Plant enrichment effects of quantum dots in agroecosystems: a double-edged sword
Hao
Cheng
 a,
Yanxing
Xu
a,
Abdelrahman
Ibrahim
a,
Yanzheng
Gao
a,
Yanxing
Xu
a,
Abdelrahman
Ibrahim
a,
Yanzheng
Gao
 a,
Hefei
Wang
a,
Ahmed
Mosa
b and
Wanting
Ling
*a
a,
Hefei
Wang
a,
Ahmed
Mosa
b and
Wanting
Ling
*a
aInstitute of Organic Contaminant Control and Soil Remediation, College of Resources and Environmental Sciences, Nanjing Agricultural University, Weigang Road 1, Nanjing 210095, P.R. China. E-mail: lingwanting@njau.edu.cn
bSoils Department, Faculty of Agriculture, Mansoura University, Mansoura 35516, Egypt
First published on 24th October 2025
Abstract
Quantum dots (QDs) are increasingly used in diverse fields, and thus they inevitably spread unintentionally into the farmland ecosystem through (i) environmental release and (ii) intentional application in fertilizers, pesticides or growth promoters in agriculture. Several studies have shown that QDs can enter plants through leaf and root absorption and translocate throughout the plant, potentially affecting plant growth and development. At appropriate concentrations, QDs have been found to stimulate plant growth, enhance nutritional quality, improve resilience to abiotic stressors, and facilitate disease management. However, inappropriate concentrations of QDs, particularly those containing heavy metals or functional moieties such as hydroxyl and amino groups, may exert adverse effects including oxidative stress, cellular damage, growth retardation, and genetic toxicity. This review synthesizes the enrichment effect of QDs on plants in the farmland ecosystem from aspects such as the absorption pathway, transport mechanism, and its impact on plant growth, photosynthesis, stress resistance and yield. Accordingly, we propose that future research should be based on this “double-edged effect” to develop agricultural applications of QDs. Focus should be on elucidating the specific uptake and transport mechanisms of different types of QDs in different plant species, refining the preparation methods and application technologies of QDs, and rigorously assessing their ecological risks, to provide a sound scientific basis for the safe and effective use of QDs in agroecosystems aligned with determining their full agricultural potential.
Environmental significanceThe increase in production and application has resulted in a significant influx of quantum dots (QDs) into farmland ecosystems, potentially influencing plant growth and development. Crops are capable of absorbing these nanoparticles via leaves and roots and subsequently transporting them throughout their systems. At appropriate concentrations, QDs can stimulate crop growth, enhance nutritional quality, strengthen resistance to abiotic stress, and facilitate disease management. The strategic development and rational utilization of this beneficial effect could reduce reliance on agricultural chemicals, thereby minimizing traditional agricultural pollution. This approach offers precise and environmentally friendly solutions for plant disease management, addressing the challenges of food security and environmental sustainability in the context of climate change. |
1 Introduction
Quantum dots (QDs) are semiconductor nanocrystals composed of elements from groups II–IV or III–V in the periodic table, with typical diameters ranging from 2 to 20 nanometers.1 Based on composition, QDs can be broadly classified into metal-based QDs, carbon-based QDs, and perovskite QDs, each exhibiting distinct physicochemical and environmental behaviors.2 Key physicochemical properties, such as particle size, crystal structure, surface functionalization and composition, determine their characteristics like emission wavelength, quantum yield and biocompatibility, all of which are crucial to their behavior in the ecosystems.3 For instance, surface functionalization with groups such as carboxyl, amino, or hydroxyl can enhance water dispersibility and reduce potential toxicity. Furthermore, QDs demonstrate strong size-tunable optical properties, characterized by narrow emission peaks and high quantum yields, which make them widely used in bioimaging, sensing, medicine and agriculture.4–6 As the utilization of QDs has increased, global production reached 55 tons per year in 2012,7 and projections indicate a projected annual growth rate of 200% over the next few decades.8 However, the increased production of QDs unavoidably results in their prevalence in the environment due to the nature of the manufacturing process and the potential improper disposal.1 A probabilistic analysis of material flow in electronic devices suggests that the majority of the QDs employed in these devices may be released into their surrounding environments during recycling. In contrast, about 20% of the QDs used in medical devices may eventually accumulate in soil at levels ranging from ng kg−1 to μg kg−1.9 Furthermore, based on the effectiveness of QDs in promoting plant growth and improving stress resistance, the intentional application of QD-based fertilizers, pesticides, or growth promoters inevitably results in the introduction of QDs into the farmland ecosystem. Therefore, soil serves as a potential destination for engineered nanomaterials (ENMs),10 and, likely, QDs will eventually accumulate either in soil or even in agricultural fields.Plants are a vital component of terrestrial ecosystems11 and significantly influence the environmental fate of various QDs. Kong et al. highlighted the crucial pathways for QDs and their ubiquitous transfer in the environment represented in plant uptake and bioaccumulation of QDs.12 QDs could enter whole plants and seedlings through multiple plant sites (including plant leaves and roots) and reach each other through plant uptake and translocation.13 Concurrently, QDs exhibiting distinct surface attributes can modulate many plant-associated pathways such as physiological, biochemical, and physicochemical processes.14 This, in turn, could detrimentally influence plant growth, products, and metabolism. Consequently, when QDs enter farmland, a plant enrichment effect inevitably ensues, impacting the morphology and physiology of the plant,15 and may impact crop growth, yield, and the resistance to pests and diseases.
Thus, we review the environmental content and environmental fate of QDs and discuss the uptake and translocation of QDs by plants, considering both root-to-leaf and leaf-to-root perspectives. Subsequently, we examined the documented effects of QDs on plants in recent years, illustrating both beneficial and detrimental outcomes. Finally, a proposal was put forth regarding the rational utilization of the beneficial effects of QDs to regulate the growth of crops in agroecosystems, thereby mitigating any associated environmental risks.
2 Environmental content and environmental fate of QDs
2.1 Environmental content of QDs
According to Gallagher et al., the potential for environmental release exists throughout the entire life cycle of QD or QD-enabled products, such as encompasses synthesis, manufacturing, application, and end-of-life stages.16 The environmental concentration of QDs was expected to increase as the range of applications for QDs expands and the lifespan of specific QD species and devices using QDs lengthens.17 Nevertheless, owing to limitations in analytical capabilities, there have been no reports of experimentally derived environmental concentrations of nanoparticles.18 However, model-based estimates and predictions have been published in the relevant literature. For example, Gottschalk et al. employed a modeling approach to predict concentrations of QDs between various samples of water as follows: 0.2 and 45 μg kg−1 in freshwater sediments, between 0.04 and 2 μg kg−1 in seawater sediments, and 0.0001–0.013 μg kg−1 QDs in sludge-treated soils, in Denmark, for the years 2000–2014.19 Based on accumulation of data from 1990 to 2014, Wang et al. projected that the range of QD concentrations in surface waters across seven European regions may fall within the range of 9.6–530 fg L−1.9 On a global scale, the aggregate QD input to wastewater treatment facilities was estimated to range from 0.0057 to 0.456 metric tons per annum.Furthermore, given the indiscriminate disposal of electronic products containing QDs, soil seems to be the most significant environmental sink for these particles.20 Indeed, global emissions of QDs into the soil have been estimated to range between 6.1 and 30.9 tonnes per year as of 2021.8 However, the amount of QDs discharged from wastewater and deposited in sludge is considerably greater, with global emissions estimated to reach 55.29 tons per year by 2021.8 The disposal of QD-containing devices in landfill sites and their incineration have resulted in the expansion of landfills into a significant repository for QDs. During the combustion process, cadmium selenide quantum dots (CdSe-QDs) are released in the form of particles, and a proportion of the QDs are retained in the bottom ash or residue produced following incineration.21 More recently, Chopra et al. have demonstrated that products containing QDs, when discarded in landfills, result in the release of approximately 0.077 μg L−1 QDs into the surrounding environment.20 Moreover, the model developed by Wang et al. indicated that compared with natural soil (0.003–0.027 ng kg−1), sewage-treated soils presented considerably elevated concentrations of QDs (0–17 ng kg−1).9 Therefore, these findings suggest that QDs may be introduced into terrestrial ecosystems through treated sewage and sludge. However, the environmental concentrations of these expected QDs are orders of magnitude lower than those of most other commonly used nanoparticles (e.g., carbon nanotubes, TiO2, ZnO, and Ag) due to the yield of QDs relative to other ENMs, lower solubility in the natural environment, and lower minimized environmental exposures designed into the use of QD products.22,23 Consequently, it is noteworthy that the present concentration of QDs in the environment may exceed the predicted concentration.
2.2 Environmental fate of QDs
QDs that are released into or remain in the surrounding environment may be ingested by organisms and undergo dissolution in their bodies.24 For instance, some QDs that enter freshwater environments could enter algal plants through macropinocytosis and be solubilized due to redox reactions, electrostatic attraction, or hydrogen bonding between QDs and biomolecules.25 Furthermore, QDs present in the soil may be absorbed by root-bearing plants during nutrient uptake or water intake, subsequently accumulating in their bodies. For example, Whiteside et al. reported that after introducing QDs into fungus-inoculated bluegrass, the QDs were subsequently transported into the plant root tissues through the organic nitrogen uptake pathway facilitated by the plant root system.26 For their part, Al-Salim et al. found that soil water-soluble QDs (cadmium selenide/zinc sulfide core–shell quantum dots (CdSe/ZnS-QDs)) could be taken up by onions, ryegrass, and chrysanthemums along with limited transport observed in the vascular system of their cut stems.27Concurrently, QDs with particular coatings may interact with compounds produced within plant tissues, such as proteins, amino acids, organic acids, sugars, and phenols, thus increasing aggregation. For instance, CdSe/Cd/ZnS-QDs with anionic coatings exhibit a relatively stable and uniform distribution in Arabidopsis leaves after uptake by Arabidopsis leaf axes and roots, whereas cationic and relatively neutral-coated QDs accumulate in Arabidopsis tissues.28 Sun et al., in contrast, discovered that negatively charged polyethylene glycol-carboxyl-functionalized CdS/ZnS quantum dots (PEG-COOH-CdS/ZnS-QDs) as well as QDs functionalized with a neutral coating were able to undergo significant aggregation and form aggregates on the surfaces of primary, lateral, and fibrous roots of maize seedlings.4
Moreover, researchers found that after caterpillars were fed Arabidopsis leaves containing QDs, fluorescence from the QDs was detected in both the bodies and the debris of the caterpillars.28 Similarly, Al-Salim et al. detected the fluorescence of QDs in the intestines and hemolymph of four different Lepidoptera after feeding on QD-containing plants for 2–4 days.27 Lee et al. also observed the tertiary trophic transfer of QDs from protozoa to zooplankton to fish using bioimaging.29 These results suggest that consumers have ingested QDs from water, plants, or animals through the food chain, which may be another home for QDs in the environment.
Additionally, the QDs that enter the environment may also be degraded by microorganisms or enzymes. For example, functionalized QDs may be degraded by fungal processes, specifically via the Fenton reaction.30 In addition to fungi, researchers have shown that the human myeloperoxidase enzyme, expressed by neutrophils and eosinophils, could also degrade graphene quantum dots (GQDs).31 At the same time, carbon quantum dots (CQDs) could also be catalytically degraded in the presence of lipases.32 Nevertheless, few in-depth studies have evaluated the biodegradability of QDs within living organisms.
3 Uptake, translocation and biotransformation of QDs by plants
The specialized structures of terrestrial plants (such as stomata in leaves, pores, and fissures in roots) permit the exchange of gases and the uptake of nutrients from the external environment, thereby enabling plant growth and development33 and providing potential avenues for the entry of QDs into land plants. Terrestrial plants are in direct contact with soil, water, and even the atmosphere, which represent the primary environmental components of agroecosystem QDs. Consequently, terrestrial plants may be exposed to QDs from many sources. Airborne QDs could be deposited on the surface of plant leaves or other air-contacted plant parts, where they may accumulate and enter the plant tissue through stomatal channels.6,10 In contrast, QDs adsorbed on soil and sediment can penetrate root epidermal cell walls and cell membranes, enter the plant's vascular bundles (xylem) through a complex series of processes, and move toward the columnar structure through sympathetic transport and then into the leaf, as shown in (Fig. 1).34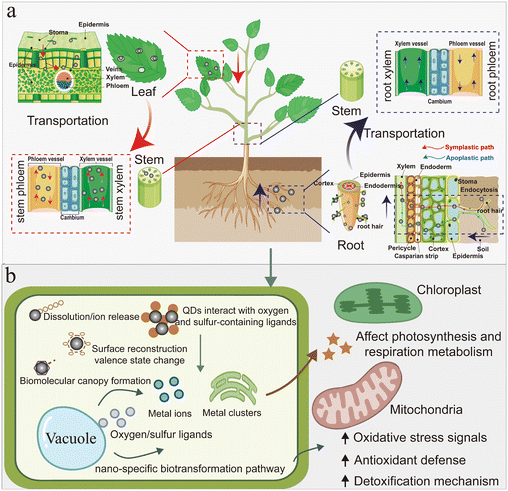 | ||
| Fig. 1 QDs penetration in plant tissues and biotransformation pathways in plant cells. (a) Penetration pathway of QDs via plant tissue. (b) Biotransformation pathways of QDs in plant cells. | ||
3.1 Root uptake and root-to-leaf translocation
The epidermal cells that constitute the root cell wall exhibit semipermeability, as evidenced by the presence of minute pores that impede the passage of nanoparticles with a diameter greater than a certain threshold. As a result, some QDs may stimulate the development of novel stomata within the epidermal cell wall, thereby facilitating their entry.39 However, the restricted pore size of QDs restricts their access to cells via this pathway. Therefore, plant cells can internalize large-sized QDs through endocytosis. Endocytosis could result in the invagination of the plant cell membrane, thereby facilitating the internalization of QDs into the cell. For instance, plant protoplasts have been shown to take up QDs with a diameter less than 1 μm via endocytosis.40 Li et al. reported that the root surface cells of woody plants facilitate the penetration of cadmium sulfide/zinc sulfide quantum dots (CdS/ZnS-QDs) through the plant cell wall/membrane via endocytosis, thus enabling their cellular uptake.7 Moreover, the increased specific surface area of QDs allows them to form complexes with different carrier proteins or root secretions and carry them inside the root cells.36 For example, as demonstrated by Lian and Majumdar et al., the specific transporters present in soybean and rice plants can facilitate the effective translocation of cadmium sulfide quantum dots (CdS-QDs) and cadmium telluride quantum dots (CdTe-QDs) within the plants themselves.41,42
Apoplastic and symplastic pathways are the two main routes for nanoparticle transport in the roots of land plants,44 with the apoplast comprising the cell walls, intercellular spaces, and vascular tissues. QDs could migrate through extraplastidial plasmodesmata, traversing root cell walls, intercellular spaces, and xylem lumens, ultimately reaching the vascular bundle.45 Nevertheless, when QDs are transported through the epidermis to the endodermis, they encounter Casparian bands, which impede their progression. Some QDs are deposited in the endodermis, whereas others undergo radial transportation along the Casparian strip, but not across it. However, the absence of fully developed Casparian strips at the lateral root junction resulted in reliance on the vascular system for QD transport.46 An additional pathway is symplastic transport, which is how nanoparticles are transported between plant cells. Notwithstanding the relative autonomy and resilience of plant cells, cytoplasmic hyphae can establish intercellular connections across cell walls. Furthermore, the utility of cytoplasmic hyphae enables the transportation of QDs to continuous root cells and into endothelial cells.47
3.2 Foliar uptake and leaf-to-root translocation
The diameter range of the hydrophilic and lipophilic channels is between 0.6 nm and 4.8 nm.50 Hydrophilic channels permit the diffusion of hydrophilic QDs with a diameter of less than 4.8 nm; lipophilic channels, in contrast, allow lipophilic QDs to enter plant leaves through diffusion and osmosis.51 In a recent study, Hu et al. demonstrated that CQDs with a diameter of less than 2 nm could enter cotton leaves via the epidermal pathway.13 Nevertheless, the absorptive capacity of plants for QDs through the epidermal layer is limited by the narrow dimensions of the pore channels within the cuticle. Accordingly, it has been proposed that QDs may be taken up by plants through the stomatal pathway (Fig. 1).
Stomata are located on the surface of leaves and serve as gas exchange organs in terrestrial plants, allowing for the efficient transfer of ENPs from the leaf surface into chloroplasts.52 For example, Sun et al. discovered that GQDs primarily enter leaves through stomata, forming aggregates that gradually increase in size within the stomata.53 In addition, Wang et al. employed laser confocal scanning microscopy to observe the entry of CdTe-QDs into plants via stomata, subsequently demonstrating their diffusion into anthers and ovules through vascular bundles.54 Typically, the diameter of a single stomatal pore ranges from 10 to 100 μm on average, with considerable variation observed among different plant species. Nevertheless, the specific size exclusion limits for stomatal apertures and QD diffusion remain uncertain because of the distinctive geometry and physiological function of these structures.55
3.3 Biotransformation of QDs by plants
Once internalized, QDs are not inert entities; instead, during their translocation across roots, stems, and leaves, they undergo a cascade of physicochemical transformations, including changes in chemical speciation, surface reconstruction, and the formation of biomolecular coronas.62 These transformations critically determine their bioavailability, tissue distribution, toxicological outcomes, and ecological risks while also influencing perturbations to photosynthetic and respiratory metabolism.63 For instance, Marmiroli et al. demonstrated that CdS-QDs, upon interacting with oxygen- and sulfur-containing ligands such as organic acids, sulfur metabolites, proteins, and secondary metabolites, undergo progressive lattice destabilization.64 This process generates trace Cd(II) and CdSn clusters, which subsequently form stable complexes with thiol-rich molecules like glutathione and phytochelatins; the complexes are further compartmentalized in vacuoles, depicting a nano-specific biotransformation pathway distinct from the fate of ionic Cd. Furthermore, Pagano et al. emphasized that the core–shell architecture and surface ligand stability of QDs are decisive factors in determining whether particles undergo partial dissolution, surface redox reactions, or ligand rearrangements in planta.65 Such transformations are intimately linked to the activation of oxidative stress signaling, induction of antioxidant defenses, and detoxification mechanisms, particularly those involving glutathione metabolism. Overall, the interplay between QD integrity and plant defense capacity determines biotransformation outcomes, and surface engineering offers a viable path toward safer agricultural applications.4 Effects of QDs on plants
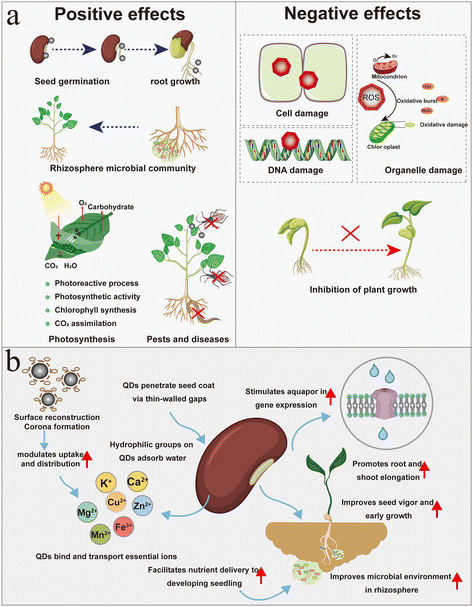
Once internalized by plants, QDs interact with cellular and subcellular structures, thereby altering plant morphology and physiological status.66 As highlighted by Ma et al. in their discussion of plant responses to nanoparticle exposure—which is also applicable to QD–plant interactions—the effects of nanoparticles are inherently biphasic.67 At low or optimal concentrations, they may promote seed germination,68 enhance photosynthesis, stimulate growth,69 and improve tolerance to abiotic stresses (Fig. 2). Conversely, at higher concentrations or in combination with certain chemical constituents, they can induce oxidative stress, DNA damage, chromosomal aberrations, and growth inhibition. Moreover, the magnitude and direction of these responses are strongly governed by the physicochemical properties of the nanoparticles, including their size, concentration, surface charge, and surface coatings as well as the developmental stage or species of the host plant. A unifying mechanistic basis underlying phytotoxicity involves the overproduction of reactive oxygen species (ROS) and subsequent metabolic reprogramming, which can disrupt both primary and secondary metabolism. For example, exposure to CdSe-QDs can lead to oxidative stress within plants, which in turn can result in physical damage70 including cytotoxicity and genotoxicity.714.1 Positive effects
In recent years, researchers have studied the positive effects of QDs on soil plants, such as rice, corn, and wheat. The positive effects include the promotion of seed germination and root growth, enhancement of photosynthesis and biomass accumulation, and improvement of plant resistance to abiotic stresses and diseases,72 as described in Table 1.| Type of QDs | Plant species | Effects of QDs on plant | QD concentration applied | Mechanism of action | Reference |
|---|---|---|---|---|---|
| CQDs | Rice | Improved disease resistance and food production | 0.56 g L−1 | Increased expression of thionin genes; degradation of CQDs to form phytohormone analogs and CO2; 42% increase in RuBisCO enzyme activity | 73 |
| CQDs | Rice | Promoted photosynthesis in rice plants, resulting in a significant increase in plant growth | 300 mg L−1 | The electron transport rate and photosynthetic efficiency of photosystem II exhibit a notable increase, reaching 29.81% and 29.88%, respectively; the chlorophyll content and RuBisCO enzyme activity demonstrate a significant rise, reaching 64.53% and 23.39%, respectively | 74 |
| CQDs | Rice | In the presence of CQDs, photosynthetic rate and stomatal conductance were increased by 56% and 18% in rice and maize, respectively | 150 mg L−1 | Promoted electron transfer rate during photosynthesis and increased the fluorescence quantum efficiency and CO2 assimilation rate and stomatal conductance were also improved | 75 |
| Magnesium–nitrogen co-doped carbon dots (Mg,N-CQDs) | Rice | The height, fresh biomass, and chlorophyll a and b content of rice seedlings were significantly increased | 300 mg L−1 | The expression of genes associates with the biosynthesis of chlorophyll is increased; the levels of chlorophyll a and b are elevated by 14.39% and 26.54%, respectively; and the activity of the enzyme RuBisCO exhibits a 46.62% increase | 76 |
| Water-soluble carbon nano-dots (wsCND) | Wheat | Increased root and shoot growth | 0.75 g L−1 | Easily crosses biological barriers in seedlings, carrying more nutrients and water for wheat plant growth | 77 |
| Cerium-doped carbon dots “”(CDs:Ce) | Wheat | Promoted the growth and development of wheat in the concentration range of 0.01–0.4 mg mL−1, resulting in a 45% increase in root number, a 57% increase in root length, a 28% increase in leaf length and a 46% increase in plant height | 0.01–0.025 g L−1 | Increased the accumulation of chlorophyll, enhanced light absorption, promoted photosynthesis, reduced MDA content, and improve POD activity | 78 |
| N-CQDs | Maize | Increased maize photosynthesis by 44.48%, maize yield by 24.50% and 1000 kernel weight by 15.03% | 5–50 mg L−1 | Resulted in a significant increase in light conversion efficiency, chlorophyll content, psbA gene expression, ATPase activity, and NADPH production in maize; N doping led to a high separation efficiency of electrons and holes, thereby increasing light conversion and electron supply during photosynthesis | 79 |
| CQDs | Maize | Resulted in 20.9% and 39.6% increase in height and spike weight, respectively, as well as an increase in yield | 150 mg L−1 | The hydroxyl and carboxyl groups on the surface of CQDs provided abundant binding sites for water molecules to enter the plant together with the CQDs and increased the maize CO2 assimilation rate and stomatal conductance by 16% and 18%, respectively | 75 |
| Orange carbon dots (O-CDs) | Maize | Increased the net photosynthetic rate of maize | 1 mg L−1 foliarly; 5 mg L−1 rootly | O-CDs can increase the photosynthetic parameters of maize leaves and enhance photosynthetic pigments | 80 |
| N-CQDs | Maize | Increased the biomass of maize, promoted root growth, ensured the survival of maize under drought stress, and enhanced the drought tolerance of maize | 5 mg L−1 | Increased superoxide dismutase activity and decreased malondialdehyde enzyme activity; improved light use efficiency, upregulated the expression of psbA gene, and promoted the rapid synthesis of D1 protein, thus repairing photosystem II under drought stress | 81 |
| CQDs | Maize | Increased root exudates, improved photosynthesis, increased the abundance of rhizosphere microorganisms, promoted nutrient uptake, and enhanced drought tolerance | 5 mg L−1 | Improved the photo use efficiency and ability to contribute electrons to promote plant photosynthesis, scavenge ROS, upregulated the gene encoding isocitrate dehydrogenase, increased the synthesis of succinic acid | 69 |
| Silicon quantum dots (Si-QDs) | Cucumber | Increased the total fresh weight of seedlings by 51.91% and root water absorption by 74.6% | 0.01–0.3 g L−1 | Induced a significant increase in the expression of water channel protein genes in the root system of cucumber seedlings and improved hydraulic conductance and transport in the root system | 82 |
| Zinc oxide quantum dots (ZnO-QDs) | Lettuce | Promoted the absorption and accumulation of Ca, Mg, Fe, Mn, Zn, and B in lettuce; increased the soluble sugar content of lettuce; and improved the biomass and nutritional quality of lettuce | 50–200 mg L−1 | The low concentration of ZnO-QD treatment significantly increased lettuce soluble protein and soluble sugar content, induced antioxidant, improved plant resistance, promoted chlorophyll synthesis, and facilitated lettuce nutrient uptake | 83 |
| Si-QDs | Lettuce | Root length, seedling height and biomass of lettuce seedlings were significantly increased when the concentration of Si-QDs was less than 30 mg L−1 | <30 mg L−1 | Concentrations of Si-QDs less than 30 mg L−1 resulted in increased chlorophyll production in lettuce, enhanced water and nutrient uptake by the root system, promoted photosynthesis in lettuce, and maintained the concentration of reactive oxygen species at a beneficial level | 84 |
| La2O3 + CdS-QDs | Zucchini | The stem length of zucchini was increased by 13–25% | 500 mg L−1 | La2O3 stimulated cell elongation | 85 |
Wang et al. demonstrated that CQDs could facilitate mung bean seed development by increasing the permeability of the seed coat and enhancing seedling hydration.89 Moreover, hydrophilic groups, namely –OH and –COOH, which are present on the QD surface, have been demonstrated to be involved in multiple crucial processes during seed germination including the development of roots, seed water content, and seedling length.86,90 The presence of hydrophilic groups provides numerous binding sites for water, which is subsequently absorbed by the plant. Accordingly, sufficient water uptake could facilitate seed germination and accelerate seedling growth.73,90 Also, Li et al. discovered that the hydrophilic groups on the surface of CQDs could readily bind to water molecules, facilitating transportation to rice seeds and ensuring adequate water levels in the seeds to promote germination and accelerate the growth of rice plants.73
In addition to functioning as water adsorption sites, QDs may also stimulate aquaporin-related gene expression, facilitate cell growth by regulating the cell cycle, and thereby enhance seed germination and plant growth.91 Kou et al. observed that the hydrophilic groups of CQDs may facilitate enhanced water and mineral element uptake, promote root and hypocotyl elongation in seedlings, and accelerate seed germination by increasing the expression of genes associated with aquaporins.92 Therefore, it can be surmised that QDs may promote plant uptake of mineral nutrients in addition to promoting water absorption. The hydrophilic groups present on the surface of QDs can adsorb a diverse array of nutrient ions (including K+, Ca2+, Mg2+, Cu2+, Zn2+, Mn2+, and Fe3+) that are indispensable for optimal plant growth. These hydrophilic groups engage in both hydrogen bonding and electrostatic interactions with the nutrient ions, subsequently facilitating their adsorption onto the surface of the QDs. The nutrient ions enter the plant together with the QDs, resulting in an increased concentration of nutrient ions within the plant.93 Li et al. elevated levels of nutrient ions in Arabidopsis treated with CQDs in comparison with the control, indicating that CQDs facilitate plant growth by adsorbing water and metal ions through their surface functional groups (–OH and –COOH) and transporting them to the plant.90 Hu et al. demonstrated that the effect of CQDs was significantly dose-dependent. They reported notable increases in the levels of soluble sugars, proteins, vitamin C, minerals, and plant yields after being subjected to CQDs at concentrations exceeding 30 mg L−1.94
In conclusion, QDs have the potential to activate water channel proteins and lower the inter-root pH, which in turn could facilitate the uptake of water and nutrients, enhance the inter-root microbial environment, and stimulate seed germination and growth.95 Nevertheless, the specific molecular mechanisms by which enzymes and phytohormones control seed germination remain unidentified. Consequently, future studies should focus on investigating alterations in related enzymes and phytohormones at the molecular level.
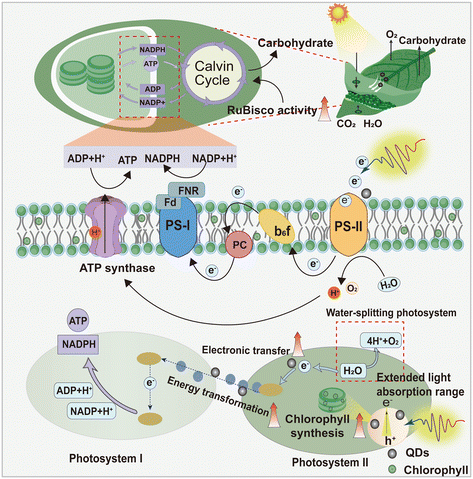
In general, QDs, and CQDs in particular, exhibit exceptional luminescence properties, typically demonstrating pronounced absorption in the UV region (200–400 nm). The light absorption range of these materials can extend into the visible range due to the specific characteristics of their surface groups and the abundance of oxygen and nitrogen present in their carbon nuclei. Additionally, in the 500–800 nm range, QDs demonstrate longwave absorption, which has the potential to convert UV light that is not utilized by plants into visible light.97,98 Therefore, QDs can enhance solar efficiency, increase the concentration of light-trapping pigments, and expedite electron transfer.99 For example, in 2010, Nabiev et al. discovered that CdTe-QDs possess an “antenna effect” that could enhance the efficiency of chlorophyll photosynthesis by approximately threefold through resonant energy transfer with photopigments.100 Additional research by Li et al. discovered that Si-QDs increased the contents of chlorophyll a and chlorophyll b in lettuce by 41.04% and 114.83%, respectively, and augmented the photosynthetic activity and electron transfer rate of chloroplasts, thus enhancing photosynthesis in lettuce.84 Furthermore, QDs, as highly efficacious electron donors and acceptors, could augment plant photosynthesis through accelerated electron transfer efficiency, whereby electrons are transported to chloroplasts and subsequently to light-trapping complexes.87 For instance, amine-functionalized CQDs are markedly coupled at the surface of chloroplasts, facilitating photon uptake and electron transfer to the chloroplasts, thereby accelerating the electron transfer chain within the photosynthetic reaction and consequently enhancing photosynthetic activity.101 Also, in 2021, Wang et al. demonstrated that N-doped CQDs are efficacious light-converting materials and electron donors that can augment light conversion and electron supply during maize photosynthesis, which could lead to a substantial increase of 122.80% in the photoelectron transfer rate.79
In addition to facilitating the processes of photosynthesis that occur in the presence of light, QDs have been demonstrated to accelerate the dark reactions of this metabolic pathway. Carbon assimilation is understood to be the process by which CO2 is reduced to sugar through absorptive forces (ATP and NADPH), which are the result of light reactions. This process is similarly crucial for plant growth. Ribulose bisphosphate carboxylase oxygenase (RuBisCO) is a pivotal enzyme within the Calvin cycle that has a decisive influence on the dark reaction of photosynthesis.102 Consequently, the activity of this enzyme directly impacts the rate of photosynthesis. It has been demonstrated that QDs could enhance their activity, accelerate the carbon reaction process, and improve their photosynthetic efficiency, thereby increasing their carbohydrate content.103 According to Wang et al., the activity of the RuBisCO enzyme in mung bean seedlings treated with CQDs was 30.9% greater than that in mung bean seedlings than in the control group.89 Similarly, CQDs were found to increase RuBisCo enzyme activity in other plants, including rice and A. thaliana. This increase in activity coincided with an increase in photosynthesis in these plants.73,90 Li et al. reported that when various dicotyledonous plants were treated with CQDs, the activity of the RuBisCO enzyme increased by 30.9%, the quantity of carbohydrates increased, and the total yield of the plants increased by 20%.90 Consistent with the findings of Zhang et al., their study examined the effects of distinct chiral CQDs on mung bean and found that D-chiral carbon quantum dots (D-CQDs) increased RuBisCo enzyme activity by 67.5%, which in turn enhanced carbohydrate production and promoted plant growth.104 Furthermore, QDs could undergo degradation in plants, forming phytohormone analogs and releasing CO2. The degradation of these hormones could promote growth in plants, while the released CO2 could be incorporated into carbohydrates through the Calvin cycle, leading to an increase in carbohydrate accumulation.90
The enhanced production of ROS constitutes a crucial mechanism by which abiotic stresses affect plant growth, and the accumulation of ROS within cells frequently results in damage to essential biomolecules, including proteins, lipids, carbohydrates, and DNA.108 QDs enriched by plants may act as antioxidants with the ability to scavenge excess free radicals generated by abiotic stressors. As shown by Farhangi-Abriz et al., CQDs accumulation under drought stress induces a decrease in ROS production, lipid peroxidation, and osmotic stress in soybean, leading to an increase in the concentration of proline, soluble carbohydrates, and proteins, which in turn leads to improved osmoregulation and increased yield in soybean.109 Concurrently, QDs have the potential to enhance the activity of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), while simultaneously reducing the levels of ROS and malondialdehyde (MDA).110 In their investigations into the mitigating effects of CQDs on heat stress in lettuce, Wang et al. discovered that CQDs enhanced antioxidant enzyme activities and osmoregulation while simultaneously reducing lipid peroxidation damage to plant cells, thus improving the heat tolerance of the plant.111 Thereafter, in 2022, Wang et al. discovered that under conditions of drought, CQDs could upregulate the GmNRT (mediates nitrate uptake in soybean roots), GmAMT (involved in ammonium uptake, especially during nodulation), GmLB (facilitates O2 transport/homeostasis in root nodules), and GmAQP (regulates water and some solutes transport under stress) genes within the root system of soybean crops, leading to a notable increase in the N content and water uptake capacity of soybeans, thus enhancing the drought tolerance of soybean plants.112 Additional research by Chai et al. illustrated that ZnO-QDs could reduce the levels of H2O2 and MDA, increase antioxidant enzyme activity, reduce the Na/K ratio, and modulate the expression of genes related to stress response pathways under salt stress in Salvia miltiorrhiza, thus jointly alleviating salt stress.113
Recent studies have indicated that the presence of QDs in plants may also enhance their resistance to pests and diseases, thereby reducing the overall incidence of yield loss and associated environmental risks. For instance, Wang et al. observed that ZnO-QDs induced the production of hydroxyl radicals in pathogenic bacteria, amplified the expression of genes associated with oxidative stress, and compromised intracellular defenses, culminating in cell lysis and death of the bacteria.114 This led to a substantial decrease in the prevalence of bacterial fruit blotch disease in melons. Luo et al. discovered that foliar uptake of N-CQDs induced the expression of jasmonic acid- and salicylic acid-dependent resistance pathways in tomato plants, which effectively inhibited pathogen growth and led to a 71.19% reduction in the symptoms associated with greening disease.115
Furthermore, studies have shown that QDs exhibit diverse effects on plant and microbial systems, which are highly dependent on their composition, concentration, and surface functionalization characteristics. At high concentrations or when using heavy metal-based QDs (e.g., CdTe-QDs, CdSe-QDs), QDs can generate ROS or induce photodynamic effects under light activation, causing broad-spectrum antibacterial and antifungal activity by disrupting cell membranes, damaging microbial DNA/RNA, and impairing cell wall integrity.116,117 Such effects have been observed against both Gram-positive and Gram-negative pathogens.118 In contrast, at low or environmentally relevant concentrations, particularly for carbon-based (e.g., CQDs) and N-CQDs, these nanomaterials have demonstrated beneficial effects on plants and the rhizobiome. For instance, Luo et al. reported that foliar application of 10 mg L−1 N-CQDs significantly suppressed tomato bacterial wilt by 71%, enhanced fresh shoot biomass by ∼56%, activated ROS scavenging systems, induced salicylic acid- and jasmonic acid-dependent systemic acquired resistance (SAR), and stimulated the tricarboxylic acid (TCA) cycle and fatty acid synthesis in tomato cells.115 Similarly, Kou et al. showed that spray treatment with CQDs at 240 mg L−1 reduced the incidence of gray mold in Nicotiana benthamiana and tomato by 44% and 12%, respectively, and under simulated sunlight (480 mg L−1), achieved broad-spectrum antifungal activity—up to 100% inhibition of Botrytis cinerea—by damaging fungal hyphae and spore structures while activating antioxidant enzyme responses in treated tissues.119
A healthy plant-associated microbiome has been shown to enhance many important plant functions, including growth, nutrient uptake, and resistance to pathogens.121 In recent years, researchers have reported that the enrichment of QDs in plants can affect plant growth by modulating soil microbial activity at the level of root-to-root interactions. On the one hand, QDs exposed to the plant root system can act directly on the microbial community. For example, the presence of Si-QDs in radish rhizosphere soil, by enriching microorganisms mainly associated with the nitrogen cycle, can change the structure of soil bacterial communities and establish a healthy inter-root microenvironment, thus providing sufficient nitrogen resources for plant growth and defense against subterranean herbivores.122 A study by Yin et al. revealed that tomato rhizosphere exposed to 2.5 mg kg−1 selenium-doped carbon quantum dots (Se-CQDs) showed a significant increase in the soil Actinobacteria and mycorrhizal phyla increased significantly in diversity and prevalence, while the inter-root soil microenvironment was also optimized to enhance the uptake of selenium (and other mineral nutrients), which ultimately promotes root development and plant growth.123
On the other hand, the uptake of QDs by plants through leaves may affect the inter-root microbial community and soil metabolism, thereby improving soil microbiology and promoting plant growth. For example, Xu et al. observed that when pumpkin leaves were exposed to ZnO-QDs, the inter-root fungal community was altered through coordinated host–microbe and microbe–microbe interactions.124 This resulted in the accumulation of beneficial microorganisms in endophytic and inter-root environments, which promoted plant growth, nutrient uptake, and adversity tolerance.125,126 Furthermore, experimental evidence has demonstrated that foliar exposure to QDs results in a decrease in inter-root organic acid abundance. This phenomenon, in turn, has been shown to promote the growth of soil microorganisms associated with carbon and nitrogen, thereby exerting an effect on soil fertility and plant health.127
4.2 Negative effects
As demonstrated in Table 2, the adverse impact of QDs on plants, particularly those with heavy metals, hydroxyl and amino functional groups, or relatively high concentrations, cannot be overlooked. The toxicity of QDs may cause damage to plants via several mechanisms including the stimulation of oxidative stress in the plant, which could result in physical damage, or the induction of oxidative damage, which might have toxic effects on the plant, thereby inhibiting growth and development.128 Recent studies have consistently demonstrated that especially CdS-QDs exhibit phytotoxic effects across a wide range of concentrations, indicating that their toxicity is not limited to high-dose exposures. Multiple studies have reported that CdS-QDs disrupt fundamental metabolic pathways such as glutathione metabolism, the tricarboxylic acid cycle, and amino acid biosynthesis in soybean and other crops, leading to oxidative stress, impaired growth, and cellular damage.42 Similar observations have been made in wheat and maize, where CdS-QD exposure resulted in DNA damage, alterations in antioxidant enzyme activity, and inhibition of seed germination regardless of the applied concentration.4,129 Similarly, Lian et al. reported that rice plants exposed to CdS QDs exhibited inhibited root development, altered antioxidative enzyme activity, and elevated cadmium accumulation regardless of the applied concentration.41 In A. thaliana, Marmiroli et al. discovered that CdS-QD toxicity followed a mechanism distinct from ionic cadmium toxicity, involving unique gene expression responses in resistant mutants, such as alterations in photosynthetic regulatory genes.130 Further, Marmiroli et al. reported that 40–60 mg L−1 CdS-QDs induced oxidative stress in A. thaliana, reduced chlorophyll and carotenoid content, and caused alterations in plant morphology.131| Type of QDs | Plant species | Effects of QDs on plant | QD concentration applied | Mechanism of action | Reference |
|---|---|---|---|---|---|
| CdTe-QDs | Wheat | Plant height, root length and total biomass were significantly reduced | 25–400 mg L−1 | Causes antioxidant defense and programmed cell death in roots and shoots of wheat seedlings, leading to apoptotic body formation and DNA breaks | 132 |
| GQDs | Wheat | The contents of globulin, lamin, amylose, amylopectin and mineral elements in wheat grains decreased | 0.5–50 μg kg−1 | Downregulation of the levels of proteins with nutrient reserve activity thus leads to changes in the protein structure of wheat grain and downregulation of α-amylase inhibitors, thus interfering with metabolic processes | 133 |
| CdS-QDs | Soybean | QDs accumulate in soybean roots and shoots and cause phytotoxicity | 200 mg L−1 | Disrupts major metabolic pathways such as glutathione metabolism, the tricarboxylic acid cycle, glycolysis, fatty acid oxidation, and the biosynthesis of phenylpropanes and amino acids in soybeans | 42 |
| ZnO-QDs | Lettuce | When the concentration of ZnO-QDs increased (up to 500 mg L−1), plant biomass decreased | 500 mg L−1 | High concentrations of ZnO-QDs induced stress and altered the activity of antioxidant enzymes in lettuce, leading to membrane lipid peroxidation as well as an increase in MDA content, which disrupted the chloroplast membranes and led to a decrease in chlorophyll content | 83 |
| Si-QDs | Lettuce | At concentrations greater than 100 mg L−1, the plants become greener but photosynthesis was also inhibited which in turn inhibits seedling growth and biomass production | >30 mg L−1 | When the concentration of Si-QDs was greater than 100 mg L−1, the water uptake capacity of seedlings was inhibited, which led to oxidative damage and increased SOD activity and MDA content | 84 |
| Amino-functionalized graphene quantum dots (N-GQDs) | Lettuce | At a concentration of 50 mg L−1, N-GQDs reduced the root elongation degree by 40%. When the concentration was increased to 100 mg L−1, N-GQDs reduced the root elongation degree by 32% | 50 mg L−1, 100 mg L−1 | Causes oxidative damage, disrupts mineral and organic nutrient homeostasis, impairs plant photosynthesis and regulates phytohormone levels and causes light-triggered production of reactive oxygen species and oxidation of antioxidants in plants | 134 |
| Carboxyl-functionalized graphene quantum dots (C-GQDs) | At a concentration of 50 mg L−1, C-GQDs reduced the root elongation degree by 36%, the root dry weight by 39%, the stem dry weight by 44%, the root fresh weight by 55%, and the leaf moisture by 74%, and the leaf moisture by 75%. When the concentration was increased to 100 mg L−1, C-GQDs reduced the root elongation degree by 41% | 50 mg L−1, 100 mg L−1 | |||
| Hydroxyl-functionalized graphene quantum dots (O-GQDs) | When the concentrations were 1 and 10 mg L−1, O-GQDs reduced the stem dry weight by 36% and 47% respectively, the root fresh weight by 74%, and the leaf moisture by 76%. When the concentration was 50 mg L−1, O-GQDs reduced the root elongation by 41%, the stem elongation by 39%, the seed germination rate by 39%, the root dry weight by 43%, the stem dry weight by 55%, the fresh root weight by 31%, the stem fresh weight by 89%, and the leaf moisture by 90%. When the concentration was increased to 100 mg L−1, the root elongation degree decreased by 85%, the stem elongation degree decreased by 88%, and the seed germination rate decreased by 71% | 1 mg L−1, 10 mg L−1, 50 mg L−1, 100 mg L−1 | |||
| CdTe/CdS/ZnS-QDs | Allium cepa L. | The adsorption of QDs on the surface of plant roots caused cadmium to accumulate in the roots and caused a reduction in plant root growth | 30 μM, 100 μM | Cd-based QDs lack chemical stability when subjected to prolonged exposure. The release of Cd from QDs into the exposure medium occurs in the form of Cd2+, which is subsequently absorbed and utilized by onion roots. The internalization of nuclear/shell QDs into plants is limited or negligible due to the plant cell wall thickness, the large size and aggregation of QDs, and the lack of endocytic capacity in the selected plant species | 135 |
| CdS-QDs | Zucchini | The fresh weight of the above-ground part was significantly reduced by approximately 21.9% | 100 mg L−1 | High accumulation and transport of Cd lead to excessive generation of ROS and downregulation of photosynthetically related genes, thereby inhibiting carbon assimilation | 85 |
| CeO2 + CdS-QDs | Zucchini fresh weight was reduced by 23.4% | CeO2 500 mg L−1 CdS-QDs 100 mg L−1 | Cd toxicity was dominant, and the transport pattern of Ce was significantly altered (Ce in the stem increased to 576 mg kg−1), exacerbating metabolic interference |
In addition to their genotoxic effects, QDs have been demonstrated to induce ROS and trigger hormonal changes in plants. Presumably in all aerobic organisms, plant cells could be stimulated by environmental changes, thereby activating the production of ROS.137 When ROS levels surpass the capacity of defensive mechanisms, a state of “oxidative stress” is initiated within the cellular milieu, resulting in indiscriminate damage to proteins and nucleic acids.138 Verma et al. mentioned that QDs have been demonstrated to induce oxidative stress and ultimately lead to programmed cell death in plant systems.139 For example, research by Shi et al. discovered that CdTe-QDs can decompose into highly toxic Cd within rice root cells, disrupting the redox homeostasis of the roots and leading to excessive accumulation of ROS and subsequent cell death.129 This ultimately results in the inhibition of root and shoot growth. Recently, researchers evaluated the detrimental impacts of CdS-QDs on soybean plants and indicated that CdS-QDs induced oxidative stress and impaired amino acid metabolism in soybean leaves, resulting in the formation of lignin and hardening of the cell wall and reduced root growth.140 Additionally, high concentrations of QDs may induce apoptosis in plant cells, which could have a detrimental impact on plant growth and development.131 Also, in 2010, Hu et al. showed that the internalization of QDs by plants predominantly influences their oxidative stress processes, and that high concentrations of QDs (>312.8 μM) exhibit cytotoxicity, specifically affecting maize seed germination and root growth.141
Furthermore, after entering plant cells, QDs may disrupt the integrity of the cellular membrane, leading to the leakage of ions and an increase in membrane permeability. In response to the oxidative stress induced by QDs, plant cells typically initiate the antioxidant defense system and enhance the activity of antioxidant enzymes. However, this process results in the exhaustion of substantial quantities of antioxidant resources, which can impair cellular function and, consequently, plant growth and development.131 Additionally, it has been demonstrated that the presence of heavy metal QDs in soil may lead to a reduction in chlorophyll levels in plants.131,132 As a consequence of this reduction, plant photosynthesis is adversely affected, which consequently affects plant growth and development.
5 Perspectives and future outlook
QDs are now widely detected in agroecosystems, and their enrichment by plants has become an unavoidable reality. In recent years, substantial advances have already been made in clarifying the uptake and transport pathways of QDs in plants, their regulatory effects on photosynthesis and stress tolerance, and their interactions with rhizosphere microbial communities. Likewise, numerous studies have revealed both the beneficial roles of QDs in stimulating seed germination, promoting growth, and enhancing stress resistance, and their adverse effects involving oxidative stress, DNA damage, and growth inhibition. These findings suggest that QD–plant interactions are not simply prospective issues but represent an active research field with a rapidly growing body of evidence.Despite these advances, a critical gap remains in defining the precise conditions under which QDs exert positive versus negative effects on plant agroecosystems. Factors such as QDs' concentration, particle size, functionalization, and environmental context are decisive in shaping outcomes. Moreover, the outcome of QD exposure is strongly species-dependent, as an enhancement of photosynthetic efficiency or stress resilience in one crop species may, in another species, result in toxicity and growth inhibition. This plant-specific variability highlights the urgent need for systematic, comparative studies across representative crops to establish boundary conditions for safe and beneficial use.
Future research should therefore prioritize disentangling the molecular and biochemical mechanisms by which QDs regulate enzyme activity, phytohormone signaling, and DNA repair or cell cycle processes. At the same time, there is a pressing need to investigate the long-term transport, transformation, and ecological fate of QDs in soils and water bodies as well as their impacts on non-target organisms such as soil microbiota and insects. Rigorous risk assessments, including both acute and chronic toxicity tests, will be indispensable to ensure the safe deployment of QDs in agroecosystems. By integrating these mechanistic insights with ecological evaluations, future work can move beyond proof-of-concept studies to establish a rational framework for exploiting the beneficial effects of QDs while minimizing environmental risks.
Author contributions
Hao Cheng: writing – original draft, data curation, methodology. Yanxing Xu: writing – review & editing. Abdelrahman Ibrahim: writing – review & editing. Yanzheng Gao: writing – review & editing, conceptualization, supervision, funding acquisition. Hefei Wang: writing – review & editing, methodology, supervision. Ahmed Mosa: writing – review & editing, methodology, supervision. Wanting Ling: writing – review & editing, methodology, supervision.Conflicts of interest
There are no conflicts of interest to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Acknowledgements
This work was supported by the National Natural Science Foundation of China (42261144738, U22A20590, 42207025, 42430703, 42577012).References
- M. A. Mimona, M. I. H. Rimon, F. T. Zohura, J. M. Sony, S. I. Rim, M. M. R. Arup and M. H. Mobarak, Quantum dot nanomaterials: Empowering advances in optoelectronic devices, Chem. Eng. J. Adv., 2025, 21, 100704 CrossRef CAS
.
- D. Ozyurt, M. A. Kobaisi, R. K. Hocking and B. Fox, Properties, synthesis, and applications of carbon dots: A review, Carbon Trends, 2023, 12, 100276 CrossRef CAS
.
- J. Sanmartín-Matalobos, P. Bermejo-Barrera, M. Aboal-Somoza, M. Fondo, A. M. García-Deibe, J. Corredoira-Vázquez and Y. Alves-Iglesias, Semiconductor Quantum Dots as Target Analytes: Properties, Surface Chemistry and Detection, Nanomaterials, 2022, 12, 2501 CrossRef
.
- H. Sun, M. Wang, C. Lei and R. Li, Cell wall: An important medium regulating the aggregation of quantum dots in maize (Zea mays L.) seedlings, J. Hazard. Mater., 2021, 403, 123960 CrossRef CAS
.
- P. Sun, Z. Xing, Z. Li and W. Zhou, Recent advances in quantum dots photocatalysts, Recent advances in quantum dots photocatalysts, Chem. Eng. J., 2023, 458, 141399 CrossRef CAS
.
- L. Wei, J. Liu and G. Jiang, Nanoparticle-specific transformations dictate nanoparticle effects associated with plants and implications for nanotechnology use in agriculture, Nat. Commun., 2024, 15, 7389 CrossRef CAS
.
- R. Li, H. Sun, S. Wang, Y. Wang and K. Yu, Retention of CdS/ZnS Quantum Dots (QDs) on the Root Epidermis of Woody Plant and Its Implications by Benzo[a]pyrene: Evidence from the in Situ Synchronous Nanosecond Time-Resolved Fluorescence Spectra Method, J. Agric. Food Chem., 2018, 66, 814–821 CrossRef CAS
.
- M. S. Giroux, Z. Zahra, O. A. Salawu, R. M. Burgess, K. T. Ho and A. S. Adeleye, Assessing the environmental effects related to quantum dot structure, function, synthesis and exposure, Environ. Sci.:Nano, 2022, 9, 867–910 RSC
.
- Y. Wang and B. Nowack, Dynamic probabilistic material flow analysis of nano-SiO2, nano iron oxides, nano-CeO2, nano-Al2O3, and quantum dots in seven European regions, Environ. Pollut., 2018, 235, 589–601 CrossRef CAS
.
- D. V. Francis, A. K. Abdalla, W. Mahakham, A. K. Sarmah and Z. F. R. Ahmed, Interaction of plants and metal nanoparticles: Exploring its molecular mechanisms for sustainable agriculture and crop improvement, Environ. Int., 2024, 190, 108859 CrossRef CAS PubMed
.
- L. Yin, X. Wen, D. Huang, C. Du, R. Deng, Z. Zhou, J. Tao, R. Li, W. Zhou, Z. Wang and H. Chen, Interactions between microplastics/nanoplastics and vascular plants, Environ. Pollut., 2021, 290, 117999 CrossRef CAS
.
- W. Kong, X. Hou, L. Wei, W. Chen, J. Liu, J. L. Schnoor and G. Jiang, Accumulation, translocation, and transformation of two CdSe/ZnS quantum dots in rice and pumpkin plants, Sci. Total Environ., 2023, 864, 161156 CrossRef CAS PubMed
.
- P. Hu, J. An, M. M. Faulkner, H. Wu, Z. Li, X. Tian and J. P. Giraldo, Nanoparticle Charge and Size Control Foliar Delivery Efficiency to Plant Cells and Organelles, ACS Nano, 2020, 14, 7970–7986 CrossRef PubMed
.
- J. I. Kwak and Y. An, The current state of the art in research on engineered nanomaterials and terrestrial environments: Different-scale approaches, Environ. Res., 2016, 151, 368–382 CrossRef PubMed
.
- J. Lv, P. Christie and S. Zhang, Uptake, translocation, and transformation of metal-based nanoparticles in plants: recent advances and methodological challenges, Environ. Sci.:Nano, 2019, 6, 41–59 RSC
.
- M. J. Gallagher, J. T. Buchman, T. A. Qiu, B. Zhi, T. Y. Lyons, K. M. Landy, Z. Rosenzweig, C. L. Haynes and D. H. Fairbrother, Release, detection and toxicity of fragments generated during artificial
accelerated weathering of CdSe/ZnS and CdSe quantum dot polymer composites, Environ. Sci.:Nano, 2018, 5, 1694–1710 RSC
.
- R. Hardman, A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors, Environ. Health Perspect., 2006, 114, 165–172 CrossRef
.
- D. G. Goodwin Jr., A. S. Adeleye, L. Sung, K. T. Ho, R. M. Burgess and E. J. Petersen, Detection and Quantification of Graphene-Family Nanomaterials in the Environment, Environ. Sci. Technol., 2018, 52, 4491–4513 CrossRef
.
- F. Gottschalk, C. Lassen, J. Kjoelholt, F. Christensen and B. Nowack, Modeling Flows and Concentrations of Nine Engineered Nanomaterials in the Danish Environment, Int. J. Environ. Res. Public Health, 2015, 12, 5581–5602 CrossRef
.
- S. S. Chopra, Y. Bi, F. C. Brown, T. L. Theis, K. D. Hristovski and P. Westerhoff, Interdisciplinary collaborations to address the uncertainty problem in life cycle assessment of nano-enabled products: case of the quantum dot-enabled display, Environ. Sci.:Nano, 2019, 6, 3256–3267 RSC
.
- J. Gigault, C. Guilmette, H. Cai, C. Carrier-Belleau, M. Le Bagousse, A. Luthi-Maire, M. Gibaud, A. Decaulne and M. Alam, Waste Combustion Releases Anthropogenic Nanomaterials in Indigenous Arctic Communities, Environ. Sci. Technol., 2024, 58, 15170–15180 Search PubMed
.
- S. Mahendra, H. Zhu, V. L. Colvin and P. J. Alvarez, Quantum Dot Weathering Results in Microbial Toxicity, Environ. Sci. Technol., 2008, 42, 9424–9430 CrossRef
.
- S. Zhang, Y. Jiang, C. Chen, J. Spurgin, K. A. Schwehr, A. Quigg, W. Chin and P. H. Santschi, Aggregation, Dissolution, and Stability of Quantum Dots in Marine Environments: Importance of Extracellular Polymeric Substances, Environ. Sci. Technol., 2012, 46, 8764–8772 CrossRef PubMed
.
- Q. Liu, X. Ding, Y. Pang, Y. Cao, J. Lei, J. Wu and T. Zhang, New insights into the safety assessment of quantum dots: potential release pathways, environmental transformations, and health risks, Environ. Sci.:Nano, 2022, 9, 3277–3311 RSC
.
- Y. Wang, A. Miao, J. Luo, Z. Wei, J. Zhu and L. Yang, Bioaccumulation of CdTe quantum dots in a freshwater alga Ochromonas danica: a kinetics study, Environ. Sci. Technol., 2013, 47, 10601–10610 CrossRef PubMed
.
- M. D. Whiteside, K. K. Treseder and P. R. Atsatt, The brighter side of soils: quantum dots track organic nitrogen through fungi and plants, Ecology, 2009, 90, 100–108 CrossRef PubMed
.
- N. Al-Salim, E. Barraclough, E. Burgess, B. Clothier, M. Deurer, S. Green, L. Malone and G. Weir, Quantum dot transport in soil, plants, and insects, Sci. Total Environ., 2011, 409, 3237–3248 CrossRef PubMed
.
- Y. Koo, J. Wang, Q. Zhang, H. Zhu, E. W. Chehab, V. L. Colvin, P. J. J. Alvarez and J. Braam, Fluorescence Reports Intact Quantum Dot Uptake into Roots and Translocation to Leaves of Arabidopsis thaliana and Subsequent Ingestion by Insect Herbivores, Environ. Sci. Technol., 2015, 49, 626–632 CrossRef PubMed
.
- W. M. Lee and Y. J. An, Evidence of three-level trophic transfer of quantum dots in an aquatic food chain by using bioimaging, Nanotoxicology, 2015, 9, 407–412 CrossRef CAS PubMed
.
- K. M. Metz, A. N. Mangham, M. J. Bierman, S. Jin, R. J. Hamers and J. A. Pedersen, Engineered Nanomaterial Transformation under Oxidative Environmental Conditions: Development of an in vitro Biomimetic Assay, Environ. Sci. Technol., 2009, 43, 1598–1604 CrossRef CAS PubMed
.
- C. Martín, G. Jun, R. Schurhammer, G. Reina, P. Chen, A. Bianco and C. Ménard-Moyon, Enzymatic Degradation of Graphene Quantum Dots by
Human Peroxidases, Small, 2019, 15, 1905405 CrossRef
.
- I. Srivastava, D. Sar, P. Mukherjee, A. S. Schwartz-Duval, Z. Huang, C. Jaramillo, A. Civantos, I. Tripathi, J. P. Allain, R. Bhargava and D. Pan, Enzyme-catalysed biodegradation of carbon dots follows sequential oxidation in a time dependent manner, Nanoscale, 2019, 11, 8226–8236 RSC
.
-
B. B. Buchanan, W. Gruissem and R. L. Jones, Biochemistry and Molecular Biology of Plants, Science Press, Beijing, 2002, vol. 35, pp. 105–106 Search PubMed
.
- H. Zhu, J. Han, J. Q. Xiao and Y. Jin, Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants, J. Environ. Monit., 2008, 10, 713–717 RSC
.
- R. Nair, S. H. Varghese, B. G. Nair, T. Maekawa, Y. Yoshida and D. S. Kumar, Nanoparticulate material delivery to plants, Plant Sci., 2010, 179, 154–163 CrossRef CAS
.
-
M. A. U. Khan, H. Arshad and A. Majid, Quantum Dots for Plant Systems, Springer International Publishing, Cham, 2022, pp. 103–136 Search PubMed
.
- C. Peng, D. Duan, C. Xu, Y. Chen, L. Sun, H. Zhang, X. Yuan, L. Zheng, Y. Yang, J. Yang, X. Zhen, Y. Chen and J. Shi, Translocation and biotransformation of CuO nanoparticles in rice (Oryza sativa L.) plants, Environ. Pollut., 2015, 197, 99–107 CrossRef CAS
.
- V. Rajput, T. Minkina, M. Mazarji, S. Shende, S. Sushkova, S. Mandzhieva, M. Burachevskaya, V. Chaplygin, A. Singh and H. Jatav, Accumulation of nanoparticles in the soil-plant systems and their effects on human health, Ann. Agric. Sci., 2020, 65, 137–143 CrossRef
.
- M. H. Wong, R. P. Misra, J. P. Giraldo, S. Kwak, Y. Son, M. P. Landry, J. W. Swan, D. Blankschtein and M. S. Strano, Lipid Exchange Envelope Penetration (LEEP) of Nanoparticles for Plant Engineering: A Universal Localization Mechanism, Nano Lett., 2016, 16, 1161–1172 CrossRef CAS PubMed
.
- X. Ma and J. Yan, Plant uptake and accumulation of engineered metallic nanoparticles from lab to field conditions, Curr. Opin. Environ. Sci. Health, 2018, 6, 16–20 CrossRef
.
- F. Lian, C. Wang, C. Wang, S. Gu and X. Cao, Variety-dependent responses of rice plants with differential cadmium accumulating capacity to cadmium telluride quantum dots (CdTe QDs): Cadmium uptake, antioxidative enzyme activity, and gene expression, Sci. Total Environ., 2019, 697, 134083 CrossRef CAS PubMed
.
- S. Majumdar, L. Pagano, J. A. Wohlschlegel, M. Villani, A. Zappettini, J. C. White and A. A. Keller, Proteomic, gene and metabolite characterization reveal the uptake and toxicity mechanisms of cadmium sulfide quantum dots in soybean plants, Environ. Sci.:Nano, 2019, 6, 3010–3026 RSC
.
- Y. Su, V. Ashworth, C. Kim, A. S. Adeleye, P. Rolshausen, C. Roper, J. White and D. Jassby, Delivery, uptake, fate, and transport of engineered nanoparticles in plants: a critical review and data analysis, Environ. Sci.:Nano, 2019, 6, 2311–2331 RSC
.
- Z. Azim, N. B. Singh, A. Singh, N. Amist, S. Niharika, R. K. Khare, C. Bano Yadav and V. Yadav, A review summarizing uptake, translocation and accumulation of nanoparticles within the plants: current status and future prospectus, J. Plant Biochem. Biotechnol., 2023, 32, 211–224 CrossRef
.
- A. Singh, N. B. Singh, S. Afzal, T. Singh and I. Hussain, Zinc oxide nanoparticles: a review of their biological
synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants, J. Mater. Sci., 2018, 53, 185–201 CrossRef
.
- F. Schwab, G. Zhai, M. Kern, A. Turner, J. L. Schnoor and M. R. Wiesner, Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants--Critical review, Nanotoxicology, 2016, 10, 257–278 CrossRef PubMed
.
-
H. Tombuloglu, G. Tombuloglu, E. Al-Suhaimi, A. Baykal and K. R. Hakeem, Molecular Impacts of Nanoparticles on Plants and Algae, Academic Press, 2024, pp. 41–63 Search PubMed
.
- P. Sharma, S. L. Kothari, M. Rathore and V. Gour, Properties, variations, roles, and potential applications of epicuticular wax: A review, Turk. J. Bot., 2018, 2, 135–149 CrossRef
.
- C. Yang, C. A. Powell, Y. Duan, R. Shatters and M. Zhang, Antimicrobial Nanoemulsion Formulation with Improved Penetration of Foliar Spray through Citrus Leaf Cuticles to Control Citrus Huanglongbing, PLoS One, 2015, 10, e0133826 CrossRef
.
- H. Kohay, J. Wielinski, J. Reiser, L. A. Perkins, K. Ristroph, J. P. Giraldo and G. V. Lowry, Nanocarrier foliar uptake pathways affect delivery of active agents and plant physiological response, Environ. Sci.:Nano, 2025, 12, 660–674 RSC
.
- P. Bussières, Estimating the number and size of phloem sieve plate pores using longitudinal views and geometric reconstruction, Sci. Rep., 2014, 4, 4929 CrossRef PubMed
.
- A. Avellan, J. Yun, B. P. Morais, E. T. Clement, S. M. Rodrigues and G. V. Lowry, Critical Review: Role of Inorganic Nanoparticle Properties on Their Foliar Uptake and in Planta Translocation, Environ. Sci. Technol., 2021, 55, 13417–13431 CrossRef PubMed
.
- H. Sun, M. Wang, J. Wang and W. Wang, Surface charge affects foliar uptake, transport and physiological effects of functionalized graphene quantum dots in plants, Sci. Total Environ., 2022, 812, 151506 CrossRef PubMed
.
- J. Wang, Y. Gong, X. Yan, R. Han and H. Chen, CdTe-QDs Affect Reproductive Development of Plants through Oxidative Stress, Toxics, 2023, 11, 585 CrossRef PubMed
.
- H. Zhang and W. Su, Classification, uptake, translocation, and detection methods of nanoparticles in crop plants: a review, Environ. Sci.:Nano, 2024, 11, 1847–1870 RSC
.
- M. Djanaguiraman, V. Anbazhagan, O. P. Dhankher and P. V. V. Prasad, Uptake, Translocation, Toxicity, and Impact of Nanoparticles on Plant Physiological Processes, Plants, 2024, 13, 3137 CrossRef PubMed
.
- J. Y. Cornu, S. Bussière, C. Coriou, T. Robert, M. Maucourt, C. Deborde, A. Moing and C. Nguyen, Changes in plant growth, Cd partitioning and xylem sap composition in two sunflower cultivars exposed to low Cd concentrations in hydroponics, Ecotoxicol. Environ. Saf., 2020, 205, 111145 CrossRef PubMed
.
- P. Miralles, T. L. Church and A. T. Harris, Toxicity, Uptake, and Translocation of Engineered Nanomaterials in Vascular plants, Environ. Sci. Technol., 2012, 46, 9224–9239 CrossRef
.
- X. Xu, C. Zhao, K. Qian, M. Sun, Y. Hao, L. Han, C. Wang, C. Ma, J. C. White and B. Xing, White Physiological responses of pumpkin to zinc oxide quantum dots and nanoparticles, Environ. Pollut., 2022, 296, 118723 CrossRef PubMed
.
- R. Dong, W. Li, Y. Kang, X. Yang, S. Qu and X. Zhang,
et al., Uptake, translocation and toxicity of fluorescent carbon dots in oyster mushroom (Pleurotus ostreatus), J. Lumin., 2021, 235, 118010 CrossRef
.
- X. Yan, J. Wang, D. Li, J. Feng, Q. Xu, H. Chen and R. Han, Response of primary root to nitrogen-doped carbon dots in Arabidopsis thaliana: alterations in auxin level and cell division activity, Environ. Sci.:Nano, 2021, 8, 1352–1363 RSC
.
- L. Pagano, R. Rossi, J. C. White, N. Marmiroli and M. Marmiroli, Nanomaterials biotransformation: In planta mechanisms of action, Environ. Pollut., 2023, 318, 120834 CrossRef
.
- L. Pagano, M. Marmiroli, M. Villani, J. Magnani, R. Rossi, A. Zappettini, J. C. White and N. Marmiroli, Engineered Nanomaterial Exposure Affects Organelle Genetic Material Replication in Arabidopsis thaliana, ACS Nano, 2022, 16, 2249–2260 CrossRef PubMed
.
- M. Marmiroli, G. O. Lepore, L. Pagano, F. D'Acapito, A. Gianoncelli, M. Villani, L. Lazzarini, J. C. White and N. Marmiroli, The fate of CdS quantum dots in plants as revealed by extended X-ray absorption fine structure (EXAFS) analysis, Environ. Sci.:Nano, 2020, 7, 1150–1162 RSC
.
- L. Pagano, E. Maestri, J. C. White, N. Marmiroli and M. Marmiroli, Quantum dots exposure in plants: Minimizing the adverse response, Curr. Opin. Environ. Sci. Health, 2018, 6, 71–76 CrossRef
.
- M. R. Khan, V. Adam, T. F. Rizvi, B. Zhang, F. Ahamad, I. Jośko, Y. Zhu, M. Yang and C. Mao, Nanoparticle–Plant Interactions: Two-Way Traffic, Small, 2019, 15, 1901794 CrossRef PubMed
.
- C. Ma, J. C. White, J. Zhao, Q. Zhao and B. Xing, Uptake of Engineered Nanoparticles by Food Crops: Characterization, Mechanisms, and Implications, Annu. Rev. Food Sci. Technol., 2018, 9, 129–153 CrossRef
.
- B. Guo, G. Liu, W. Li, C. Hu, B. Lei, J. Zhuang, M. Zheng and Y. Liu, The role of carbon dots in the life cycle of crops, Ind. Crops Prod., 2022, 187, 115427 CrossRef
.
- H. Yang, C. Wang, F. Chen, L. Yue, X. Cao, J. Li, X. Zhao, F. Wu, Z. Wang and B. Xing, Foliar carbon dot amendment modulates carbohydrate metabolism, rhizospheric properties and drought tolerance in maize seedling, Sci. Total Environ., 2022, 809, 151105 CrossRef
.
- M. Kolackova, A. Moulick, P. Kopel, M. Dvorak, V. Adam, B. Klejdus and D. Huska, Antioxidant, gene expression and metabolomics fingerprint analysis of Arabidopsis thaliana treated by foliar spraying of ZnSe quantum dots and their growth inhibition of Agrobacterium tumefaciens, J. Hazard. Mater., 2019, 365, 932–941 CrossRef
.
- R. Banerjee, P. Goswami, M. Chakrabarti, D. Chakraborty, A. Mukherjee and A. Mukherjee, Cadmium selenide (CdSe) quantum dots cause genotoxicity and oxidative stress in Allium cepa plants, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2021, 865, 503338 CrossRef PubMed
.
- N. Andleeb, S. Zafar, Z. Rahim, M. M. Iqbal, R. Lal and M. A. Farooq, Carbon quantum dots as versatile nanomaterials for improving soil health and plant stress tolerance: a comprehensive review, Planta, 2025, 262, 44 CrossRef PubMed
.
- H. Li, J. Huang, F. Lu, Y. Liu, Y. Song, Y. Sun, J. Zhong, H. Huang, Y. Wang, S. Li, Y. Lifshitz and S. Lee, Impacts of Carbon Dots on Rice Plants: Boosting the Growth and Improving the Disease Resistance, ACS Appl. Bio Mater., 2018, 1, 663–672 CrossRef
.
- Y. Li, X. Pan, X. Xu, Y. Wu, J. Zhuang, X. Zhang, H. Zhang, B. Lei, C. Hu and Y. Liu, Carbon dots as light converter for plant photosynthesis: Augmenting light coverage and quantum yield effect, J. Hazard. Mater., 2021, 410, 124534 CrossRef
.
- T.
L. Tan, N. A. Zulkifli, A. S. K. Zaman, M. B. Jusoh, M. N. Yaapar and S. A. Rashid, Impact of photoluminescent carbon quantum dots on photosynthesis efficiency of rice and corn crops, Plant Physiol. Biochem., 2021, 162, 737–751 CrossRef
.
- Y. Li, X. Xu, B. Lei, J. Zhuang, X. Zhang, C. Hu, J. Cui and Y. Liu, Magnesium-nitrogen co-doped carbon dots enhance plant growth through multifunctional regulation in photosynthesis, Chem. Eng. J., 2021, 422, 130114 CrossRef
.
- S. Tripathi and S. Sarkar, Influence of water soluble carbon dots on the growth of wheat plant, Appl. Nanosci., 2015, 5, 609–616 CrossRef
.
- Y. Gong and Z. Dong, Transfer, transportation, and accumulation of cerium-doped carbon quantum dots: Promoting growth and development in wheat, Ecotoxicol. Environ. Saf., 2021, 226, 112852 CrossRef
.
- C. Wang, H. Yang, F. Chen, L. Yue, Z. Wang and B. Xing, Nitrogen-Doped Carbon Dots Increased Light Conversion and Electron Supply to Improve the Corn Photosystem and Yield, Environ. Sci. Technol., 2021, 55, 12317–12325 CrossRef
.
- I. Milenković, M. Borišev, Y. Zhou, S. Z. Spasić, R. M. Leblanc and K. Radotić, Photosynthesis Enhancement in Maize via Nontoxic Orange Carbon Dots, J. Agric. Food Chem., 2021, 69, 5446–5451 CrossRef
.
- C. Wang, Y. Yao, L. Yue, F. Chen, X. Cao, J. Li, H. Yang, N. Zhang, T. Liu, Z. Wang and B. Xing, Regulation Mechanisms of Nitrogen-Doped Carbon Dots in Enhanced Maize Photosynthesis under Drought Stress, ACS Agric. Sci. Technol., 2023, 3, 181–189 CrossRef
.
- Y. Li, W. Li, H. Zhang, R. Dong, D. Li, Y. Liu, L. Huang and B. Lei, Biomimetic preparation of silicon quantum dots and their phytophysiology effect on cucumber seedlings, J. Mater. Chem. B, 2019, 7, 1107–1115 RSC
.
- Z. Liang, X. Pan, W. Li, E. Kou, Y. Kang, B. Lei and S. Song, Dose-Dependent Effect of ZnO Quantum Dots for Lettuce Growth, ACS Omega, 2021, 6, 10141–10149 CrossRef CAS
.
- Y. Li, W. Li, H. Zhang, Y. Liu, L. Ma and B. Lei, Amplified light harvesting for enhancing Italian lettuce photosynthesis using water soluble silicon quantum dots as artificial antennas, Nanoscale, 2020, 12, 155–166 RSC
.
- L. Pagano, F. Pasquali, S. Majumdar, R. De la Torre-Roche, N. Zuverza-Mena, M. Villani, A. Zappettini, R. E. Marra, S. M. Isch, M. Marmiroli, E. Maestri, O. P. Dhankher, J. C. White and N. Marmiroli, Exposure of Cucurbita pepo to binary combinations of engineered nanomaterials: physiological and molecular response, Environ. Sci.:Nano, 2017, 4, 1579–1590 RSC
.
- A. D. E. S. Pereira, H. C. Oliveira, L. F. Fraceto and C. Santaella, Nanotechnology Potential in Seed Priming for Sustainable Agriculture, Nanomaterials, 2021, 11, 267 CrossRef PubMed
.
- A. Maholiya, P. Ranjan, R. Khan, S. Murali, R. C. Nainwal, P. S. Chauhan, N. Sathish, J. P. Chaurasia and A. K. Srivastava, An insight into the role of carbon dots in the agriculture system: a review, Environ. Sci.:Nano, 2023, 10, 959–995 RSC
.
- W. Li, Y. Zheng, H. Zhang, Z. Liu, W. Su, S. Chen, Y. Liu, J. Zhuang and B. Lei, Phytotoxicity, Uptake, and Translocation of Fluorescent Carbon Dots in Mung Bean Plants, ACS Appl. Mater. Interfaces, 2016, 8, 19939–19945 CrossRef PubMed
.
- H. Wang, M. Zhang, Y. Song, H. Li, H. Huang, M. Shao, Y. Liu and Z. Kang, Carbon dots promote the growth and photosynthesis of mung bean sprouts, Carbon, 2018, 136, 94–102 CrossRef
.
- H. Li, J. Huang, Y. Liu, F. Lu, J. Zhong, Y. Wang, S. Li, Y. Lifshitz, S. Lee and Z. Kang, Enhanced RuBisCO activity and promoted dicotyledons growth with degradable carbon dots, Nano Res., 2019, 12, 1585–1593 CrossRef
.
- M. N. Khan, M. Mobin, Z. K. Abbas, K. A. Almutairi and Z. H. Siddiqui, Role of nanomaterials in plants under challenging environments, Plant Physiol. Biochem., 2017, 110, 194–209 CrossRef PubMed
.
- E. Kou, Y. Yao, X. Yang, S. Song, W. Li, Y. Kang, S. Qu, R. Dong, X. Pan, D. Li, H. Zhang and B. Lei, Regulation Mechanisms of Carbon Dots in the Development of Lettuce and Tomato, ACS Sustainable Chem. Eng., 2021, 9, 944–953 CrossRef
.
- Y. Zheng, G. Xie, X. Zhang, Z. Chen, Y. Cai, W. Yu, H. Liu, J. Shan, R. Li, Y. Liu and B. Lei, Bioimaging Application and Growth-Promoting Behavior of Carbon Dots from Pollen on Hydroponically Cultivated Rome Lettuce, ACS Omega, 2017, 2, 3958–3965 CrossRef PubMed
.
- J. Hu, W. Jia, X. Yu, C. Yan, J. C. White, J. Liu, G. Shen, S. Tao and X. Wang, Carbon dots improve the nutritional quality of coriander (Coriandrum sativum L.) by promoting photosynthesis and nutrient uptake, Environ. Sci.:Nano, 2022, 9, 1651–1661 RSC
.
- M. Farshbaf, S. Davaran, F. Rahimi, N. Annabi, R. Salehi and A. Akbarzadeh, Carbon quantum dots: recent progresses on synthesis, surface modification and applications, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 1331–1348 CrossRef PubMed
.
- G. Li, J. Xu and K. Xu, Physiological Functions of Carbon Dots and Their Applications in Agriculture: A Review, Nanomaterials, 2023, 13, 2684 CrossRef PubMed
.
- J. Liu, Y. Geng, D. Li, H. Yao, Z. Huo, Y. Li, K. Zhang, S. Zhu, H. Wei, W. Xu, J. Jiang and B. Yang, Deep Red Emissive Carbonized Polymer Dots with Unprecedented Narrow Full Width at Half Maximum, Adv. Mater., 2020, 32, 1906641 CrossRef
.
- S. Zhu, Q. Meng, L. Wang, J. Zhang, Y. Song, H. Jin, K. Zhang, H. Sun, H. Wang and B. Yang, Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging, Angew. Chem., Int. Ed., 2013, 52, 3953–3957 CrossRef PubMed
.
- M. Hatami, K. Kariman and M. Ghorbanpour, Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants, Sci. Total Environ., 2016, 571, 275–291 CrossRef PubMed
.
- I. Nabiev, A. Rakovich, A. Sukhanova, E. Lukashev, V. Zagidullin, V. Pachenko, Y. P. Rakovich, J. F. Donegan, A. B. Rubin and A. O. Govorov, Fluorescent Quantum Dots as Artificial Antennas for Enhanced Light Harvesting and Energy Transfer to Photosynthetic Reaction Centers, Angew. Chem., Int. Ed., 2010, 49, 7217–7221 CrossRef PubMed
.
- S. Chandra, S. Pradhan, S. Mitra, P. Patra, A. Bhattacharya, P. Pramanik and A. Goswami, High throughput electron transfer from carbon dots
to chloroplast: a rationale of enhanced photosynthesis, Nanoscale, 2014, 6, 3647–3655 RSC
.
- M. Paul, Photosynthesis: Plastid biology, energy conversion and carbon assimilation, Ann. Bot., 2013, 111, 3 CrossRef
.
- S. Dhiman, N. Debnath and S. Das, Carbon Based Photosynthetic Biohybrid System: A New Approach to Energy Conversion, Bionanoscience, 2024, 14, 4910–4929 CrossRef
.
- M. Zhang, L. Hu, H. Wang, Y. Song, Y. Liu, H. Li, M. Shao, H. Huang and Z. Kang, One-step hydrothermal synthesis of chiral carbon dots and their effects on mung bean plant growth, Nanoscale, 2018, 10, 12734–12742 RSC
.
- G. R. Cramer, K. Urano, S. Delrot, M. Pezzotti and K. Shinozaki, Effects of abiotic stress on plants: a systems biology perspective, BMC Plant Biol., 2011, 11, 163 CrossRef PubMed
.
- R. S. Taha, M. F. Seleiman, A. Shami, B. A. Alhammad and A. H. A. Mahdi, Integrated Application of Selenium and Silicon Enhances Growth and Anatomical Structure, Antioxidant Defense System and Yield of Wheat Grown in Salt-Stressed Soil, Plants, 2021, 10, 1040 CrossRef
.
- J. Yang, J. Zhang, Z. Wang, Q. Zhu and W. Wang, Remobilization of carbon reserves in response to water deficit during grain filling of rice, Field Crops Res., 2001, 71, 47–55 CrossRef
.
- F. K. Choudhury, R. M. Rivero, E. Blumwald and R. Mittler, Reactive oxygen species, abiotic stress and stress combination, Plant J., 2017, 90, 856–867 CrossRef PubMed
.
- S. Farhangi-Abriz, K. Ghassemi-Golezani, S. Torabian, S. Rahimzadeh, F. Osati and H. Safarpour, Response of Soybean Plants to the Foliar Application of Carbon Quantum Dots Under Drought Stress: A Field Study, J. Plant Growth Regul., 2024, 44, 621–631 CrossRef
.
- L. Su, X. Ma, K. Zhao, C. Shen, Q. Lou, D. Yin and C. Shan, Carbon Nanodots for Enhancing the Stress Resistance of Peanut Plants, ACS Omega, 2018, 3, 17770–17777 CrossRef
.
- H. Wang, Y. Kang, H. Li, S. Huang, W. Li, M. Zheng, R. Huang, B. Lei and X. Yang, Salvia miltiorrhiza Derived Carbon Dots and Their Heat Stress Tolerance of Italian Lettuce by Promoting Growth and Enhancing Antioxidant Enzyme Activity, ACS Omega, 2021, 6, 32262–32269 CrossRef PubMed
.
- C. Wang, Y. Ji, X. Cao, L. Yue, F. Chen, J. Li, H. Yang, Z. Wang and B. Xing, Carbon Dots Improve Nitrogen Bioavailability to Promote the Growth and Nutritional Quality of Soybeans under Drought Stress, ACS Nano, 2022, 16, 12415–12424 CrossRef
.
- S. Chai, Z. Yang, X. Deng, L. Wang, Y. Jiang, J. Liao, R. Yang, X. Wang and L. Zhang, ZnO quantum dots alleviate salt stress in Salvia miltiorrhiza by enhancing growth, scavenging reactive oxygen species, and modulating stress-responsive genes, Environ. Pollut., 2024, 344, 123363 CrossRef
.
- H. Wang, C. Qian, H. Jiang, S. Liu, D. Yang and J. Cui, Visible-Light-Driven Zinc Oxide Quantum Dots for the Management of Bacterial Fruit Blotch Disease and the Improvement of Melon Seedlings Growth, J. Agric. Food Chem., 2023, 71, 2773–2783 CrossRef PubMed
.
- X. Luo, X. Cao, C. Wang, L. Yue, X. Chen, H. Yang, X. Le, X. Zhao, F. Wu, Z. Wang and B. Xing, Nitrogen-doped carbon dots alleviate the damage from tomato bacterial wilt syndrome: systemic acquired resistance activation and reactive oxygen species scavenging, Environ. Sci.:Nano, 2021, 8, 3806–3819 RSC
.
- X. Hua, Y. Bao, Z. Chen and F. Wu, Carbon quantum dots with intrinsic mitochondrial targeting ability for mitochondria-based theranostics, Nanoscale, 2017, 9, 10948–10960 RSC
.
- S. Alfei, G. C. Schito, A. M. Schito and G. Zuccari, Reactive Oxygen Species (ROS)-Mediated Antibacterial Oxidative Therapies: Available Methods to Generate ROS and a Novel Option Proposal, Int. J. Mol. Sci., 2024, 25, 7182 CrossRef CAS PubMed
.
- Y. Li, X. Xu, Y. Wu, J. Zhuang, X. Zhang, H. Zhang, B. Lei, C. Hu and Y. Liu, A review on the effects of carbon dots in plant systems, Mater. Chem. Front., 2020, 4, 437–448 RSC
.
- E. Kou, Z. Luo, J. Ye, X. Chen, D. Lu, M. P. Landry, H. Zhang and H. Zhang, Sunlight-sensitive carbon dots for plant immunity priming and pathogen defence, Plant Biotechnol. J., 2025, 23, 2150–2161 CrossRef CAS
.
- C. Merten, R. Schoonjans, D. Di Gioia, C. Peláez, Y. Sanz, D. Maurici and T. Robinson, Editorial: Exploring the need to include microbiomes into EFSA's scientific assessments, EFSA J., 2020, 18, e18061 Search PubMed
.
- P. Trivedi, J. E. Leach, S. G. Tringe, T. Sa and B. K. Singh, Plant–microbiome interactions: from community assembly to plant health, Nat. Rev. Microbiol., 2020, 18, 607–621 CrossRef CAS
.
- N. Fan, C. Zhao, Z. Chang, L. Yue, F. He, Z. Xiao and Z. Wang, Silicon quantum dots promote radish resistance to root herbivores without impairing rhizosphere microenvironment health, Environ. Sci.:Nano, 2023, 10, 2232–2244 RSC
.
- K. Yin, Q. Bao, J. Li, M. Wang, F. Wang, B. Sun, Y. Gong and F. Lian, Molecular mechanisms of growth promotion and selenium enrichment in tomato plants by novel selenium-doped carbon quantum dots, Chemosphere, 2024, 364, 143175 CrossRef CAS PubMed
.
- X. Xu, A. Liang, H. Li, H. Shang, K. Qian, W. Jia, J. C. White, C. Ma and B. Xing, Foliar Applied ZnO Quantum Dots Boost Pumpkin (Cucurbita moschata Duch.) Growth and Positively Alter Endophytic and Rhizosphere Microbial Communities, ACS Sustainable Chem. Eng., 2023, 11, 8503–8516 CrossRef CAS
.
- H. Zhang, T. Zheng, Y. Wang, T. Li and Q. Chi, Multifaceted impacts of nanoparticles on plant nutrient absorption and soil microbial communities, Front. Plant Sci., 2024, 15, 1497006 CrossRef PubMed
.
- J. Prokisch, D. H. H. Nguyen, A. Muthu, A. Ferroudj, A. Singh, S. Agrawal, V. D. Rajput, K. Ghazaryan, H. El-Ramady and M. Rai, Carbon Nanodot–Microbe–Plant Nexus in Agroecosystem and Antimicrobial Applications, Nanomaterials, 2024, 14, 1249 CrossRef CAS
.
- M. Wang, C. Mu, X. Lin, W. Ma, H. Wu, D. Si, C. Ge, C. Cheng, L. Zhao, H. Li and D. Zhou, Foliar Application of Nanoparticles Reduced Cadmium Content in Wheat (Triticum aestivum L.) Grains via Long-Distance “Leaf–Root–Microorganism” Regulation, Environ. Sci. Technol., 2024, 58, 6900–6912 CrossRef CAS PubMed
.
- X. Yan, Q. Xu, D. Li, J. Wang and R. Han, Carbon dots inhibit root growth by disrupting auxin biosynthesis and transport in Arabidopsis, Ecotoxicol. Environ. Saf., 2021, 216, 112168 CrossRef CAS PubMed
.
- Q. Shi, T. Li, H. Xiang, Y. Wu, S. Yuan, M. Yuan, S. Haider and Y. Chen, Cysteine Alleviates the Toxicity of Cadmium Telluride Quantum Dots (CdTe QDs) by Modulating the Antioxidant System and Root Metabolic Pathways in Rice, Physiol. Plant., 2025, 177, e70493 CrossRef CAS PubMed
.
- M. Marmiroli, L. Pagano, M. L. Savo Sardaro, M. Villani and N. Marmiroli, Genome-Wide Approach in Arabidopsis thaliana to Assess the Toxicity of Cadmium Sulfide Quantum Dots, Environ. Sci. Technol., 2014, 48, 5902–5909 CrossRef CAS
.
- M. Marmiroli, F. Mussi, L. Pagano, D. Imperiale, G. Lencioni, M. Villani, A. Zappettini, J. C. White and N. Marmiroli, Cadmium sulfide quantum dots impact Arabidopsis thaliana physiology and morphology, Chemosphere, 2020, 240, 124856 CrossRef CAS PubMed
.
- H. Chen, Y. Gong and R. Han, Cadmium Telluride Quantum Dots (CdTe-QDs) and Enhanced Ultraviolet-B (UV-B) Radiation Trigger Antioxidant Enzyme Metabolism and Programmed Cell Death in Wheat Seedlings, PLoS One, 2014, 9, e110400 CrossRef
.
- X. Li, L. Mu, D. Li, S. Ouyang, C. He and X. Hu, Effects of the size and oxidation of graphene oxide on crop quality and specific molecular pathways, Carbon, 2018, 140, 352–361 CrossRef CAS
.
- P. Zhang, X. Wu, Z. Guo, X. Yang, X. Hu and I. Lynch, Stress Response and Nutrient Homeostasis in Lettuce (Lactuca sativa) Exposed to Graphene Quantum Dots Are Modulated by Particle Surface Functionalization, Adv. Biol., 2021, 5, 2000778 CrossRef CAS
.
- P. Modlitbová, P. Pořízka, K. Novotný, J. Drbohlavová, I. Chamradová, Z. Farka, H. Zlámalová-Gargošová, T. Romih and J. Kaiser, Short-term assessment of cadmium toxicity and uptake from different types of Cd-based Quantum Dots in the model plant Allium cepa L, Ecotoxicol. Environ. Saf., 2018, 153, 23–31 CrossRef PubMed
.
- G. Marslin, C. J. Sheeba and G. Franklin, Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst, Front. Plant Sci., 2017, 8, 832 CrossRef
.
- P. M. Mullineaux, M. Exposito-Rodriguez, P. P. Laissue and N. Smirnoff, ROS-dependent signalling pathways in plants and algae exposed to high light: Comparisons with other eukaryotes, Free Radical Biol. Med., 2018, 122, 52–64 CrossRef CAS
.
- R. Biczak, A. Telesiński and B. Pawłowska, Oxidative stress in spring barley and common radish exposed to quaternary ammonium salts with hexafluorophosphate anion, Plant Physiol. Biochem., 2016, 107, 248–256 CrossRef CAS PubMed
.
- S. K. Verma, A. K. Das, M. K. Patel, A. Shah, V. Kumar and S. Gantait, Engineered nanomaterials for plant growth and development: A perspective analysis, Sci. Total Environ., 2018, 630, 1413–1435 CrossRef CAS PubMed
.
- S. Majumdar, C. Ma, M. Villani, N. Zuverza-Mena, L. Pagano, Y. Huang, A. Zappettini, A. A. Keller, N. Marmiroli, O. P. Dhankher and J. C. White, Surface coating determines the response of soybean plants to cadmium sulfide quantum dots, NanoImpact, 2019, 14, 100151 CrossRef
.
- Y. Hu, J. Li, L. Ma, Q. Peng, W. Feng, L. Zhang, S. He, F. Yang, J. Huang and L. Li, High efficiency transport of quantum dots into plant roots with the aid of silwet L-77, Plant Physiol. Biochem., 2010, 48, 703–709 CrossRef CAS
.
- P. Modlitbová, P. Pořízka, S. Střítežská, Š. Zezulka, M. Kummerová, K. Novotný and J. Kaiser, Detail investigation of toxicity, bioaccumulation, and translocation of Cd-based quantum dots and Cd salt in white mustard, Chemosphere, 2020, 251, 126174 CrossRef
.
| This journal is © The Royal Society of Chemistry 2026 |