Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of a quinoidal dithieno[2,3-d;2′,3′-d]benzo[2,1-b;3,4-b′]-dithiophene based open-shell singlet biradicaloid†
Debin
Xia‡
ab,
Ashok
Keerthi‡
bc,
Cunbin
An
b and
Martin
Baumgarten
*b
aMIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, 150001 Harbin, P. R. China
bMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: martin.baumgarten@mpip-mainz.mpg.de
cSchool of Physics and Astronomy Condensed Matter Physics Group, the University of Manchester, M13 9PL Manchester, UK
First published on 31st October 2016
Abstract
A fused heteroacene derivative, bis(dicyanomethylene)-end-capped-dithieno[2,3-d;2′,3′-d]benzo[2,1-b;3,4-b′]-dithiophene (4CN-DTmBDT) was synthesized. Its open-shell biradical character in the ground state is studied by a combination of electron paramagnetic resonance (EPR) and nuclear magnetic resonance (NMR). Structural assignment was verified with single crystal X-ray diffraction analysis. A highly stable biradicaloid with a low energy gap and low LUMO level is achieved.
Open-shell singlet biradicaloids have been attracting tremendous interest due to their unique electronic, optical and magnetic properties for their applications in nonlinear optics, molecular spintronics, and energy storage devices.1–5 Recently open-shell polycyclic aromatic hydrocarbons (PAHs) such as tetrabenzo[a,f,j,o]perylene,6 cethrene,7 zethrenes,1,8 indenofluorene9–12 and quinoidal rylenes13–15 with a singlet biradical ground state have been developed. However, the open-shell structure of these molecules is vulnerable to degradation, which hampers their applications. To date, only a few molecular electronic and spintronic devices based on open-shell PAHs have been investigated.16,17 Thus, the exploration of novel synthetic designs towards stable open-shell PAHs with tunable magnetic properties is imperative.
It is noted that the stability of the open-shell PAHs is strongly related to the additional sextet rings gained in the biradical resonance form, according to the theoretical predictions and experimental results.1–3,18,19 Nevertheless, the synthesis of stable open-shell quinoidal oligothiophene derivatives is rarely reported.20–22
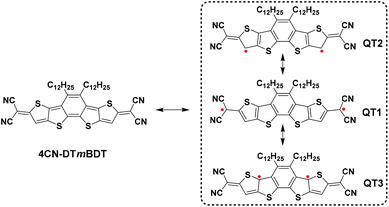
Recently, Ponce Ortiz and coworkers discovered that a quinoidal oligothiophene presents biradical character when the number of central thiophene rings is four or above, otherwise showing closed-shell quinoidal structures.20 Therefore, considering the lack of synthesis protocols and challenges in thermal stability of such biradical character, we have designed a molecule 4CN-DTmBDT (Scheme 1) using the planar heteroacene building block, dithieno[2,3-d;2′,3′-d]benzo[2,1-b;3,4-b′]-dithiophene (DTmBDT).23 Three biradical resonant structures are sketched in Scheme 1. Among them, QT1 is dominant due to the recovery of one aromatic benzene ring and two more aromatic thiophene rings. Thus 4CN-DTmBDT is expected to be a stable molecule with an open-shell singlet ground state.
Herein, we report the facile synthesis of a thieno[3,2-b]thiophene based quinoidal 4CN-DTmBDT and its biradical character is systematically investigated. As the quinoidal backbone is planar and thus having low solubility, dodecyl alkyl chains are chosen to improve the processability. Optical absorption and cyclic voltammetry (CV) measurements were applied to reveal the narrow energy gap. The variable-temperature NMR and EPR were carried out thoroughly to prove our assumption that 4CN-DTmBDT possesses an open-shell singlet ground state.
