Reductive amination of triglycerides to fatty amines over a titanium oxide-supported Pt–Mo catalyst†
Katsumasa
Sakoda
a,
Harumi
Furugaki
a,
Sho
Yamaguchi
 abc,
Takato
Mitsudome
abc,
Takato
Mitsudome
 abc and
Tomoo
Mizugaki
abc and
Tomoo
Mizugaki
 *abd
*abd
aDepartment of Materials Engineering Science, Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan. E-mail: mizugaki.tomoo.es@osaka-u.ac.jp
bInnovative Catalysis Science Division, Institute for Open and Transdisciplinary Research Initiatives (ICS-OTRI), Osaka University, Suita, Osaka 565-0871, Japan
cPRESTO, Japan Science and Technology Agency (JST), 4-1-8 Honcho, Kawaguchi, Saitama 333-0012, Japan
dResearch Center for Solar Energy Chemistry, Graduate School of Engineering Science, Osaka University, 1-3 Machikaneyama, Toyonaka, Osaka 560-8531, Japan
First published on 6th January 2025
Abstract
The reductive amination of naturally abundant triglycerides is a promising approach for the synthesis of fatty amines. However, existing catalytic systems for this transformation typically require harsh reaction conditions. Herein, we present a titanium oxide-supported platinum–molybdenum (Pt–Mo/TiO2) catalyst that promotes the reductive amination of triglycerides to fatty amines. The Pt–Mo/TiO2 catalyst exhibits a high activity under milder conditions, specifically at 1 MPa of H2, surpassing the performance of previously reported catalysts. A wide range of triglycerides, including cooking oils, are successfully converted into the corresponding fatty amines in high yields. The Pt–Mo/TiO2 catalyst is reusable and applicable to gram-scale reactions, demonstrating the high potential of Pt–Mo/TiO2 for green and sustainable fatty amine production.
Introduction
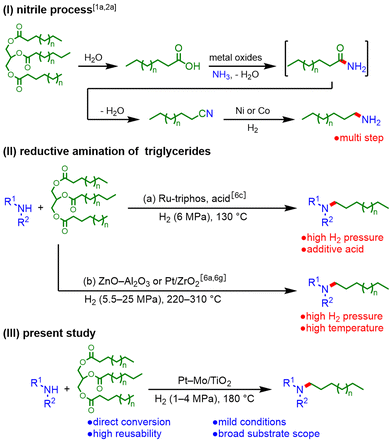
The conversion of biomass-derived renewable carbon resources into valuable chemicals and fuels, instead of relying on fossil resources, has been recognized as a strategy to mitigate the global environmental burden caused by carbon dioxide emissions.1 Among plant-based biomass resources, triglycerides, which are the main component of vegetable oils and fats, are used for the production of fatty acids, fatty alcohols, fatty acid alkyl esters, hydrocarbons, and fatty amines.2 In this context, fatty amines are important due to their diverse applications, including use as fabric softeners, mineral processing reagents, corrosion inhibitors, and lubricants.3 One of the well-known methods for producing fatty amines from triglycerides is the nitrile process.1a,2a This process involves multiple steps: hydrolysis to fatty acids, amidation followed by dehydration to form nitriles, and hydrogenation to produce fatty amines. It requires substantial amounts of solvent and energy for the synthesis and purification of intermediates (Scheme 1(I)). Therefore, the development of a more efficient and greener method for the direct conversion of triglycerides to fatty amines is desired.4The reductive amination of triglycerides under H2 is a straightforward method for producing fatty amines.5,6 Beller et al. reported a homogeneous catalyst system consisting of a Ru–triphos complex with an additive acid working under 6 MPa H2 at 130 °C (Scheme 1(IIa)).6c In addition, there have been two reports on heterogeneous catalyst systems (Scheme 1(IIb)). The ZnO–Al2O3 catalyst successfully promoted direct reductive amination at 25 MPa H2 and 310 °C.6a Shimizu et al. reported a Pt/ZrO2 catalyst capable of operating under 5.5 MPa H2 and 220 °C.6g However, these catalytic systems require harsh reaction conditions, and their reusability has not yet been investigated. Hence, the development of highly active and reusable catalysts is desired to achieve the green and sustainable production of fatty amines.
Herein, we present the direct reductive amination of triglycerides to fatty amines using a titanium oxide-supported platinum–molybdenum (Pt–Mo/TiO2) catalyst (Scheme 1(III)). The key features of the catalyst system include: (1) mild conditions, such as 1 MPa H2 and 180 °C, in comparison with previously reported catalyst systems; (2) high reusability without loss of activity; and (3) a broad substrate scope, extending to real cooking oil.
Results and discussion
Catalyst performance
Pt–Mo/TiO2 and the other supported catalysts listed in Table 1 were prepared using the co-impregnation method. Catalytic activities were evaluated for the reaction between piperidine (1) and trilaurin (2) under 4 MPa H2 at 180 °C for 6 h (Table 1 and Scheme S1†). The Pt–Mo/TiO2 catalyst afforded 1-dodecylpiperidine (3) in a 35% yield, 1-(1-piperidinyl)-1-dodecanone (4) in a 4% yield, and 1-propylpiperidine (5) in a 3% yield along with unidentified compounds (Table 1, entry 1 and Scheme S2†). When the reaction time was prolonged to 16 h, 3 was obtained in a 70% yield (Table 1, entry 2). The combination of other platinum-group metals (Rh, Ru, and Pd) with Mo, as well as Pt with other metals (Re, W, and V), resulted in lower yields of 3 compared with those of the Pt–Mo catalyst (Table 1, entries 4–9). The support materials for the Pt–Mo catalysts were also tested. ZrO2 and γ-Al2O3 supports were effective in producing 3, although the yields were slightly lower compared with those obtained with TiO2 (Table 1, entries 10 and 11). The use of WO3 and Nb2O5 resulted in a low yield of 3 (Table 1, entries 12 and 13). Single-metal catalysts (Pt/TiO2 and Mo/TiO2) and bare TiO2 were ineffective in the reductive amination (Table 1, entries 14–16), with amide 4 being obtained as the major product. These results demonstrated that the combination of Pt, Mo, and TiO2 was important for efficient reductive amination of triglycerides.| Entry | Catalyst | H2 (MPa) | Time (h) | Conv. of 1b (%) | Yieldb (%) | ||
|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | |||||
| a Reaction conditions: catalyst (0.15 g, Pt: 7 mol% and Mo: 2 mol%), 1 (1 mmol), 2 (0.5 mmol), n-hexane (3 mL), 180 °C. b Determined by gas chromatography-flame ionization detection (GC-FID) using an internal standard method. Yield was based on 1. | |||||||
| 1 | Pt–Mo/TiO2 | 4 | 6 | 54 | 35 | 4 | 3 |
| 2 | Pt–Mo/TiO2 | 4 | 16 | 99 | 70 | 2 | 7 |
| 3 | Pt–Mo/TiO2 | 1 | 60 | >99 | 61 | 1 | 11 |
| 4 | Rh–Mo/TiO2 | 4 | 6 | 35 | 17 | 2 | 1 |
| 5 | Ru–Mo/TiO2 | 4 | 6 | 62 | 8 | 24 | <1 |
| 6 | Pd–Mo/TiO2 | 4 | 6 | 79 | 1 | 67 | 1 |
| 7 | Pt–Re/TiO2 | 4 | 6 | 61 | 26 | 21 | 1 |
| 8 | Pt–W/TiO2 | 4 | 6 | 95 | 14 | 53 | 1 |
| 9 | Pt–V/TiO2 | 4 | 6 | 67 | 14 | 41 | <1 |
| 10 | Pt–Mo/ZrO2 | 4 | 6 | 53 | 32 | 3 | 2 |
| 11 | Pt–Mo/γ-Al2O3 | 4 | 6 | 54 | 30 | 2 | 3 |
| 12 | Pt–Mo/WO3 | 4 | 6 | 23 | 7 | 6 | <1 |
| 13 | Pt–Mo/Nb2O5 | 4 | 6 | 21 | 4 | 2 | 0 |
| 14 | Pt/TiO2 | 4 | 6 | 80 | 16 | 49 | 1 |
| 15 | Mo/TiO2 | 4 | 6 | 59 | 0 | 47 | 0 |
| 16 | TiO2 | 4 | 6 | 70 | 0 | 61 | 0 |
The solvents used in the reductive amination were also investigated (Table S1†). Nonpolar n-hexane was effective for the reductive amination, while the use of tetrahydrofuran as a polar solvent resulted in poor yield of 3. When ethanol was used as the solvent, 1 reacted with ethanol to give 1-ethylpiperidine in >99% yield. The high performance of the Pt–Mo/TiO2 catalyst was further demonstrated in a reaction under 1 MPa H2. The Pt–Mo/TiO2 catalyst promoted the reductive amination under 1 MPa of H2 to afford 3 in a 61% yield (Table 1, entry 3), outperforming previously reported catalysts (Table S2†).6a,c,g The Pt–Mo/TiO2 catalyst was applicable to the gram-scale reaction (Scheme 2); the reaction of 1.0 g of 1 with 3.5 g of 2 produced 1.9 g of 3 in a 62% isolated yield. Furthermore, the reusability of the heterogeneous catalysts is one of their key advantages over homogeneous catalysts. After reductive amination, the Pt–Mo/TiO2 catalyst was recovered by centrifugation and reused in the next reaction without pretreatment. The high activity of the Pt–Mo/TiO2 catalyst was maintained until the tenth cycle (Fig. 1 and Table S3†). Inductively coupled plasma atomic emission spectroscopy analyses of the used Pt–Mo/TiO2 catalyst showed similar Pt and Mo loadings to those of the fresh sample, confirming that the leaching of Pt and Mo into the reaction solution was negligible (Table S4†). These results strongly support the high durability and reusability of the Pt–Mo/TiO2 catalyst.
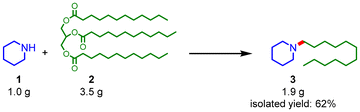 | ||
| Scheme 2 Gram-scale reductive amination. Reaction conditions: Pt–Mo/TiO2 (1.0 g, Pt: 4.1 mol% and Mo: 1.2 mol%), n-hexane (15 mL), 180 °C, H2 (4 MPa), 60 h. Yield was based on 1. | ||
Substrate scope
The substrate scope of the Pt–Mo/TiO2 catalyst was investigated using various amines and 2 (Table 2 and Table S5†). Cyclic amines (1 and azepane) were alkylated to afford the corresponding tertiary amines (3 and 1-dodecylazepane) in yields of 70% and 60%, respectively (Table 2, entries 1 and 2). The Pt–Mo/TiO2 catalyst could be applied to the reductive amination of 2 with primary amines; hexylamine and dodecylamine were alkylated to N-hexyldodecylamine and didodecylamine in 35 and 70% yields, respectively (Table 2, entries 3 and 4). Moreover, NH3 gas was used for the reductive amination of 2, and didodecylamine was obtained in a 74% yield (Table 2, entry 5). The reductive amination using pyridine and aniline proceeded along with hydrogenation of aromatic rings, providing 3 and N-dodecylcyclohexanamine as the major products in 44% and 46% yields, respectively (Table 2, entries 6 and 7).| Entry | Amine | Triglyceride | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: Pt–Mo/TiO2 (0.15 g, Pt: 7 mol% and Mo: 2 mol%), amine (1 mmol), triglyceride (0.5 mmol), n-hexane (3 mL), 180 °C, H2 (4 MPa). b Determined by GC-FID using an internal standard method. Yield was based on amine. c NMR yield. d Pt–Mo/TiO2 (0.15 g, Pt: 4.8 mol% and Mo: 1.4 mol% with respect to the ester moiety), NH3 (0.7 MPa). Yield was based on 2. e Isolated yield. f n-Hexane (5 mL). g Cooking oil (0.5 g). The carboxylic acid contents of the cooking oil are shown in Table S6.† | |||||
| 1 |

|

|

|
16 | 70 |
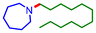
| 2 |

|

|
|
36 | 60c |
| 3 |

|

|

|
36 | 35 |
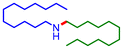
| 4 |

|

|
|
36 | 70 |
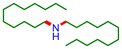
| 5d |

|

|
|
36 | 74 |
| 6 |

|

|

|
24 | 44 |
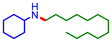
| 7 |

|

|
|
24 | 46 |
| 8 |

|
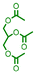
|
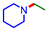
|
12 | 79 |
| 9 |

|
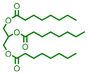
|

|
24 | 62e |
| 10f |

|

|

|
48 | 63e |
| 11f |

|

|

|
48 | 61e |
| 12f,g |

|

|

|
48 | 65c |
The applicability of the Pt–Mo/TiO2 catalyst in the reductive amination of triglycerides with different carbon chain lengths was explored. Pt–Mo/TiO2 promoted the amination of triacetin, tricaprylin, tripalmitin, and tristearin, yielding N-alkylated piperidine in over 61% yield (Table 2, entries 8–11). The utility of the Pt–Mo/TiO2 system was also investigated in a reaction using real cooking oil, the composition of which is shown in Table S6,† without pretreatment. The cooking oil was successfully aminated to the corresponding fatty amines (1-hexadecylpiperidine and 1-octadecylpiperidine) in a 65% total yield (Table 2, entry 12), demonstrating the versatility of Pt–Mo/TiO2.
Reaction pathway
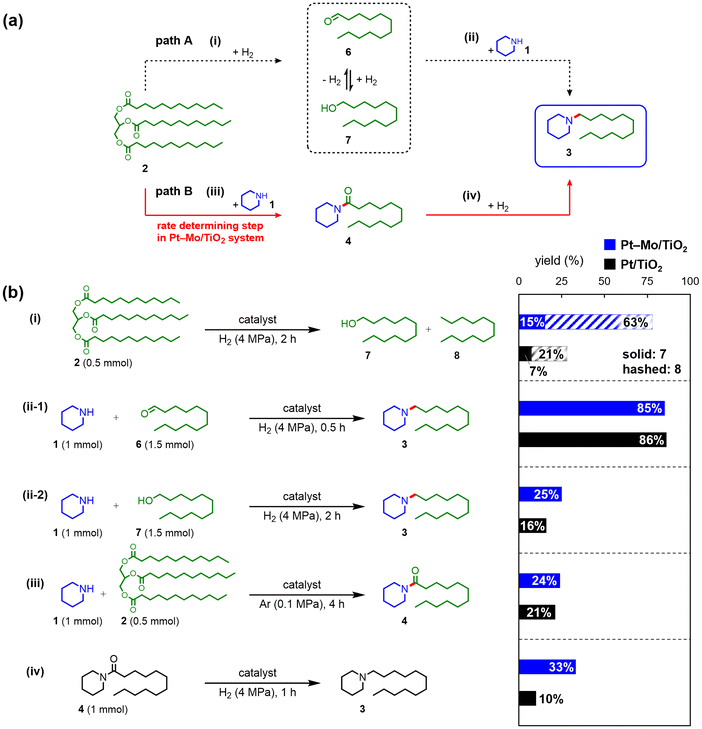
The reaction pathways in the Pt–Mo/TiO2-catalyzed reductive amination of triglycerides were investigated. A time-course experiment was performed to obtain information on intermediates. The yield of 1-dodecanol (7) increased to 10% over 6 h and then decreased, whereas the formation of 4 was observed in a less than 4% yield throughout the reaction (Fig. S1†). In addition, various TiO2-supported catalysts and bare TiO2 promoted the amidation of 2 with 1 to 4 (Table 1, entries 1–9 and 14–16). These results suggested that 7 and 4 were the potential intermediates in this reaction. Based on the above results, two reaction pathways were proposed: hydrogenation of triglycerides to aldehydes or alcohols followed by amination (path A), and amidation of triglycerides followed by hydrogenation of amides to amines (path B) (Fig. 2a).6d,f,gCatalysis by Pt–Mo
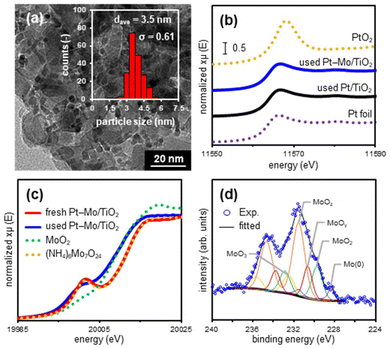
To gain insight into the high activity of Pt–Mo/TiO2, its activity in each reaction step was evaluated and compared with that of Pt/TiO2 as a reference (Fig. 2b and Table S7†). The Pt–Mo/TiO2 catalyst promoted the hydrogenation of 2 to afford 7 and n-dodecane (8) in yields of 15% and 63%, respectively, whereas Pt/TiO2 afforded considerably lower yields than Pt–Mo/TiO2 (Fig. 2b(i)). In the hydrogenation of 4, the Pt–Mo/TiO2 catalyst showed a higher activity towards 3 compared with using Pt/TiO2. The yield of 3 was three times higher with Pt–Mo/TiO2 (33%) than that obtained with Pt/TiO2 (10%) (Fig. 2b(iv)). Other control experiments, such as the reductive amination of 1-dodecanal (6) (Fig. 2b(ii-1)), amination of 7 (Fig. 2b(ii-2)), and amidation of 2 (Fig. 2b(iii)), did not show significant differences in yields. Therefore, control experiments demonstrated that cooperative catalysis between Pt and Mo promoted the reaction steps of hydrogenation of triglycerides and amides more efficiently.Furthermore, the structure of the catalyst during reductive amination was investigated. TEM images of the used Pt–Mo/TiO2 and Pt/TiO2 revealed the presence of Pt nanoparticles (NPs) with mean diameters of 3.5 and 4.2 nm, respectively, on the TiO2 surface (Fig. 3a and S2†). Elemental mapping using high-angle annular dark-field scanning TEM (HAADF-STEM) coupled with energy-dispersive X-ray spectroscopy (EDX), confirmed dispersion of Mo species in the used Pt–Mo/TiO2 (Fig. S3†). X-ray absorption near-edge structure (XANES) analysis was used to investigate the chemical states of the Pt and Mo species (Fig. 3b, c, S4, and S5†). The white-line intensities of the Pt L3-edge XANES spectra of the used Pt–Mo/TiO2 and Pt/TiO2 were similar to those of the Pt foil, suggesting the presence of zero-valent Pt in both catalysts (Fig. 3b, S4, and S6†). Fig. 3c shows the Mo K-edge XANES spectra of fresh and used Pt–Mo/TiO2. The absorption edge of the Mo K-edge XANES spectrum shifted towards a lower energy after reductive amination, indicating that the Mo species were reduced in situ. To obtain detailed information on the valence state of the Mo species, X-ray photoelectron spectroscopy (XPS) analysis of used Pt–Mo/TiO2 was conducted. The deconvolution of the XPS spectrum of the used Pt–Mo/TiO2 in the Mo 3d region displayed multiple peaks corresponding to Mo(0) and Mo oxides with valence states ranging from 4+ to 6+ (Fig. 3d and Table S8†). These Mo K-edge XANES and XPS results demonstrated the formation of reduced Mo (MoOx) species during the reaction (Fig. S7†).
To investigate the interaction between MoOx and carbonyl compounds, temperature-programmed desorption infrared (TPD-IR) study was performed using acetone as a probe (Fig. S8†). The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of acetone adsorbed on the Pt/TiO2 and Pt–Mo/TiO2 catalysts at 50 °C exhibited a peak at approximately 1690 cm−1.7 As temperature increased, the peak decreased in intensity and completely disappeared at 170 °C for Pt/TiO2 (Fig. S8a†). In contrast, the spectrum of Pt–Mo/TiO2 showed a strong peak, and it retained even at 200 °C (Fig. S8b†). Thus, the results of TPD-IR, Mo K-edge XANES, and Mo 3d XPS suggest that the carbonyl moieties of the substrates are strongly adsorbed on the MoOx sites.
O stretching vibration of acetone adsorbed on the Pt/TiO2 and Pt–Mo/TiO2 catalysts at 50 °C exhibited a peak at approximately 1690 cm−1.7 As temperature increased, the peak decreased in intensity and completely disappeared at 170 °C for Pt/TiO2 (Fig. S8a†). In contrast, the spectrum of Pt–Mo/TiO2 showed a strong peak, and it retained even at 200 °C (Fig. S8b†). Thus, the results of TPD-IR, Mo K-edge XANES, and Mo 3d XPS suggest that the carbonyl moieties of the substrates are strongly adsorbed on the MoOx sites.
Support effect
The role of support materials was then investigated. Pt–Mo catalysts supported on TiO2, ZrO2, and γ-Al2O3 facilitated the reductive amination, whereas WO3- and Nb2O5-supported catalysts showed a lower conversion of 1 (Table 1, entries 1 and 10–13). Moreover, bare TiO2 efficiently converted 1 to 4 (Table 1, entry 16), indicating that the support materials are important for promoting amidation step (iii) in Fig. 2a. We further performed control experiments on the amidation of 2 under Ar; the Pt–Mo/TiO2 and Pt–Mo/γ-Al2O3 catalysts provided 4 in 24% and 21% yields, respectively, which were much higher than that obtained with Pt–Mo/Nb2O5 (Table S9†). NH3-TPD measurements showed that the acid amount of Pt–Mo/TiO2 and Pt–Mo/γ-Al2O3 were significantly higher than that of Pt–Mo/Nb2O5 (Fig. S9†). It has been reported that acid property of metal oxides facilitates amidation reactions.8 Hence, support materials with high acid amount effectively promote amidation, resulting in a high yield of 3 in the reductive amination of triglycerides. This result suggests that path B including amidation is likely to be a major reaction pathway.Proposed reaction mechanism
In the time-course data shown in Fig. S1,†3 was formed without an induction period, and the yield of 4 was kept low (<4%) throughout the reaction. Moreover, control experiments on the hydrogenation of amide using Pt–Mo/TiO2 afforded 3 in 33% yield for 1 h (Fig. 2b(iv)), which is higher than the yield of 4 (24%) in the amidation for 4 h (Fig. 2b(iii)). These results reveal that the amidation is the rate-determining step in the present catalyst system. Based on the aforementioned results, including control experiments and physicochemical characterizations, the high catalytic activity of Pt–Mo/TiO2 can be explained as follows: the carbonyl moiety of triglycerides is activated on the acid site of the TiO2 surface and transformed to amides, which is the rate-determining step.8 H2 is dissociated on the Pt NPs,9 and spillover hydrogen reduces MoO3 to form MoOx10 (Fig. S5†). The carbonyl moiety of amides is then adsorbed onto MoOx and deoxygenated.11 Thus, the combination of Pt NPs, MoOx, and TiO2 efficiently facilitated the reductive amination of triglycerides.Conclusions
The direct synthesis of fatty amines by the reductive amination of triglycerides was achieved using a Pt–Mo/TiO2 catalyst. The Pt–Mo/TiO2 catalyst could be operated under milder reaction conditions, such as 1 MPa H2 and 180 °C, compared with those reported in the previous studies. The high activity of Pt–Mo/TiO2 was maintained even after being recycled ten times, demonstrating its excellent durability and reusability. A wide range of amines and triglycerides, including cooking oil, was applied to the catalytic system to afford the corresponding fatty amines. The high activity of Pt–Mo/TiO2 was attributed to cooperative catalysis of Pt NPs, MoOx, and TiO2, where TiO2 facilitate amidation of triglycerides and Pt–Mo effectively hydrogenate amide intermediate. This study provides a green and sustainable method for fatty amine production.Author contributions
K. S. and H. F. designed the experiments, conducted the catalytic activity tests, and characterized the catalysts. S. Y. and T. Mitsudome discussed the experiments and results. T. Mizugaki directed and conceived the project. K. S. and S. Y. co-wrote the manuscript with input from all authors. All authors commented on the manuscript and approved the final version.Data availability
The experimental data supporting this article have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by a JSPS Research Fellowship for Young Scientists (grant number 23KJ1473), JSPS KAKENHI (grant numbers: 23K26454 and 24K01255), JST PRESTO (grant numbers: JPMJPR21Q9 and JPMJPR24MB), Shorai Foundation for Science and Technology, and the Joint Usage/Research Center for Catalysis (24DS0575). This study was partially supported by the JST-CREST (grant number: JPMJCR21L5). Part of the experimental analysis was supported by the “Advanced Research Infrastructure for Materials and Nanotechnology in Japan” (grant number: JPMXP1222HK0062) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT). The synchrotron radiation experiments for the XANES measurements were performed at the BL01B1 beamline at SPring-8 with the approval of JASRI (2024B1602). This study used research equipment shared through the MEXT Project aimed at promoting the public utilization of advanced research infrastructure, as part of a program for supporting the construction of core facilities (grant numbers: JPMXS0441200023 and JPMXS0441200024). Finally, we thank the Shimadzu Corporation for performing the XPS measurements, and Dr Satoshi Suganuma and Mr Takashi Tanioka (Hokkaido University) for HAADF-STEM and EDX mapping measurements.References
- (a) A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef PubMed; (b) P. Sudarsanam, R. Zhong, S. Van den Bosch, S. M. Coman, V. I. Parvulescu and B. F. Sels, Chem. Soc. Rev., 2018, 47, 8349–8402 RSC; (c) Y. S. Yun, C. E. Berdugo-Díaz and D. W. Flaherty, ACS Catal., 2021, 11, 11193–11232 CrossRef; (d) G. Li, R. Wang, J. Pang, A. Wang, N. Li and T. Zhang, Chem. Rev., 2024, 124, 2889–2954 CrossRef PubMed.
- (a) P. Foley, A. Kermanshahi-pour, E. S. Beach and J. B. Zimmerman, Chem. Soc. Rev., 2012, 41, 1499–1518 RSC; (b) P. Munkajohnpong, C. Kesornpun, S. Buttranon, J. Jaroensuk, N. Weeranoppanant and P. Chaiyen, Biofuels, Bioprod. Biorefin., 2020, 14, 986–1009 CrossRef; (c) X. Yao, T. J. Strathmann, Y. Li, L. E. Cronmiller, H. Ma and J. Zhang, Green Chem., 2021, 23, 1114–1129 RSC; (d) F. Long, W. Liu, X. Jiang, Q. Zhai, X. Cao, J. Jiang and J. Xu, Renewable Sustainable Energy Rev., 2021, 148, 111269 CrossRef; (e) J. Citoler, W. Finnigan, H. Bevinakatti and N. J. Turner, ChemBioChem, 2022, 23, e202100578 CrossRef PubMed; (f) Z. Rahmawati, L. Santoso, W. N. W. Abdullah, A. Hamid, N. L. A. Jamari, D. Sugiarso, Y. L. Ni'mah and A. A. Widati, RSC Adv., 2024, 14, 28827–28843 RSC.
- (a) P. Roose, K. Eller, E. Henkes, R. Rossbacher and H. Höke, Amines, Aliphatic, Ullmann's Encyclopedia of Industrial Chemistry, Wiley, 2015 Search PubMed; (b) R. A. Gonçalves, K. Holmberg and B. Lindman, J. Mol. Liq., 2023, 121335 CrossRef.
- (a) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed; (b) M. J. Climent, A. Corma, S. Iborra and M. J. Sabater, ACS Catal., 2014, 4, 870–891 CrossRef CAS.
- For reviews on the reductive amination of esters, see: J. R. Cabrero-Antonino, R. Adam and M. Beller, Angew. Chem., Int. Ed., 2019, 58, 12820–12838 CrossRef CAS PubMed.
- For the reductive amination of esters including triglycerides and other esters, see: (a) H. Rutzen, DE Pat, 1288595B, 1969 Search PubMed; (b) J. Barrault, S. Brunet, N. Suppo-Essayem, A. Piccirilli and C. Guimon, J. Am. Oil Chem. Soc., 1994, 71, 1231–1238 CrossRef; (c) R. Adam, J. R. Cabrero-Antonino, K. Junge, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2016, 55, 11049–11053 CrossRef; (d) Y. Shi, P. C. J. Kamer, D. J. Cole-Hamilton, M. Harvie, E. F. Baxter, K. J. C. Lim and P. Pogorzelec, Chem. Sci., 2017, 8, 6911–6917 RSC; (e) T. Toyao, S. M. A. H. Siddiki, Y. Morita, T. Kamachi, A. S. Touchy, W. Onodera, K. Kon, S. Furukawa, H. Ariga, K. Asakura, K. Yoshizawa and K. Shimizu, Chem. – Eur. J., 2017, 23, 14848–14859 CrossRef PubMed; (f) Y. Shi, P. C. J. Kamer and D. J. Cole-Hamilton, Green Chem., 2017, 19, 5460–5466 RSC; (g) M. A. R. Jamil, S. M. A. H. Siddiki, A. S. Touchy, M. N. Rashed, S. S. Poly, Y. Jing, K. W. Ting, T. Toyao, Z. Maeno and K. Shimizu, ChemSusChem, 2019, 12, 3115–3125 CrossRef PubMed; (h) R. Coeck and D. E. De Vos, Green Chem., 2020, 22, 5105–5114 RSC; (i) R. Coeck, J. Meeprasert, G. Li, T. Altantzis, S. Bals, E. A. Pidko and D. E. De Vos, ACS Catal., 2021, 11, 7672–7684 CrossRef.
- Y. Nakamura, K. Kon, A. S. Touchy, K. Shimizu and W. Ueda, ChemCatChem, 2015, 7, 921–924 CrossRef.
- H. Lundberg, F. Tinnis, N. Selander and H. Adolfsson, Chem. Soc. Rev., 2014, 43, 2714–2742 RSC.
- (a) S. Nishimura, Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis, John Wiley & Sons Inc., 2001 Search PubMed; (b) L. Zhang, M. Zhou, A. Wang and T. Zhang, Chem. Rev., 2020, 120, 683–733 CrossRef.
- (a) Y. Kuwahara, Y. Yoshimura, K. Haematsu and H. Yamashita, J. Am. Chem. Soc., 2018, 140, 9203–9210 CrossRef CAS; (b) K. Sakoda, S. Yamaguchi, K. Honjo, Y. Kitagawa, T. Mitsudome and T. Mizugaki, Green Chem., 2024, 26, 2571–2576 RSC.
- (a) T. Toyao, S. M. A. H. Siddiki, K. Kon and K. Shimizu, Chem. Rec., 2018, 18, 1374–1393 CrossRef CAS; (b) K. Sakoda, S. Yamaguchi, T. Mitsudome and T. Mizugaki, JACS Au, 2022, 2, 665–672 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob01843e |
| This journal is © The Royal Society of Chemistry 2025 |