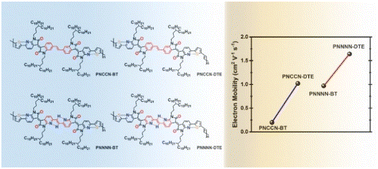
The synthesis and characterization of four ethylene-bridged bisisoindigo (NCCN and NNNN)-based conjugated polymers are reported. In these polymers, 2,2′-bithiophene or (E)-1,2-di(thiophen-2-yl)ethene (DTE) is used as the electron donor. Compared to NCCN, NNNN has two additional sp2-nitrogen atoms adjacent to its vinyl group. The sp2-nitrogen atoms endow the two NNNN-based polymers PNNNN-BT and PNNNN-DTE with not only improved backbone planarity due to the formation of intramolecular five-ring intramolecular CH⋯N hydrogen bonds, but also slightly lowered frontier orbital energy levels. Combined with the more rigid backbone of DTE, PNNNN-DTE showed the highest electron mobility (μe) of 1.64 cm2 V−1 s−1 in 1,2-dichlorobenzene (DCB)-processed ambipolar field-effect transistors and even a slightly increased μe in its DCB/1-chloronaphthalene (with v/v of 99.2/0.8) bi-component solvent-processed ones. Microstructural analyses indicated that the PNNNN-DTE thin films have more ordered and denser molecular packing, which is well in accordance with the change tendency of the electron mobility of these bisisoindigo-based conjugated polymers.