Oxidative stress modulating nanomaterials and their biochemical roles in nanomedicine
Kapil D.
Patel
 *abcdef,
Zalike
Keskin-Erdogan
*abcdef,
Zalike
Keskin-Erdogan
 fgh,
Prasad
Sawadkar
fgh,
Prasad
Sawadkar
 in,
Nik Syahirah Aliaa
Nik Sharifulden
in,
Nik Syahirah Aliaa
Nik Sharifulden
 g,
Mark Robert
Shannon
g,
Mark Robert
Shannon
 abc,
Madhumita
Patel
abc,
Madhumita
Patel
 j,
Lady Barrios
Silva
g,
Rajkumar
Patel
j,
Lady Barrios
Silva
g,
Rajkumar
Patel
 k,
David Y. S.
Chau
k,
David Y. S.
Chau
 g,
Jonathan C.
Knowles
g,
Jonathan C.
Knowles
 efg,
Adam W.
Perriman
*abc and
Hae-Won
Kim
*deflm
efg,
Adam W.
Perriman
*abc and
Hae-Won
Kim
*deflm
aJohn Curtin School of Medical Research, Australian National University, Canberra, ACT 2601, Australia. E-mail: kapildpatel20@gmail.com; chawp@bristol.ac.uk
bResearch School of Chemistry, Australian National University, Canberra, ACT 2601, Australia
cSchool of Cellular and Molecular Medicine, University of Bristol, BS8 1TD, UK
dInstitute of Tissue Regeneration Engineering (ITREN), Dankook University, Cheonan, 31116, Republic of Korea. E-mail: kimhw@dku.edu
eDepartment of Nanobiomedical Science & BK21 PLUS NBM Global Research Center for Regenerative Medicine Research Center, Dankook University, Cheonan, 31116, Republic of Korea
fUCL Eastman-Korea Dental Medicine Innovation Centre, Dankook University, Cheonan, 31116, Republic of Korea
gDivision of Biomaterials and Tissue Engineering, UCL Eastman Dental Institute, University College London, Royal Free Hospital, Rowland Hill Street, NW3 2PF, London, UK
hDepartment of Chemical Engineering, Imperial College London, Exhibition Rd, South Kensington, SW7 2BX, London, UK
iDivision of Surgery and Interventional Science, UCL, London, UK
jDepartment of Chemistry and Nanoscience, Ewha Women University, 52 Ewhayeodae-gil, Seodaemun-gu, Seoul 03760, Republic of Korea
kEnergy & Environment Sciences and Engineering (EESE), Integrated Sciences and Engineering Division (ISED), Underwood International College, Yonsei University, 85 Songdongwahak-ro, Yeonsungu, Incheon 21938, Republic of Korea
lDepartment of Biomaterials Science, School of Dentistry, Dankook University, Cheonan 31116, Republic of Korea
mCell & Matter Institute, Dankook University, Cheonan 31116, Republic of Korea
nThe Griffin Institute, Northwick Park Institute for Medical Research, Northwick Park and St Mark's Hospitals, London HA1 3UJ, UK
First published on 10th July 2024
Abstract
Many pathological conditions are predominantly associated with oxidative stress, arising from reactive oxygen species (ROS); therefore, the modulation of redox activities has been a key strategy to restore normal tissue functions. Current approaches involve establishing a favorable cellular redox environment through the administration of therapeutic drugs and redox-active nanomaterials (RANs). In particular, RANs not only provide a stable and reliable means of therapeutic delivery but also possess the capacity to finely tune various interconnected components, including radicals, enzymes, proteins, transcription factors, and metabolites. Here, we discuss the roles that engineered RANs play in a spectrum of pathological conditions, such as cancer, neurodegenerative diseases, infections, and inflammation. We visualize the dual functions of RANs as both generator and scavenger of ROS, emphasizing their profound impact on diverse cellular functions. The focus of this review is solely on inorganic redox-active nanomaterials (inorganic RANs). Additionally, we deliberate on the challenges associated with current RANs-based approaches and propose potential research directions for their future clinical translation.
1. Introduction
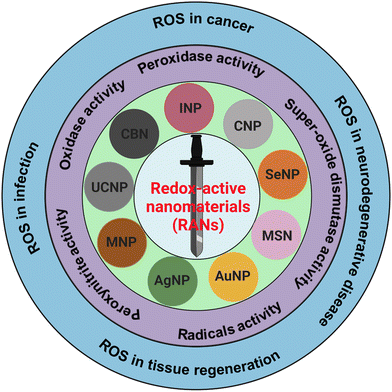
In the past few decades, nanomaterials with redox-activities (oxidant and antioxidant) have gained massive attention for their therapeutic purposes in deadly diseases such as cancer, strokes, osteoarthritis, and other neurodegenerative disorders such as Alzheimer's, Huntington's, and Parkinson's disease. The ability of nanomaterials to generate reactive oxygen species (ROS) is oxidant-activity, and scavenging of ROS is antioxidant-activity. Moreover, ROS are generated in normal biological processes and can decrease or increase pathological conditions.1–3 Therefore, the dual actions of redox-active foreign particles/nanoparticles as ROS generators and scavengers, can act like a double-edged sworsd to control the ROS in various pathological conditions as presented in schematic Fig. 1. The generation and scavenging of ROS are two important properties of redox-active nanoparticles which have tremendous potential for application in biomedicine as ROS scavengers to aid ischemia-reperfusion injury, stroke, skeletal conditions, myocardial infarction, neurodegeneration, and diabetes.4–7 However, controlling the properties of redox-active nanomaterials (RANs) in vivo and under pathological conditions is challenging.Reactive oxygen species (ROS) include free oxygen radicals and other molecules with at least one oxygen atom and one or more unpaired electrons that can exist independently.8 The presence of an unpaired electron in these species makes them extremely unstable and reactive. Radicals can act as oxidants or reductants, depending on whether they give or take an electron. Several common biological functions, such as aerobic metabolism and pathogenic defense mechanism, produce radicals. Furthermore, external exposures including radiation, pollution, dust particles, and smoke from cigarettes can also cause free radical production. Antioxidants are compounds that inhibit oxidation and work in two different ways to combat radicals.9 Enzymatic antioxidants function by oxidizing damaging ROS to produce H2O2, which is then converted to water. Superoxide dismutase (SOD) catalyzes the formation of oxygen and H2O2 from two superoxide anions. Furthermore, vitamin E, vitamin C, and glutathione are examples of non-enzymatic antioxidants that function by directly interacting with radical. For instance, glutathione has a free sulfhydryl group, which makes it a desirable target for radical attacks. The enzyme glutathione reductase then squelches the free radical and recycles the oxidized glutathione.
Redox-active nanomaterials are mainly classified as inorganic, organic, or composite based on their chemical composition. Inorganic redox-responsive nanosystems offer unique physicochemical properties such as robustness, cost-effectiveness, stability, and ease of synthesis and modification.10 These systems can achieve controlled drug release by incorporating reduction- or oxidation-responsive bonds and are generally easier to prepare and modify compared to organic ones.11 Organic redox nanomaterials, on the other hand, are typically more biodegradable and biocompatible.12 Composite redox-responsive nanomaterials, which integrate both inorganic and organic components, combine the strengths of each type, enhancing their physicochemical properties and often exhibiting synergistic effects.13 In this study, our focus will mainly be on inorganic redox-active nanomaterials.
Various types of nanoparticles with a ROS generating or scavenging propensity have recently been synthesized and are currently at different stages of manufacturing. These are also being scrutinized for potential clinical translational application in nanomedicine. These nanomaterials are defined as RANs that include metallic/metallic oxide nanoparticles, carbon-based nanomaterials (CBNs) and some other sources of nanomaterials. Metallic nanoparticles of silver, gold, iron, zinc, palladium, platinum, and ruthenium, as well as nonmetal selenium nanoparticles are known to have intrinsic antioxidant properties, thus, these nanoparticles do not require any functional modification for additional antioxidant function. To enhance the antioxidant activities of these metallic nanoparticles, strategies such as oxygen moiety grafting,14–16 peptide coating,17,18 ligand-exchange,19–21 and chemical conjugation of functional groups have been applied.14,22 Metallic nanoparticles such as magnesium oxide (MgO), titanium dioxide (TiO2), vanadium oxide (V2O5), manganese oxide (MnO2), iron oxide (Fe3O4), copper oxide (CuO), zinc oxide (ZnO), gadolinium oxide (Gd2O3), and cerium oxide (CeO2) have been tested for better antioxidant and catalytic activities. CBNs including fullerenes, carbon nanotubes (CNTs), carbon nanodots and metal-doped carbon nanodots, graphene oxide (GO), reduced graphene oxide (rGO), graphene quantum dots (GQDs) and few layer graphene (FLG), and metal nanoparticles conjugated GO have also been investigated for their antioxidant and redox-activities.23–26 Lately, up-conversion nanoparticles (UCNPs) have also gained significant attention for antioxidant and redox-active applications in nanomedicine.
This review paper highlights oxidative stress in physiological and pathological conditions, and the roles of RANs-associated ROS linked to cellular and molecular mechanisms. The redox-active mechanism of RANs and their roles in oxidase, peroxidase, SOD, radical, and peroxynitrile activities have thoroughly been explored and summarized. Finally, the roles of ROS generated by RANs in various diseases such as cancer, neurodegeneration, infection, and other conditions, along with their role in tissue engineering, have also been discussed in detail.
In recent years, the therapeutic applications of RANs to target ROS have been intensively studied. Current research has emphasized the roles of ROS in some pathological conditions and suggested ROS-based nanomedicine.27–30 However, the review papers mainly focused on ROS's roles in certain diseases’ pathological condition. In this review, we discuss the broad range of nanomaterials and their ROS generating or scavenging properties and underlying mechanisms of ROS in vitro and in vivo disease models. We have also listed nanomaterials with their application in various diseases and regenerative medicine. The family of RANs, various redox-activities and their roles in diseases and regenerative medicine are depicted in Fig. 1.
2. Oxidative stress in biology and medicine
The impact of oxidative stress has been intensively studied in the field of biomedicine over the last few decades.27,31,32 An imbalance between ROS and antioxidants is known as oxidative stress, this can be caused by any form of free radical, oxygen-containing molecules such as superoxide ion/radical, hydrogen peroxide and peroxynitrate. These radicals contain an uneven number of electrons that easily react with proteins/lipids disrupting redox reaction/signalling and eventually causing molecular damage in the body.33 Oxidative stress plays a vital role in normal physiology and the pathophysiology of many life-threatening diseases. Essentially all complex organisms are affected by oxidative stress and free radicals. Although oxidative stress in biology and medicine has been studied for decades, its functional and mechanistic diversity in varying microenvironments has attracted huge attention in the last few years.34–37 Oxidative stress in biology and medicine is part of research of human and animal biology at both the cellular and molecular level.36 Redox homeostasis-based strategy to control the ROS in production has gained great attention due to controllable scale. Lin et al. have developed a radiotherapy-mediated redox homeostasis-controllable nanomedicine for amplifying ferroptosis sensitivity in tumor therapy.38 This strategy can achieve high efficacy of ROS production and modulate the tumor cell microenvironment antioxidant to amplify ferroptosis. Moreover, many of the biological consequences of vitamin and selenium deficiency or excess radiation exposure are thought to be the result of oxidative damages.39 Several reports have also highlighted the role of oxidative damage in human diseases such as cancer, osteoarthritis, chronic inflammatory diseases, neurodegenerative diseases, and retinopathy of prematurity.2.1. The oxygen paradox
A variety of biological processes, including cell viability/death, cell signalling, differentiation, and the creation of inflammation-related factors, are naturally triggered by ROS during aerobic respiration in the cell. Radicals and non-radicals are two subcategories of biologically significant ROS. A list of free radicals in human biology, its origin, roles, and applications are summarized in Table 1. In a living organism, ROS are created as a result of regular cellular metabolism and external influences including metal toxicity, cigarette smoke, air pollutant, salinity and drought.40| Name of free radicals | Chemical formula | Origin and role | ROS concentration level and disease | Ref. |
|---|---|---|---|---|
| Superoxide radical | O2˙− | • Superoxide radical generated as by product of cellular respiration (mitochondrial respiratory chain) and can lead to the formation of other types of ROS. | • Diffusion-limited rate 2 × 109 M−1 s−1. | 41 and 42 |
| • It plays a dual role, at physiological balance level, by product of O2 reduction for the cellular signalling. | • Superoxide radical-based ROS level in normal cell is in nanomolar (nM) range, while in cancer cell range is in micromolar (μM) | |||
| • At pathological level, induces cellular apoptosis, necrosis, ferroptosis, pyroptosis, and cell death. | ||||
| Hydroxyl radical | ˙OH | • Hydroxyl radical is formed from water during various biochemical reactions. | • Diffusion limit rate for hydroxyl radical is 1.9 × 1010 M−1 s−1. | 43–45 |
| • In presence of hydrogen peroxide and iron ions produced hydroxyl radical via Haber–Weiss reaction. | • Hydroxyl radical concentration range in normal cell is very low nanomolar. | |||
| • It is highly reactive, and can damage the DNA, proteins, and lipids. | • However, in cancer, inflammation, and neurodegenerative diseases it is in mid high nanomolar to micromolar range. | |||
| • Hydroxyl radical induces polymerization of human fibrinogen. | • In infection, high nanomolar to low micromolar range. | |||
| Peroxyl radical | ROO˙ | • Peroxyl radical commonly formed during oxidation of lipids. | • Diffusion limit rate for hydroxyl radical is 1.9 × 1010 M−1 s−1. | 46–49 |
| • They form as a natural byproduct during the various cellular events including metabolism of lipids, and chain reaction of lipid peroxidation. | • Peroxyl radical concentration range in normal cell is very low nanomolar. | |||
| • It plays main role in lipid peroxidation, cellular damage, oxidative stress, antioxidant defense, and cell signalling. | • However, in cancer, inflammation, and neurodegenerative diseases it is in mid high nanomolar to micromolar range. | |||
| • In infection, high nanomolar to low micromolar range. | ||||
| Hydroperoxyl radical | HO2˙ | • Hydroperoxyl radical generate in biological systems during the dismutation of superoxide. | • Diffusion limit rate for hydroperoxyl radical is 2.3 × 108 M−1 s−1. | 45 and 50–52 |
| • Excessive level of hydroperoxyl radicals can leads to oxidative damage to biomolecules including lipids in cell membranes. | • Hydroperoxyl radical concentration for normal cell is low nanomolar range. | |||
| • Hydroperoxyl radical is associated with various pathological conditions and contribute to the aging process. | • However for cancer cell, inflammation, are mid to high nanomolar to low micromolar. | |||
| • Hydroperoxyl radicals are also associated with the immune's system defense against pathogens. | • Neurodegenerative diseases mid to high nanomolar to micromolar range. | |||
| Alkoxyl radical | RO˙ | • Alkoxyl radicals are generated during the breakdown of peroxides. | • Diffusion limit rate for hydroperoxyl radical is 1 × 109 M−1 s−1. | 53–55 |
| • Alkoxyl radicals are reactive and participate in redox reactions. | • Alkoxyl radical concentration range for normal cell is low nanomolar. | |||
| • They involved in the lipid peroxidation process, free radicals damage lipids in cell membranes. | • However, for cancer and inflammation range is mid to high nanomolar to low micromolar. | |||
| • For infection, high nanomolar to low micromolar. | ||||
| • Neurodegenerative diseases, mid to high nanomolar to micromolar range. | ||||
| Carbonate radical | CO3˙− | • The carbonate radicals can be formed through different pathways including reaction involving peroxides and other ROS. | • Diffusion limit rate for carbonate radical is 5.27–7.89 × 105 M−1 s−1. | 56–60 |
| • It can generate in presence of hydrogen peroxide and bicarbonate ions. | • Carbonate radical concentration for normal cell is low picomolar range. | |||
| • Carbonate radicals are highly reactive and can oxidized organic and inorganic molecules and leads to the modification of biomolecular and cellular structure. | • For cancer, it is low to mid nanomolar. | |||
| • Potentially contribute the oxidative stress in the biological system, | • For inflammation, it is mid to high nanomolar range. | |||
| • Infection, it is high nanomolar range. | ||||
| • Neurodegenerative diseases, it is mid to high nanomolar to low micromolar range. | ||||
| Nitric oxide radical | NO˙ | • Nitric oxide radicals are synthesized endogenously by various cell types, primarily through the action of enzyme called nitric oxide synthases (NOS). | • Diffusion limit rate for nitric oxide radical is 6.7 × 109 M−1 s−1. | 61–64 |
| • Nitric oxide radicals act as a cell signalling molecules in various physiological process. | • Nitric oxide radical for normal cells is 1 to 100 nanomolar range. | |||
| • It is involved in blood vessel dilation in cardiovascular system, and relaxation in smooth muscle cells. | • For cancer, it is from 20 to 500 nanomolar range. | |||
| • Act as neurotransmission in nervous system, body defense system against pathogens, anti-inflammatory. | • For inflammation, it is 100 nanomolar to 1 micromolar range. | |||
| • Infection, it is 100 nanomolar to 1 micromolar range. | ||||
| • Neurodegenerative diseases, it is 50 nanomolar to 1 micromolar range. | ||||
| Thiyl radical | RS˙− | • Thiyl radicals are formed through the homolytic cleavage of sulphur–hydrogen bond. | • Diffusion limit rate for thiyl radical is 1 × 108 M−1 s−1. | 65–68 |
| • In biological system, thiyl radicals are generated during the oxidative stress, and redox reactions involving thiol-containing biomolecules. | • Thiyl radical concentration range for normal cells is picomolar to low nanomolar range. | |||
| • Thiyl radicals serve as intermediates in the transfer of electrons during various cellular processes including antioxidant defense, cellular signalling, and redox balance in cellular process. | • Cancer cell, low to mid nanomolar range. | |||
| • Dysregulation of thiyl radicals and thiol redox balance are associated with various diseases including neurodegenerative disorder, and cardiovascular diseases. | • Inflammation condition, mid to high nanomolar. | |||
| • Infection, it is high nanomolar to low micromolar range. | ||||
| • Neurodegenerative diseases, it is mid to high nanomolar to micromolar range. |
ROS are highly reactive, thus excessively produced ROS can rapidly bind to cell membrane proteins or lipids, nucleic acids, and carbohydrates leading to irreversible structural alteration. Thus, controlling the reducing and oxidizing (redox) states of ROS is critical for cellular activation, viability, proliferation and, ultimately, organ function. Endogenous antioxidant systems exist, in that, enzymatic and non-enzymatic mechanisms normally operate to chelate ROS in healthy normal organisms. Nevertheless, overproduction of ROS in the pathological conditions mentioned above often leads to an imbalance of oxidant and antioxidant levels, resulting in an oxidative stress condition. Highly reactive oxygen radicals can also affect gene expression by up-regulation of redox-sensitive transcription factors and chromatic remodeling through modulation of histone acetylation and deacetylation.69,70
In healthy physiology, the simultaneous oxidation and reduction of O2−˙, and ˙OH form H2O2 which is then broken down by the enzyme glutathione peroxidase in the presence of metals in the reduced state. For instance, mitochondria produce about one-third of the liver's total glutathione peroxidase activity.71 O2−˙, the mediator in an oxidative chain reaction and the product of the one electron reduction of oxygen (from 1O2) molecules, is the precursor to the majority of ROS. Additionally, superoxide, which can be reduced to H2O or ˙OH, catalyzes the dismutation of O2 to produce H2O2.72 Among normal biological processes, most ROS are produced as by-products of the interaction of oxygen with the leaking electrons from the electron transport chain (ETC), in particular protein complexes CI and CIII of the mitochondrial respiratory chain.73 Additionally, metal-catalyzed oxidation reactions generate ROS as intermediates. In the outer shell of the oxygen atom, there are two unpaired electrons. The sequential reduction of oxygen by adding electrons results in the formation of excessive ROS summarized in Fig. 2(a). The breadth of ROS generated by oxygen reduction is demonstrated by the application of photosensitive gold nanoparticles in photodynamic cancer therapy. Fig. 2(b) highlights that the stepwise oxidation and reduction processes triggered by photocatalyst absorption can produce ROS from H2O2 or O2, respectively.
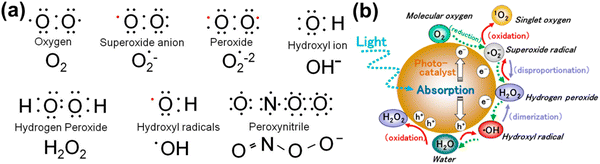 | ||
Fig. 2 (a) Electronic structure of some common ROS. The structure of chemical formula and corresponding name are provided, and unpaired electron are designated as a red dot ( ). (b) Reactive oxygen species generated in the photocatalytic redox-reactions, and steps into O2 and H2O2. ROS in free-radical form such as superoxide radicals, hydrogen peroxide, and hydroxyl radicals may be produced sequentially from molecular oxygen (O2) via a stepwise reduction mechanism, and reversibly from hydroxyl radicals, hydrogen peroxide, superoxide radicals, and singlet oxygen as well from water (H2O) via a stepwise oxidation mechanism. Reproduced with permission from Nosaka et al.29 [Copyright 2019, American Chemical Society]. ). (b) Reactive oxygen species generated in the photocatalytic redox-reactions, and steps into O2 and H2O2. ROS in free-radical form such as superoxide radicals, hydrogen peroxide, and hydroxyl radicals may be produced sequentially from molecular oxygen (O2) via a stepwise reduction mechanism, and reversibly from hydroxyl radicals, hydrogen peroxide, superoxide radicals, and singlet oxygen as well from water (H2O) via a stepwise oxidation mechanism. Reproduced with permission from Nosaka et al.29 [Copyright 2019, American Chemical Society]. | ||
Ranking ROS in terms of their toxicity in mammalian systems involves considering their reactivity, stability, and potential to cause cellular damage. Table 2 is the general ranking of ROS based on their toxicity from most to least harmful:
| ROS species | Toxicity level | Description | Ref. |
|---|---|---|---|
| Hydroxyl radical (˙OH) | Extremely high | • It is one of most reactive ROS, can cause significant damage to DNA, proteins, and lipid due to high reactivity and lac of selectivity. | 74 and 75 |
| • Even at very low concentration, can induce severe oxidative damage. | |||
| Peroxynitrite (ONOO−) | Very high | • Peroxynitrite is a potent oxidant formed from the reaction of nitric oxide (NO˙) with superoxide (O2˙−). | 76 and 77 |
| • It can nitrate tyrosine residues in proteins, leading to functional alteration and damage. | |||
| • Highly damaging to the cells and tissue. | |||
| Superoxide anion (O2˙−). | High | • Superoxide is a primarily ROS that can lead to the formation of other, or more reactive species like hydrogen peroxide, hydroxyl radicals. | 78 and 79 |
| • It is less reactive than hydroxyl radicals with significant toxicity. | |||
| • It can disrupt the cellular functions and contribute to the formation of other toxic ROS. | |||
| Alkoxyl radical (RO˙) | Moderate | • Alkoxy radicals are intermediate in reactivity. | 80 and 81 |
| • It is generated from decomposition of organic peroxides and can propagate lipid peroxidation. | |||
| Hydroperoxyl radical (HO2˙) | Moderate | • Hydroperoxyl radicals are in equilibrium with superoxide and involved in lipid peroxidation. | 50 and 82 |
| • It is less reactive than hydroxyl radicals but still contribute to oxidative stress. | |||
| Carbonate radical (CO3˙−) | Moderate | • Carbonate radicals can oxidize biomolecules but are generally less reactive than hydroxyl and peroxynitrite radicals. | 83 and 84 |
| • It is formed during the reactions involved peroxynitrite and bicarbonate. | |||
| Nitric oxide radical (NO˙−) | Low to moderate | • Nitric oxide radical is less reactive than many other ROS. | 61 and 85 |
| • It has physiological importance such as vasodilation, and cell signalling. | |||
| • However, in high concentration or in combination with superoxide, it forms peroxynitrite, which is highly toxic. |
In summary, superoxide and peroxynitrite both are harmful to mammalian cells. Peroxynitrite is generally considered as more toxic due to its reactivity and potential to cause significant damage to the cell membrane. The impact of upregulated ROS levels should be evaluated in the context of the overall redox balance and the cell's ability to neutralize the ROS with antioxidants.
2.2. Molecular switches in oxidative stress
Eukaryotic cells have evolved to harness energy in the form of adenosine triphosphate (ATP). Enzymatic reduction of ATP (catabolic) enables the generation of macromolecular precursor nucleotides, and amino acids (anabolic). ROS are generated within the electron transport chain (ETC) in mitochondria which facilitates this energy utilization, with about 0.1–0.2% of the total O2 consumed through ETC type I and III complexes generating ROS.86,87 Additionally, in the process of energy metabolism, the signalling molecule known as the mammalian target of rapamycin complex 1 (mTORC1) receives signals from both amino acids and glucose.88 Eukaryotic cells frequently include mTORC1 downstream signalling, serving as an important signalling node that connects nutrition sensing and metabolic control. Since cellular metabolism and cell survival are tightly related, signalling pathways for metabolic activity and autophagy may interact despite being functionally independent.89 According to a study by Dibble et al., mTORC1 may serve as a link between the anabolic process and the circumstances that promote cellular development.90 Moreover, mTORC1 signals are integrated with systemic signals, including secreted growth factors, as well as intracellular signals, such as amino acids, glucose, oxygen, and ATP.Autophagy, a catabolic process responsible for clearing out damaged organelles and unnecessary dysfunctional components in cells, occurs in normal and stressed conditions including viral infection, nutrient deprivation, and genotoxic effects. Recent studies have reported that the oxidative stress created by ROS and reactive nitrogen species (RNS) might converge to trigger sustained autophagy.91 Furthermore, autophagy is closely linked to redox homeostasis and metabolic networks, which involve both nitrosative and oxidative stress.
Thiol-containing proteins can undergo reversible post-translational changes, which are known to be damaging to both biological biomolecules and signal mediators.92 Protein activity is regulated by these thiol-based redox switches, which are also essential for cellular ROS response and adaptation to local and global changes. First responder proteins control redox levels via ROS-specific transcription factors, chaperones, or metabolic enzymes to protect cells from increasing amounts of oxidants, repair the damage, and restore redox homeostasis. In addition, phosphatases and kinases are regulated by redox-regulation, resulting in ROS generation that is considered to be a crucial second messenger in growth, development and differentiation.93 ROS are essential for cell growth and differentiation, and excessive ROS production in the cell causes apoptosis.94 Several studies have reported the roles of ROS during the differentiation of embryonic stem cells (ESC).95 For example, ROS are transiently elevated during the G2/M period of the cell cycle, differing from other differentiated mature cells. It is of interest to highlight that ESCs produce little ATP due to their immature mitochondria, leading ESCs to be presumably resistant to the oxidative stress condition.5 To meet energy demand, ESCs mainly utilize glycolysis instead of mitochondrial oxidative phosphorylation to avoid ROS production. Thus, the produced nicotinamide adenine dinucleotide phosphate (NADPH) from glycolysis can maintain thioredoxin and glutathione to support scavenging ROS.96,97 ROS also plays a crucial role in the differentiation of embryonic hematopoietic stem cells and cardiomyocytes.95,98 Adult stem cells (ASC) have the capability to regenerate in injured tissues throughout their whole life span, retaining a propensity to differentiate into specific lineages. ASC such as neural and mesenchymal stem cells also maintain low levels of ROS by utilizing glycolysis with suppression of mitochondrial oxidative phosphorylation.99–101 The mechanism of ROS scavenging changes under hypoxic conditions; hypoxia-inducible factor-1α (HIF-1α) is produced through oxidative phosphorylation in the presence of elevated ROS. In ASC, Meis Homeobox 1 (MEIS1) regulates HIF-1α; Kocabas et al. have demonstrated that MEIS1 knockout in mice is entirely mediated through ROS and treatment of MEIS1 with the scavenger N-acetylcysteine maintains ASC function.102 However, HIF-1α enhances glycolytic metabolism from oxidative phosphorylation to glycolysis by upregulating gene expression with pyruvate dehydrogenase kinase (PDK1), glucose transporter 1 (GLUT1) and acetate dehydrogenase A (LDHA).103,104 These all factors suggest that the glycolytic metabolism and hypoxia signalling are crucial for ROS regulation.
2.3. Cellular mechanisms of oxidative stress in human health
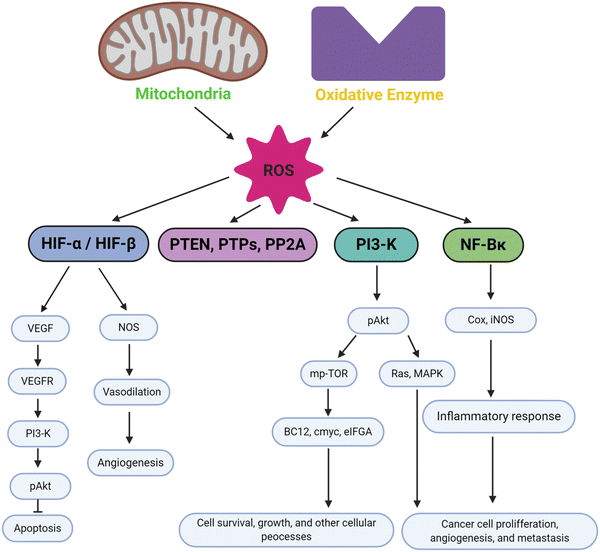
Oxidative stress plays a significant part in a wide range of diseases, including cancer, inflammation, wound healing, and neurological disorders. An imbalance in the generation and clearance of ROS can lead to oxidative stress, thus directly affecting cellular functions. If the imbalance is severe enough and not controlled by redox homeostasis, then it can lead to serious injuries in the cell and possibly cell death, either by apoptosis or necrosis.105,106 This condition may involve the initiation and progression of certain disease pathologies. In response to tissue injury, cells develop various responses induced by ROS and activate repair mechanisms via modulation of downstream signalling molecules. In this section, we will discuss the mechanisms of oxidative stress within cancer pathology, inflammation, wound healing, and neurodegenerative disease.Oxidative stress is more prevalent in cancer cells than normal cells.107,108 As mechanisms for normal cell repair and division fail during tumorigenic progression, the cycle of cancer cells speeds up resulting in higher energy demands for fast growth, uncontrolled cell division and cellular migration. The high cellular metabolism and oxygen consumption required to keep these energetic demands result in a higher accumulation of ROS leading to oxidative stress being more prevalent in cancer cells compared to normal cells.109 The radicals and ROS produced in cancer cells induce several effects in the body, such that low concentrations of ROS mediate cell proliferation and tumor progression to the metastatic stage resulting in aggregation of tumor cells. On the other hand, high level of ROS induce a more contradictory outcome, activating cell death pathways in cancers as well as mediating cancer recurrence.110 In the case of cell death initiation, the antioxidant system in cancer cells fail to control excessive ROS generation during oxidative stress resulting in cell death within the tumor. Thus, deciding how to implement ROS to affect tumors is challenging. Their use as part of combination therapies has been suggested, for example, using ROS modulating antioxidants together with chemotherapy could be more effective to deal with the different stages of tumor progression.111
In cancer cells, oxidative stress can be induced by oncogenic signalling, mitochondrial activation, metabolic activity, and increased enzyme activity.112,113 Additionally, cytokines and growth factors such as insulin, platelet-derived growth factor (PDGF), transforming growth factor (TGF), tumor necrosis factor (TNF), and epidermal growth factor (EGF) can drive cancer cells to create intracellular ROS.114–116 Under the hypoxic conditions observed during the intermediate stage of tumor development due to inadequate vascularization, ROS-induced transcription of HIF-1α, a fundamental member of hypoxia-inducing factors, stabilizes the encoded protein, which should be hydroxylated within five minutes by iron-dependent prolyl 4-hydroxylase (PDH), a HIF-1α degrading enzyme.117 As a consequence of HIF-1α activation, several essential genes in cancer progression, such as vascular endothelial growth factor (VEGF) and VEGF-receptors, are induced.118 It has been shown that epidermal growth factor receptors (EGF-receptors) and PDGF-receptors, along with activating mutations in K-ras, can initiate Akt signalling mediated by oxidative stress. In addition, hydrogen peroxide activates Akt either directly or via ROS-induced activation of phosphoinositide-dependent kinase 1 (PDK1), its upstream kinase.119 Additionally, mutations of downstream growth factor receptors, like Kars-RAS 77, 78, can also result in an increase in superoxide generation.120,121 Another downstream effector of numerous growth factor receptors, including EGF receptors and c-Mets, is the tiny Rho GTPase Rac-1.122 Chavda et al. have recently summarized the role of ROS and the molecular mechanism of oxidative stress in cancer and brain stroke.123 It has been well established that oxidative stress plays an important role in tumorigenesis via inflammation, immune evasion, autophagy and apoptosis control through signalling pathway regulation, angiogenesis, and drug resistance. The mitochondrial ROS cause of cell apoptosis and the oxidative-stress-mediated molecular mechanism of cancer progression are presented in Fig. 3.
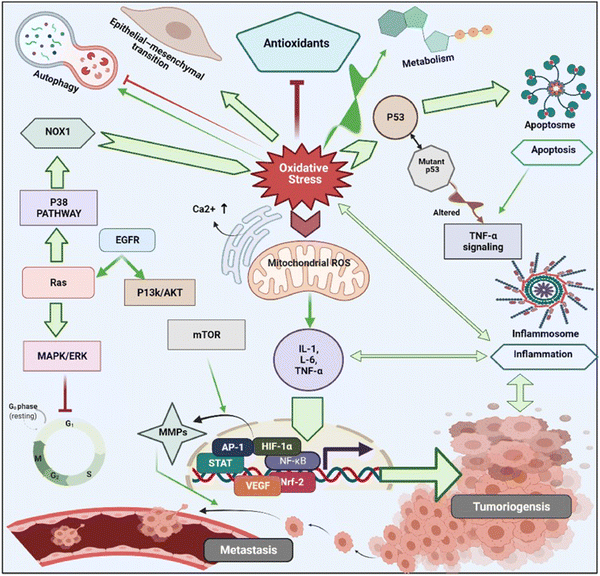 | ||
| Fig. 3 Schematic illustration of various cell signalling pathways associated to oxidative stress mediated progression of tumorigenesis and metastasis. Under oxidative stress, intracellular ROS activates cancer cell surviving signalling cascades such as MAPK/ERK1/2, p38, JNK, PI3K/Akt, which leads to activation of transcription factors such as NF-κB, MMPs, AP-1, HIF-1α, STAT, Nrf2, VEGF. These transcriptional factors cause imperative pathophysiologies aggravating carcinogenesis, and cancer metastasis. Reproduced with permission from Chavda et al.123 [Copyright 2022, Elsevier]. | ||
The primary function of inflammation is to protect the body from infectious pathogens. It is usually not a disease condition itself but is commonly observed in various pathological conditions such as hepatitis B & C, malaria, dengue, and tuberculosis (TB) infections, autoimmune diseases, radiation, or toxic chemical damage, and even in obesity. Inflammation also occurs in non-pathological processes, including tissue rearrangement, elimination of cellular waste, and tissue regeneration. Recent investigations have shown that the progression of many chronic diseases is closely related to the situation of oxidative stress, where the resulting protein oxidation accelerates inflammatory responses.124,125 Protein-oxidation induces the release of inflammatory signalling molecules including peroxiredoxins 2 (PRDX2).126 During this response, proinflammatory mediators like tumor necrosis factor-α (TNF-α) are released through activation of disintegrin, metalloproteinase-17 (ADAM-17), and PRDX2, a ubiquitous redox-active intracellular enzyme which also acts as a redox-dependent inflammatory mediator to activate macrophages after the release of TNF-α. It is of note that chronic inflammatory responses are commonly observed in insulin resistance, type 2 diabetes mellitus (T2DM), and cardiovascular diseases.
Wound healing is another redox-regulated biological process involving continuous and extending phases of homeostasis, inflammatory related events, cell proliferation, and new tissue formation.127 Immediately after blood vessel injury, platelet aggregation and activation are initiated, forming blood clots that temporarily seal the wound site. The subsequent inflammatory response involves different immune cells such as neutrophils and monocytes that are recruited into the wound. These immune cells secrete proteolytic enzymes and proinflammatory cytokines together with excessive ROS, which are essential to kill invading bacteria and other microorganisms. During normal aging or oxidative-stress-related pathological conditions such as diabetes, alcohol abuse, smoking, or infectious disease, the normal inflammatory response can be delayed or impaired.128 Some interesting studies have suggested that ROS might be crucial regulators involved in all stages of wound healing process. Although ROS function as important regulators during wound healing, over production of ROS could cause molecular damage, disrupting the wound healing process resulting in the formation of chronic wounds. In fact, many studies have suggested that antioxidant strategies are effective and beneficial during the wound healing inflammatory response. Antioxidant strategies such as mitochondrial-targeted peptides like elamipretide, can protect against mitochondrial dysfunction and inflammation by activating NOD-like family receptors, including the pyrin domain 3 (NLRP3) inflammasome and inhibiting the nuclear factor-kappa B (NF-κB) signalling pathway, and the nuclear factor (erythroid derived 2)-like 2 (Nrf2).129 Furthermore, sustained oxidative stress accelerates the inflammatory response in the chronic period of wound healing via ROS-stimulated chemotaxis and migration of neutrophil and macrophage cells to the wound area by expressing adhesion molecules in blood vessels. ROS can directly affect cell migration, proliferation, and extracellular matrix (ECM) production in fibroblast and keratinocytes.130
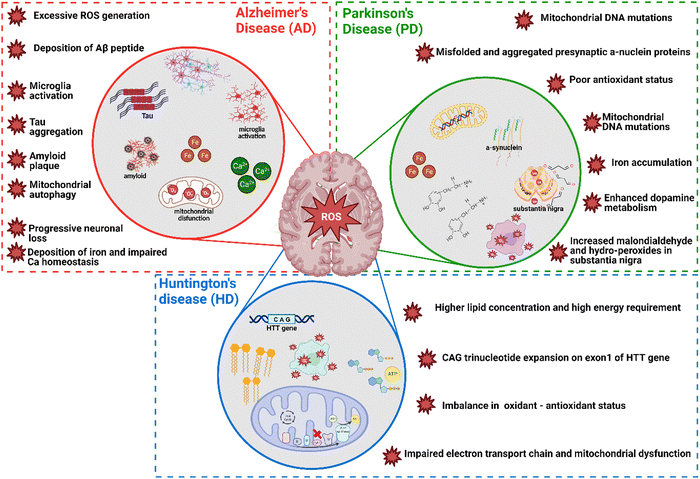
In neurodegenerative disorders including AD, PD, HD, and ALS, the ROS-induced oxidative stress is extremely high with comparatively low levels of antioxidants. Particularly in AD among other neurodegenerative disorders mentioned above, ROS-induced oxidative stress plays a critical role in the accumulation and deposition of β-amyloid peptide (Aβ peptide). The aggregation of Aβ peptides can lead to mitochondrial dysfunction and energy failure prior to plaque formation in the brain via impairing the electron transport system complex I and IV.131,132 Similarly, α-synuclein, a presynaptic protein in PD that forms Lewy bodies (LBs), is misfolded and aggregated causing a decrease of mitochondrial functions.133,134 Importantly, the PD-related genes such as PINK1, DJ-1, LRRK2, and PARK2 (Parkin), are all involved in homeostasis of mitochondrial ROS.135,136 Moreover, the induction of mitochondrial recruitment of Parkin by mitochondrial ROS plays an important role in the PINK1/Parkin-related mitophagy, as well as mutations or deficiency of PINK1.137 HD is caused by a mutation in the Huntingtin (HTT) gene located on chromosome 4 (4p63).138 This mutation leads to the expansion of CAG trinucleotide repeats, causing the aggregation of Huntingtin proteins and eventually neuronal death in the brain at the early stages of HD. Oxidative damage is suggested as one of the major pathological mechanisms due to the higher lipid concentrations and high energy requirement in the HD brain.139 Mutant HTT proteins serve as of the initiator of ROS, due to oxidized proteins in partially purified mHTT aggregates.140 Thus, oxidative damage has been suggested as one of the major pathological mechanisms in the early stages of HD progression.141 The roles of oxidative stress in neurodegenerative diseases such as AD, PD, and HD and corresponding cellular and molecular details are summarized in Fig. 4.
Reactive oxygen species play a crucial role in the ageing process as pathogenic components. These extremely reactive chemicals, such as superoxide (a free radical) and hydrogen peroxide (a non-radical molecule), are naturally produced during cellular metabolism, especially in the mitochondria. Under normal physiological conditions cells have a homeostatic equilibrium in between ROS and presence of antioxidant mechanisms. As we age this equilibrium shifts towards higher levels of ROS as a result of a decrease in mitochondrial activity and antioxidant capability.
Elevated levels of ROS are detrimental and can result in significant harm to cellular and organelle membranes, DNA, and proteins.142 Gradual oxidation over a period of time and decline in ATP production cause damage to cells and tissues which is one of the causes of aging. ROS are involved in the development of other age-related illnesses, including neurological conditions like Alzheimer's and Parkinson's diseases, cardiovascular diseases, and malignancies.143,144 These molecules have the ability to initiate and alter various cellular signalling pathways that result in apoptosis, inflammation and cellular senescence, hence affecting the process of ageing and the progression of diseases.145
ROS have a crucial impact on the ageing process and the emergence of age-related ailments through the initiation of oxidative harm and the disturbance of cellular equilibrium. Gaining a comprehensive understanding of the processes involved in the generation and reduction of ROS is essential for the development of effective treatment approaches to address the effects of ageing and its related diseases.
3. Nanomaterials possessing antioxidant and redox-activities
Antioxidants are molecules/compounds/materials that can react with radicals by donating an electron,8 radical addition,46 H-atom donation,146 as well as regeneration by other reducing agents,147 preventing unfavorable biochemical chain reactions by converting them to nontoxic metabolites. In other words, antioxidants can bind to oxidizable molecules, protecting cells by delaying or inhibiting their autoxidation.148 Antioxidants can therefore reduce oxidative stress, playing a key role in the mediation and control of ROS induced deadly diseases. They can be categorized as preventive antioxidants and chain breaking antioxidants. Preventative antioxidants are a heterogeneous class of molecules/compounds including metal chelators,149 hydroperoxide-decomposing agents,150 and glutathione peroxidase;151 their main role is to interrupt the initiation rate of ROS generation.152 On the other hand, the main role of chain-breaking antioxidants is to slow down (or inhibit) the autooxidation. The antioxidants that break chains are also known as radical-trapping antioxidants. For example, phenols are the best-known chain-breaking antioxidant as they can rapidly trap 2-preoxyl radicals per molecule. Tocopherols (vitamin E), ascorbate (Vitamin C) flavonoids, and stilbenes are some other examples of chain-breaking antioxidants.153Some best practices to measure oxidative-stress induced by nanoparticles, it is important to have strong and sensitive assays to detect reactive oxygen species and related oxidative damage.8,145 There are several potential assays that can be used, including the dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay, the thiobarbituric acid reactive substances (TBARS) assay, and the glutathione (GSH/GSSG) assay. The DCFH-DA assay is commonly used to measure oxidative stress and assess ROS generation.154–156 The TBARS assay measures malondialdehyde (MDA), a byproduct of lipid peroxidation caused by nanoparticles.157,158 Additionally, the GSH/GSSG assay measures reduced (GSH) and oxidized (GSSG) glutathione levels, which can give insight into the cellular redox state.159,160 According to the previous report, it is important to include both positive (e.g., H2O2 treatment) and negative controls (untreated cells) when conducting these assays.155
In the last several decades, numerous nanomaterials have been developed and evaluated for their antioxidant properties to see if they can provide defense against oxidative damage.153 Nanomaterials such as metals/nonmetals (Cu, Ag, Au, Pt, and Se), metal oxides (TiO2, ZnO, Fe3O4, CeO2), carbon-based nanomaterials (fullerenes, CNTs, GO, GQDs, nanodiamonds), nanomaterials developed as delivery tools (Ce@SiO2, Ce@GO), and UCNP have shown intrinsic redox-activities like superoxide dismutase (SOD) or catalase-like activities often associated in radicals trapping. Grafting or modifying nanomaterials with low molecular weight antioxidants can sometimes make them antioxidants. In this section, we have categorized the above nanomaterials and discussed their antioxidant/redox-activities and mechanism of action in different physiological conditions.
3.1. Metallic nanomaterials
Metallic nanoparticles (silver, gold, palladium, platinum, and ruthenium) and nonmetal (selenium) mostly possess intrinsic antioxidant properties and these nanoparticles do not require any functional modification for having antioxidant properties. These nanoparticles do not need to be functionally modified in order to exhibit antioxidant activities and they exhibit oxidase-like activity under acidic conditions and in the presence of oxidizing agents like 2,2-azinobis(3-ethylbenzothizoline-6-sulfonic acid) and 3,3′,5,5′-tetramethylbenzidine (TMB).161–165 Unlike antioxidant activity, which only occurs at neutral or basic pH levels, this redox-activity occurs at acidic pH levels, similar to that of peroxidase enzymes. A Fenton-like reaction on the surface releases OH radicals, resulting in the peroxidase activity.29Several studies have further reported AgNPs-induced ROS generation in mouse fibroblasts and human hepatocytes, showing reduced membrane potential of mitochondria with subsequent release of cytochrome C into the cytosol followed by JNK activation and Bax translocation.176,177 Contrary to this result antiapoptotic protein Bcl2 is highly expressed in HCT116 cells (human colon cancer cells) that are resistant to AgNPs.176 Ag+ from AgNPs directly mediates the synthesis of ROS, such as superoxide, hydroxyl radicals and hydrogen peroxide, in free-cell environments.178,179 This report has been further supported by Mendis et al. showing ROS generated from AgNPs can lead to cell membrane disruption, mitochondrial dysfunction, and DNA damage.180 Another study by Chang et al. has suggested the antibacterial properties of AgNPs result from the formation of multiple forms of ROS, observing a reduction in antibacterial activity on addition of the ROS scavenging enzymes super oxide dismutase and catalase.181 Inoue et al. have found that the bacterial activity induced by the introduction of Ag+ into zeolite is mediated by four forms of ROS under aerated conditions, the activity of which can again specifically be decreased by scavenger addition.182
Despite evidence for ROS generation from AgNPs and its antibacterial effect, the potential toxicity of Ag+, the structure of AgNPs, and their combinatorial effect with other factors confounds a clear understanding of AgNPs functional mechanisms. Jones et al. have investigated the possible reactions involved in ROS generation by AgNPs and the potential interaction between Ag+ and ROS once they have been generated.183 Henglein group has reported a possible reformation of AgNPs by O2˙− as a result of O2˙− mediated charging of AgNPs.184,185 A schematic illustration of AgNPs acting as an electron pool during ROS generation is shown in Fig. 5(a), proposing potential interactions among AgNPs, Ag+, H2O2 and O2˙−. A second source of ROS is the leakage of O2˙− through cell membranes, which can be neutralized by natural antioxidants as shown in Fig. 5(b). Since AgNPs and Ag+ have a strong affinity for thiol groups (–SH) in cysteine residues, it is conceivable for AgNPs to be internalized and disrupt mitochondrial function through altered membrane permeability, disruption of the electron transfer chain, and disruption of mitochondrial membrane proteins.
