Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ionic liquids: not always innocent solvents for cellulose†
Matthew T.
Clough
a,
Karolin
Geyer
*b,
Patricia A.
Hunt
a,
Sunghee
Son
b,
Uwe
Vagt
b and
Tom
Welton
*a
aDepartment of Chemistry, Imperial College London, London, SW7 2AZ, UK. E-mail: t.welton@imperial.ac.uk
bBASF SE, Ludwigshafen, Germany. E-mail: karolin.geyer@basf.com
First published on 31st October 2014
Abstract
The decomposition pathways of a series of carbohydrates dissolved in carboxylate ionic liquids have been investigated in detail using a broad range of thermal and chromatographic techniques. Mixtures of the carboxylate ionic liquid 1-ethyl-3-methylimidazolium acetate with carbohydrates were found to undergo reaction of the C2 carbon of the imidazolium ring with the aldehyde functionality on the open chain sugar, yielding an imidazolium adduct with a hydroxylated alkyl chain. Subsequently, degradation of the hydroxyalkyl chain occurs by sequential loss of formaldehyde units, to yield a terminal adduct species, 1-ethyl-2-(hydroxymethyl)-3-methylimidazolium acetate. Identities of the final and intermediate adduct species, and the reaction mechanisms connecting adducts, were elucidated by NMR, HPLC and LCMS techniques. Factors affecting the rate and quantity of adduct formation were explored. Changing the ionic liquid cation and anion, the acid number, sugar concentration and temperature influenced the rate of formation and relative quantities of the adduct species. Formation of adducts could not be entirely prevented when employing carboxylate ionic liquids. By contrast, 1-butyl-3-methylimidazolium chloride was identified as an ionic liquid capable of dissolving a significant quantity of cellulose, yet without reacting with carbohydrates.
Introduction
Ionic liquids are molten at, or close to, room temperature, and are typically constituted of polyatomic, asymmetrical and charge-diffuse ions.1–3 Ionic liquids are characterised by their high densities and viscosities,4–6 and differ from their neutral small-molecule solvent counterparts by their low vapour pressures.7–14 Throughout the past twenty years, applications of ionic liquids have become varied and far-reaching, foremost as solvents for sustainable synthetic processes,1,15 as battery electrolytes,16 in the capture of CO2,17–19 and in the deconstruction of lignocellulosic biomass.20–23 The organic halide salts 1-ethylpyridinium chloride, [C2py]Cl,24 and 1-butyl-3-methylimidazolium chloride, [C4C1im]Cl,25 are able to dissolve cellulose, and this has initiated a great deal of research into cellulose dissolution procedures.23Cellulose, a linear carbohydrate polymer of repeating glucopyranose residues, held together by β-1,4-glycosidic bonds, contributes between 35 and 50% of the dry weight of biomass. It is the most prevalent of the three lignocellulosic polymers, and the world's most abundant renewable feedstock.26,27 However, numerous strong intermolecular bonding interactions bind together the individual cellulose strands, rendering the polymer insoluble in the majority of conventional solvents; this recalcitrant behaviour of cellulose presents a major challenge to the utilization of this abundant resource.28–30
Existing technologies for the dissolution of cellulose often employ the solvent N-methylmorpholine N-oxide (NMMO) in the ‘Lyocell process’,31,32 or phosphoric acid.33 Alternatively, the ‘Viscose process’ entails the chemical functionalization of hydroxyl residues along the cellulose backbone with carbon disulphide to form xanthate esters, greatly improving the solubility. Each of these technologies carries a significant drawback; NMMO suffers from poor thermal stability,34 and the Viscose process generates two kilograms of waste per kilogram of cellulose obtained.35
Ionic liquids represent a promising alternative to existing cellulose-dissolving solvents because of their higher thermal stabilities relative to NMMO,34 and the purported non-derivatizing nature of cellulose dissolution with ionic liquids.
The ability of an ionic liquid to dissolve cellulose correlates with hydrogen-bond basicity, β, of the anion; hydrogen bonds between the ionic liquid anion and the cellulose chain are necessary to separate the individual cellulose strands.28–30 Hence, ionic liquids incorporating halide,25 dialkylphosphate/dialkylphosphonate36 and, in particular, carboxylate37–39 anions have been identified as promising candidates for cellulose processing.
The inability to distil ionic liquids on a practical scale, together with their high cost, leads to their recycling being a particular challenge for any proposed large scale application.40,41 Recycling of a carboxylate ionic liquid may be hindered via a number of plausible pathways: (i) thermal degradation of the ionic liquid and subsequent volatilisation of thermal decomposition products;42–47 (ii) vaporisation of the intact ionic liquid;11 or (iii) reaction of the ionic liquid with the cellulose. The reactivity of carboxylate ionic liquids towards carbohydrates was first demonstrated by Ebner and co-workers, who described the room-temperature reaction of the imidazolium C2 carbon of the ionic liquid 1-butyl-3-methylimidazolium acetate, [C4C1im][OAc], with the reducing aldehyde end of glucose (acting as a model compound for cellulose), generating an imidazolium adduct bearing a C2 hydroxyalkyl substituent.48 They also observed the addition of a fluorescent imidazolium cation to cellulose itself and that the reaction was faster in the presence of base, which was subsequently supported by calculations of Wei et al.49 Ebner proposed that this reaction was reversible and did not explore any further reactivity of the adduct.
The ability of dialkylimidazolium acetate ionic liquids to form N-heterocyclic carbenes (NHCs)50–52 at the C2 position of the ring is well established.42,53–62 Transfer of this proton from the cation to the anion is facile, and the NHC may be trapped by addition to CO2.63 The acidic nature of this C2 proton, with a pKa ∼ 21–23, is likely to play a key role in the reaction mechanisms of carboxylate ionic liquids with solvated cellulose.
Altogether, the reaction of solvated cellulose with an ionic liquid solvent presents potential problems for both laboratory and industrial processes, due to the influence of new chemical species on both the physical and rheological properties of the mixture, and the potential damaging or fibrilation of the reprocessed cellulose material. It is crucial, therefore, to understand factors contributing towards unwanted side reactions, and how they might be prevented or controlled.
Herein, we investigate mixtures of a series of ionic liquids initially with solvated cellulose, and subsequently with simple carbohydrate model compounds, at elevated temperatures similar to those used in processing cellulose and lignocellulosic biomass. Employing the archetypal carboxylate ionic liquid, [C2C1im][OAc], a sequence of intermediates are identified originating from an initial imidazolium-carbohydrate adduct which undergoes sequential loss of formaldehyde (HCHO) units from the hydroxyalkyl chain to yield the final product of 1-ethyl-2-(hydroxymethyl)-3-methylimidazolium acetate. Factors affecting the rate and extent of adduct formation are explored, including the temperature, the acidity and the carbohydrate concentration. The study was then extended to other ionic liquids in which both cation and anion are changed. One plausible mechanism for formation of the adduct intermediates is proposed.
Experimental
Ionic liquids included in this investigation, 1–11, are shown in Fig. 1. Their syntheses are fully described in the ESI.† Two different samples of 1-ethyl-3-methylimidazolium acetate, [C2C1im][OAc], 1, were obtained by different synthetic routes, and are herein denoted 1a and 1b. This enables the impact of any residual impurities in either sample to be assessed. Ionic liquids 1a and 3 were synthesized via anion exchange employing commercial 1-ethyl-3-methylimidazolium ethyl sulfate, [C2C1im][EtSO4], to yield aqueous 1-ethyl-3-methylimidazolium hydroxide, [C2C1im][OH]. Subsequent neutralization with the conjugate acid of the desired anion, acetic acid and methanesulfonic acid, respectively, yielded ionic liquids 1a and 3. Ionic liquids 1b and 2 are commercial samples, included for comparative purposes. Ionic liquids 4–10 were made according to known literature procedures.42,64,65 The synthesis of the mixed inorganic salt, 11, is described in the ESI.†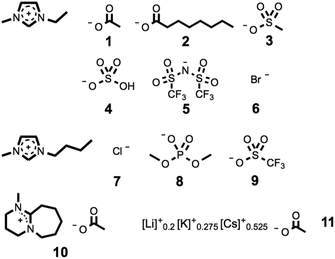 | ||
| Fig. 1 Ionic liquids used in this study, incorporating the [C2C1im]+ (1–6), [C4C1im]+ (7–9), and [Me-DBU]+ (10) cations. Compound 11 is a low-melting mixed inorganic eutectic salt. | ||
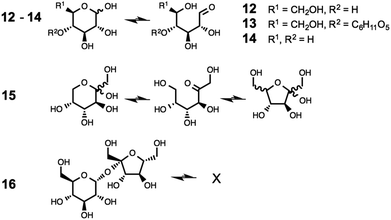
The five carbohydrate model compounds employed in this investigation, 12–16, are shown in Fig. 2, in both cyclic and acyclic forms where applicable. The carbohydrates were purchased from Sigma-Aldrich in anhydrous form, and were used without further purification.
Ionic liquid–sugar mixtures were initially prepared by the addition of an aqueous solution of the sugar to the neat ionic liquid with known measured water content. It was observed that when anhydrous sugar was added to the neat ionic liquid, new peaks were observable by HPLC of ≤5% of the integration of the parent [C2C1im]+ cation, suggesting that the ionic liquid and sugar had already reacted. By contrast, addition of an aqueous sugar solution to the neat ionic liquid, followed by removal of the water under vacuum at 70 °C for one hour, resulted in the formation of significantly smaller peaks (<2% of the integration of the [C2C1im]+ peak), or no peak at all, for new chemical species. Therefore, this second method was employed for preparing the reaction mixtures.
Procedures for preparation and decomposition experiments of the ionic liquid + 5 wt% cellulose mixtures, and the 10 wt% sugar model compound mixtures, are described below. Procedures for the ionic liquid + 25 or 100 wt% sugar model compound mixtures are listed in the ESI.†
HPLC procedures
HPLC experiments were performed on an ‘Agilent 1100 Series’ HPLC spectrometer, using two ‘Sielc’ brand ‘Primesep 200’ (250 mm × 3.2 mm) HPLC columns in series to give improved separation of the charged species. A mobile phase of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 vol/vol H2O–MeCN + 0.2 mol% H3PO4, and an injection volume of 6 μl were employed. The temperature was 25 °C and the flow rate was 0.5 ml min−1. Samples were prepared by diluting 0.06 ± 0.02 g of the ionic liquid–sugar mixture in 25 ml of the mobile phase solution in a volumetric flask. Vials were then prepared using approximately 1 ml of this solution. The absolute concentration, in wt%, of the [C2C1im]+, [C4C1im]+, 1-ethyl-2-(hydroxymethyl)-3-methylimidazolium, [C2C1(HO)C12im]+, and 1-butyl-2-(hydroxymethyl)-3-methylimidazolium, [C4C1(HO)C12im]+, cations could be determined from pre-generated calibration curves. For all other species, without calibration data, the percentage of that peak relative to the total integration of HPLC peaks is quoted as the ‘HPLC%’.
40 vol/vol H2O–MeCN + 0.2 mol% H3PO4, and an injection volume of 6 μl were employed. The temperature was 25 °C and the flow rate was 0.5 ml min−1. Samples were prepared by diluting 0.06 ± 0.02 g of the ionic liquid–sugar mixture in 25 ml of the mobile phase solution in a volumetric flask. Vials were then prepared using approximately 1 ml of this solution. The absolute concentration, in wt%, of the [C2C1im]+, [C4C1im]+, 1-ethyl-2-(hydroxymethyl)-3-methylimidazolium, [C2C1(HO)C12im]+, and 1-butyl-2-(hydroxymethyl)-3-methylimidazolium, [C4C1(HO)C12im]+, cations could be determined from pre-generated calibration curves. For all other species, without calibration data, the percentage of that peak relative to the total integration of HPLC peaks is quoted as the ‘HPLC%’.
LCMS procedures
LCMS spectra were obtained on a ‘Sielc’ brand ‘Primesep 200’ (250 mm × 3.2 mm) HPLC column. A mobile phase of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 vol/vol H2O + 0.1% HCO2H–MeCN, a flow rate of 0.8 ml min−1, and a temperature of 40 °C were employed. Eluent peaks were analysed with electrospray ionisation (ESI) mass spectrometry.
20 vol/vol H2O + 0.1% HCO2H–MeCN, a flow rate of 0.8 ml min−1, and a temperature of 40 °C were employed. Eluent peaks were analysed with electrospray ionisation (ESI) mass spectrometry.
NMR procedures
NMR spectra were recorded on Bruker Avance-400 NMR spectrometers, with 1H and 13C spectra recorded at 400 and 100 MHz, respectively. Chemical shifts are reported downfield of tetramethylsilane, in units of ppm (referenced against the DMSO-d6 residual peak at 2.50 ppm).Water measurements
Water content measurements were carried out on a ‘Metrohm 787 Titrino’ Karl Fischer titrometer, with an integrated ‘Metrohm 703 Ti Stand’. Typically, 0.2 ± 0.1 g of the material was titrated against a ‘Hydranal® Composite 5’ solution. Water contents were recorded in units of wt%.Acid number measurements
Acid numbers, in units of mmol H+ kg−1 ionic liquid (‘IL’), were measured on a ‘Metrohm 848 Titrino Plus’ titrometer, with integrated ‘Metrohm 804 Ti Stand’. Samples of 0.2 ± 0.1 g were titrated against tetrabutylammonium hydroxide, [nBu4N][OH] (0.1 mol L−1 in isopropanol–methanol).General procedure: ionic liquid + 5 wt% cellulose mixtures
Prior to experiments, the ionic liquids were dried under high vacuum with gentle heating of ≤60 °C, until the water concentration was found to be below 0.3 wt% as determined by Coulometric Karl Fischer titration. The ionic liquid–cellulose mixtures (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) were prepared by the addition of dry ‘Sigmacell’ cellulose (0.05 ± 0.001 g) to a sample of the ionic liquid (1.00 ± 0.01 g) in a 100 ml glass pressure tube containing a magnetic stirrer bar. The pressure tube was then partially submerged into an oil bath with integrated thermostat, set at a constant temperature of 120 °C, and was maintained, with stirring, at this temperature for 48 hours. The apparatus was positioned behind a protective blast screen as a safety precaution. Subsequently, the mixture was allowed to cool to room temperature and deionized water (∼0.1 ml) was added to precipitate cellulose. The suspension was filtered to remove solid material, and the filtrate was examined by 1H NMR spectroscopy.
1 w/w) were prepared by the addition of dry ‘Sigmacell’ cellulose (0.05 ± 0.001 g) to a sample of the ionic liquid (1.00 ± 0.01 g) in a 100 ml glass pressure tube containing a magnetic stirrer bar. The pressure tube was then partially submerged into an oil bath with integrated thermostat, set at a constant temperature of 120 °C, and was maintained, with stirring, at this temperature for 48 hours. The apparatus was positioned behind a protective blast screen as a safety precaution. Subsequently, the mixture was allowed to cool to room temperature and deionized water (∼0.1 ml) was added to precipitate cellulose. The suspension was filtered to remove solid material, and the filtrate was examined by 1H NMR spectroscopy.
General procedure: ionic liquid + 10 wt% sugar mixtures
Ionic liquid–sugar mixtures (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) were prepared by the addition of an aqueous sugar solution (1.00 ± 0.01 g in 5 ml deionized water) to a portion of the ionic liquid (10.0 ± 0.1 g) in a 50 ml round-bottomed flask (the two liquids were readily miscible). Water was then removed by rotary evaporation for one hour; a pressure of 8 mbar was employed, and the water bath was set to a temperature of 70 °C. The sample was analysed by Karl Fischer titration in order to confirm the water content was <5 wt%. In addition, an HPLC chromatogram was obtained, so as to confirm that no significant decomposition had occurred prior to the primary heating period. The round-bottomed flask was then fitted with an adaptor for an oil bubbler and was partially submerged in an oil bath with integrated thermostat and magnetic stirrer function, set at a constant temperature (120 or 100 °C). The mixture was maintained at this temperature for 24 hours under a gentle flow of nitrogen gas, with a stirring rate of 150 rpm. Small aliquots (0.06 ± 0.02 g) were removed at regular intervals (‘tx’, where x = 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6 and 24 hours) and were diluted with 25 ml of the mobile phase solvent. Small portions of each solution were measured into vials for HPLC analysis.
1 w/w) were prepared by the addition of an aqueous sugar solution (1.00 ± 0.01 g in 5 ml deionized water) to a portion of the ionic liquid (10.0 ± 0.1 g) in a 50 ml round-bottomed flask (the two liquids were readily miscible). Water was then removed by rotary evaporation for one hour; a pressure of 8 mbar was employed, and the water bath was set to a temperature of 70 °C. The sample was analysed by Karl Fischer titration in order to confirm the water content was <5 wt%. In addition, an HPLC chromatogram was obtained, so as to confirm that no significant decomposition had occurred prior to the primary heating period. The round-bottomed flask was then fitted with an adaptor for an oil bubbler and was partially submerged in an oil bath with integrated thermostat and magnetic stirrer function, set at a constant temperature (120 or 100 °C). The mixture was maintained at this temperature for 24 hours under a gentle flow of nitrogen gas, with a stirring rate of 150 rpm. Small aliquots (0.06 ± 0.02 g) were removed at regular intervals (‘tx’, where x = 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6 and 24 hours) and were diluted with 25 ml of the mobile phase solvent. Small portions of each solution were measured into vials for HPLC analysis.
The temperature of the oil bath fluctuated by no more than ±2 °C during the course of the experiment. 1H NMR spectra were recorded after the 24 hour heating period. In addition, the water content was measured at the end of the 24 hour heating period.
