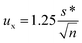Determination of total uranium and uranium isotope ratios in human urine by ICP-MS:results of an interlaboratory study
John G.
Arnason
ab,
Christine N.
Pellegri
a and
Patrick J.
Parsons
*ab
aLaboratory of Inorganic and Nuclear Chemistry, Wadsworth Center, New York State Department of Health, P.O. Box 509, Albany, NY 12201-0509, USA. E-mail: patrick.parsons@health.ny.gov; Fax: +1 518 473-7586; Tel: +1 518 474-7161
bDepartment of Environmental Health Sciences, School of Public Health, The University at Albany, P.O. Box 509, Albany, NY 12201-0509, USA
First published on 17th September 2014
Abstract
In biomonitoring studies, the very low levels of uranium [U] found in urine and the complexity of the urine matrix present challenges for accurate isotope ratio determination. The principal goals of this study were to evaluate current interlaboratory performance and measurement reproducibility for determining [U] and uranium isotope ratios in human urine, and to produce an archive of well-characterized urine reference materials for future method validation and quality control purposes. Six stabilized pools of urine were prepared following the same protocol that is used to prepare materials for the New York State Department of Health (NYSDOH) Proficiency Testing (PT) Program. The candidate reference materials were characterized by their [U] content, isotopic ratios, as well as for homogeneity, and stability. The interlaboratory study design was modeled on the PT program for trace elements in human urine operated by the NYSDOH's Wadsworth Center. Among the nine laboratories that returned results, sample preparation methodologies included simple dilution, acid digestion, column extraction, or a combination of these techniques. For [U] determination, four laboratories used SF-ICP-MS and five used Q-ICP-MS, whereas, for isotope ratios, five used SF-ICP-MS and four Q-ICP-MS. Sample introduction was achieved using either desolvation-nebulization systems or a nebulizer and spray chamber (ambient temperature or Peltier-cooled). Certified reference materials were used by six laboratories to validate [U], isotope ratios, or both. In general for values of [U] > 5 ng L−1, the assigned target values are in good agreement with predicted values and the standard uncertainties are relatively small. For 235U/238U, there was generally good agreement between the target and predicted values, however the relative standard uncertainties for isotope measurements were higher than for [U]. For the minor isotope ratios 234U/238U and 236U/238U, there was generally poor agreement between target and predicted values and the standard uncertainties were also relatively high. These results suggest that accurate and precise measurements of [U] in urine can be achieved using a wide range of ICP-MS instrument types and methodologies. In contrast, measurement of uranium isotope ratios in urine is more strongly dependent on instrumentation and analytical methodology.
Introduction
Determination of uranium isotopes in human urine is critical for distinguishing between exposure to natural and anthropogenic sources of uranium. Traditionally, uranium isotope amount ratios or activity ratios are measured by Thermal Ionization Mass Spectrometry (TIMS) or Alpha Spectrometry (AS), respectively. TIMS typically yields highly accurate and precise measurements for the commonly measured isotope ratios (234U/238U, 235U/238U, and 236U/238U) but sample preparation is time-consuming and instrumentation costly. AS is capable of precise 234U/238U measurements but it is not suitable for measuring 235U/238U or 236U/238U due to the combination of low abundance and low activity of these isotopes in most biological and environmental samples.Over the last two decades, there has been increasing interest in measuring uranium isotope ratios in urine by ICP-MS. New methods have been developed and validated for quadrupole (Q)-ICP-MS,1–8 Dynamic Reaction Cell (DRC)-ICP-MS,2 flow injection extraction chromatography (FI-SPE)-ICP-MS,9 Sector Field (SF)-ICP-MS,10–19 and Multi-collector (MC)-ICP-MS.20,21 Applications for uranium isotope “bioassay” by ICP-MS include studies of nuclear workers;4,16 military veterans exposed to depleted uranium (DU) through retained shrapnel;1,14,22 reference populations of veterans or civilians not exposed to DU;1,9,14,17,23–25 workers at DU or nuclear factories or civilians living near those factories;4,26 and rescue workers at an air disaster.27 With the exception of some nuclear workers and veterans with retained shrapnel the urine uranium concentrations for these populations are generally at biomonitoring levels (1–50 ng L−1). In the US, a population reference interval has been established for total U in urine based on the National Health and Nutrition Examination Surveys (NHANES), and updated tables with ranges are published by the CDC in their National Exposure Reports. Currently, the upper limit of the reference interval, defined as the 95th percentile, is 36 ng L−1 for a spot urine specimen, or 29 ng g−1 creatinine.28
A common issue for many of these studies is the lack of suitable certified reference materials (CRMs) with assigned values for uranium isotopes in urine, along with a statement of uncertainty, that could be used for method validation and quality control purposes. There are available sources of certified isotopic reference materials for uranium in the form of aqueous solutions or uranium oxides (e.g. Institute for Reference Materials and Measurement (IRMM), New Brunswick Laboratory), but there are very few available with a urine matrix. In 2004, eight synthetic urine reference materials were produced and distributed for the concurrent Comité Consultatif pour la Quantité de Matière (CCQM)-P48 and Nuclear Signatures Inter-laboratory Measurement Evaluation Programme (NUSIMEP)-4 interlaboratory studies.29,30 These materials contain approximately 5 ng U g−1 urine. While this [U] concentration ensures sample stability, it is 100–1000 times above the upper limit of the reference interval for human urine and is therefore not ideal for use with biomonitoring studies of the type described above.
Another issue affecting these measurements is a paucity of external quality assurance (EQA) schemes or proficiency testing (PT) programs for assessing routine laboratory performance. At least two schemes currently exist that include measurements of total uranium in urine. These include the Québec Multielement External Quality Assessment Scheme (QMEQAS) and the New York State Department of Health's (NYSDOH) PT program for trace elements in urine. The NYSDOH program consists of three challenges per year for total uranium (and other trace elements) in human urine at concentrations ranging from 100–1500 ng L−1. However, none of the current EQA/PT programs include specific uranium isotopes in urine, as recommended by the authors of a 2002 U.S. Department of Defense Study.31 To the best of our knowledge, only four previous interlaboratory studies have been conducted on the uranium isotopic composition of urine.
The U.S. Department of Defense Study31 used a synthetic urine matrix containing natural uranium (NU) and DU, and included six laboratories that had previously determined [U] in urine specimens from military veterans or Defense Department personnel. The levels of [U] in the study materials ranged from 0.1 to 10 μg L−1, levels that would most frequently be found in individuals with acute occupational exposure or with embedded DU shrapnel. Instrumentation included ICP-MS, Kinetic Phosphorescence Analysis (KPA), and AS. Only the ICP-MS and KPA methods were able to measure [U] accurately at the 0.1 μg L−1 level. In general, all laboratories had considerable difficulty measuring individual uranium isotopes, particularly at the lower concentrations.
The NUSIMEP-4 (ref. 29) and CCQM-P48 (ref. 30) studies were conducted concurrently and involved synthetic urine matrices supplemented with two and eight isotopic enrichment levels, respectively, with total uranium mass fractions [U] of 5 ng U g−1 urine. The NUSIMEP-4 study involved 21 participants employing a range of measurement techniques, including AS, Q-ICP-MS, SF-ICP-MS, MC-ICP-MS, TIMS, and Accelerator Mass Spectrometry (AMS). The authors concluded that there was substantial difficulty for many labs in measuring uranium isotope ratios in the simulated urine, especially for 236U/238U. In addition, the capabilities of radiometric techniques were deemed limited except for measurement of 234U/238U. In contrast, the CCQM-P48 study, which included 5 national metrology institutes (NMIs) and 10 invited laboratories, involved only inorganic mass spectrometric techniques. Stated uncertainties for 234U/238U, 235U/238U, and 236U/238U were generally 3.0, <0.7, and 20% relative, around the assigned values for the majority of participating laboratories.
In the Ough et al. (2006) study, twelve (12) synthetic urine pools containing 25–770 ng L−1 [U] and a range of isotopic compositions were analyzed for [U] and 235U/238U in four laboratories using four different techniques: SF-ICP-MS, Q-ICP-MS, TIMS, and Instrumental Neutron Activation Analysis (INAA). The two sets of ICP-MS results closely matched the predicted values for [U] and 235U/238U, whereas the TIMS and NAA results did not.
In a study by Parrish et al. (2006), two laboratories using MC-ICP-MS and one using SF-ICP-MS analyzed 50–400 mL aliquots of human urine containing approximately 1.2 to 6 ng U L−1 with NU and DU isotopic compositions. All three laboratories were able to measure 235U/238U to within 0.5 to 4.0% RSD. Only the MC-ICP-MS techniques were able to measure 236U/238U accurately at the ∼20 fg L−1 level. For each of these analyses, 400 mL of urine was required, a volume comparable to a 24 hour urine specimen. This study is notable for the capability of the techniques to measure uranium isotope ratios at very low [U] with high degrees of precision and accuracy.
The present study was motivated by the need to measure uranium isotopes in random (spot) urine specimens (10–20 mL) at biomonitoring levels ([U] = 1–50 ng L−1), including the general lack of available CRMs needed to validate such measurements. In previous work,18 we developed a method to determine uranium isotope ratios in random urine specimens of <50 mL, from former workers and nearby residents of the NL Industries uranium processing plant that was previously located in Colonie, NY, USA. Between 1958 and 1982, the factory produced components containing enriched uranium (EU) and DU. Uranium milling waste was burned, producing micron-size uranium oxide particles that contaminated the work site and surrounding area via atmospheric deposition.32–34 As part of our method validation study, we produced urine quality control materials (QCMs) from human base urine, supplemented with U isotopes to produce DU and NU at low (5 ng L−1), medium (10 ng L−1) and high (50 ng L−1) concentrations.
The present study is distinguished from previous interlaboratory studies in that it addresses the current capability of laboratories to measure the uranium isotopic composition of random (spot) urine specimens (<50 mL) at total U concentrations that encompass the current US reference interval for human urine (∼1–50 ng L−1). The principal objectives of the current study were to (1) evaluate current interlaboratory performance and measurement reproducibility for [U] and the isotope ratios 234U/238U, 235U/238U, and 236U/238U in stabilized pools of human urine; and (2) as a result of the former, to produce an archive of well-characterized urine reference materials for future method validation and quality control studies.
Experimental
Source of study materials
Six urine reference materials were prepared following a standard protocol that is routinely used to prepare urine materials for the NYSDOH PT Program for Trace Elements in Urine. Base urine was collected from laboratory personnel into pre-cleaned, plastic bottles. Urine aliquots from each container were screened to ensure total uranium [U] was <3 ng L−1. Screened urines were pooled, acidified, and frozen at −80 °C to achieve precipitation of urate salts. After thawing and centrifugation to separate particulates, the pool was analyzed for background [U] and uranium isotope ratios. It was then sub-divided into six portions and each was spiked with calculated amounts of solutions containing NU and DU in order to bring [U] and 235U/238U to the desired target values for each material. Following spiking, urine pools were thoroughly mixed, dispensed into cryo-tubes, and stored at −80 °C.Homogeneity and stability of study materials
Homogeneity and stability for [U] and isotope ratios was established by the coordinating laboratory, following the method described in the International Organization for Standardization (ISO) Guide, entailing analysis of 20 vials selected at random from each level for homogeneity, and 6 vials for stability.35 Samples for homogeneity testing were analyzed within 6 hours of thawing, whereas samples for stability testing were kept at room temperature for 2 weeks prior to testing. For homogeneity and stability testing, [U] and isotope ratios were determined using a Thermo Scientific Element 2 ICP-MS equipped with a Cetac Aridus® II desolvation-nebulization system by the method described previously.18All six candidate reference materials met the assessment criterion for homogeneity of ss < 0.3σ, where ss is the between-sample standard deviation (SD) and σ is less than or equal to the PT SD typical for urine U. The factor 0.3 assures that the between sample SD contributes no more than ∼10% of the SD. In addition, all six materials met the criteria for stability testing.
Interlaboratory study design
The study design was modeled on the PT program for trace elements in human urine operated by the NYSDOH Wadsworth Center. In March 2013, an open invitation was posted on the Plasmachem and Element/Axiom HR-ICP-MS e-mail list-server sites, and thirteen laboratories agreed to participate in the study. Government, private, and academic institutions from 6 countries were represented. Each laboratory was asked to report total U and uranium isotope ratios as well as analytical method details. In July 2013, 20 mL of each material was shipped frozen, with a transit time of between 1 and 7 days, depending on the destination. Laboratories were given approximately 2 months to complete the analyses.Analytical methods
Each participating laboratory used its “in-house” method or methods for analyzing the reference materials. The participants filled out a questionnaire about the analytical method and instrumentation used in their laboratory. The results of the questionnaires are summarized in Table 1. Among the nine laboratories that returned results, sample preparation methodologies included simple dilution, acid digestion, column extraction, precipitation, or a combination of these techniques. For [U] determination, four laboratories used SF-ICP-MS and five Q-ICP-MS, whereas, for isotope ratios, five used SF-ICP-MS and four Q-ICP-MS. Sample introduction was achieved with desolvation-nebulization systems (ESI APEX® or CETAC Aridus®) in five cases, with nebulizer and spray chamber (ambient temperature or Peltier-cooled) in the remainder. CRMs were used by seven of the nine laboratories to validate measurements for [U], isotope ratios, or both. Two laboratories applied external corrections for some or all isotope ratios, one corrected 236U/238U only, and majority did not apply isotope ratio corrections. Each laboratory reported uncertainty according to their method with the majority of labs determining uncertainty from replicate measurements.| Category | Response (number of responses) |
|---|---|
| a 1 + 4 and 1 + 9 in 2–5% (V/V) HNO3. b U-TEVA (2), TRU(1), DOWEX(1). c 3 g of urine with 1 mL of concentrated HNO3 was digested in microwave (closed vessel) at 230 °C and made up to 20 g. d Precipitated by ammonium hydroxide, supernatants discarded, and precipitates diluted with 2% (V/V) HNO3. | |
| Sample preparation | Dilutiona (4); column extractionb (4); digestionc (1); precipitationd (1) |
| Sample introduction | Desolvation/nebulization system (5); solution nebulizer & quartz spray chamber (3); peltier cooled spray chamber (2) |
| Instrument | SF-ICP-MS (5); Q-ICP-MS (5) |
| Internal standard | U-233 (4); Ir-193 (3); In-115 (1); Tl (1); none (1) |
| Source of reference materials | NBS/NBL (5); NIST or NIST-traceable (3); HP (2); SPEX (2); none (2); Ecker & Ziegler (1); REIMEP-18 (1), IRMM (1) |
| Isotope ratio corrections | None (7); external (2); U-236 only (1) |
| Uncertainty calculation | Replicates (6); counting statistics for single analysis (3); sum of all sources (1) |
Statistical methods
Assigned target values and standard uncertainties for [U] were calculated from the robust mean and robust standard deviation of data from all the laboratories using Algorithm A of the International Organization for Standardization (ISO) Guide.35 The standard uncertainty (ux) was determined using the equation.where s* is the robust SD and n is the number of measurements (ISO 13528, Section 5.6). The expanded uncertainty (U) was obtained by multiplying the standard uncertainty by a coverage factor equal to the two-tailed value of the Student's t for the number of degrees of freedom and at the 95% confidence level. For the isotope ratio measurements, robust mean, robust SD, combined uncertainty, and expanded uncertainty were calculated for a subset of laboratories using chemical separation for the measurements. This group of laboratories is denoted as “separation” in the tables.
Also shown in the tables are the arithmetic mean and SD of the participating laboratories and the long-term (>1 year) arithmetic mean and SD for repeated measurements of each reference material in the coordinating laboratory at the Wadsworth Center, using the method of Arnason et al. (2013).18 Outliers, as determined by the Grubbs test, were omitted from calculation of the arithmetic mean and SD and are indicated by (†) in the tables.
Results and discussion
Interlaboratory results for total uranium in urine
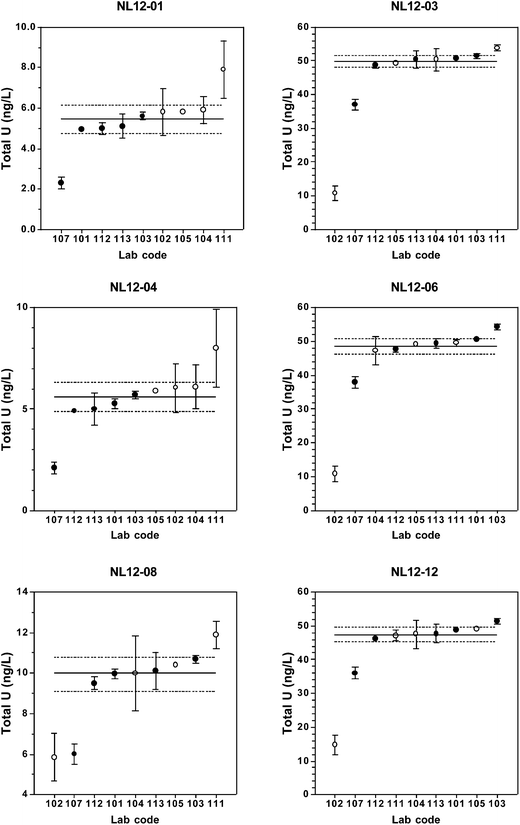
Total [U] results returned by the participating laboratories are presented in Table 2 and Fig. 1. Table 2 also includes summary statistics for long-term replicate measurements of each material as measured in the coordinating laboratory and the predicted values.| Lab Code | Candidate reference material | |||||
|---|---|---|---|---|---|---|
| NL12-01 | NL12-03 | NL12-04 | NL12-06 | NL12-08 | NL12-12 | |
| a †= outlier as determined by the Grubbs test. | ||||||
| 101 | 4.9567 | 50.6423 | 5.2596 | 50.5831 | 9.9671 | 48.7814 |
| 102 | 5.80 | 10.8† | 6.05 | 10.8† | 5.85 | 14.8† |
| 103 | 5.6 | 51.4 | 5.7 | 54.4 | 10.7 | 51.4 |
| 104 | 5.9 | 50.4 | 6.1 | 47.3 | 10 | 47.6 |
| 105 | 5.81 | 49.29 | 5.88 | 49.13 | 10.39 | 49.04 |
| 107 | 2.3 | 37 | 2.1 | 38 | 6.0 | 36 |
| 111 | 7.9 | 53.8 | 8.0 | 49.6 | 11.9 | 47.1 |
| 112 | 5.0 | 48.8 | 4.9 | 47.7 | 9.5 | 46.3 |
| 113 | 5.1 | 50.4 | 5.0 | 49.5 | 10.1 | 47.7 |
| All participants (excluding outliers) | ||||||
| Arithmetic mean | 5.4 | 49.0 | 5.4 | 48.3 | 9.4 | 46.7 |
| SD | 1.5 | 5.1 | 1.6 | 4.7 | 2.1 | 4.6 |
| RSD | 27.1% | 10.3% | 28.5% | 9.7% | 22.1% | 9.9% |
| n | 9 | 8 | 9 | 8 | 9 | 8 |
| All participants | ||||||
| Robust mean | 5.46 | 49.9 | 5.58 | 48.7 | 9.95 | 47.4 |
| Robust SD (s*) | 0.72 | 1.79 | 0.74 | 2.4 | 0.84 | 2.2 |
| u x | 0.30 | 0.75 | 0.31 | 1.0 | 0.35 | 0.91 |
| Relative ux (%) | 5.5 | 1.5 | 5.5 | 2.1 | 3.5 | 1.9 |
| U | 0.70 | 1.72 | 0.71 | 2.33 | 0.80 | 2.11 |
| n | 9 | 9 | 9 | 9 | 9 | 9 |
| Coordinating laboratory long-term data | ||||||
| Arithmetic mean | 5.44 | 49.6 | 5.29 | 49.0 | 10.05 | 48.0 |
| SD | 0.28 | 1.1 | 0.24 | 1.9 | 0.45 | 1.4 |
| RSD | 5.2% | 2.3% | 4.6% | 4.0% | 4.5% | 2.9% |
| n | 27 | 26 | 27 | 37 | 27 | 34 |
| Predicted | ||||||
| Mean | 4.84 | 48.10 | 4.99 | 50.04 | 10.10 | 49.96 |
For the low-level (5 ng L−1) urine materials NL12-01 and NL12-04, there is a bias between the laboratories that used chemical separation and those that used dilution or digestion (lab#102), with the latter group reporting systematically higher [U] values (Fig. 1). Potential causes of a positive bias for [U] are laboratory contamination, polyatomic interferences on 238U, and/or matrix effects. Urine contains several elements and organic molecules that may cause positive interferences on one or more of the uranium isotopes that result in apparently higher uranium concentrations, although this effect is more significant for the minor isotopes.36,37 Urine diluted 1 + 4 or 1 + 9 v/v has relatively high total dissolved solids content that may result in a significant matrix effect. In contrast, chemical separation removes those ions that cause polyatomic interferences and reduces the total dissolved solid concentration that can result in matrix effects. Use of matrix-matched calibration standards would alleviate these problems in the dilution and digestion methods. An alternative explanation for the observed positive bias is that chemical separation could lead to incomplete U recovery and lower apparent uranium concentrations. For the U-TEVA column extraction method used by the coordinating laboratory, U recovery was typically >95%.18 In one case (lab #107), however, incomplete analyte recovery could account for the low [U] result. Regardless of the cause, the bias between separation and dilution/digestion methods is relatively small, as it is not present in the urine materials containing [U] ≥ 10 ng L−1.
In general, [U] results from at least 6 of the 9 laboratories fall within the ranges of the assigned values ± the expanded uncertainty at all concentration ranges. The relative standard uncertainties range from 1.5–5.5% with uncertainty inversely related to concentration. At [U] = 50 ng L−1, there are no discernable differences among results obtained by the different sample preparation methods or instrumentation type (SF-ICP-MS vs. ICP-QMS). At 5 ng L−1, however, dilution-only methods yield consistently higher results than chemical separation or digestion methods.
Interlaboratory results for uranium isotopes in urine
| Lab Code | Candidate reference material | |||||
|---|---|---|---|---|---|---|
| NL12-01 | NL12-03 | NL12-04 | NL12-06 | NL12-08 | NL12-12 | |
| a † = outlier; Separation methods indicated in bold. DL = detection limit. | ||||||
| 101 | 0.0000837 | 0.0000559 | 0.0000836 | 0.0000554 | 0.0000609 | 0.0000260 |
| 102 | 0.0000520 | 0.0000540 | 0.0000525 | 0.0000545 | 0.0000535 | 0.0000520 |
| 103 | <DL | 0.000050 | <DL | 0.000052 | <DL | <DL |
| 104 | 0.04918 | 0.00489 | 0.02941 | 0.002 | 0.02391 | 0.01005 |
| 105 | 0.00195924 | 0.000464 | 0.001526 | 0.000151 | 0.0012 | 0.000353 |
| 107 | 0.00023 | 0.000079 | 0.00020 | 0.000072 | 0.00012 | 0.000053 |
| 111 | 0.1153† | 0.0161† | 0.1809† | 0.0183† | 0.0895† | 0.0201† |
| 112 | 0.000061 | 0.000063 | 0.000065 | 0.000053 | 0.000036 | 0.000028 |
| 113 | 0.0000551 | 0.000052 | 0.0000564 | 0.0000552 | 0.0000349 | 0.000023 |
| All participants (excluding outliers) | ||||||
| Mean | 0.007375 | 0.000714 | 0.004485 | 0.000312 | 0.003631 | 0.001512 |
| SD | 0.018448 | 0.001694 | 0.011004 | 0.000683 | 0.008952 | 0.003767 |
| RSD | 250.2% | 237.3% | 245.4% | 219.2% | 246.6% | 249.1% |
| n | 7 | 8 | 7 | 8 | 7 | 7 |
| All participants | ||||||
| Robust mean | 0.000205 | 0.000071 | 0.000183 | 0.000058 | 0.000118 | 0.000066 |
| Robust SD (s*) | 0.000182 | 0.000022 | 0.000153 | 0.000005 | 0.000094 | 0.000048 |
| u x | 0.000081 | 0.000009 | 0.000068 | 0.000002 | 0.000042 | 0.000021 |
| U | 0.000190 | 0.000021 | 0.000160 | 0.000005 | 0.000098 | 0.000050 |
| n | 8 | 9 | 8 | 9 | 8 | 8 |
| Separation methods | ||||||
| Robust mean | 0.000076 | 0.000058 | 0.000077 | 0.000055 | 0.000052 | 0.000027 |
| Robust SD | 0.000026 | 0.000009 | 0.000024 | 0.000003 | 0.000023 | 0.000005 |
| u x | 0.000016 | 0.000005 | 0.000015 | 0.000002 | 0.000015 | 0.000003 |
| Relative ux (%) | 21 | 8.6 | 19 | 3.6 | 29 | 11 |
| U | 0.000051 | 0.000014 | 0.000048 | 0.000005 | 0.000046 | 0.000009 |
| n | 4 | 5 | 4 | 5 | 4 | 4 |
| Coordinating laboratory long-term data | ||||||
| Mean | 0.000054 | 0.000053 | 0.000056 | 0.000054 | 0.000031 | 0.000021 |
| SD | 0.000014 | 0.000008 | 0.000017 | 0.000005 | 0.000013 | 0.000008 |
| RSD | 26.1% | 15.1% | 29.6% | 9.9% | 42.9% | 39.5% |
| n | 27 | 28 | 29 | 38 | 27 | 34 |
| Predicted | ||||||
| Mean | 0.000052 | 0.000052 | 0.000053 | 0.000053 | 0.000032 | 0.000021 |
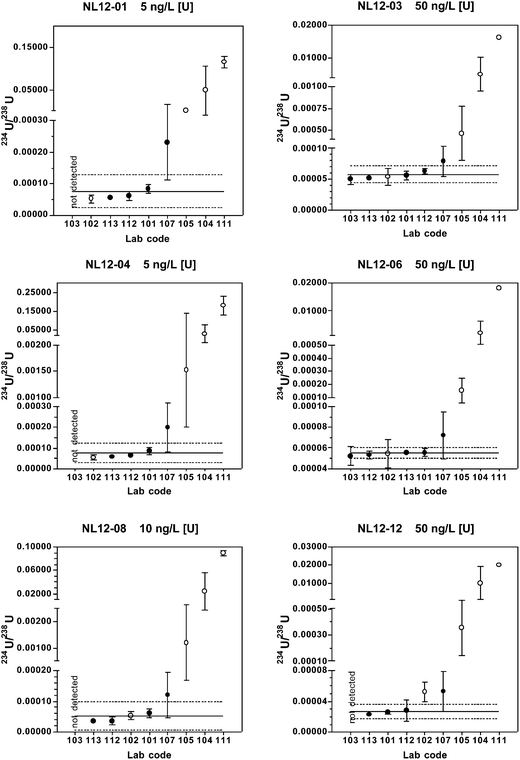
When only chemical separation methods are included, 75–80% of the reported 234U/238U results fall within the expanded uncertainty range for all reference materials. Relative standard uncertainties are 3.6–29%, with uncertainty increasing with decreasing 234U abundance. The predicted values and long-term mean values from the coordinating laboratory also fall within the expanded uncertainty range for all materials.
| Lab Code | Candidate Reference Material | |||||
|---|---|---|---|---|---|---|
| NL12-01 | NL12-03 | NL12-04 | NL12-06 | NL12-08 | NL12-12 | |
| a †= outlier; Separation methods indicated by bold lab code. | ||||||
| 101 | 0.0073379 | 0.0072175 | 0.0073110 | 0.0072920 | 0.0049456 | 0.0034789 |
| 102 | 0.007390 | 0.0072 | 0.00734 | 0.00730 | 0.00734 | 0.00670 |
| 103 | 0.0072 | 0.0072 | 0.0072 | 0.0072 | 0.0047 | 0.0034 |
| 104 | 0.0328 | 0.01272 | 0.01959 | 0.00903 | 0.01915 | 0.00761 |
| 105 | 0.022032 | 0.009004 | 0.0345 | 0.010243 | 0.0137 | 0.00504 |
| 107 | 0.0066 | 0.0071 | 0.0073 | 0.0072 | 0.0040 | 0.0034 |
| 111 | 0.1395† | 0.0245† | 0.1738† | 0.0302† | 0.0898† | 0.0211† |
| 112 | 0.007130 | 0.00713 | 0.00726 | 0.00729 | 0.004681 | 0.003285 |
| 113 | 0.0071294 | 0.0070705 | 0.0072677 | 0.0072161 | 0.0047349 | 0.0033774 |
| All participants (excluding outliers) | ||||||
| Mean | 0.012200 | 0.008085 | 0.012226 | 0.007852 | 0.007912 | 0.004536 |
| SD | 0.009825 | 0.001982 | 0.009977 | 0.001149 | 0.005539 | 0.001732 |
| RSD | 80.5% | 24.5% | 81.6% | 14.6% | 70.0% | 38.2% |
| n | 8 | 8 | 8 | 8 | 8 | 8 |
| All participants | ||||||
| Robust mean | 0.00738 | 0.00722 | 0.00736 | 0.00733 | 0.00567 | 0.00362 |
| Robust SD (s*) | 0.00039 | 0.00012 | 0.00014 | 0.00012 | 0.00144 | 0.00032 |
| u x | 0.00016 | 0.00005 | 0.00006 | 0.00005 | 0.00060 | 0.00013 |
| U | 0.00038 | 0.00011 | 0.00014 | 0.00012 | 0.00138 | 0.00031 |
| n | 9 | 9 | 9 | 9 | 9 | 9 |
| Separation methods | ||||||
| Robust mean | 0.007141 | 0.007151 | 0.007275 | 0.007249 | 0.004712 | 0.003388 |
| Robust SD | 0.000101 | 0.000067 | 0.000065 | 0.000047 | 0.000063 | 0.000082 |
| u x | 0.000057 | 0.000037 | 0.000036 | 0.000027 | 0.000035 | 0.000046 |
| Relative ux (%) | 0.80 | 0.52 | 0.49 | 0.37 | 0.74 | 1.4 |
| U | 0.000157 | 0.000104 | 0.000100 | 0.000074 | 0.000097 | 0.000128 |
| n | 5 | 5 | 5 | 5 | 5 | 5 |
| Coordinating laboratory long-term data | ||||||
| Mean | 0.007097 | 0.007124 | 0.007284 | 0.007261 | 0.004723 | 0.003381 |
| SD | 0.000044 | 0.000026 | 0.000032 | 0.000029 | 0.000066 | 0.000052 |
| RSD | 0.6% | 0.37% | 0.45% | 0.40% | 1.39% | 1.53% |
| n | 27 | 28 | 29 | 38 | 27 | 34 |
| Predicted | ||||||
| Mean | 0.007100 | 0.007100 | 0.007262 | 0.007262 | 0.004661 | 0.003340 |
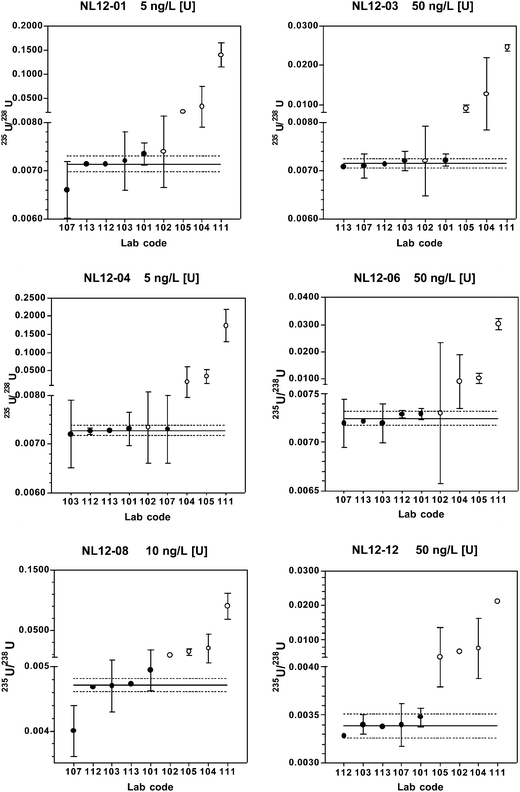
When only the chemical separation method results are considered, the predicted and coordinating lab long-term mean 235U/238U values fall within the expanded uncertainty range of the robust mean for each material. At 5–10 ng L−1 [U], 60–100% of the reported results are within the expanded uncertainty range, and at 50 ng L−1 [U], 100% of the results are within this range. Relative standard uncertainties are 0.37–1.4%.
| Lab Code | Candidate Reference Material | |||||
|---|---|---|---|---|---|---|
| NL12-01 | NL12-03 | NL12-04 | NL12-06 | NL12-08 | NL12-12 | |
| a †= outlier; Separation methods indicated by bold lab code. NR = not reported. DL = detection limit. | ||||||
| 101 | 0.0000704 | 0.0000215 | 0.0000863 | 0.0000052 | 0.0001300 | 0.0001547 |
| 102 | NR | NR | NR | NR | NR | NR |
| 103 | <DL | 0.000015 | <DL | <DL | 0.00010 | 0.00014 |
| 104 | 0.07377 | 0.00391 | 0.08823 | 0.001 | 0.01435 | 0.01332 |
| 105 | 0.00435 | 0.000652 | 0.005839 | 0.0007 | 0.00198 | 0.00074 |
| 107 | NR | NR | NR | NR | 0.000099 | 0.00014 |
| 111 | 0.1222 | 0.0172† | 0.1876 | 0.0198† | 0.0983† | 0.0204 |
| 112 | <DL | 0.000012 | <DL | <DL | 0.000097 | 0.000146 |
| 113 | 0.000005 | 0.0000049 | <DL | <DL | 0.0000901 | 0.0001374 |
| All participants (excluding outliers) | ||||||
| Arithmetic mean | 0.040084 | 0.000769 | 0.070429 | 0.000568 | 0.002407 | 0.004395 |
| SD | 0.055602 | 0.001560 | 0.087856 | 0.000510 | 0.005313 | 0.007921 |
| RSD (%) | 138.7 | 202.8% | 124.7% | 89.8% | 220.7% | 180.3% |
| n | 5 | 6 | 4 | 3 | 7 | 8 |
| All participants | ||||||
| Robust mean | 0.006491 | 0.000033 | 0.059807 | 0.000915 | 0.000127 | 0.000157 |
| Robust SD (s*) | 0.008041 | 0.000028 | 0.078993 | 0.000918 | 0.000039 | 0.000025 |
| u x | 0.00449 | 0.00001 | 0.04937 | 0.00057 | 0.00002 | 0.00001 |
| U | 0.01248 | 0.00003 | 0.15712 | 0.00183 | 0.00004 | 0.00003 |
| n | 5 | 7 | 4 | 4 | 8 | 8 |
| Separation methods | ||||||
| Robust mean | 0.000038 | 0.000013 | — | — | 0.000099 | 0.000138 |
| Robust SD | 0.000052 | 0.000008 | — | — | 0.000006 | 0.000004 |
| u x | 0.000046 | 0.000005 | — | — | 0.000003 | 0.000002 |
| Relative ux (%) | 121 | 38 | — | — | 3.0 | 1.4 |
| U | 0.000589 | 0.000016 | — | — | 0.000009 | 0.000006 |
| n | 2 | 4 | 1 | 1 | 5 | 5 |
| Coordinating laboratory long term data | ||||||
| Mean | <DL | 0.000008 | <DL | <DL | 0.000094 | 0.000144 |
| SD | — | 0.000003 | — | — | 0.000007 | 0.000005 |
| RSD | — | 32.3% | — | — | 7.3% | 3.5% |
| n | 27 | 28 | 29 | 38 | 27 | 34 |
| Predicted | ||||||
| Mean | 0.000006 | 0.000006 | <0.0000010 | <0.0000010 | 0.000096 | 0.000144 |
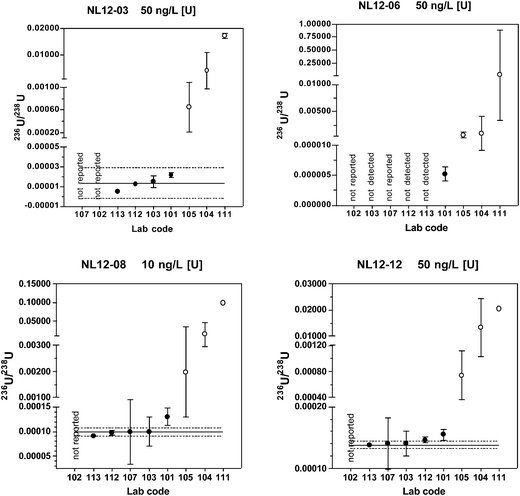
Of the five laboratories using chemical separation methods, four either did not report a result or reported a non-detect result for the materials with natural isotopic ratios (NL12-04 and NL12-06). These findings are consistent with the predicted 236U/238U value of < 0.000001 and the coordinating lab long-term mean value below the detection limit of 0.000010. The true value 236U/238U for these two materials is probably close to the certified value of certified reference material IRMM-3184 (236U/238U = 0.00000012446(17)) that was used to spike the base urine. A much larger volume of urine than used in this study and MC-ICP-MS or AMS instrumentation would be required to quantify 236U at these low levels. Because only one of the laboratories using chemical separation reported detectable results, robust statistics were not determined for these two materials.
For the low level, depleted material NL12-01, only two laboratories reported detectable values. One result (236U/238U = 0.000005) is very close to the predicted value of 0.000006, whereas the other result is anomalously high (236U/238U = 0.000070). For the high level depleted material NL12-03, four laboratories reported results. The predicted value (0.000006) and coordinating laboratory long-term mean (0.000008) fall within the expanded uncertainty of the robust mean (0.000013 ± 0.000016), however this range includes zero.
For the two most depleted urine materials NL12-08 and NL12-12, the robust means are in good agreement with both the predicted and long-term coordinating laboratory values with relative standard uncertainties of 3.0 and 1.4%, respectively. For the laboratories using chemical separation, 60–80% of the results are within the range defined by the robust mean ± the extended uncertainty.
Effect of polyatomic species on uranium isotope ratios measurements
As described above, laboratories using dilution-only methods have significant positive biases in the measured 234U/238U, 235U/238U, and 236U/238U values relative to those that used chemical separation or digestion. Positive biases are most likely due to isobaric and polyatomic interferences on the minor isotopes. Potential polyatomic interferences on the isotopes of uranium are given in Table 6. The concentrations of the interfering species listed in Table 6 that are present in the urine reference materials have not been measured, but urine reference ranges from the literature are available. In its most recent survey (2009–2010) the National Health and Nutrition Examination Survey (NHANES) of the US CDC lists geometric mean urinary concentrations for several of these heavy metals, including W (81 ng L−1), Tl (144), and Pb (458).28,38 The most recent reported geometric mean for Hg (443 ng L−1) is from the 2007–2008 NHANES report.39 Urinary Pt was also measured in both 2007–2008 and 2009–2010, but the majority of values were below the limit of detection of 9 ng L−1 in both years, and the geometric means were reported as “<LOD”. The surveys do not provide reference ranges for Re, Ir, and Au in urine. In a study of 161 Italian police officers, the mean urinary Pt concentration was found to be 4.45 and 4.56 ng L−1 in the exposed and control groups, respectively.40 Using platinum polyatomic interferences as an example, the urinary Pt concentrations are comparable to [U] in the urine reference materials NL12-01 and NL12-04. Since the relative abundance of 234U is only 0.005% of [U], formation of 194Pt40Ar+, 195Pt40Ar+, and 196Pt40Ar+ at only 0.005% of total Pt would produce significant positive interferences on 234U, 235U, and 236U, respectively. As Hg and Pb are typically even more abundant than Pt in urine, polyatomic interferences containing Hg or Pb would also expected to be significant.| m/z | Polyatomic Species |
|---|---|
| 234 | 193Ir40ArH, 194Pt40Ar, 198Pt36Ar, 197Au37Cl, 199Hg35Cl, 202HgO2, 201HgO2H, 198Hg36Ar, 203TlNOH, 204PbNO, 206Pb12CO |
| 235 | 186WO3H, 187ReO3, 195Pt40Ar, 194Pt40ArH, 200Hg35Cl, 198Hg37Cl, 202HgO2H, 199Hg36Ar, 203TlO2, 205TlNO, 204PbNOH, 207Pb12CO, 206Pb12COH |
| 236 | 187ReO3H, 196Pt40Ar, 195Pt40ArH, 201Hg35Cl, 199Hg37Cl, 204HgO2, 200Hg36Ar, 203TlO2H, 205TlNOH, 206PbNO, 204PbO2, 208Pb12CO, 235UH |
The digestion method (lab# 102) yields 234U/238U values that are within the expanded uncertainty range for all reference material except NL12-12, and 235U/238U values are within the expanded uncertainty range for all materials except NL12-08 and NL12-12. These materials have the lowest relative abundances of 234U and 235U, and, therefore, these isotope ratios are most sensitive to polyatomic interferences. The efficacy of the digestion method for many of the reference materials suggests that at least some of the interferences contain volatile components driven off during digestion, most notably organic C. Removal of organic carbon through digestion would eliminate potential interferences such as 206PbCO, 207PbCO, 206Pb12COH, and 208PbCO. Other potentially volatile elements present in the polyatomic interferences in Table 6 include Hg and Pb.
Another well-known interference that is independent of the sample preparation procedure is that of 235UH+ on 236U+. This interference can be significantly reduced by removing the solvent prior to sample introduction to the plasma with a desolvation system. All laboratories that employed chemical separation also used a desolvation system, except lab #101 which used a Peltier-cooled spray chamber. The positive bias in 236U/238U of laboratory #101 compared to the others may be due to 235UH+ interference.
Conclusions
For measurement of total U in urine, all sample preparation methods and instrumental techniques employed produced accurate and precise results at [U] concentrations above 5 ng L−1. At 5 ng L−1, however, dilution-only methods yielded results with a positive bias compared to the other methods.Sample preparation methods employing only urine dilution result in inaccurate isotope ratio measurements having large positive biases. The positive bias is most likely caused by polyatomic interferences on the minor U isotopes. Urine digestion improved isotope ratio accuracy over dilution-only methods, but chemical separation of U from the urine matrix provided the most reliable results. In addition, accurate measurement of 236U/238U requires use of a desolvation system to reduce interference from 235UH+ on 236U.
Assigned target values and standard uncertainties for [U] were calculated from the robust means and robust standard deviations of data reported by all the participants. In general, [U] assigned target values are in very good agreement with the predicted values and the standard uncertainties are typically below 6% (range 1.5–5.5%).
For isotope ratios, assigned target values and standard uncertainties were calculated from the robust means and standard deviations according to Algorithm A using the data taken only from those laboratories using chemical separation techniques. For 235U/238U, there was good agreement between the target and predicted values, and the relative standard uncertainties were below 2% (range: 0.4–1.3%). For the minor isotope ratios, there was generally poor agreement between target and predicted values and the standard uncertainties were relatively high. These data suggest that accurate and precise measurements of [U] in urine can be achieved using a wide range of ICP-MS instrumentation and analytical methodologies. In contrast, measurement of U isotope ratios in urine is more strongly dependent on ICP-MS instrumentation and analytical methodology.
Acknowledgements
This study was supported in part by funding under cooperative agreement No. U38EH000464-01 from the Centers for Disease Control and Prevention (CDC) to the Wadsworth Center. Use of trade names is for informational purposes only, and does not imply an endorsement by the NYSDOH.References
- J. W. Ejnik, A. J. Carmichael, M. M. Hamilton, M. McDiarmid, K. Squib, P. Boyd and W. Tardiff, Health Phys., 2000, 79, 143–146 CrossRef.
- J. W. Ejnik, T. I. Todorov, F. G. Mullick, K. Squibb, M. A. McDiarmid and J. A. Centeno, Anal. Bioanal. Chem., 2005, 382, 73–79 CrossRef CAS PubMed.
- B. G. Ting, D. C. Paschal and K. L. Caldwell, J. Anal. At. Spectrom., 1996, 11, 339–342 RSC.
- E. J. Wyse, J. A. MacLellan, C. W. Lindenmeier, J. P. Bramson and D. W. Koppenaal, J. Radioanal. Nucl. Chem., 1998, 234, 165–171 CrossRef CAS.
- C. Bouvier-Capely, N. Baglan, A. Montègue, J. Ritt and C. Cossonnet, Health Phys., 2003, 85, 216–219 CrossRef CAS.
- W. S. Kinman, S. P. Lamont and R. E. Steiner, J. Radioanal. Nucl. Chem., 2009, 282, 1027–1030 CrossRef CAS PubMed.
- C. S. Westphal, J. A. McLean, S. J. Hakspiel, W. E. Jackson, D. E. McClain and A. Montaser, Appl. Spectrosc., 2004, 58, 1044–1050 CrossRef CAS PubMed.
- S. L. Maxwell III and V. D. Jones, Talanta, 2009, 80, 143–150 CrossRef PubMed.
- S. Y. Tolmachyov, J. Kuwabara and H. Noguchi, J. Radioanal. Nucl. Chem., 2004, 261, 125–131 CrossRef CAS.
- P. Schramel, J. Chromatogr. B, 2002, 778, 275–278 CrossRef CAS.
- R. S. Pappas and D. C. Paschal, J. Anal. At. Spectrom., 2006, 21, 360–361 RSC.
- R. S. Pappas, B. G. Ting and D. C. Paschal, J. Anal. At. Spectrom., 2003, 18, 1289–1292 RSC.
- J. S. Becker, M. Burow, S. F. Boulyga, C. Pickhardt, R. Hille and P. Ostapczuk, At. Spectrosc., 2002, 23, 177–182 CAS.
- R. H. Gwiazda, K. Squibb, M. McDiarmid and D. Smith, Health Phys., 2004, 86, 12–18 CrossRef CAS PubMed.
- P. K. Krystek and R. R. Ritsema, Anal. Bioanal. Chem., 2002, 374, 226–229 CrossRef CAS PubMed.
- Y. Shi, X. Dai, R. Collins and S. Kramer-Tremblay, Health Phys., 2011, 101, 148–153 CrossRef CAS PubMed.
- I. Trešl, G. D. Wannemacker, C. R. Quétel, I. Petrov, F. Vanhaecke, L. Moens and P. D. P. Taylor, Environ. Sci. Technol., 2004, 38, 581–586 CrossRef.
- J. G. Arnason, C. N. Pellegri and P. J. Parsons, J. Anal. At. Spectrom., 2013, 28, 1410–1419 RSC.
- P. J. Gray, L. Zhang, H. Xu, M. McDiarmid, K. Squibb and J. A. Centeno, Microchem. J., 2012, 105, 94–100 CrossRef CAS PubMed.
- R. R. Parrish, M. F. Thirlwall, C. Pickford, M. Horstwood, A. Gerdes, J. Anderson and D. Coggon, Health Phys., 2006, 90, 127–138 CrossRef CAS.
- Z. Karpas, A. Lorber, H. Sela, O. Paz-Tal, Y. Hagag, P. Kurttio and L. Salonen, Health Phys., 2005, 89, 315–321 CrossRef CAS.
- M. A. McDiarmid, S. M. Engelhardt, C. D. Dorsey, M. Oliver, P. Gucer, P. D. Wilson, R. Kane, A. Cernich, B. Kaup, L. Anderson, D. Hoover, L. Brown, R. Albertini, R. Gudi and K. S. Squibb, J. Toxicol. Environ. Health, Part A, 2009, 72, 14–29 CrossRef CAS PubMed.
- D. Bland, R. J. Rona, D. Coggon, J. Anderson, N. Greenberg, L. Hull and S. Wessely, Occup. Environ. Med., 2007, 64, 834–838 CrossRef CAS PubMed.
- DUOB, Depleted Uranium Oversight Board, 2007.
- U. Oeh, N. D. Priest, P. Roth, K. V. Ragnarsdottir, W. B. Li, V. Höllriegl, M. F. Thirlwall, B. Michalke, A. Giussani, P. Schramel and H. G. Paretzke, Sci. Total Environ., 2007, 381, 77–87 CrossRef CAS PubMed.
- R. R. Parrish, M. Horstwood, J. G. Arnason, S. Chenery, T. Brewer, N. S. Lloyd and D. O. Carpenter, Sci. Total Environ., 2008, 390, 58–68 CrossRef CAS PubMed.
- J. A. Bijlsma, P. Slottje, A. C. Huizink, J. W. R. Twisk, G. B. Van Der Voet, F. A. De Wolff, F. Vanhaecke, L. Moens and T. Smid, Nephrol., Dial., Transplant., 2008, 23, 249–255 CrossRef CAS PubMed.
- CDC, Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, September 2013, Centers for Disease Control and Prevention, 2013 Search PubMed.
- A. Stolarz, A. Alonso, W. D. Bolle, A. Moens, E. Ponzevera, C. Quétel, S. Richter, A. Verbruggen and R. Wellum, NUSIMEP 4: Uranium isotopic abundance in simulated urines, DG Joint Research Center, Institute for Reference Materials and Measurements, 2005 Search PubMed.
- C. R. Quétel, N. Lévêque, W. D. Bolle and E. Ponzevera, CCQM-P48: Uranium isotope ratio measurements in simulated biological/environmental materials, Office for Official Publications of the European Communities, Luxembourg, 2007 Search PubMed.
- U. S. Department of Defense, Office of the Special Assistant for Military Deployments, ed. U. S. D. o. Defense, Falls Church, 2002, vol. 2014 Search PubMed.
- J. G. Arnason and B. A. Fletcher, Environ. Pollut., 2003, 123, 383–391 CrossRef CAS.
- N. S. Lloyd, S. R. N. Chenery and R. R. Parrish, Sci. Total Environ., 2009, 408, 397–407 CrossRef CAS PubMed.
- D. Lo, R. L. Fleischer, E. A. Albert and J. G. Arnason, J. Environ. Radioact., 2006, 89, 240–248 CrossRef CAS PubMed.
- ISO-Guide-13528, Statistical Methods for use in Proficiency Testing by Interlaboratory Comparisions, International Organization for Standardizations, Switzerland, 2005 Search PubMed.
- S. Y. Oh, S. A. Lee, J. H. Park, M. Lee and K. Song, Mass Spectrom. Lett., 2012, 3, 54–57 CrossRef CAS.
- C. C. Shen, R. L. Edwards, H. Cheng, J. A. Dorale, R. B. Thomas, S. B. Moran, S. E. Weinstein and H. N. Edmonds, Chem. Geol., 2002, 185, 165–178 CrossRef CAS.
- CDC, Fourth National Report on Human Exposure to Environmental Chemicals, U. S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, Atlanta, GA, 2009 Search PubMed.
- CDC, Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, February 2012, U. S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, Atlanta, GA, 2012 Search PubMed.
- I. Iavicoli, B. Bocca, F. Petrucci, O. Senofonte, G. Carelli, A. Alimonti and S. Caroli, Occup. Environ. Med., 2004, 61, 636–639 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |