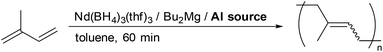Open Access Article
Open Access ArticleSynthesis of stereodiblock polyisoprene consisting of cis-1,4 and trans-1,4 sequences by using a neodymium catalyst: change of the stereospecificity triggered by an aluminum compound†
Ryo
Tanaka
*,
Kaede
Yuuya
,
Hiroki
Sato
,
Peter
Eberhardt
,
Yuushou
Nakayama
and
Takeshi
Shiono
*
Graduate School of Engineering, Hiroshima University, 1-4-1 Kagamiyama, Higashi-hiroshima, Hiroshima, 739-8527 Japan. E-mail: rytanaka@hiroshima-u.ac.jp
First published on 12th January 2016
Abstract
The first example of the synthesis of stereodiblock polyisoprene which consists of cis-1,4 and trans-1,4 sequences was achieved by using a neodymium catalyst. The stereospecificity was controlled by the ratio of Bu2Mg and modified methylaluminoxane (MMAO), and by sequential addition of Bu2Mg and MMAO, a geometrically well-defined stereodiblock polymer was obtained.
A stereoblock polymer, which contains multiple different stereosequences in one polymer chain, changes its physical properties from the original stereoregular polymer. One strategy to synthesize such a polymer is exchanging a propagating chain between two different metal species by equilibrium. Sita applied activated cationic zirconium for the isospecific propylene polymerization and unactivated neutral zirconium species for the racemization of stereocenters which are in equilibrium, giving an isotactic–atactic stereomultiblock polypropylene.1 This stereomultiblock polypropylene shows elastomeric properties, whereas isotactic polypropylene shows crystallinity. More simply, mixing two different polymerization catalysts with a chain transfer reagent can also afford the isotactic-capped syndiotactic polystyrene2 and isotactic–syndiotactic stereomultiblock polypropylene.3 Sometimes a isostructural catalyst performs as an equilibrium of two kinds of catalysts with different symmetries by occasional flipping of the polymer chain or the rotation of the ligand during propylene polymerization, resulting in the formation of isotactic and atactic polypropylene blocks.4,5
Another way to synthesize stereoblock polymers is using a living polymerization system and changing the selectivity by external stimuli during polymerization (Scheme 1). In this synthetic strategy, the lengths of each block can be controlled precisely by the polymerization time. We have previously reported the synthesis of di- or triblock polypropylene which consists of syndiotactic and atactic sequences by changing the reaction temperature,6 solvent7 or monomer concentration.8 However, none of them have control over two different high stereoregularities. Namely, they just control stereospecific polymerization and non-stereospecific polymerization.
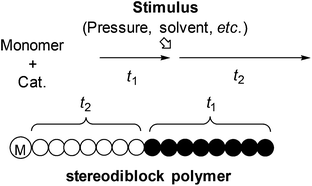 | ||
| Scheme 1 Synthetic strategy of stereodiblock polymers by changing the stereospecificity of living polymerization with an external stimulus. | ||
Additives are among the most fundamental tools to control the selectivity of the metal-catalyzed reaction. In the coordination polymerization of olefins, the stereospecificity strongly depends on the symmetry of the catalyst and therefore it is difficult to control with additives. However, in the polymerization of conjugated dienes, a small amount of additives sometimes dramatically change the stereospecificities. For example, a cis/trans- or 1,4/1,2-specificity of butadiene polymerization can be controlled by the ratio of phosphine and metal precursors such as Ni(tfa)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and CoCl2
9 and CoCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10,11 activated by methylaluminoxane (MAO). In addition, main group metal alkyls, which can coordinate to transition metal with σ-bond bridges, are also applied as additives. The cis/trans-1,4 specificity of the isoprene polymerization catalyzed by Nd(BH4)3(thf)3
10,11 activated by methylaluminoxane (MAO). In addition, main group metal alkyls, which can coordinate to transition metal with σ-bond bridges, are also applied as additives. The cis/trans-1,4 specificity of the isoprene polymerization catalyzed by Nd(BH4)3(thf)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12–14 or Y(allyl)2Cl(MgCl2)2(thf)4
12–14 or Y(allyl)2Cl(MgCl2)2(thf)4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 is varied with activators such as Bu2Mg, Ph3CB(C6F5)4, iBu3Al and MAO. Switching 3,4- and cis-1,4 specificities by the addition of Me3Al to the yttrium amidinate catalyst is also reported.16 The application of these additive effects to living polymerization systems would open up the way to the synthesis of stereodiblock polymers. As the first example, we were motivated to investigate the synthesis of geometrically well-defined stereodiblock17 polyisoprene using a neodymium catalyst activated by Bu2Mg, controlling cis-1,4 and trans-1,4 specificities in the living manner.
15 is varied with activators such as Bu2Mg, Ph3CB(C6F5)4, iBu3Al and MAO. Switching 3,4- and cis-1,4 specificities by the addition of Me3Al to the yttrium amidinate catalyst is also reported.16 The application of these additive effects to living polymerization systems would open up the way to the synthesis of stereodiblock polymers. As the first example, we were motivated to investigate the synthesis of geometrically well-defined stereodiblock17 polyisoprene using a neodymium catalyst activated by Bu2Mg, controlling cis-1,4 and trans-1,4 specificities in the living manner.
Nd(BH4)3(thf)3 activated by Bu2Mg can promote the trans-1,4 specific isoprene polymerization in a living manner (Table 1, run 1). For the synthesis of stereodiblock polyisoprene, the trans-1,4 specificity should be shifted to cis-1,4 in a living manner. Therefore, we first investigated the effect of several aluminum additives on the stereospecificity of isoprene polymerization. The 1,4/3,4 specificity was determined by 1H NMR and the cis/trans specificity was determined by 13C NMR.18
| Run | Nd (μmol) | Al source | Al/Mg | Toluene (mL) | Temp. (°C) | Yield (%) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (103) b (103) |
PDIb | Cis/trans/3,4c (mol%) |
|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: [isoprene]/[Nd] = 625, [Bu2Mg]/[Nd] = 1, time = 60 min, and solvent = toluene. b Determined by GPC calibrated with the polystyrene standard. c Determined by 1H and 13C NMR. d Not determined. e The amount of Bu2Mg was 2 equiv. of Nd. f 1 equiv. of tBuCl was added and the time was 10 h. g 0.25 equiv. of Me2SiCl2 was added. h Bu2Mg was not added. | |||||||||
| 1 | 38 | None | — | 1.3 | 40 | 15 | 11 | 1.1 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 93 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 2 | 25 | MMAO | 10 | 1.4 | r.t. | Trace | n.d.d | n.d.d | n.d.d |
| 3e | 25 | MMAO | 20 | 1.7 | r.t. | 97 | 78 | 1.6 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 4 | 25 | MMAO | 40 | 1.2 | r.t. | 89 | 121 | 1.5 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 5 | 18 | MMAO | 100 | 3.1 | r.t. | 49 | 55 | 1.7 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 6f | 33 | MMAO | 100 | 3.4 | 40 | 25 | 24 | 1.4 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 7g | 50 | dMMAO | 100 | 2.9 | r.t. | 22 | 34 | 1.4 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 8 | 50 | iBu3Al | 100 | 2.3 | r.t. | 43 | 3 | 1.3 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 9 | 45 | MAO | 100 | 2.9 | r.t. | 4 | 10 | 1.5 | n.d.d |
| 10h | 25 | MMAO | 100 | 1.8 | r.t. | 0 | n.d.d | n.d.d | n.d.d |
An excess amount of modified methylaluminoxane (MMAO), which was prepared by the partial hydrolysis of iBu3Al and Me3Al greatly increased the cis-1,4 specificity and activity so that the reaction proceeded at room temperature. The cis-1,4 ratio increased according to the Al/Mg ratio, whereas the 3,4-selectivity remained unchanged (runs 1–5). The molecular weight distribution became narrower and the cis-1,4 specificity was improved by the addition of tBuCl, although the polymerization rate was decreased (run 6). The addition of Me2SiCl2 to dried MMAO (dMMAO) also gave a narrow molecular weight polymer (run 8). The use of iBu3Al gave small molecular weight poly(cis-1,4-isoprene), which is because of the frequent chain transfer between neodymium and aluminium (run 8). Such a tendency was also observed when Cp*La(BH4)2(thf)2 was used as a catalyst precursor, although the change of selectivity from trans to cis did not take place.19 Using MAO, which contains 30 mol% of Me3Al (run 9), instead of MMAO decreased the activity probably because small Me3Al can suppress the polymerization by coordinating to the cationic neodymium active species. MMAO alone did not promote the polymerization, indicating that cationic neodymium-alkyl species was not generated only by MMAO (run 10).
The 13C NMR spectrum of polyisoprene obtained by using the Nd–Mg/Al system (Table 1, run 4, cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans = 59
trans = 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37) in the region of carbon at the 1-position (31–41 ppm) is shown in Fig. 1. Four peaks are observed corresponding to the tactic dyads, which are assigned to cis–trans (23%), trans–trans (16%), cis–cis (38%), and trans–cis (23%). The integral ratios of all the four peaks follow the statistical probability, showing that the cis–trans unit is randomly distributed in this polyisoprene.
37) in the region of carbon at the 1-position (31–41 ppm) is shown in Fig. 1. Four peaks are observed corresponding to the tactic dyads, which are assigned to cis–trans (23%), trans–trans (16%), cis–cis (38%), and trans–cis (23%). The integral ratios of all the four peaks follow the statistical probability, showing that the cis–trans unit is randomly distributed in this polyisoprene.
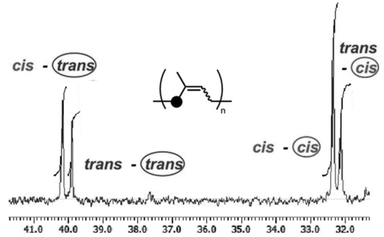 | ||
| Fig. 1 A part of the 13C NMR spectrum of polyisoprene synthesized by using the Nd–Mg/Al catalyst system (125 MHz, in CDCl3, Table 1, run 4). | ||
In general, the cis/trans stereospecificity in the coordination polymerization of isoprene is determined as follows: when isoprene coordinates to the metal center in a cis-η4-manner, the following insertion gives anti-π-allyl species. Normally, the syn-π-allyl configuration which gives the trans-1,4 sequence is more stable than the anti-π-allyl configuration which gives the cis-1,4 sequence because of the steric hindrance of the polymer chain. Therefore, the trans-1,4 sequence is obtained when the anti–syn configurational equilibrium is fast enough compared with the propagation. The cis-1,4 sequence is obtained when the propagation is faster than the anti–syn equilibrium, namely, as a kinetic product. When isoprene coordinates in a trans-η4-manner, the syn-π-allyl intermediate, which gives the trans-1,4 sequence, would be obtained regardless of the rate of the anti–syn equilibrium.20
Alkylmagnesium is considered as an alkylating reagent and the NMR investigation implies that Nd–Mg bimetallic species is formed in the previously reported Nd–Bu2Mg system.12,21 Taube reported trans-1,4 specific polymerization of butadiene using (C3H5)3Nd and the stereospecificity is because of the steric hindrance of the propagating chain forcing the butadiene monomer to bind in a trans-η4 or η2 manner.22 The trans-specificity of Nd(BH4)3(thf)3–Bu2Mg can also be derived by following this mechanism: the isoprene coordinates to the neutral neodymium species in a trans-η4 or η2 manner because of the steric hindrance of alkylmagnesium, and the following syn-π-allyl intermediate would give the trans-1,4 sequence.
On the other hand, a cationic neodymium catalyst from Nd(BH4)3(thf)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 or neutral dialkyl neodymium (C3H5)2NdCl24 can promote the cis-1,4 specific polymerization of conjugated dienes. Excess alkylaluminum can form a dimer with magnesium and a naked neodymium is generated, which can derive the enhancement of the propagation rate and allow isoprene to coordinate in a cis-η4 manner. As a result, a kinetic cis-1,4 product would be obtained. In our mixed cocatalyst system, a fast equilibrium between Nd–Mg bimetallic species and Mg–Al multimetallic species would propagate the cis/trans random sequences (Fig. 2). The relatively broad molecular weight distribution when MMAO is applied is probably because of the deactivation of the unstable naked cationic active species. The addition of chloride would prevent the deactivation by coordinating to the neodymium center.25,26
23 or neutral dialkyl neodymium (C3H5)2NdCl24 can promote the cis-1,4 specific polymerization of conjugated dienes. Excess alkylaluminum can form a dimer with magnesium and a naked neodymium is generated, which can derive the enhancement of the propagation rate and allow isoprene to coordinate in a cis-η4 manner. As a result, a kinetic cis-1,4 product would be obtained. In our mixed cocatalyst system, a fast equilibrium between Nd–Mg bimetallic species and Mg–Al multimetallic species would propagate the cis/trans random sequences (Fig. 2). The relatively broad molecular weight distribution when MMAO is applied is probably because of the deactivation of the unstable naked cationic active species. The addition of chloride would prevent the deactivation by coordinating to the neodymium center.25,26
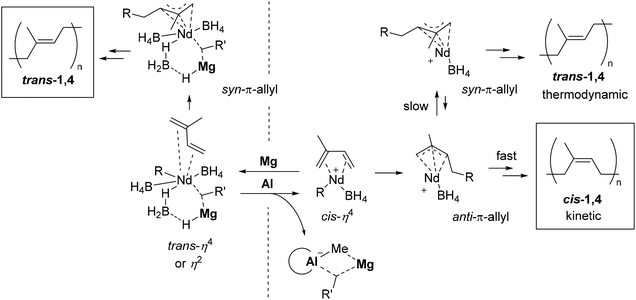 | ||
| Fig. 2 Estimated active species and the mechanism of the change of stereospecificity in the polymerization of isoprene using the Nd–Mg/Al system. | ||
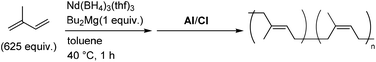
The synthesis of stereodiblock polymers by the sequential addition of Bu2Mg and MMAO/tBuCl or dMMAO/Me2SiCl2 was performed (Table 2). In both systems, a polymer with a narrow molecular weight distribution was obtained in high yield. The GPC trace of the obtained polymer shifted to higher molecular weight from the prepolymer obtained using the Nd–Bu2Mg system with a narrow molecular weight distribution (Fig. 3). This result strongly suggested that the polymer was obtained in a living manner. The integral ratios of cis–cis and trans–trans dyads from 13C NMR were much higher than the others, indicating the formation of a stereodiblock polymer which consists of cis- and trans-polyisoprene sequences (Fig. 4).
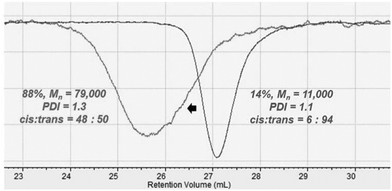 | ||
| Fig. 3 GPC traces of trans-1,4-polyisoprene (right, Table 1, run 1) and trans-1,4-b-cis-1,4-polyisoprene (left, Table 2, run 2) measured at 150 °C in 1,2,4-trichlorobenzene. | ||
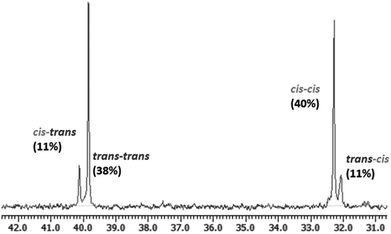 | ||
| Fig. 4 A part of the 13C NMR spectrum of trans-1,4-b-cis-1,4-polyisoprene obtained in Table 2, run 2 (125 MHz, in CDCl3). | ||
| Run | Al/Cl source | Time (h) | Yield (%) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (103) b (103) |
PDIb | Cis/transc |
|---|---|---|---|---|---|---|
| a Reaction conditions for the first block: [isoprene]/[Nd] = 625, [Bu2Mg]/[Nd] = 1, temp. = 40 °C, time = 1 h, and solvent = toluene. b Determined by GPC calibrated with the polystyrene standard. c Determined by 1H and 13C NMR. d Nd = 37 mol, [Al]/[Nd] = 100, [Cl]/[Nd] = 1, temp. = 40 °C. e Nd = 50 mol, [Al]/[Nd] = 100, [Cl]/[Nd] = 0.25, temp. = r.t. | ||||||
| 1 | None | 1 | 15 | 11 | 1.1 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 93 93 |
| 2d | MMAO/tBuCl | 1 + 10 | 88 | 79 | 1.3 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| 3e | dMMAO/Me2SiCl2 | 1 + 1 | 59 | 34 | 1.3 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61 61 |
| 4e | dMMAO/Me2SiCl2 | 1 + 5 | 78 | 50 | 1.4 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
The synthesized cis–trans stereodiblock polyisoprene in Table 2, run 2 showed a melting point (Tm) at 33 °C, which was almost the same value with trans-1,4-polyisoprene obtained in Table 1, run 1 (37 °C), although the melting enthalpy (ΔH) was much smaller compared with trans-1,4-polyisoprene (7 vs. 62 J g−1). The decrease of the melting enthalpy was probably because of the high miscibility of cis-1,4 and trans-1,4 blocks.27 The tensile modulus of the stereodiblock polymer was 1.7 MPa, which was the typical value of an elastomeric material. Therefore, the synthesized stereodiblock polymer had both the crystallinity and elastomeric properties.
Conclusions
In conclusion, we succeeded in controlling the cis/trans-1,4 selectivity of isoprene polymerization initiated by Nd(BH4)3(thf)3 by the addition of Bu2Mg and MMAO. The cis-1,4 specificity reached 90% when the Al/Mg ratio was 100. The stereodiblock polymer which consists of cis-1,4 and trans-1,4 blocks was successfully synthesized by sequential addition of alkylmagnesium and MMAO in the presence of a monomer.Experimental section
General
All manipulations were performed under nitrogen gas using standard Schlenk techniques. Modified methylaluminoxane (MMAO, 6.5 wt% Al, 2.17 M in toluene) was donated by Tosoh-Finechem Co. Dried MMAO was prepared according to the literature.28 Isoprene was distilled over CaH2 and stored with 4A molecular sieves. Bu2Mg (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of s-butyl and n-butyl, 1.0 M in heptane) was purchased and used as received. Nd(BH4)3(thf)3 was synthesized according to the literature.29 Dry toluene was purchased from Kanto Chemical Co., Inc., and further dried over sodium metal. 1H and 13C NMR spectra of the polymer were measured by using a Varian 500 MHz spectrometer using CDCl3 as a solvent. The obtained spectra were calibrated by the peaks originating from the solvent (1H: 7.26 ppm, 13C: 77.16 ppm). The number-average molecular weight (Mn) of the polymer was determined by using a Viscotec 350HT-GPC chromatograph at 150 °C using 1,2,4-trichlorobenzene as an eluent calibrated with a RI/Viscometer/MALS triple detector, or Tosoh HLC-8320 at 40 °C using THF as an eluent calibrated with the PS standard. The polymer concentration of the injecting solution was 1 mg mL−1 and the injection volume was 0.2 mL. DSC analyses of the polymer were performed by using a SII EXSTAR6000 system. The tensile modulus of the polymer was measured by using an Olientec RTC-1210A instrument following ISO 527-3/1B at 25 °C with a drawing rate of 50 mm min−1.
1 mixture of s-butyl and n-butyl, 1.0 M in heptane) was purchased and used as received. Nd(BH4)3(thf)3 was synthesized according to the literature.29 Dry toluene was purchased from Kanto Chemical Co., Inc., and further dried over sodium metal. 1H and 13C NMR spectra of the polymer were measured by using a Varian 500 MHz spectrometer using CDCl3 as a solvent. The obtained spectra were calibrated by the peaks originating from the solvent (1H: 7.26 ppm, 13C: 77.16 ppm). The number-average molecular weight (Mn) of the polymer was determined by using a Viscotec 350HT-GPC chromatograph at 150 °C using 1,2,4-trichlorobenzene as an eluent calibrated with a RI/Viscometer/MALS triple detector, or Tosoh HLC-8320 at 40 °C using THF as an eluent calibrated with the PS standard. The polymer concentration of the injecting solution was 1 mg mL−1 and the injection volume was 0.2 mL. DSC analyses of the polymer were performed by using a SII EXSTAR6000 system. The tensile modulus of the polymer was measured by using an Olientec RTC-1210A instrument following ISO 527-3/1B at 25 °C with a drawing rate of 50 mm min−1.
Isoprene polymerization was carried out using the Nd(BH4)3(thf)3–Bu2Mg/MMAO system (Table 1, run 5)
To a 20 mL Schlenk flask, Nd(BH4)3(thf)3 (18 mg, 44 μmol) and Bu2Mg in toluene (44 μmol, 40 mM, 1.1 mL), and MMAO (2.0 mL, 4.4 mmol) were charged and stirred at room temperature for 3 minutes. To the yellow solution, isoprene (2.8 mL, 28 mmol) was added. The reaction mixture was stirred at room temperature for 1 hour. The resulting solution was poured into acidic methanol containing 1 wt% of BHT and the precipitated solid was recovered. The polymer was dried under vacuum overnight until constant weight. 909 mg (49%) of a colorless viscous polymer was obtained.Synthesis of stereodiblock polyisoprene using the Nd(BH4)3(thf)3–Bu2Mg/MMAO/tBuCl system (Table 2, run 2)
To a 20 mL Schlenk flask, Nd(BH4)3(thf)3 (15 mg, 37 μmol) and Bu2Mg in toluene (37 μmol, 40 mM, 0.93 mL) were charged and stirred at room temperature for 3 minutes. To the yellow solution, isoprene (2.34 mL, 23.4 mmol) was added. The reaction mixture was warmed to 40 °C and stirred for 1 hour. To the mixture was added the mixture of toluene solution of MMAO (2.3 mL, 4.9 mmol) and tBuCl (4.0 μL, 37 μmol) and further stirred for 10 hours. The resulting solution was poured into acidic methanol containing 1 wt% of BHT and the precipitated solid was recovered. The polymer was dried under vacuum overnight until constant weight. 1.40 g (88%) of a colorless polymer was obtained.We are grateful to Tosoh Finechem Co. for generous donation of chemicals. We also gratefully acknowledge the Natural Science Center for Basic Research and Development (N-BARD), Hiroshima University for the high temperature NMR measurement.
Notes and references
- M. B. Harney, Y. Zhang and L. R. Sita, Angew. Chem., Int. Ed., 2006, 45, 2400–2404 CrossRef CAS PubMed.
- R. Po and S. Spera, Polym. J., 2010, 42, 416–418 CrossRef CAS.
- C. Descour, T. Macko, D. Cavallo, M. Parkinson, G. Hubner, A. Spoelstra, M. Villani and R. Duchateau, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 1422–1434 CrossRef CAS.
- D. T. Mallin, M. D. Rausch, Y.-G. Lin, S. Dong and J. C. W. Chien, J. Am. Chem. Soc., 1990, 112, 2030–2031 CrossRef CAS.
- G. W. Coates and R. M. Waymouth, Science, 1995, 267, 217–219 CrossRef CAS PubMed.
- Z. Cai, Y. Nakayma and T. Shiono, Macromolecules, 2008, 41, 6596–6598 CrossRef CAS.
- Z. Cai, Y. Nakayma and T. Shiono, Kinet. Catal., 2006, 47, 274–277 CrossRef CAS.
- Z. Cai, Y. Nakayma and T. Shiono, Macromol. Res., 2010, 18, 737–741 CrossRef CAS.
- P. Hadjiandrreou, M. Julemont and P. Teyssie, Macromolecules, 1984, 17, 2455–2456 CrossRef.
- D. C. D. Nath, T. Shiono and T. Ikeda, Macromol. Chem. Phys., 2003, 204, 2017–2022 CrossRef CAS.
- Z. Cai, M. Shinzawa, Y. Nakayama and T. Shiono, Macromolecules, 2009, 42, 7642–7643 CrossRef CAS.
- F. Bonnet, M. Visseaux, A. Pereira and D. Barbier-Baudry, Macromolecules, 2005, 38, 3162–3169 CrossRef CAS.
- M. Terrier, M. Visseaux, T. Chenal and A. Mortreux, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 2400–2409 CrossRef CAS.
- F. Bonnet, C. E. Jones, S. Semlali, M. Bria, P. Roussel, M. Visseaux and P. L. Arnold, Dalton Trans., 2013, 42, 790–801 RSC.
- N. Ajellal, L. Furlan, C. M. Thomas, O. L. Casagrande and J.-F. Carpentier, Macromol. Rapid Commun., 2006, 27, 338–343 CrossRef CAS.
- L. Zhang, M. Nishiura, M. Yuki, Y. Luo and Z. Hou, Angew. Chem., Int. Ed., 2008, 47, 2642–2645 CrossRef CAS PubMed.
- Currently the use of term ‘geometrical isomer’ was strongly discouraged and the cis–trans isomer was categorized as a stereoisomer. Thus, these kinds of block polymers should be called as ‘stereoblock polymers’, although there is no stereogenic center in the polymer. See: (a) G. P. Moss, Pure Appl. Chem., 1996, 68, 2193–2222 CrossRef CAS; (b) A. D. Jenkins, Pure Appl. Chem., 1981, 53, 733–752 Search PubMed.
- M. W. Duch and D. M. Grant, Macromolecules, 1970, 3, 165–174 CrossRef CAS.
- A. Valente, P. Zinck, M. J. Vitorino, A. Mortreux and M. Visseaux, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4640–4647 CrossRef CAS.
- R. Taube, H. Windisch and S. Maiwald, Macromol. Symp., 1995, 89, 393–409 CrossRef CAS.
- F. Bonnet, H. E. Dye, Y. El Kinani, C. Dietz, P. Roussel, M. Bria, M. Visseaux, P. Zinck and P. Mountford, Dalton Trans., 2015, 44, 12312–12325 RSC.
- S. Maiwald, H. Weissenborn, C. Sommer and R. Taube, J. Organomet. Chem., 2001, 640, 1–9 CrossRef CAS.
- M. Visseaux, M. Mainil, M. Terrier, A. Mortreux, P. Roussel, T. Mathivet and M. Destarac, Dalton Trans., 2008, 4558–4561 RSC.
- S. Maiwald, C. Sommer, G. Müller and R. Taube, Macromol. Chem. Phys., 2002, 203, 1029–1039 CrossRef CAS.
- G. Kwag, H. Lee and S. Kim, Macromolecules, 2001, 34, 5367–5369 CrossRef CAS.
- H. Guo, J. Bi, J. Wang, X. Zhang, S. Jiang and Z. Wu, Dalton Trans., 2015, 44, 9130–9139 RSC.
- The properties of blended natural rubber and trans-1,4-polyisoprene are well investigated. See: J.-S. Song, B.-C. Huang and D.-S. Yu, J. Appl. Polym. Sci., 2001, 82, 81–89 CrossRef CAS.
- H. Hagimoto, T. Shiono and T. Ikeda, Macromol. Rapid Commun., 2002, 23, 73–78 CrossRef CAS.
- S. M. Cendrowski-Guillaume, G. Le Gland, M. Nierlich and M. Ephritikhine, Organometallics, 2000, 19, 5654–5660 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra and DSC traces of the obtained polymer. See DOI: 10.1039/c5py01872b |
| This journal is © The Royal Society of Chemistry 2016 |