Results and discussion
Characterisation of ionic liquid–sugar adducts
Following the key discoveries of Ebner48,66 and Wei,49 the reactivity of cellulose with a variety of available ionic liquids was initially investigated. Nine dialkylimidazolium ionic liquids, 1–9, and one ionic liquid with the organic N-methyl-diazabicycloundecenium cation 10 were selected. This was in order to cover a broad range of hydrogen bond basicities, β (a key determinant in cellulose solubility), including compounds that are known good solvents for cellulose, 1, 2, 7 and 8, and equally those that are not, 3–6 and 9. Mixtures of an ionic liquid, 1–10, with 5 wt% of cellulose added, were prepared according to the method detailed in the Experimental section above, and were heated at 120 °C for 48 hours. Cellulose has only a very low solubility in some ionic liquids (e.g.3–5), and in these cases mixtures took the form of suspensions rather than solutions. Following the heating period, the mixtures were analysed using 1H NMR spectroscopy (shown for 2 in graphical form in the ESI, Fig. E2†) to assess formation of new chemical species.Interestingly, new peaks were observed in the 1H NMR spectra for the mixtures incorporating both of the carboxylate ionic liquids, 1 and 2. For dimethyl phosphate ionic liquid 8, tiny peaks were observed, which were more significant after extending the heating period to one week. Of particular note, a singlet at δ 4.79 ppm (in DMSO-d6) was observed for the mixtures of cellulose with liquids 1 or 2, and at δ 4.73 ppm for the mixture with [C4C1im]+ ionic liquid 8. By contrast, the mixtures incorporating ionic liquids with methanesulfonate, 3, hydrogen sulfate, 4, bis(trifluoromethanesulfonyl)imide, 5, halide, 6 and 7, and triflate, 9, anions exhibited no new peaks in the NMR spectrum. The carboxylate ionic liquid [Me-DBU][OAc], 10, did exhibit new peaks, although they originated from degradation of the ionic liquid itself, and not primarily from direct reaction with the cellulose.
In order to explore the reaction between the ionic liquids and cellulose more closely, and to elucidate factors affecting the formation of new chemical species, cellulose was replaced with a series of smaller carbohydrate model compounds. Mixtures were prepared with ionic liquids 1, 2, 3, 7 and 8 (all good cellulose-dissolving ionic liquids, with the exception of 3), and model compounds 12–16. The mixtures were heated to 100–120 °C, temperatures typical of industrial cellulose dissolution processes, and changes in chemical composition were monitored by HPLC, LCMS, and 1H NMR techniques. In addition, the acid number was measured at t0 and t24 (0 and 24 hours heating) for some of the mixtures.
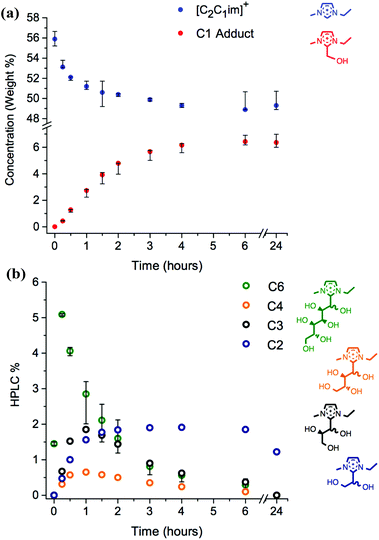
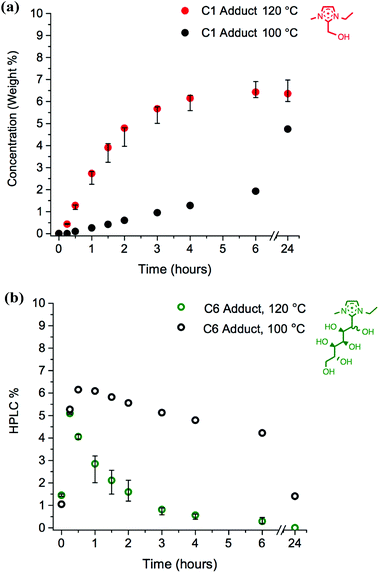
Initially, mixtures of the ionic liquid [C2C1im][OAc], 1a, with 10 wt% of D-(+)-glucose, 12, were heated at 120 °C for 24 hours. Periodically, aliquots of the mixture were analysed by reverse-phase HPLC, the traces of which demonstrated the appearance of new small peaks of higher polarity (lower retention time) than the parent [C2C1im]+ ion peak. Moreover, the absolute concentration of [C2C1im]+ reduced over the course of the 24 hours, suggesting that the new peaks were derived from the ionic liquid cation (Fig. 3a).
LCMS was employed to identify the chemical species responsible for these new peaks (Fig. 4). Notably, the peak of highest polarity exhibited a strong, single mass signal at m/z 291, equal to the mass of [C2C1im]+ + D-(+)-glucose, 12. This species was present in fairly small quantity (<2 HPLC%) before the 120 °C heating period. Therefore, this species was assigned as the equivalent adduct to that observed by Ebner and co-workers,48 formed from the reaction at the ring C2 imidazolium substituent with the reducing end of the sugar molecule. This adduct is herein denoted as the ‘C6’ adduct, referring to the six-carbon hydroxyalkyl substituent at the C2 position of the imidazolium ring. The same notation, ‘Cn’ is used hereafter to refer to other observed adducts, where ‘n’ corresponds to the number of carbons in the C2 substituent chain, excluding the C2 carbon itself.
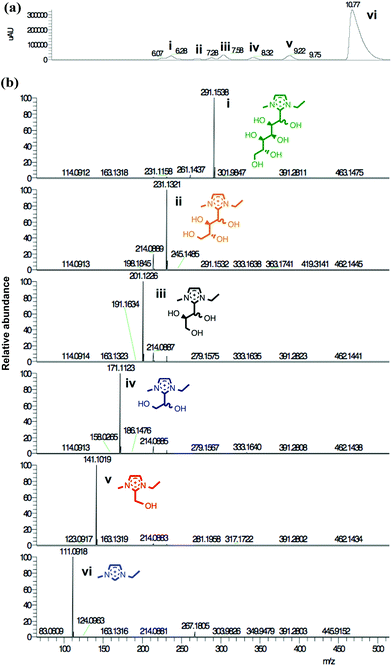 | ||
| Fig. 4 LCMS investigation of adduct species formed from [C2C1im][OAc], 1a + 10 wt% D-(+)-glucose, 12, heated to 120 °C for one hour (t1): (a) survey of peaks i–vi; (b) ESI mass spectra of peaks i–vi. | ||
The other new HPLC peaks were identified, all with lower masses than the C6 adduct, each separated by increments of 30 m/z. This mass corresponds to a difference of a one-carbon CH2O unit in the hydroxyalkyl backbone, or a formaldehyde molecule, HCHO. Hence, C4, C3, C2 and C1 adducts were observed at m/z 231, 201, 171 and 141, respectively (Fig. 3b and 4). The C5 adduct was absent from both the HPLC and LCMS spectra, and there are several possible explanations for this observation: (i) the C5 adduct is not formed at all; (ii) the C5 adduct does form but is unstable and rapidly converts to a smaller adduct; or (iii) the C5 is present but the retention times of the C6 and C5 adducts are so similar that the two could not be resolved. HPLC% concentrations of the C6–C2 adduct peaks were plotted as a function of time (Fig. 3b).
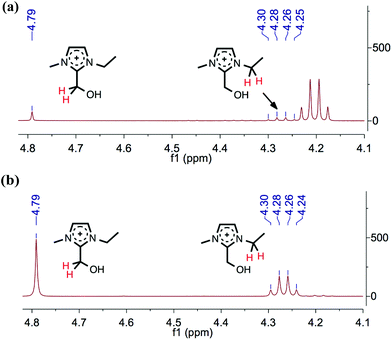
For confirmation of its identity, the C1 adduct compound, 1-ethyl-2-(hydroxymethyl)-3-methylimidazolium acetate, 17, was directly synthesised from the reaction of [C2C1im][OAc], 1a, with paraformaldehyde at 80 °C for 24 hours (Fig. 5). The 1H NMR spectrum of the synthesised compound 17 perfectly matched the new peaks observed from the mixture of 1a and 12, after 24 hours of heating at 120 °C (Fig. 6), and the HPLC retention times matched. Therefore, the ‘C1’ adduct observed in the HPLC experiments, and at m/z 141 in the LCMS experiments, was confirmed as [C2C1(HO)C12im][OAc], 17. Recently, similar 2-hydroxymethyl-functionalised ionic liquid compounds, incorporating halide anions, were demonstrated by Wang et al. to behave as catalysts for the synthesis of cyclic carbonates from epoxides and CO2.67 Moreover, these ionic liquids were shown to be stable under basic conditions.
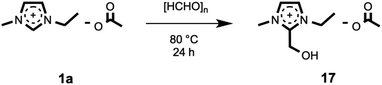 | ||
| Fig. 5 Preparation of [C2C1(HO)C12im][OAc], 17, by heating [C2C1im][OAc], 1a, in the presence of paraformaldehyde. | ||
By retroactively examining the preliminary NMR spectra for the mixtures of ionic liquids with cellulose, the observed singlet peak at δ 4.79 ppm for compounds 1 and 2, and at δ 4.73 ppm for 8, can be assigned to the respective C1 adducts. HPLC calibration curves were constructed; subsequently, the wt% of the C1 adduct was displayed alongside [C2C1im]+ (Fig. 3a).
The change in the HPLC% and wt% of the C6–C1 adduct species reveals key information about their relative stabilities (Fig. 3). The C6 adduct is generated rapidly, reaching the maximum concentration at t0.25 before gradually disappearing. No C6 adduct is present after 24 hours (t24), so it has been completely converted into further products. By contrast, the C1 adduct only reaches maximum concentration by t6, and is not diminished after 24 hours. Indeed, the C1 adduct appears to be metastable at the operating temperature of 120 °C, with no net loss or gain in concentration between the t6 and t24 time points (Fig. 3a). The comparatively slow and gradual formation of the smaller adducts (C2 and C1), coupled with the early rise and subsequent decrease of the larger adducts (C6, C4 and C3) indicates that the stability of the adducts increases with decreasing C2-substituent size. Moreover, the concentrations as a function of time, t, suggest that the C6 adduct forms initially; sequential elimination of CH2O units from the hydroxyalkyl chain follows, to yield successively more stable, smaller species with longer lifetimes.
The ‘acid number’, defined as the concentration of H+ (titrated against aqueous [nBu4N][OH]), in units of mmol H+ kg−1 IL), was measured for the mixture of [C2C1im][OAc], 1a, +10 wt% D-(+)-glucose, 12, at the same time points as the HPLC samples. This acid number value was then plotted as a function of heating time, and was overlaid with the wt% concentration of the C1 cation, [C2C1(HO)C12im]+ (Fig. 7).
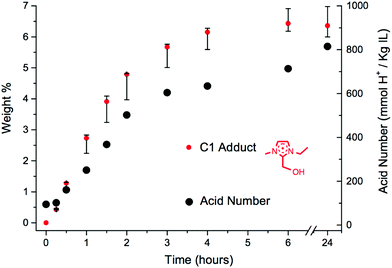 | ||
| Fig. 7 Relationship between acid number and wt% of the C1 adduct, [C2C1(HO)C12im]+, for the mixture of [C2C1im][OAc], 1a + 10 wt% D-(+)-glucose, 12, heated at 120 °C for 24 hours. | ||
The experiments revealed a gradual increase in acidity of the system during the 24 hour heating period, from ∼100 to 800 mmol H+ kg−1 IL. The curvature of the two plots in Fig. 7 indicates that increasing acid concentration is, to some extent, linked to the formation of the C1 adduct. However, there is a notable increase of approximately 100 mmol H+ kg−1 IL between t6 and t24, whereas the C1 adduct cation has reached its maximum concentration by t6. Moreover, acid number measurements on synthesised [C2C1(HO)C12im][OAc], 17 (Fig. 5), reveal that it is not itself responsible for the increase in acid number.
The number of moles of D-(+)-glucose, 12, incorporated into the mixture (∼505 mmol kg−1 IL), is marginally lower than the number of moles of [C2C1im]+ consumed (∼595 mmol kg−1 IL). The moles of C1 adduct present in the t24 mixture (∼450 mmol kg−1 IL), account for approximately 75% of the moles of [C2C1im]+ consumed. [C2C1im][OAc] exhibits slow decomposition at 120 °C,42,68 which will contribute partially to the loss of [C2C1im]+. There is a measurable quantity of the C2 adduct (∼1.2 HPLC%) still present in the mixture after 24 hours heating (Fig. 3b). A longer heating period would likely lead to total conversion of the C2 adduct to the C1 adduct. Therefore, it is not certain whether a stoichiometric 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction is occurring, but it is possible that one mole of the [C2C1im]+ cation reacts with one mole of D-(+)-glucose (12), initiating a sequence of reactions that eventually yields approximately one mole of the C1 cation, [C2C1(HO)C12im]+.
1 reaction is occurring, but it is possible that one mole of the [C2C1im]+ cation reacts with one mole of D-(+)-glucose (12), initiating a sequence of reactions that eventually yields approximately one mole of the C1 cation, [C2C1(HO)C12im]+.
However, it is highly plausible that the C1 adduct exists in an equilibrium with the parent [C2C1im]+ cation. By taking t24 mixtures of 1a + 10 wt% D-(+)-glucose, 12, and heating them at the higher temperature of 180 °C for four hours, a detectable reduction in concentration of [C2C1(HO)C12im]+ and a gradual increase in the concentration of the original [C2C1im]+ cation occurred. This indicates that the equilibrium is forced towards the ionic liquid side, with the evaporation of formaldehyde at this higher temperature.
The molar increase in acid number (∼700 mmol H+ kg−1 IL, Fig. 7), was greater than stoichiometric. The sequence of adduct-forming reactions between 1a and 12 appeared to terminate in an equilibrium concentration of the ‘C1’ adduct, [C2C1(HO)C12im]+; at 120 °C; the maximum quantity was reached by t6 and had not diminished by t24 (Fig. 3a). In order to assess its thermal stability, the synthesised sample of [C2C1(HO)C12im][OAc], 17, was further analysed at higher temperatures.
Temperature-ramped Thermogravimetric Analysis (TGA) experiments were performed on ionic compound 17, in the temperature range 80–700 °C, using a heating rate of 10 °C min−1. The TGA data is represented in graphical form in the ESI (Fig. E5a†). Complete TGA experimental conditions are described in the ESI.† The Tonset temperature for compound 17, 221 °C, was very similar to that of unsubstituted ionic liquid 1b, measured as 216 ± 2 °C employing identical experimental conditions. The derivative weight curve did not reveal any distinguishable weight loss event corresponding to loss of the C2-hydroxyalkyl substituent.
Recently, we investigated the long-term thermal stability of the typical carboxylate ionic liquid, [C2C1im][OAc], 1, and established Arrhenius parameters for thermal decomposition of this compound.42 Herein, we expanded this investigation to the C1 adduct compound, [C2C1(HO)C12im][OAc], 17, recording an isothermal TGA thermograph for 17 at 120 °C, for 24 hours (Fig. E5b†). Similar to ionic liquid 1, compound 17 yielded a straight isotherm, suggesting pseudo zeroth-order kinetics. Comparing rates of thermal decomposition (change in molar proportion over time, dα/dt), at the temperature of 120 °C, degradation of ionic liquid 17 is marginally faster (dα/dt = 7.3 × 10−3 h−1) than that of parent ionic liquid 1 (dα/dt = 5.7 × 10−3 h−1), because 17 is likely to incorporate a contribution to mass loss from both the regeneration of [C2C1im]+ and loss of formaldehyde, and from subsequent thermal decomposition of [C2C1im][OAc], occurring simultaneously.
Hence, attempting to regenerate [C2C1im]+ simply by high temperature treatment is not a suitable strategy for preventing the accumulation of the C1 adduct cation [C2C1(HO)C12im]+ in solutions of carbohydrates in carboxylate ionic liquids.
Elucidation of adduct-forming mechanisms
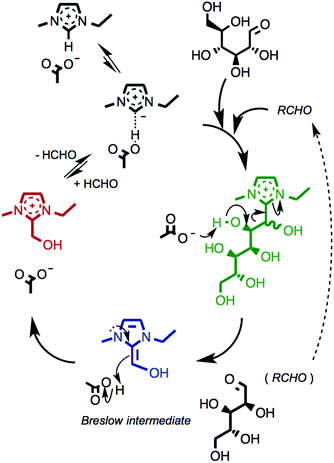
A proposed reaction mechanism for the formation and inter-conversion of the observed intermediates is shown in Fig. 8. It accounts for the key observations: (i) the concentration of the ionic liquid cation, [C2C1im]+, is markedly reduced following the 24 hour heating, and D-(+)-glucose, 12, is no longer present in the 1H NMR spectrum. Therefore, adducts are formed from reaction of [C2C1im]+ with 12; (ii) C6, C4, C3 and C2 adducts were all observed as intermediates and, moreover, their concentrations as a function of time suggest that the smaller adducts appear later and are more stable than the larger analogues; (iii) the concentration of the C1 adduct, [C2C1(HO)C12im]+, appears to reach an equilibrium by the end of the 24 hour heating; (iv) the successive reactions are accompanied by a progressive increase in the acid number of the mixture, although the C1 adduct is not itself the source of H+, and the increase in mmol H+ is not stoichiometric.The postulated mechanism involves initial abstraction of the C2 imidazolium proton by the basic acetate anion generating an NHC, which undergoes nucleophilic addition to the formyl group of glucose in its open chain form. The C6 adduct that forms then cleaves a five-carbon aldehyde fragment to form the C1 adduct via an established ‘Breslow’ intermediate.69 Subsequent recondensation of the liberated aldehyde fragment with a further ionic liquid ion pair yields the next homologue of the series, and reaction repeats to account for each of the observed C4, C3 and C2 intermediate adducts. Thus, in effect, the reaction constitutes gradual digestion of the carbohydrate by releasing one-carbon formaldehyde (HCHO) fragments in turn, bound up in the form of the C1 adduct species.
One apparent disparity between the postulated mechanism and the experimental observations is that reaction of one mole of D-(+)-glucose, 12, should eventually generate six moles of the C1 adduct species. This predicted quantity is far greater than the quantity observed. However, as discussed above, there is likely to be an equilibrium between this adduct and the parent ionic liquid cation. At the temperatures of these reactions (100–120 °C), it is probable that formaldehyde will largely be lost from the reaction mixture by evaporation. Moreover, it has so far been assumed that all the consumed glucose is via this mechanistic pathway. Formation of various ‘caramelisation’ products from the burning of the sugar, and other reactions, may account for some substantial loss of glucose. These products would not be clearly observed by spectroscopic means, yet the darkening of the reaction mixtures would support this hypothesis. Each of these two explanations would contribute to the disparity between the expected and observed quantity of [C2C1(HO)C12im]+ in the t24 mixtures.
The increase in acid number could, in principle, arise from oxidation of the liberated formaldehyde into formic acid, but no peak for this was observed in the 1H NMR spectrum for the mixture of 1a + 12 after the 24 hour heating period. Another possible cause for the increase in acid number could be the oxidation of C1 into the 1-ethyl-3-methylimidazolium-2-carboxylate cation, a species known to form between the [C2C1im]+ cation and CO2.63 This acidic zwitterionic species is present in the HPLC spectrum of the mixture of 1a + 12 prior to heating, in low concentration (<1 HPLC%), as a residual impurity from the ionic liquid synthesis and present in the HPLC of the pure ionic liquid. A very small increase (+0.36 ± 0.04 HPLC%) in the concentration of this cation was observed over the course of the 24 hour heating period. However, this is unlikely to be sufficient to account for all of the measured acid number increase of the experiments.
Reaction with other sugar compounds
The investigation was extended to the other sugar model compounds, 13–16 (see ESI†). The formation of adduct species was observed for mixtures of 1a with D-(+)-cellobiose, 13, D-(+)-xylose, 14, and D-(−)-fructose, 15, accompanied by a significant blackening of each mixture.In contrast, no adduct species were observed from HPLC analysis of the mixture 1a + 10 wt% D-(+)-sucrose, 16, heated to 120 °C for 24 hours. Instead, only a slight darkening of the mixture was observed, and the wt% of the [C2C1im]+ cation remained constant. Critically, D-(+)-sucrose, 16, is the only studied carbohydrate model compound lacking an open chain form. Therefore, we conclude that it is necessary for the saccharide to have an open chain structure for reaction with [C2C1im]+ to occur.
D-(+)-Cellobiose, 13, is an improved model compound for cellulose relative to D-(+)-glucose, 12, due to the presence of the two glucopyranose units held together by a β-1,4-glycosidic link. Upon heating [C2C1im][OAc], 1a, with D-(+)-cellobiose, 13, at 120 °C, adducts of higher polarity than [C2C1im]+ were observed by reverse-phase HPLC, as for the mixtures of 1a with 12. Several of the components present after 0.25 hours of heating were analysed by LCMS (Fig. E6†). The peak of highest polarity by HPLC was found to represent three separate chemical species in the LCMS. Three sharp signals were observed in the mass spectra, at m/z 453, 423 and 393 (each differing by m/z 30, CH2O). Using a similar nomenclature to products of 1a with D-(+)-glucose, 12, these masses were assigned to the ‘C12’, ‘C11’ and ‘C10’ adducts, respectively (Fig. 9). The C11 adduct, observed when studying mixtures with D-(+)-cellobiose, is analogous to the unseen C5 adduct in the experiments with D-(+)-glucose. Therefore, it is likely that the C5 adduct does form, but that the rapid conversion of adducts, or similarity in the HPLC retention times, explains why it is not formally distinguished.
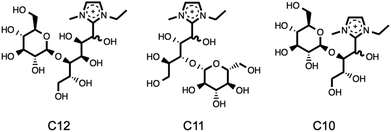 | ||
| Fig. 9 Assigned C12 (m/z 453), C11 (m/z 423) and C10 (m/z 393) adducts, observed from the mixture [C2C1im][OAc], 1a + 10 wt% D-(+)-cellobiose, 13, following heating at 120 °C for 0.25 hours. | ||
A small peak of the same retention time for the C6 adduct from the glucose experiment (m/z 291) was also observed, suggesting that both glucopyranose residues of 13 had reacted with [C2C1im]+ to eventually yield [C2C1(HO)C12im]+. This is directly supported by the formation of measurable quantities of the C1 adduct compound in the initial experiments with 5 wt% cellulose.
It remains unclear whether the reaction of both of the glucopyranose units of cellobiose occurs independently, or after a single addition to the imidazolium ring. Nevertheless, these results carry important implications for the dissolution of cellulose in carboxylate ionic liquids; adduct-forming reactions are likely to extend beyond the terminal glucopyranose residue, and employing carboxylate ionic liquids in the dissolution of cellulose for long periods of time will bring about a reduction in the degree of polymerisation of the cellulose, a diminished quality of the cellulose fibres, and the gradual accumulation of unwanted by-products.
Sugars D-(+)-xylose, 14, and D-(−)-fructose, 15, are model compounds for hemicellulose, also one of the major polymer components of lignocellulosic biomass (ca. 25 dry wt%) and a minor component (<10 wt%) of a typical cellulose pulp. Mixtures were prepared with [C2C1im][OAc], 1a, and 10 wt% quantities of 14 or 15. These mixtures were heated to 120 °C for a period of 24 hours, as for previous experiments with model compounds 12 and 13. In an analogous manner to D-(+)-glucose, 12, the mixture of 1a + 10 wt% D-(+)-xylose, 14, yielded a sequence of HPLC peaks, the most polar corresponding to the parent ‘C5’ adduct (xylose is a pentose sugar, Fig. 2), with a similar pattern of intermediate adducts finishing at the C1 compound, [C2C1(HO)C12im]+ (Fig. E7†).
Similarly, the mixture of 1a + 10 wt% D-(−)-fructose, 15 (a structural isomer of 12) demonstrated new adduct peaks, which were assigned to the expected Cn (n = 1–6) adducts, on the basis of the nearly identical pattern of HPLC peaks compared to the D-(+)-glucose experiment. The C1 adduct was assigned unambiguously from the 1H NMR data.
Therefore, all of the sugar model compounds that exhibit an open-chain form (12–15) undergo reaction at the ring C2 position of [C2C1im][OAc], 1a, yielding a common sequence of hydroxyalkyl-decorated adduct species.
Reduction in temperature
Although 120 °C is a very commonly used temperature for biomass and cellulose processing,23 it would be expected that the rates of by-product-forming reactions would be lower at reduced temperatures. The investigation of reactions of 1a + 10 wt% 12/13 were repeated at the reduced temperature of 100 °C. A comparison of the quantities of C6 and C1 adduct compounds (in HPLC% and wt%, respectively), between the two temperatures is displayed for 1a + 12 in Fig. 10. A comparison of the intermediate C4, C3 and C2 adduct species is shown in the ESI (Fig. E4†).Clearly, the same chemical species are formed at the lower temperature. The initial formation of the C6 adduct remains rapid at 100 °C (Fig. 10b), but the subsequent reactions are far slower, exemplified by the sluggish accumulation of the C1 adduct cation [C2C1(HO)C12im]+; at 120 °C, the maximum concentration is reached by t6, whereas at 100 °C this is not reached by t24 (Fig. 10a).
Thus, reducing the temperature of the cellulose solvation system appears to be a sensible strategy for diminishing the rate of by-product formation, although the generation of by-products is by no means entirely halted. However, the lower temperature limit of an industrial cellulose dissolution process will likely be dictated by the rate at which cellulose dissolves. Moreover, high viscosities of ionic liquid–cellulose solutions present a more significant problem at lower temperatures. Therefore, another process variable must be modified.
