Challenges in aromaticity: 150 years after Kekulé's benzene
Nazario
Martín
*ab and
Lawrence T.
Scott
*cde
aDepartamento de Química Orgánica, Facultad de Química, Universidad Complutense de Madrid, Av. Complutense s/n, E-28040 Madrid, Spain. E-mail: nazmar@ucm.es
bIMDEA Nanociencia, C/ Faraday 9, Ciudad Universitaria de Cantoblanco, E-28049 Madrid, Spain
cMerkert Chemistry Center, Department of Chemistry, Boston College, 2609 Beacon St., Chestnut Hill, MA 02467-3860, USA. E-mail: lawrence.scott@bc.edu
dDepartment of Chemistry/216, University of Nevada, Reno, NV 89557-0020, USA. E-mail: lawrence.scott@unr.edu
eJST-ERATO Project, Department of Chemistry, Graduate School of Science, Nagoya University, Furo-cho, Chikusa, Nagoya 464-8602, Japan
Although some aromatic compounds were known before the 1820s, it was Michael Faraday who first identified the parent benzene from compressed oil gas, a non-aromatic source, in 1825 (Fig. 1). It was twenty-five years later when A. W. Hofmann recognized benzene and its derivatives as members of the great family of the so-called aromatic compounds. At that time, investigations on benzene and its derivatives had a lot of experimental problems typically stemming from the presence of impurities, which resulted in compounds being difficult to characterize. These drawbacks were eventually solved by the use of fractional distillation, formerly introduced by Charles Mansfield in 1849. Interestingly, just nine years after the isolation of benzene by Faraday, the hydrocarbon was prepared independently by Eilhardt Mitscherlich from aromatic sources by heating benzoic acid with lime. He determined its molecular formula, and the obtained hydrocarbon was named as benzin and later benzene, the name currently accepted by the IUPAC.
These new aromatic compounds generated much excitement when Kekulé and others decided to apply the most recent structural theory to unveil their chemical structure. Since the empirical formula of benzene, C6H6, as well as the carbon bonding rules were already known, a variety of proposals to describe the benzene molecule were published before Kekulé's solution in 1865. In this regard, it is fair to mention the proposals by Archibald Scott Couper and Josef Loschmidt with the diallene structure, which had no experimental evidence. Furthermore, although Loschmidt reported the chemical structure of benzene as a circle to note the unknown and elusive structure in his Chemischen Studien in 1861, it was considered by Kekulé to be a misunderstanding.
It was in this context that Kekulé considered a hexagon as the solution, matching all the experimental evidences and the structural theory (Fig. 2). Whether this was the result of a dream while travelling on a bus in London or not is a matter for debate. However, there can be no doubt that the formula stemmed from a deductive method resulting in the new ring concept. As stated by Kekulé at the ceremony in Berlin in 1890 to celebrate the twenty-fifth anniversary of his proposal of benzene's structure, “Let us learn to dream, gentlemen, then perhaps we shall find the truth. But let us beware of publishing our dreams until they have been put to the proof by the waking understanding”.
In January 1865, the recently founded Société Chimique de Paris published its “bulletin mensual” containing Kekulé's first paper entitled “Sur la constitution des substances aromatiques”. In this paper he represented benzene as a closed chain of alternating single and double bonds in a linear sausage-shape formula applicable to all the aromatic compounds. Although a bare hexagon was later used by Adolph Claus in 1866, it was eventually Hofmann who, since 1868, used the hexagon extensively to represent benzene in his position as the editor of Berichte. Eventually, the chemical structure of benzene was unambiguously established by Bragg's colleague Kathleen Lonsdale by X-ray crystallography at the Royal Institution in 1929 (Fig. 3).
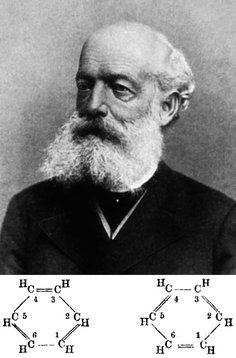 | ||
| Fig. 2 Friedrich August Kekulé von Stradonitz and his chemical structures for benzene. Image reproduced from https://en.wikipedia.org/wiki/August_Kekulé. | ||
 | ||
| Fig. 3 The molecular structure of benzene was unambiguously established by Kathleen Lonsdale by X-ray crystallography using this apparatus at the Royal Institution in 1929. | ||
Since the very beginning, the unravelling and establishment of the concept of aromaticity has ranked among the most important issues concerning chemistry in general and organic chemistry in particular. In his Kekulé Memorial Lecture to the London Chemical Society in 1898, Francis Japp declared Kekulé's benzene theory to be “the most brilliant piece of scientific production to be found in the whole of organic chemistry… three-fourths of modern organic chemistry is directly or indirectly the product of this theory.”
With the perspective of time, more than a century after this proclamation by Japp, his words seem quite modest. Nowadays, aromaticity has a wider meaning referring to those compounds having planar or quasiplanar cyclic conjugated structures obeying Hückel's rule of (4n + 2) π electrons. Aromatic compounds are thus no longer related with the former pleasant odour but to those compounds showing a higher level of stability than that expected from their formulation as conjugated cyclohexatrienes. Although free benzene does not occur naturally, its industrial production is huge, and the impact of its derivatives is tremendous, not only at a scientific level but, most importantly, at a societal level. The wide variety of compounds having benzene as a constituent have been used for applications in areas such as biochemistry, pharmacy, medicine, and materials science, including plastics, engineering, etc., with a remarkable societal impact.
In this special issue devoted to the current challenges in aromaticity, we commemorate the 150th anniversary of Kekulé's benzene structure. For this purpose, we have selected a host of prominent scientists engaged in aromaticity in the broadest sense. All of them consider benzene and aromaticity as common elements to address fascinating molecules that would have been difficult to conceive until recent times. Altogether, they provide a timely and realistic mosaic of important current topics in chemistry stemming from a molecule as simple as C6H6 but affording complex and sophisticated molecules whose only limit is the imagination of the chemist.
Thus, with the title “New advances in nanographene chemistry” (DOI: 10.1039/c5cs00183h), Klaus Müllen and co-workers present the very latest progress in their “bottom-up” and atomically precise fabrication of nanographenes using the tools of modern synthetic organic chemistry. These recent advances have been classified in four categories: (1) non-conventional methods, (2) structures incorporating seven- or eight-membered rings, (3) selective heteroatom doping, and (4) direct edge functionalization. Of particular relevance are the advances in the construction of graphene nanoribbons (GNRs), by extending nanographene synthesis to longitudinally extended polymeric systems. Thus, GNRs have been accessed by both solution-mediated and surface-assisted cyclodehydrogenation, and even by a graphitization process from well-known polyphenylenes.
With the paper entitled “Carbo-aromaticity and novel carbo-aromatic compounds” (DOI: 10.1039/c5cs00244c), Valérie Maraval, Remi Chauvin and co-workers bring to the attention of the readership that while the concept of aromaticity is being more narrowly defined, the term “aromatic compounds” is expanding to embrace more, different compounds. This means that “aromaticity” refers to a wider variety of compounds and, more specifically, to recently proposed two-membered rings of the acetylene and butatriene molecules, as representative examples of the so-called “carbo-aromaticity”, involving a new family of novel and intriguing aromatic compounds. These carbo-mers are π-electron-enriched compounds such as σ,π-macrocyclic carbo-benzene archetypes. Other non-aromatic references of carbo-benzenes and pro-aromatic congeners are also discussed. Importantly, these carbo-mers exhibit interesting properties, such as linear or nonlinear optical properties and conductance at the single molecule level.
Jesús M. Ugalde and co-workers report on “Recent developments and future prospects of all-metal aromatic compounds” (DOI: 10.1039/c5cs00341e). In this interesting paper, the authors address both the concepts of aromaticity and antiaromaticity to foresee the electronic properties and stabilities of small inorganic rings as well as those of all-metal rings. Particularly appealing is the passivation of these rings to prevent the formation of further aggregates and attack by environmental molecules.
In the paper by Renana Gershoni-Porannea and Ammon Stanger on “Magnetic criteria of aromaticity” (DOI: 10.1039/c5cs00114e), the authors show the state of the art on the magnetic criteria of aromaticity. The fundamentals of ring currents in aromatic and antiaromatic systems as well as associated experimental and computational tools: NMR, diamagnetic susceptibility exaltation, current density analyses (CDA), and nucleus independent chemical shifts (NICS) are briefly discussed. A more detailed discussion focuses on the NICS and CDA by means of some representative examples.
Graham J. Bodwell and co-workers report on “Cyclophanes containing large polycyclic aromatic hydrocarbons” (DOI: 10.1039/c5cs00274e). In this review, the authors show the wide structural diversity and singular behaviour of cyclophanes. Interestingly, whereas a large number of cyclophanes are known, only a few of them contain polycyclic aromatic systems with more than four rings. The structural features as well as the main general synthetic approaches to these king-size cyclophanes are reviewed. Eleven PAHs with different size aromatic systems, involving pyrene and hexabenzocoronene among other PAHs, are discussed in the framework of cyclophanes.
With the provocative title “Pro-aromatic and anti-aromatic π-conjugated molecules: an irresistible wish to be diradicals” (DOI: 10.1039/c5cs00051c), Juan Casado, Jishan Wu and co-workers use the concept of aromaticity to understand the stability and physical properties of π-conjugated molecules. Actually, pro-aromatic and anti-aromatic molecules exhibit an irresistible tendency to become diradicals in the ground state. Molecules with diradical character show different properties from those of closed-shell π-conjugated systems. Recent examples of different stable/persistent diradicaloids based on pro-aromatic and anti-aromatic compounds are discussed mainly in terms of structure–property relationships and potential applications in materials science.
In their review article entitled “The excited state antiaromatic benzene ring: a molecular Mr Hyde?” (DOI: 10.1039/c5cs00057b), Raffaello Papadakis and Henrik Ottosson describe theoretical and computational studies that examine and confirm Baird's rule on reversal in the electron count for aromaticity and antiaromaticity of annulenes in their lowest triplet states as compared to Hückel's rule for the ground state (S0). In the second part of the article, the photochemical reactions of benzene derivatives from the excited state antiaromatic character of the benzene ring are discussed. The authors argue that benzene can be viewed as a molecular “Dr Jekyll and Mr Hyde” with its largely unknown excited state antiaromaticity representing its “Mr Hyde” character.
In their article “Non-alternant non-benzenoid Kekulenes: the birth of a new Kekulene family” (DOI: 10.1039/c5cs00185d), Yoshito Tobe and co-workers present “Kekulene” as a doughnut-like or racetrack shaped polycyclic aromatic hydrocarbon consisting of cyclically arrayed benzene rings. It represents an ideal model to study conjugation circuits of π electrons, in particular, whether they delocalize locally in benzene rings or globally throughout the molecule. This article shows the theoretical and experimental aspects of Kekulene-related molecules, focusing on three types: benzenoid Kekulenes, yet unknown anti-Kekulene, and non-alternant non-benzenoid Kekulenes.
With the title “Quantifying aromaticity with electron delocalisation measures” (DOI: 10.1039/c5cs00066a), Ferran Feixas, Eduard Matito, Jordi Poater and Miquel Solà show that quantification of the concept “aromaticity” cannot be done directly and, therefore, is done indirectly from the measure of different properties exhibited by aromatic compounds, resulting in the multiple indices of aromaticity known to date. Based on electron delocalisation measures, new indicators of aromaticity such as the PDI, FLU, ING, and INB have been defined by their group. In this article, the authors compare and summarise a number of applications of electronic-based indices for the analysis of aromaticity in solving chemical problems.
In his article “Chemistry at the interior atoms of polycyclic aromatic hydrocarbons” (DOI: 10.1039/c4cs00479e), Lawrence T. Scott describes the rare chemical reactivity that PAHs exhibit at their interior atoms. The first compound with no edges, C60, was the first polycyclic carbon π-system observed to exhibit such reactivity; however, some fragments of C60, known as “geodesic polyarenes”, have subsequently been found to exhibit “fullerene-type chemistry” at their interior carbon atoms. Although no examples of σ-bond-forming reactions at interior carbons of planar, benzenoid PAHs have yet been reported, Scott speculates that such reactions may also be observed someday.
With the title “Aromaticity of metallabenzenes and related compounds” (DOI: 10.1039/c5cs00004a), Israel Fernández, Gernot Frenking and Gabriel Merino discuss the concept of aromaticity as focused on a particular family of organometallic compounds known as metallabenzenes, which are characterized by the formal replacement of a CH group in benzene by an isolobal transition metal fragment. In this article, the aromaticity of related compounds such as heterometallabenzenes is also revised by means of available experimental data as well as by computational methods developed as descriptors for aromaticity presented in a critical manner.
Masahiko Iyoda and Hideyuki Shimizu present a tutorial devoted to “Multifunctional π-expanded oligothiophene macrocycles” (DOI: 10.1039/c5cs00388a), wherein they summarize recent progress in the design, synthesis, and multifunctional properties of fully conjugated macrocyclic π-systems. They mainly focus on π-expanded oligothiophene macrocycles. In particular, the π-expanded oligothiophene macrocycle containing 2,5-thienylenes, ethynylenes, and vinylenes is one of the most widely applicable macrocycles for constructing multifunctional π-systems. These π-expanded oligothiophene macrocycles with different ring sizes can be efficiently prepared. Since these macrocycles have inner and outer domains, specific information concerning structural, electronic, and optical properties as well as applications are discussed.
In their tutorial entitled “The dynamic, size-dependent properties of [5]–[12]cycloparaphenylenes” (DOI: 10.1039/c5cs00143a), Evan R. Darzi and Ramesh Jasti present [n]cycloparaphenylenes (or “carbon nanohoops”) as cyclic fragments of carbon nanotubes that consist of n para-linked benzene rings, whose properties largely depend on their size. In this tutorial, the structural, optical, and electronic properties of the family [5]–[12]CPPs are discussed in a clear and systematic way that will be highly welcomed by the wide readership.
In their tutorial entitled “π,π-Interactions in carbon nanostructures” (DOI: 10.1039/c5cs00578g), Emilio M. Pérez and Nazario Martín reveal the essential role of π,π interactions as the dominating supramolecular forces in carbon nanostructures, which inherently constitute large conjugated π-systems. After a systematic introduction to the main carbon nanostructures and their supramolecular forces presented according to their presence in these nanoforms of carbon, the authors describe in more detail the π,π-interactions in the most important carbon nanostructures, namely fullerenes, carbon nanotubes and graphene. Interestingly, the skilful use of these π,π-interactions has allowed the construction of fascinating supramolecular ensembles, presented throughout the article, thus opening a new avenue in carbon chemistry.
References
- W. H. Brock, The Norton History of Chemistry, The Triumph of Structural Theory, 1992, pp. 257–269 Search PubMed.
- K. Hafner, August Kekulé – The Architect of Chemistry. Commemorating the 150th Anniversary of His Birth, Angew. Chem., Int. Ed. Engl., 1979, 18, 641–651 CrossRef PubMed.
- D. H. Wilcox Jr. and F. R. Greenbaum, Kekulé's Benzene Ring Theory, J. Chem. Educ., 1965, 42, 266–267 CrossRef.
- A. Kekulé, Sur la Constitution des Substances Aromatiques, Bull. Soc. Chim. Paris, 1865, 98–108 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |