Mesostructured material based on [Re6Te8(CN)6]4−cluster and Mn2+: a rational synthesis of hexagonal nonoxidic mesoscale material†
Vo
Vien
a,
Min-Jung
Suh
a,
Seong
Huh
ab,
Youngmee
Kim
a and
Sung-Jin
Kim
*a
aDepartment of Chemistry and Nano Science, Ewha Womans University, Seoul, 120-750, Korea. E-mail: sjkim@ewha.ac.kr; Fax: +82 2 32773419; Tel: +82 2 3277 2350
bDepartment of Chemistry and Protein Research Center for Bio-Industry, Hankuk University of Foreign Studies, Yongin, 449-791, Korea
First published on 5th December 2008
Abstract
A new nonoxidic mesostructured material with highly ordered hexagonal symmetry, [C16H33N(CH3)3]3Mn0.5[Re6Te8(CN)6], has been obtained through supramolecular assembly of [Re6Te8(CN)6]4−clusters in the presence of a transition metal cation, Mn2+, with cetyltrimethylammonium bromide as a liquid crystal template under hydrothermal conditions.
In 1992, Mobil researchers used long-chain organic liquid crystal templates to assemble inorganic silicate structures. Since then this method has become a powerful tool in the synthesis of ordered nanoporous solids.1 This approach is used to prepare a wide range of mesoporous oxides2 and phosphates.3 The preparation of materials with nanoscale features is of great importance because of their potential applications to, for example, nanoscale electronic and magnetic devices.
Recently, the surfactant templating route has been extended to nonoxidic mesoporous materials based on tetrahedral metal chalcogenide ions, such as [MQ4]4−, [M2Q6]4− and [M4Q10]4− (M = Ge, Sn; Q = S, Se, Te), and various linking transition metal ions.4 Having low band gaps, a salient semiconducting property,4d these new materials are very attractive.
The basic chemistry related to these frameworks differs slightly from that used in the silicate system. For example, these nonoxidic systems form predominantly through a controlled electrostatic assembly mechanism in formamide instead of water or alcohol. The authors emphasize the need for a nonaqueous solvent, such as formamide, which plays an important role in the synthesis of highly ordered phases.4 The chalcogenide building units are linked together through the bridging of metal ions to cover the surface of the charged template assembly, which is used as the structure-directing agent. In general, because the template assembly processes occur immediately and the assembled species are stabilized over a very narrow range of experimental conditions, controlling the overall symmetry and organization of the template in these materials is difficult.4c Although there have been significant advances in the design and synthesis of the oxidic or nonoxidic mesoporous materials with tetrahedral [MQ4]4− building units, synthesis of ordered mesostructured materials with other nonoxidic metal clusters remains a significant challenge.
The chemistry of hexarhenium chalcogenide clusters, [Re6Q8]2+/3+ (Q = S, Se, Te), has developed rapidly, producing an increasing number of structurally well-defined solid architectures.5 Some of them with [Re6Q8]2+/3+ (Q = S, Se, Te) cores can allow ligand-exchange reactions occurring at each ReIII apex under normal conditions to form [Re6Q8(CN)6]3−/4−.5 By careful control of reaction conditions, such octahedral cluster anions with various metal cations or organic ligands could yield expanded porous frameworks with interesting electronic and luminescence properties. Therefore, such hexarhenium chalcogenide clusters have been attractive building blocks for the design of numerous materials having interesting structures and useful functionalities.5c However, to our knowledge, the use of these octahedral clusters with the aid of mesoscale templates to assemble ordered mesoscale materials is rare.6 Here we present a rational synthesis of a new mesostructured metal chalcogenide material prepared by a surfactant-templated assembly of [Re6Te8(CN)6]4− and Mn2+ under hydrothermal conditions.7
The title compound, [C16H33N(CH3)3]3Mn0.5[Re6Te8(CN)6], was obtained from the hydrothermal reaction between the aqueous solutions of MnCl2·4H2O (0.024 g, 0.123 mmol), cetyltrimethylammonium bromide, or CTAB, (0.15 g, 0.42 mmol) and [(CH3)4N]4[Re6Te8(CN)6]† (0.3 g, 0.123 mmol). The total volume of the solution was 15 mL. A yellow brown solid was formed within a few seconds after mixing the three solutions. The mixture was further stirred for 2 h and divided into three equal portions. The first portion was immediately filtered to retrieve the solid (L–Re/Te/Mn). It was washed with warm water and dried at 70 °C. The second filtrate (HL–Re/Te/Mn) and the rest (H–Re/Te/Mn) were transferred to two separate Teflon-lined high pressure bombs, placed in an oven and heated to the respective temperatures of 75 °C and 120 °C for 48 h. The solid products were isolated by filtration, washed with warm water and dried at 70 °C. The yield for the synthesis of H–Re/Te/Mn was about 70% based on the cluster [(CH3)4N]4[Re6Te8(CN)6].
The powder X-ray diffraction, or XRD, patterns are shown in Fig. 1(a). The four peaks at low angles with 2θ = 2.52°, 4.38°, 5.04°, and 6.70° of the 120 °C H–Re/Te/Mn (red, top) can be indexed to (100), (110), (200), and (210) reflections, indicative of a two-dimensional hexagonal space group with p6mm symmetry. The hexagonal lattice parameter, calculated from the powder XRD pattern, is a = 40.5 Å. These peaks are well resolved and the intense peaks indicate a long-range ordered structure, characteristic of template-based mesostructuring. In addition, extra peaks appear at higher angles, which can be attributed to the degree of molecular scale ordering of [Re6Te8(CN)6]4− cores in the wall. To the best of our knowledge, this is the first example showing a hexagonally ordered mesostructure formed by hexarhenium chalcogenide clusters in aqueous solution.
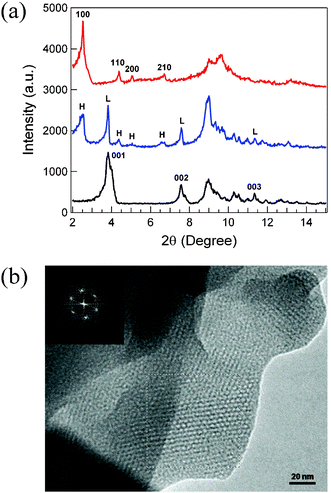 | ||
| Fig. 1 (a) Powder XRD patterns of samples prepared at room temperature (L–Re/Te/Mn in black), at 75 °C (HL–Re/Te/Mn in blue); and at 120 °C (H–Re/Te/Mn in red). For the HL–Re/Te/Mn, H- denotes hexagonal phase and L- denotes lamellar phase. (b) TEM image of H–Re/Te/Mn. The inset shows the Fourier-transformed diffraction pattern of the boxed area. | ||
The intermediate phases (75 °C HL–Re/Te/Mn) were also isolated and their powder XRD patterns are shown in Fig. 1(a) (blue). Clearly, the phase obtained at room temperature (black) is lamellar, and the 75 °C HL–Re/Te/Mn (blue) is a mixed phase of hexagonal and lamellar phases. For 120 °C HL–Re/Te/Mn (red), only a hexagonal phase is obtained. This implies that there is a phase transition from lamellar to hexagonal between 25 and 120 °C under our synthetic conditions. Therefore, a hydrothermal condition in aqueous solution at 120 °C is required to obtain a phase-pure hexagonal mesoscale material.
The pore size and periodicity in the mesostructure of H–Re/Te/Mn were confirmed directly by high-resolution transmission electron microscopy, or HR-TEM. The HR-TEM image for H–Re/Te/Mn is a highly ordered hexagonal phase Fig. 1(b). Both the perpendicular and parallel channels relative to the longitudinal axis are observed. The approximate interpore spacing of ∼40 Å is close to the value calculated from the XRD pattern, or aH∼40.5 Å. The pore size estimated from the HR-TEM image is approximately 27 Å. The wall thickness was also estimated from the HR-TEM image to be about 13 Å, approximately the dimension of the [Re6Te8(CN)6]4−cluster anion revealed by single crystal XRD. Therefore, the wall is consisted of a single layer of the [Re6Te8(CN)6]4−cluster anions.
H–Re/Te/Mn was analyzed using inductively coupled plasma-atomic emission spectrometry (ICP-AES) to investigate its chemical composition. The result showed that the Mn : Re : Te molar ratio was 0.51 : 6.00 : 7.87. The relative Re : Te ratio agreed well with the value expected in [Re6Te8(CN)6]4−clusters. This shows that the cluster core [Re6Te8]2+ remains intact in the product. This conclusion is further supported by the fact that the [Re6Q8]2+ (Q = S, Se, Te) cores are structurally robust under most solution-phase reaction conditions.5 To probe the bonding features of the framework, [(CH3)4N]4[Re6Te8(CN)6], CTAB and H–Re/Te/Mn were also characterized by infrared spectroscopy, or IR, and the results are shown in Fig. 2.
![IR spectrum of compound H–Re/Te/Mn (a), crystalline CTAB (b), [(CH3)4N]4[Re6Te8(CN)6] (c).](/image/article/2009/CC/b816000g/b816000g-f2.gif) | ||
| Fig. 2 IR spectrum of compound H–Re/Te/Mn (a), crystalline CTAB (b), [(CH3)4N]4[Re6Te8(CN)6] (c). | ||
Curve (c) in Fig. 2 shows a sharp band at 2085 cm−1, corresponding to the stretching vibration mode of the cyanide group in the cluster complexes.8 Most of the bands from CTA+ [Fig. 2(b)] appear in the spectrum for H–Re/Te/Mn [Fig. 2(a)]. In addition, the ratio of peak intensities of C–H bonds to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonds in H–Re/Te/Mn is higher than that found in [(CH3)4N]4[Re6Te8(CN)6]. From this we inferred that more C–H bonds originating from surfactants are in H–Re/Te/Mn. This confirms the presence of CTA+ in the product. Worth noting is that the characteristic band of cyanide at 2092 cm−1 in the spectrum for H–Re/Te/Mn indicates that the cyanide group is intact in the material. Compared with the pristine cluster anion, the material exhibits a vibration mode of cyanide that shifts to a higher frequency by 7 cm−1. However, it is only slightly different from the value, 2090 cm−1, observed for the previous layered material [C16H33N(CH3)3]4[Re6Te8(CN)6].6 Nevertheless, this shift may be attributed to the coordination of the cyanide ligands to the Mn2+ ions in the material. An intense peak in the region of 3200–3500 cm−1 is characteristic of O–H stretching, which may originate from the absorbed water.
N bonds in H–Re/Te/Mn is higher than that found in [(CH3)4N]4[Re6Te8(CN)6]. From this we inferred that more C–H bonds originating from surfactants are in H–Re/Te/Mn. This confirms the presence of CTA+ in the product. Worth noting is that the characteristic band of cyanide at 2092 cm−1 in the spectrum for H–Re/Te/Mn indicates that the cyanide group is intact in the material. Compared with the pristine cluster anion, the material exhibits a vibration mode of cyanide that shifts to a higher frequency by 7 cm−1. However, it is only slightly different from the value, 2090 cm−1, observed for the previous layered material [C16H33N(CH3)3]4[Re6Te8(CN)6].6 Nevertheless, this shift may be attributed to the coordination of the cyanide ligands to the Mn2+ ions in the material. An intense peak in the region of 3200–3500 cm−1 is characteristic of O–H stretching, which may originate from the absorbed water.
The thermal behavior of H–Re/Te/Mn was studied using thermogravimetric analysis, or TGA. Fig. 3(a) shows a small weight loss from room temperature to 200 °C. The most weight loss occurs in the region between 225 and 500 °C with a maximum weight loss rate at about 275 °C. The loss (∼26%) corresponds to the weight loss of template CTA+ and the cyanide group in the material. The color of the residue after TGA was black. The powder XRD pattern of the residue does not show the hexagonal symmetry, indicating a collapse of the original mesostructure. A combination of IR, ICP-AES, EDX analysis (see ESI Fig. S1†), elemental analysis and TGA suggests that the chemical formula of H–Re/Te/Mn is Mn0.5(CTA)3[Re6Te8(CN)6].9 Based on the previously reported crystal structure of the rhenium cluster–Mn complex, [Mn(DMF)3]2[Re6Se8(CN)6],10 we can suggest a binding feature for Mn0.5(CTA)3[Re6Te8(CN)6]. Each Mn2+ ion is coordinated to three cyano ligands of rhenium clusters, forming Mn[Re6Te8(CN)6]310− and further condensed to form Mn3[Re6Te8(CN)6]618−. These clusters lie on the wall of cylindrical rod micelles because of the electrostatic interactions between the cationic surfactant heads and anionic metal clusters such as (CTA)6Mn[Re6Te8(CN)6]2, compensating for the charges.
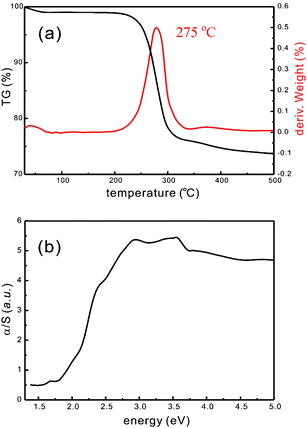 | ||
| Fig. 3 (a) Thermogravimetric and differential thermogravimetric curves of H–Re/Te/Mn under nitrogen flow (heating rate 10 °C min−1). (b) Solid state UV/Vis spectrum of H–Re/Te/Mn. Absorption data were calculated from the reflectance data using the Kubelka–Munk function; α/S = (1 −R)2/2R, where R is the reflectance at a given wavenumber, α is the absorption coefficient, and S is the scattering coefficient. The band gap energy is 1.92 eV. | ||
The absorption property of the solid was also investigated with reflectance solid-state ultraviolet–visible spectroscopy. In general, chalcogenide materials have narrower energy band gaps than oxides.11 The optically observed band gap of H–Re/Te/Mn is 1.92 eV (Fig. 3(b)).
We have obtained a new hybrid mesostructured material by employing the hydrothermal method using octahedral [Re6Te8(CN)6]4−clusters in the presence of a transition metal and surfactant template. The hydrothermal method with the aid of the liquid crystal template provides an efficient route for the highly ordered mesostructured phases. This new type of material has a narrow energy band gap, making it potentially suitable for application in electrical and photo electronic devices.
This research was supported by the SRC program of the Korea Science and Engineering Foundation (KOSEF) through the Center for Intelligent Nano-Bio Materials (grant R11-2005-008-00000-0) and by the Korea Research Foundation Grant (KRF-2004-005-C00093).
Notes and references
- (a) C. T. Kresge, M. Leonowicz, W. J. Roth, J. C. Vartuli and J. C. Beck, Nature, 1992, 359, 710 CrossRef CAS; (b) J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS.
- (a) D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS; (b) S. Inagaki, S. Guan, T. Ohsuna and O. Terasaki, Nature, 2002, 416, 304 CrossRef CAS; (c) T. Asefa, M. J. MacLachlan, N. Coombs and G. A. Ozin, Nature, 1999, 402, 867 CAS; (d) P. T. Tanev and T. J. Pinnavaia, Science, 1995, 267, 865 CrossRef CAS.
- B. Tian, X. Liu, B. Tu, C. Yu, J. Fan, L. Wang, S. Xie, G. D. Stucky and D. Zhao, Nat. Mater., 2003, 2, 159 CrossRef CAS.
- (a) M. J. MacLachlan, N. Coombs and G. A. Ozin, Nature, 1999, 397, 681 CrossRef CAS; (b) P. N. Trikalitis, K. K. Rangan, T. Bakas and M. G. Kanatzidis, Nature, 2001, 410, 671 CrossRef CAS; (c) A. E. Riley and S. H. Tolbert, J. Am. Chem. Soc., 2003, 125, 4551 CrossRef CAS; (d) P. N. Trikalitis, T. Bakas and M. G. Kanatzidis, J. Am. Chem. Soc., 2005, 127, 3910 CrossRef CAS; (e) S. D. Korlann, A. E. Riley, B. L. Kirsch, B. S. Mun and S. H. Tolbert, J. Am. Chem. Soc., 2005, 127, 12516 CrossRef; (f) M. G. Kanatzidis, Adv. Mater. (Weinheim, Ger.), 2007, 19, 1165 CrossRef CAS.
- (a) J. C. P. Gabriel, K. Boubekeur, S. Uriel and P. Batail, Chem. Rev., 2001, 101, 2037 CrossRef CAS; (b) H. D. Selby, B. K. Roland and Z. Zheng, Acc. Chem. Res., 2003, 36, 933 CrossRef CAS; (c) H. D. Selby and Z. Zheng, Comments Inorg. Chem., 2005, 26, 75 CrossRef CAS; (d) Y. V. Mironov, N. G. Naumov, S. G. Kozlova, S.-J. Kim and V. E. Fedorov, Angew. Chem., Int. Ed., 2005, 44, 6867 CrossRef CAS.
- M.-J. Suh, V. Vien, S. Huh, Y. Kim and S.-J. Kim, Eur. J. Inorg. Chem., 2008, 686 CrossRef CAS.
- Y. V. Mironov, J. A. Cody, T. E. Albrecht-Schmitt and J. A. Ibers, J. Am. Chem. Soc., 1997, 119, 493 CrossRef CAS.
- T. G. Gray, C. M. Rudzinski, E. E. Meyer, R. H. Holm and D. G. Nocera, J. Am. Chem. Soc., 2003, 125, 4755 CrossRef CAS.
- Our formulation, Mn0.5(CTA)3[Re6Te8(CN)6], was based on the following elemental analysis and EDX results. Anal. Calcd. for C63H126Mn0.5N9Re6Te8: C 23.81, H 4.00, N 3.97%; Found: C 22.68, H 4.18, N 3.82%. EDX indicated the following atomic ratio: Mn : Re : Te = 0.5 : 6.00 : 8.20.
- S. Kim, Y. Kim, Y. Kal and S.-J. Kim, Inorg. Chim. Acta, 2007, 360, 1870 CrossRef CAS.
- P. N. Trikalitis, K. K. Rangan, T. Bakas and M. G. Kanatzidis, Nature, 2001, 410, 671 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and EDX spectrum of the H-Re/Te/Mn. See DOI: 10.1039/b816000g |
| This journal is © The Royal Society of Chemistry 2009 |
