bWood Technology, TVM, Luleå University of Technology, 931 87 Skellefteå, Sweden
First published on 7th August 2017
Abstract
Changes in both formaldehyde legislations and voluntary requirements (e.g. Germany RAL) are currently the driving factors behind research on alternatives to amino-based adhesives; moreover, consumer interest in healthy and sustainable products is increasing in bio-based adhesives. Sources of formaldehyde emissions in wood-based panels as well as different emission test methods have been discussed, and the main focus of this review is on the research conducted on sustainable bio-based adhesive systems for wood panels. Lignin, tannin, protein, and starch have been evaluated as both raw materials and adhesive alternatives to existing amino-based thermosetting adhesives. Adhesion improving modifications of these bio-based raw materials as well as the available and experimental crosslinkers have also been taken into account.
1. Introduction
The wood panel industry uses almost exclusively synthetic, petroleum-derived thermosetting adhesives, which are mainly based on the reaction of formaldehyde with urea, melamine, phenol, or co-condensates.1 The low-cost and good adjustable properties of these adhesives have made it difficult for new bio-based alternatives (e.g. lignin, tannin, starch, protein) to enter this market. Sustainable adhesives should not only be available at low costs, but also need to be easily distributable, fast reacting, and have a long pot life.1 The mechanical strength of manufactured panels and especially their moisture tolerance are additional crucial parameters the new, bio-based adhesives have yet to fulfil satisfactorily.2There are two main factors driving the trend to move away from using formaldehyde-based synthetic resins for wood-based panel manufacturing: formaldehyde emissions and sustainability of raw materials and final products. In the panel industry, sustainability and petroleum independency cannot, as of yet, justify the increase in cost due to new bio-based adhesives. Thus, concern about formaldehyde emissions from panels, especially in indoor applications, is currently the most important driving factor.3 Legislations concerning both work environment and final product emissions have steadily become stricter over time. In 2004, the International Agency for Research on Cancer (IARC) re-classified formaldehyde from “probable human carcinogen” to “known human carcinogen”.4 The California Air Resources Board (CARB) also formulated regulations with new limits for formaldehyde emissions from composite wood products.5 It is unclear how the limits of formaldehyde emissions for wood panels will change in different parts of the world in the future. The wood-based panel industry has so far reacted by applying appropriate formaldehyde scavengers (catchers) by developing low-emission melamine fortified urea-formaldehyde (MUF) adhesives and by employing other synthetic or bio-based adhesives that do not contain formaldehyde.
As a volatile organic compound (VOC), most of the formaldehyde is normally emitted from panels during production. There are two formaldehyde sources when producing wood panels: formaldehyde that might be contained in the adhesive and that in the wood material itself. The emissions can be reduced by either using formaldehyde scavengers or by decreasing free formaldehyde in the adhesive and number of formaldehyde emitting groups during and after curing (e.g. reducing formaldehyde/urea molar ratio).2 The most typical scavenger for wood-based panels is urea, but other compounds like ammonia and ammonium salts can also be used.6 They can be added directly to the synthetic resin or wood particles. Some organic scavengers, such as tannin powder, wheat flour and charcoal have also been shown to reduce formaldehyde emissions.7 By loading urea onto the nano-mesoporous structure of an inorganic additive (diatomaceous earth), an improved scavenging function of urea has been shown.8 Additionally, various types of carbon based nanomaterials such as activated nano-carbon, carbon nanotubes, exfoliated graphite nanoparticles are used as formaldehyde adsorbents in formaldehyde-based adhesives.9–12
Synthetic diphenylmethane diisocyanates (MDIs) offer non-formaldehyde emitting solutions for panel producers. The most commonly used MDI for panel production is polymeric MDI (pMDI). The produced panels have better mechanical (i.e. stiffness) and physical (resistance towards humidity) properties.2 In North America, MDIs are commonly used for construction panels (oriented strand board, OSB) and to some extent for other panel types such as particleboards (PBs), high density fibreboards (HDFs) and medium density fibreboards (MDFs). Using MDIs for these boards is possible because their cost, with reduced application amounts, is comparable to that of melamine fortified urea-formaldehyde adhesives (MUFs). However, in Europe, prices for MDIs exceed those for UFs and MUFs. Also, extra safety control is required due to toxicity of isocyanate during production. This makes transition to the use of MDIs slower and is thus more difficult to implement on a large scale in Europe. Except for lower adhesive amounts, MDIs offer no clear advantage with respect to sustainability or environmental friendliness in panel products.
“Bio-based product” is defined as “a commercial or industrial product (other than food or feed) that is composed, in whole or in significant part, of biological products or renewable domestic agricultural materials (including plant, animal, and marine materials) or forestry materials”.13 Only a few bio-based industrial adhesive products exist for panels, and these have a high cost. They can be used to produce premium priced panels, but so far, they are not economically feasible for mainstream panel production. For these adhesives and for the ones still in development, a synthetic cross-linker is usually required to reach the required properties at reasonable cost. Soy protein is among the first bio-based adhesives to be launched commercially for plywood manufacturing, and the most promising research is around different kinds of proteins.14 Although in limited capacity, industrial applications also exist for panel adhesives based on tannin and starch. Lignin-based adhesives are also of interest as new types of bio-refineries increasingly bring new types of lignin to the market.
This article presents a review of the research undertaken on various bio-based adhesive systems usable for wood panels as alternatives to existing amino-based thermosetting adhesives. The first part focuses on the structure and availability of different sustainable raw materials that can be used to formulate adhesives, such as lignin, starch, protein and tannin, as presented in Table 1.15 The second part focuses on the suitability of these raw materials as adhesives. Bio-based adhesives tested on veneer and solid wood are included, as this testing approach is quite common in the initial stages of wood-based panel adhesive development. Future prospects of promising adhesives for the wood-based panel industry are also given, taking into account recent opportunities and challenges caused by emerging bio-refineries and food shortage policies.
| Source | Biopolymer type | Industrial uses |
|---|---|---|
| Trees, plants, plant biomass, plant waste and by-products from bio-processing | Cellulose | Textiles, wood manufacturing, and composites |
| Trees, plants, recovered from pulping processes | Lignin | Adhesives, coatings, paints, and plastics |
| Corn, potato, cassava, wheat, etc. | Starch | Adhesives, foams, food, plastics, gums and pharmaceutics |
| Soybean, vegetables, fruits and animals | Protein | Plastics, adhesives, and composites |
| Soybean, vegetable crops and specialty crops | Oils and waxes | Adhesives, resins, coatings and paints |
| Shell fish, fin fish and fish waste | Chitin | Gums, foods, pharmaceutics and cosmetics |
| Citrus fruits and their waste | Pectin | Food, gum, emulsifiers, pharmaceutics and cosmetics |
| Rubber trees or guayule shrubs | Latex | Aerospace, medical, plastics and adhesives |
2. Wood-based panels (WBPs) and adhesives
Wood-based panels (WBPs) are composite products manufactured by effective bonding of wood materials (fibers, flakes, particles, chips, wood powder, veneers, etc.) with various adhesives. WBPs are classified by usage for structural or non-structural panels, to the exterior or interior grade panels, and by the type of wood and materials used ranging from fiberboards to laminated beams. Maloney (1977)16 classifies WBPs according to the type of raw materials used and process of manufacturing (dry and wet). Further, he proposes the division of panels according to their density and specific gravity, as shown in Fig. 1.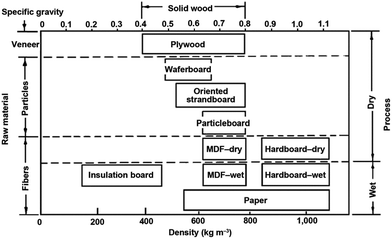 | ||
| Fig. 1 Classification of wood-based panels.17 | ||
Urea-formaldehyde (UF) resins are extensively used as adhesives in the production of WBPs like medium density fiberboards (MDFs), particleboards and plywood. MDFs and particleboards consume 68% of UF resins produced in the world, while 23% is used in plywood manufacturing. Other types of adhesives predominantly used in the manufacturing of WBPs are phenol-formaldehyde (PF), melamine-urea-formaldehyde (MUF) and polymeric 4,4-diphenylmethane diisocyanate (pMDI). These synthetic adhesives have certain advantages and disadvantages as mentioned in Table 2.
| Properties | Adhesives | |||
|---|---|---|---|---|
| UF | MUF | PF | pMDI | |
| Price | Low | Medium to high | Medium | High |
| Cure temperature | Low | Medium | High | Medium |
| Pressing time | Short | Medium | Medium to long | Short |
| Susceptibility against wood species | High | Medium | Low | High |
| Efficiency | Low | Medium to high | Medium to high | High |
| Compatibility with bio-based raw-materials | Medium | Medium | Medium to high | High |
| Manipulations | Easy | Easy | Easy | Difficult |
| Resistant against hydrolysis | No | Medium to high | High | High |
| Use in wet conditions | No | Partially yes | Yes | Yes |
| Formaldehyde emission | E1. CRAB I5 possible | E1. CARB II5 possible | Very low emission | No |
3. Formaldehyde emissions from WBPs
WBPs and flooring materials are the main sources of formaldehyde emissions inside buildings.18 Various WBPs like particleboards, plywood, medium density fibreboards and high density fibreboards have become increasingly popular and are being used for manufacturing of furniture, cabinets and various building products. These products are mainly bonded with formaldehyde-based adhesives (UF, MUF and phenol-formaldehyde), which are the primary sources of formaldehyde. UF resin has the highest formaldehyde emission rate because it contains a large amount of incompletely cured UF, which results in free formaldehyde after the hydrolysis of the cured UF resin.19 However, formaldehyde release can also be reduced by adding formaldehyde-binding substances (“scavengers”) to the resin, such as formaldehyde-binding paraffin20 or by adding urea, propylamine, methylamine, ethylamine, and cyclopentylamine solution to the UF resin.20 It has been shown that within a certain range of molar ratios, there is an almost linear relationship between molar ratio, formaldehyde release and extractable formaldehyde content. A decrease in formaldehyde content in the resin leads to a decrease in formaldehyde emission rate in the finished product.21Different factors are associated with formaldehyde and VOC emissions from WBPs. During particleboard production, formaldehyde emissions increase with pressing temperature and time, mat resin content and moisture content, and board density.22 It is also stated that VOCs increased with pressing temperature linearly. This increase in VOC emission is mainly due to the increase in emissions of terpenes and aldehydes. A linear relationship of formaldehyde emissions with pressing temperature also exists.23 Temperature and humidity can influence formaldehyde emissions from WBPs that are produced using UF-type adhesives.24 For example, when increasing the temperature from 25.2 to 50.6 °C, the initial emittable formaldehyde from dry building materials is increased significantly by about 507%.25 In general, formaldehyde emissions from WBPs can be influenced by exogenous and endogenous factors.26 The exogenous factors include temperature, humidity, air movement over the panel surface, air change rate and local formaldehyde concentration within the space where the material is placed. The endogenous factors include wood species, moisture content of wood material, type and chemical composition of the adhesive binder used, additives (e.g. catalysts and formaldehyde scavengers), arrangement of multi-layer board, surface treatment, density of the board and manufacturing conditions (e.g. temperature and duration of the hot pressing process).27 There is a small amount of free formaldehyde in the liquid resin (generally less than 0.1%), which is used during cross-linking of the resin. Free formaldehyde is present in various forms in the manufactured panel. It can react with moisture present in the wood to form methylene glycol, polymethylene glycol, polyoxymethylene hemiacetal, etc., and can undergo labile binding to the wood or to the polymer resin.27 Free formaldehyde will in time migrate and be released to the environment, especially under high temperature and when exposed to a well-ventilated environment.26 Table 3 provides an overview of the different international standards used for calculation/estimation of formaldehyde emissions.
| Region | Method | Test sample | Conditioning | Test conditions | ||
|---|---|---|---|---|---|---|
| Size loading factor | Edge sealing (m open edge m−2) | Temp/RH | Temp/RH | Air exchange/hour | ||
| EU | EN 717-1 0.225, 1 or >12 m3 chamber | 1 m2 m−3 | Partly (1.5 m m−2) | 23 °C/45% | 23 °C/45% | 1 |
| EN 717-2 gas analysis 41 chamber | 0.4 × 0.05 m | Yes | Not stated | 60 °C/≤3% | 15 | |
| EN 717-3 500 mL flask | 0.025 × 0.025 m, 20 g | No (80 m m−2) | Not stated | 40 °C/∼100% | No | |
| EN 120 perforator | 0.025 × 0.025 m, 110 g | No | Not stated | Toluene extraction at 110 °C | No | |
| Japan | JIS A 1901 0.1-1m3 chamber | 2.2 m2 m−3 | Yes | 28 °C/50% | 28 °C/50% | 0.5 |
| JIS A 1469 9-111 desiccator | 0.18 m2 | No (27 m m−2) | 20 °C/65% | 20 °C/0–80% | No | |
| JAS 233 9-111 desiccator | 0.18 m2 | No (27 m m−2) | No | No | ||
| Global | ISO/CD 12460-1 m3 chamber | 1 m2 m−3 | Partly (1.5 m m−2) | 23 °C/50% | 23 °C/50% | 1 |
| CARB/EPA | ASTM E 1333, ≥22m3 chamber | 0.13–0.95 m2 m−3 | No | 24 °C/50% | 25 °C/50% | 0.5 |
| ASTM D 6007-02, 0.02–1 m3 chamber | 0.13–0.95 m2 m−3 | Yes | 24 °C/50% | 25 °C/50% | 0.526 to 3.846 m h−1 ventilation volume to emission surface | |
| China | GB 18580 perforator | 0.020 × 0.020 m | No | Not stated | Toluene extraction at 110 °C | No |
| 105–110 g | ||||||
| GB 18580, 9-11 L desiccator | 0.15 × 0.05 m, 10 pieces | No | Not stated | 20 °C | No | |
| GB 18580, 40 L desiccator | 0.045 m2 | Yes | 20 °C in vinyl resin bag | 20 °C | No | |
| GB 18580, 1 m3 environmental chamber | 1 m2 m−3 | Yes | Not stated | 23 °C/45% | 1 | |
European panel producers prefer the perforator method (EN 120) as a quality control method due to the very short testing time (2.5 h), cheap equipment, and available data. However, toluene used is a concern for the work environment and the correlation to chamber methods is poor (R2 = 0.8731).28,29 The perforator measures formaldehyde content, while most legislations focus on emissions. Desiccator (JIS A 1469, JAS 233, GB 18580) is an emission method with cheap equipment, but the testing time is longer (24 h testing and seven days preconditioning). Flask method is another quick and inexpensive emission measurement method, but accuracy and correlation to the chamber methods are difficult to achieve due to small sample sizes, no air exchange, and high relative humidity (correlation to 1 m3 chamber is R2 = 0.67). Gas analysis method (EN 717-2) is an accelerated emission test method with relatively short testing time (4 h). The high temperature and air exchange rate cause variations with respect to chamber methods, and it is not officially approved for uncoated board materials (PBs, OSBs, MDFs). The correlation to the 1 m3 chamber method is however still better than that of the methods mentioned above (R2 = 0.859). Chamber methods (EN 717-1, ASTM D 6007, ASTM E 1333, JIS A 1901, GB 18580) have the benefit of mimicking final use conditions for furniture. The bigger sample size also helps against errors caused by variations in material. However, they are expensive and have long testing times (test period 10–28 days), making them unsuitable for factory quality control. It should be noted that variables between test methods stated in Table 3 can lead to significant differences between chamber test methods.27
4. Sustainable resources for adhesives for WBPs
4.1. Lignin
Historically, most of the available lignin comes as a by-product of the pulping process. These lignin derived fragments have low value and usually serve as fuel for the recovery boiler of pulp and paper mills. They are very heterogeneous in their structure with structural units that range from almost native to highly degraded.30 The structure of lignin plays a key role in the required modifications and crosslinking to allow for better adhesive properties of the derived adhesive. Commercial lignin types from different processes are divided into two main categories (Fig. 2). Sulfur containing lignin (mainly kraft lignin found in the black liquor of kraft pulping process and lignosulfonate lignin in the sulphite liquor of sulfite pulping process) and non-sulfur biorefinery lignin (soda, organosolv, steam explosion, hydrolysis, diluted acid, pyrolytic, high-pressure refining, ammonia-fiber-expansion lignin, etc.).31,32 The main chemical changes occurring during processing of kraft lignin, soda lignin and ethanol/water process lignin are demonstrated in Fig. 3.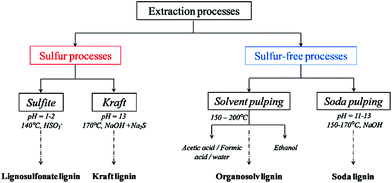 | ||
| Fig. 2 Different extraction processes to separate lignin from lignocellulosic biomass and corresponding productions of technical lignin (reproduced from ref. 33 with permission from Elsevier Ltd). | ||
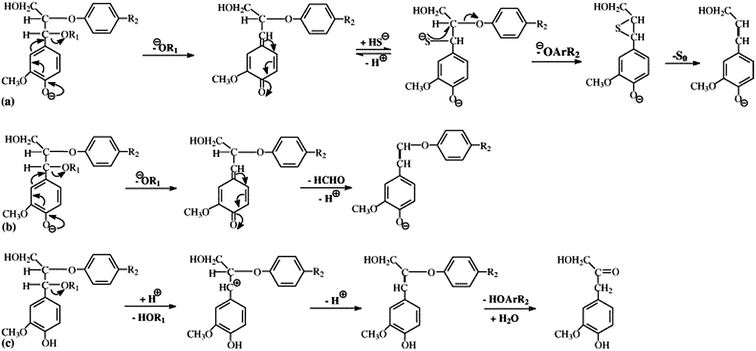 | ||
| Fig. 3 Schematic representation of the main changes occurring in lignin structure during the (a) kraft, (b) soda and (c) ethanol/water processes (reproduced from ref. 34 with permission from Elsevier Ltd). | ||
Lignin is composed of cross-linked phenolic C6C3 units. The main repeating units are presented in Fig. 4.35 The major chemical functional groups in lignin include hydroxyl, methoxyl, carbonyl and carboxyl groups. Methods used for identification of the chemical groups in lignin include Fourier transform infrared (FTIR) and UV/vis spectroscopy, pyrolysis-GC/MS, liquid chromatography, elemental analysis, and wet chemistry methods, such as thioacidolysis, methoxyl content analysis and nitrobenzene oxidations.36–38 1H–13C correlation 2D NMR spectroscopy techniques are often used instead of 1D NMR, due to overlapping signals of irregular lignin structures with carbohydrate, cellulose, and protein impurities often found in residual lignin.
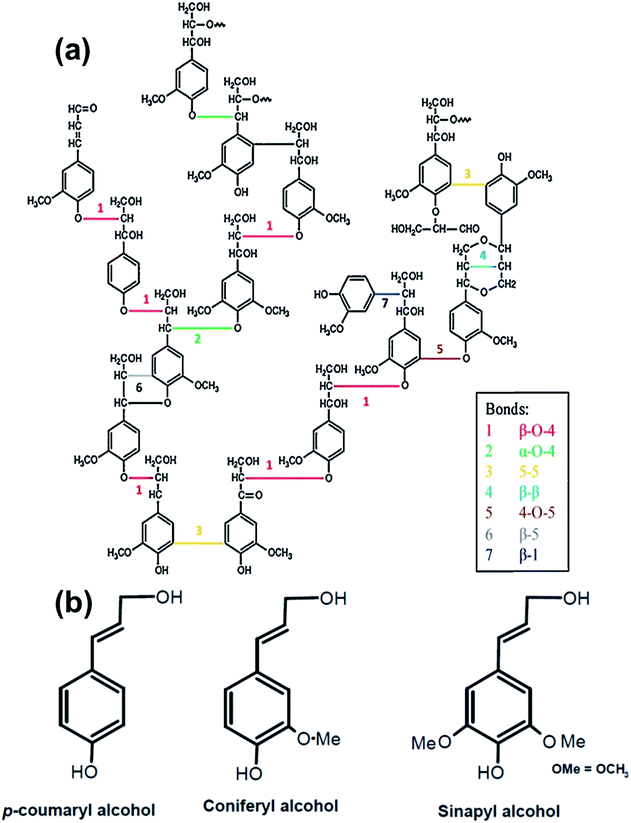 | ||
| Fig. 4 Main linkage of softwood lignin (a) (reproduced from ref. 45 with permission from Elsevier Ltd) and structural units of lignin (b). | ||
There is a big difference in properties and water solubility of lignin derived from different processes. Lignin residue from sulfur-free extraction processes, such as all alkaline processes, is typically of relatively high quality. It has higher amounts of functional groups than sulfur lignin as the treatments are milder.39,40 However, detailed structural information is still missing for most types of biorefinery lignin. The suitability of a lignin type for incorporation into phenolic adhesives is partly defined by the presence of chemical features that can be involved in polymerization reactions; the two most important being the phenolic hydroxyl and aliphatic hydroxyl groups. Phenolic hydroxyl groups increase the reactivity of lignin towards formaldehyde due to activation of the aromatic ring in o-position and provide the possibility to form quinone methide intermediates, which could be a starting point for further condensation with other phenolic units.39,41 Kraft lignin is water-insoluble and mostly solvent-insoluble except for in highly alkaline environments (pH > 11). Soda lignin and organosolv lignin are also essentially water-insoluble, while lignosulphonates are water-soluble in the presence of a suitable counter ion.42,43 Biorefinery lignin is expected to be available in larger amounts in the future as a result of the growth in biomass-to-biofuel and biomass-to-sugar conversion industries. Oxidative ways of turning biorefinery lignin into valuable platform chemicals, which can later be turned into adhesives, has been reviewed by Ma et al. (2015).44
4.2. Tannins
Tannins occur naturally in bark, wood, leaves and fruits of plants. Tannins are used in various industrial applications, mainly in the manufacturing of inks, textile dyes and as a corrosion inhibitor. Although tannin occurs in many plant species, only a few have high enough concentration to make its extraction worthwhile. Tannin can, for example, be extracted from pine, quebracho, oak, chestnut, wattle, eucalyptus, myrtle, maple, birch and willow.46 Different extraction methods are used, the most common ones being maceration, Soxhlet extraction supercritical CO2 and percolation. Other rare techniques such as microwave and ultrasound assisted extraction has also been studied to increase tannin yield.47 The extraction method affects adhesive properties of tannin extracts. The extraction agent affects degree of polymerization, sugar concentration and number of functional groups (e.g. hydroxyl groups).48,49 In the extraction solution, other components such as starch, polymeric carbohydrates and amino acids are also present. Purification steps to remove other components are typically not performed at an industrial scale, and tannin is typically sold as spray dried powders.1Characterization of plant tannin is done by using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), FTIR spectroscopy and nuclear magnetic resonance (NMR) spectroscopy.49 Tannins are loosely divided into two main categories based on their phenolic nature: condensed and hydrolysable tannins (Fig. 5).50
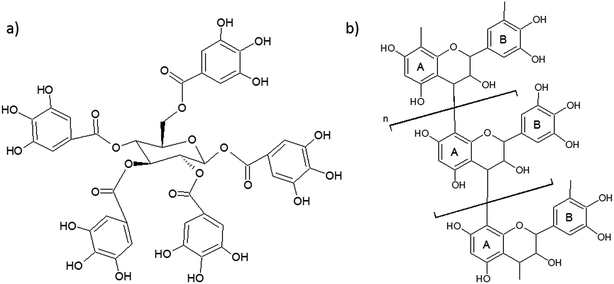 | ||
| Fig. 5 (a) Hydrolysable tannin (the simplest one with gallic acid groups esterified to glucose) and (b) condensed tannin. | ||
Condensed tannins, as their name suggests, possess a condensed and complex chemical structure made of hydroxylated C-15 flavonoid units with variations in the sites at which bonds between flavonoid units are formed. In the basic structure of condensed tannin, the A-ring can be of a resorcinol or phloroglucinol type and the B-ring of pyrogallol, catechol or sometimes phenol type. Catechol is the only B-ring structure capable of cross-linking. Phloroglucinol tannin type is obtained in low yields during extraction of most pines and has higher reactivity than resorcinol type towards formaldehyde, leading to short pot lives. Condensed tannins with a low degree of polymerization are soluble in polar solvents, and those with high degree of polymerization are soluble in dilute alkali solutions.51 Certain condensed tannins, such as from quebracho and wattle, are produced commercially from wood and bark and have been used to make wood adhesives since the 1970s. The polycyclic structure of condensed tannins leads to fast curing rates but also to high viscosity of adhesives.1
Hydrolysable tannins, as well as gallotannins and ellagitannins, are esters of carboxylic acids and sugars. Gallotannins are polymeric esters of gallic acid and normally associated with sugars. However, a study by Pizzi et al.52 shows that some of them do have an extensive polymeric structure based on pentagalloyl glucose repeating units. Hydrolysable tannins are readily soluble in water and easily hydrolyzed, resulting in benzoic acid derivates and sugars. In the natural state, hydrolysable tannins allow a low level of phenol substitution and have low nucleophilicity.
4.3. Proteins
The most common source of proteins is the mechanical or solvent extraction of oils, soy, palm, canola, cottonseed and sunflower oils making up the biggest markets. In Europe, protein from wheat gluten is also widely available as a by-product from bioethanol production.53 Other sources of proteins are zein from maize seeds,54 casein from milk and animal blood and feather. Soybeans are the largest single source of edible oil and account for approximately 52% of the total oil seed production in the world, USA and Brazil accounting for most of it. Soybean seed processing includes cleaning, drying, cracking, de-hulling, flaking and extraction of oil by using hexane. Around 4.5 tons of soybean meal is produced for each ton of crude soybean oil. The protein content of this soybean meal is around 44–50%, which is typically higher than that of the oil.55 The residual soybean meal is used for production of soy flour (SF), soy protein concentrates and soy protein isolates (SPI). Soy flour is the cheapest option but also the one with the lowest protein content. The most commonly produced isolates have a protein content of 80–90%. Protein content greatly affects the properties of the final adhesive. The adhesive performance of soybean proteins is also affected by particle size, nature of the surface, structure of the protein, viscosity and pH.56 The high viscosity of soybean proteins results from increased intermolecular interactions due to uncoiling of molecules. Ionic environments weaken these electrostatic interactions, so treatment with salts or reducing agents can reduce viscosity without affecting adhesive strength. Other ways to reduce viscosity include enzymatic or alkaline hydrolysis. High pH increases rate of hydrolysis and consequently increases adhesive strength and water resistance, but shortens pot life. The optimal viscosity of the binder varies according to material to be glued.57Proteins are complex macromolecules consisting of amino acid monomers, which are chemically linked together to form polypeptide chains. The chemical links are mainly amide bonds that are stable but can be degraded using strong acids. Proteins have a secondary structure of α-helices and β-sheets and a three-dimensional tertiary structure where α-helices and β-sheets fold into compact globules that interact with the surfaces of other globules, forming a quaternary structure (Fig. 6). Physical and chemical properties of proteins are influenced by this complex structure as well as the order and presence of amino acids, as shown in Fig. 7.
 | ||
| Fig. 6 Folding of protein (reproduced from ref. 56 with permission from American Chemical Society). | ||
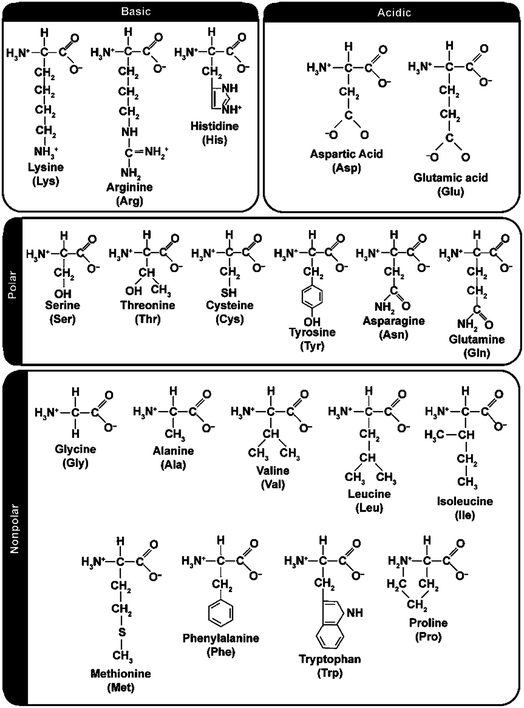 | ||
| Fig. 7 Amino acids grouped as hydrophobic, hydrophilic or polar vs. non-polar as well as acidic or basic. (reproduced from ref. 56 with permission from American Chemical Society). | ||
The structure of protein molecules can be determined using methods such as crystallography, nuclear magnetic resonance (NMR) and infrared spectroscopy (IR). Besides these, methods such as atomic force microscopy (AFM) have shown to provide valuable information on the structure of modified proteins.53,58
Soy protein is a combination of about 18 different amino acids that include functional groups such as –OH, –NH2, –COOH and –SH. Each one of these functionalities are polar in nature, which leads to water sensitivity of soy proteins. The major components of soy protein are 7S globulin (β-conglycinin) and 11S globulin (glycinin), and minor factions 2S and 15S. S stands for Svedberg unit that describes sedimentation rate. The effect of the ratio of 7S and 11S has been studied previously for physicochemical properties of soy protein adhesives. Hydration capacity is found to be better for systems with more 7S.59 The water solubility of soy protein is affected by pH. The minimum solubility is at pH 4.2–4.6, which is the isoelectric region of soy proteins taken as a whole.57 At the isoelectric point, the concentration of the ammonium ion (NH3+CHRCOOH) equals that of the carboxylate ion (NH2CHRCOO−) in the amino acids. Zein, the main storage protein of maize, on the other hand, has a high percentage of non-polar amino acids (leucine, alanine and proline), making it one of the few hydrophobic water-insoluble biopolymers.54
Although soybean protein is the most widely studied protein for use in wood adhesives, partly due to support from American United Soybean Board, other proteins have shown interesting qualities for wood bonding. The properties of other proteins, such as canola, wheat gluten, zein, casein, pea, mussel, whey and cottonseed can differ greatly due to the different composition of amino acid groups. This different composition can give adhesives produced with their unique properties such as the higher water tolerance of mussel proteins.60 Due to the high cost of mussel adhesives as compared to other wood adhesives, other approaches have been taken. As an example, soy proteins have been modified to resemble mussel proteins to increase moisture tolerance.61
The basic structure and denaturation mechanism of other proteins are similar to that of natural soy protein, although reaction to denaturation chemicals can vary among proteins. Wheat gluten is readily available as the by-product of starch production for bio-ethanol. It is also extracted from wheat flour to produce gluten-free products. Gluten is mainly used in the bakery industry. Wheat gluten is a hydrophobic protein due to its high amount of non-polar amino acids. Its isoelectric point is 7.3, and it is dispersible in alkali and acid but not in water. Wheat gluten consists mainly of storage protein that can be divided into two groups: glutenin (elastic properties) and gliadin (viscous properties), the former being dispersible in acids or bases and the latter being soluble in alcohol. Rest of wheat gluten is polysaccharides, lipids, and minerals. Wheat gluten is more hydrophobic than soy protein. Wheat gluten, as well as hydrolyzed gluten proteins have been studied as adhesives for wood-based panels.53,62,63
4.4. Starch
Starch is a polysaccharide derived from the seeds, roots and leaves of plants. It acts mainly as the energy storage unit of plants and can be found in large quantities in corn, wheat, potato, rice, tapioca and sago. In pure form, it is insoluble in cold water. Starches can be dry roasted in the presence of an acid catalyst to form dextrins. Depending on roasting time, dextrins can be divided into white dextrins, canary (yellow) dextrins and British gums. Unlike starches, dextrins are soluble in water, and the viscosity of dextrin solution is easier to adjust than that of starches.Starch consists of glucose units joined by glucosidic bonds. The two fractions of starch are amylose and amylopectin. Amylose is a linear helical chain molecule composed of α-(1,4)-bonds and amylopectin is a branched molecule composed of the same α-(1,4)-linkages except at the branch point, which are α-(1,6)-bonds (Fig. 8). Amylopectin and amylose are assembled to form semi-crystalline granules. The size and shape of the granule and amylose/amylopectin ratio vary among plant species from which the starch is obtained.64 Also, molecular weights of amylose fractions greatly affect the final properties of different starches. Technically, if not economically, it is possible to fractionate linear and helical amylose and branched amylopectin by, for example, using methanol and butanol solvents according to Schoech's preferential precipitation method.65 Methods used for starch analysis include X-ray diffraction (XRD), TGA, and DSC, as for other bio-based materials. Additionally, titration to determine amylose content is important, as the amylose–amylopectin ratio greatly affects properties of starches.66
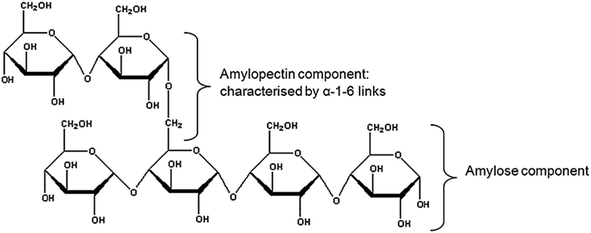 | ||
| Fig. 8 Starch co-polymer with amylose and amylopectin component (reproduced from ref. 67 with permission from Elsevier Ltd). | ||
The Stein-Hall process in the 1930s was the first major commercial process for producing starch adhesives for corrugated boxes. A gelatinized starch adhesive, the carrier, is formed in the first cooking phase. In the second phase, the carrier is mixed with starch, water, and borax to form the finished adhesive.68 This combination of gelatinized and un-gelatinized starch forms together with a good corrugating adhesive is still important in the packaging industry today. Starch has a large number of glucosidic and hydroxyl groups spread across the polymer chain, which due to their polar nature, have high hydrogen-bonding capability. They are also very hydrophilic, making water resistance of native starch adhesives poor.69 Starch also has relatively low bonding strength, making it unsuitable for wood-based panel products in its native form. Thus, starch needs to be highly modified or cross-linked when used in the wood industry. The main types of modifications for starch-based adhesives are chemical, physical, enzymatic and genetic.70 The main chemical modifications, oxidation, esterification and etherification, have been reviewed previously67 and are presented in Fig. 9. Starch adhesives have very high viscosity due to entanglement of high molecular weight macromolecules. High viscosity makes it very difficult to use them for industrial applications. One way of reducing viscosity is by decreasing the number of entanglements per chain by adding small molecules to swell the polymer network. Another method is by shear refinement. The molecules orient themselves under flow, which decreases the number of entanglements in polymer melts.71
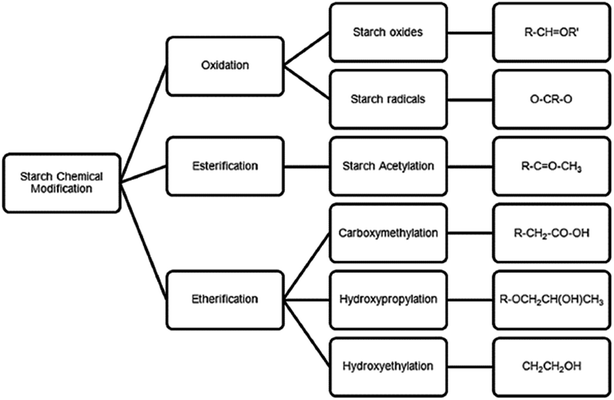 | ||
| Fig. 9 Schematic summarizing classical chemical methods for starch modification (reproduced from ref. 67 with permission from Elsevier Ltd). | ||
5. Development of bio-based adhesives for WBPs
5.1. Lignin
Lignin-based adhesive systems have been the subject of several reviews.15,72,73 Self-bonding properties of wood particles and improvement of those by enzyme treatment has been well reviewed in literature.43 Kai et al.,74 in their review paper, discussed potential lignin functionalization for the development of sustainable materials such as biopolymers as reinforcement fillers, antioxidants, UV adsorbents, antimicrobial agents, carbon precursors and biomaterials for tissue engineering and gene therapy. Recently, Zhao et al.75 and Wen et al.76 have reported the fundamental understanding about lignin solubilization using small-angle neutron scattering (SANS) and nuclear magnetic resonance (NMR) and it will be a very useful tool for analysis of functional lignin for adhesive synthesis.Lignocellulosic ethanol residue (ER), which is the by-product of lignocellulosic ethanol production and rich in active lignin, has also been studied.79 Best results are obtained with the replacement of 30% and 50% of phenol and the exterior grade plywood produced thereof fulfils the relevant Chinese standard. ER shows a good potential as it has a lower content of polysaccharide, higher content of hydroxyl groups and lower molecular weight than conventional technical lignin. Alkaline, de-alkaline, and sulfonate lignin are liquefied in phenol with H2SO4 or HCl catalysts and used to produce resol type PF resins.80 The resins can be prepared at lower temperatures and in shorter times and have higher reactivity than normal PF resin. Biorefinery-based technical lignin has also been used in preparing lignin-phenol-formaldehyde synthesis for wood based panel applications.81–83 Zhao and Abu-Omar84 have performed lignin modifications through deprotection, phenolation and phenol-formaldehyde reactions and have successively improved the uniformity and mechanical and thermal properties of bio-based thermosets.
The presence of guaiacyl-type (G) units in both lignins confirms that both lignins have potential active sites for polymerization (Fig. 10a), similar to phenol-formaldehyde condensation reactions (Fig. 10b): phenol structure has three reactive sites, 2-, 4- and 6-positions, and typical lignin structure has 1 reactive site, 3-position. The second stage of the reaction involves the reaction of methylol groups with other available phenol or methylolphenol, leading first to the formation of linear polymers and then to the formation of hard cure and a highly branched structure.
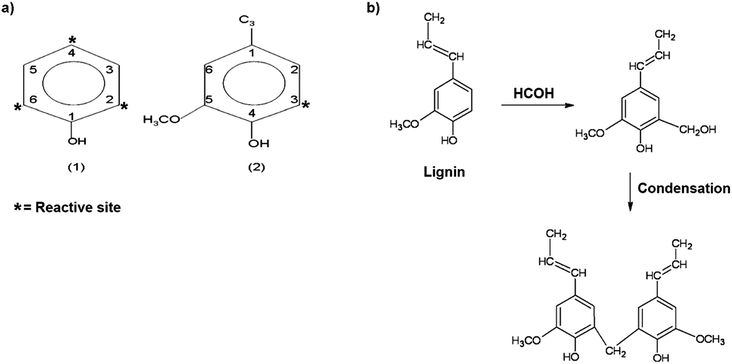 | ||
| Fig. 10 (a) (1) Phenol structure with three reactive sites, 2-, 4- and 6-position, and (2) typical lignin structure with 1 reactive site, 3-position. (b) Crosslinking between lignin and formaldehyde (reproduced from ref. 85 with permission from Elsevier Ltd). | ||
The similarity between G-type unit of lignin and phenol (Fig. 10a) reveals that lignin can also react with formaldehyde and can be cross-linked with formaldehyde in the same way as in phenol-formaldehyde condensation reaction, as shown in Fig. 10b. Thus, the free 3-position of G-type units in both lignins give significant values compared to the S-type unit, which has both the 3- and 5-position attached to the methoxyl group, preventing the occurrence of a polymerization reaction.
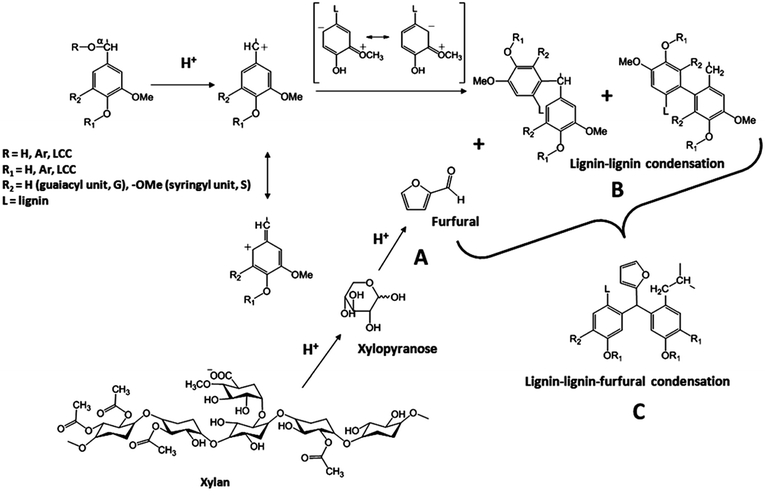 | ||
| Fig. 11 Proposed mechanism of lignin-furfural condensation products: (A) xylose-based carbohydrates breakdown to furfural; (B) lignin–lignin condensation takes place; and (C) lignin–lignin-furfural condensation product (reproduced from ref. 86 with permission from MDPI AG (Basel, Switzerland)). | ||
Phenolated organosolv LPF resins are proved to perform better (e.g. improved hardening time) than resins with addition of unmodified lignin.92 In this case, the adhesive is formed in two steps. In the first step, the lignin is allowed to react with phenol to increase the number of potential reactive sites. The phenolated lignin is then combined with formaldehyde. Up to 30% of phenolated organosolv lignin can be used to replace phenol and produce particleboards with mechanical properties comparable to those of particleboards bonded with PF resin.93 It is shown that 50 wt% of phenol can be replaced by phenolated lignin from sources such as eucalyptus bark lignin,94 bagasse alkali lignin95 and groundnut shells96 and still improve adhesion strength on teak wood specimens compared with PF resins. According to these findings, the phenolation step before polymerizing LPF resin is critical in order to successfully increase lignin portion of the final resin. In that respect, lignin concentration, formaldehyde-to-phenol molar ratio, catalyst concentration, reaction time and reaction temperature are optimized.94
As methyl groups of methoxyl groups block potentially reactive aromatic hydroxyl groups, reactivity of lignin and its ability to form crosslinks can be improved by demethylating lignin aromatic rings. Demethylation can be done chemically or enzymatically (e.g. laccase, peroxidase).97 Laccase reacts on phenolics via non-specific oxidation reactions. Oxidative degradation is also an established method of depolymerization of lignin present in residues from pulp mills. However, an oxidation step leads to additional cost and a significant increase in lignin reactivity cannot always be guaranteed.98 Various fungi can be potentially used to modify lignin. Brown-rot fungi can demethylate lignin and partially oxidize side-chains.
| Combination of natural glue components | Panel | Pressing time (min)/Pressing tem. (°C) | Density (kg m−3) | IB (MPa) | Dry shear strength (MPa) | Sources or commercial supplier name | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|
| GL/pMDI/MT | PB | 7.5/195 | 708 | 0.36 | — | Lignin (Protobind 100SA), Commercial mimosa tannin from Tanac SA (Montenegro, Brazil) | Two types of glyoxylated tannin (GL), commercial and heat treated | 103 |
| 55/25/20 | 733 | |||||||
| 55/30/15 | 694 | 0.36 | ||||||
| 60/25/15 | 674 | 0.31 | ||||||
| 60/40/0 | 733 | 0.46 | ||||||
| 55/25/20 (heat treated GL) | 0.31 | |||||||
| CS + 0% silane | PW | — | — | — | 4.2 | Cornstarch (CS) was provided by Changchun Dacheng Corn Co. (China); silane coupling agent (CH2CHSi(OC2H5)3, A-151) was obtained from Nanjing Forward Chemical Co. (China) | A graft-copolymerized starch-based wood adhesive was prepared as follows: 10 g dried corn starch and 100 mL distilled water were placed in a four-necked round-bottom flask and stirred at 80 °C for 30 min. After the starch gelatinization, the reaction temperature was cooled to 55 °C, following by the addition 0.1 g ferrous sulfate and H2O2. Oxidation was conducted after 1 h, and the temperature was again raised to 80 °C, and 3 g PVA was dropped into the mixture in the four-necked round-bottom flask. The mixture was stirred after 0.5 h and was cooled to 60 °C, and 0.1 g of SDS, 0.05 g of APS and silane coupling agent were added | 155 |
| CS + 1% silane | 5.8 | |||||||
| CS + 2% silane | 6.0 | |||||||
| CS + 3% silane | 7.3 | |||||||
| CS + 4% silane | 7.0 | |||||||
| CS + 5% silane | 6.5 | |||||||
| Starch/monomer | PW | 24 h/25 | — | — | Cornstarch [Changchun Dacheng Corn Co. (China)] | A starch-based wood adhesive was synthesized via graft copolymerization of oxidized starch with a silane coupling agent and butyl acetate and vinyl acetate monomer | 161 | |
| 1/0.5 | 4.42 | |||||||
| 1/1 | 5.45 | |||||||
| 1/1.5 | 5.38 | |||||||
| 1/2 | 7.88 | |||||||
| 1/2.5 | 10.05 | |||||||
| SP (soy protein) | PW | 10/170 | — | — | 6.4 | Kraft Lignin (L) (no. 370959) with particle sizes of 35.66 ± 21.42 (L), 19.13 ± 22.90 (M), and 10.26 ± 9.98 (S) μm; Soy flour | Improved water resistance due to addition of lignin in soy protein (SP) and also studied the influence of lignin size on properties of adhesives | 141 |
| SP/LL | 6.5 | |||||||
| SP/ML | 6.3 | |||||||
| SP/SL | 6.4 | |||||||
| Corn TMP | HDF | 7/230 | 917 | 0.21 | — | Corn residue and Cationic starch, colloidal silica (La Tallada d’ Emporda, Girona, Spain); and Black liquor pulp (Zaragoza, Spain) for kraft lignin | Lignin addition to corn starch improved mechanical properties of HDF and 0.5% cationic starch & 0.8% colloidal silica were used as retention agents | 167 |
| Corn + 5% lignin | 1063 | 0.32 | ||||||
| Corn + 9% lignin | 1107 | 0.29 | ||||||
| Corn + 13% lignin | 1108 | 0.43 | ||||||
| Corn + 21% lignin | 1135 | 0.42 | ||||||
| Corn + 25% lignin | 1098 | 0.45 | ||||||
| Corn + 29% lignin | 1128 | 0.31 | ||||||
| Tannin | PB | 7.5/195 | — | 0.85 | — | Commercial tannins from pine (Pinus pinaster L.) and Cellulose nanofibers (Intelligent Chemicals Pty Ltd (China)) | The addition of cellulose nanofibers to tannin adhesives is an effective method for the production of high performance particleboards | 168 |
| Tannin + 1% CNF | 0.96 | |||||||
| Tannin + 2% CNF | 0.98 | |||||||
| Tannin + 3% CNF | 0.86 | |||||||
| Bark tannin + vanillin | PB | 7.5/220 | 660 to 680 | 0.45 to 0.50 | Purified maritime pine (Pinus pinaster) bark tannin extract (Phenopyn) [DRT (Derives Resiniques et Terpeniques, Dax, France)]; Vanillin (Sigma Aldrich) | 100 g of purified maritime pine (Pinus pinaster) tannin dissolved in water/ethanol 50/50 by weight to yield a concentration of 40–45% solids. The pH was corrected to 9.5 with NaOH solution at 33% concentration. 20 g (20% solids on solids) vanillin in ethanol | 169 | |
| MSP/SL | PW | 10/210 | — | — | 6.18 | Sorghum lignin (SL) and soy protein adhesives (SPAs), soy protein isolates (SPI) or modified soy protein (MSP) Cargill (Cedar Rapids, IA) | MSP: NaHSO3 modified soy protein, Dry shear strength of all blends of SPI and MSP with SL or ESL showed 100% wood cohesive failure | 170 |
| 100/0 | 5.70 | |||||||
| 90/10 | 6.40 | |||||||
| 80/20 | 5.12 | |||||||
| 70/30 | 4.86 | |||||||
| 60/40 | 5.84 | |||||||
| 50/50 | ||||||||
| MSP/ESL | PW | 10/210 | — | — | Extruded sorghum lignin (ESL) and modified soy protein (MSP) | |||
| 90/10 | 5.68 | |||||||
| 80/20 | 5.94 | |||||||
| 70/30 | 4.25 | |||||||
| 60/40 | 5.36 | |||||||
| 50/50 | 4.76 | |||||||
| SWKL (15%) | HDF | 16/160 | 1336 | 0.35 | — | Softwood kraft lignin (SWKL), soda wheat straw lignin (SoWhStL) and hydrolysis wheat straw lignin (HWhStL) supplied by Chemtex | — | 171 |
| SoWhStL (15%) | 1297 | 0.61 | ||||||
| HWhStL (15%) | 1313 | 0.66 | ||||||
| SWKL (25%) | 1345 | 0.30 | ||||||
| SoWhStL (25%) | 1296 | 0.56 | ||||||
| HWhStL (25%) | 1327 | 0.60 | ||||||
| MT + 50% FA | PB | 7.5/195 | 716 | 0.34 | — | Mimosa tannin (MT) (Acacia mearnsii) extract or pine tannin (PT) (Pinus radiata) and furfuryl alcohol (FA) | Tannin-furfuryl alcohol resins reacted under alkaline conditions to minimize self-condensation of furfuryl alcohol and forced its reaction with tannins, proving to be another alternative for formaldehyde-free, environment friendly adhesives from renewable materials | 172 |
| PT + 50% FA | 697 | 0.35 | ||||||
| PT + 50% FA | 715 | 0.40 | ||||||
| 10% tannin in PB | PB | 20/200 | — | Tannic acid powder having molecular formula of C76H52O56 with molecular weight of 1701.2 g mol−1 (Sigma Aldrich) and Rhizophora spp. Trunks particles sizes (a) ≥210 μm, (b) 104–210 μm and (c) ≤104 μm | 10% tannin was added during manufacturing of particleboard of Rhizophora spp. | 173 | ||
| (a) ≥210 μm, | 1011 | 0.21 | ||||||
| (b) 104–210 μm | 1058 | 0.38 | ||||||
| (c) ≤104 μm | 1006 | 0.49 | ||||||
| T (40%)/HA | PW | 6/150 | 461 | 0.74 | Commercial condensed tannin (T) of quebracho wood (Fontenay-sous-Bois, France) | Aqueous tannin solutions were prepared at 40% and 50% (w/w), and NaOH solution (50% w/w aqueous solution) was added to modify the pH up to 10. To make tannin/hexamine glues, 6% hexamine (HA) based on the tannin solids content was used as hardener (33% w/w hexamine aqueous solution) and 20% PMDI resin was added | 174 | |
| T (50%)/HA | 451 | 0.84 | ||||||
| T (40%)/HA/PMDI | 455 | 0.96 | ||||||
| T (50%)/HA/PMDI | 470 | 0.98 | ||||||
| Citric acid: sucrose (wt%) | PB | 10/200 | 900 | — | Citric acid and sucrose supplied from Weifang Ensign Industry Co. Ltd. (China) | Citric acid and sucrose were dissolved in water with target concentration of 59–60 wt% | 175 | |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
0.22 | |||||||
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
0.21 | |||||||
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
0.23 | |||||||
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
0.21 | |||||||
00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.37 | |||||||
| Tannin–hexamine | MDF | 6-8/160-180 | 700–800 | — | Tannin extracted from the bark of mimosa (Acacia mearnsii [De wild.]) was purchased from Silvateam S.p.a. (Italy). Hexamine and sodium hydroxide were provided by Sigma-Aldrich, Switzerland | The tannin–hexamine adhesive was prepared as a 40% w/w solution of mimosa tannin in water. A hexamine aqueous solution (40% w/w) was then added up to reach a dry weight ratio between hexamine and tannin equal to 5% w/w. Sodium hydroxide solution (50% w/v) was finally added to set the desired pH | 176 | |
| Unmodified pH | 0.15 | |||||||
| pH 9 | 0.45 | |||||||
| pH 10 | 0.65 | |||||||
| OL20 | PB | 4/220 | 700 | 0.78 | — | Organosolv lignin (OL20 and OL40), phenolated organosolv lignin (pOL20 and pOL40), or lignosulfonate (LS40 and pLS40), (2.75 mL, 0.051 mol) at 110 °C for 20 min. Softwood ammonium lignosulfonate (LS) was obtained from Tembec Avebé ne SAS (France) | Beech organosolv lignin (OL) was produced and phenolated as reported previously.19 An up-scaled phenolation of beech organosolv lignin (pOL) was performed with phenol (50 g, 0.53 mol), lignin (25 g, ∼0.13 mol C9 units), and H2SO4 (2.75 mL, 0.051 mol) at 110 °C for 20 min | 177 |
| OL40 | 0.08 | |||||||
| pOL20 | 0.72 | |||||||
| pOL40 | 0.68 | |||||||
| LS40 | 0.45 | |||||||
| pLS40 | 0.60 | |||||||
| WCSM content (%) | PW | 20/100 | — | — | Water-washed cottonseed meal (WCSM), citric acid (CA), sodium dodecyl sulfate (SDS) and guanidine hydrochloride (GdmCl) | Adhesive slurries were prepared by mixing WCSM with appropriate volumes of deionized water to produce the required solid content for 2 h. As needed, 1% or 3% (total weight) of CA or SDS was added into the slurry | 178 | |
| 11 | 4.06 | |||||||
| 20 | 3.90 | |||||||
| 30 | 3.97 | |||||||
| WCSM (%) + 1% SDS | ||||||||
| 11 | 3.13 | |||||||
| 20 | 4.48 | |||||||
| 30 | 3.78 |
Another very interesting aspect has also emerged in the area of lignin self-crosslinking.105,106 The in situ treatment of wood fibers with laccase shows distinct increases in molecular weight of lignin directly due to formation of lignin covalent inter-bonding, as shown in Fig. 12.106 Fabrication of MDF using this process has been reported in various articles,106–108 and this area is continuously developing towards industrial-scale production.
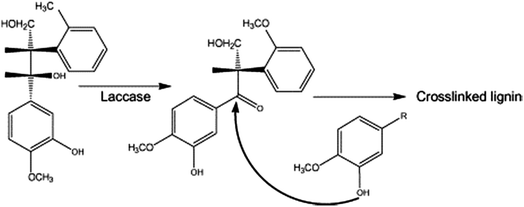 | ||
| Fig. 12 Laccase-mediated oxidation of lignin model compound and putative further reaction leading to lignin crosslinking (reproduced from ref. 105 with permission from Elsevier Ltd). | ||
Recently, for the first time, Prof. Sun's research group reported the self-crosslinking of industrial alkali lignins for green wood–lignin composite fabrication via heat-treatment.109 They reported the decrease of β-O-4′ linkages content, accompanied by the formation of β-β′, β-5′, and β-1′ linkages under mild heat treatment temperatures (130–170 °C), and most of the β-O-4′, β-β′, β-5′, and β-1′ linkages nearly disappeared at a higher temperature (180 °C). A self-crosslinking mechanism was proposed as shown in Fig. 13.
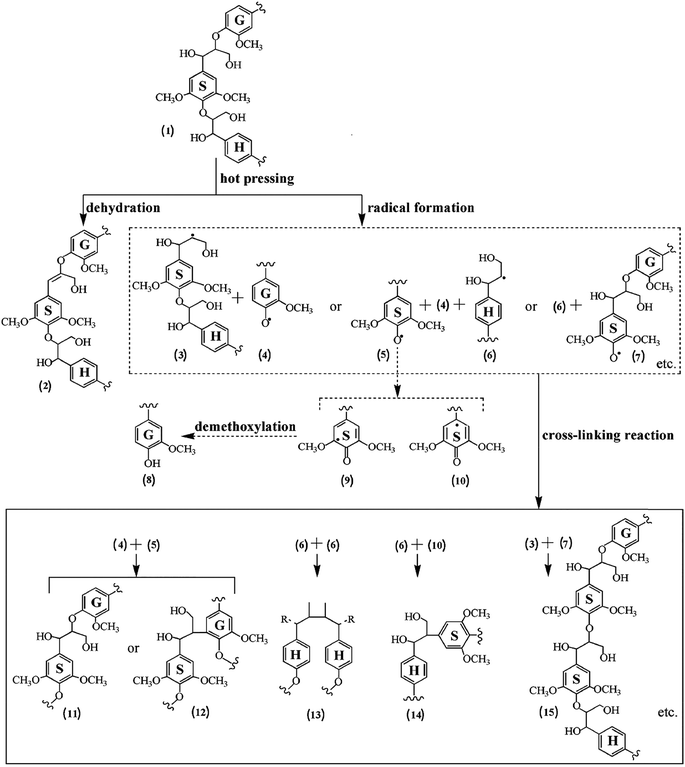 | ||
| Fig. 13 Proposed self-bonding mechanism of lignin (reproduced from ref. 109 with permission from American Chemical Society). | ||
5.2. Tannin
It has been mentioned already that tannin has been used together with lignin for different types of wood adhesives. If no formaldehyde is used, lignin can be glyoxalated before mixing with tannin and a hardener (e.g. hexamine). The formaldehyde emissions of particleboards glued with the studied mimosa tannin and glyoxalated wheat straw lignin according to EN 717-3 is 0.92–1.12 mg kg−1.100 Also, other bio-based materials are suitable to be used with tannins to form adhesives. For example, corn starch-mimosa tannin with hexamine as the hardener has been used as an adhesive for plywood. One improvement and research area on starch-tannin adhesives is the reduction of high viscosity that limits their industrial possibilities.112
Tannin adhesives due to their phenolic nature have very low formaldehyde emissions. The emissions have been even further reduced by using non-emitting hardeners or by using tannins that are cured by auto-condensation in the absence of aldehydes.89 The autocatalytic hardening can occur in highly reactive tannins, such as in procyanidins, without the need of an external catalyst. For slower-reacting tannins, such as prorobinetinidins, autocondensation occurs when a small amount of alkaline SiO2 is present at high pH.113
In auto-condensation, the O1–C2 link of the flavonoid repeating unit is opened, which then drives auto-condensation between the reactive C2 of the open chain and free sites in C6 or C8 of the flavonoid unit in another polymer chain. Auto-condensation can also occur at room temperature at higher pH, increasing the viscosity of the tannin adhesive. Different tannins have different auto-condensation behaviors and require different pH, e.g. for mimosa tannins, auto-condensation occurs at alkaline pH (ref. 114) and for Acacia Nilotica spp. Tannins, at their initial acidic pH.115
Increasing tannin content in PF-tannin adhesives reduces formaldehyde emissions proportionally. However, high concentrations of tannin in PF resins lead to higher viscosity and shorter pot lives, because tannins are more reactive than phenol towards formaldehyde due to resorcinol and/or phloroglucinol rings in their structure.113 Jahanshaei et al.116 replace up to 30% of phenol in PF with condensed Quercus castaneifolia bark tannin. Replacement above 30% deteriorates mechanical properties and increases water absorption and swelling thickness of particleboards. Valonea tannin (VT)-modified phenol-formaldehyde resin has been prepared by Li et al.117 via copolycondensation mechanism; FTIR and 13C NMR analytical results confirm that VT is composed primarily of galloyl groups, hexahydroxydiphenol groups and glucose. Table 4 shows some latest literature about tannin-based adhesives and their application in WBPs.
Hexamethylenetetramine (hexamine) has been used as a cross linking agent (hardener) for tannin adhesives since the 1950s, when it could only be used for interior particleboards due to moisture sensitivity. Unlike formaldehyde, hexamine cannot react with tannin unless intimately mixed with it or unless it decomposes to formaldehyde and ammonia, in which case the panel properties are further lowered. It has been proven that with very reactive nucleophilic sites, such as those of highly reactive phenols (tannin), amines, amides or anions, hexamine is not a formaldehyde-yielding compound. This is because slow hexamine decomposition forms reactive imines that react very fast with nucleophiles and do not allow hexamine to reach its final decomposition products of ammonia and formaldehyde.120 Hexamine was used as a hardener for tannin adhesives used in industrial panels produced in Chile during 1993–2002. The good potential of tannin adhesives for industrial use have been reported by Valenzuela et al. (2012).121
Methylolated nitroparaffins, such as the simplest and least expensive tris(hydroxymethyl)nitromethane (TRIS), function well as crosslinking agents for tannin adhesives. It has been shown to lower formaldehyde emissions and increase pot life when used as a proportional substitution of other hardeners for tannin adhesives.122 However, it also requires much higher curing temperatures than other hardeners.118 Other curing agents, such as fatty amides based on vegetable oils, have also been tested as crosslinkers for tannin adhesives. Patel et al.123 have tested N,N-bis(2-hydroxyethyl) fatty amide (HEFA) from Karanja oil and rice bran oil as cross-linkers for tannin. H2SO4, NaOH, and NH4Cl are used as curing catalysts. NaOH gives slightly lower joint strength for both rice bran oil and Karanja oil. The increase in HEFA content increases chemical resistance, tensile strength and impact strength of the bonded joints. HEFA from Karanja oil gives slightly better results, because the reactivity of methylol groups in it is higher than in rice bran oil. Recently, it has been reported that a suitable mixture of tannin and EPL (30% tannin and 13% EPL) gives an adhesive that passes the EN 314 class 1 standard, with respect to both water resistance and adhesive strength.124 A chemical route for possible crosslinking of tannin and protein has also been proposed, as shown in Fig. 14.
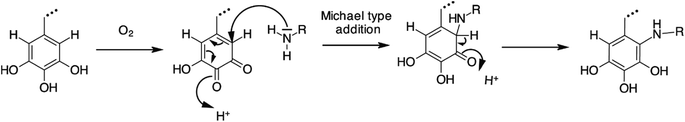 | ||
| Fig. 14 Plausible reaction mechanism for tannin-based adhesives. Terminal gallic acid (top left) is oxidized to a diketone (top right). The diketone then reacts with the amine group of the protein, which results in tannin and protein becoming cross-linked (reproduced from ref. 124 with permission from Elsevier Ltd). | ||
5.3. Soy protein
A detailed review on the use of soy protein as wood adhesive can be found in literature.16,56,57,125 The use of soy protein as an adhesive dates back to the ancient times but its commercial use in plywood production began only in the 1930s.126 Back then, soy protein adhesives were used for wood and paper and as binders in coatings and paints. The soy proteins used as plywood adhesives were typically denaturized by caustic treatment. The products had typically short pot lives, poor biological stability, low solid content, slow pressing times and very poor water resistance, which limited them to mainly interior applications.127 In the 1960s, most soy-based adhesives were replaced with synthetic adhesives, such as phenol-formaldehyde (PF) and urea-formaldehyde (UF) adhesives.The new developed soy adhesives have higher moisture tolerances and are stronger than those known before the 1960s. As a wood adhesive, soy protein is inexpensive, easy to handle, has low pressing temperatures and can bond wood with relatively high moisture content. However, soy protein adhesives have high viscosities and short pot lives and the wood composites bound with them have relatively low strength, low water tolerance, and are sensitive to biological degradation.14 Protein adhesives are also quite sensitive to changes in temperature, pH, ionic strength and pressing conditions. The adhesive properties highly depend on protein content.128 The high viscosity of soy adhesives can be lowered by using low solids' content or by hydrolysis of the protein. Hydrolysis breaks the protein macromolecules into small fragments, which also leads to inferior bond strengths. Hydrolysis can be done using hot caustic or enzymatic treatment reactions.129 Another less evaluated aspect of using soy adhesives is in particleboards produced from straw instead of wood. Soy protein adhesives have been proposed to be more suitable than formaldehyde based adhesives for most straw boards since the straw surface is hydrophobic due to silica and wax components.130
It is possible to unfold protein complexes with urea, as its oxygen and hydrogen atoms interact with hydroxyl groups of the proteins and break down the hydrogen bonds in the protein body.132 Urea at a very high concentration also breaks the secondary structure of the protein, but this can have a negative effect on adhesive properties of the protein. Soy proteins can also be treated with sodium dodecyl sulfonate (SDS), sodium dodecyl benzene sulfonate (SDBS) and enzymes to break apart the quaternary protein structure while still retaining some part of the secondary structure.133 Guanidine hydrochloride (GuHCl) can also denature protein. In a fiberboard study by Zhong et al.,134 the best GuHCl concentration for denaturing soy protein isolates (SPIs) is 1 M. Increasing pressing temperature is the major physical method to cause denaturation of protein molecules, and temperatures above 75 °C can cause further denaturation of SPI.134
Sodium hydroxide has been shown to denature higher molecular weight soy protein better than sodium carbonate. After denaturation, it is important to stabilize the protein by adding, for example, formaldehyde and phenol. Some crosslinking in the protein occurs after the addition of formaldehyde. The reaction between soy flour and formaldehyde and the possibilities to replace PF resins has been studied by Lorenz et al.135 using gel permeation chromatography. The chemical modification of soy proteins is shown to increase its adhesion properties, but such methods often require the use of more expensive SPIs. Maleic anhydride (MA) is one way to modify soy protein. It reacts more readily with amino groups than with hydroxyl groups in the soy protein. However, this modification alone does not increase dry shear strength or water tolerance enough. Combining MA-grafted SPIs with polyethyleneimine (PEI) has been noted to improve adhesive performance. The highest shear strengths in the tested plywood samples are achieved with 20% PEI content.136 The particleboard pressing conditions for soy flour-PEI–MA–NaOH adhesives are optimized by Gu and Li.137 The largest increase in MOR, MOE and IB is detected when the temperature is increased from 160 °C to 170 °C. Above this temperature, only minor changes are detected in MOR. Thus, for SF–PEI–MA–NaOH adhesive, the pressing temperature of 170 °C and pressing time of 270 s for a 17 mm three-layer board is found to be the most desirable. Poly(glycidyl methacrylate) (GMA) contain both methacrylic and epoxy groups that react readily with many different functional groups. Grafting GMA into canola protein has been shown to be possible and is known to increase adhesive strength and water tolerance of the adhesive when tested on veneer sheets.58 Table 4 shows some latest literature about soy protein based adhesives and their application in WBPs.
Soy protein has also been tested in polyketone-based veneer adhesives. High molecular weight polyketones show many interesting properties such as biodegradability; photo-degradability; chemical resistance to acids, bases and solvents; and stability against electrolytic corrosion. Replacing some of the polyketones with soy protein does not affect the performance of the wood adhesive and it is also economical due to the lower price of soy protein.138
Proteins from different sources react differently to modifications and additives. For example, in a study by Cheng et al.,139 cotton seed protein adhesive has been found to benefit from modifiers with anionic charge in them (e.g. glutamic acid, acetic acid, butyric acid), while no positive change is detected in soy proteins adhesive formulations. Similarly, it has been found that adhesive properties and water resistance of soy protein isolates is significantly better than that of wheat gluten protein after alkali modification.140 Lignin-modified soy protein adhesives with improved mechanical and water resistance properties have been reported by Pradyawong et al.141 The water resistance of soy adhesives is also improved by the addition of soy-oil based waterborne polyurethane.142
Historically, replacement of synthetic resins partially with soy protein has shown to decrease reactivity of resin and increase viscosity compared to synthetic adhesives. Adding soy protein as a modifier to UF resin is not found to reduce formaldehyde emissions.143 When compared to 100% soy adhesives, the properties are however improved when combining cheap soy flour with a synthetic adhesive such as MUF and UF. In soy-MUF adhesives, methylene bridges are formed between soy and MUF molecules. It is found that the addition of MUF increases water tolerance and wet shear strength of soy adhesives, but MUF content has to be above 20% for the adhesive to pass the three-cycle water-tolerance test.144
Using soy proteins to partially replace PF adhesives is problematic, since the amine groups of soy proteins do not react well under basic conditions of PF formulations.110 Wescott et al.127 have used soy-PF resin containing 40% soy in the production of strandboards showing good water tolerance properties, even withstanding 2 h of boil tests. Based on water extraction and elemental analysis results, 55–86% of previously water-soluble soy flour is converted to water-insoluble material through co-polymerization with resole PF resin.
Soy protein is modified by alkaline calcium, the active hydrophilic groups chelated with calcium or ions to form insoluble calcite or aragonite crystals. During the adhesion process with wood substrate at high pH, the modified soy protein formed ionic bonding.147 A schematic model for the adhesive phenomenon (Fig. 15) has been proposed, and it is adopted from naturally occurring biomimetic hybrid adhesives in gecko and mussel adhesion.
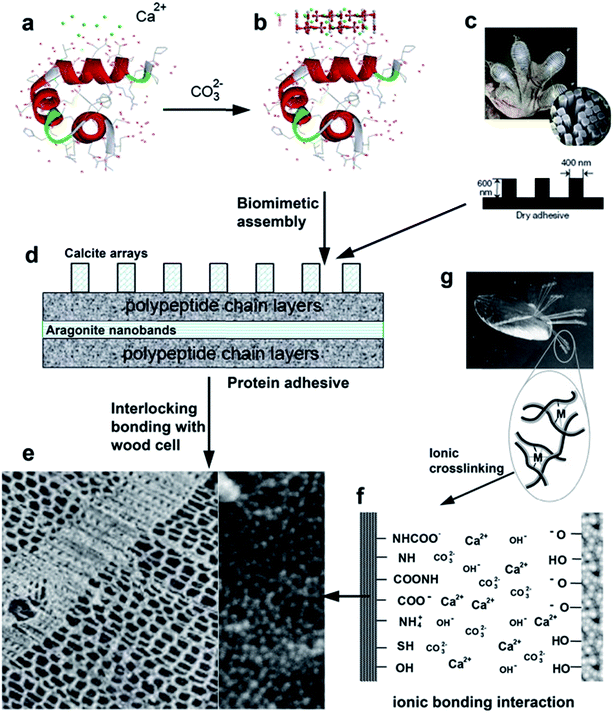 | ||
| Fig. 15 Schematic describing the inspiration of gecko and mussel adhesives (c and g, respectively), route of synthesis for soy protein hybrid adhesives (a, b, and d), and an illustration of adhesion interface structure and mechanical interlocking and ionic crosslinking interaction (e and f, respectively) (reproduced from ref. 147 with permission from Elsevier Ltd). | ||
5.4. Starch
The adhesive bonding strength of natural starch is not strong enough to glue wood, and the amount of research done is not as great as for some of the other bio-based adhesives. Most recent starch adhesive research focuses on replacement or extender for solid wood dispersion adhesives to reduce material cost and increase viscosity.148,149 The studies on the use of starch as a wood adhesive have focused on corn starch in starch/polyvinyl alcohol,150 starch/isocyanates151 and starch/tannin71 adhesives. Also, rice, rye, wheat and potato starch have been evaluated for use in particleboards. The adhesive properties of starch vary greatly depending on where it is derived from.64,66Starch adhesives rely on hydrogen bonding forces, which are much weaker than chemical bonds. They also easily form hydrogen bonds with water molecules, leading to poor water resistance. Higher bonding strength and better water resistance can be achieved by crosslinking starch using crosslinking agents such as sodium borate, epoxy chloropropane, hexamethoxymethylmelamine, formaldehyde, and isocyanates.151
Starch is not soluble in water and it is easily precipitated due to the high amount of intra- and intermolecular hydrogen bonding. Thus, starch adhesives tend to crystallize upon drying, resulting in reduced contact area and loss of adhesion when used for veneer gluing in plywood manufacturing. Crystalline particle formation of starch chains can be disrupted by heating in water. First, in this gelatinization, the heating breaks hydrogen bonds in the starch helices, allowing water to penetrate into the structure. Starch paste is formed when the heating is continued with excess water. The paste consists of dissolved amylose and starch granule fragments (D'Amico et al., 2010). Other modifications to open tightly bound starch granules are acid treatment, alkali treatment, derivatization, oxidation and enzyme treatment.152 Chemical, physical, enzymatic and genetic modifications of starch for different applications have been thoroughly reviewed by Kaur et al.70 Wang et al.153 have studied the influence of starch hydrolysis on various characteristics of starch-based wood adhesives. Up to two hours of acid hydrolysis improves grafting reaction and reduces steric hindrance of adhesives. Two hours of acid hydrolysis of starch can inhibit retrogradation of starch molecules by preventing starch chains from re-arranging and locking water molecules in the adhesive system.
Another universal synthetic adhesive/crosslinker that can be used as a crosslinker of starch and other bio-based adhesives is epoxy resin. Epoxy resins have been tested mainly for veneer gluing, e.g. in combination with polyvinyl acetate (PVAc) grafted starch adhesives. Epoxy groups form three-dimensional networks that provide good shear strength in both dry and humid conditions.152
An innovative starch-based adhesive has been formulated by the addition of a silane coupling agent (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–Si (OC2H5)3, A-151, as a cross-linking agent), an olefin monomer (butyl acetate and vinyl acetate as a co-monomer) and hydrogen peroxide (as an oxidant).155 Fig. 16 shows the reaction pathway for the synthesis of this starch-based adhesive.
CH–Si (OC2H5)3, A-151, as a cross-linking agent), an olefin monomer (butyl acetate and vinyl acetate as a co-monomer) and hydrogen peroxide (as an oxidant).155 Fig. 16 shows the reaction pathway for the synthesis of this starch-based adhesive.
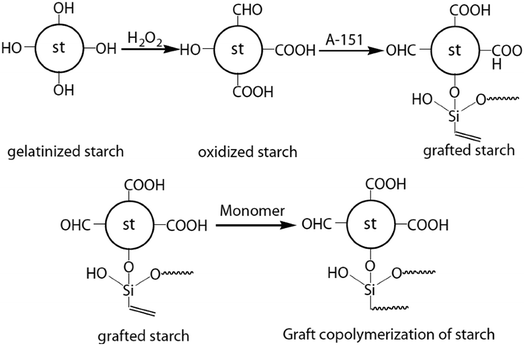 | ||
| Fig. 16 Reaction pathway for the synthesis of starch-based adhesive (reproduced from ref. 155 with permission from Elsevier Ltd). | ||
A starch/PVOH adhesive has been studied for birch veneers and the production conditions have been optimized accordingly.156 Hexamethoxymethylmelamine is found to be an effective crosslinker through transetherification reactions between the methoxy groups of the crosslinker and the hydroxyl groups of wood, starch and PVOH. Furthermore, latex is found to increase moisture resistance properties of starch. Crosslinking of starch with PVOH and the presence of latex also prevent microbial growth on the adhesive. Additives, such as citric acid, can be added to the starch/PVOH adhesives to potentially increase adhesion.157 Oxidized corn starch/PVA copolymers with silane coupling agent have also given good results when used as solid wood adhesives.155 Sucrose molecules are relatively small compared with amylose and amylopectins of starch and extremely soluble in water. It has been proposed that sucrose can form hydrogen bonds with longer molecules of starch. Sucrose is caramelized at 160 °C and the bonding strength of the starch/sucrose adhesive for plywood is found to increase greatly above this temperature. Although sucrose increases the bonding strength for plywood when combined with starch, the best results are achieved when sucrose/tannin (20/80 wt%) is combined with NaOH and tannin. NaOH and high temperature are supposed to catalyse starch and sucrose interactions. Starch can be denatured with NaOH to create less organized agglomerates that are more soluble in water and more easily penetrable to wood.158 Zheng et al.149 have studied the effects of starch heat pre-treatment at 70, 80 and 90 °C on graft copolymerization reaction with vinyl acetate (VAc) and the performance of the resulting starch-based wood adhesive (SWA).
For low density particleboards, self-expanding cassava sour starch adhesive has been tested in a more recent study. Chitosan in propionic acid solution is added to the starch solution, before fibres and glycerol are added to the mixture. The panel properties of this preliminary study are promising.159
Borate additives can form inter-chain linkages through a borate anion structure, and their introduction into starch has shown to increase its bonding ability and resistance to biodegradation. Cyano functions induced by these additives can form a donor–acceptor bond with the contacting phase and increase attachment of low surface energy polymers, such as starch. Derivatives of starch hydroxyl groups by cyanoethylation disrupt starch crystallites, causing starch to be easily dispersed in water. Nwokocha et al. (2011)163 have studied the performance of a cyanoethyl cassava starch adhesive by varying the amounts of cyano groups, solid content, pH and environmental moisture conditions. It is found that the adhesive strength is dependent on the degree of substitution. However, this cyano-ethyl starch adhesive has poor moisture tolerance.163 Chen et al.164 use a silane coupling agent (KH570) as an additive to improve bonding performance and water stability of a starch-based wood adhesive. Sun et al.164 have proposed a new method of preparing a cassava starch-based wood adhesive with high performance using hydrogen peroxide, acrylamide, butyl acrylate (BA) and an organic siloxane as an oxidant, hard co-monomer, soft co-monomer and crosslinking agent, respectively.
6. Outlooks and future propsects
Bio-based adhesives provide a sustainable solution to indoor air quality and formaldehyde concerns. All adhesive raw materials discussed above can significantly reduce emissions (formaldehyde and VOCs) when replacing UF and MUF adhesives currently used in wood-based panel industry. At the same time, they can help the industry be less fossil-fuel dependent. However, bio-based adhesives suffer from several different issues that hinder their usage industrially. Be it availability for tannins, lack of adhesion for starches, poor water resistance for hydroxyl group enriched materials or viscosity for long-molecule chain polymers. Some of the main performance points are presented in Table 5.| Biopolymer | Potential for adhesives | Crosslinker examples |
|---|---|---|
| Lignin | Available, low cost, low reactivity, requires modification, low moisture resistance | Aldehydes (e.g. glyoxal), MDI, tannin |
| Tannin | Good adhesion, fast curing, high viscosity, good water resistance, poor geographical availability | Hexamine, glyoxal, TRIS |
| Protein | Available, low pressing temperature, high viscosity, low water resistance (mostly), denaturation required | Polyamines, PAE, PEI, MDI, (ketones) |
| Starch | Medium cost, low reactivity, low water resistance, modification/grafting required | Epoxies, MDI, tannin, chitosan |
Tannin differs from other bio-adhesives. It provides good adhesion and can be used to make panels with higher moisture tolerance. Thus, its main advantage is that it does not need any reinforcement from synthetic petroleum-based adhesives, while suitable crosslinkers have already been identified at both lab and industrial scale. As tannin is highly reactive with short pot life, the purpose of new crosslinkers is to be less reactive than formaldehyde. Thus, hexamine, glyoxal, and tris(hydroxylmethyl)nitromethane are well suited for tannin-based adhesives, but rarely can be used for other bio-based adhesives for panel production.113,118 Even if extraction methods are developed, tannin extraction rates are not economically profitable for most wood species because tannins are not globally available for industrial use. Additionally, tannins have high viscosity, a dark colour and varying composition that depends on the species, growth conditions and time of harvesting.51 The modifications of tannins focus on decreasing viscosity for easier handling, increasing pot life and on creating better crosslinking.113 In regions where tannin is readily available, tannin provides an industrially viable alternative for synthetic wood composite adhesives. For the other bio-based adhesives, a common problem seems to be exist based on all the research done so far: a lack of economically viable crosslinkers for bio-based adhesives that would increase reactivity, mechanical properties, and humidity stability.
For lignin adhesives, the main problem is their extremely low reactivity, that leads to long pressing times and thus increased production costs in panel manufacturing. Pressing factors mentioned in the literature (+30 s mm−1) are three times higher than what is even considerable for lab scale particleboard testing. The industrial success in using these materials has therefore been small, though lignin has probably been the most intensely researched raw material for wood adhesive applications.89 Most of the research has been done on industrial lignin from pulping processes. Best results have been achieved by replacing phenol in PF resins. Industrial lignin has been used to replace up to 30% of synthetic phenol in the final resin without resulting in unsatisfactory properties of the produced adhesive. Increasing the percentage of industrial lignin in the final resin has been attempted through different modifications, such as methylolation, phenolation, demethylation and oxidation.72 Use of a combination of tannin/lignin to replace phenol and different crosslinkers to replace formaldehyde has also been reported in literature.100 Crosslinking agents that have been and can be considered include aromatic aldehydes, glyoxal, furfuryl alcohol, caprolactam, glycol compounds and hexamine.99 Lignin from biorefinery processes have been less researched. These types of lignin are typically closer to their natural form than those from pulping processes.41 There are plenty unexplored modifications and ways to use them as adhesives. However, current methods are not strong enough to increase the reactivity of lignin to the level it needs to be so as to work as a wood adhesive. This is especially true for panels that require fast curing times, such as particleboards and fibreboards. Some effort has been put into turning biorefinery lignins into platform chemicals44 but a cost-efficient way of doing this is still lacking.
Soy protein adhesives, on the other hand, have a promising future. Development of new crosslinkers and curing agents has enabled soy proteins to become commercially available in the North American market. Although they are so far only used in higher cost premium “green” panels, there is further potential due to the relatively low price and wide availability of soy protein as a by-product. They are environmentally friendly, relatively easy to handle, and have low pressing temperatures that enable lower production costs.137 However, the usage of soy adhesives has long been limited by their low water resistance, sensitivity to biological degradation and relatively low strength of the wood composites bound using them.14 For all protein-based adhesives, controlling denaturation and creating good crosslinking in an economical way are the key parameters to create industrially viable solutions.
Starch-based adhesives provide many advantages for solid wood and plywood industries, as they are easy to handle, are low cost and have low formaldehyde emissions. However, the lack of reactivity, bonding strength, storage stability and water tolerance of starch-based adhesives makes them challenging when industrial board applications are considered. Proper modification combined with crosslinking is needed to reach the required bonding strength. So far, no economically viable bio-based crosslinkers are available on the market, and starch adhesives rely on synthetic crosslinkers, such as isocyanates and epoxides.
Developing good properties for bio-based adhesives other than tannin is challenging. Typically, adhesion is heavily hydrogen-bonding dependent, and the cross-linker needs to both increase adhesion and water resistance. Often there is a desire for the cross-linker to also be bio-based, but as of now, this is not possible mainly for economic reasons. Among the potential synthetic crosslinkers, isocyanates seem to be the most popular for bio-based applications close to commercialization. However, it should be noted and remembered that isocyanates can be used as panel adhesives on their own as well, even though they are expensive in Europe and require alterations in production due to safety issues. For panel production, when isocyanate-containing adhesives are used, special release agents are required to protect the pressing belts. Thus, it seems that in order to find industrially viable bio-based solutions, adhesive research needs to focus more on developing novel reactive crosslinkers.
The sustainable raw materials mentioned in this article belong to the most researched and well-known ones for bio-based adhesives. Our knowledge on the modification of these natural materials has increased and new raw material options have emerged.
Acknowledgements
Venla Hemmilä and Stergios Adamopoulos would like to thank the Knowledge Foundation for financial support (project titled “New environment-friendly board materials”, 2015–2019). The authors are grateful to all publishers (for example, Elsevier Ltd., American Chemical Society, Royal Society of Chemistry, Taylor & Francis and PMDI) who permitted to use figures and tables from their publications.References
- Adhesives in the wood industry, ed. M. Dunky, 2003 Search PubMed.
- L. F. Zhao, Y. Liu, Z. D. Xu, Y. Z. Zhang, F. Zhao and S. B. Zhang, For. Stud. China, 2011, 13, 321–326 CrossRef CAS.
- P. Navarrete, Z. Kebbi, F. Michenot, J. Lemonon, C. Rogaume, E. Masson, Y. Rogaume and A. Pizzi, J. Adhes. Sci. Technol., 2013, 27, 748–762 CrossRef CAS.
- IARC, Press Release No. 153, 2004.
- CARB, California Code of Regulations, section 93120-93120.12, title 17, 2007.
- N. A. Costa, J. Pereira, J. Ferra, P. Cruz, J. Martins, F. D. Magalhães, A. Mendes and L. H. Carvalho, Wood Sci. Technol., 2013, 47, 1261–1272 CrossRef CAS.
- S. Kim, H. J. Kim, H. S. Kim and H. H. Lee, Macromol. Mater. Eng., 2006, 291, 1027–1034 CrossRef CAS.
- M. Funk, R. Wimmer and S. Adamopoulos, Wood Mater. Sci. Eng., 2017, 12, 92–97 CAS.
- A. Kumar, PhD, Universiti Malaysia Pahang, 2013.
- A. Kumar, A. Gupta and K. V. Sharma, Holzforschung, 2015, 69, 199–205 CrossRef CAS.
- A. Kumar, K. Sharma, A. Gupta, J. Tywoniak and P. Hajek, Eur. J. Wood Wood Prod., 2017, 75, 203–213 CrossRef CAS.
- A. Kumar, A. Gupta, K. Sharma, M. Nasir and T. A. Khan, Int. J. Adhes. Adhes., 2013, 46, 34–39 CrossRef CAS.
- Congress Bill, H.R.2646 (107th) Congress of the United States of America, 2002.
- K. Li, S. Peshkova and X. Geng, J. Am. Oil Chem. Soc., 2004, 81, 487–491 CrossRef CAS.
- S. H. Imam, C. Bilbao-Sainz, C. Bor-Sen, G. M. Glenn and W. J. Orts, J. Adhes. Sci. Technol., 2013, 27, 1972–1997 CrossRef CAS.
- T. M. Maloney, Modern particleboard and dry-process fiberboard manufacturing, Miller Freeman Pub., San Francisco, Calif. (USA), 1977 Search PubMed.
- Fiberboard manufacturing practices in the United States, ed. O. Suchsland and G. E. Woodson, 1987 Search PubMed.
- M. Baharoğlu, G. Nemli, B. Sarı, S. Bardak and N. Ayrılmış, Composites, Part B, 2012, 43, 2448–2451 CrossRef.
- M. Böhm, M. Z. Salem and J. Srba, J. Hazard. Mater., 2012, 221, 68–79 CrossRef PubMed.
- B. Sundin, B. Mansson and E. Endrody, Particleboard with different contents of releasable formaldehyde. A comparison on the board properties, including results from different formaldehyde tests, in Proceedings of the 21st Particleboard Symposium, Washington State University, Pullman, 1997, pp. 139–186 Search PubMed.
- R. Marutzky, E. Roffael and L. Ranta, Eur. J. Wood Wood Prod., 1979, 37, 303–307 CrossRef CAS.
- D. Corneau and R. Lovell, For. Prod. J., 2000, 50, 35 Search PubMed.
- J. J. Wolcott, W. K. Motter, N. K. Daisy, S. C. Tenhaeff and W. D. Detlefsen, For. Prod. J., 1996, 46, 62 CAS.
- C. R. Frihart, J. M. Wescott, T. L. Chaffee and K. M. Gonner, For. Prod. J., 2012, 62, 551–558 CAS.
- J. Xiong and Y. Zhang, Indoor Air, 2010, 20, 523–529 CrossRef CAS PubMed.
- C. Yu, D. Crump and R. Squire, Indoor Built Environ., 1999, 8, 287–292 CrossRef CAS.
- Formaldehyde release from wood products, ed. B. Meyer, B. K. Andrews and R. M. Reinhardt, American Chemical Society, 1986 Search PubMed.
- T. Salthammer, S. Mentese and R. Marutzky, Chem. Rev., 2010, 110, 2536–2572 CrossRef CAS PubMed.
- T. Salthammer and S. Mentese, Chemosphere, 2008, 73, 1351–1356 CrossRef CAS PubMed.
- K. Eriksson, C. Heitner, D. Dimmel and J. Schmidt, Lignin Lignans, 2010, 495–520 CAS.
- N.-E. E. Mansouri and J. Salvadó, Ind. Crops Prod., 2006, 24, 8–16 CrossRef.
- H. Chung and N. R. Washburn, Green Mater., 2013, 1, 137–160 CrossRef CAS.
- S. Laurichesse and L. Avérous, Prog. Polym. Sci., 2014, 39, 1266–1290 CrossRef CAS.
- A. Tejado, C. Pena, J. Labidi, J. Echeverria and I. Mondragon, Bioresour. Technol., 2007, 98, 1655–1663 CrossRef CAS PubMed.
- D. Watkins, M. Nuruddin, M. Hosur, A. Tcherbi-Narteh and S. Jeelani, J. Mater. Res. Technol., 2015, 4, 26–32 CrossRef CAS.
- M. V. Alonso, J. J. Rodríguez, M. Oliet, F. Rodríguez, J. García and M. A. Gilarranz, J. Appl. Polym. Sci., 2001, 82, 2661 CrossRef CAS.
- E. A. Capanema, M. Y. Balakshin, C. Chen-Loung, J. S. Gratzl and H. Gracz, Holzforschung, 2001, 55, 302 CAS.
- C. G. da Silva, S. Grelier, F. Pichavant, E. Frollini and A. Castellan, Ind. Crops Prod., 2013, 42, 87–95 CrossRef CAS.
- N.-E. E. Mansouri and J. Salvadó, Ind. Crops Prod., 2006, 24, 8–16 CrossRef.
- R. J. A. Gosselink, M. H. B. Snijder, A. Kranenbarg, E. R. P. Keijsers, E. de Jong and L. L. Stigsson, Ind. Crops Prod., 2004, 20, 191–203 CrossRef CAS.
- W. Zhang, Y. Ma, Y. Xu, C. Wang and F. Chu, Int. J. Adhes. Adhes., 2013, 40, 11–18 CrossRef CAS.
- J. H. Lora and W. G. Glasser, J. Polym. Environ., 2002, 10, 39 CrossRef CAS.
- P. Widsten and A. Kandelbauer, Biotechnol. Adv., 2008, 26, 379–386 CrossRef CAS PubMed.
- R. Ma, Y. Xu and X. Zhang, ChemSusChem, 2015, 8, 24–51 CrossRef CAS PubMed.
- E. Windeisen and G. Wegener, Lignin as Building Unit for Polymers-10.15, 2012, 2012 Search PubMed.
- E. T. N. Bisanda, W. O. Ogola and J. V. Tesha, Cem. Concr. Compos., 2003, 25, 593–598 CrossRef CAS.
- E. Aspé and K. Fernández, Ind. Crops Prod., 2011, 34, 838–844 CrossRef.
- P. Lan, A. Pizzi, G. Zhou Ding and N. Brosse, Ind. Crops Prod., 2012, 40, 13–20 CrossRef.
- G. Vázquez, A. Pizzi, M. S. Freire, J. Santos, G. Antorrena and J. González-Álvarez, Wood Sci. Technol., 2013, 47, 523–535 CrossRef.
- M. Engstrom, M. Karonen, J. Ahern, N. Baert, B. Payré, H. Hoste and J.-P. Salminen, J. Agric. Food Chem., 2016, 64, 840–851 CrossRef CAS PubMed.
- R. M. Rowell, Handbook of Wood Chemistry and Wood Composites, CRC Press, 2005 Search PubMed.
- A. Pizzi, J. Adhes., 2009, 85, 57–68 CrossRef CAS.
- P. Nordqvist, N. Nordgren, F. Khabbaz and E. Malmström, Ind. Crops Prod., 2013, 44, 246–252 CrossRef CAS.
- A. R. Patel and K. P. Velikov, Curr. Opin. Colloid Interface Sci., 2014, 19, 450–458 CrossRef CAS.
- Z. Berk, Food and A. O. o. t. U. Nations, Technology of Production of Edible Flours and Protein Products from Soybeans, Food and Agriculture Organization of the United Nations, 1992 Search PubMed.
- C. R. Frihart and M. J. Birkeland, in Soy-Based Chemicals and Materials, ACS Publications, 2014, pp. 167–192 Search PubMed.
- R. Kumar, V. Choudhary, S. Mishra, I. K. Varma and B. Mattiason, Ind. Crops Prod., 2002, 16, 155–172 CrossRef CAS.
- C. Wang, J. Wu and G. M. Bernard, Ind. Crops Prod., 2014, 57, 124–131 CrossRef CAS.
- G. Qi, N. Li, D. Wang and S. X. Sun, J. Am. Oil Chem. Soc., 2012, 89, 301–312 CrossRef CAS.
- Y. H. Song, J. H. Seo, Y. S. Choi, D. H. Kim, B.-H. Choi and H. J. Cha, Int. J. Adhes. Adhes., 2016, 70, 260–264 CrossRef CAS.
- F. Y. García-Becerra, E. J. Acosta and D. Grant Allen, J. Am. Oil Chem. Soc., 2012, 89, 1315–1323 Search PubMed.
- H. Lei, A. Pizzi, P. Navarrete, S. Rigolet, A. Redl and A. Wagner, J. Adhes. Sci. Technol., 2010, 24, 1583–1596 CrossRef CAS.
- S. Khosravi, P. Nordqvist, F. Khabbaz and M. Johansson, Ind. Crops Prod., 2011, 34, 1509–1515 CrossRef CAS.
- C. E. Ferrández-García, J. Andreu-Rodríguez, M. T. Ferrández-García, M. Ferrández-Villena and T. García-Ortuño, BioResources, 2012, 7, 2012 CrossRef.
- F. N. Emengo, S. E. R. Chukwu and J. Mozie, Int. J. Adhes. Adhes., 2002, 22, 93–100 CrossRef CAS.
- K. M. Salleh, R. Hashim, O. Sulaiman, S. Hiziroglu, W. Noor Aidawati Wan Nadhari, N. Abd Karim, N. Jumhuri and L. Z. P. Ang, J. Adhes. Sci. Technol., 2015, 29, 319–336 CrossRef CAS.
- N. Masina, Y. E. Choonara, P. Kumar, L. C. du Toit, M. Govender, S. Indermun and V. Pillay, Carbohydr. Polym., 2017, 157, 1226–1236 CrossRef CAS PubMed.
- Industrial uses of starch and its derivatives, (No. 664.22 R35), ed. J. A. Radley, Applied Science Publishers, London, 1976 Search PubMed.
- S. D'Amico, M. Hrabalova, U. Müller and E. Berghofer, Ind. Crops Prod., 2010, 31, 255–260 CrossRef.
- B. Kaur, F. Ariffin, R. Bhat and A. A. Karim, Food Hydrocolloids, 2012, 26, 398–404 CrossRef CAS.
- A. Moubarik, N. Causse, T. Poumadere, A. Allal, A. Pizzi, F. Charrier and B. Charrier, J. Adhes. Sci. Technol., 2011, 25, 1701–1713 CrossRef CAS.
- L. Hu, H. Pan, Y. Zhou and M. Zhang, BioResources, 2011, 6, 1 Search PubMed.
- F. Ferdosian, Z. Pan, G. Gao and B. Zhao, Polymers, 2017, 9, 70 CrossRef.
- D. Kai, M. J. Tan, P. L. Chee, Y. K. Chua, Y. L. Yap and X. J. Loh, Green Chem., 2016, 18, 1175–1200 RSC.
- W. Zhao, L.-P. Xiao, G. Song, R.-C. Sun, L. He, S. Singh, B. A. Simmons and G. Cheng, Green Chem., 2017, 19, 3272–3281 RSC.
- J.-L. Wen, S.-L. Sun, B.-L. Xue and R.-C. Sun, Materials, 2013, 6, 359–391 CrossRef CAS.
- B. Danielson and R. Simonson, J. Adhes. Sci. Technol., 1998, 12, 923 CrossRef CAS.
- R. K. Gothwal, M. K. Mohan and P. Ghosh, J. Sci. Ind. Res., 2010, 69, 390–395 CAS.
- W. Zhang, Y. Ma, C. Wang, S. Li, M. Zhang and F. Chu, Ind. Crops Prod., 2013, 43, 326–333 CrossRef CAS.
- W.-J. Lee, K.-C. Chang and I. M. Tseng, J. Appl. Polym. Sci., 2012, 124, 4782–4788 CAS.
- S. Yang, Y. Zhang, T. Q. Yuan and R. C. Sun, J. Appl. Polym. Sci., 2015, 132, 42493 Search PubMed.
- S. Yang, J.-Q. Wu, Y. Zhang, T.-Q. Yuan and R.-C. Sun, J. Biobased Mater. Bioenergy, 2015, 9, 266–272 CrossRef CAS.
- S. Yang, J.-L. Wen, T.-Q. Yuan and R.-C. Sun, RSC Adv., 2014, 4, 57996–58004 RSC.
- S. Zhao and M. M. Abu-Omar, ACS Sustainable Chem. Eng., 2017, 5, 5059–5066 CrossRef CAS.
- M. N. M. Ibrahim, N. Zakaria, C. S. Sipaut, O. Sulaiman and R. Hashim, Carbohydr. Polym., 2011, 86, 112–119 CrossRef CAS.
- P. Dongre, M. Driscoll, T. Amidon and B. Bujanovic, Energies, 2015, 8, 7897–7914 CrossRef CAS.
- J. Li and G. Gellerstedt, Ind. Crops Prod., 2008, 27, 175–181 CrossRef CAS.
- K. Lundquist, Low-molecular weight lignin hydrolysis products, in Applied Polymer Symposium, 1976, vol. 28, pp. 1393–1407 Search PubMed.
- A. Pizzi, J. Adhes. Sci. Technol., 2006, 20, 829–846 CrossRef CAS.
- P. Benar, A. R. Gonçalves, D. Mandelli and U. Schuchardt, Bioresour. Technol., 1999, 68, 11–16 CrossRef CAS.
- G. Vázquez, J. González, S. Freire and G. Antorrena, Bioresour. Technol., 1997, 60, 191–198 CrossRef.
- N. S. Çetin and N. Özmen, Int. J. Adhes. Adhes., 2002, 22, 477–480 CrossRef.
- N. S. Çetin and N. Özmen, Int. J. Adhes. Adhes., 2002, 22, 481–486 CrossRef.
- M. A. Khan, S. M. Ashraf and V. P. Malhotra, J. Appl. Polym. Sci., 2004, 92, 3514 CrossRef CAS.
- M. A. Khan, S. M. Ashraf and V. P. Malhotra, Int. J. Adhes. Adhes., 2004, 24, 485–493 CrossRef CAS.
- M. A. Khan and S. M. Ashraf, Indian J. Chem. Technol., 2006, 13, 347–352 CAS.
- A. R. Gonçalves and P. Benar, Bioresour. Technol., 2001, 79, 103–111 CrossRef.
- S. Kawai, M. Asukai, N. Ohya, K. Okita, T. Ito and H. Ohashi, FEMS Microbiol. Lett., 1999, 170, 51–57 CAS.
- N.-e. El Mansouri, A. Pizzi and J. Salvadó, Eur. J. Wood Wood Prod., 2007, 65, 65–70 CrossRef.
- P. Navarrete, A. Pizzi, S. Tapin-Lingua, B. Benjelloun-Mlayah, H. Pasch, K. Rode, L. Delmotte and S. Rigolet, J. Adhes. Sci. Technol., 2012, 26, 1667–1684 CAS.
- A. Tejado, G. Kortaberria, C. Peña, J. Labidi, J. M. Echeverria and I. Mondragon, Ind. Crops Prod., 2008, 27, 208–213 CrossRef CAS.
- P. Navarrete, A. Pizzi, S. Tapin-Lingua, B. Benjelloun-Mlayah, H. Pasch, K. Rode, L. Delmotte and S. Rigolet, J. Adhes. Sci. Technol., 2012, 26, 1667–1684 CAS.
- H. Lei, A. Pizzi and G. Du, J. Appl. Polym. Sci., 2008, 107, 203–209 CrossRef CAS.
- H. Mansouri, P. Navarrete, A. Pizzi, S. Tapin-Lingua, B. Benjelloun-Mlayah, H. Pasch and S. Rigolet, Eur. J. Wood Wood Prod., 2011, 69, 221–229 CrossRef CAS.
- D. Stewart, Ind. Crops Prod., 2008, 27, 202–207 CrossRef CAS.
- C. Felby, L. G. Thygesen, A. Sanadi and S. Barsberg, Ind. Crops Prod., 2004, 20, 181–189 CrossRef CAS.
- M. Nasir, A. Gupta, M. D. H. Beg, G. K. Chua and A. Kumar, Int. J. Adhes. Adhes., 2013, 44, 99–104 CrossRef CAS.
- M. Nasir, A. Gupta, M. Beg, G. K. Chua, M. Jawaid, A. Kumar and T. A. Khan, BioResources, 2013, 8, 3599–3608 CrossRef.
- Y. Zhang, J.-Q. Wu, H. Li, T.-Q. Yuan, Y.-Y. Wang and R.-C. Sun, ACS Sustainable Chem. Eng., 2017 DOI:10.1021/acssuschemeng.7b01485.
- A. Pizzi, in Handbook of Adhesive Technology, Revised and Expanded, CRC Press, 2003 Search PubMed.
- S. Spina, X. Zhou, C. Segovia, A. Pizzi, M. Romagnoli, S. Giovando, H. Pasch, K. Rode and L. Delmotte, Int. Wood Prod. J., 2013, 4, 95–100 CrossRef.
- A. Moubarik, A. Allal, A. Pizzi, F. Charrier and B. Charrier, Eur. J. Wood Wood Prod., 2010, 68(4), 427–433 CrossRef CAS.
- A. Pizzi, J. Adhes., 2009, 85, 57 CrossRef CAS.
- C. Peña, K. Caba, A. Retegi, C. Ocando, J. Labidi, J. Echeverria and I. Mondragon, J. Therm. Anal. Calorim., 2009, 96, 515 CrossRef.
- Z. Osman, J. Therm. Anal. Calorim., 2012, 107, 709 CrossRef CAS.
- S. Jahanshaei, T. Tabarsa and J. Asghari, Pigm. Resin Technol., 2012, 41, 296–301 CrossRef CAS.
- C. Li, W. Wang, Y. Mu, J. Zhang, S. Zhang, J. Li and W. Zhang, J. Polym. Environ., 2017, 1–13 Search PubMed.
- G. Vázquez, J. Santos, M. Freire, G. Antorrena and J. González-Álvarez, J. Therm. Anal. Calorim., 2012, 108, 605 CrossRef.
- M. Basso, A. Pizzi, J. P. Maris, L. Delmotte, B. Colin and Y. Rogaume, Ind. Crops Prod., 2017, 95, 621–631 CrossRef CAS.
- F. Pichelin, C. Kamoun and A. Pizzi, Holz Roh- Werkst., 1999, 57, 305 CrossRef CAS.
- J. Valenzuela, E. von Leyser, A. Pizzi, C. Westermeyer and B. Gorrini, Eur. J. Wood Wood Prod., 2012, 70, 735–740 CrossRef CAS.
- A. Trosa and A. Pizzi, Holz Roh- Werkst., 2001, 59, 266 CrossRef CAS.
- D. S. Patel, S. D. Toliwal and J. V. Patel, J. Adhes. Sci. Technol., 2012, 26, 2217–2227 CAS.
- S. Omura, Y. Kawazoe and D. Uemura, Int. J. Adhes. Adhes., 2017, 74, 35–39 CrossRef CAS.
- D. Vnučec, A. Kutnar and A. Goršek, J. Adhes. Sci. Technol., 2017, 31, 910–931 CrossRef.
- K. S. Liu, Soybeans: Chemistry, Technology and Utilization, Springer, 1997 Search PubMed.
- J. M. Wescott, C. R. Frihart and A. E. Traska, J. Adhes. Sci. Technol., 2006, 20, 859–873 CrossRef CAS.
- R. M. Rowell, F. Caldeira and J. K. Rowell, Sustainable development in the forest products industry, Universidade Fernando Pesoa, Portugal, 2010 Search PubMed.
- J. M. Wescott and C. R. Frihart, Competitive soybean flour/phenol-formaldehyde adhesives for oriented strandboard, 2004 Search PubMed.
- X. Mo, J. Hu, X. S. Sun and J. A. Ratto, Ind. Crops Prod., 2001, 14, 1–9 CrossRef CAS.
- Y. Wang, D. Wang and X. S. Sun, J. Am. Oil Chem. Soc., 2005, 82, 357–363 CrossRef CAS.
- X. Sun and K. Bian, J. Am. Oil Chem. Soc., 1999, 76, 977 CrossRef CAS.
- W. Huang and X. Sun, J. Am. Oil Chem. Soc., 2000, 77, 101 CrossRef CAS.
- Z. Zhong, X. Susan Sun, X. Fang and J. A. Ratto, Int. J. Adhes. Adhes., 2002, 22, 267–272 CrossRef CAS.
- L. Lorenz, C. R. Frihart and J. M. Wescott, Presented in part at the Wood adhesives 2005, San Diego, California, USA, 2005 Search PubMed.
- K. Li and Y. Liu, Int. J. Adhes. Adhes., 2007, 27, 59–67 CrossRef.
- K. Gu and K. Li, J. Am. Oil Chem. Soc., 2011, 88, 673–679 CrossRef CAS.
- A. I. Hamarneh, H. J. Heeres, A. A. Broekhuis, K. A. Sjollema, Y. Zhang and F. Picchioni, Int. J. Adhes. Adhes., 2010, 30, 626–635 CrossRef CAS.
- H. N. Cheng, C. Ford, M. K. Dowd and Z. He, Int. J. Adhes. Adhes., 2016, 68, 156–160 CrossRef CAS.
- P. Nordqvist, F. Khabbaz and E. Malmström, Int. J. Adhes. Adhes., 2010, 30(2), 72–79 CrossRef CAS.
- S. Pradyawong, G. Qi, N. Li, X. S. Sun and D. Wang, Int. J. Adhes. Adhes., 2017, 75, 66–73 CrossRef CAS.
- H. Liu, C. Li and X. S. Sun, Int. J. Adhes. Adhes., 2017, 73, 66–74 CrossRef CAS.
- L. F. Lorenz, A. H. Conner and A. W. Christiansen, For. Prod. J., 1999, 49, 73 CAS.
- D.-B. Fan, T.-F. Qin and F.-X. Chu, J. Adhes. Sci. Technol., 2011, 25, 323–333 CrossRef CAS.
- Y. Jang, J. Huang and K. Li, Int. J. Adhes. Adhes., 2011, 31, 754–759 CrossRef CAS.
- C. Yuan, M. Chen, J. Luo, X. Li, Q. Gao and J. Li, Carbohydr. Polym., 2017, 169, 417–425 CrossRef CAS PubMed.
- D. Liu, H. Chen, P. R. Chang, Q. Wu, K. Li and L. Guan, Bioresour. Technol., 2010, 101, 6235–6241 CrossRef CAS PubMed.
- L. Cheng, H. Guo, Z. Gu, Z. Li and Y. Hong, Int. J. Adhes. Adhes., 2017, 72, 92–97 CrossRef CAS.
- X. Zheng, L. Cheng, Z. Gu, Y. Hong, Z. Li and C. Li, Int. J. Biol. Macromol., 2017, 96, 11–18 CrossRef CAS PubMed.
- S. H. Imam, L. Mao, L. Chen and R. V. Greene, Starch/Staerke, 1999, 51, 225–229 CrossRef CAS.
- Z. Qiao, J. Gu, S. Lv, J. Cao, H. Tan and Y. Zhang, J. Adhes. Sci. Technol., 2015, 29, 1368–1381 CrossRef CAS.
- Y. Nie, X. Tian, Y. Liu, K. Wu and J. Wang, Polym. Compos., 2013, 34, 77 CrossRef CAS.
- Y. Wang, H. Xiong, Z. Wang and L. Chen, Int. J. Biol. Macromol., 2017, 103, 819–828 CrossRef CAS PubMed.
- H. Tan, Y. Zhang and X. Weng, Procedia Eng., 2011, 15, 1171–1175 CrossRef CAS.
- Y. Zhang, L. Ding, J. Gu, H. Tan and L. Zhu, Carbohydr. Polym., 2015, 115, 32–37 CrossRef CAS PubMed.
- S. H. Imam, S. H. Gordon, L. Mao and L. Chen, Polym. Degrad. Stab., 2001, 73, 529–533 CrossRef CAS.
- W. Sridach, S. Jonjankiat and T. Wittaya, J. Adhes. Sci. Technol., 2013, 27, 1727–1738 CrossRef CAS.
- G. Tondi, S. Wieland, T. Wimmer, T. Schnabel and A. Petutschnigg, Eur. J. Wood Wood Prod., 2012, 70, 271–278 CrossRef CAS.
- S. Monteiro, J. Martins, F. D. Magalhães and L. Carvalho, Polymers, 2016, 8, 354 CrossRef.
- Z. Li, J. Wang, C. Li, Z. Gu, L. Cheng and Y. Hong, Carbohydr. Polym., 2015, 115, 394–400 CrossRef CAS PubMed.
- Z. Li, J. Wang, L. Cheng, Z. Gu, Y. Hong and A. Kowalczyk, Carbohydr. Polym., 2014, 99, 579–583 CrossRef CAS PubMed.
- Z. Wang, Z. Gu, Y. Hong, L. Cheng and Z. Li, Carbohydr. Polym., 2011, 86, 72–76 CrossRef CAS.
- L. M. Nwokocha, J. Adhes. Sci. Technol., 2011, 25, 893–902 CAS.
- L. Chen, Y. Wang, P. Fei, W. Jin, H. Xiong and Z. Wang, Int. J. Biol. Macromol., 2017, 104A, 137–144 CrossRef PubMed.
- S. Peshkova and K. Li, J. Biotechnol., 2003, 102, 199–207 CrossRef CAS PubMed.
- A. K. Patel, P. Michaud, E. Petit, H. de Baynast, M. Grédiac and J. D. Mathias, J. Appl. Polym. Sci., 2013, 127, 5014–5021 CrossRef CAS.
- D. Theng, N.-E. El Mansouri, G. Arbat Pujolràs, B. Ngo, M. Delgado Aguilar, M. À. Pèlach Serra, P. Fullana i Palmer and P. Mutjé Pujol, BioResources, 2017, 12, 2379–2393 CAS.
- J. Cui, X. Lu, X. Zhou, L. Chrusciel, Y. Deng, H. Zhou, S. Zhu and N. Brosse, Ann. For. Sci., 2015, 72, 27–32 CrossRef.
- F. Santiago-Medina, G. Foyer, A. Pizzi, S. Caillol and L. Delmotte, Int. J. Adhes. Adhes., 2016, 70, 239–248 CrossRef CAS.
- Z. Xiao, Y. Li, X. Wu, G. Qi, N. Li, K. Zhang, D. Wang and X. S. Sun, Ind. Crops Prod., 2013, 50, 501–509 CrossRef CAS.
- R. Tupciauskas, J. Gravitis, J. Abolins, A. Veveris, M. Andzs, T. Liitia and T. Tamminen, Holzforschung, 2017, 71(7–8), 555–561 CAS.
- U. H. B. Abdullah and A. Pizzi, Eur. J. Wood Wood Prod., 2013, 71, 131–132 CrossRef CAS.
- M. F. M. Yusof, R. Hashim, A. A. Tajuddin, S. Bauk and O. Sulaiman, Ind. Crops Prod., 2017, 95, 467–474 CrossRef CAS.
- D. Efhamisisi, M.-F. Thevenon, Y. Hamzeh, A.-N. Karimi, A. Pizzi and K. Pourtahmasi, ACS Sustainable Chem. Eng., 2016, 4, 2734–2740 CrossRef CAS.
- R. Widyorini, P. A. Nugraha, M. Z. A. Rahman and T. A. Prayitno, BioResources, 2016, 11, 4526–4535 CrossRef CAS.
- J. Fitzken Da Vinci M. Niro, M. Kyriazopoulos, S. Bianchi, I. Mayer, D. A. Eusebio, J. R. Arboleda, M. Lanuzo and F. Pichelin, Int. Wood Prod. J., 2016, 7(4), 208–214 CrossRef.
- J. Podschun, A. Stücker, R. I. Buchholz, M. Heitmann, A. Schreiber, B. Saake and R. Lehnen, Ind. Eng. Chem. Res., 2016, 55, 5231–5237 CrossRef CAS.
- Z. He and H. N. Cheng, J. Adhes. Sci. Technol., 2017, 1–10 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |