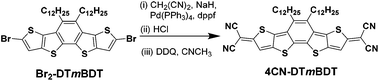
The synthesis of the tetracyano quinoidal derivative, 4CN-DTmBDT was carried out through the palladium-catalyzed Takahashi coupling reaction of the precursor Br2-DTmBDT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 with malononitrile followed by oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) as shown in Scheme 2 with an overall yield of 68%. The dodecyl alkyl chains enable 4CN-DTmBDT to have good solubility in common organic solvents such as tetrahydrofuran, dichloromethane, toluene and N-methylpyrrolidone. The product was characterized by 1H-NMR in deuterated tetrachloroethane (C2D2Cl4) and high-resolution MALDI-TOF MS. Thermogravimetric analysis (TGA) indicated excellent stability, with 5% weight loss occurring at 420 °C (Fig. S1†).
23 with malononitrile followed by oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) as shown in Scheme 2 with an overall yield of 68%. The dodecyl alkyl chains enable 4CN-DTmBDT to have good solubility in common organic solvents such as tetrahydrofuran, dichloromethane, toluene and N-methylpyrrolidone. The product was characterized by 1H-NMR in deuterated tetrachloroethane (C2D2Cl4) and high-resolution MALDI-TOF MS. Thermogravimetric analysis (TGA) indicated excellent stability, with 5% weight loss occurring at 420 °C (Fig. S1†).
Single crystals of 4CN-DTmBDT suitable for X-ray crystallographic analysis were obtained by slow evaporation of tetrachloroethane solution. The molecular structure was further confirmed by single crystal XRD analysis at a temperature of 193 K (Fig. 1). The backbone of the molecule is completely flat and packed in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (triclinic) space group. The molecules are arranged in a slipped π-stacking fashion with a π-face separation of 3.46 Å. It is found that there are significant bond length alternations in the structure. On closer inspection of such a conjugated system, only distinct double-bond character is observed rather than delocalized partial double bonds.16 This might suggest that the ground electronic state exists mainly in the form of a closed-shell quinoidal structure at 193 K.
(triclinic) space group. The molecules are arranged in a slipped π-stacking fashion with a π-face separation of 3.46 Å. It is found that there are significant bond length alternations in the structure. On closer inspection of such a conjugated system, only distinct double-bond character is observed rather than delocalized partial double bonds.16 This might suggest that the ground electronic state exists mainly in the form of a closed-shell quinoidal structure at 193 K.
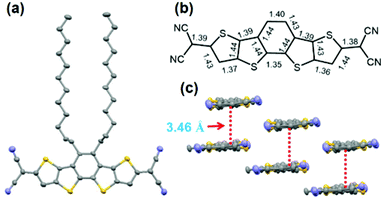 | ||
| Fig. 1 Molecular structure of 4CN-DTmBDT. Top view (a), (b) selected bond lengths (Å), and (c) crystal packing. | ||
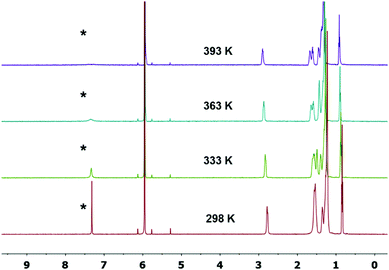
Intrigued by the single crystal XRD analysis, variable-temperature 1H-NMR spectra of 4CN-DTmBDT were recorded in C2D2Cl4 to investigate temperature dependent differences (Fig. 2). The marked (*) resonance signal at 7.33 ppm is well-assigned to the proton of the DTmBDT unit. This peak appeared sharply at 298 K but became broader as the temperature was increased step by step. This resonance almost disappeared at 393 K and no new peak was observed suggesting transformation to the biradical NMR inactive form. This process is entirely reversible upon cooling the sample back to 298 K, thus suggesting the high thermal stability of 4CN-DTmBDT. These findings indicate a radical feature, which has also been observed in phenalenyl-, zethrene- and tetrabenzo[a,f,j,o]perylene-based biradicals, and is caused by a thermally excited triplet species.3,6
To further confirm the biradical nature of 4CN-DTmBDT, electron paramagnetic resonance (EPR) measurement was carried out in the solid state (Fig. 3a) and in dilute solutions of toluene (Fig. S2†). As shown in Fig. 3a, 4CN-DTmBDT exhibited increasing EPR signal intensities upon warming the sample from 220 to 320 K, which was in accordance with the variable-temperature 1H-NMR measurements. The g value is 2.0028 indicating a carbon based radical. The temperature dependence of the EPR signal intensity (Fig. S2†) was analyzed using the Bleaney and Bowers equation.24,25 The estimated singlet–triplet energy gap (ΔEST) is 2.65 kcal mol−1. Consequently, both the NMR and EPR results demonstrate that compound 4CN-DTmBDT has the biradical feature.