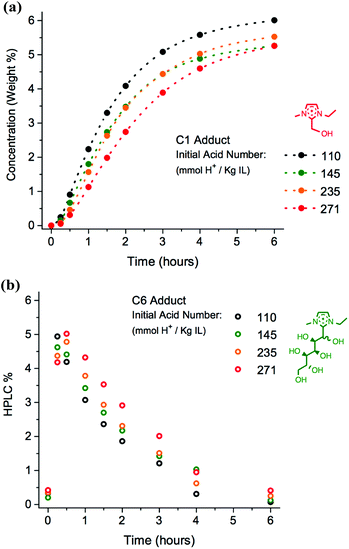
Starting acidity
The acid number of the mixture at the outset of the reaction was investigated as another possible variable which could be modified to limit reaction of the ionic liquid with sugars. Samples of ionic liquid [C2C1im][OAc], 1a, were treated with small aliquots of acetic acid, before being blended into mixtures with the addition of 10 wt% D-(+)-glucose, 12. The acid numbers of the mixtures were then determined (110–271 mmol H+ kg−1 IL), and the mixtures were heated at 120 °C for a period of 24 hours with HPLC analysis at the same regular time points (t0–t24) as for the experiments described above. Complete acid number data is listed in the ESI (Table E1b†). A comparison of the rate of formation of C6 and C1 for mixtures of 1a + 10 wt% 12, at differing initial acid numbers, is shown in Fig. 11.Increasing the acid number of the mixture [C2C1im][OAc], 1a + 10 wt% D-(+)-glucose, 12, reduces the rate of formation of [C2C1(HO)C12im]+ to a small extent, and this is most pronounced at the middle time points, t1–t4 (Fig. 11a). A corresponding reduction in the rate of disappearance of the C6 adduct is observed at higher acid numbers; maximum concentration of the C6 adduct appears to have occurred before t0.25 for the experiment with acid number 110 mmol H+ kg−1 IL, whereas maximum concentration is nearer t0.5 for the experiment with 271 mmol H+ kg−1 IL.
Nevertheless, differences in the rate of adduct formation appear to be minimal, at least for the acid number range and reaction mixture we have studied. A key implication of these results is that the mechanism of formation of the initial adducts, as well as their subsequent inter-conversion, is not significantly acid-catalysed. Regardless of the initial acid number, the measured increase in acid number was approximately equivalent over the 24 hour heating period, at +750 ± 50 mmol H+ kg−1 IL.
Sugar concentration
Subsequently, mixtures were studied with [C2C1im][OAc], 1a, and higher concentrations of sugar model compounds D-(+)-glucose, 12 and D-(+)-xylose, 14 (25 wt% and 100 mol%, relative to the ionic liquid), in an effort to prepare and isolate intermediate adducts for structural analysis. Mixtures were prepared in an analogous way to the 10 wt% mixtures, by addition of an aqueous sugar solution to the neat ionic liquid, followed by drying of the resultant solution under reduced pressure for one hour at 70 °C to yield a highly viscous liquid. Full procedures are described in the ESI.† Graphs representing the change in wt% of the [C2C1im]+ and [C2C1(HO)C12im]+ cations as a function of time, for the 25 wt% mixtures, are displayed in the ESI (Fig. E3†).Upon increasing the initial sugar quantity in the example of [C2C1im][OAc], 1a + 25 wt% D-(+)-glucose, 12, the pattern of observed adducts was equivalent to the former mixtures. HPLC% quantities of the intermediate adducts and wt% of the final adduct, [C2C1(HO)C12im]+, were markedly increased, although the increase was far from proportional to the increase in sugar concentration; only a marginal increase of [C2C1(HO)C12im]+ concentration was observed, from 6.5 ± 0.5 wt% up to 7.89 wt%. The 1H NMR spectrum of the mixture at t24 did not show any residual D-(+)-glucose, 12. However, the unaccounted concentration of D-(+)-glucose had perhaps undergone thermal decomposition, a suggestion supported by the observed darkening of the mixture and odour of caramel.
Increasing the sugar loading up to 100 mol% (relative to the ionic liquid, i.e. equimolar mixtures) yielded highly viscous solutions. Upon heating to 120 °C, the mixture exhibited no distinguishable HPLC adduct peaks, instead showing a broad, shapeless shoulder of retention time 3–10 minutes. These equimolar mixtures of [C2C1im][OAc], 1a + D-(+)-glucose, 12/D-(+)-xylose, 14, again exhibited a strong caramel odour after 24 hours of heating, suggesting that thermal decomposition of the carbohydrate had occurred.
Changing the ionic liquid
1-Ethyl-3-methylimidazolium acetate, [C2C1im][OAc], 1, has most commonly been used for the dissolution of cellulose and is highly effective. However, other ionic liquids have also been used. The solubility of cellulose in an ionic liquid is dependent on hydrogen-bond basicity, requiring β > 0.8 for dissolution to occur.23,70 However, the same basic behaviour of the ionic liquid anion has been associated with generating an N-heterocyclic carbene (NHC) from a dialkylimidazolium cation.1 We propose that this is the property that initiates the sequence of reactions culminating in formation of [C2C1(HO)C12im]+ (Fig. 8).In an effort to explore the breadth and scope of the adduct-forming mechanism between dialkylimidazolium ionic liquids and carbohydrate model compounds, the ionic liquid cation and anion were independently varied. Initially, a homologous carboxylate ionic liquid, [C2C1im][CH3(CH2)6CO2], 2, was studied to determine the impact of extending the anion alkyl chain length. Subsequently, other ionic liquids with similarly high β values were investigated ([C4C1im]Cl, 7, β = 0.83, [C4C1im][(CH3)2PO4], 8, β = 1.13).64 Furthermore, the ionic liquid [C2C1im][CH3SO3], 3, was studied, anticipated to be marginally below the β = 0.8 cut-off for cellulose solubility (β = 0.77 for the analogous [C4C1im][CH3SO3]).64
Finally, an all-inorganic eutectic mixture was investigated, which cannot form an NHC or subsequent adduct products, namely the mixture of lithium, potassium and cesium acetates, [Li]0.2[K]0.275[Cs]0.525[OAc], 11, reported first by Diogenov et al. in 1965,71 and more recently by Bajus and co-workers.72,73 As before, these were studied in mixtures with added 10 wt% D-(+)-glucose, 12, with heating for 24 hours at 120 °C.
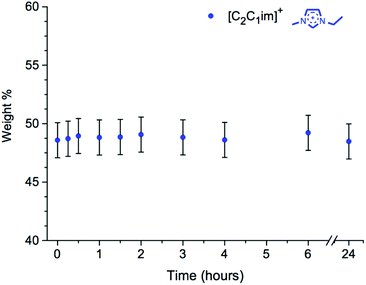
The two ionic liquids with lowest values of β, 3 and 7, exhibited no reaction between the cation and D-(+)-glucose. Furthermore, the change in acid number for these mixtures, where measured, was minimal (∼±10 mmol H+ kg−1 IL). The absolute concentration of the [C2C1im]+ or [C4C1im]+ cation was unchanged in each example after the 24 hour heating period (Fig. 12), and the colour changes of these mixtures were far less substantial than had been seen for the mixtures with [C2C1im][OAc], 1.
Hence, [C2C1im][CH3SO3], 3, and [C4C1im]Cl, 7, appear to be inert solvents with respect to the carbohydrate model compound D-(+)-glucose, 12, at the temperature of 120 °C. Crucially, [C4C1im]Cl was the first recognised example of a cellulose-dissolving ionic liquid;25 therefore, this apparent lack of adduct-forming reactions is highly significant.
By contrast, the mixture of [C4C1im][(CH3)2PO4], 8, with D-(+)-glucose, 12, did exhibit several new HPLC peaks of higher polarity than the [C4C1im]+ cation. However, only four peaks formed on the timescale of the experiment, which were slow to appear in comparison to those for the mixtures of 1 + 10 wt% 12. The size of the [C4C1(HO)C12im]+ peak at the t24 time point was considerably lower than the analogous peak for 1 + 10 wt% 12.
LCMS data for the mixture [C4C1im][(CH3)2PO4], 8, with D-(+)-glucose, 12, at the t24 time point is shown in the ESI (Fig. E8†). The handful of peaks that were present at t24 could be comfortably assigned as the equivalent C6, C2 and C1 products, derived from the [C4C1im]+ cation (m/z 319, 199 and 169, respectively). The two peaks of shortest retention time (highest polarity) were of identical mass (m/z 319), and therefore were assigned as the two diastereoisomers of the C6 adduct. The quantity of the C2 adduct was vanishingly small. The C4 and C3 intermediate adducts were absent.
While it is possible that the reaction of D-(+)-glucose with [C4C1im][(CH3)2PO4], 8, is mechanistically different to that of [C2C1im][OAc], 1a, this is unlikely and it is probable that the intermediates C5, C4 and C3 do form, but are extremely unstable in this ionic liquid and rapidly convert to the smaller and more stable C2 and C1 products. What is clear is that the rate and quantity of the products formed are significantly lower than for the carboxylate ionic liquid [C2C1im][OAc], 1a.
Finally, the mixture of the inorganic acetate eutectic, [Li]0.2[K]0.275[Cs]0.525[OAc], 11, with D-(+)-glucose, 12, was prepared as for all other mixtures, by the addition of an aqueous solution of the sugar to the ionic compound. This resulted in a viscous, glassy mixture, with poor homogeneity; an accurate and reproducible water content measurement could therefore not be obtained. Upon heating the mixture to the experimental temperature of 120 °C for 0.25 hours, a drastic colour change was observed, from colourless/white to deep orange/brown. The solution had a strong odour of burnt caramel, as for the equimolar mixtures of ionic liquid [C2C1im][OAc], 1a with carbohydrates 12 or 14, described previously. Moreover, HPLC analysis at the t0.25 time point revealed an absence of new distinct adduct peaks, suggesting instead that thermal decomposition of the sugar had occurred. Therefore, the eutectic mixture [Li]0.2[K]0.275[Cs]0.525[OAc], 11, despite lacking the dialkylimidazolium cation necessary for adduct formation, is not an appropriate solvent for cellulose.
In summary, the screening of different ionic liquid cation and anion species, with respect to their reactivity with D-(+)-glucose, 12, highlighted several interesting phenomena. For dialkylimidazolium based ionic liquids, the high β value that is required to dissolve cellulose seems also to lead to reactions between the cation and sugar compounds. However, the reactions in the ionic liquid with the highest value of β, [C4C1im][(CH3)2PO4], 8 (β = 1.13) were slower than in the carboxylate ionic liquids. Dimethyl phosphate, (CH3)2HPO4, has a pKa of 1.29, relative to 4.76 for acetic acid and 4.89 for octanoic acid.74 pKa values of hydrogen chloride and methanesulfonic acid, both strong acids, are ≪1. Therefore, Brønsted basicity may be a better measure of the likelihood of adduct formation than hydrogen-bond basicity, β, measured from Kamlet–Taft experiments. This is worthy of further study.
Recent investigations have shown the tendency of chloride ionic liquids to cause hydrolytic cleavage and degradation of cellulose during dissolution processes,75–77 yielding a mixture of cellooligosaccharides, cellobiosan and glucose when water content is above a certain threshold.78 Careful exclusion of water (concentration <0.3 wt%), and the high temperature of our experiments (120 °C rather than 100 °C, ensuring more water was in the vapour phase) are the likely explanations for the absence of these hydrolysis reactions being observed for our mixtures of ionic liquids with cellulose.
The precise explanation for the differences in reactivity of carboxylate (1 and 2), chloride (7) and dimethyl phosphate (8) ionic liquids with D-(+)-glucose, 12, is unknown. However, what is striking is that anhydrous [C4C1im]Cl, 7, an effective solvent for cellulose (albeit less so than [C2C1im][OAc], 1), does not exhibit the undesirable sequence of adduct-forming pathways occurring for anhydrous carboxylate ionic liquids.
Conclusions
Dialkylimidazolium ionic liquids incorporating carboxylate anions react with cellulose and low molecular weight sugars to generate a series of intermediates leading eventually to cations with a hydroxymethyl substituent at the C2 position of the ring, e.g. the 1-ethyl-2-(hydroxymethyl)-3-methylimidazolium cation, [C2C1(HO)C12im]+. Substituting with the dimethyl phosphate anion slows, but does not prevent, these reactions with the model sugar compounds. By contrast, the investigated chloride and methanesulfonate ionic liquids are sufficiently less reactive towards the model sugar D-(+)-glucose that no measureable quantity of reaction products was observed after 24 hours at 120 °C. However, of these two, only the chloride-based ionic liquid can dissolve cellulose to a significant concentration.The series of reactions begins with the addition of the aldehyde of the open chain form of the sugar to the NHC of the cation. This is then followed by sequential elimination of formaldehyde units until the final product is reached. The observed reactivity of D-(+)-cellobiose demonstrated that these reactions are not limited to the terminal glucopyranose residue. Although the rate of formation of these by-products may be partly reduced by lowering the operating temperature or increasing the initial acidity of the system, their formation cannot be entirely prevented.
Given the preference for carboxylate-anion ionic liquids in the dissolution of cellulose and particularly the popularity of [C2C1im][OAc], this behaviour has the potential to prevent successful implementation of cellulose dissolution processes employing these ionic liquids. The accumulation of ionic liquid derived by-products will affect rheological properties and prevent the crucial recycling of the expensive ionic liquid component, greatly increasing process costs. The degradation, fibrilation and shortening of the cellulose fibres are also key concerns for the reduction of the quality and quantity of the cellulose product.
One obvious strategy would be the modification of the ionic liquid cation. The adduct-forming reaction mechanism for [C2C1im][OAc] clearly involves the reactive C2 position of the imidazolium ring. Unfortunately, simply substituting this proton for a methyl group yields an ionic liquid with a higher viscosity, higher melting point and limited thermal stability.42 Moreover, recent investigations have highlighted the importance of the cation in cellulose dissolution,79–81 when previously it was considered to have only a secondary role. Therefore, whilst modification of the ionic liquid cation may provide a feasible solution to the undesired adduct-forming reactions, it is not a trivial problem.
When employing dialkylimidazolium ionic liquids, anions of sufficiently low basicity are required to inhibit formation of N-heterocyclic carbenes. However, such anions also lead to a reduction of the solubility of cellulose in the ionic liquid. Until alternatives can be identified, only anhydrous ionic liquids incorporating the chloride anion have been shown to be able to both dissolve cellulose and to avoid undesirable reaction of the dialkylimidazolium cations with the cellulose. Further investigation into the relationships between ionic liquid structure and reactivity towards cellulose is required.
Acknowledgements
The authors thank Michael Klein, Sven Holzmann and Günter Forster of BASF SE, for assistance with the experimental work. The authors gratefully acknowledge the help of Gilbert de Gregorio of Imperial College, London, with HPLC method development, and BASF SE for the sponsorship of Matthew T. Clough. The authors thank Prof. Alan Spivey of Imperial College, London, for useful discussions relating to chemical mechanisms, and also Paul Corbett, Dr Andy Dolan and Dr Heiko Niedermeyer of Imperial College, London, for their donation of ionic liquids.References
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- H. Weingaertner, Angew. Chem., Int. Ed., 2008, 47, 654–670 CrossRef CAS PubMed.
- I. Krossing, J. M. Slattery, C. Daguenet, P. J. Dyson, A. Oleinikova and H. Weingartner, J. Am. Chem. Soc., 2006, 128, 13427–13434 CrossRef CAS PubMed.
- P. Wasserscheid and T. Welton, Ionic liquids in synthesis, Wiley Online Library, 2008 Search PubMed.
- Ionic Liquids Database (ILThermo). NIST Standard Reference Database 147. http://ilthermo.boulder.nist.gov/ILThermo/.
- N. E. A. Cousens, L. J. Taylor Kearney, M. T. Clough, K. R. J. Lovelock, R. G. Palgrave and S. Perkin, Dalton Trans., 2014, 43, 10910–10919 RSC.
- M. J. Earle, J. Esperanca, M. A. Gilea, J. N. C. Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831–834 CrossRef CAS PubMed.
- A. Deyko, K. R. J. Lovelock, J. A. Corfield, A. W. Taylor, P. N. Gooden, I. J. Villar-Garcia, P. Licence, R. G. Jones, V. G. Krasovskiy, E. A. Chernikova and L. M. Kustov, Phys. Chem. Chem. Phys., 2009, 11, 8544–8555 RSC.
- A. W. Taylor, K. R. J. Lovelock, A. Deyko, P. Licence and R. G. Jones, Phys. Chem. Chem. Phys., 2010, 12, 1772–1783 RSC.
- K. R. J. Lovelock, A. Deyko, P. Licence and R. G. Jones, Phys. Chem. Chem. Phys., 2010, 12, 8893–8901 RSC.
- J. Esperanca, J. N. C. Lopes, M. Tariq, L. Santos, J. W. Magee and L. P. N. Rebelo, J. Chem. Eng. Data, 2010, 55, 3–12 CrossRef CAS.
- S. P. Verevkin, D. H. Zaitsau, V. N. Emeĺyanenko and A. Heintz, J. Phys. Chem. B, 2011, 115, 12889–12895 CrossRef CAS PubMed.
- F. Heym, B. J. M. Etzold, C. Kern and A. Jess, Phys. Chem. Chem. Phys., 2010, 12, 12089–12100 RSC.
- S. P. Verevkin, V. N. Emel'yanenko, D. H. Zaitsau, R. V. Ralys and C. Schick, J. Phys. Chem. B, 2012, 116, 4276–4285 CrossRef CAS PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed.
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621–629 CrossRef CAS PubMed.
- Z. Lei, C. Dai and B. Chen, Chem. Rev., 2013, 114, 1289–1326 CrossRef PubMed.
- J. F. Brennecke and B. E. Gurkan, J. Phys. Chem. Lett., 2010, 1, 3459–3464 CrossRef CAS.
- X. Zhang, X. Zhang, H. Dong, Z. Zhao, S. Zhang and Y. Huang, Energy Environ. Sci., 2012, 5, 6668–6681 CAS.
- S. Zhu, Y. Wu, Q. Chen, Z. Yu, C. Wang, S. Jin, Y. Ding and G. Wu, Green Chem., 2006, 8, 325–327 RSC.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS PubMed.
- A. Brandt, J. Grasvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC.
- C. Gränacher, U.S. Patent, 1,943,176, 1934 Search PubMed.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- J. Pickett, Sustainable biofuels: prospects and challenges, The Royal Society, 2008 Search PubMed.
- R. D. Perlack, L. L. Wright, A. F. Turhollow, R. L. Graham, B. J. Stokes and D. C. Erbach, Biomass as feedstock for a bioenergy and bioproducts industry: the technical feasibility of a billion-ton annual supply, DTIC Document, 2005 Search PubMed.
- A. C. O'Sullivan, Cellulose, 1997, 4, 173–207 CrossRef.
- X. Qian, S.-Y. Ding, M. R. Nimlos, D. K. Johnson and M. E. Himmel, Macromolecules, 2005, 38, 10580–10589 CrossRef CAS.
- Y. Nishiyama, P. Langan and H. Chanzy, J. Am. Chem. Soc., 2002, 124, 9074–9082 CrossRef CAS PubMed.
- C.-H. Kuo and C.-K. Lee, Bioresour. Technol., 2009, 100, 866–871 CrossRef CAS PubMed.
- H. P. Fink, P. Weigel, H. J. Purz and J. Ganster, Prog. Polym. Sci., 2001, 26, 1473–1524 CrossRef CAS.
- Y.-H. P. Zhang, S.-Y. Ding, J. R. Mielenz, J.-B. Cui, R. T. Elander, M. Laser, M. E. Himmel, J. R. McMillan and L. R. Lynd, Biotechnol. Bioeng., 2007, 97, 214–223 CrossRef CAS PubMed.
- S. Dorn, F. Wendler, F. Meister and T. Heinze, Macromol. Mater. Eng., 2008, 293, 907–913 CrossRef CAS.
- T. Liebert, Cellulose Solvents: For Analysis, Shaping and Chemical Modification, American Chemical Society, 2010 Search PubMed.
- Y. Fukaya, K. Hayashi, M. Wada and H. Ohno, Green Chem., 2008, 10, 44–46 RSC.
- A. W. T. King, J. Asikkala, I. Mutikainen, P. Järvi and I. Kilpeläinen, Angew. Chem., Int. Ed., 2011, 50, 6301–6305 CrossRef CAS PubMed.
- A. Xu, J. Wang and H. Wang, Green Chem., 2010, 12, 268–275 RSC.
- K. Ohira, Y. Abe, M. Kawatsura, K. Suzuki, M. Mizuno, Y. Amano and T. Itoh, ChemSusChem, 2012, 5, 388–391 CrossRef CAS PubMed.
- L. Chen, M. Sharifzadeh, N. Mac Dowell, T. Welton, N. Shah and J. P. Hallett, Green Chem., 2014, 16, 3098–3106 RSC.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- M. T. Clough, K. Geyer, P. A. Hunt, J. Mertes and T. Welton, Phys. Chem. Chem. Phys., 2013, 47, 20480–20495 RSC.
- Y. Cao and T. Mu, Ind. Eng. Chem. Res., 2014, 53, 8651–8664 CrossRef CAS.
- C. Maton, N. De Vos and C. V. Stevens, Chem. Soc. Rev., 2013, 42, 5963–5977 RSC.
- S. Sowmiah, V. Srinivasadesikan, M.-C. Tseng and Y.-H. Chu, Molecules, 2009, 14, 3780–3813 CrossRef CAS PubMed.
- K. J. Baranyai, G. B. Deacon, D. R. MacFarlane, J. M. Pringle and J. L. Scott, Aust. J. Chem., 2004, 57, 145–147 CrossRef CAS.
- T. J. Wooster, K. M. Johanson, K. J. Fraser, D. R. MacFarlane and J. L. Scott, Green Chem., 2006, 8, 691–696 RSC.
- G. Ebner, S. Schiehser, A. Potthast and T. Rosenau, Tetrahedron Lett., 2008, 49, 7322–7324 CrossRef CAS PubMed.
- X. Wei, Z. Han and D. Zhang, Carbohydr. Res., 2013, 374, 40–44 CrossRef CAS PubMed.
- N. Marion, S. Díez-González and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988–3000 CrossRef CAS PubMed.
- M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed.
- A. J. Arduengo, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361–363 CrossRef CAS.
- S. T. Handy and M. Okello, J. Org. Chem., 2005, 70, 1915–1918 CrossRef CAS PubMed.
- J. Dupont and J. Spencer, Angew. Chem., Int. Ed., 2004, 43, 5296–5297 CrossRef CAS PubMed.
- T. L. Amyes, S. T. Diver, J. P. Richard, F. M. Rivas and K. Toth, J. Am. Chem. Soc., 2004, 126, 4366–4374 CrossRef CAS PubMed.
- O. Holloczki, D. Gerhard, K. Massone, L. Szarvas, B. Nemeth, T. Veszpremi and L. Nyulaszi, New J. Chem., 2010, 34, 3004–3009 RSC.
- O. Holloczki and L. Nyulaszi, Org. Biomol. Chem., 2011, 9, 2634–2640 CAS.
- Z. Kelemen, O. Holloczki, J. Nagy and L. Nyulaszi, Org. Biomol. Chem., 2011, 9, 5362–5364 CAS.
- H. Rodriguez, G. Gurau, J. D. Holbrey and R. D. Rogers, Chem. Commun., 2011, 47, 3222–3224 RSC.
- O. Hollóczki, D. S. Firaha, J. Friedrich, M. Brehm, R. Cybik, M. Wild, A. Stark and B. Kirchner, J. Phys. Chem. B, 2013, 117, 5898–5907 CrossRef PubMed.
- M. Thomas, M. Brehm, O. Hollóczki and B. Kirchner, Chem. – Eur. J., 2014, 20, 1622–1629 CrossRef CAS PubMed.
- O. Hollóczki, Inorg. Chem., 2013, 53, 835–846 CrossRef PubMed.
- G. Gurau, H. Rodríguez, S. P. Kelley, P. Janiczek, R. S. Kalb and R. D. Rogers, Angew. Chem., Int. Ed., 2011, 50, 12024–12026 CrossRef CAS PubMed.
- M. A. Ab Rani, A. Brant, L. Crowhurst, A. Dolan, M. Lui, N. H. Hassan, J. P. Hallett, P. A. Hunt, H. Niedermeyer, J. M. Perez-Arlandis, M. Schrems, T. Welton and R. Wilding, Phys. Chem. Chem. Phys., 2011, 13, 16831–16840 RSC.
- H. Niedermeyer, C. Ashworth, A. Brandt, T. Welton and P. A. Hunt, Phys. Chem. Chem. Phys., 2013, 15, 11566–11578 RSC.
- M. Schrems, G. Ebner, F. Liebner, E. Becker, A. Potthast and T. Rosenau, in Cellulose Solvents: For Analysis, Shaping and Chemical Modification, American Chemical Society (ACS), 2010, pp. 149–164 Search PubMed.
- J.-Q. Wang, W.-G. Cheng, J. Sun, T.-Y. Shi, X.-P. Zhang and S.-J. Zhang, RSC Adv., 2014, 4, 2360–2367 RSC.
- A. W. T. King, A. Parviainen, P. Karhunen, J. Matikainen, L. K. J. Hauru, H. Sixta and I. Kilpelainen, RSC Adv., 2012, 2, 8020–8026 RSC.
- R. Breslow, J. Am. Chem. Soc., 1958, 80, 3719–3726 CrossRef CAS.
- T. V. Doherty, M. Mora-Pale, S. E. Foley, R. J. Linhardt and J. S. Dordick, Green Chem., 2010, 12, 1967–1975 RSC.
- G. G. Diogenov and G. S. Sergeeva, Zl. Neorg. Khim., 1965, 10, 292–294 CAS.
- S. Bajus, A. Deyko, A. Bosmann, F. Maier, H.-P. Steinruck and P. Wasserscheid, Dalton Trans., 2012, 41, 14433–14438 RSC.
- A. Deyko, S. Bajus, F. Rietzler, A. Bösmann, P. Wasserscheid, H.-P. Steinrück and F. Maier, J. Phys. Chem. C, 2013, 117, 22939–22946 CAS.
- W. D. Kumler and J. J. Eiler, J. Am. Chem. Soc., 1943, 65, 2355–2361 CrossRef CAS.
- O. M. Gazit and A. Katz, ChemSusChem, 2012, 5, 1542–1548 CrossRef CAS PubMed.
- J. Vitz, T. Erdmenger, C. Haensch and U. S. Schubert, Green Chem., 2009, 11, 417–424 RSC.
- E. Ohno and H. Miyafuji, J. Wood Sci., 2013, 59, 221–228 CrossRef CAS.
- E. Ohno and H. Miyafuji, J. Wood Sci., 2014, 1–10 Search PubMed.
- H. Zhao, G. A. Baker, Z. Song, O. Olubajo, T. Crittle and D. Peters, Green Chem., 2008, 10, 696–705 RSC.
- T. G. A. Youngs, C. Hardacre and J. D. Holbrey, J. Phys. Chem. B, 2007, 111, 13765–13774 CrossRef CAS PubMed.
- R. S. Payal and S. Balasubramanian, Phys. Chem. Chem. Phys., 2014, 16, 17458–17465 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4gc01955e |
| This journal is © The Royal Society of Chemistry 2015 |