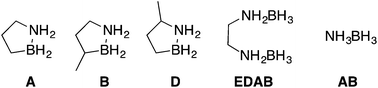
Mixtures of hydrogen storage materials containing the elements of boron, nitrogen, carbon, i.e., isomers of BN cyclopentanes are examined to find a ‘fuel blend’ that remains a liquid phase throughout hydrogen release, maximizes hydrogen storage density, minimizes impurities and remains thermally stable at ambient temperatures. We find that the mixture of ammonia borane dissolved in 3-methyl-1,2-dihydro-1,2-azaborolidine (compound B) provide a balance of these properties and provides ca. 5.6 wt% hydrogen. The two hydrogen storage materials decompose at a faster rate than either individually and products formed are a mixture of molecular trimers. Digestion of the product mixture formed from the decomposition of the AB + B fuel blend with methanol leads to the two corresponding methanol adducts of the starting material and not a complex mixture of adducts. The work shows the utility of using blends of materials to reduce volatile impurities and preserve liquid phase.