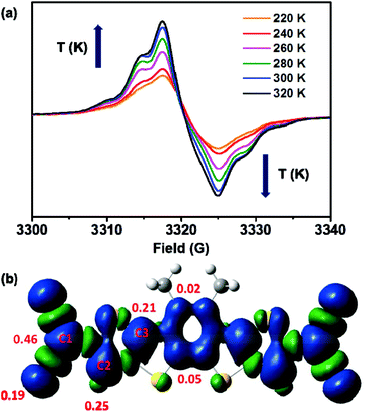 | ||
| Fig. 3 (a) Variable-temperature EPR spectra of 4CN-DTmBDT in the solid state. (b) Spin-density of the triplet state of 4CN-DTmBDT calculated with DFT using the U3BLY/6-31G(d) level of theory. | ||
To gain insight into the electronic structure of 4CN-DTmBDT, DFT calculations were performed with the geometry of single crystal structure to obtain the singlet–triplet energy gap (ΔEST) and to compare the spin density distribution. The ΔEST = −6.88 kcal mol−1 was calculated at the DFT unrestricted broken symmetry (BS) B3LYP/6-31g(d) level of theory26,27 (Table S1 and Fig. S3†). As shown in Fig. 3b, C1 and C2 have the largest spin densities, as predicted in Scheme 1. Thus, the calculation results also indicate that 4CN-DTmBDT exist in resonance forms between the Kekulé and biradical structures. The closed-shell DFT calculations for the quinoidal structure were also carried out to predict the frontier molecular orbital distributions and its absorption spectra through B3LYP/6-31(d) and TD-SCF B3LYP/6-31G(d), respectively. The HOMO and LUMO were completely distributed all over the molecule with an energy gap of 1.34 eV (Fig. S4†). The simulated absorption spectra had two peaks at 660 nm (λmax) and 318 nm with onset absorption extended over 1000 nm (Fig. S5†).
Fig. 4a illustrates the UV-vis absorption spectra of 4CN-DTmBDT in dilute toluene solution. The absorption maximum appeared at 679 nm, which was red shifted around ∼80 nm in comparison with the quinoidal benzo[1,2-b:4,5-b′]dithiophene derivative DHB-QDTB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 (for the chemical structure, see ESI, Scheme S1†), which is due to the enlarged conjugation length. The absorption spectrum of 4CN-DTmBDT had another two red-shifted shoulder peaks at 722 nm and 776 nm, respectively. The onset of absorption is related to an optical gap of 1.4 eV. The high energy peak around 320 nm was assigned to the conjugated backbone of DTmBDT. Moreover, 4CN-DTmBDT showed very weak photoluminescence, further indicating the presence of biradicaloid character. Cyclic voltammetry (Fig. 4b) was employed to investigate the electrochemical properties of 4CN-DTmBDT. It presented a well-resolved reversible oxidation peak at 1.12 V and two reversible reduction waves at −0.33 V and −0.47 V respectively. The ionization potential (IP) and electron affinity (EA) of 4CN-DTmBDT were thus estimated to be −5.70 eV and −4.52 eV, respectively, from the onsets of the 1st oxidation and reduction peak potentials. Notably, the electrochemical energy gap (1.18 eV) of 4CN-DTmBDT was similar to its analogue dicyanomethylene-substituted quinoidal dithieno[2,3-d;2,3-d]benzo[1,2-b;4,5-b′]dithiophene,29 while the experimental values were in well agreement with the calculated HOMO and LUMO levels of the molecule (Fig. S4†).
28 (for the chemical structure, see ESI, Scheme S1†), which is due to the enlarged conjugation length. The absorption spectrum of 4CN-DTmBDT had another two red-shifted shoulder peaks at 722 nm and 776 nm, respectively. The onset of absorption is related to an optical gap of 1.4 eV. The high energy peak around 320 nm was assigned to the conjugated backbone of DTmBDT. Moreover, 4CN-DTmBDT showed very weak photoluminescence, further indicating the presence of biradicaloid character. Cyclic voltammetry (Fig. 4b) was employed to investigate the electrochemical properties of 4CN-DTmBDT. It presented a well-resolved reversible oxidation peak at 1.12 V and two reversible reduction waves at −0.33 V and −0.47 V respectively. The ionization potential (IP) and electron affinity (EA) of 4CN-DTmBDT were thus estimated to be −5.70 eV and −4.52 eV, respectively, from the onsets of the 1st oxidation and reduction peak potentials. Notably, the electrochemical energy gap (1.18 eV) of 4CN-DTmBDT was similar to its analogue dicyanomethylene-substituted quinoidal dithieno[2,3-d;2,3-d]benzo[1,2-b;4,5-b′]dithiophene,29 while the experimental values were in well agreement with the calculated HOMO and LUMO levels of the molecule (Fig. S4†).
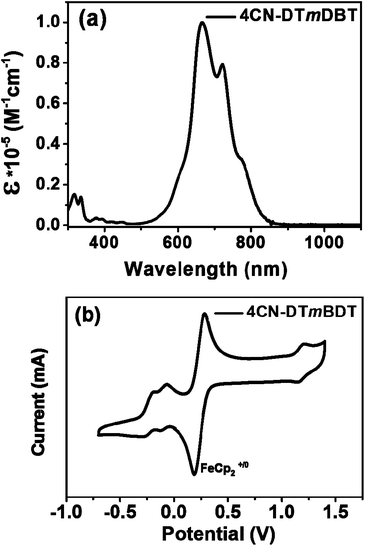 | ||
| Fig. 4 (a) UV-vis absorption spectrum of 4CN-DTmBDT in CH2Cl2 and (b) the cyclic voltammogram of 4CN-DTmBDT in CH2Cl2 with Bu4NPF6 as the supporting electrolyte, at a scan rate of 100 mV s−1. | ||
Fascinated by the high thermal stability of 4CN-DTmBDT from the TGA analysis, we have investigated possible phase transitions using differential scanning calorimetry (DSC) in the temperature range of 20 °C to 350 °C under a nitrogen atmosphere. Surprisingly, there was an exothermic phase transition at 275 °C apart from the endothermic peak at 126 °C which might be assigned to segmental melting or rearrangement. The high temperature phase transition was not reversible in the cooling cycle and did not occur in subsequent heating–cooling cycles suggesting a permanent change in the 4CN-DTmBDT material. This black coloured “transformed product” from the heating cycle up to 350 °C was not soluble in any solvent even under sonication and heating conditions. MALDI-TOF analysis did not provide any clues about the “transformed product”. In another experiment, heating–cooling curves for 4CN-DTmBDT were recorded in the range of 20 °C to 190 °C and the endothermic phase transition (at 126 °C) was found to be reversible. Interestingly this product is quite stable which is confirmed by 1H-NMR, MALDI-TOF and FT-IR analysis and in complete agreement with 4CN-DTmBDT. According to our observations (Fig. S6 and S7†) and Seki's recent work,30 we tentatively predict that 4CN-DTmBDT might go through radical polymerization at 275 °C to yield the “transformed product”. The tentative polymer structure P1 is presented in Fig. S8† by considering the following aspects: (i) in the IR spectrum (Fig. S9†), the peak representing the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching frequency at ∼2204 cm−1 has not changed in both 4CN-DTmBDT and the polymer; (ii) the stretching frequency of aromatic C–H (ν
N stretching frequency at ∼2204 cm−1 has not changed in both 4CN-DTmBDT and the polymer; (ii) the stretching frequency of aromatic C–H (ν![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H 3089 cm−1) has disappeared in the polymer spectra; and (iii) the solid did not lose color thus supporting the polymerization reaction through the C2 position.29,30
C–H 3089 cm−1) has disappeared in the polymer spectra; and (iii) the solid did not lose color thus supporting the polymerization reaction through the C2 position.29,30
In summary, we have successfully synthesized the conjugation elongated quinoidal molecule (4CN-DTmBDT) with 1.2 eV narrow energy gap. The exhibited open-shell biradical properties were confirmed by variable-temperature 1H NMR and EPR analysis. The biradical 4CN-DTmBDT demonstrated good thermal and electrochemical stability. Together with its planarity, efficient intermolecular π–π interaction and low singlet–triplet energy gap (ΔEST), it is expected that 4CN-DTmBDT can be an ideal material for molecular electronic devices.
Acknowledgements
We acknowledge Dr Dieter Schollmeyer at Johannes Gutenberg-University for crystal structure analysis and Dr Prince Ravat at Basel University for fitting the singlet triplet energies. This work was financially supported by Transregio TR49 and the Natural Science Foundation of China (no. 51603055). We are grateful to the King Abdulaziz City of Science and Technology (KACST) for financial support.Notes and references
- Z. Sun, Z. Zeng and J. Wu, Acc. Chem. Res., 2014, 47, 2582–2591 CrossRef CAS PubMed.
- Z. Zeng, X. Shi, C. Chi, J. T. Lopez Navarrete, J. Casado and J. Wu, Chem. Soc. Rev., 2015, 44, 6578–6596 RSC.
- Z. Sun and J. Wu, J. Mater. Chem., 2012, 22, 4151–4160 RSC.
- C. Herrmann, G. C. Solomon and M. A. Ratner, J. Am. Chem. Soc., 2010, 132, 3682–3684 CrossRef CAS PubMed.
- F. Hinkel, J. Freudenberg and U. H. F. Bunz, Angew. Chem., Int. Ed., 2016, 55, 9830–9832 CrossRef CAS PubMed.
- J. Liu, P. Ravat, M. Wagner, M. Baumgarten, X. Feng and K. Müllen, Angew. Chem., Int. Ed., 2015, 54, 12442–12446 CrossRef CAS PubMed.
- P. Ravat, T. Šolomek, M. Rickhaus, D. Häussinger, M. Neuburger, M. Baumgarten and M. Juríček, Angew. Chem., Int. Ed., 2016, 55, 1183–1186 CrossRef CAS PubMed.
- Z. Sun, S. Lee, K. H. Park, X. Zhu, W. Zhang, B. Zheng, P. Hu, Z. Zeng, S. Das, Y. Li, C. Chi, R.-W. Li, K.-W. Huang, J. Ding, D. Kim and J. Wu, J. Am. Chem. Soc., 2013, 135, 18229–18236 CrossRef CAS PubMed.
- A. Shimizu, R. Kishi, M. Nakano, D. Shiomi, K. Sato, T. Takui, I. Hisaki, M. Miyata and Y. Tobe, Angew. Chem., Int. Ed., 2013, 52, 6076–6079 CrossRef CAS PubMed.
- H. Miyoshi, S. Nobusue, A. Shimizu, I. Hisaki, M. Miyata and Y. Tobe, Chem. Sci., 2014, 5, 163–168 RSC.
- D. T. Chase, B. D. Rose, S. P. McClintock, L. N. Zakharov and M. M. Haley, Angew. Chem., Int. Ed., 2011, 123, 1159–1162 CrossRef.
- D. T. Chase, A. G. Fix, S. J. Kang, B. D. Rose, C. D. Weber, Y. Zhong, L. N. Zakharov, M. C. Lonergan, C. Nuckolls and M. M. Haley, J. Am. Chem. Soc., 2012, 134, 10349–10352 CrossRef CAS PubMed.
- Z. Zeng, S. Lee, M. Son, K. Fukuda, P. M. Burrezo, X. Zhu, Q. Qi, R.-W. Li, J. T. L. Navarrete, J. Ding, J. Casado, M. Nakano, D. Kim and J. Wu, J. Am. Chem. Soc., 2015, 137, 8572–8583 CrossRef CAS PubMed.
- Z. Zeng, Y. M. Sung, N. Bao, D. Tan, R. Lee, J. L. Zafra, B. S. Lee, M. Ishida, J. Ding, J. T. López Navarrete, Y. Li, W. Zeng, D. Kim, K.-W. Huang, R. D. Webster, J. Casado and J. Wu, J. Am. Chem. Soc., 2012, 134, 14513–14525 CrossRef CAS PubMed.
- X. Zhu, H. Tsuji, K. Nakabayashi, S.-i. Ohkoshi and E. Nakamura, J. Am. Chem. Soc., 2011, 133, 16342–16345 CrossRef CAS PubMed.
- G. E. Rudebusch, J. L. Zafra, K. Jorner, K. Fukuda, J. L. Marshall, I. Arrechea-Marcos, G. L. Espejo, R. Ponce Ortiz, C. J. Gómez-García, L. N. Zakharov, M. Nakano, H. Ottosson, J. Casado and M. M. Haley, Nat. Chem., 2016, 8, 753–759 CrossRef CAS PubMed.
- H. Zhang, H. Dong, Y. Li, W. Jiang, Y. Zhen, L. Jiang, Z. Wang, W. Chen, A. Wittmann and W. Hu, Adv. Mater., 2016, 28, 7466–7471 CrossRef CAS PubMed.
- M. Randić, Chem. Phys. Lett., 2014, 601, 1–5 CrossRef.
- Z. Sun, Z. Zeng and J. Wu, Chem. – Asian J., 2013, 8, 2894–2904 CrossRef CAS PubMed.
- R. Ponce Ortiz, J. Casado, S. Rodríguez González, V. Hernández, J. T. López Navarrete, P. M. Viruela, E. Ortí, K. Takimiya and T. Otsubo, Chem. – Eur. J., 2010, 16, 470–484 CrossRef CAS PubMed.
- C. Wang, Y. Zang, Y. Qin, Q. Zhang, Y. Sun, C.-a. Di, W. Xu and D. Zhu, Chem. – Eur. J., 2014, 20, 13755–13761 CrossRef CAS PubMed.
- A. Mishra, C.-Q. Ma and P. Bäuerle, Chem. Rev., 2009, 109, 1141–1276 CrossRef CAS PubMed.
- A. Keerthi, C. An, M. Li, T. Marszalek, A. G. Ricciardulli, B. Radha, F. D. Alsewailem, K. Müllen and M. Baumgarten, Polym. Chem., 2016, 7, 1545–1548 RSC.
- B. Bleaney and K. D. Bowers, Philos. Trans. R. Soc. London, Ser. A, 1952, 214, 451–465 CrossRef CAS.
- P. Ravat and M. Baumgarten, Phys. Chem. Chem. Phys., 2015, 17, 983–991 RSC.
- K. Yamaguchi, F. Jensen, A. Dorigo and K. N. Houk, Chem. Phys. Lett., 1988, 149, 537–542 CrossRef CAS.
- M. Shoji, K. Koizumi, Y. Kitagawa, T. Kawakami, S. Yamanaka, M. Okumura and K. Yamaguchi, Chem. Phys. Lett., 2006, 432, 343–347 CrossRef CAS.
- S. Wang, M. Wang, X. Zhang, X. Yang, Q. Huang, X. Qiao, H. Zhang, Q. Wu, Y. Xiong, J. Gao and H. Li, Chem. Commun., 2014, 50, 985–987 RSC.
- J. Li, X. Qiao, Y. Xiong, H. Li and D. Zhu, Chem. Mater., 2014, 26, 5782–5788 CrossRef CAS.
- T. Kobashi, D. Sakamaki and S. Seki, Angew. Chem., Int. Ed., 2016, 55, 8634–8638 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis, characterization, single crystal data, DFT calculations and NMR spectra. CCDC 1414138. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00543h |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2017 |