DOI:
10.1039/D5CE00129C
(Paper)
CrystEngComm, 2025,
27, 3229-3237
Hollow architectures of CoNi2S4/NiS2 with boosted performance for supercapacitors†
Received
5th February 2025
, Accepted 6th April 2025
First published on 7th April 2025
Abstract
Hollow architectures have great prospects in supercapacitors due to their rich active surface area, good structural stability and convenient transmission channels for ions/electrons. Herein, we present a self-engaged template process to prepare hybrid CoNi2S4/NiS2 hollow architectures. Ni–Co glycerate solid spheres are used as templates and transformed to CoNi2S4/NiS2via a sulfidation procedure. The supercapacitor’s performance evaluation unveils that these CoNi2S4/NiS2 hollow architectures manifest a high capacitance of 1309.2 F g−1 at 4.0 A g−1 and remarkable cyclability with 1147.6 F g−1 retention (only 4.2% decay) for 5000 cycles at 6.0 A g−1. Furthermore, a two-electrode cell assembly of the CoNi2S4/NiS2 and activated carbon, displays an energy density of 47.5 W h kg−1 at 3301 W kg−1 and impressively durable cycle life. These results provide a simple avenue to design an efficient electrode material for supercapacitors based on metal sulfides.
1. Introduction
Supercapacitors with long cycle lives, ideally large power density and high reliability, have received intense research interest in recent years.1 Exploring potential electrode materials is crucial to achieve the desirable performance of supercapacitors.2 To date, many explorations have been made to obtain suitable electrode materials for supercapacitors. Among the various alternatives, transition metal sulfides are deemed to be promising candidates due to their abundant active sites, significant capacity and efficient electric conductivity.3–5 In comparison with single metal sulfides, construction of different metal sulfides into one advanced architecture is valid for promoting the electrochemical features with boosted conductivity, electroactivity and structural stability, owing to the synergetic effects of each metal sulfide.6–8 For instance, Ni3S2@Co3S4 composites were designed to exhibit high specific capacitance, exceptional rate capacity and cycling behavior for supercapacitors.9 A Co9S8@Ni3S2/ZnS composite formed via a template assisted method has been reported; the unique composite and architecture endow Co9S8@Ni3S2/ZnS with remarkable electrochemical properties.10 As a result, exploring metal sulfides with complex components is highly significant for high performance supercapacitors.
Many facts have affirmed that electrode materials with tunable architectures have a significant effect on supercapacitor performance.11,12 In this context, an effective way is to fabricate desirable electrode material architectures for high performance. Hollow architectures possess attractive features of rich inner space, a short ion permeation channel, large surface area and incremental electrolyte–electrode contact area.13–15 For example, a hollow NiCo2S4/Co9S8 spindle electrode material that shows high pseudocapacitance and energy density has been reported.16 Zhao et al. explored a NiS2/CuS hollow microsphere that manifested outstanding pseudocapacitive as well as exceptional cycling life and rate behavior.17 Therefore, hollow architectures are considered as versatile electrode materials for electrochemical energy storage.
Ni–Co sulfides have higher conductivity and richer redox reactions and are promising candidates for supercapacitor electrode materials. In particular, CoNi2S4 is an outer layer material,18 which exhibits high conductivity and can boost electron transport and initiate ionic reactions. Moreover, NiS2, featuring large theoretical capacity is another promising candidate for supercapacitors. Therefore, some beneficial CoNi2S4 or NiS2 supercapacitor electrode materials have been reported. However, hybrid CoNi2S4/NiS2 hollow architectures are rarely reported. Herein, we exploit a self-engaged template method to prepare hybrid CoNi2S4/NiS2 hollow architectures, as shown in Scheme 1. As a result of the architecture and components, the CoNi2S4/NiS2 demonstrates remarkable performance, delivering a high capacitance of 1309.2 F g−1 at 4.0 A g−1 and excellent durability with 1147.6 F g−1 retention (only 4.2% loss) over 5000 cycles at 6.0 A g−1. Moreover, the constructed two-electrode device with CoNi2S4/NiS2 and activated carbon (AC) shows a high energy density of 47.5 W h kg−1 at 3301 W kg−1 and superb cycle life. These results suggest its great potential for electrochemical energy storage.
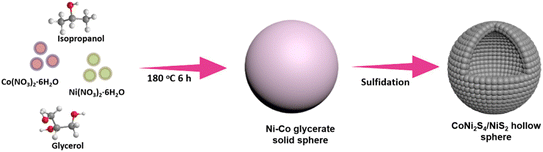 |
| | Scheme 1 Schematic synthesis procedure toward CoNi2S4/NiS2 hollow architectures. | |
2. Experimental
2.1 Preparation of Ni–Co glycerate precursor
In a typical precursor preparation, Ni(NO3)2·6H2O (0.40 mmol) and Co(NO3)2·6H2O (0.10 mmol) are solubilized in a mixture of 8.0 mL glycerol and 40 mL isopropanol under stirring. Then the solution is placed into an autoclave and heated at 180 °C for 6.0 h. Finally, the resulting solution is centrifuged, purified with ethanol and dried, providing the precursor of the Ni–Co glycerate.
2.2 Synthesis of CoNi2S4/NiS2 hollow architectures
Typically, 30 mg precursor is dispersed in 20 mL ethanol under stirring. Then 50 mg thioacetamide (TAA) is added into the dispersion and further stirred for about 30 min. The solution is sealed in an autoclave and heated at 160 °C for 6.0 h. After centrifugation, purification with ethanol and drying, the Ni–Co sulfide product is obtained.
2.3 Sample characterization
The architecture observation was performed using a scanning electron microscope (SEM; Hitachi, SU8010) and transmission electron microscope (TEM; Tecnai, G2F30). To acquire the sample composition, X-ray diffraction (XRD) patterns were obtained using a Rigaku D/max 2500 diffractometer. The chemical states of each element in the Ni–Co sulfide were characterized using an X-ray photoelectron spectrometer (XPS; Thermo, Escalab250Xi). Nitrogen adsorption–desorption characterization was conducted using an ASAP 2020 Micromeritics instrument.
2.4 Electrochemical tests
Electrochemical tests were performed on a CHI 660D electrochemical workstation using a three-electrode system in 3.0 M KOH electrolyte, in which Hg/HgO/sat. KCl electrode and platinum foil acted as the reference electrode and counter electrode. The Ni–Co sulfide, acetylene black and polyvinylidene difluoride (mass ratio = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) were polished in isopropanol for about 30 min and further dropped on a nickel foam as the working electrode. The mass loading of the CoNi2S4/NiS2 hollow architectures electrode material for the three electrode system is about 5 mg.
5) were polished in isopropanol for about 30 min and further dropped on a nickel foam as the working electrode. The mass loading of the CoNi2S4/NiS2 hollow architectures electrode material for the three electrode system is about 5 mg.
The asymmetric supercapacitor tests were measured in 3.0 M KOH electrolyte with a two-electrode system: an AC negative electrode and Ni–Co sulfide positive electrode. AC, acetylene black and polyvinylidene difluoride (mass ratio = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) were added in isopropanol. After grinding the mixture for 30 min, the resultant slurry was coated on a nickel foam to provide the negative electrode. Similarly, the fabrication of the positive electrode resembles the process for the negative electrode except the AC was replaced by the Ni–Co sulfide. The mass ratio between CoNi2S4/NiS2 and AC was around 1
5) were added in isopropanol. After grinding the mixture for 30 min, the resultant slurry was coated on a nickel foam to provide the negative electrode. Similarly, the fabrication of the positive electrode resembles the process for the negative electrode except the AC was replaced by the Ni–Co sulfide. The mass ratio between CoNi2S4/NiS2 and AC was around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The energy density (E) and power density (P) of the asymmetric supercapacitor can be calculated, as shown in eqn (1) and (2),
2. The energy density (E) and power density (P) of the asymmetric supercapacitor can be calculated, as shown in eqn (1) and (2),
| |  | (1) |
| | 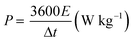 | (2) |
where
C (F g
−1) and Δ
V (V) stand for the specific capacitance and working voltage, Δ
t (s) denotes discharging process time.
3. Results and discussion
As analyzed by FESEM and TEM (Fig. 1a–c), the Ni–Co glycerate forms solid spheres with a diameter of about 1.0 μm and smooth surfaces. The obtained Ni–Co glycerate is subsequently sulfurized by reacting with TAA under solvothermal conditions. Eventually, the Ni–Co sulfide is synthesized. The hollow architectures can be distinctly observed for the Ni–Co sulfide. A careful observation of the shell of the Ni–Co sulfide, we see that the hollow architecture is constructed by nanoparticles (Fig. 1d–g). To illustrate the hollow architecture formation process, samples prepared at various time intervals were observed by TEM, as shown in Fig. S1.† The samples still form solid spheres but possess a rough surface after 0.5 h (Fig. S1a†), demonstrating the occurrence of sulfidation reactions on the Ni–Co glycerate precursor surface. After 1.0 h, a core–shell structured is formed (Fig. S1b†). Extending the sulfidation time to 3.0 h, the core becomes smaller (Fig. S1c†). Further increasing the sulfidation time to 6 h, the core is consumed completely, and the hollow architecture is finally formed (Fig. 1f and g).The XRD result (Fig. S2†) proves that the derived Ni–Co sulfide includes CoNi2S4 (JCPDS No. 24-0334) and NiS2 (JCPDS No. 11-0099). A high resolution TEM (HRTEM) image shows lattice distances of 0.15 nm and 0.33 nm (Fig. 1h), which conform to the (321) plane of NiS2 and (220) plane of CoNi2S4, respectively. As shown in the elemental mapping images of a Ni–Co sulfide (Fig. 1i), the Co, Ni and S elements are evenly distributed in the hollow architecture. Moreover, the electronic states of Ni, Co and S elements in the CoNi2S4/NiS2 were further characterized using XPS. In the Ni 2p spectra (Fig. 2a), peaks at 853.5 and 871 eV are associated with Ni2+ Ni 2p3/2 and 2p1/2,19 while peaks emerging at 856.3 and 874.4 eV, are attributed to the Ni3+ Ni 2p3/2 and 2p1/2.20 The Co 2p spectra (Fig. 2b) shows pronounced peaks at 778.6 and 793.6 eV, which ascribe to Co3+ Co 2p3/2 and 2p1/2.21 Another two peaks at 781.5 and 797.3 eV are assigned to Co2+ Co 2p3/2 and 2p1/2.22 The S 2p spectra of the Ni–Co glycerate derivative presents the peaks at 162.6 and 168.5 eV, which are correlated with Co/Ni–S and S–O bonds (Fig. 2c).23 These above analyses confirm the successful preparation of the CoNi2S4/NiS2 hollow architecture.
 |
| | Fig. 1 (a and b) FESEM and (c) TEM images of Ni–Co glycerate, (d and e) FESEM and (f and g) TEM images of CoNi2S4/NiS2 hollow architectures, (h and i) HRTEM and TEM elemental mapping images of CoNi2S4/NiS2 hollow architectures. | |
 |
| | Fig. 2 (a) Ni 2p, (b) Co 2p and (c) S 2p XPS spectra of the CoNi2S4/NiS2 hollow architectures; (d) N2 adsorption/desorption isotherms and the corresponding pore size distributions (inset) of the CoNi2S4/NiS2 hollow architectures. | |
Due to the special structural characteristics, a large surface area of 75.4 m2 g−1 with mesoporous structure for Ni–Co sulfide hollow architectures illustrates the existence of considerable active sites that can boost the electrochemical performance (Fig. 2d). The supercapacitor behavior of the Ni–Co sulfide electrode material is firstly studied in the three-electrode cell. Fig. 3a shows the cyclic voltammetry (CV) curves of the Ni–Co sulfide electrode measured at a scan rate of 5.0–30 mV s−1. The distinct redox peaks can be discerned, revealing the Faradaic features of the Ni–Co sulfide. The capacitive effect analysis is further evaluated using eqn (3):24
where
i corresponds to the current response at oxidation/reduction peaks and
v represents the scan rate, while
a and
b stand for the experimental parameters. The parameter
b is 0.5, signifying that ionic diffusion is dominant. A
b value of 1.0 denotes the electrochemical reaction is mainly due to capacitive behavior. As shown in
Fig. 3b,
b values of 0.546 and 0.575 for Ni–Co sulfide suggest the charging process is governed by both ionic diffusion and capacitive behavior. The Faradaic redox process could be expressed using the following equations:
18,25| | | CoNi2S4 + OH− + H2O ⇌ CoSOH + 2NiSOH + e− | (4) |
| | | CoSOH + OH− ⇌ CoSO + H2O + e− | (5) |
| | | NiSOH + OH− ⇌ NiSO + H2O + e− | (6) |
| | | NiS2 + OH− ⇌ NiS2OH + e− | (7) |
To further quantitatively analyse the contribution fraction of ionic diffusion and capacitive behavior , the following
eqn (8) is applied:
where
i(
V) is the response current
versus operating voltage of
V,
k1v and
k2v1/2 represent the capacitive and ionic diffusion in the redox processes, with
k1 and
k2 acquired from the relationship
i(
V)/
v1/2versus v1/2.
26 As illustrated in
Fig. 3c and Fig. S3,
† a 52.8% capacitive contribution for Ni–Co sulfide is obtained at 5.0 mV s
−1, and the capacitive contribution increases from 60.3 to 86.3 as the scan rate varies from 10 to 30 mV s
−1.
 |
| | Fig. 3 Electrochemical behavior of the CoNi2S4/NiS2 hollow architectures as supercapacitors: (a) CV curves at scanning rates of 5.0 to 30 mV s−1; (b) the linear relationships between log (peak currents) and log (scanning rates); (c) capacitive contribution ratios at various scanning rates; (d) GCD; and specific capacitance profiles (e) at various current densities; (f) continuous cycling performance at 6.0 A g−1 after 5000 cycles. | |
Representative galvanostatic charge/discharge (GCD) profiles of the Ni–Co sulfide at current densities from 4.0 to 25 A g−1 are displayed in Fig. 3d. Also, Ni–Co sulfide electrode shows charge/discharge platforms, further indicating the Faradaic behavior of the material,27 which is in accordance with the CV characterizations. As shown in Fig. 3e, the Ni–Co sulfide electrode acquires a high capacitance of 1309.2 F g−1 at 4.0 A g−1. At 25 A g−1, a capacitance of 525 F g−1 still remains. Certainly, results of cycling measurements uncover that the Ni–Co sulfide exhibits remarkable electrochemical stability (Fig. 3f), and 1147.6 F g−1 is retained after 5000 cycles at 6.0 A g−1. The decay of electrochemical stability after cycling can be ascribed to the hollow architecture volume variation associated with repeated charge/discharge. The impedance plots of the CoNi2S4/NiS2 hollow architectures are shown in Fig. S4.† The Rs values for before and after 5000 cycles are determined to be 0.17 and 0.55 Ω, confirming that the Rs changed slightly after the cycles. Moreover, there is not much change in the CV and GCD curves after the cycles (Fig. S5†). Of note, the CoNi2S4/NiS2 electrode with hollow architecture structure is preserved after continuous cycles (Fig. S6†), demonstrating the stability of the CoNi2S4/NiS2 hollow architectures electrode material for long term cycling. So it is assumed that the desired cycling of CoNi2S4/NiS2 is attributed to the unique hollow architectures that store electrolytes and shorten ion diffusion routes, and enhance the conductivity and optimize electron transfer of the material. Also, compared with previously reported hybrid metal sulfide electrodes for supercapacitors, the Ni–Co sulfide hollow architectures show comparable or obvious superiority (Table 1).13,28–37
Table 1 Comparison of the electrochemical performance of the CoNi2S4/NiS2 hollow architectures with previously reported counterparts
| Electrode materials |
Morphology |
Current density |
Specific capacitance |
Capacitance retention |
Reference |
| Mo-doped CoS hollow nanocages |

|
0.5 A g−1 |
781 F g−1 |
46.8% after 5000 cycles |
28
|
| Hollow dodecahedral Co4S3/MoS2/MnS |

|
1.0 A g−1 |
590 F g−1 |
90% after 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
29
|
| Hollow MnS@MoS2 cubes |
|
1.0 A g−1 |
1713.4 F g−1 |
96.8% after 1000 cycles |
30
|
| Hollow CuCo2S4 cages |

|
0.5 A g−1 |
1096.27 F g−1 |
— |
13
|
| Hollow urchin-like CuCo2S4 |

|
1.0 A g−1 |
1069 F g−1 |
93.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
31
|
| Hollow NiCo2S4 ellipsoids |

|
10 A g−1 |
495 F g−1 |
— |
32
|
| Carbon coated NiCo2S4 hollow spheres |

|
1.0 A g−1 |
935 F g−1 |
103.1% after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles 000 cycles |
33
|
| Hollow core–shell NiCo2S4@MoS2 |

|
1.0 A g−1 |
860 F g−1 |
71.9% after 1000 cycles |
34
|
| Rod-shaped CoNi2S4 |

|
2.0 mA cm−2 |
7.54 F cm−2 |
71.25% after 5000 cycles |
35
|
| Yolk–shell (NiCo)9S8/GC spheres |

|
1.0 A g−1 |
1367 F g−1 |
89.2% after 6000 cycles |
36
|
| Double-shelled hollow MnCo2S4/CoS1.097 spheres |

|
1.0 A g−1 |
1006 F g−1 |
91.3% after 5000 cycles |
37
|
|
Hollow CoNi
2
S
4
/NiS
2
hollow spheres
|

|
4.0 A g
−1
|
1309.2 F g
−1
|
95.9% after 5000 cycles
|
This work
|
To delve into its practical application, we assembled a two-electrode configuration in which 3.0 M KOH, Ni–Co sulfide and AC served as the electrolyte, positive and negative electrodes, respectively. The CV profiles were performed at different scan rates from 0 to 1.6 V. As is noticeably displayed in Fig. 4a, the profiles feature an asymmetrical rectangle-like shape with broad and weak redox peaks, confirming that the device has double layer capacitive and pseudocapacitive peculiarities. Moreover, the original shape of the CV profiles is retained at scan rates from 5 to 100 mV s−1, unveiling the remarkable rate capabilities.38 The GCD profiles (Fig. 4b) are virtually symmetric in the current densities of 4.0 to 20 A g−1, which implies its excellent electrochemical reversibility.39Fig. 4c reveals that the device achieves 125.6, 118.6, 100.6 and 67.8 F g−1 from 4.0 to 20 A g−1. The determined Ragone plots show that the energy density of the device is 47.5 W h kg−1 at 3301 W kg−1, and reaches 26 W h kg−1 at 16481 W kg−1 (Fig. 4d), which is better than reported asymmetric supercapacitor devices, such as Co3S4@FeCo2S4/NF//NF/rGO (43.6 W h kg−1 at 770 W kg−1),40 C/NiCo-LDH/Co9S8//CNT (39.0 W h kg−1 at 2400 W kg−1),41 Fe–Ni–S/rGO//AC (30.5 W h kg−1 at 800 W kg−1),42 Ni–Co–S@NC//AC (43.6 W h kg−1 at 770 W kg−1),43 SnxNi3−xS2//AC (29.13 W h kg−1 at 700 W kg−1),44 NiCo2S4/ACC//AC/ACC (30.1 W h kg−1 at 800.2 W kg−1),45 NiCo2S4//AC (35.3 W h kg−1 at 750 W kg−1),46 and NiCo2S4@CC//NC@CC (37.5 W h kg−1 at 2250 W kg−1).47 Remarkably, as shown in Fig. 4e, the device retains its performance over 3000 charge/discharge cycles with about a 3.0% decline, under 6.0 A g−1. Moreover, the device can retain a Coulombic efficiency of ∼99.6% during the cycles, suggesting its remarkable reversibility. Above all, these results show that the developed hybrid CoNi2S4/NiS2 hollow architecture has very promising application potential in energy storage.
 |
| | Fig. 4 Electrochemical performance of the two-electrode system: (a) CV profiles at scanning rates of 5.0 to 100 mV s−1; (b) GCD profiles in current densities of 4.0 to 20 A g−1; (c) specific capacitance at various current densities; (d) Ragone plots; and (e) cycling stability for 3000 cycles at 6.0 A g−1. | |
The remarkable capacitive behaviors can be accounted for by the following advantages. First, the hollow architectures with structural stability not only endow considerable electroactive sites but also provide convenient transmission channels for ions/electrons, which generate the high capacitance and excellent cyclability. Second, the synergistic effect combining the advantages of CoNi2S4 and NiS2 renders the hybrid CoNi2S4/NiS2 with the desired electrochemical performance. In short, profiting from its architecture and the properties of its component parts, the hollow architecture of CoNi2S4/NiS2 is deemed to be an ideal candidate for supercapacitor materials.
4. Conclusions
In summary, hybrid CoNi2S4/NiS2 hollow architectures are developed as a potentially excellent supercapacitor material. The CoNi2S4/NiS2 is obtained via a self-engaged template route to synthesize Ni–Co glycerate, followed by a sulfidation procedure. Profiting from the hollow architectures and complex components, the CoNi2S4/NiS2 shows a high capacitance of 1309.2 F g−1 at 4.0 A g−1, and remarkable durability with 1147.6 F g−1 maintained over 5000 cycles under 6.0 A g−1. Furthermore, a two-electrode configuration with the CoNi2S4/NiS2 and AC achieves a high energy density of 47.5 W h kg−1 at 3301 W kg−1 and excellent cycle life. This work would offer a valid avenue to design high performance supercapacitor materials.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was financed by the Major Science and Technology Special Project of Anyang City (2023A02GX009).
Notes and references
- A. L. Narayana, N. Attarzadeh, V. Shutthanandan and C. V. Ramana, High-performance NiCo2O4/Graphene quantum dots for asymmetric and symmetric supercapacitors with enhanced energy efficiency, Adv. Funct. Mater., 2024, 34, 2316379 CrossRef.
- Q. H. Wu, T. Q. He, Y. K. Zhang, J. L. Zhang, Z. J. Wang, Y. Liu, L. Zhao, Y. Z. Wu and F. Ran, Cyclic stability of supercapacitors: materials, energy storage mechanism, test methods, and device, J. Mater. Chem. A, 2021, 9, 24094–24147 RSC.
- R. Tian, Y. Zhou, H. N. Duan, Y. P. Guo, H. Li, K. F. Chen, D. F. Xue and H. Z. Liu, MOF-derived hollow Co3S4 quasi-polyhedron/MWCNT nanocomposites as electrodes for advanced lithium ion batteries and supercapacitors, ACS Appl. Energy Mater., 2018, 1, 402–410 CrossRef CAS.
- H. T. Niu, Y. Liu, B. D. Mao, N. Xin, H. Jia and W. D. Shi, In-situ embedding MOFs-derived copper sulfide polyhedrons in carbon nanotube networks for hybrid supercapacitor with superior energy density, Electrochim. Acta, 2020, 329, 135130 CrossRef CAS.
- M. Y. Zhu, X. Li, C. Y. Tu, Q. Luo, Y. J. Nie, J. M. Pan and S. J. Li, Ethylene glycol assisted self-template conversion approach to synthesize hollow NiS microspheres for a high performance all-solid-state supercapacitor, Mater. Chem. Front., 2022, 6, 203–212 RSC.
- C. P. Yu, Y. Li, Y. Wang, J. W. Cui, T. Y. Zhu, D. B. Yu, X. Shu, Y. Zhang, D. R. MacFarlane and Y. C. Wu, Enhanced energy storage performance of 3D hybrid metal sulfides via synergistic engineering of architecture and composition, ACS Sustainable Chem. Eng., 2020, 8, 11491–11500 CrossRef CAS.
- Y. Wang, X. S. Wang, X. J. Dai, K. L. Li, Z. H. Bao, H. Y. Li, H. W. Tian, P. A. Yang, H. Zhou, H. Chen, Y. L. Yu, P. Yan and Y. X. Zhang, Structural evolution and sulfuration of nickel cobalt hydroxides from 2D to 1D on 3D diatomite for supercapacitors, CrystEngComm, 2021, 23, 5636–5644 RSC.
- J. C. Qi, H. Y. Duan, Z. L. Peng, J. Wang, B. X. Ma, Z. H. Yuan and H. F. Zhang, Cobalt-nickel mixed sulfide hollow nanocages with enhanced surface induced capacitive storage for hybrid supercapacitor, Energy Fuels, 2024, 38, 13379–13389 CrossRef CAS.
- Q. Zhang, Q. S. Shi, Y. Yang, Q. Zhang, Z. Y. Xiao, X. H. Zhang and L. Wang, 2D nanosheet/3D cubic framework Ni–Co sulfides for improved supercapacitor performance via structural engineering, Dalton Trans., 2020, 49, 8162–8168 RSC.
- H. M. Chen, J. J. Zhou, Q. Li, S. H. Zhao, X. B. Yu, K. Tao, Y. P. Hu and L. Han, MOF-assisted construction of a Co9S8@Ni3S2/ZnS microplate array with ultrahigh areal specific capacity for advanced supercapattery, Dalton Trans., 2020, 49, 10535–10544 RSC.
- X. T. Mei, C. Yang, F. Y. Chen, Y. T. Wang, Y. Zhang, Z. M. Man, W. Y. Lu, J. H. Xu and G. Wu, Interfacially ordered NiCoMoS nanosheets arrays on hierarchical Ti3C2Tx Mxene for high-energy-density fiber-shaped supercapacitors with accelerated pseudocapacitive kinetics, Angew. Chem., Int. Ed., 2024, 63, e202409281 CrossRef CAS PubMed.
- X. Cui, F. W. Ma, G. P. Lei, W. Jiang, X. Y. Yang, Z. Y. Liu, J. F. Wan and Y. F. Liu, Trisodium citrate as a double-edged sword: selective etching Prussian blue analog nanocubes into orthogonal frustums and their derivatives for supercapacitors, Small, 2024, 20, 2403732 CrossRef CAS PubMed.
- J. Q. Wang, Y. L. Quan, G. X. Wang, D. Z. Wang, J. Xiao, S. P. Gao, H. F. Xu, S. Liu and L. Cui, 3D hollow cage copper cobalt sulfide derived from metal-organic frameworks for high-performance asymmetric supercapacitors, CrystEngComm, 2021, 23, 7385–7396 RSC.
- Y. X. Zeng, Z. H. Pei, D. Y. Luan and X. W. David Lou, Atomically dispersed zincophilic sites in N, P-codoped carbon macroporous fibers enable efficient Zn metal anodes, J. Am. Chem. Soc., 2023, 145, 12333–12341 CrossRef CAS PubMed.
- H. Tong, L. Li, C. Q. Wu, Z. Tao, J. H. Fang, C. Y. Guan and X. G. Zhang, Sea urchin-like NiCo-LDH hollow spheres anchored on 3D graphene aerogel for high-performance supercapacitors, ChemSusChem, 2024, 17, e202400142 CrossRef CAS PubMed.
- L. R. Hou, Y. Y. Shi, S. Q. Zhu, M. Rehan, G. Pang, X. G. Zhang and C. Z. Yuan, Hollow mesoporous hetero-NiCo2S4/Co9S8 submicro-spindles: unusual formation and excellent pseudocapacitance towards hybrid supercapacitors, J. Mater. Chem. A, 2017, 5, 133–144 RSC.
- W. X. Zhao, X. D. Wang, X. Q. Ma, L. C. Yue, Q. Liu, Y. L. Luo, Y. Liu, A. M. Asiri and X. P. Sun,
In situ tailoring bimetallic-organic framework-derived yolk-shell NiS2/CuS hollow microspheres: an extraordinary kinetically pseudocapacitive nanoreactor for an effective sodium-ion storage anode, J. Mater. Chem. A, 2021, 9, 15807–15819 RSC.
- J. J. Hu, L. Sun, F. Xie, Y. R. Qu, H. K. Tan, X. C. Shi, J. L. Qian, K. Wang and Y. H. Zhang, A sandwich-like CoNiLDH@rGO@CoNi2S4 electrode enabling high mass loading and high areal capacitance for solid-state supercapacitors, J. Mater. Chem. A, 2022, 10, 21590–21602 RSC.
- H. Z. Wang, X. Gao, Y. Q. Xie, E. J. Guo, H. Bai, F. Jiang, Q. Li and H. Y. Yue, Design and fabrication of island-like CoNi2S4@NiCo-LDH/biomass carbon heterostructure as advanced electrodes for high-performance hybrid supercapacitors, Adv. Energy Mater., 2024, 14, 2400493 CrossRef CAS.
- Q. Hu, Y. C. Bai, Y. L. Fang, L. Guo and W. P. Li, Acid- and alkaline-induced phase and structure reconstruction of nickel sulfide toward enhanced electrochemical performance, CrystEngComm, 2024, 26, 5393–5404 RSC.
- X. F. Lu, S. L. Zhang, W. L. Sim, S. Y. Gao and X. W. David Lou, Phosphorized CoNi2S4 yolk-shell spheres for highly efficient hydrogen production via water and urea electrolysis, Angew. Chem., Int. Ed., 2021, 60, 22885–22891 CrossRef CAS PubMed.
- C. L. Lv, J. H. Wei, F. Hu, L. M. Bian and Q. Y. Ouyang, Effect of sulfur vacancies of CoNi2S4 on its electrochemical performance in hybrid supercapacitors, Langmuir, 2024, 40, 27386–27395 CrossRef CAS PubMed.
- Z. Wang, J. Jian, X. Y. Wang, Y. Qiao, M. T. Wang, S. Gao, P. Nie and L. M. Chang, CoNi2S4@CoNi-LDH heterojunction grown on SSM as a highly efficient trifunctional catalyst for water splitting and Zn-air batteries, J. Mater. Chem. C, 2023, 11, 16384–16389 RSC.
- J. J. Ban, X. H. Wen, H. H. Lei, G. Q. Cao, X. H. Liu, C. Y. Niu, G. S. Shao and J. H. Hu, In-plane grain boundary induced defect state in hierarchical NiCo-LDH and effect on battery-type charge storage, Nano Res., 2023, 16, 4908–4916 CrossRef CAS.
- J. J. Hu, L. Sun, F. Xie, Y. R. Qu, H. K. Tan and Y. H. Zhang, An urchin-like Co-doped NiS2/C nanorod array with enriched sulfur vacancies for asymmetric supercapacitors, J. Mater. Chem. A, 2023, 11, 8380–8391 RSC.
- G. B. Bhanuse, S. Kumar, C. C. Yu and Y. P. Fu, Nanostructured ZnCo2O4@NiMn-LDH electrodes for supercapacitor and zinc-air battery application, ACS Appl. Nano Mater., 2024, 7, 13649–13663 CrossRef CAS.
- H. L. Jiang, Q. Ke, X. H. Qiu, J. Z. Chen, P. H. Chen, S. Wang, X. B. Luo and B. Y. Rao, NiCo layered double hydroxide nanocages for high performance asymmetric supercapacitors, Inorg. Chem. Front., 2023, 10, 2154–2164 RSC.
- Z. Yang, Q. X. Ma, L. Han and K. Tao, Design of Mo-doped cobalt sulfide hollow nanocages from zeolitic imidazolate frameworks as advanced electrodes for supercapacitors, Inorg. Chem. Front., 2019, 6, 2178–2184 RSC.
- X. H. Wang, G. H. Ren, B. N. Chen, J. Liu, X. X. Lv, A. Li, Y. J. Han, J. G. Tao, J. H. Dong and D. Nan, Built-in electric field enhanced electrochemical performance of metal-organic frameworks (MOFs)-derived hollow dodecahedral Co4S3/MoS2/MnS heterostructures, J. Phys. Chem. C, 2024, 128, 15829–15842 CrossRef CAS.
- Q. N. Zhou, W. Li, H. Z. Xu, M. Y. Gao, X. C. Dong and J. J. Lin, Fabrication of hierarchical integrated 3D hollow MnS@MoS2 microcubes via a template-controlled synthesis for asymmetric supercapacitors, J. Mater. Chem. A, 2022, 10, 9370–9379 RSC.
- H. N. Jia, Y. F. Cai, Z. Y. Wang, X. H. Zheng, C. Li, H. Y. Liang, J. L. Qi, J. Cao, J. C. Feng and W. D. Fei, Sea urchin-like CuCo2S4 microspheres with a controllable interior structure as advanced electrode materials for high-performance supercapacitors, Inorg. Chem. Front., 2020, 7, 603–609 RSC.
- L. R. Hou, R. Q. Bao, Z. Y. Chen, M. Rehan, L. N. Tong, G. Pang and C. Z. Yuan, Comparative investigation of hollow mesoporous NiCo2S4 ellipsoids with enhanced pseudo-capacitances towards high-performance asymmetric supercapacitors, Electrochim. Acta, 2016, 214, 76–84 CrossRef CAS.
- Z. B. Wang, Y. H. Hao, W. J. Zhang, J. Y. Fang and C. N. Chen, Facile fabrication of carbon-coated spinel nickel-cobalt-sulfide hollow spheres to achieve high-performance supercapacitors, New J. Chem., 2021, 45, 12150–12158 RSC.
- X. Z. Song, F. F. Sun, Y. L. Meng, Z. W. Wang, Q. F. Su and Z. Q. Tan, Hollow core-shell NiCo2S4@MoS2 dodecahedrons with enhanced performance for supercapacitors and hydrogen evolution reaction, New J. Chem., 2019, 43, 3601–3608 RSC.
- Q. H. Chen, W. N. Zhao, Z. H. Huang, G. C. Li, K. Tao and L. Han, Controlled preparation of CoNi2S4 nanorods derived from MOF-74 nanoarrays involving an exchange reaction for high energy density supercapacitors, Dalton Trans., 2023, 52, 9346–9355 RSC.
- Y. L. Liu, C. Yan, G. G. Wang, F. Li, Y. Shang, H. Y. Zhang, J. C. Han and H. Y. Yang, Self-templated formation of (NiCo)9S8 yolk-shelled spheres for high-performance hybrid supercapacitors, Nanoscale, 2020, 12, 23497–23505 RSC.
- X. Yu, J. L. Yu, L. Hou, A. Gagnoud, Y. Fautrelle, Z. M. Ren and X. Li, Double-shelled hollow hetero-MnCo2S4/CoS1.097 spheres with carbon coating for advanced supercapacitors, J. Power Sources, 2018, 408, 65–73 CrossRef CAS.
- G. R. Reddy, B. Sravani, N. Jung, G. R. Dillip and S. W. Joo, Engineering rich-cation vacancies in CuCo2O4 hollow spheres with a large surface area derived from a template-free approach for ultrahigh capacity and high-energy density supercapacitors, ACS Appl. Mater. Interfaces, 2023, 15, 36500–36511 CrossRef CAS PubMed.
- D. Yao, F. L. Wang, W. Lei, Y. Hua, X. F. Xia, J. P. Liu and Q. L. Hao, Oxygen vacancies boosting ultra-stability of mesoporous ZnO-CoO@N-doped carbon microspheres for asymmetric supercapacitors, Sci. China Mater., 2020, 63, 2013–2027 CrossRef CAS.
- K. Le, M. J. Gao, W. Liu, J. R. Liu, Z. Wang, F. L. Wang, V. Murugadoss, S. D. Wu, T. Ding and Z. H. Guo, MOF-derived hierarchical core-shell hollow iron-cobalt sulfides nanoarrays on Ni foam with enhanced electrochemical properties for high energy density asymmetric supercapacitors, Electrochim. Acta, 2019, 323, 134826 CrossRef CAS.
- G. Yilmaz, K. M. Yam, C. Zhang, H. J. Fan and G. W. Ho, In situ transformation of MOFs into layered double hydroxide embedded metal sulfides for improved electrocatalytic and supercapacitive performance, Adv. Mater., 2017, 29, 1606814 CrossRef PubMed.
- X. Q. Zeng, M. Y. Yang, J. Zhao, J. J. Shao and Z. Ding, Iron-doped nickel sulfide nanospheres anchored on reduced graphene oxide for high performance supercapacitors, Mater. Chem. Front., 2024, 8, 1816–1826 RSC.
- M. J. Yi, C. Q. Zhang, C. Cao, C. Xu, B. S. Sa, D. P. Cai and H. B. Zhan, MOF-derived hybrid hollow submicrospheres of nitrogen-doped carbon-encapsulated bimetallic Ni–Co-S nanoparticles for supercapacitors and lithium ion batteries, Inorg. Chem., 2019, 58, 3916–3924 CrossRef CAS PubMed.
- S. N. Fu, L. Ma, M. Y. Gan, J. Shen, T. T. Li, X. L. Zhang, W. Zhan, F. Xie and J. Yang, Sn-doped nickel sulfide (Ni3S2) derived from bimetallic MOF with ultra high capacitance, J. Alloys Compd., 2021, 859, 157798 CrossRef CAS.
- W. Zhao, Y. W. Zheng, L. Cui, D. D. Jia, D. Wei, R. K. Zheng, C. Barrow, W. R. Yang and J. Q. Liu, MOF derived Ni–Co-S nanosheets on electrochemically activated carbon cloth via an etching/ion exchange method for wearable hybrid supercapacitors, Chem. Eng. J., 2019, 371, 461–469 CrossRef CAS.
- P. F. Cai, T. Liu, L. Y. Zhang, B. Cheng and J. G. Yu, ZIF-67 derived nickel cobalt sulfide hollow cages for high-performance supercapacitors, Appl. Surf. Sci., 2020, 504, 144501 CrossRef CAS.
- D. Wang, L. Y. Tian, J. Y. Huang, D. W. Li, J. Y. Liu, Y. Xu, H. Z. Ke and Q. F. Wei, “One for two” strategy to prepare MOF-derived NiCo2S4 nanorods grown on carbon cloth for high-performance asymmetric supercapacitors and efficient oxygen evolution reaction, Electrochim. Acta, 2020, 334, 135636 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  *abc,
Cheng
Cheng
abc,
Weichen
Lin
abc,
Chenxi
Li
abc,
Weimin
Du
*abc,
Cheng
Cheng
abc,
Weichen
Lin
abc,
Chenxi
Li
abc,
Weimin
Du
 abc,
Kaige
Du
abc and
Shuo
Shan
bc
abc,
Kaige
Du
abc and
Shuo
Shan
bc
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) were polished in isopropanol for about 30 min and further dropped on a nickel foam as the working electrode. The mass loading of the CoNi2S4/NiS2 hollow architectures electrode material for the three electrode system is about 5 mg.
5) were polished in isopropanol for about 30 min and further dropped on a nickel foam as the working electrode. The mass loading of the CoNi2S4/NiS2 hollow architectures electrode material for the three electrode system is about 5 mg.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) were added in isopropanol. After grinding the mixture for 30 min, the resultant slurry was coated on a nickel foam to provide the negative electrode. Similarly, the fabrication of the positive electrode resembles the process for the negative electrode except the AC was replaced by the Ni–Co sulfide. The mass ratio between CoNi2S4/NiS2 and AC was around 1
5) were added in isopropanol. After grinding the mixture for 30 min, the resultant slurry was coated on a nickel foam to provide the negative electrode. Similarly, the fabrication of the positive electrode resembles the process for the negative electrode except the AC was replaced by the Ni–Co sulfide. The mass ratio between CoNi2S4/NiS2 and AC was around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The energy density (E) and power density (P) of the asymmetric supercapacitor can be calculated, as shown in eqn (1) and (2),
2. The energy density (E) and power density (P) of the asymmetric supercapacitor can be calculated, as shown in eqn (1) and (2),
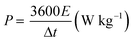


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles
000 cycles


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles
000 cycles

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles
000 cycles









