Anion receptor chemistry
Philip A.
Gale
School of Chemistry, University of Southampton, Southampton, SO17 1BJ, UK. E-mail: philip.gale@soton.ac.uk; Fax: +44 (0)23 8059 6805; Tel: +44 (0)23 8059 3332
First published on 3rd June 2010
Abstract
This article looks back at advances made in anion complexation since the turn of the millennium and ahead to the application of receptors in areas such as organocatalysis and nanotechnology.
 Philip A. Gale | Phil Gale is Professor of Supramolecular Chemistry at the University of Southampton. A major focus of his research concerns the molecular recognition, sensing and lipid bilayer transport of anionic species. |
The field of anion receptor chemistry initiated in the late 1960s by Shriver and Biallas1 and by Park and Simmons2 was slow to develop in the subsequent two decades. However in the 1990s and particularly in the last ten years, inspired by the early work of Graf and Lehn3 and of Schmidtchen,4 the field has blossomed5 and has moved on from the development of simple neutral receptors which complex anions in organic solvents6 and protonated aza-crown ethers which bind anions in water.7 More recent advances include the production of neutral receptors capable of complexing anions selectively in aqueous solvent mixtures,8 compounds designed to bind and sense anions of biological interest in vivo,9 compounds that can detect ppm levels of pollutants in water,10 carriers and channels for transporting anions across cell membranes11 and extractants to complex particular anionic species and transfer them from aqueous to organic solution selectively against the anion's intrinsic Hofmeister bias12 or to selectively precipitate a particular salt from solution in the presence of a mixture of charged species in solution.13 Additionally progress has been made in the assembly both of anion receptors from metals and simple organic components,14 and the generation of interlocked species using anionic templates.15 The roles of OH16 and CH groups (in a variety of systems including 1,2,3-triazoles and imidazolium groups)17 as hydrogen bond donors to bind anions have begun to be explored in addition to complexation via anion⋯π interactions.18 In this article, particular milestones from the noughties will be highlighted.
The realisation that very simple organic compounds can function as selective and effective anion receptor systems has led to work on studying the ability of simple off-the-shelf compounds such as catechol,16 and of a wide range of commercially available compounds containing NH and OH hydrogen bond donor groups,19 as selective anion sensors. In this latter case, the sensing mechanism may be selective complexation or may be related to the basicity of the anionic guest triggering proton transfer and subsequent colour change. Of course this process is not limited to off-the-shelf compounds containing hydrogen bond donors, and receptors designed to complex anions have sometimes been found to instead undergo a deprotonation process upon addition of anions such as fluoride (driven by the production of the very stable HF2−).20 The realisation that anions may deprotonate neutral hosts containing NH or OH groups has recently been complemented by the finding that the pKa of protonated oxo-anions bound to receptors containing multiple hydrogen bond donor groups may be perturbed to the extent that the bound anion is deprotonated by the same anion free in solution, a finding21 which may go some way to explain the multiple equilibria sometimes observed upon complexation of protonated oxo-anions in organic solution. This finding offers the prospect of enhancing the reactivity of bound species using anion receptors—effectively using these compounds as organocatalysts.22
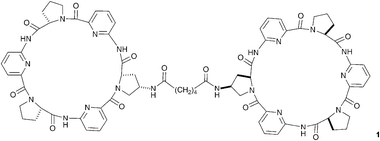
Effective design has allowed neutral hydrogen bond donor receptor species to bind anions in aqueous solution, so overcoming the enthalpic penalty that must be paid to dehydrate even the most strongly hydrated anionic guest prior to complexation. Some of the most elegant examples come from Kubik and co-workers who have developed a new class of anion receptor based on cyclic peptide subunits.8 Compounds containing two linked cyclic peptides such as compound 1, dubbed molecular oysters, are capable of encapsulating and complexing sulfate with high stability constants (e.g. 3.5 × 105 M−1 in 1 ∶ 1 water/methanol solution with compound 1). These receptors bind under such competitive solvent conditions by virtue of the hydrophobic interactions between the cyclic peptide rings contributing to the stability of the hydrogen bonded complex in solution.23 Dynamic combinatorial chemistry has been employed successfully to optimise the length and geometry of the linking group in these systems.24
In the charged receptor arena the guanidiniocarbonylpyrrole motif developed by Schmuck and co-workers has proven to be one of the most effective carboxylate receptors available, being employed in a variety of systems designed to recognise biomolecules containing carboxylate groups.25
Much effort over the last decade has gone into the design of sensors for the detection of anions. Ground breaking work in this area includes the development of displacement assays for anion recognition by Nguyen and Anslyn26 and on the uses of arrays of non-specific anion receptors combined with principal component analysis techniques to achieve recognition by Anslyn and co-workers27 and Anzenbacher and co-workers.28
Gunnlaugsson and co-workers have reported remarkable thiourea-based systems capable of colorimetric anion sensing in water.29
Recently, there has been somewhat of a renaissance in the development of anion selective chemodosimeters (compounds that react with anionic analytesvia covalent bond formation resulting in a change in colour or fluorescence). For example Martínez-Máñez, Sancenón and co-workers have employed 2,4,6-triarylthiopyrylium cations containing an amine substituent in the para-position as selective cyanide sensors (Scheme 1 and Fig. 1).30
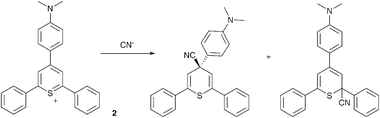 | ||
| Scheme 1 The proposed products obtained from the reaction of CN− with cation 2. | ||
 | ||
| Fig. 1 Colour changes observed in acetonitrile solutions of chemodosimeter 2 (1.0 × 10−4 mol dm−3) in the presence of 10 equiv. of the corresponding anions (except for cyanide; 1 equiv.). From left to right: no anion, Cl−, Br−, I−, NO3−, AcO−, H2PO4−, HSO4−, NCS− and CN−. Reproduced with permission from ref. 30. | ||
Another significant achievement in the last decade has been the development of systems for in vivo binding and sensing of anionic species. A recent example from Hamachi's group is shown in Scheme 2. In the absence of a polyphosphate guest, the conjugation of the xanthene ring in zinc complex 3 is broken by attack of zinc bound water.9 Complexation of ATP to the zinc atoms reverses this reaction so regenerating the fluorescent conjugated xanthene ring (Scheme 2). This complex has been used to image the intracellular stores of ATP in Jurkat cells. Other approaches include Parker's europium based luminescence assays for lactate and citrate which have potential future use in cancer screening biological fluid samples.31
 | ||
| Scheme 2 Turn-on fluorescence sensing of ATP by zinc complex 3 functions in vivo. | ||
Transmembrane transport of anions by carriers and channels is another area in which supramolecular chemistry can be applied in vivo. Driven by the need to find future treatments for diseases such as cystic fibrosis that are caused by mis-regulation of chloride transport through epithelial cell membranes, a range of discrete molecular carriers and channels have been developed for anion transport across lipid bilayers.11 Innovative approaches to the design of transmembrane anion channels have included Gokel's channel forming peptides that have been shown to alter chloride transport in epithelial cells32 and Matile's anion–π slides based on oligomeric naphthalenediimides.33
Smith and co-workers have attached urea groups to phosphatidylcholine to produce a transporter that functions via a relay mechanism with the functionalised lipid ‘handing’ an anionic guest onto a receiving functionalised lipid on the opposite membrane leaflet.34
Amongst the most efficient chloride transport systems developed so far are Davis's cholapods, cholic acid derivatives functionalised with hydrogen bond donor groups to bind anions.35 These systems have been shown to mediate anion transport across polarised epithelia.36 The latest systems reported, including compound 4, introduce a further degree of preorganisation into the binding site by introducing a link between hydrogen bond donor groups and have been named “cholaphanes”.37 These systems transport chloride at a faster rate than the previous generation of compounds which the authors believe may be due to a decreased propensity to complex lipid head groups and increased shielding from the external environment as compared to the previous generation cholapods.
Simpler receptors can also function as very effective chloride transporters, including compound 5 reported by Davis, Gale, Quesada and co-workers.38 This compound, preorganised by intramolecular OH⋯O hydrogen bonds, has been shown to facilitate the antiport of chloride and the more hydrophilic bicarbonate across POPC bilayers.39 Other approaches to chloride transport include employing proton gradients across membranes to drive transport40—a process that mimics the behaviour of the natural product prodigiosin.41
Anion receptors may play future roles in medicine. Here the exemplar is sapphyrin, an expanded porphyrin shown by Sessler to bind anions such as fluoride, chloride and dihydrogen phosphate.42 Functionalised sapphyrins have been shown to have anti-cancer activity which may be related to their affinity for the phosphate groups present in DNA.43
Significant advances have been made in the separation of anionic species, by both selective crystallisation and extraction processes. Sulfate is strongly solvated and a difficult challenge is to remove it selectively from aqueous solution. Custelcean and co-workers have reported a series of sulfate selective tris-urea compounds based upon the tren scaffold and found that the compounds bind sulfate in a 2 ∶ 1 receptor ∶ anion stoichiometry via 12 hydrogen bonds.44 Custelcean et al. have recently crystallised receptor 6 with a variety of MSO4 salts (M = Zn, Cd, Co and Mg) from 1 ∶ 1 H2O/MeOH solution. The authors found that, regardless of the nature of M, all the crystals formed are isostructural.45 This compound can be used to selectively crystallise sulfate from an aqueous solution containing a 100-fold excess of nitrate. Tasker, Schröder and co-workers have used similar tren based tris-ureas, tris sulfonamides and tris-amides as extractants for [PtCl6]2− from an aqueous acid chloride feed solution to an organic phase.46
Macrocyclic species have been employed by Moyer, Sessler, Bowman-James and co-workers, together with traditional quaternary ammonium extractants, in liquid–liquid extraction processes.12 Compounds 7–9 allow the selective extraction of sulfate by tricaprylmethylammonium nitrate to be achieved in the presence of excess nitrate (so overcoming Hofmeister bias). A dicationic expanded porphyrin, cyclo[8]pyrrole, proved even more effective for this purpose.47
By using metals as directing elements or templates it has proven possible to arrange simple organic components into three-dimensional convergent arrays of hydrogen bond donors producing receptors with high selectivity for particular anions.48 This is exemplified by receptor (Pt-104)2+, reported by Bondy, Gale and Loeb, formed by complexation of four urea-functionalised iso-quinoline ligands 10 with platinum, resulting in a complex with a high affinity for oxo-anions and in particular sulfate (Fig. 2).14
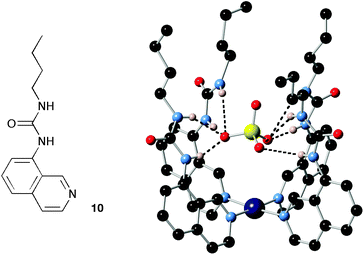 | ||
| Fig. 2 The structure of compound 10 and the X-ray crystal structure of the tetrakis Pt(II) complex of this compound binding sulfate. | ||
The use of anions themselves as templates for the formation of new molecular ensembles including interlocked species such as rotaxanes and catenanes has been a fruitful area of research. Early examples of the use of anions as templates for the formation of tetrameric mercuracarborands49 and pentameric circular helicates50 have provided inspiration in this area. Beer and co-workers have developed a series of interlocked catenanes (e.g.11) and rotaxanes templated around chloride51 (Fig. 3) and more recently sulfate anions.52
![A chloride-templated [2]-catenane 11.](/image/article/2011/CC/c0cc00656d/c0cc00656d-f3.gif) | ||
| Fig. 3 A chloride-templated [2]-catenane 11. | ||
The roles anions can play in controlling the conformational properties of acyclic molecules have also begun to be explored. Jeong and co-workers have employed alkynyl-linked biindole oligomers as anion templated foldamers.53Proton NMR studies with the compound 12 in CD3CN in the absence and presence of one equivalent of chloride showed significant upfield shifts of the aromatic protons in compounds upon addition of the anion. The authors attribute these changes to the formation of a helical complex with the acyclic receptor wrapping around the chloride anion (Scheme 3).
 | ||
| Scheme 3 Chloride induced helix formation by compound 12. | ||
Looking forward
There have been many advances over the last decade in anion receptor chemistry in moving from systems functioning in organic solvent to functioning in aqueous solution, in moving from discrete sensors to arrays, pattern recognition and highly specific chemodosimeters, in our understanding of hydrogen bonding vs.proton transfer in complexation processes and of the thermodynamics of anion complexation54 and in the development of transporters and channels that function in cells. The next ten years offer the prospect of similar leaps forward and can expect to see further applications of supramolecular systems in biology, the use of compounds designed as anion receptors employed as organocatalysts,55 exploitation of anions as conformational and functional controllers for complex systems and soft materials,56 in nanoscale systems for binding and sensing,57 in the development of systems for active transport of anions across membranes and the commercial development of the breakthroughs in anion sensing in the last decade. It's an exciting prospect.Notes and references
- D. F. Shriver and M. J. Biallas, J. Am. Chem. Soc., 1967, 89, 1078 CrossRef CAS.
- C. H. Park and H. E. Simmons, J. Am. Chem. Soc., 1968, 90, 2431 CrossRef CAS.
- E. Graf and J.-M. Lehn, J. Am. Chem. Soc., 1975, 97, 5022 CrossRef CAS; E. Graf and J.-M. Lehn, J. Am. Chem. Soc., 1976, 98, 6403 CrossRef CAS.
- F. P. Schmidtchen, Angew. Chem., Int. Ed. Engl., 1977, 16, 720 CrossRef; F. P. Schmidtchen, Chem. Ber., 1980, 113, 864 CAS; F. P. Schmidtchen, Chem. Ber., 1981, 114, 597 CrossRef CAS; F. P. Schmidtchen and G. Muller, J. Chem. Soc., Chem. Commun., 1984, 1115 RSC.
- J. L. Sessler, P. A. Gale and W.-S. Cho, Anion Receptor Chemistry, ed. J. F. Stoddart, RSC, Cambridge, 2006 Search PubMed; P. A. Gale, Chem. Soc. Rev., 2010 10.1039/c001871f.
- K. Kavallieratos, S. R. de Gala, D. J. Austin and R. H. Crabtree, J. Am. Chem. Soc., 1997, 119, 2325 CrossRef CAS.
- A. Bianchi, M. Micheloni and P. Paoletti, Inorg. Chim. Acta, 1988, 151, 269 CrossRef CAS.
- S. Kubik, R. Kirchner, D. Nolting and J. Seidel, J. Am. Chem. Soc., 2002, 124, 12752 CrossRef CAS.
- A. Ojida, I. Takashima, T. Kohira, H. Nonaka and I. Hamachi, J. Am. Chem. Soc., 2008, 130, 12095 CrossRef CAS.
- Y. Kim and F. P. Gabbaï, J. Am. Chem. Soc., 2009, 131, 3363 CrossRef CAS.
- A. P. Davis, D. N. Sheppard and B. D. Smith, Chem. Soc. Rev., 2007, 36, 348 RSC.
- C. J. Fowler, T. J. Haverlock, B. A. Moyer, J. A. Shriver, D. E. Gross, M. Marquez, J. L. Sessler, M. A. Hossain and K. Bowman-James, J. Am. Chem. Soc., 2008, 130, 14386 CrossRef CAS.
- R. Custelcean, Curr. Opin. Solid State Mater. Sci., 2009, 13, 68 CrossRef CAS.
- C. R. Bondy, P. A. Gale and S. J. Loeb, J. Am. Chem. Soc., 2004, 126, 5030 CrossRef CAS.
- M. R. Sambrook, P. D. Beer, J. A. Wisner, R. L. Paul and A. R. Cowley, J. Am. Chem. Soc., 2004, 126, 15364 CrossRef CAS.
- K. J. Winstanley, A. M. Sawyer and D. K. Smith, Org. Biomol. Chem., 2006, 4, 1760 RSC.
- Y. Li, M. Pink, J. A. Karty and A. H. Flood, J. Am. Chem. Soc., 2008, 130, 17293 CrossRef CAS; Z. Xu, S. K. Kim and J. Yoon, Chem. Soc. Rev., 2010, 39, 1457 RSC.
- B. P. Hay and V. S. Bryantsev, Chem. Commun., 2008, 2417 RSC.
- H. Miyaji and J. L. Sessler, Angew. Chem., Int. Ed., 2001, 40, 154 CrossRef CAS.
- S. Camiolo, P. A. Gale, M. B. Hursthouse, M. E. Light and A. J. Shi, Chem. Commun., 2002, 758 RSC; T. Gunnlaugsson, P. E. Kruger, P. Jensen, F. M. Pfeffer and G. M. Hussey, Tetrahedron Lett., 2003, 44, 8909 CrossRef CAS; D. Esteban-Gòmez, L. Fabbrizzi, M. Licchelli and E. Monzani, Org. Biomol. Chem., 2005, 3, 1495 RSC.
- P. A. Gale, J. R. Hiscock, S. J. Moore, C. Caltagirone, M. B. Hursthouse and M. E. Light, Chem.–Asian J., 2010, 5, 555 CrossRef CAS.
- Z. Zhang and P. R. Schreiner, Chem. Soc. Rev., 2009, 38, 1187 RSC; P. R. Schreiner, Chem. Soc. Rev., 2003, 32, 289 RSC . For a recent key example see: S. J. Zuend and E. N. Jacobsen, J. Am. Chem. Soc., 2009, 131, 15358 Search PubMed.
- S. Otto and S. Kubik, J. Am. Chem. Soc., 2003, 125, 7804 CrossRef CAS.
- Z. Rodriguez-Docampo, S. I. Pascu, S. Kubik and S. Otto, J. Am. Chem. Soc., 2006, 128, 11206 CrossRef CAS.
- C. Schmuck and P. Wich, Angew. Chem., Int. Ed., 2006, 45, 4277 CrossRef CAS; C. Schmuck, D. Rupprecht, M. Junkers and T. Schrader, Chem.–Eur. J., 2007, 13, 6864 CrossRef CAS.
- B. T. Nguyen and E. V. Anslyn, Coord. Chem. Rev., 2006, 250, 3118 CrossRef CAS.
- S. C. McCleskey, M. J. Griffin, S. E. Schneider, J. T. McDevitt and E. V. Anslyn, J. Am. Chem. Soc., 2003, 125, 1114 CrossRef CAS; A. T. Wright and E. V. Anslyn, Chem. Soc. Rev., 2006, 35, 14 RSC.
- M. A. Palacios, R. Nishiyabu, M. Marquez and P. Anzenbacher Jr., J. Am. Chem. Soc., 2007, 129, 7538 CAS.
- T. Gunnlaugsson, P. E. Kruger, P. Jensen, J. Tierney, H. D. P. Ali and G. M. Hussey, J. Org. Chem., 2005, 70, 10875 CrossRef CAS.
- T. Ábalos, S. Royo, R. Martínez-Máñez, F. Sancenon, J. Soto, A. M. Costero, S. Gil and M. Parra, New J. Chem., 2009, 33, 1641 RSC.
- R. Pal, D. Parker and L. C. Costello, Org. Biomol. Chem., 2009, 7, 1525 RSC.
- G. A. Cook, R. Pajewski, M. Aburi, P. E. Smith, O. Prakash, J. M. Tomich and G. W. Gokel, J. Am. Chem. Soc., 2006, 128, 1633 CrossRef CAS; R. Pajewski, R. Garic-Medina, S. L. Brody, W. M. Leevy, P. H. Schlesinger and G. W. Gokel, Chem. Commun., 2006, 329 RSC.
- V. Gorteau, G. Bollot, J. Mareda, A. Perez-Velasco and S. Matile, J. Am. Chem. Soc., 2006, 128, 14788 CrossRef CAS; J. Mareda and S. Matile, Chem.–Eur. J., 2009, 15, 28 CrossRef CAS.
- B. A. McNally, E. J. O'Neil, A. Nguyen and B. D. Smith, J. Am. Chem. Soc., 2008, 130, 17274 CrossRef CAS.
- B. A. McNally, A. V. Koulov, T. N. Lambert, B. D. Smith, J. B. Joos, A. L. Sisson, J. P. Clare, V. Sgarlata, L. W. Judd, G. Magro and A. P. Davis, Chem.–Eur. J., 2008, 14, 9599 CrossRef CAS.
- H. Y. Li, G. Magro, J. B. Joos, J. P. Clare, A. L. Sisson, L. W. Judd, P. Brotherhood, L. K. Hughes, D. N. Sheppard and A. P. Davis, J. Physiol. Sci., 2009, 59, 492 Search PubMed.
- L. W. Judd and A. P. Davis, Chem. Commun., 2010, 46, 2227 RSC.
- P. V. Santacroce, J. T. Davis, M. E. Light, P. A. Gale, J. C. Iglesias-Sánchez, P. Prados and R. Quesada, J. Am. Chem. Soc., 2007, 129, 1886 CrossRef.
- J. T. Davis, P. A. Gale, O. A. Okunola, P. Prados, J. C. Iglesias-Sanchez, T. Torroba and R. Quesada, Nat. Chem., 2009, 1, 138 Search PubMed.
- J. L. Sessler, L. R. Eller, W. S. Cho, S. Nicolaou, A. Aguilar, J. T. Lee, V. M. Lynch and D. J. Magda, Angew. Chem., Int. Ed., 2005, 44, 5989 CrossRef CAS; P. A. Gale, J. Garric, M. E. Light, B. A. McNally and B. D. Smith, Chem. Commun., 2007, 1736 RSC; P. A. Gale, M. E. Light, B. McNally, K. Navakhun, K. E. Sliwinski and B. D. Smith, Chem. Commun., 2005, 3773 RSC.
- S. Ohkuma, T. Sato, M. Okamoto, H. Matsuya, K. Arai, T. Kataoka, K. Nagai and H. H. Wasserman, Biochem. J., 1998, 334, 731 CAS.
- J. L. Sessler and J. M. Davis, Acc. Chem. Res., 2001, 34, 989 CrossRef CAS.
- Z. Wang, P. S. Lecane, P. Thiemann, Q. Fan, C. Cortez, X. Ma, D. Tonev, D. Miles, L. Naumovski, R. A. Miller, D. Magda, D.-G. Cho, J. L. Sessler, B. L. Pike, S. M. Yeligar, M. W. Karaman and J. G. Hacia, Mol. Cancer, 2007, 6, 9 CrossRef.
- R. Custelcean, B. A. Moyer and B. P. Hay, Chem. Commun., 2005, 5971 RSC.
- R. Custelcean, P. Remy, P. V. Bonnesen, D. Jiang and B. A. Moyer, Angew. Chem., Int. Ed., 2008, 47, 1866 CrossRef.
- K. J. Bell, A. N. Westra, R. J. Warr, J. Chartres, R. Ellis, C. C. Tong, A. J. Blake, P. A. Tasker and M. Schröder, Angew. Chem., Int. Ed., 2008, 47, 1745 CrossRef CAS; R. J. Warr, A. N. Westra, K. J. Bell, J. Chartres, R. Ellis, C. Tong, T. G. Simmance, A. Gadzhieva, A. J. Blake, P. A. Tasker and M. Schröder, Chem.–Eur. J., 2009, 15, 4836 CrossRef CAS.
- L. R. Eller, M. Stepien, C. J. Fowler, J. T. Lee, J. L. Sessler and B. A. Moyer, J. Am. Chem. Soc., 2007, 129, 14523 CrossRef CAS.
- D. R. Turner, E. C. Spencer, J. A. K. Howard, D. A. Tocher and J. W. Steed, Chem. Commun., 2004, 1352 RSC.
- T. J. Wedge and M. F. Hawthorne, Coord. Chem. Rev., 2003, 240, 111 CAS.
- B. Hasenknopf, J. M. Lehn, B. O. Kneisel, G. Baum and D. Fenske, Angew. Chem., Int. Ed. Engl., 1996, 35, 1838 CrossRef CAS.
- M. S. Vickers and P. D. Beer, Chem. Soc. Rev., 2007, 36, 211 RSC; M. R. Sambrook, P. D. Beer, J. A. Wisner, R. L. Paul and A. R. Cowley, J. Am. Chem. Soc., 2004, 126, 15364 CrossRef CAS.
- K. M. Mullen and P. D. Beer, Chem. Soc. Rev., 2009, 38, 1701 RSC.
- K.-J. Chang, B.-N. Kang, M.-H. Lee and K.-S. Jeong, J. Am. Chem. Soc., 2005, 127, 12214 CrossRef CAS.
- F. P. Schmidtchen, Coord. Chem. Rev., 2006, 250, 2918 CrossRef CAS.
- S. J. Zuend and E. N. Jacobsen, J. Am. Chem. Soc., 2009, 131, 15358 CrossRef CAS.
- G. O. Lloyd and J. W. Steed, Nat. Chem., 2009, 1, 437 Search PubMed.
- J. Massue, S. J. Quinn and T. Gunnlaugsson, J. Am. Chem. Soc., 2008, 130, 6900 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |