Rapid humification of biomass via hydrothermal conversion: a comprehensive review
Yangjiuzhou
Wang†
ab,
Changbin
Yuan†
abc,
Kai
Zhang
abc,
Jinyu
Tong
ab,
Ningjie
Ma
d,
Mahmoud M.
Ali
ab,
Yongdong
Xu
 *abc and
Zhidan
Liu
*abc and
Zhidan
Liu
 *abc
*abc
aCollege of Water Resources and Civil Engineering, China Agricultural University, Beijing 100083, China. E-mail: zdliu@cau.edu.cn; xuydenvelope@163.com; Tel: +86-13810431521
bLaboratory of Environment-Enhancing Energy (E2E), Key Laboratory of Agricultural Engineering in Structure and Environment of Ministry of Agriculture and Rural Affairs, Beijing 100083, China
cState Key Laboratory of Efficient Utilization of Agricultural Water Resources, Beijing 100083, China
dXi'an Kedagaoxin University, Xi'an 710109, China
First published on 27th December 2024
Abstract
Humic acid (HA), a crucial substance for maintaining soil fertility and health, plays a vital role in sustainable agricultural development and environmental remediation. Hydrothermal humification (HTH) offers the advantage of producing HA analogues from biomass in a significantly shorter timeframe compared with natural processes, thereby enhancing carbon efficiency. This approach aligns with green chemistry principles by promoting the sustainable utilization of resources while minimizing environmental impacts. However, research on the hydrothermal production of HA is still in its early stages, with the underlying conditions, influencing factors, and conversion mechanisms remaining unclear. Furthermore, the potential applications of hydrothermal HA are not yet fully understood. Drawing from nearly a decade of research, this article addresses the mechanism of hydrothermal conversion of biomass into HA and discusses the impacts of diverse HTH operating parameters such as reaction time, biomass composition, reaction solvent, and reaction temperature on the humification process. Given the current lack of research on the applications of hydrothermal HA, we demonstrated the potential applications and challenges of hydrothermal HA by exploring the use of HA from various other sources in diverse scenarios, including agriculture, environmental protection, functional material preparation and animal husbandry. Furthermore, the challenges and research directions for the commercial application of hydrothermal HA are discussed, aiming to provide a reference for studies on HA derived from biomass via hydrothermal conversion.
Green foundation1. Hydrothermal humification (HTH) offers the advantage of generating humic acid (HA) analogues from biomass in a much shorter timeframe than the natural process. This enhances carbon efficiency and aligns with green chemistry principles by promoting sustainable biomass utilization while minimizing environmental impact. However, the mechanisms underlying the hydrothermal conversion process remain unclear, and the potential applications of hydrothermal HA are not yet well understood.2. We explore the potential applications and challenges of hydrothermal HA by exploring the use of HA derived from various sources in different scenarios, including agriculture, environmental protection, functional material preparation and animal husbandry. 3. The challenges and research directions for the commercial application of hydrothermal HA are discussed, aiming to provide a reference for the study of HA production from biomass via hydrothermal conversion. |
1. Introduction
Humic substances in soil play a crucial role in soil fertility and sustainable agricultural development. These substances are predominantly derived from the decomposition and transformation of animal and plant residues through microbial processes and a series of chemical reactions.1 Humic substances comprise complex organic polymers, including humic acid (HA), fulvic acid and humins, which are primarily composed of aromatic and aliphatic rings as well as functional groups, such as phenolic hydroxyls, ether bonds, and carboxylic acid groups.2 Humic substances in soil and water influence soil structural stability, promote complexation of heavy metals and facilitate transformation of pollutants in terrestrial and aquatic environments.3–5 HA is the most abundant substance in humic substances. Research has demonstrated that natural HA can alter soil properties in agriculture, reduce soil heavy metal content, enhance fertilizer efficiency, protect plant growth and development, improve crop quality, and optimize pesticide efficacy.6 In addition to its agricultural uses, HA has been found to have significant applications in environmental protection, electrochemistry, medicine, and animal husbandry.7,8The wide availability of HA from renewable natural sources has led to increased attention on HA. Consequently, investigating the source and transformation processes of HA is of utmost importance to achieve maximum humification. Currently, there are four widely accepted molecular models of HA: the fulvic acid model, classical HA model, two-dimensional model of HA, and three-dimensional model of the HA structure.9 There are three conversion pathways for HA formation: natural sedimentation, microbial methods, chemical oxidation and thermochemical methods.10 These methods require varying conversion times. Natural sedimentation takes decades to centuries, while microbial methods involve weeks or months of composting and microbial metabolism. Thermochemical methods, such as pyrolysis and hydrothermal treatment, require hours or days for the reaction and extraction of HA from materials such as corn stover, sludge, and animal manure.11
Compared to natural sedimentation and biological conversion, thermochemical conversion demonstrates the key advantage of rapid conversion.12,13 The hydrothermal humification (HTH) process stands out due to its ability to shorten the reaction period and increase the yield of HA.14,15 However, HTH reactions are complex and variable, depending on numerous reaction parameters, such as reaction time, and reaction temperature. The selection and control of the reaction conditions, as well as the dynamic changes during the reaction process, can significantly impact the humification results, such as HA yield and characteristics. There are lots of emerging studies about HTHs from biomass, including reports covering the operational process,10 transformation pathway,16 and the characteristics of hydrothermal HA, key influencing factors of the HTH process, and the potential applications of HA.6
In recent years, a few reviews on the HTH of biomass have emerged. The synthesis technology and application of artificial humic substances (A-HSs) were emphasized along with the application of A-HSs synthesized by these technologies in agriculture and environmental remediation.17 Many reviews have summarized the pathways of preparing HA by biological and abiotic methods and the influence of key factors within those processes, and introduced the differences between compost and hydrothermal treatment in the production of humic acid, including the main mechanism of the humification process and the influence of the reaction factors.9 Other reviews have described the mechanism of hydrothermal humic acid production, the influence of hydrothermal conditions on humic acidification, the properties of hydrothermal humic acid, and its applications in agriculture and the environment field.11 Current reviews have focused on the effects of the reaction temperature and time on HTH, but lack comprehensive discussions on other factors, particularly the conversion mechanisms and pathways of the various components in the feedstock. Researchers have shown that artificial HA possesses a richer array of functional groups, offering promising prospects for agricultural and environmental applications. It can improve soil properties, promote plant growth, and mitigate the toxicity of pollutants such as heavy metals. However, research on artificial HA is still in its early stages, and the potential applications of hydrothermal HA remain largely unexplored, leading to an incomplete understanding of its full application potential.17–19
Based on the articles mainly published from 2010 to 2024, this review provides a comprehensive introduction of HA from biomass, which evaluates the impact of diverse HTH operating parameters and process schemes on the mechanism, yield, and characteristics of HA production. Furthermore, it delves into the practical applications of HA across multiple domains, including agriculture, environmental protection, electrochemistry, healthcare, industry, and animal husbandry. This work can serve as a valuable reference for the research and application prospects of HA from HTH and aid in better regulating the formation of HA during biomass waste conversion.
2. Conversion mechanism and influencing factors of hydrothermal humification
Hydrothermal treatment, a thermochemical conversion process conducted under subcritical or supercritical water conditions (180–360 °C, 2–20 MPa),20 can rapidly valorize bio-waste within hours or minutes, transforming it into valuable materials such as bio-oil, HA, hydrochar, and chemicals.21,22 This promising technology shortens reaction cycles, enhances catalytic efficiency, reduces energy input (compared to pyrolysis), and minimizes secondary pollution.23 Recent advancements have demonstrated its feasibility for converting bio-waste into hydrothermal humic substances,24,25 simulating natural humification through a mild chemical process under both acidic and alkaline conditions.26 In the hydrothermal process for producing HAs, solvent environment facilitates the dissolution of humic substances and humic acid extraction. Notably, hydrothermal treatment stands out with its various beneficial features, like a wide adaptability to biomass feedstocks, short reaction cycles, and reduced greenhouse gas emissions, allowing turning waste management into a source of economic benefits.27 A study indicated that the yield of HAs in the hydrothermal process is twice that of composting.28 Although hydrothermal treatment offers many advantages, it also faces several challenges. First, the hydrothermal process for HA production involves complex chemical reactions and various chemical substances, which pose difficulties in the purification, identification, and quantification of HAs.29 Second, the hydrothermal process requires a significant amount of energy input, with the process often operating at temperatures above 160 °C. Third, catalysts are commonly used to minimize the generation of undesired solid byproducts, shorten reaction times, and reduce reaction risks.27,30 However, the effect and mechanism of catalysts in hydrothermal conversion are not straightforward and require further understanding. Additionally, the scale of hydrothermal processing for biomass waste is still limited to laboratory settings. Before large-scale application, the unstable HA yields in hydrothermal treatment still need to be addressed as current research in this area is still insufficient.92.1. Conversion mechanism of humic acid
Currently, there are several theories regarding the generation of HAs in the natural environment, including the Maillard reaction (also known as the sugar–amine condensation theory), the lignin theory, and the polyphenol theory.31 Additionally, there are several other theoretical hypotheses, such as the microbial synthesis hypothesis, the cell autolysis hypothesis, and the polyphenol hypothesis originating from lignin.32 The process of hydrothermal conversion to produce HAs is complex and involves a series of chemical reactions and continuous component transformations (Fig. 1). Generally, during the humification process, the feedstocks undergo hydrolysis and condensation reactions. In biochemical processes for HA production, these reactions are mostly driven by microorganisms. In abiotic oxidation processes, such as catalytic oxidation polymerization, alkali–oxygen reactions, and HTH, these reactions are mostly induced by heating, oxidation, and pressure. Under low-temperature conditions, biomass undergoes hydrolysis reactions, primarily involving components such as cellulose, hemicellulose, lignin, lipids, and proteins. These components rapidly hydrolyze into monosaccharides, such as xylose, arabinose, and glucose, as well as small molecules, such as furans, phenols, and organic acids. These are the key precursors for the formation of HAs.16,33 As the temperature increases, the precursors of HAs gradually decompose or degrade. For example, arabinose and xylose decompose into furfural, while glucose degrades into 5-hydroxymethylfurfural, acetic acid, or γ-valerolactone, which catalyzes the later generation of hydrothermal HA.34 With further temperature increases, the Maillard reaction or aldehyde–alcohol reaction becomes dominant, leading to complex reactions, such as molecular rearrangement in HA.35,36 This results in changes in the structure and molecular weight of HA, leading to the formation of high-molecular-weight HA (Fig. 1). However, when the temperature exceeds a certain threshold, the Maillard reaction is inhibited, resulting in a decrease in the content of HA, ultimately forming humic aggregates in the thermal environment.9,18,37 There are three conversion pathways for HA: (1) one-step HTH. Here, the pretreated biomass raw materials are heated in an alkaline solution and cooled to room temperature to obtain liquid products; then HA is obtained by acidification treatment; (2) acid–base two-step HTH. Here, the acidic solution is selected as the dissolved solution (first step), and then an alkaline solution is selected for the humification using degradation products as the feedstock (second step); and finally the liquid product is acidified after the two stages to obtain HA; (3) hydrochar-based two-step HTH. Here, the raw materials go through a high-temperature hydrothermal carbonization process to generate hydrocarbons (first step), and then an alkaline solution is selected for the humification using the hydrocarbons as the feedstock (second step); and finally acid precipitation is used to extract HA38 (Fig. 2). Additionally, research suggests that reactions involving free radicals and photosensitization under frozen conditions may contribute to the formation of humic-like substances by promoting the oxidation and polymerization of aromatic compounds. By simulating natural low-temperature processes, it has been observed that aromatic and phenolic compounds can transform into polymers with humic-like structures under freezing conditions.39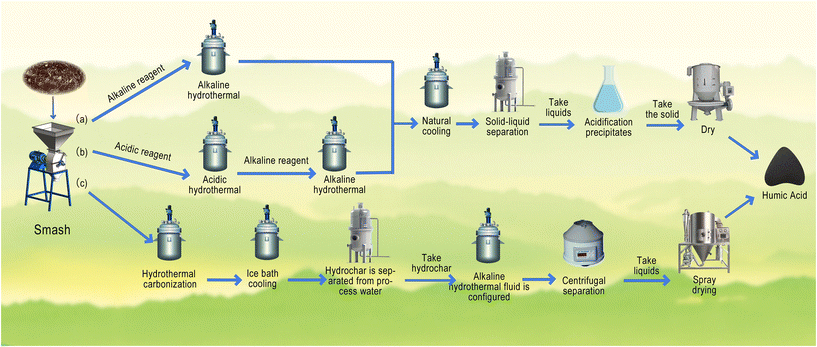 | ||
| Fig. 2 HA production pathways: (a) one-step alkaline solution method, (b) two-step hydrothermal acid–base solution method, and (c) hydrothermal carbonization method (revised from ref. 10 with permission from Elsevier, Copyright 2023). | ||
2.2. Influencing factors in humic acid conversion
The production of HA via the hydrothermal process is influenced by various operational parameters. These parameters include the raw materials’ characteristics, reaction temperature, reaction time, reaction rate, pressure, gas atmosphere, solution environment, catalyst, and extraction methods used for product separation (Fig. 3). Each of these factors can affect the composition of the substances and the yield of HAs.10 Among these, the reaction raw materials, temperature, and reaction time have been found to have the most significant influences on the production process.40 Therefore, this chapter will particularly focus on the impact of these three variables, as well as covering the solution acidity/alkalinity and the choice of catalysts, on the production of HAs. | ||
| Fig. 3 (a) Factors influencing hydrothermal humification, including time, temperature, catalyst, feedstock and pH. (b) Effect of pH on humic acid yield (reproduced and revised from ref. 42 with permission from Elsevier, Copyright 2022). (c) Effect of the catalyst on humic acid yield (reproduced and revised from ref. 42 with permission from Elsevier, Copyright 2022). (d) Effect of time on humic acid yield (reproduced and revised from ref. 42 with permission from Elsevier, Copyright 2022). (e) Effect of temperature on humic acid yield (reproduced and revised from ref. 42 with permission from Elsevier, Copyright 2022). | ||
3. Application potential of humic acid
HAs offer a wide range of possible applications in various fields (Fig. 4). The applications of HA are extensive, ranging from pollution control and soil fertility enhancement to environmental bioremediation and renewable energy production. These versatile compounds hold great promise for addressing carbon efficiency, environmental challenges, and promoting sustainable practices in various industries.53 However, there are still few studies that have paid attention to the application of HA from HTH because most of the current studies have tended to focus on the formation mechanism. Compared with HAs obtained from natural microbial transformation, artificially synthesized HAs from HTH contain high-water-solubility compounds, such as carbohydrates and amino acids, suggesting that HAs from HTH can be easily used in soil by plants.54 HA synthesized by hydrothermal reactions between sludge and biomass can recover the phosphorus elements from sludge into liquid products while heavy metals are enriched in solid products, which indicates that it may exhibit less environmental toxicity.55,56 In this chapter, the role and function of HA in various fields are summarized, which can reflect the strong application prospects of hydrothermal HA.3.1. Application potential in agriculture
HA can improve the soil property, enhance nutrient utilization efficiency, and promote plant physiological activity (Fig. 5). HAs play an effective role in improving soil structure and porosity, water retention, and nutrient content owing to their rich active groups, such as phenolic hydroxyl, carboxyl, carbonyl, and alcoholic hydroxyl groups.57 The colloidal properties of HA enable soil particles to form stable aggregates, improving the soil porosity and hence its nutrient retention capacity.58,59 The presence of active hydrophilic groups allows forming hydrogen bonds with water, which imparts HA with strong water-absorption and -retention capabilities, enhancing the soil water-holding capacity.60 A highly water-absorbent resin was prepared using HA that demonstrated excellent water-absorption and -retention capabilities.61 Drought-resistant products based on HAs can effectively promote photosynthesis in plants.18 Based on the multifunctional role of HA in soil, its combined application with other fertilizers, such as nitrogen fertilizers, can significantly improve the soil's physicochemical properties.62 Additionally, it has been shown that HAs can promote microbial activity, achieving stable soil organic carbon sequestration and reducing the soil bulk density.63,64 HAs also play a significant role in optimizing the soil environment, whereby the active groups in Has, such as hydroxyl and carboxyl groups, can undergo adsorption, redox, and ion-exchange reactions with soluble salts and heavy metal ions, improving the physical state of soils (Fig. 5).65,66 | ||
| Fig. 5 (a) Principle of HA to improve plant nutrient utilization. (b) Principle of HA to enhance the physiological activity of plants. (c) Principle of HA to improve soil properties. (d) Behavior of plants and soil with and without humic acid (reproduced from ref. 59, published under Creative Commons licence; reproduced from ref. 58 with permission from Elsevier, Copyright 2020; reproduced from ref. 75, published under Creative Commons licence). | ||
HAs can also enhance the nutrient utilization efficiency and promote the growth of plants. On the one hand, HA can directly serve as a nutrient source for C, N, and S for supporting the growth of horticultural plants.67 On the other hand, HA can enhance the utilization efficiency of nutrients in soil. It was found that HA increased the uptake of Na, Ba, P, N, and K ions from soil.68 HA also stimulates the activity of H-ATPase in plants and promotes the formation of root hairs and lateral roots.69 The expansion of the plant root system increases the surface area for nutrient uptake, thus strengthening the absorption, assimilation, and cycling of nutrients and ensuring the availability of nutrients.17,70 Additionally, research indicated that HA chelates various nutritional elements, which can improve some plant growth parameters, including the plant height, panicle length, and seed number.71,72 Moreover, it can regulate soil microbial populations and activity,73 and enhance the transformation of nitrogen and carbon to stimulate plant growth and accelerate nutrient uptake.74 HA can directly participate in the intracellular biochemical and biophysical metabolic processes of plants, such as cell development, photosynthesis, and hormone synthesis, thereby improving plant physiological activity and promoting plant growth and development.61,75 In another study, it was found that the addition of HA in soil increased the absorption of N, P, K, Ca, and Mg elements (in pineapple leaves) by 52%, 71%, 50%, 58%, and 59%, respectively.76
HAs from both HTH and natural microbial transformation are amorphous colloids that contain various functional groups, such as carboxyl, hydroxyl, quinone, aliphatic groups, aromatic hydrocarbon groups, and nitrogenous groups.77–80 It can be seen that HA from HTH can also exhibit the function of improving the soil property because of its rich and similar functional group structure to natural HA. More importantly, the richer functional groups in hydrothermal humic acid may have even more pronounced effects.11,81 Some reports have indicated that the hydrothermal humification of sludge has good potential for nitrogen and phosphorus fixation.82,83 The nitrogen content of HA prepared by the hydrothermal humification of sludge was high as 15%, and about 90% of the phosphorus could be recovered from the sludge through a hydrothermal oxidation precipitation method. The phosphorus content of the solid product obtained was 9.47%. Therefore, the HTH of sludge can promote the recovery of nitrogen and phosphorus elements. A previous study produced HA from corn straw using HTH, which demonstrated that HA from HTH improved soil quality, water retention, nutrient slow-release, and plant growth in pot experiments, suggesting its potential use in sustainable agriculture and soil remediation.84 Another study showed that preparation of HA (from hydrothermal conversion)–urea fertilizers with high aromaticity or a low molecular weight is an effective way to improve nitrogen use efficiency in the early stage of soil cultivation and maintain nitrogen fixation in the later stage of soil cultivation.1 In another article, the effect of hydrothermal HA on lettuce was studied, and experiments showed that adding HA to soil could significantly increase the wet and dry weights of lettuce.81
3.2. Application potential in environment protection
The functional groups in HA, such as phenols, carboxyl, hydroxyl, quinone, aliphatic groups, aromatic hydrocarbon groups, and nitrogenous and sulfur-containing groups, have various effects in environment protection and remediation, including combining with heavy metals, organic pollutants, radionuclides, and other pollutants, and participating in the photolysis of pollutants (Fig. 6).80,85–88HA can serve as an electron medium for chemical reactions and microbial metabolic processes, undergoing degradation or reduction reactions with pollutants in water. The amphiphilic structure and redox capabilities of HAs contribute to their interactions with heavy metals.53 HAs are considered environmentally friendly alternatives for water pollution control and have been proven to be effective for water environmental remediation.18,89
As previously mentioned, HAs have colloidal properties and are rich in various functional groups, such as carboxyl, hydroxyl, and amino groups. They can interact with sulfur oxides, nitrogen oxides, ammonia, etc., to reduce environmental pollution. Research has confirmed that HA has anti-smog effects by acting on secondary particles of PM2.5, such as ammonium nitrate and ammonium sulfate.90 In a previous study, HA inhibited the conversion of acetyl coenzyme A in methanogenic bacteria by electron competition, thus affecting methane generation.91 It was found in sludge test experiments that when adding HA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VSS (volatile suspended solids) at 15%, the methane-generation-inhibition efficiency was 35.1%; when HA
VSS (volatile suspended solids) at 15%, the methane-generation-inhibition efficiency was 35.1%; when HA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VSS was 20%, the inhibition efficiency of methane generation was 74.3%.92 Given the binding ability of functional groups and heavy metals in HAs, HA can be used for the soil remediation of heavy metal pollution. For example, HAs have been reported to reduce the toxicity of metal elements in soil by altering valence states through redox processes, while the functional groups, such as carboxyl groups, on HA molecules interact with ionic metals to form spherical HA–metal chelates, leading to precipitation adsorption and hence a reduction in toxicity.3,93 Previous studies found that modified HA–ATP composites not only showed enhanced adsorption effects on polycyclic aromatic hydrocarbons (PAHs) but could also avoid secondary pollution of the soil by harmful chemicals.94
VSS was 20%, the inhibition efficiency of methane generation was 74.3%.92 Given the binding ability of functional groups and heavy metals in HAs, HA can be used for the soil remediation of heavy metal pollution. For example, HAs have been reported to reduce the toxicity of metal elements in soil by altering valence states through redox processes, while the functional groups, such as carboxyl groups, on HA molecules interact with ionic metals to form spherical HA–metal chelates, leading to precipitation adsorption and hence a reduction in toxicity.3,93 Previous studies found that modified HA–ATP composites not only showed enhanced adsorption effects on polycyclic aromatic hydrocarbons (PAHs) but could also avoid secondary pollution of the soil by harmful chemicals.94
The rich oxygen-containing functional groups in HA from hydrothermal conversion can increase the adsorption sites and affinity of HA and regulate the surface charge of HA, and their adsorption has been proven to have great potential in environmental protection applications. For example, HA prepared by the alkaline hydrothermal conversion of biomass using oxygen had an adsorption capacity of 24.4% for divalent cadmium.81 In addition, HA from hydrothermal conversion can be used in combination with other materials to enhance the overall adsorption performance.95 For example, it was reported that composite materials synthesized from HHA and iron could adsorb various heavy metals,96 whereby the maximum adsorption capacities for Cd2+, Pb2+, and Ni2+ from water were 290.81, 374.99, and 296.85 mg g−1, respectively. These results highlight the advantages of hydrothermal HAs in environmental applications. In summary, HA from HTH as an adsorbent has broad application prospects.
3.3. Application potential in electrochemistry
The rich functional groups in HA give it high application value in the electrochemical field. For instance, HA can be used to prepare porous carbon materials for energy storage and humidity sensors (Fig. 7), indicating the high potential of HA from HTH in electrochemical applications.97–100 HA has also been used to modify electrodes, whereby it was found that HA-modified electrodes can interact with water and metal ions, thus amplifying electrical signals through electron transfer.101 In addition, HA-modified electrodes can interact with pollutants to detect their electrical activities.7 Due to the hydrophilic functional groups of HA, HA-modified electrodes can also be used as humidity sensors, exhibiting a decrease in impedance with increasing relative humidity.102 Moreover, HA also has applications in microbial fuel cells. It was reported that an HA-modified bioanode could reduce the activation energy of ion oxidation, thereby improving the electron-transfer efficiency between microorganisms and electrodes and enhancing the electrochemical performance.103 Research has shown that HA-based energy-storage materials have excellent rate capabilities and capacitance performance.100 A hybrid capacitor prepared using a combination of HA and activated carbon104 and an asymmetric capacitor prepared using HA and nickel sulfide105 both showed high power density and energy density. In another study, a concentrated battery was constructed based on reduced graphene oxide membranes and HA, which demonstrated high stability, durability, maximum open circuit voltage maximum power values, and a longer duration than general batteries.106 In another study, graphene–SnO2 HA nanocomposites were synthesized, and exhibited an ultra-high reversible specific capacity.107 In another study, reduced graphene oxide and HA composite materials were used to prepare supercapacitor electrodes.108 However, there is currently no research on the application performance evaluation of HA prepared by a hydrothermal method for storing electrical energy and preparing capacitors. | ||
| Fig. 7 (a) Applications of HA in the electrochemical field. (b) Coulombic efficiency and oxygen demand removal efficiency of microbial fuel cells in the absence of HA, HA, and HA@Fe3O4. (c-1) Undoped anode. (c-2) Anode containing HA. (c-3) Anode containing HA@Fe3O4 (reproduced from ref. 130 with permission from Elsevier, Copyright 2019). | ||
3.4. Application potential in healthcare and animal husbandry
HAs have a wide range of functions in healthcare. Many researchers have studied the pharmacological properties of HA and found that it has antibacterial, anti-inflammatory, antiviral, and analgesic properties, with the additional advantages of having no side effects on the body and causing no irritation to the skin.109 HA molecules have a negative charge in neutral and alkaline solution environments and can inhibit the replication of viruses (including COVID-19, vaccine viruses, and cytomegalovirus) by binding to the cationic domain of viruses, exerting antioxidant effects, and enhancing the permeability of cell membranes (Fig. 8).110–113 Besides, it has been found that the addition of HA can inhibit the infection of human lymphocytes in vitro and its anti-HIV activity was also confirmed.114 The anti-mutagenicity activity of HA has also been observed,115,116 suggesting that HA can be used as a mutagenesis inhibitor. HA also provides new ideas for its use in drug development. Sodium humate, a product of the combination of HA and sodium hydroxide, was investigated as an effective clinical medicine for use in veterinary practices for the treatment of various animal diseases.117 In other experiments, HA was found to be able to alleviate neuronal damage and reduce brain tissue death in rats, indicating its potential application in hypoxic-ischemic brain injury.5 HA was also used investigated as an active ingredient in efficacy masks to prevent the secondary activation of viruses after chemical facial treatments.118 Additionally, HA has the potential to be used as a component in lipsticks and lip balms.119,120 Therefore, HA has extensive application value in healthcare. Furthermore, in an animal husbandry study, it was found that HA could reduce the levels of mycotoxins and improve the gastrointestinal health of animals, aiding maintaining normal metabolism, thereby enhancing animal production.121 As a feed additive, HA can accelerate the efficiency of animals’ absorption of feed, thus expediting the metabolism of livestock, thereby enabling increasing the production speed, and improving product quality.122 It was found that the nitrogen element in HA molecules can stimulate feed protein into muscle protein, thereby improving the growth capacity of lean meat.123 Meanwhile, studies have shown that when HA is used as a dispersant in ceramic suspensions, it can effectively reduce viscosity.124 The excellent dispersing effect of HA can play a key role in the preparation of composite materials. For instance, a novel HA-g-PSSNa dispersant based on HA not only demonstrated good dispersibility but also exhibited excellent slurry manufacturability.125 | ||
| Fig. 8 (a) Antiviral pathway of HA. (b) Images of the jejunum and liver of mice (reproduced from ref. 117, with permission from Elsevier, Copyright 2023). (c) Inhibition curves of humic substances against SARS-CoV-2 virus B1.1.7 variants (reproduced from ref. 131, published under Creative Commons licence). (d) Spleen index of mice (reproduced from ref. 117, with permission from Elsevier, Copyright 2023). (e) Mouse jejunal villi length (reproduced from ref. 117, with permission from Elsevier, Copyright 2023). (C is the control group; LPS is the structural damage group induced by lipopolysaccharide; L-HNa, M-HNa, H-HNa are the experimental groups receiving 0.1%, 0.3%, 0.5% sodium humate, respectively). | ||
4. Challenges and perspectives for the application of hydrothermal humic acid
Previous studies have shown that hydrothermal humic acids (HTHAs) possess significant agricultural, soil, environmental, and material functions.18,19,52 However, the development of HTHAs remains in its early stages. The complexity of the raw materials’ properties and reaction conditions has so far limited the ability to obtain stable and uniform products. While current research has attributed the application potential of HTHAs primarily to their abundance of functional groups, their specific mechanisms of action remain unclear. Presently, the production of HTHAs is still confined to laboratory-scale studies, with limited yields and a lack of large-scale production facilities and practical applications. Furthermore, the hydrothermal conversion process often requires highly alkaline solutions and substantial energy input, raising concerns about operational safety and environmental impacts, which require further assessment.4.1. Obtaining high-quality and stable humic acid via hydrothermal humification
To achieve the production of HA from biomass for application in various fields, the first step should be obtaining high-quality and stable HA via hydrothermal humification. Producing HAs through hydrothermal conversion represents a sustainable and effective method for obtaining these compounds from various organic materials. The feasibility of the hydrothermal conversion of HA has been confirmed, but the current production of HA through hydrothermal conversion is still in the early stage.126 It is still necessary to further deepen the research on the generation mechanism of HA, and to optimize the reaction conditions and reactors used with the original biological waste to obtain high HA yields. The reaction mechanism of HA production through hydrothermal conversion has not yet been completely elucidated. Further detailed studies of the reaction mechanism could help better understand the reaction process and regulation of the reaction conditions. This includes studying the reaction kinetics, reaction thermodynamics, and reaction pathways to explore how to improve the production efficiency and purity of HA. It is already known that the reaction conditions for hydrothermal conversion, such as reaction temperature, additives, pH value, reaction medium, and reaction time, have significant effects on the production efficiency and purity of HA.29 In addition, considering the different functions of hydrothermal HA, it is also important to explore the targeted preparation of functional HA materials by regulating the preparation process of hydrothermal HA. Therefore, it is particularly important to study how to optimize the reaction conditions to further improve the production efficiency and purity of HA.4.2. Understanding the application effects and mechanisms of hydrothermal humic acid
To achieve the production of HA from biomass for applications in various fields, the second step should be to comprehensively understand the application effects and mechanisms of hydrothermal HA. The application potential of HA has been gradually tapped in the past decade, but research on the application of hydrothermal HA remains limited. Although hydrothermal HA appears to have greater application potential based on a comparison of its functional groups, there is still a lack of case studies with hydrothermal HA. In the field of agriculture, HA is usually used in the form of a compound agent, which has been shown to improve the physical and chemical properties of the soil and improve the ability of plants to resist biological stresses as a regulator, but the overapplication of such agents can cause problems, such as soil acidification, microbial community imbalance, and leaf burns, so the rational use of HA is key.69 In the field of environmental protection, HA mainly relies on the redox ability of its functional groups to reduce environmental pollution, but the specific contribution of the various functional groups is still unknown. Therefore, in the face of different sources of HA, there will be different environmental remediation effects in environmental treatment, and future research should focus on the specific mechanism of HA redox pollutants.3,18 Besides, it is necessary to consider the purity and product specifications required for specific applications, and to develop appropriate quality control, certification and labeling procedures.127,1284.3. Developing large-scale equipment for hydrothermal humic acid production
To achieve the production of HA from biomass for application in various fields, the third step should be developing large-scale equipment for hydrothermal HA production. Current research related to HA applications are based on laboratory studies, and lack large-scale application verification. Promoting the commercial application of hydrothermal HA and studying the design and scale-up of reactors for hydrothermal conversion are also important aspects that should receive special focus. The material selection, structural design, and operating parameters of the reactor can affect the production efficiency of HA.129 Therefore, studying how to design an efficient and stable reactor and achieving scale-up from small-scale laboratory studies to industrial production is crucial. HA has already demonstrated potential for use in multiple areas, such as preparing fertilizers, soil conditioners, coatings, cosmetics, and others. It is further necessary to study how to improve the stability and safety of HA in these areas and how to further utilize the generated HA for resource maximization.4.4. Evaluating the environmental and economic benefits of hydrothermal humic acid
To achieve the production of HA from biomass for application in various fields, evaluating the environmental and economic benefits of hydrothermal HA is required. During the process of hydrothermal conversion to produce HA, some environmental issues, such as waste gas, wastewater, chemical reagents, and solid waste, may arise.93 Therefore, it is necessary to evaluate the environmental impacts of the hydrothermal conversion to produce HA and formulate corresponding environmental protection strategies based on the actual impact level. Although artificially synthesized HA has excellent performance, its impact on the structure and function of ecosystems as an exogenous substance still needs in-depth research. For example, the impact of HA on the original biological communities in water bodies, sediments, and soil environments, as well as the secondary pollution and environmental risks generated during the remediation process, are key issues that urgently need to be explored before promoting the application of HAs in the field of environmental remediation. Additionally, the cost of raw materials, energy, and equipment is an important factor that needs to be considered for the commercialization of this process. When compared with existing biological conversion methods for producing HA, it is necessary to evaluate its technical–economic performance and safety to ensure the development of economically feasible conversion technologies, as well as effective methods for dealing with byproducts to make the production of HA competitive with existing methods.5. Conclusions
The hydrothermal humification of biomass is an effective approach for producing humic acid (HA) to improve the carbon efficiency of biomass utilization. This article explored the conversion mechanisms involved in hydrothermal HA production, compared the potential of various raw materials for HA conversion, and examined how factors such as the reaction time, temperature, pH, and additives influence the conversion efficiency. With significant application potential in agriculture, environmental protection, and functional material production, HA has gained much attention, indicating the high application of hydrothermal HA. Given the currently unclear mechanisms of hydrothermal HA transformation and the few available application studies that have been performed, achieving high-quality and consistent-quality HA through hydrothermal methods, understanding the application effects and mechanisms, and developing large-scale equipment for hydrothermal HA production are critical steps toward commercializing HA. At the same time, evaluating the environmental and economic benefits of hydrothermal HA is required. This review offers a comprehensive reference for ongoing research on hydrothermal HA.Author contributions
Yangjiuzhou Wang: Conceptualization, data curation, formal analysis, writing – original draft, writing – review & editing. Changbin Yuan: Conceptualization, data curation, formal analysis, writing – original draft, writing – review & editing. Kai Zhang: Writing – original draft. Jinyu Tong: Writing – original draft. Ningjie Ma: Visualization. M. M. Ali: Writing – original draft. Yongdong Xu: Conceptualization, data curation, formal analysis, writing – original draft, investigation, supervision, writing – review & editing. Zhidan Liu: Conceptualization, data curation, formal analysis, funding acquisition, project administration, supervision, writing – review & editing.Data availability
No primary research results, software or code has been included and no new data were generated or analyzed as part of this review.Conflicts of interest
The authors declare no Competing Financial or Non-Financial Interests.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (U21A20162; 52261145701) and the 2115 Talent Development Program of China Agricultural University, China.References
- Y. Jin, X. Zhang, Y. Yuan, Y. Lan, K. Cheng and F. Yang, J. Environ. Manage., 2023, 344 DOI:10.1016/j.jenvman.2023.118377.
- L. M. Stancampiano, M. Verrillo, S. Cangemi, I. Meignant, R. Spaccini, A. Piccolo and M. C. Bridoux, Environ. Chem. Lett., 2023, 21, 2489–2498 Search PubMed.
- X. X. Peng, S. Gai, K. Cheng and F. Yang, J. Hazard. Mater., 2022, 435, 129070 CrossRef CAS PubMed.
- P. Xu, X. Zhu, H. Tian, G. Zhao, Y. Chi, B. Jia and J. Zhang, J. Cleaner Prod., 2022, 337, 130510 Search PubMed.
- S. Hriciková, I. Kožárová, N. Hudáková, A. Reitznerová, J. Nagy and S. Marcinčák, Life, 2023, 13, 858 CrossRef PubMed.
- O. Vioratti Telles de Moura, R. Luiz Louro Berbara, D. França de Oliveira Torchia, H. Fernanda Oliveira Da Silva, T. Augusto van Tol de Castro, O. Carlos Huertas Tavares, N. Fernandes Rodrigues, E. Zonta, L. Azevedo Santos and A. Calderín García, J. Saudi Soc. Agric. Sci., 2023, 22 DOI:10.1016/J.JSSAS.2023.05.001.
- C. Wang, T. Cheng, D. Zhang and X. Pan, Sci. Total Environ., 2023, 863, 160755 CrossRef CAS PubMed.
- B. A. G. De Melo, F. L. Motta and M. H. A. Santana, Mater. Sci. Eng., C, 2016, 62, 967–974 Search PubMed.
- S. Wei, Z. Li, Y. Sun, J. Zhang, Y. Ge and Z. Li, Renewable Sustainable Energy Rev., 2022, 170, 112984 CrossRef CAS.
- M. Wang, Y. Li, H. Peng, J. Wang, Q. Li, P. Li, J. Fan, S. Liu and G. Zheng, Renewable Sustainable Energy Rev., 2023, 187, 113771 CrossRef CAS.
- Y. Shao, Z. Li, Y. Long, J. Zhao, W. Huo, Z. Luo and W. Lu, Sci. Total Environ., 2024, 908, 168232 CrossRef CAS PubMed.
- S. Jha, J. A. Okolie, S. Nanda and A. K. Dalai, Chem. Eng. Technol., 2022, 45, 791–799 CrossRef CAS.
- H. Choi, Y. T. Kim, Y. F. Tsang and J. Lee, Korean J. Chem. Eng., 2023, 40, 1815–1821 CrossRef CAS.
- V. S. Santos, B. R. Moura, I. C. Constantino, G. Metzker, M. Boscolo, M. L. Cornélio, O. P. Ferreira, J. L. S. Mounier, H. Hajjoul, M. C. Bisinoti, F. H. S. Junior and A. B. Moreira, Environ. Technol. Innovation, 2021, 23, 101688 CrossRef CAS.
- E. Efremenko, N. Stepanov, O. Senko, I. Lyagin, O. Maslova and A. Aslanli, Biomimetics, 2023, 8, 613 CrossRef CAS PubMed.
- Y. Shao, W. Huo, R. Ye, Y. Liu, M. Ajmal and W. Lu, Chem. Eng. J., 2023, 457, 141180 CrossRef CAS.
- F. Yang, C. Tang and M. Antonietti, Chem. Soc. Rev., 2021, 50, 6221–6239 RSC.
- F. Yang and M. Antonietti, Adv. Sci., 2020, 7, 1–7 Search PubMed.
- F. Yang and M. Antonietti, Prog. Polym. Sci., 2020, 100, 101182 CrossRef CAS.
- Y. Xu and Z. Liu, Prog. Chem., 2021, 33, 2150–2162 CAS.
- Y. Xu, Y. Wang, Z. Liu, C. Yuan, J. Lu, Z. Wang and Z. Liu, J. Cleaner Prod., 2023 DOI:10.1016/j.jclepro.2023.136971.
- Y. Xu, J. Lu, Y. Wang, C. Yuan and Z. Liu, J. Hazard. Mater., 2022 DOI:10.1016/j.jhazmat.2021.127162.
- C. Wang, Z. Wang, X. Wang, N. Li, J. Tao, W. Zheng, B. Yan, X. Cui, Z. Cheng and G. Chen, Processes, 2022, 10, 2439 CrossRef CAS.
- X. Peng, S. Gai, K. Cheng and F. Yang, Green Chem., 2023, 25, 1503–1512 RSC.
- Y. Shao, M. Bao, W. Huo, R. Ye, M. Ajmal and W. Lu, Chem. Eng. J., 2023, 452, 139172 CrossRef CAS.
- Y. Shao, J. Zhao, Y. Long and W. Lu, Front. Environ. Sci. Eng., 2023, 17, 119 CrossRef CAS.
- W. Sui, S. Li, X. Zhou, Z. Dou, R. Liu, T. Wu, H. Jia, G. Wang and M. Zhang, Molecules, 2021, 26, 3841 CrossRef CAS PubMed.
- C. Kaya, M. Şenbayram, N. A. Akram, M. Ashraf, M. N. Alyemeni and P. Ahmad, Sci. Rep., 2020, 10, 1–13 CrossRef PubMed.
- H. Zhang, N. Xie, Z. Fan, T. Xu and C. Tang, E3S Web Conf., 2022, 341, 01012 Search PubMed.
- M. Elhassan, R. Abdullah, M. R. R. Kooh and Y. F. Chou Chau, Bioresour. Technol. Rep., 2023, 21, 101314 CrossRef CAS.
- V. K. H. Bui, H. B. Truong, S. Hong, X. Li and J. Hur, J. Cleaner Prod., 2023, 402, 136832 CrossRef CAS.
- G. XiaoXia, L. HongTao, C. ZhiZhou, T. XiuPing, J. HongMei, D. HongMin and Z. ZhiPing, J. Ecol. Rural Environ., 2018, 34, 489–498 Search PubMed.
- W. Junzhe, T. Lihua, G. Jianxin, W. Junzhe, T. Lihua and G. Jianxin, Chin. J. Environ. Eng., 2017, 11, 578–581 Search PubMed.
- Y. Meng, Y. Zhou, Y. Shao, D. Zhou, D. Shen and Y. Long, Fuel, 2021 DOI:10.1016/j.fuel.2021.121769.
- N. Yang, S. Yang, L. Yang, Q. Song and X. Zheng, Environ. Res., 2023, 217, 114814 CrossRef CAS PubMed.
- J. G. Lee, H. Y. Yoon, J. Y. Cha, W. Y. Kim, P. J. Kim and J. R. Jeon, Biotechnol. Adv., 2019, 37, 107416 CrossRef CAS PubMed.
- A. Jokic, A. I. Frenkel, M. A. Vairavamurthy and P. M. Huang, Geophys. Res. Lett., 2001, 28, 3899–3902 CrossRef CAS.
- Y. Shao, Z. Luo, M. Bao, W. Huo, R. Ye, M. Ajmal and W. Lu, Chem. Eng. J., 2023, 145634 CrossRef CAS.
- D. W. Min, K. Kim, K. H. Lui, B. Kim, S. Kim, J. Cho and W. Choi, Environ. Sci. Technol., 2019, 53, 7410–7418 CrossRef CAS PubMed.
- Z. T. Hu, W. Huo, Y. Chen, Q. Zhang, M. Hu, W. Zheng, Y. Shao, Z. Pan, X. Li and J. Zhao, Front. Bioeng. Biotechnol., 2022, 10, 878686 CrossRef PubMed.
- G. Cheng, Z. Niu, C. Zhang, X. Zhang and X. Li, Appl. Sci., 2019, 9, 1356 CrossRef CAS.
- P. Chen, R. Yang, Y. Pei, Y. Yang, J. Cheng, D. He, Q. Huang, H. Zhong and F. Jin, Sci. Total Environ., 2022, 828, 154440 CrossRef CAS PubMed.
- D. Shu, C. Xiong and Y. Chi, Huanjing Kexue Xuebao, 2016, 36, 2563–2570 CAS.
- H. Li, Q. Zeng, J. Zhu, Y. Zhu and Y. Xu, Ind. Crops Prod., 2023, 205, 117514 CrossRef CAS.
- D. Lachos-Perez, P. César Torres-Mayanga, E. R. Abaide, G. L. Zabot and F. De Castilhos, Bioresour. Technol., 2022, 343, 126084 CrossRef CAS PubMed.
- S. Li, X. Qi, M. Chen, W. Yang, K. Sun and Y. Li, Acta Pedol. Sin., 2023, 60, 345–354 Search PubMed.
- Y. Zhao, K. Lu, H. Xu, L. Zhu and S. Wang, Renewable Sustainable Energy Rev., 2021, 139, 110706 CrossRef CAS.
- Y. Wu, H. Wang, H. Li, X. Han, M. Zhang, Y. Sun, X. Fan, R. Tu, Y. Zeng, C. C. Xu and X. Xu, Renewable Energy, 2022, 196, 462–481 CrossRef CAS.
- F. Yang, S. Zhang, K. Cheng and M. Antonietti, Sci. Total Environ., 2019, 686, 1140–1151 CrossRef CAS PubMed.
- Y. Zhi, X. Li, F. Lian, C. Wang, J. C. White, Z. Wang and B. Xing, Sci. Total Environ., 2022, 848, 157536 CrossRef CAS PubMed.
- X. X. Peng, S. Gai, Z. Liu, K. Cheng and F. Yang, ChemSusChem, 2023, e202301227 Search PubMed.
- Y. Shao, M. Bao, W. Huo, R. Ye, Y. Liu and W. Lu, J. Cleaner Prod., 2022, 335, 130302 CrossRef CAS.
- X. Zhu, J. Liu, L. Li, G. Zhen, X. Lu, J. Zhang, H. Liu, Z. Zhou, Z. Wu and X. Zhang, Chemosphere, 2023, 312, 137193 CrossRef CAS PubMed.
- Y. Yao, X. Wang, Y. Yang, T. Shen, C. Wang, Y. Tang, Z. Wang, J. Xie, L. Liu, S. Hou, B. Gao, Y. C. Li and Y. Wan, Environ. Sci. Technol., 2019, 53, 14752–14760 CrossRef CAS PubMed.
- S. Zhang, Q. Du, K. Cheng, M. Antonietti and F. Yang, Chem. Eng. J., 2020 DOI:10.1016/j.cej.2020.124832.
- S. Zhang, J. Song, Q. Du, K. Cheng and F. Yang, Chemosphere, 2020 DOI:10.1016/j.chemosphere.2020.126606.
- Z. Ennan, Y. Zhu, J. Hu and T. Xu, Nat., Environ. Pollut. Technol., 2022, 21, 1243–1249 CrossRef.
- M. Liu, C. Wang, X. Liu, Y. Lu and Y. Wang, Appl. Soil Ecol., 2020, 156, 103705 CrossRef.
- N. R. Wandansari, S. Soemarno, R. Suntari and S. Kurniawan, J. Degraded Min. Lands Manage., 2023, 10, 4245–4254 CrossRef.
- X. Yu, Z. Wang, J. Liu, H. Mei, D. Yong and J. Li, Mater. Today Commun., 2019, 19, 124–130 CrossRef CAS.
- L. Liang, Y. Guo, H. Wang, Z. Liao, J. Zhang, L. Wei and K. Hou, J. Appl. Polym. Sci., 2023, 140, e53390 CrossRef CAS.
- M. Rostami, A. Shokouhian and M. Mohebodini, Int. J. Fruit Sci., 2022, 22, 203–214, DOI:10.1080/15538362.2021.2022566.
- C. Tang, Y. Li, J. Song, M. Antonietti and F. Yang, iScience, 2021, 24, 102647 CrossRef CAS PubMed.
- H. Zhang, S. Xie, Z. Bao, H. Tian, E. J. M. Carranza, W. Xiang, L. Yao and H. Zhang, J. Soils Sediments, 2020, 20, 109–121 CrossRef CAS.
- M. Rashad, M. Hafez and A. I. Popov, J. Plant Nutr., 2022, 45, 1072–1122 CrossRef CAS.
- Y. Li, F. Fang, J. Wei, X. Wu, R. Cui, G. Li, F. Zheng and D. Tan, Sci. Rep., 2019, 9, 1–9 CrossRef.
- L. Zanin, N. Tomasi, S. Cesco, Z. Varanini and R. Pinton, Front. Plant Sci., 2019, 10, 675 CrossRef.
- X. Xia Guo, H. tao Liu and S. Biao Wu, Sci. Total Environ., 2019, 662, 501–510 CrossRef PubMed.
- J. Tiwari, A. L. Ramanathan, K. Bauddh and J. Korstad, Pedosphere, 2023, 33, 237–249 CrossRef CAS.
- J. Gerke, J. Plant Nutr. Soil Sci., 2021, 184, 329–338 CrossRef CAS.
- A. Hayati, S. M. Hosseini, M. M. Rahimi and A. Kelidari, Commun. Soil Sci. Plant Anal., 2022, 53, 1744–1755 CrossRef CAS.
- A. M. M. A. Tahoun, M. M. A. El-Enin, A. G. Mancy, M. H. Sheta and A. Shaaban, J. Soil Sci. Plant Nutr., 2022, 22, 2857–2871 CrossRef CAS PubMed.
- Q. Li, D. Zhang, H. Cheng, L. Ren, X. Jin, W. Fang, D. Yan, Y. Li, Q. Wang and A. Cao, J. Environ. Manage., 2022, 309, 114666 CrossRef CAS PubMed.
- S. Nardi, A. Ertani and O. Francioso, J. Plant Nutr. Soil Sci., 2017, 180, 5–13 CrossRef CAS.
- L. P. Canellas and F. L. Olivares, Chem. Biol. Technol. Agric., 2014, 1, 1–11 CrossRef.
- L. E. B. Baldotto, M. A. Baldotto, V. B. Giro, L. P. Canellas, F. L. Olivares and R. Bressan-Smith, Rev. Bras. Cienc. Solo, 2009, 33, 979–990 CrossRef CAS.
- T. Li, Y. He, J. Wang, H. Xiang, X. Xu, C. Li and Z. Wu, Sci. Total Environ., 2023, 888, 164246 CrossRef CAS PubMed.
- L. Han, Z. Zhao, J. Li, X. Ma, X. Zheng, H. Yue, G. Sun, Z. Lin and S. Guan, Sci. Total Environ., 2023, 859, 160315 CrossRef CAS PubMed.
- Q. Chen, Z. Qu, G. Ma, W. Wang, J. Dai, M. Zhang, Z. Wei and Z. Liu, Agric. Water Manage., 2022, 263, 107447 CrossRef.
- A. Deng, X. Wu, C. Su, M. Zhao, B. Wu and J. Luo, Chem. Geol., 2021, 583, 120473 CrossRef CAS.
- Y. Shao, Y. Geng, Z. Li, Y. Long, M. Ajmal, W. Lu and J. Zhao, Chem. Eng. J., 2023, 472, 145098 CrossRef CAS.
- M. Malhotra and A. Garg, Waste Manage., 2020, 117, 114–123 CrossRef CAS PubMed.
- J. Wang and Y. Yin, Renewable Sustainable Energy Rev., 2018, 92, 284–306 CrossRef CAS.
- F. Deng, Z. Cao, Y. Luo, R. Wang, H. Shi and D. Li, J. Environ. Manage., 2023, 345, 118845 CrossRef CAS PubMed.
- B. Xing, M. Ouyang, N. Graham and W. Yu, Chem. Eng. J., 2020, 393, 124730 CrossRef CAS.
- V. Cozzolino, A. De Martino, A. Nebbioso, V. Di Meo, A. Salluzzo and A. Piccolo, Environ. Sci. Pollut. Res. Int., 2016, 23, 11312–11322 CrossRef CAS PubMed.
- Y. Sun, D. Bai, L. Lu, Z. Li, B. Zhang, Y. Liu, L. Zhuang, T. Yang and T. Chen, Colloids Surf., A, 2023, 658, 130771 CrossRef CAS.
- Y. Li, C. Zhang and Z. Hu, Water Res., 2021, 189, 116628 CrossRef CAS PubMed.
- K. Tang, M. Escola Casas, G. T. H. Ooi, K. M. S. Kaarsholm, K. Bester and H. R. Andersen, Int. J. Hyg. Environ. Health, 2017, 220, 604–610 CrossRef CAS PubMed.
- T. Zhang, Z. Shen, L. Zhang, Z. Tang, Q. Zhang, Q. Chen, Y. Lei, Y. Zeng, H. Xu and J. Cao, Atmos. Res., 2020, 234, 104784 CrossRef CAS.
- K. Liu, Y. Chen, N. Xiao, X. Zheng and M. Li, Environ. Sci. Technol., 2015, 49, 4929–4936 CrossRef CAS PubMed.
- J. Li, X. Hao, M. C. M. van Loosdrecht, Y. Luo and D. Cao, Water Res., 2019, 155, 431–443 CrossRef CAS PubMed.
- O. T. Ore, A. O. Adeola, O. Fapohunda, D. T. Adedipe, A. A. Bayode and F. M. Adebiyi, Environ. Sci. Pollut. Res., 2023, 30, 59106–59127 CrossRef CAS PubMed.
- N. Sun, J. Liu, B. W. Qi, L. L. Lu, H. L. Du, S. Li, C. Q. Li, S. W. Jiang, Z. J. Wang, A. P. Yang, G. L. Zhu, T. Y. Wang, S. M. Wang and Q. Fu, Chemosphere, 2023, 329, 138555 CrossRef CAS PubMed.
- Q. Du, G. Li, S. Zhang, J. Song, Y. Zhao and F. Yang, J. Hazard. Mater., 2020, 383 DOI:10.1016/j.jhazmat.2019.121170.
- X. Zhang, Q. Jia, F. Wu, L. Zhu and L.-Z. Huang, J. Hazard. Mater., 2023, 458 DOI:10.1016/j.jhazmat.2023.131872.
- B. Xing, R. Yuan, C. Zhang, G. Huang, H. Guo, Z. Chen, L. Chen, G. Yi, Y. Zhang and J. Yu, Fuel Process. Technol., 2017, 165, 112–122 CrossRef CAS.
- P. Y. Zhao, B. J. Yu, S. Sun, Y. Guo, Z. Z. Chang, Q. Li and C. Y. Wang, Electrochim. Acta, 2017, 232, 348–356 CrossRef CAS.
- Y. Zhu, M. Chen, Q. Li, C. Yuan and C. Wang, Carbon, 2017, 123, 727–734 CrossRef CAS.
- Y. Li, X. Jia, X. Li, P. Liu, X. Zhang and M. Guo, Waste Manage., 2023, 162, 55–62 CrossRef CAS PubMed.
- A. El-Ghenymy, M. Alsheyab, A. Khodary, I. Sirés and A. Abdel-Wahab, Chemosphere, 2020, 246, 125674 CrossRef CAS PubMed.
- E. S. M. Duraia and G. W. Beall, Sens. Actuators, B, 2015, 220, 22–26 CrossRef CAS.
- C. Gonzalez-Nava, J. Manríquez, L. A. Godínez and F. J. Rodríguez-Valadez, Bioelectrochemistry, 2022, 144, 108003 CrossRef CAS PubMed.
- C. Wang, P. Wang, D. Ouyang, N. Xue, X. Xue, Y. Zhang, H. Zhu and J. Yin, Energy Fuels, 2022, 36, 12807–12815 CrossRef CAS.
- F. Hekmat, M. Shahi and S. Shahrokhian, Sustainable Energy Fuels, 2021, 5, 4869–4881 RSC.
- Y. He, Z. Wang, J. Zhang and L. Wei, J. Mater. Chem. A, 2017, 5, 21130–21133 RSC.
- E. S. M. Duraia, S. Niu, G. W. Beall and C. P. Rhodes, J. Mater. Sci.: Mater. Electron., 2018, 29, 8456–8464 CrossRef CAS.
- Y. M. Shulga, S. A. Baskakov, Y. V. Baskakova, A. S. Lobach, E. N. Kabachkov, Y. M. Volfkovich, V. E. Sosenkin, N. Y. Shulga, S. I. Nefedkin, Y. Kumar and A. Michtchenko, J. Alloys Compd., 2018, 730, 88–95 CrossRef CAS.
- P. Goel, M. Dhingra, P. Goel and M. Dhingra, Humic Substances, 2021, ch. 4 Search PubMed.
- O. I. Klein, N. A. Kulikova, A. I. Konstantinov, M. V. Zykova and I. V. Perminova, Polymers, 2021 DOI:10.3390/POLYM13193262.
- Y. Zhernov, J. Allergy Clin. Immunol., 2018, 141, AB233, DOI:10.1016/j.jaci.2017.12.737.
- P. Hajdrik, B. Pályi, Z. Kis, N. Kovács, D. S. Veres, K. Szigeti, F. Budán, I. Hegedüs, T. Kovács, R. Bergmann and D. Máthé, Foods, 2022, 11, 694 CrossRef CAS PubMed.
- R. Klöcking and B. Helbig, Biopolymers Online, 2001 DOI:10.1002/3527600035.BPOL1013.
- Y. V. Zhernov, A. I. Konstantinov, A. Zherebker, E. Nikolaev, A. Orlov, M. I. Savinykh, G. V. Kornilaeva, E. V. Karamov and I. V. Perminova, Environ. Res., 2021, 193, 110312 CrossRef CAS PubMed.
- G. Ferrara, E. Loffredo, N. Senesi and R. Marcos, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2006, 603, 27–32 CrossRef CAS PubMed.
- I. Marova, J. Kucerik, K. Duronova, A. Mikulcova and Z. Vlckova, Environ. Chem. Lett., 2011, 9, 229–233 CrossRef CAS.
- Y. He, D. Wang, K. Liu, S. Deng and Y. Liu, Mol. Immunol., 2023, 161, 61–73 CrossRef CAS PubMed.
- U. Wollina, J. Cutaneous Aesthetic Surg., 2009, 2, 17 CrossRef PubMed.
- X. Tian, Y. Zhang, H. Li, Y. Jiao, Q. Wang, Y. Zhang, N. Ma and W. Wang, Environ. Geochem. Health, 2022, 44, 4235–4251 CrossRef CAS PubMed.
- M. Drobnik and T. Latour, Rocz. Panstw. Zakl. Hig., 2011, 62, 225–231 CAS.
- P. C. Aristimunha, R. D. Mallheiros, P. R. Ferket, K. M. Cardinal, A. L. B. M. Filho, E. T. Santos, D. T. Cavalcante and A. M. L. Ribeiro, J. Appl. Poult. Res., 2020, 29, 85–94 CrossRef CAS.
- O. Bezuglova and A. Klimenko, Agronomy, 2022, 12, 584 CrossRef CAS.
- F. Nabi, D. Shi, Q. Wu and D. M. Baloch, Front. Vet. Sci., 2023, 10, 1171987 CrossRef PubMed.
- F. De Souza and S. R. Bragança, J. Mater. Res. Technol., 2017, 7, 254–260 CrossRef.
- W. Zhang, J. Luo, Y. Huang, C. Zhang, L. Du, J. Guo, J. Wu, X. Zhang, J. Zhu and G. Zhang, Fuel, 2020, 262, 116576 CrossRef CAS.
- I. Kozyatnyk, K. G. Latham and S. Jansson, ACS Sustainable Chem. Eng., 2019, 7, 2585–2592 CrossRef CAS.
- S. Salati, G. Papa and F. Adani, Biotechnol. Adv., 2011, 29, 913–922 CrossRef CAS PubMed.
- C. Urdiales, M. P. Sandoval, M. Escudey, C. Pizarro, H. Knicker, L. Reyes-Bozo and M. Antilén, J. Environ. Manage., 2018, 227, 117–123 CrossRef CAS PubMed.
- E. Sarlaki, A. Sharif Paghaleh, M. H. Kianmehr and K. Asefpour Vakilian, Renewable Energy, 2021, 163, 105–122 CrossRef CAS.
- B. Huang, G. Fu, C. He, H. He, C. Yu and X. Pan, J. Electroanal. Chem., 2019, 851, 113464 CrossRef CAS.
- P. Hajdrik, B. Pályi, Z. Kis, N. Kovács, D. S. Veres, K. Szigeti, F. Budán, I. Hegedüs, T. Kovács, R. Bergmann and D. Máthé, Foods, 2022, 11, 694 CrossRef CAS PubMed.
Footnote |
| † These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |