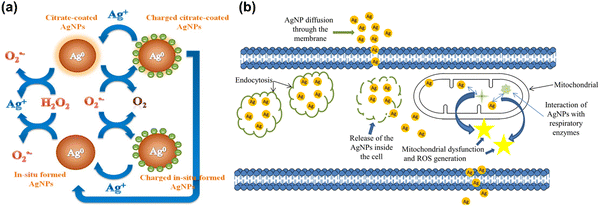 | ||
| Fig. 5 (a) Schematic illustration of the action of silver nanoparticles in generation of ROS in the form of radical. Reproduced with permission from He et al.179 [Copyright 2012, American Chemical Society]. (b) Interactions of AgNPs with cellular membranes and mitochondria and generation of ROS, causing cellular apoptosis. Reproduced with permission from Flores-López et al.186 [Copyright 2018, Wiley]. | ||
Gold nanoparticles (AuNPs) have been employed extensively in nanomedicine applications, for example biosensing,187 drug delivery,188 theranostics,189 biolabeling,190 wound healing,191,192 and medicine.193 The development of plant extract and bio-object derived green synthesized AuNPs with high redox-ability has started to gain interest among researchers. Green synthesized AuNPs are mainly derived from bacteria, virus, yeast, fungi, algae, and plants.194,195 Plant extract-based nanoparticles (also known as phytosynthesized NPs) are more effective compared to microorganism sources, and their preparation method requires fewer additional reagents.196–198 Stozhko et al. have recently introduced a phytosynthetic method to create AuNPs using leaf extract (phyto-AuNPs) and demonstrated their antioxidant activity together with details of the phytosynthesis kinetics, particle size, and dispersibility of the created nanoparticles.199 They found that smaller phyto-AuNPs produced a higher antioxidant activity with an increase of the absolute value of zeta-potential. Nie et al. has developed antioxidant-functionalized AuNPs using self-assembly of thiol ligands derived from Trolox (Vitamin E analogue).19 Surprisingly, the Vitamin E (tocopherol) analogue-functionalized AuNPs has shown strong reactivity towards 2,2-diphenyl-1-picrylhydrazyl radicals (DPPH˙), about eight times higher than AuNPs alone. Hamelian et al. have developed Thyme-derived green AuNPs as a reducing agent, exhibiting antibacterial and antioxidant activity specifically for DPPH˙.200 Quercetin capped AuNPs have also been synthesized using a green route by Milanezi et al. for antioxidant, antibacterial application. The antioxidant activity of quercetin both free and on AuNPs has been proven by free radical scavenging methods using ABTS, DPPH, and nitric oxide. In addition, quercetin-capped AuNPs (IR50 0.37 μg mL−1) demonstrated higher antioxidant activity than free quercetin (IR50 0.57 μg mL−1) in nitric oxide free radical scavenging method.201 In a recent study by Nieves et al. have reviewed silver chalcogenide-based hybrid nanoparticles for its synthesis methodologies, and thorough biomedical applications including bio-imaging, theranostic agents, and biosensors.202 For example, Mantri et al. synthesized iodine-doped silver shell/gold core metal nanorod for measuring the oxidative stress in vivo via photoacoustic imaging.203
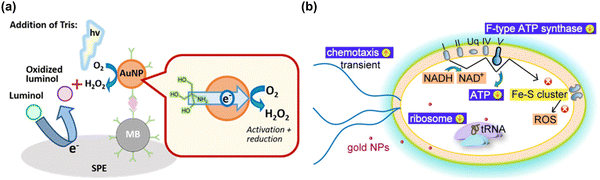 | ||
| Fig. 6 (a) A schematic illustration of gold nanoparticle-generated ROS controlled on a disposable screen-printed electrode using an enzyme-free electrochemiluminescence-based immunoassay. Reproduced with permission from Higashi et al.214 [Copyright 2018, American Chemical Society]. (b) Schematic diagram illustrating the of cellular mechanism of bactericidal gold nanoparticles on E. coli, and subsequent gold nanoparticles inducing the reduction of oxidative phosphorylation pathway (F-type ATP synthase and ATP level) and ribosome pathways, and the transient up-regulation of chemotaxis. During the process, the gold nanoparticles do not induce any change in ROS-related processes. Reproduced with permission from Cui et al.217 [Copyright 2012, Elsevier]. | ||
In addition to the ROS generating effect of AuNPs, many research groups have reported a ROS independent effect of AuNPs. AuNPs have been shown to be capable modulating cell interaction, and to have an apoptotic effect on Escherichia coli via a redox imbalance followed by decreased GSH without ROS generation.35,217 By transcriptomic and proteomic approaches, the Cui group has investigated the molecular mechanism by which AuNPs exert their antibacterial activity against multidrug-resistant Gram-negative bacteria.218 The antibacterial actions by AuNPs could occur through two routes; first, by changing membrane potential and inhibiting ATP synthase causing a subsequent metabolic decline through decreased ATP; second, by inhibiting ribosome binding to tRNA, causing a collapse of biological processes as demonstrated in Fig. 6(b). The antibacterial activity by AuNPs seems to be a bactericidal antibiotic effect that is independent from ROS.219 Mateo et al. have investigated the cytotoxic role of oxidative stress in human tumor cells. which is suggested to involve an AuNP-induced decrease of superoxide dismutase activity.220 Overall, AgNPs and AuNPs have potential for some important biological applications including for antibiotic effect, drug delivery, biosensing, and cancer theranostics, however they are not very effective catalytically due to low coordination numbers and so frequently require complex surface modifications with biologically active molecules.
Selenium is known to be involved in various biological processes including the immune response. Some pathological conditions associated with the immune system have also been affected by selenium content level, and its different salt forms. In the past, concerns have been raised about the metabolism of selenium compounds into metabolites and potential hereditary factors influencing their utilization. Due to low levels of selenium in soils and food, selenium deficiency is uncommon in the United States and Canada,243 while it is also common in some regions of China, New Zealand, and portions of Europe and Russia.244 Recent studies have observed selenium deficiency in immune-related diseases and suggested selenium supplementation to solve the health issues associated with selenium deficiency, although the cellular and molecular mechanisms underlying the effects of selenium in immune-related diseases are not fully understood.245 One possible mechanism by selenium is to activate leukocytes in an immune response including adherence, migration, phagocytosis, and cytokine secretion at an optimal dose. The redox-activities of selenoproteins seem very important in modulating cell signalling in these immune responses. Thus, the redox-activities of selenium-based materials could be a promising therapy for immunity-related pathologies including chronic inflammation, by generating reduced forms of thioredoxin to balance the reduced and oxidized molecules within the cell.245–247
The range of oxidation states accessible to selenium (2−, 0, 2+, 4+, 6+) and its electronegativity are the two properties which are important in redox biology. Because selenium possesses different oxidation states, it has interesting redox activities and therefore a capability to generate ROS. In proteins, selenium can be incorporated in the place of sulfur in cysteine residues forming selenocysteine (Se-Cysteine), the electronegativity of which is lower than cysteine (−0.23 V Cysteine, −0.38 V Se-Cysteine). This incorporation occurs by the action of diverse antioxidant enzymes like thioredoxin reductase, glutathione peroxidase, and selenoprotein.248 Selenium acts as a redox center for all of these enzymes which are essential for biochemical activities. Compared to disulfide, diselenide has a much lower binding energy (H–Se–Se–H, 172 kJ mol−1) than disulfide (H–S–S–H, 240 kJ mol−1).249 The lower binding energy of diselenide bond allows having redox-activity companying with visible light radiation in an effective manner.250–252 Diselenide can produce seleninic acid or selenol under redox conditions by the cleavage of the diselenide bond, and selenium radicals under stress conditions such as under irradiation or heating could be generated. Selenium can be reduced by thiol compounds or oxidized by oxygen leading to ROS production in both reactions and subsequently to apoptotic cell death. However, studies have reported that selenium has a narrow therapeutic window for clinical application.253–255
SeNPs made from sodium selenite (Na2SeO3) can be an alternative form to modulate oxidative stress, presenting an advantage for therapeutic purposes. The ionization of sodium selenite to sodium ions and selenite (SeO32−) is the most common fabrication method (eqn (1)). Selenite has a high affinity with glutathione (GSH), producing selenodiglutathione (GS–Se–GS) as reported previously (eqn (2)).256
 | (1) |
| SeO32− + 4GSH + 2H+ → GS–Se–GS + GSSG + 3H2O | (2) |
| GS–Se–GS + GSH → GS–SeH + GSSG | (3) |
| GS–Se–H → Se + GSH | (4) |
Selenium has been tested in several disease models including ischemic cerebral stroke, an acute brain degeneration with a high mortality rate and no appropriate treatment so far.257,258 Amani et al. have developed biodegradable SeNPs to target ischemic brain stroke, demonstrating a dramatic effect. They found that SeNPs reduced brain edema, protected axons and promoted axonal growth, and enhanced remyelination in the hippocampal area.257 The group has also suggested an effective delivery of SeNPs to target a specific area with minimal side effects. SeNPs possibly modulate cellular signalling pathways in inflammatory and metabolic responses including the ubiquitin-proteasome system (ERK5), Tsc1/Tsc2 complex, biquitin-proteasome system (ERK5), FoxO1, and wnt/β-catenine. The activation of JAC2/STAT3 and Adamts1 are important in inflammatory responses. Studies suggest that SeNPs are promising therapeutics for cerebral stroke via its antioxidant and anti-inflammatory properties.
Redox homeostasis is critical in living organisms and excessive ROS damage cellular biomolecules during oxidative stress. GSH is a tripeptide of L-glutamate, glycine, and L-cysteine, which plays a critical role in many biological functions in mammals. GSH is a major nonprotein thiol in organisms that is required for intracellular redox homeostasis. Importantly, GSH and ˙OH are natural counterparts functioning as reducing and oxidizing agents, respectively. Yang et al. have developed selenium-conjugated graphene quantum dots (Se-GQDs) based on an ultrasensitive reversible redox-fluorescent switch for detecting ˙OH and reductive GSH in aqueous solution and living cells.259 They found that the fluorescence of Se-GQDs is statically quenched by ˙OH, causing a condition known as fluorescence OFF condition that is brought on by Se–Se groups right after reduction of Se–Se groups to C–Se groups upon GSH addition, and fluorescence can be turned ON in the reverse process. This fluorescence-based switch can be useful for detecting redox-activity in cells.
Although the cytotoxic effects of selenium against many cancer cells are thought to be due to its ROS producing activities,260–262 the biochemical mechanism involved in tumor suppression by selenium remains to be studied. Wang et al. have developed biodegradable and pH-responsive selenium-doped hydroxyapatite (SeHA) nanoparticles for osteosarcoma treatment, using in vitro and in vivo osteosarcoma models.263 The molecular mechanism involved in suppression of osteosarcoma is presented in Fig. 7(a) and (b). In another study, Li et al. have demonstrated selenium-containing amphiphiles reduced and stabilized AuNPs suggesting that the selenium in the particles possibly induces high levels of ROS, leading to cancer cell death.264 Zheng et al. have further investigated the therapeutic use of SeNPs in a co-delivery system of selenium and small interfering RNAs (siRNAs).265 This approach aims to overcome drug resistance in breast cancer therapy mostly caused by P-glycoprotein (P-gp) and class III β-tubulin. For co-delivery of selenium and siRNAs (anti-P-gp and anti-β-tubulin III), they have designed layered double hydroxide (LDH) nanoparticles and found a more efficient gene-silencing effect than siRNA alone by a significant downregulation of P-gp and β-tubulin III expression. In addition, apoptotic cells undergo morphological change with an increase of intracellular ROS and altered signalling pathways such as Bcl-2/Bax, caspase-3, PI2K/AKT/mTOR and MAPK/ERK pathways. A similar trial was also performed for dual delivery of siRNAs and cisplatin (DDD) to A549/DDP cells, a breast cancer cell line exhibiting multidrug resistance (MRD). The co-delivery of gene and drug (siRNA and DDP) in A549/DDP cells showed a synergistic effect in anti-cancer therapy, leading to a decreased expression of P-gp and MRP.266 Although selenium conjugated nanomaterials have been intensively explored in many disease conditions, the diagnostic or therapeutic applications of these NPs require more investigation. Furthermore, the exact mechanisms of SeNPs in certain pathological conditions remain unclear. Redox-activity and biocompatibility are considered as the main properties of selenium-based NPs that are applicable for clinical applications. The current challenges of SeNP-based therapies in clinical applications are limiting due to a lack of information on dosing accuracy, potential cytotoxicity, and metabolism in the body.
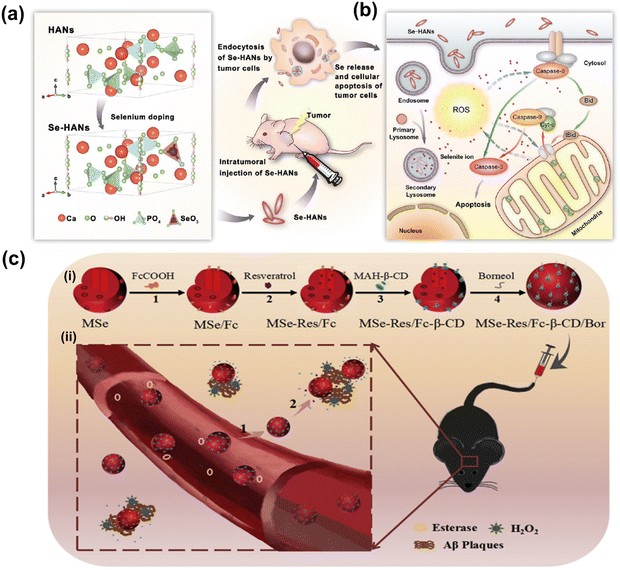 | ||
| Fig. 7 (a) Schematic chemical structural diagram represents the synthesis and acting mechanism of antitumor nanoparticles (Se-HANs) using selenite to replace phosphate groups in hydroxyapatite nanoparticles (HANs). Next, intratumorally injection of Se-HANs into xenograft osteosarcoma mice model. (b) Further, cellular schematic diagram illustrates the non-specific endocytosis of Se-HANs into tumor and subsequent rapid degradation in lysosome (acidic pH) to release selenium. Finally, selenium-induced, caspase-dependent apoptosis pathway activates the cellular apoptosis together orchestrated with the intracellular ROS generation. Reproduced with permission from Wang et al.263 [Copyright 2016, American Chemical Society]. (c) (i) Step-by-step schematic illustration of synthesis and various surface functionalization of MNSe-Res/Fc-β-CD/Bor; including (1) Ferrocene loading into mesoporous nanoselenium (MNSe/Fc), (2) Further loading with resveratrol to MNSe/Fc. (3) Then surface functionalized with MAH modifiedβ-CD on to the surface of MNSe-Res/Fc. (4) Finally, grafting of borneol onto the NPs surface via ester bond to create drug delivery carrier for AD with capability to penetrate the blood–brain-barrier. (ii) Schematic illustration of in vivo circulation of MNSe-Res/Fc-β-CD/Bor in mice: (1) the release of borneol of from the nanocarrier (MNSe-Res/Fc-β-CD/Bor) and passing the blood–brain barrier (BBB). (2) Targeting the amyloid plaques and controlled release of resveratrol by hydrogen peroxide from MNSe-Res/Fc-β-CD/Bor. Reproduced with permission from Sun et al.267 [Copyright 2019, Elsevier]. | ||
Sun et al. have created an innovative drug delivery system that targets Bor and utilizes Fc-β-CD loaded with Res to treat AD through multiple channels.267 The researchers have shown that using MNSe-Res/Fc-β-CD/Bor can effectively prevent the aggregation of Aβ proteins, reduce oxidative stress, and suppress tau hyperphosphorylation. Moreover, this treatment successfully protected neurons and restored impaired memory in APP/PS1 mice. It is interesting to note that the MSe/Fc-CD/Bor loaded with rivastigmine (Riv) displayed a higher pharmacokinetic index than Riv alone. A schematic illustration of synthesized MNSe-Res/Fc-β-CD/Bor for gradual release of drug is presented in Fig. 7(c). The in vivo therapeutic approach is illustrated in Fig. 7(c), where the compound MNSe-Res/Fc-β-CD/Bor was administered via the tail vein of mice with the APP/PS1 model. The Bor present on the nano-system's surface contains an ester bond, which can be broken down by esterases in the bloodstream. This drug delivery system utilizing nanomaterials has been demonstrated to cross the blood–brain barrier and accumulate consistently in the brain. Treatment with MNSe-Res/Fc-β-CD/Bor effectively restored the diminished cognitive function in APP/PS1 mice, accompanied by a decrease in the total levels of Aβ, hyperphosphorylated tau, and the loss of neurons in the brain.
3.2. Metal oxide nanomaterials
Metal oxide nanoparticles such as magnesium oxide (MgO), titanium dioxide (TiO2), vanadium oxide (V2O5), manganese oxide (MnO2), iron oxide (Fe3O4), copper oxide (CuO), zinc oxide (ZnO), gadolinium oxide (Gd2O3), and cerium oxide (CeO2) have antioxidant and catalytic properties. By developing pH-responsive oxide nanomaterials, we can modulate oxidative stress inside the cells through redox-chemistry.268 It is important to note that the reaction mechanisms for these types are not well understood.29,269 The reaction of these metal oxide NPs can be modulated by a given condition, which can be a major factor to favour either antioxidant or redox reaction. Understanding the detailed conditions will provide important information for the use of metal oxide NPs as redox-regulators, however the exact reaction mechanisms of metal oxide NPs need to be thoroughly investigated.29,268,269 In this section, we will discuss and detail the mechanisms of their antioxidant and redox activities on the physiology and pathology of living organisms.A recent study has further investigated the underlying mechanisms of anti-cancer effects induced by magnetite nanoparticles, demonstrating that ROS produced by magnetite NPs lead to mitochondrial damage and genotoxic effects in A549 cells. Mathias et al. have further demonstrated the cytotoxic mechanisms of magnetite-mediated ROS generation using A549 and H1299 human lung cancer cells.340 The study has clearly shown that ROS generated by magnetite nanoparticles induce gene mutations, without a direct effect on cell death. Hauser et al. have utilized ROS produced by iron oxide nanoparticles to enhance the efficacy of radiation therapy, as a combination treatment.343 In this study, they used iron oxide nanoparticles coated with TAT, a cell penetrating peptide, to avoid degradation by lysosomes after being internalized by cancer cells, enabling intracellular hydroxyl radical formation. In addition, TAT-coated MNPs have been shown to produce significantly more ROS compared to uncoated MNPs in A549 lung carcinoma cells. Combined treatment of TAT-functionalized MNPs and radiation therapy resulted in a synergistic effect due to the increased lysosomal permeability, ROS generation, and loss of mitochondrial integrity and function.344 In particular, when greater amounts of superoxide anions are generated under increased cellular respiration in mitochondria, MNPs may show synergic effects on the formation of the highly reactive hydroxyl radicals as described in Fig. 8. Aranda et al. have also suggested that the magnetite nanoparticles might generate higher levels of ROS and oxidative stress due to magnetite possessing both Fe3+ and Fe2+ ions, while maghemite has mostly ferric iron ions (Fe3+).154 For this reason, magnetite nanoparticles have been more frequently used to generate intracellular ROS.345–348
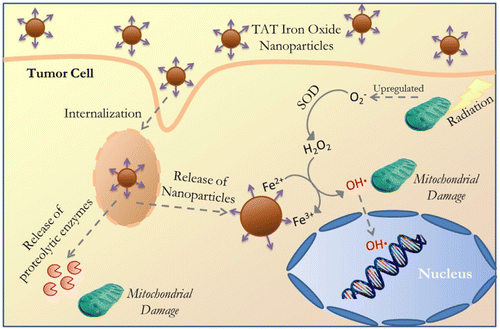 | ||
| Fig. 8 Schematic illustration of the cellular internalization of TAT-conjugated iron oxide via lysosomal membrane permeabilization followed by NP and proteolytic enzyme delivery into the cytoplasm and subsequent interactions with organelles (nucleus and mitochondria) and catalytic reaction to produce the hydroxyl radical. Reproduced with permission from Hauser et al.344 [Copyright 2016, Elsevier]. | ||
CeO2NPs are well known to act as antioxidants and to have redox-activity in tissue repair and regeneration. The relatively stable surface of peroxo/hydroperoxo species of CeO2NPs is used for ROS generation.386,387 CeO2NPs with high surface Ce4+/Ce3+ ratios function more efficiently as antioxidant enzyme mimics.388 The redox-activities of CeO2NPs (i.e., switching oxidation state of Ce3+ and Ce4+) make these NPs a potential candidate for biomedical applications. The direct scavenging of ˙OH by ceria NPs is presented in process 1 of Fig. 9(a), NO˙, OONO− chelating by CeO2NPs have also been investigated.389–391 CeO2NPs have also shown superoxide dismutase-like effects (process 3 and 5 in Fig. 9(a)), that are associated with surface concentrations of Ce3+ and Ce4+, pH, and chelating ligand concentrations.388,392 The inflammatory cell signalling response to CeO2NPs, and cell apoptosis upon ROS production are shown in process 4 and 6 in Fig. 9.268,393
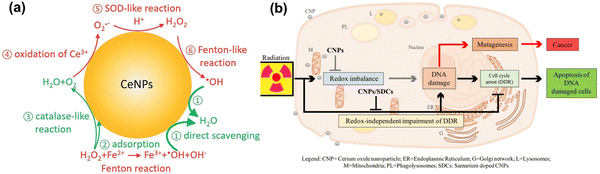 | ||
| Fig. 9 (a) Schematic illustration of Fenton reaction and reactive oxygen chemistry of ceria nanoparticles. Surface chemical reactions presented in red and green colors imply ROS generation and scavenging steps, respectively. This is a proposed model for ROS production and scavenging from ceria nanoparticles as redox-independent radio-sensitizing agents in HaCat keratinocytes cells. Reproduced with permission from Filippi et al.394 [Copyright 2019, Royal Society of Chemistry]. (b) Schematic diagram illustrate the administrative role of ceria nanoparticles (here CNPs) to promote in a redox-independent manner strengthening of the cell DNA damage response (DDR) after exposure to radiation, weakening X-ray-induced DNA lesions on one side, and strengthening the stringency of cell cycle checkpoints and forcing damaged cells to undergo apoptosis on the other, hence inhibiting radiation-induced mutagenesis. Reproduced with permission from Corsi et al.386 [Copyright 2018, Frontiers]. | ||
Recently, Filippi et al. have demonstrated the antioxidant activity of CeO2NPs and cerium nanorods (CNRs) in scavenging hydroxyl radicals.394 They have shown that CeO2NPs and CeO2NRs exhibit stronger ROS scavenging activities than ˙OH generation in phosphate buffer saline (PBS) and surrogated lung fluid (SLF). Further, CeO2NRs have a larger surface area and higher defect density than CeO2NPs, resulting in greater ˙OH scavenging activity. Mahapatra et al. have suggested that the CeO2NPs with different directional shapes (aspect ratios) are internalized in different rates in human dental pulp stem cells.395 Nanoparticles with a smaller aspect ratio (CeO2NPs and CeO2NRs) are internalized faster into the cells and are more effective at suppressing ROS either intracellularly or extracellularly upon H2O2 treatment. Their findings suggest that special attention should be paid to the resulting particle geometry during synthesis of cerium oxide-based nanomaterials, depending on their use such as for ROS-scavenging to protect from the ROS-insult environment, stem cell protection, tissue engineering and regenerative medicine applications.
Cells internalizing CeO2NPs appear to respond more effectively to DNA damage via unrelated mechanisms that reduce DNA breaks, improving apoptotic outcomes.396 Moreover, CeO2NPs help cells to restore DNA integrity, the ability of which cancer cells lose during X-ray mediated mutagenesis by acting on the intimate pathways controlling survival of injured cancer cells. The radio-sensitization has no relationship with the redox-switching of CeO2 nanoparticles because it has not been affected by Sm-doping, a strategy that prevents a switch of Ce3+/Ce4+ and provides 3+ valence by providing an antioxidant action397,398 as shown in Fig. 9(b). The mechanisms of how CeO2NPs interact with cells have been studied at cellular and molecular levels by several groups. CeO2NPs have been suggested as cytoprotective antioxidants and free radical scavengers or oxidants but have also showed cytotoxicity. CeO2NPs delayed cellular damage with ROS scavenging properties, resulting in an increase of cellular resistance to an exogenous ROS stimulation in oxidative stress condition.399 On the contrary, the pro-oxidative effect of CeO2NPs induces oxidative stress leading to cell death upon the cellular internalization of CeO2NPs. Subsequently, ROS are generated from reduction of Ce(IV) to Ce(III), and the dual nature of CeO2NPs, as an antioxidant and a pro-oxidant, could be dependent on the shape, size, dose and exposure time of the nanoparticles.395,400–402 Recently, Pota et al. have synthesized the redox-active CeO2-based hybrid nanostructure via molecular combinations of organic and inorganic semiconductor for antibacterial and biomimetic radical homeostasis applications.403
3.3. Carbon-based nanomaterials
Carbon nanotubes (CNTs), fullerenes (C60), nanodiamonds, carbon quantum dots, graphene and its derivatives are all carbon-based nanomaterials (CBNs).25,404 CBNs have unique properties including a high electrical conductivity, high mechanical strength, thermal conductivity, tunable optical behaviour, and catalytic activities which have led to significant interest in diverse fields such as biomedicine (drug delivery, tissue engineering, and regenerative medicine), energy storage, electronics, and biosensing. CBNs have recently shown a huge impact in biomedical fields, particularly applied in therapeutic delivery and cell/tissue imaging in cancer treatment. In fact, the potential use of CBNs has been reported in tissue engineering, anticancer, and anti-inflammatory treatments with their effect mainly due to ROS generation in cancer. The ROS generation by CBNs causes lysosomal and DNA damage, mitochondrial dysfunction and eventually leads to cell death via either apoptosis or necrosis. Moreover, CBNs have been intensively studied in pulmonary macrophage activation and inflammation, and the mechanisms of their immune suppression continue to be investigated.405,406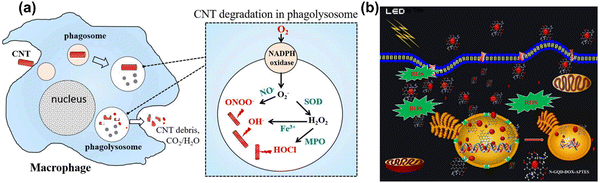 | ||
| Fig. 10 (a) A scheme illustration of CNTs degradation and generation of ROS in a macrophage cell. Reproduced with permission from Yang et al.416 [Copyright 2019, Frontiers]. (b) Light-mediated drug delivery and ROS production from GQD in breast cancer cell line MDA-MB-231 cells. Reproduced with permission from Ju et al.419 [Copyright 2019, Wiley]. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups are more active than C–O–C groups for the scavenging of radical. However, different locations and chemical environments for each surface oxygen group may contribute to the scavenging actions of GQDs. Li et al. have reported electrochemical synthesis of phosphorus-doped GQDs and tested the particles for their free radical scavenging effects.424 They found that phosphorous doping on the GQDs played a critical role in scavenging DPPH radicals, as well as oxygen groups on the surface of GQDs. Carotenoids and other intriguing compounds with lengthy conjugated C
O groups are more active than C–O–C groups for the scavenging of radical. However, different locations and chemical environments for each surface oxygen group may contribute to the scavenging actions of GQDs. Li et al. have reported electrochemical synthesis of phosphorus-doped GQDs and tested the particles for their free radical scavenging effects.424 They found that phosphorous doping on the GQDs played a critical role in scavenging DPPH radicals, as well as oxygen groups on the surface of GQDs. Carotenoids and other intriguing compounds with lengthy conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C chains have also been suggested as effective free radical scavengers.425 Similarly, other CBNs have been thoroughly investigated for their capacity to neutralize ROS and operate as free radical scavengers. For instance, fullerene derivatives exhibit high superoxide quenching activity, which is supported by a mechanism that combines electron transfer and adduct formation.426 Recently, CNT-tailored electrospun nanofibers with electrical conductivity and bi-modal nanotopography have been used for bone and neuronal tissue engineering applications.427,428 Fenogilo et al. found that MWCNTs in aqueous solution do not generate oxygen or carbon-centered radical in the presence of H2O2 and formate.429 On the contrary, purified MWCNTs are very effective scavengers for ˙OH and O2−˙, although the molecular mechanism is not fully understood. Galanio et al. have reported theoretical studies on the potential ability of SWCNTs as free radical scavengers using density functional theory calculations.430 Their findings suggest that once radicals are attached to a nanotube, additional reactions can be enhanced, indicating that SWCNTs can act as free radical sponges. In case of reaction with OH radicals, subsequent additions would lead to products in which the created OH groups are distributed in clusters rather than a homogeneous spread throughout the nanotube.
C chains have also been suggested as effective free radical scavengers.425 Similarly, other CBNs have been thoroughly investigated for their capacity to neutralize ROS and operate as free radical scavengers. For instance, fullerene derivatives exhibit high superoxide quenching activity, which is supported by a mechanism that combines electron transfer and adduct formation.426 Recently, CNT-tailored electrospun nanofibers with electrical conductivity and bi-modal nanotopography have been used for bone and neuronal tissue engineering applications.427,428 Fenogilo et al. found that MWCNTs in aqueous solution do not generate oxygen or carbon-centered radical in the presence of H2O2 and formate.429 On the contrary, purified MWCNTs are very effective scavengers for ˙OH and O2−˙, although the molecular mechanism is not fully understood. Galanio et al. have reported theoretical studies on the potential ability of SWCNTs as free radical scavengers using density functional theory calculations.430 Their findings suggest that once radicals are attached to a nanotube, additional reactions can be enhanced, indicating that SWCNTs can act as free radical sponges. In case of reaction with OH radicals, subsequent additions would lead to products in which the created OH groups are distributed in clusters rather than a homogeneous spread throughout the nanotube.
Many other CBNs including fullerenes,432 CNTs, carbon nanodots and metal-doped carbon nanodots,433–435 GQDs,436 GO,437,438 rGO,439 FLG431 and metal functionalized GO16,165,440 have been investigated for ROS and radical scavenging effects. The scavenging abilities of CBNs are due to sp2-hybridized carbon sites, structural defects, surface electrophonic effects, H-donation from surface functional groups, and adduct formation. However, other possible factors including imperfect antioxidant mechanisms, a lack of an effective method for the regulation of antioxidants, and a low antioxidant activity need to be investigated.
ROS generation and their roles have been implicated in many disease animal models. Sharma et al. have performed a set of experiments to determine SWCNT toxicity in rat epithelial cells and measured ROS generation by change in fluorescence using dichloroflurescein.441 They found that the increased ROS production upon rat epithelial cell exposure to SWCNTs occurred in a dose and time dependent manner. Additionally, the decreased level of glutathione suggested a loss of cellular defense mechanisms against ROS generation in SWCNTs treated cells. The inhibition of mitochondrial function had no change on ROS levels, suggesting the role of SWCNTs in ROS production. Manna et al. have reported the effects of SWCNTs in human keratinocytes cells, looking at toxicity, oxidative stress and cell proliferation.442 They found that the SWCNTs increased oxidative stress in keratinocytes by activating NF-κB pathway in a dose dependent manner. The NF-κB signalling seems to be activated by stress-related kinases triggered by SWCNTs in the keratinocytes. Alarifi et al. have reported the underlying mechanism of the toxic effects and ROS generation of MWCNTs in mouse fibroblasts (L929).443 They found that the MWCNTs significantly increase ROS production, lipid peroxidation, superoxide dismutase levels, and decrease glutathione concentration. Rasras et al. have examined the effects of SWCNTs and MWCNTs in rat brains, in particular focusing on mitochondria. They measured ROS activity with altered levels of malondialdehyde, glutathione and mitochondrial membrane potential.444 They found that both SWCNTs and MWCNTs may damage brain tissue through mitochondria by increasing oxidative stress and activating apoptosis-related cell death pathways. According to Hou et al. the direct photoreactivity of pristine SWCNTs is usually low under sunlight, however indirect photoreactions that generate ˙OH may play a greater role in natural aquatic environments.445 Furthermore, the reactivity of SWCNTs to ˙OH depends on their chirality as well as the surfactant used. Singh et al. have demonstrated the influence of SWCNTs on multicellular chirality, a property that describes a state of directional cell migration. They also show that incubation of SWCNTs with mouse myoblasts (C2C12) and human umbilical vein endothelial cells (hUVECs) stimulated ROS production by intra and extracellular oxidative stress in dose and time dependent manner.446 They found that the SWCNTs-mediated oxidative stress leads to a loss of multicellular chirality.
Graphene itself is not considered a good candidate for biomedical applications due to its poor dispersibility in aqueous conditions such as water, phosphate buffer and culture media, however, chemically functionalized GO or rGO are good candidates for various biomedical applications.447–449 GO and rGO have widely been studied in cell biology due to their outstanding biocompatibility, large surface area, and simple modification of the surface.450,451 GO contains hydroxyl, carbonyl, epoxy, and carboxyl groups on the base plane and edges, which promote cell affinity and a high hydrophilicity. Due to the wrinkled nature of the carbon plane, GO may increase stem cell adherence and differentiation to the osteoblast lineage.452,453 Nanosized GO sheets could serve as nanocarriers of drugs451,454,455 and nucleic acids,456 for gene transfection457 into cells or animals458 for bioimaging,459 biosensing,460,461 and therapeutic purposes.462
Graphene-based materials can modulate the metabolic activities of macrophages by increasing ROS levels resulting in mitochondrial membrane damage and apoptotic cell death. It is complicated and challenging to study the stability and susceptibility to different reagents of graphene-based nanomaterials. In the study of Hsieh et al., the degradation rate constants of graphene-based nanomaterials have been calculated using dissolved organic carbon loss and steady-state concentrations of individual ROS as an indicator of their reactivity with OH, 1O2, and O2.463 Their findings suggest that ROS distribution and GO reactivity with ROS requires long-term exposure of GO and rGO. A detailed review by Niu et al. has summarized nanocarrier-based delivery vehicles to overcome the challenge of crossing the blood–brain barrier (BBB) to cure neurodegenerative diseases.464 Ju et al. have investigated ROS using graphene quantum dots (GQD) that function as a photosensitizer with various nitrogen atom dopant concentrations, thus achieving photodynamic therapy.419 They also found that amine functionalized doxorubicin (DOX) loading on GQDs can have two functions, first, as a drug delivery carrier for nuclear targeting with photosensitizer and second, as a strong ROS generator as shown in Fig. 10(b).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, and can be considered as carbon-based nanomaterials in the sense that it contains carbon as a significant component of its structure. In crystal lattice structure, each silicon atom is covalently bonded to a carbon atom.465 Recently, SiC is growing significant interests as a potential solution for enhanced energy storage and power density.466 Moreover, the unique physical and chemical characteristics of SiC such as high temperature resistance, strong mechanical strength, excellent resistance to wear and oxidation, and chemical stability compared to carbon-based materials endow SiC an excellent nanomaterials for renewable energy storage, and portable electron applications.467
1 ratio, and can be considered as carbon-based nanomaterials in the sense that it contains carbon as a significant component of its structure. In crystal lattice structure, each silicon atom is covalently bonded to a carbon atom.465 Recently, SiC is growing significant interests as a potential solution for enhanced energy storage and power density.466 Moreover, the unique physical and chemical characteristics of SiC such as high temperature resistance, strong mechanical strength, excellent resistance to wear and oxidation, and chemical stability compared to carbon-based materials endow SiC an excellent nanomaterials for renewable energy storage, and portable electron applications.467
Silicon carbide is the second hardest-known material after diamond and is used in various industrial applications. SiC has potential applications in various fields but has been reported to have toxic effects on bacteria and mammalian cells.468,469 For example, Pourchez et al. investigated SiC nanoparticles for impact of physico-chemical features on pro-inflammatory and pro-oxidative effects on macrophage.470 They found that SiC nanoparticles generate oxidative stress in macrophages, leading to significantly enhanced and dose-dependent LDH release and pro-inflammatory response in the macrophage cell line. The cytotoxicity of SiC mostly depends on morphology of the SiC nanoparticles. Studies have shown that SiC nanostructures such as nanofibers and nanorods induce oxidative stress in Pseudomonas putida compared to micrometric SiC, likely by increasing the formation of highly reactive radicals and disrupting cell membrane integrity, leading to DNA damage.469 Similarly, research has revealed that SiC nanowires (SiCNWs) are more toxic to hMSCs than SiC nanoparticles (SiCNPs) at the same concentration. Moreover, SiCNWs negatively impact hMSCs’ cellular activities including adhesion, proliferation, migration ability, multipotency, phenotypes, and cytokine secretion. This adverse effect is likely due to an overproduction of pro-inflammatory cytokines MCP-1 and IL-8 and a deficiency in MMP-3 and C3. Furthermore, SiCNWs have a greater impact on MCP-1, IL-8, MMP-3, and C3 secretion than SiCNPs, leading to differences in cytotoxicity to hMSCs between the two.468 In summary, while SiC includes carbon as a key components, it is more accurately described as a ceramic or composite material rather than carbon-based materials.
3.4. Nanomaterials for drug delivery
RANs have also been developed for the delivery of molecules/drugs which can cause intracellular ROS generation after internalization by cells. Mesoporous silica nanoparticles (MSNs) are frequently used in drug delivery, imaging, and many biomedical applications due to their unique physical pore structure (pore size, shape, volume, and alignment), surface area, surface chemistry, biocompatibility and bio-stability.239 Their biomedical applications include targeted therapeutic delivery, biosensing, cell and tissue imaging, and photothermal or photodynamic therapy for cancer treatment. Both porous and non-porous silica nanoparticles can generate ROS in the form of ˙OH, O2−˙ and H2O2, causing an imbalance between oxidant and antioxidant states and creating intracellular oxidative stress.471 For example, Yi et al. have fabricated MSNs as redox-responsive short-chain gatekeeper nanoparticles for the controlled release of salicylic acid.472 Redox-responsive gatekeepers such as these MSNs are openable by glutathione, an endogenous reductant in cells, and thus do not require any external/exogenous agents. The versatility of MSN surface chemistry creates huge potential for the design of new multifunctional nanocarriers for more efficient delivery. An innovative redox-responsive delivery system of multifunctional nanoparticles capped with bovine serum albumin and hyaluronic acid has been developed by Chen et al. for cancer drug delivery and MRI imaging.473 An overview of the synthetic process for a multifunctional MSN for redox-responsive drug delivery and MR imaging can be found in Fig. 11. Zhao et al. have also modified MSNs with redox-responsive oligosaccharides for targeted drug delivery using disulfide bonds that are cleavable under high concentrations of glutathione (GSH).474 The covalent disulfide bond is relatively stable in the extracellular fluid and plasma, and is easily ruptured in intracellular fluid due to the increase in GSH concentration between the extracellular (2–20 μM), and intracellular (1–10 mM) fluids.475 Surface functionalized MSNs have been tested for GSH-responsive drug release in tumors. As shown in Fig. 11(b), oligomeric hyaluronic acid functionalized multifunctional MSNs (oHA-MSN) are internalized through endocytosis. The HA-mediated CD44 interaction and GSH-triggered disulfide cleavage led to drug release in the cell after cellular uptake. Singh et al. have developed cerium oxide capped MSN nanocarriers as a pH-responsive drug delivery system.25 In this system the cerium oxide nanoparticles are shown to facilitate the pro-oxidant properties of MSNs followed by increased ROS levels leading to the killing of cancer cells. Additionally, a DOX combination therapy with cerium oxide capped MSNs is more effective in ROS generation, demonstrating a cytotoxic synergism between the drug and nanoparticle in terms of ROS generation. The combined effect of the pH-triggered intracellular ROS therapy and chemotherapy caused by DOX-loaded cerium oxide capped MSNs is presented in Fig. 11(c). A proper range of ROS concentration seems to be critical in functioning as essential messengers to facilitate immune responses.476,477 ROS can be a signal for cellular danger in the immune system by the activation of dendritic cells (DCs),478,479 increasing the concentration of co-stimulating molecules like CD80 and CD86 to speed up the presentation of an antigen.480 Therefore, ROS generating nanoparticles might improve the immune response.481 For example, Lu et al. have found that mesoporous organosilica nanoparticles improve cancer immunotherapy by depleting glutathione.482 They used biodegradable glutathione-depleted dendritic mesoporous organosilica nanoparticles (GDMON) with a tetrasulfide-incorporated framework to deliver an antigen protein (ovalbumin) and a toll-like receptor 9 (TLR9) agonist into antigen-presenting cells (APCs). As a result of –S–S–/GSH redox chemistry, glutathione (GSH) levels are decreased intracellularly and ROS are generated, leading to the proliferation of cytotoxic T lymphocytes (CTLs) which inhibited tumor growth in B16- OVA cells, an aggressive melanoma model, described in Fig. 11(d). Overall, intracellularly generated ROS could be an oxidative stress and intracellular antioxidant system like GSH will be operated in nature.483 In cells, GSH is an endogenous antioxidant neutralizing excessive ROS, which can be cytotoxic.484 Farooq et al. have tested titania coated MSNPs with hydroxyl groups on the surface that can react with intracellular H2O2 producing ROS, possibly contributing to biocompatibility as well as therapeutic delivery. The MSNPs generate ROS much less than dense silica nanoparticles. In addition, MSNPs exhibit less toxicity than nonporous silica nanoparticles, whose surface modification has further improved the biocompatibility.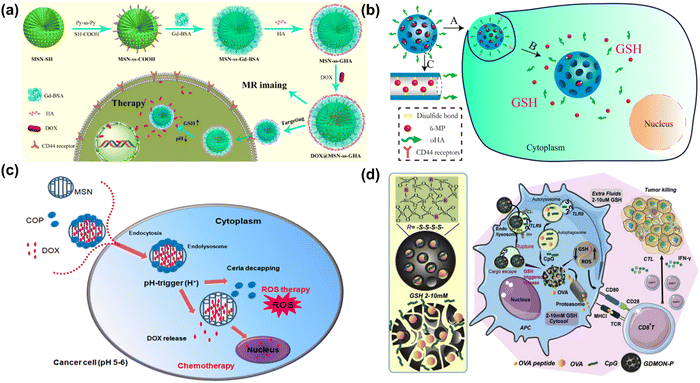 | ||
| Fig. 11 (a) Schematic diagram to illustrate the preparation method of multifunctional MSNs for redox-responsive theranostics (drug delivery and imaging). Reproduced with permission from Chen et al.473 [Copyright 2016, American Chemical Society]. (b) Schematic diagram presenting GSH-responsive drug release in the tumor cells from MSNs; (A) cellular internalization of oHA-modified MSNs via oHA mediated CD44 interaction, and (B) GSH-activated drug release into the cell, (C) Pore structure of CMS-SS-MP/oHA. Reproduced with permission from Zhao et al.474 [Copyright 2014, American Chemical Society]. (c) Schematic diagram illustrating the combined effect of intracellular ROS therapy and chemotherapy (doxorubicin), release from cerium oxide nanoparticles capped MSNs in cancer cells. Reproduced with permission from Singh et al.111 [Copyright 2019, American Chemical Society]. (d) Schematic illustration of GDMON-P + OVA + CpG enhanced cancer immunotherapy, and co-delivery of an antigen protein (ovalbumin) and CpG into antigen presenting cells (APCs) and inducing endosome escape. In the cytosol of APCs, GDMON-P diminish the intracellular glutathione (GSH) level through the –S–S–/GSH redox chemistry and up-regulating the intracellular ROS production and endowing specific cytotoxic T cell (CTL) proliferation and inducing cancer cell apoptosis. Reproduced with permission from Lu et al.482 [Copyright 2016, Elsevier]. | ||
Previous research has attributed the increase in cytoplasmic and mitochondrial ROS production during silica nanoparticles (SiNPs) exposure solely to NADPH oxidase 2 (NOX2) pathways.485 However, research teams like Cui et al. and Joshi et al. reported ROS production upon exposure of cells such as macrophages and Cos7, to silica nanoparticles could also be independent from NOX2 pathways.485,486 Reported results suggest that silica nanoparticles are able to generate ROS independently from NOX2 once they are in the phagosomal system contributing to the increase in cytoplasmic ROS and subsequently downstream cytotoxic pathways such as over expression of COX2, enzyme linked to a variety of inflammatory pathways, or including phagosome leakage.485,486 The latter is hypothesized to happen trough Fenton reaction between the free ion left on the surface of the silica particles after production of hydrogen peroxide in the phagosomes, the resulting ˙OH radicals drive the damage of the phagosomal membrane via per-oxidation leaking ROS.486
In addition to mitochondrial and NOX2 mediated ROS production, other methods for ROS production induces by silica nanoparticles include peroxisomes and xanthine oxidase.487 Furthermore, Petrache Voicu et al. have shown that silica nanoparticles induced ROS production in MRC-5 cells is also enabled via severe down regulation of the tripeptides glutathione (GSH), antioxidant mechanism in cells.488 Moreover, the abundant hydroxyl groups on the surface of silica nanoparticles together with topographic irregularties enhances the production of hydroxyl radicals, which in turn can interact in the chemical reactions, such as production of superoxide that get dismutated by extracellular superoxide dismutases to produce hydrogen peroxide, or with membrane NADPH oxidase systems to produce superoxide anion even before entering into the cells.488
3.5. Biofouling and NPs aggregation effects
Surface biofouling is a common issue with the metallic nanoparticle's therapeutics, where non-specific biomolecules bind to and accumulate at the reactive nanoparticles surface, creating a protein corona.489 The formation of a protein corona has been shown to occur rapidly, to modulate nanoparticles toxicity, and to alter their uptake.490 Furthermore, quantification of the effect of a protein corona on the kinetics of redox-reactions at a NPs surface has not been carried out to our knowledge. It may be the case that this accumulation reduces the chance of direct interaction between electron donator and acceptor molecules and the NPs, thereby lowering its therapeutics efficacy. This magnitude will likely be particle and environment specific, with potential for some proteins to perhaps improve electron transfer from NPs surface if they undergo in redox-reactions themselves.491The stability of a suspension of NPs, i.e. the likelihood of NPs aggregation, depends on its surface chemistry and the solvent its dispersed. The complex and dynamic physiological environments that a NP therapeutic may be exposed to during application, therefore present a great challenge in terms of retention activities. To overcome this, several passivation methods have been developed that preserve function and reduce NPs surface instabilities.492 Furthermore, various surface coatings approaches including polymers, lipids, and silica-based coatings have been developed to improve the stability, and functionality. Silica-based coatings introduced significant porosity at the surfaces with sustainable benefits for drug loading and controlled release,493 and lipid coating can be used to reduce cytotoxicity and improve cellular uptake due to the similarity to the cell membranes.494
3.6. Other types of nanomaterials
Up-conversion nanoparticles (UCNPs) are a distinct category of nanomaterials that are doped with rare earth metal atoms embedded in a crystalline structure. For example, lanthanide (Ln3+) can be used to dope nanocrystals. UCNPs are anti-Stokes type materials, which exhibit anti-Stokes optical properties. When these types of nanomaterials interact with infrared radiation (lower energy photons), they absorb and upconvert to emit in the visible light spectrum (higher energy photons) through a series of real as opposed to virtual levels, as in conventional two-photon dyes.495 The UCNPs can up-convert two or more lower energy photons into one high energy photon through either sequential excitation of one emitting atom or excitation actions of two different atoms and subsequent energy transfer between co-doped rare-earth atoms.496,497The applications of UCNPs as delivery and bioimaging agents have been explored enormously, for example He et al. have developed up-conversion nano-onions (UNCOs) coated with polymer, which degrade sequentially in response to NIR stimulation in the extracellular environment.28,498 These UNCNOs have a core of UCNPs functionalized with the photosensitizer rose Bengal (RB), which is conjugated with poly ethylenimine 600 (PEI-600), followed by a ROS responsive middle layer of singlet oxygen (1O2) sensitive diselenide bonds (PEI-SeSe) connected to siRNA and cell penetrating peptide R8 modifications, and an outer coating layer of negatively charged hyaluronic acid (HA) represented as (UCNPs-PEIRB-PEI-SeSe-R8-HA) as presented in Fig. 12(a). Electrostatically adsorbed siRNA molecules on the middle PEI-SeSe layer were released when the UCNP core was irradiated with light between 525 and 540 nm to activate the RB in PEI-RB's inner layer, boosting ROS production and fully decomposing the PEI-SeSe bonds as shown in Fig. 12(b). The significance of the HA coating is to prolong the circulation of the nanoparticles in the bloodstream, by preventing NP degradation during delivery process and to control UCNO transporting to target special tumor cells via cell membrane receptor CD44.499 The subsequent stripping of UCNO coating upon internalization into the cells improved siRNA delivery without leaking, thus it can be an efficient strategy for NIR-assisted gene therapy.
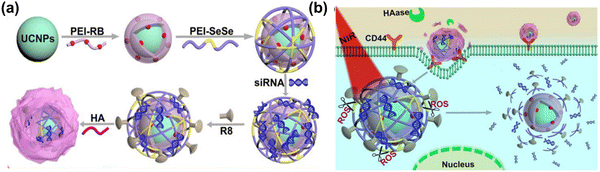 | ||
| Fig. 12 (a) Schematic illustration of step-by-step preparation and surface modification of up-conversion nanoparticles (UCNP-PEI-RB-PEI-SeSe/siRNA-R8-HA) for drug delivery and imaging applications, and (b) corresponding sequential responsive disintegration of UCNOs and NIR boosted intracellular siRNA release and reactive oxygen species therapy. Reproduced with permission from He et al.498 [Copyright 2019, Elsevier]. | ||
4. Biomedical applications of RANs
4.1. Cancer
Reactive oxygen species are natural byproducts of various cellular processes including metabolism, proliferation, and differentiation. An excessive amount of ROS can damage cells by random interaction with proteins, lipids, and DNA, ultimately leading to cell death.35,500 ROS are generated as an oxidative phosphorylation byproduct in mitochondria including superoxide, hydrogen peroxide (H2O2) and hydroxyl (OH−) radicals.28,35 ROS regulation is important within the cellular microenvironment and is mostly controlled by ROS-scavenging mechanisms such as superoxide dismutases (SOD1, SOD2, SOD3), peroxiredoxins, glutaredoxins, thioredoxins, glutathione peroxidase, and catalases.501,502 However, in the event where these unstable radicals are uncontrolled due to a reduction of intracellular ROS-scavenging, oxidative stress can result.502 Oxidative stress conditions are harmful for normal cells, and a chronic over production of ROS can induce normal cells to transform into tumor cells through genetic alterations, thus reprogramming cancer cell metabolism.110Among the pathophysiological processes that ROS are involved in, such as genomic instability, inflammation, and metabolic reprogramming, tumorigenesis is of particular interest.35 Disruption of ROS-scavenging-enzyme production can trigger several cancers signalling cascades, including cancer angiogenesis and metastasis as presented in Fig. 13. All these cellular activities mentioned above are highly dependent on the levels of ROS, which promote cell proliferation and survival at low levels, induce cell cycle arrest leading to cellular differentiation at intermediate levels and cause oxidative damage and DNA mutations at high levels potentially leading to cancer.501
ROS generation mainly occurs in mitochondria within the electron transport chain (ETC) in which one-electron reduction of oxygen happens at several major sites such as complexes I, II and III.35,501 Complexes I and II produces O2˙ in the mitochondrial matrix, and superoxide dismutase protein 2 (SOD2) will subsequently convert O2˙ into H2O2 though complex III when intermembrane O2˙ is transferred to the outer mitochondrial membranes and cytosol. Subsequently, O2˙ is converted into H2O2 by superoxide dismutase protein 1 (SOD1). Another ROS production pathway utilizes nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) located in the cell membrane, which activate the conversion of oxygen to O2˙ via electron transfer across biological membranes, which will then be further reduced by superoxide dismutases to produce H2O2.35
Over the last few decades, surface functionalized metal nanoparticles, lipids particles, liposomes particles, quantum dots, and dendrimers have been extensively applied for anticancer drug delivery and imaging applications.503 The oxidative nature of ROS generated by NPs is very useful in anticancer therapy to suppress and eliminate cancerous cells. The activation of ROS is highly dependent on the redox-related application, such as photodynamic therapy (PDT), chemodynamic therapy (CDT), radiation therapy (RT), sonodynamic therapy (SDT), controlled drug release (CDR) etc., as well as the functionalities of the nanoparticles. Meanwhile nanoparticles containing prooxidant can be internalized into cancer cells followed by ROS generation within mitochondria with subsequent activation of tumor necrotic factor and other transcription factors, which finally lead to adenosine triphosphate depletion and cell necrosis.503 Alternatively, cancer cells can be killed by cell cycle arrest via disruptions of the mitochondrial membrane, which will further increase ROS levels, exceeding body defense system, and activate effector caspases.504,505
Chen et al. have reported the mechanism of plasmon enhanced PDT based on photosensitizers (PS) that generate ROS to enhance cancer therapeutic efficiency. The PS used in the study are upconversion nanoparticles conjugated with gold nanorods that convert near-infrared (NIR) light to visible light and then excite surface plasmon resonance (SPR) locally to generate cytotoxic ROS.506 During this process, apoptotic cell death via caspases-3 activation occurs followed by the release of cytochrome c from mitochondria upon ROS production as shown in Fig. 14(a). A similar study performed by Sonkusre et al.,503 involved selenium induced necroptosis reported in PC-3 cells, where the nanoparticle activates TNF and the transcription factor IRF1, leading to ROS-mediated necroptosis through RIP1 protein as illustrated in Fig. 14(b). Selenium nanoparticles (SeNPs) have been widely studied as they show strong anticancer activity and excellent biocompatibility in a physiological environment.503 Several forms of SeNP such as chemically synthesized SeNP, sol–gel synthesized SeNPs, and co-precipitated SeNPs have been reported.504 It has been demonstrated that chemically functionalized SeNPs inhibit cell growth by suppressing the transcription and translation of androgen receptors, leading to a loss of androgen receptors via phosphorylation, ubiquitination and Akt/Mdm2 degradation pathways.507,508 Meanwhile transferrin-conjugated SeNP increases intracellular ROS and activates MAPKs pathways inducing p52-mediated apoptosis. Glucose incorporated into SeNPs is also known to induce cell apoptosis.
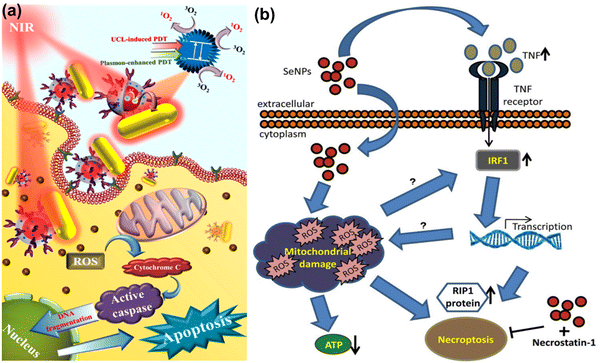 | ||
| Fig. 14 (a) Cell apoptosis pathway of plasmon-enhanced PDT treatment using upconversion nanoparticles conjugated with gold nanorods. Reproduced with permission from Chen et al.506 [Copyright 2016, American Chemical Society] (b) Selenium nanoparticles induced ROS-mediated necroptosis in PC-3 cells. Reproduced with permission from Sonkusre et al.503 [Copyright 2017, Springer]. | ||
Recently, Zhuang et al. have reported that SeNPs enhanced the permeability and retention (EPR) of NP in the cancer area, thus inhibiting tumor growth more effectively. In addition, SeNPs accumulate within the cancer causing a drastic increase of ROS and oxidative stress, thus activating cell-death pathways.509 It is of interest that SeNPs coated with human serum albumin (HSA) show enhanced mitochondrial targeting compared to unmodified SeNPs. Alternatively, Chan et al. have investigated the potential combination of SeNPs seeded with iodine-125 (125I) for chemo-radiotherapy application to be more effective and safer to target cancer.510 Sensitizer based SeNPs have shown Auger-electron effect and Compton effect by generating a high concentration of intracellular ROS with only low-dosages for long-term activation, regulating p53-mediated DNA damage apoptotic signalling pathways and MAPKs phosphorylation to prevent cancer cell growth.510
4.2. Neurodegenerative diseases
Neurodegenerative diseases are a heterogeneous group of disorders including, Parkinson disease, Huntington diseases (HD), Alzheimer's disease (AD), epilepsy, multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and strokes,511–514 defined by a decrease in the number of neurons and glial activation.515,516 Oxidative stress has been considered as a central and crucial element in the onset of these neurodegenerative conditions. Some important and comprehensive reviews have summarized ROS in neurodegenerative diseases in detail.514,517–519 The brain is far more vulnerable to excessively generated ROS than other tissues because of its high oxygen requirement and peroxidation vulnerability.The most common type of senile dementia is Alzheimer's disease (AD), which is characterized by the presence of intracellular tangles of beta amyloid (Aβ) peptides and extracellular amyloid plaques made of hyperphosphorylated tau proteins.520,521 Numerous studies have reported the relationship between ROS and amyloid plaques. High levels of ROS can induce an inflammatory response, hyperphosphorylation of tau and neural dysfunction. Thus, it has been a long therapeutic strategy for AD to find drugs to inhibit the formation of plaques and tangles and to reduce the inflammation response. In this context, Yin et al. have created sialic acid-modified selenium nanoparticles incorporating a highly effective blood–brain barrier-penetrating peptide known as B6 (referred to as B6-SA-SeNPs). These nanoparticles serve as a synthetic selenoprotein analog, designed for Alzheimer's disease therapy.522 The results of their in vitro experiments suggest that B6-SA-SeNPs exhibit effectiveness not only in preventing the aggregation and promoting the disaggregation of Aβ, but also have the capacity to shield PC12 cells from Aβ-induced damage. Moreover, they claimed the developed nanoparticles cross the blood–brain-barrier in an in vitro system of PC12 cells. In order to prevent metal-induced Aβ aggregation, Yang et al. developed modified selenium/ruthenium nanoparticles (Se/Ru NPs) containing L-cysteine.523 Se/Ru NPs suppress a Zn2+ – amyloid mediated ROS generation process, thus reducing neurotoxicity in PC12 cells. A significant reduction in intracellular peptide aggregates was observed when spherical NPs with varying surface charges were used. NPs containing Ru also inhibit the random coiling or sheeting of Aβ that disrupts the alpha helical structure of amyloid plaques. Additionally, Se/RuNPs decrease Aβ40 fibrillization intracellularly, independent from lysosomal pathways. The release of borneol (Bor), which occurs when mesoporous nanoselenium (MNSe) reacts with blood or intracellular esterase, enables the nanosystem to cross the BBB.524
At the beginning stages of AD, the buildup of Aβ causes a surge in oxidative stress, which in turn causes mitochondrial malfunction and energy failure.517,525 A decline in dopaminergic input into the brain's nigrostriatal dopaminergic pathways and a specific neuronal loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) are characteristics of the neurodegenerative disorder Parkinson's disease (PD).526–528 A genetic mutation of the Huntingtin (HTT) gene on chromosome 4 (4p63) results in HD, which is characterized by progressive loss of function in the brain and muscle, loss of neurons, uncontrollable movements, loss of intellectual ability, and emotional disorders.529 These neurogenerative disorders share common pathological mechanisms including protein aggregation, oxidative damage, and mitochondrial dysfunction. Many of the genes involved in these neurodegenerative diseases are associated with mitochondrial function.530 Therefore, it is vital to understand the underlying mechanism of mitochondrial dysfunctions when proposing RAN treatments for NDs. In addition to mitochondrial dysfunction, other cellular processes have recently been investigated for ROS generation. Activation of the peroxisome and endoplasmic reticulum, neuroinflammation, genetic mutation, and antioxidant depletion can induce ROS formation in the cell with subsequent loss of neurons by lipid peroxidation, and DNA or protein oxidation.531
Many studies have shown the roles of ROS in the development of neurodegenerative disorders.530–532 Although ROS might not be the main key factor in all events of progression of the disease developments, ROS generation in oxidative stress conditions could be a common boosting influence on cell damage. Considering that many good antioxidant candidates cannot cross the BBB, nanoparticles with antioxidant properties would be a good option for antioxidant delivery.533 Recently cerium oxide nanoparticles have been intensively studied in antioxidant delivery applications. A systematic representation of CeO2NPs and their inherent antioxidant characteristics for use as efficient neurodegeneration therapies is presented in Fig. 15(a). ROS scavenging from the injured area of the brain is critically aided by the low reduction potential of CeO2NPs and the coexistence of mixed valence states. Moreover, CeO2NPs can cross the BBB due to their nanoscale size. Considering the important role of oxidative stress, regulation of ROS concentrations is a promising treatment mechanism for the early stages of neurodegenerative disease. In this respect, numerous compounds that have antioxidant properties such as GSH, vitamin C, vitamin E and coenzyme Q10 have been studied for their potential to constrict neurodegenerative side effects in concert with NPs or using various delivery vehicles.532
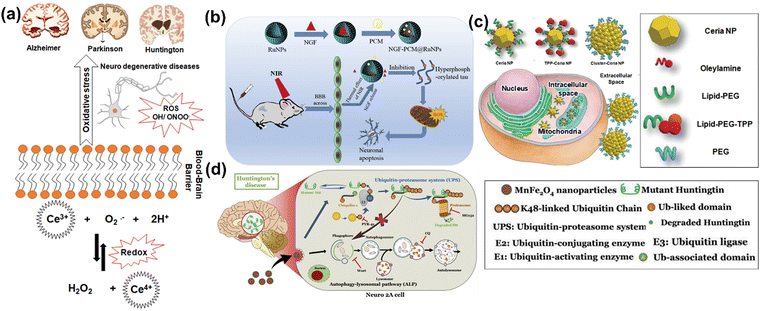 | ||
| Fig. 15 (a) Relationship between oxidative stress and neurodegenerative diseases; illustrating NP effect (cerium chosen randomly to illustrate oxidation reduction reactions). Reproduced with permission from Naz et al.534 [Copyright 2017, Future Medicine LTD]. (b) Schematic illustration of synthesis of RuNPs, their loading with nerve growth factor (NGF) and further phase change material (PCM) to create (NGF@PCM-Ru) NPs for drug delivery through the BBB under NIR-radiation in vivo and projection of thermos-responsive RuNPs to AD mice model, which subsequently down-regulated the ROS production and mitigation of neuronal damage by inhibiting tau hyperphosphorylation. Reproduced with permission from Zhou et al.,535 [Copyright 2020, Elsevier]. (c) Three different functionalized ceria nanoparticles and their cellular localization-dependent ROS scavenging in Parkinson's disease. Reproduced with permission from Kwon et al.,536 [Copyright 2018, Wiley]. (d) Schematic illustration of MnFe2O4 nanoparticles cellular internalization and accelerated clearance of mutant huntingtin (Htt) selectively through ubiquitin-proteasome (UPS) system in Huntington's disease. The rapid clearance of mutant Htt using MnFe2O4NPs via UPS rather than autophagy-lysosomal pathway (ALP). Among the enhanced degradation of Htt by UPS, K48-linked polyubiquitin chains target substrates to the proteasome and Ubiquilin-1 is the chosen ubiquitin receptor for mediating the cargo delivery. Reproduced with permission from Zhang et al.537 [Copyright 2019, Elsevier]. | ||
Other RANs have also been investigated for neurodegenerative disease treatment. For example, Zhou et al. created a thermo-responsive hollow ruthenium flower-like system to maintain the release of nerve growth factor (NGF) and prevent tau hyperphosphorylation and neuronal death, offering a potential therapeutic for AD treatment.535 The thermally controlled drug delivery system of NGF-PCM@Ru NPs not only prolonged NGF circulation time but also penetrated the BBB and inhibited tau-related AD pathogenesis via NIR irradiation as presented in Fig. 15(b). In another study, Kwon et al. developed three forms of functionalized ceria NPs for ROS scavenging in Parkinson's disease, which inhibited microglial activation, lipid peroxidation, and restored tyrosine hydroxylase expression.536 The three differently functionalized NPs, also varying in size, were localized to different subcellular parts when delivered to the cell. ROS can be detected in mitochondria, intracellularly, and extracellularly as presented in Fig. 15(c), all these ROS play a distinctive role in pathological conditions. This study represents the first biomedical application of NPs for clearing reactive oxygen by-products in Parkinson's disease demonstrating their potential as new therapeutic targets. In the future, this concept could be implemented in various neuronal disorders associated with ROS including cancer, cardiovascular diseases, and sepsis. Zhang et al. have developed biocompatible MnFe2O4 NPs and applied them to chelate the 74 glutamine repeats (Htt(Q74)) of mutant huntingtin protein (Htt), the key disease factor in Huntington disease.537 Instead of triggering the autophagy pathway, Zhang's group found that MnFe2O4NPs facilitated Htt(Q74) degradation through the ubiquitin-proteasome system (UPS). In contrast to p62/SQSTM1, MnFe2O4NPs improved the ubiquitination of GFP-Htt(Q74) associated with the Lys 48 (K48) ubiquitin chain through the ubiquitin receptor. Further, they evaluated the potential efficacy of MnFe2O4NPs in Htt protein clearance and explored the underlying mechanism for protein degradation using Neuro 2A, a neural cell line stably expressing GFP-tagged mutant Htt aggregates with 74 polyQ tracts (mHtt-GFP-Q74) Fig. 15(d). This suggests that MnFe2O4NPs are good candidate nanoparticles in nanomedicine therapy for HD.
Interestingly, exosomes, extracellular vesicles (∼30–120 nm) that are secreted by cells, have also been used for drug delivery as they can also cross the BBB.538 It has been discovered that exosomes significantly contribute to the pathways leading to dementia. For instance, they contribute to the buildup of Aβ and the development of amyloid plaques in the brains of AD patients. Furthermore, exosomes transfer pathological protein aggregates containing α-synuclein to the CNS, contributing to PD.539 The biggest hurdle for RAN-based AD treatment is the potential side-effects arising from their short and long-term use. The gradual accumulation of NPs may be harmful with serious side effects in the body; therefore, it will be necessary to optimize the conditions of their use for therapy.
A recent study highlights the potential of graphene-based acid quantum dots (GAQDs) as a new therapeutic tool in treating neurodegenerative diseases. GAQDs have shown effectively reduce ROS by 50% at a concentration of 100 μg mL−1 when exposed to a free radical generator. Moreover, GAQDs demonstrated a 70% suppression of hen egg-white lysozyme fibril production at a 5 mg mL−1 concentration. In experiments using human neuroblastoma-derived SHSY-5Y cells, GAQDs were found to be biocompatible and capable of protecting cells from rotenone-induced apoptosis.540
RANs-based nanoparticles coated with biological membranes combine synthetic and biological elements were developed to improve targeting and therapeutic effectiveness. For example, Lin et al. developed drug-loaded hybrid cell-membrane liposomes that allow for multi-drug therapy and precise targeting of inflammatory brain lesion.541 This hybridization technique involved loading of two small molecule medications (rapamycin and TPPU) and combining a platelet membrane with a membrane expressing a high level of CCR2. In vitro, cell death was prevented by the autophagy enhancer rapamycin and the soluble epoxide hydrolase (sEH) inhibitor TPPU, which also improved cognitive function in AD model mice, reduced amyloid plaque levels in their brains, and decreased neuroinflammation.
L-Molybdenum disulfide quantum dots (L-MoS2 QDs), along with a carrier material and a surface coating, were utilized as the main component to address neuronal loss in AD. The L-MoS2 QDs were more effective in inducing neural stem cells (NSCs) neuronal development when exposed to CPL NIR radiation. Moreover, when injected in vivo into the tail vein of AD mice, the L-MoS2 QDs had complementary effects such as the photothermal clearance of Aβ plaques, the production of H2, which eliminated ROS, mitochondrial protection, and an overall improvement of the brain microenvironment.542
Combining ROS scavengers with nanomaterials exhibiting photothermal properties has emerged as a recent treatment for Alzheimer's disease. Song et al. developed Prussian blue nanomaterials (PBK NPs) with outstanding antioxidant and photothermal properties. The PBK NPs displayed activities similar to multiple antioxidant enzymes, such as peroxidase, superoxide dismutase, and catalase, which can help eliminate excessive ROS and alleviate oxidative stress. When subjected to near-infrared irradiation, the PBK NPs generated localized heat, effectively breaking down Aβ fibrils in vitro. Furthermore, modifying the PBK NPs with CKLVFFAED peptide enhanced their ability to penetrate the blood–brain barrier and target Aβ. Results from in vivo studies demonstrated that the PBK NPs disintegrated Aβ plaques and reduced neuroinflammation in an AD mouse model.543 A systematic summary of therapeutic roles of RANs in various diseases are presented (Table 3).
| ROS active NMs | Application methods/surface modifications | Brief outcomes in biomedical applications | Ref. |
|---|---|---|---|
| CeO2NPs | Reverse micelle methods | • CeO2NPs reduced neuronal apoptosis by altering BDNF signalling, and decreased Aβ-aggregation in Alzheimer's disease. | 534 |
| • CeO2NPs also displayed a successful reduction of in manganese induced neuronal death in PD. | |||
| CeO2NPs | Yeast model of Parkinson's’ Disease | • The ideal amount of CeO2NPs has been shown to significantly diminish the harm caused by α-synuclein-related toxicity, primarily stemming from the buildup of α-synuclein in the cytoplasm, which leads to the repositioning of α-synuclein to the plasma membrane upon the application of NPs. | 544 |
| • CeO2NPs have demonstrated their ability to mitigate mitochondrial dysfunction induced by α-synuclein and reduce the levels of reactive oxygen species (ROS) in yeast cells. | |||
| • Through the adsorption of α-synuclein onto their surface, CeO2NPs effectively function as a stable inhibitor of α-synuclein's harmful effects rather than a radical scavenger suggested that CeO2NPs may interact co-ordinately with α-syn in vivo. | |||
| CeO2NPs | Citrate–EDTA stabilizations to CeO2NPs Murine model of ALS (SOD1G93A transgenic mice) | • The study found that administering 20 mg kg−1 of CeO2NPs intravenously twice per week increased the lifespan of SOD1G93A transgenic mice, a model for ALS. | 545 |
| • Even when treatment was initiated at the onset of muscle weakness, the mice survived longer. | |||
| • Both male and female mice benefited equally from CeO2NPs treatment suggests that CeO2NPs act as redox-agents that lower ROS levels, making them a promising therapeutic option. | |||
| Se-incorporated clioquinol compounds | Neuroblastoma | • Showed protective properties against the oxidation and aggregation of Aβ induced by Cu(II). | 251 |
| SeNPs | Transgenic Huntington Disease (HD) models of Caenorhabditis elegans | • Nano-Se has been found to reduce neuronal death in a dose-dependent manner and provide protection against neuronal dysfunction. | 546 |
| • In a HD model, treatment with Nano-Se resulted in decreased levels of ROS and HTT aggregation, indicating that Nano-Se functions as an antioxidant by regulating the expression of the histone deacetylase family and reducing polyQ aggregation. | |||
| OX26-PEG-Se NPs | To targeting the transferrin receptor. – in vivo | • Se nanoparticles coated with a polyethylene glycol (PEG) layer and treated with a monoclonal antibody (OX26) | 257 |
| • The injection of OX26-PEG-SeNPs into the peritoneal cavity reduced brain edema in Wistar rats with ischemic cerebral stroke. | |||
| Fe3O4NPs | Coated with (NIPAm-AA) and modified oleic acid/chronic Parkinson disease model | • Reduction of apoptosis and α-syn toxicity and showed neurorepair effect in vitro and in vivo models of PD. | 340 and 464 |
| AgNPs | NP–protein complex, protein-corona formation by incubating human plasma | • Exposure to AgNPs may activate pathways associated with neurotoxicity and neurodegeneration. | 547 |
| Triphenylphosphonium-conjugated ceria (TPP-ceria) | Hydrolytic sol–gel, metal chelators or DSPE-PEG coatings | The results indicated that these nanoparticles successfully decreased the buildup of mitochondrial ROS caused by Aβ in the U373 astrocyte cell line, implying that TPP-ceria NPs could potentially alleviate brain inflammation by lowering mitochondrial ROS levels. | 548 |
| Gd3N@C80 encapsulated NP | Colloidal stability in a polymer solution | • The nano-contrast agent for MRI is designed to possess colloidal stability, ROS-scavenging ability, and long-term circulation ability, making it an excellent choice for clinical diagnosis. | 549 |
| MSNP | Lipoprotein receptor (LDLR) (conjugating and PLA coating) | • The use of LDLR peptides significantly improved the transcytosis of MSNPs and facilitated their passage through the BBB. | 550 |
| • Additionally, PLA-coated MSNPs were able to release resveratrol (antioxidant) in response to artificially induced superoxide released by activated microglia. This released resveratrol effectively reduced oxidative stress. | |||
| PEG-b-poly[4-(2,2,6,6 tetra-methylpiperidine-1-oxyl)aminomethylstyrene] (PEG-b-PMNT) | 2,2,6,6-Tetramethylpiperidine-1-oxyl (TEMPO) added as side chain-oral intake to Alzheimer disease (AD) mice model | • When mice orally consumed these particles, they not only prevented the accumulation of Aβ, but also significantly improved both spatial and non-spatial memory. | 551 |
| • Additionally, the particles enhanced neuronal densities in the cortex and hippocampus by reducing the activity of glutathione peroxidase and superoxide dismutase. Furthermore, the levels of Aβ (1–40), Aβ (1–42) and gamma (γ)-secretase were reduced, ultimately leading to a reduction in Aβ plaque. | |||
| RuNPs | NGF loading, phase change material (PCM) presenting to AD mice | • The nanocomposites showed excellent choice for biosafety in AD treatment due to its good biocompatibility and high stability. | 535 |
| • With the aid of RuNPs' photothermal activity, cross-BBB movement and brain penetration was improved under NIR irradiation, leading to enhanced circulation efficiency in vivo. | |||
| • The researcher found that modified RuNPs had the ability to effectively inhibit hyperphosphorylation of tau, reduced oxidative stress, and reduced tau aggregation, restore nerve damage, and alleviate cognitive impairment in AD mice. | |||
| AuNPs | 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced rat models with PD. | • AuNPs can effectively reduce motor disorders, oxidative stress, and inflammatory cytokines, as well as inhibit TLR/NF-κB signalling in rats with PD. | 552 |
| • Additionally, the authors observed that MPTP administration in the brain increased ROS generation, while treatment with AuNPs was able to scavenge ROS in a dose-dependent manners. |
4.3. Infection and inflammation
Acute or chronic pathogenic infections by bacteria, viruses and parasites are a primary cause of human morbidity and mortality. During infection, overproduction of ROS can occur. This occurs most notably in infections caused by blood-borne viruses such as human immunodeficiency virus (HIV), hepatitis (B, C and D), influenza A, respiratory syncytial virus, Epstein-Barr virus and others.553 In liver diseases, production of ROS associated with transcriptional activation of some cytokines and growth factors leads to hepatic damage. Additionally, viral infections downregulate glutathione (GSH) in liver cells, leading to the oxidation of DNA, lipids and proteins, and the cascade of these events contributes to the development of liver cirrhosis and hepatocellular carcinoma.554 There are antioxidant strategies to react with ROS produced from the electron transport chain, including the production of non-enzymatic ROS scavengers [GSH and NAD(P)H] and antioxidant enzymes [superoxide dismutase, catalase and family members of peroxidase].555 However, temporary imbalance between antioxidants and ROS can alter cellular signalling pathways, which could be reversed by nanoparticles encapsulated with antioxidants to treat liver diseases.The versatile properties of nanoceria make it an ideal antibacterial solution against both Gram-negative and Gram-positive bacteria by generating ROS. It acts as an antioxidant in healthy cells, protecting them under normal physiological pH, while also acting as a pro-oxidant in cancer cells under low pH environments, which may lead to the death of cancer cells. Furthermore, nanoceria has been successfully utilized as a carrier for targeted drug and gene delivery in both in vitro and in vivo models. Besides, nanoceria is known to be a good candidate as an anti-diabetic, anti-inflammatory agent with protective effects against diabetes pathophysiology associated with a decrease in ROS concentration in diabetic patients. Nanoceria is also actively studied in the field of tissue engineering.556,557
ROS have two faces in normal cell responses, such that they are important in host defense, but paradoxically inhibit inflammation and immune response.558,559 In non-infectious hyper-inflammatory conditions such as chronic granulomatous disease (CGD), there are both high and low levels of ROS in the inflammatory responses.558 The lack of ROS generation by the NOX2 complex is known to be associated with a high incidence of autoimmune disease in CGD patients. A deficiency in NOX2 can lead to hyperinflammation and increased immune activation, which may have some benefits. In CGD mice, heightened inflammation has been shown to protect against pulmonary infection caused by influenza.404,560 The association between NOX2-dependent ROS generation deficiency and increased inflammation is unexpected and requires further investigation.
Oxidative stress arises when the production of reactive ROS surpasses the capacity of antioxidant defense mechanisms, potentially resulting in the development of various diseases. Hydrogen peroxide (H2O2) is a widely seen and long-lasting form of ROS that has been linked to various physiological processes including inflammation, cellular malfunction, and apoptosis. These factors ultimately have a role in the development of tissue and organ damage.561 Cellular DNA damage arises from a combination of intrinsic and extrinsic causes, encompassing ionizing radiation, chemical intake, light exposure, and radical generated during oxygen metabolism. The presence of these external stimuli leads to the production of radical, which in turn initiates a pathway that causes damage to DNA, resulting in a total of 465 instances of such damage.562 The phenomenon of DNA damage, commonly referred to as genotoxicity, is of great importance within the field of nanomaterials. Although the development of non-cytotoxic therapies is challenging, DNA damage offers a promising avenue for the creation of anti-cancer medications.563 ROS generation has been recognized as a crucial determinant in the modulation of the cellular response to chemotherapy or radiotherapy, exerting its influence on the downstream signalling pathways involved in cell survival and death.
The role of ROS in the DNA damage response is complicated and can be revealing in different ways. It is of utmost significance to distinguish between the occurrence of DNA damage resulting from oxidative stress and the following initiation of DNA damage repair (DDR). Additionally, it is crucial to consider the influence of ROS on the regulation of DDR components, encompassing both signalling and effectors. Multiple studies have indicated that the dysregulation of ROS plays a substantial role in the development of cancer, as well as in the resistance to chemotherapy and radiation therapy.564 The presence of active nanoparticles in the presence of ROS can result in the induction of DNA damage by the production of a hydroxyl radical (HO˙). This radical interacts with DNA molecules, leading to the formation of a specific DNA lesion called 8-hydroxy-2′-deoxyguanosine (8-OHdG). Ultimately, this process contributes to the occurrence of DNA damage.565 The excessive generation of radical can induce oxidative stress and inflammation, hence contributing to the development of numerous diseases.566 Certain nanoparticles, namely copper, copper oxide, iron, silver, TiO2, and nickel, exhibit pronounced toxicity. Even at low concentrations, exposure to these NPs can induce DNA damage and elicit the expression of inflammatory markers. These particles induce cellular apoptosis and inflammation through the generation of ROS, primarily targeting mitochondria and pro-oxidant enzymes.567 The utilization of various nanomaterials has been found to result in toxicity associated with the formation of ROS in multiple biological systems, such as human erythrocytes, skin fibroblasts, and various tumor cells.568 The mitochondria play a pivotal role in the production of ROS that are linked to nanoparticles. Previous studies have demonstrated that NPs have the capability to stimulate NADPH-associated enzymes, resulting in the depolarization of the mitochondrial membrane and the disruption of the electron transport chain.208,569 This inhibition of a key metabolic process would increase the quantity of ROS in cells by allowing electron transfer from respiratory carriers to oxygen.570 The mechanism implicated in NP-induced ROS production and factors affecting the ROS generation in cells are presented in Fig. 16.
There are several other diseases such as diabetes, osteoarthritis (OA), rheumatoid arthritis (RA), glaucoma, hearing loss, atherosclerosis, hypertension, restenosis, ischemia/reperfusion injury, and pulmonary and liver fibrosis that are highly affected by ROS. A potentially more favorable strategy for achieving therapeutic efficacy involves the targeted inhibition of enzymes responsible for the generation of reactive oxygen species. The occurrence of excessive formation of ROS is observed in several clinical states. Additionally, there exists a genetic factor that influences the likelihood of ROS generation, as supported by research.571
With over 170 million people worldwide affected, diabetes is a metabolic inflammatory disease that poses a significant threat to public health. Despite efforts to control the disease, its complications can only be slowed down rather than halted completely. The primary characteristic of diabetes is hyperglycemia, which initiates various metabolic signalling pathways that cause inflammation, cell death, and eventually lead to diabetic complications. The activation of diacylglycerol (DAG)–protein kinase C pathways by hyperglycemia is well-known to trigger a specific metabolic route. This signalling pathway is gaining more attention for its ability to regulate angiogenesis, reduce oxidative stress and prevent cellular death.572 It is widely believed that the disparity between the generation and removal of ROS and the resulting oxidative stress, induced by elevated blood glucose levels (hyperglycemia), is the primary factor contributing to the development of diabetic complications.573 In addition to hyperglycemia and ROS, Toll-like receptor 4 (TLR 4) activation can also trigger apoptosis. To control cardiac apoptosis in diabetes, researchers have found that silencing the TLR 4 gene or upregulating its expression in diabetic mice can be effective. This ultimately results in the reduction of oxidative stress and suppression of caspase 3 in cardiomyocytes.573 As a result, controlling ROS generation and TLR 4 downregulation may play a crucial role in managing diabetic complications.
Osteoarthritis is a degenerative joint condition that mostly impacts the geriatric population and is a significant contributor to functional impairment. The condition is distinguished by alterations in bone cells, resulting in softness, ulceration, fibrillation, degradation of articular cartilage, sclerosis of subchondral bone, inflammation of the synovial membrane, and the development of osteophytes and subchondral cysts. The mitochondria in OA produce an excessive amount of reactive oxygen species, leading to the oxidation of proteins. This oxidative process alters cell signalling pathways, favouring catabolic signalling, and ultimately contributing to cell death. A recent study has indicated that an elevated amount of ROS can interfere with the signalling pathways of IRS-1-PI-3 kinase-Akt, which is crucial for the integration of anabolic and catabolic signalling and the promotion of cell survival.574 The activation of Akt is hindered in OA chondrocytes and normal cells under oxidative stress, resulting in a decrease in matrix formation and an increase in vulnerability to cell death. Reactive oxygen species are mostly generated at minimal levels within articular chondrocytes, predominantly through the activity of NADPH oxidase. These entities play a significant role in the intracellular signalling processes that are involved in maintaining the equilibrium of cartilage. They achieve this by regulating many aspects such as chondrocyte death, gene expression, synthesis of the extracellular matrix (ECM), and cytokine production breakdown.575–577 Patients diagnosed with osteoarthritis have heightened levels of ROS generation and experience an increase in oxidative stress.578,579 The impact of interleukin-1 (IL-1) on DNA damage induced by ROS is significantly greater in cartilage afflicted by OA compared to healthy cartilage.580
Rheumatoid arthritis (RA) is a pathological condition characterized by the immune system's aberrant response, resulting in the degradation of several bodily structures, including joints, synovial tissue, cartilage, bone, and ligaments.581–583 HLA-DR-1 and HAL-DR-4 major histocompatibility complex (MHC) class II molecules exhibit a strong correlation with RA susceptibility in the human population. Additionally, DBA/1 and B10 strains have also been implicated in this association. The mice strains had I-Ag and I-Ar haplotypes, rendering them significantly vulnerable to collagen-induced arthritis (CIA), which serves as an experimental model for rheumatoid arthritis. The development of CIA in mice is associated with the expression of a limited T-cell receptor (TCR).584–586 The spontaneous development of arthritis in inbred mouse strains can be attributed to several factors, such as the expression of T cell receptor or TNF-α, as well as mutations in ZAP70 or Ncf1. There is a potential for these strains to have arthropathy, especially when exposed to specific environmental factors such as hormone imbalances, stress, or infections.587,588 There is a potential for these strains to have arthropathy, especially when exposed to specific environmental factors such as hormone imbalances, stress, or infections.589,590 Ncf1 is a constituent of the phagocyte NADPH oxidase complex, which plays a crucial role in modulating the magnitude of oxidative burst within phagocytes. Research has demonstrated that reduced levels of ROS generated by the NADPH oxidase complex have been found to exacerbate arthritis, inflammation, and immunological responses, contradicting commonly held beliefs.591
4.4. Tissue regeneration
Tissue regeneration is a dynamic process, which mainly includes cell migration and tissue reformation through morphogenesis followed by vigorous cell proliferation activity which commonly occurs at the traumatic or infected sites.592 There are complicated mechanisms underlying tissue regeneration including activation of specific cells or certain signalling molecules required for the effective recovery of injured tissues. Thus, it is important to understand intracellular and extracellular mechanisms with key mediators in tissue regeneration.593,594Radical have been implicated as an initiator of tissue damage in various diseases, such as skin aging, cardiovascular disease, chronic diabetic wounds and so on.592,595 Oxidative stress due to an imbalance of oxidative and antioxidative byproducts contributes to the development of the diseases mentioned above. On the other hand, free radical species can be beneficial, acting as a mediator in cellular signalling for tissue regeneration, in particular when there is a high demand for mitochondrial production of adenosine triphosphate (ATP) during tissue renewal.593 ROS act as secondary messenger-signalling molecules that induce a respiratory chain reaction via oxidation and reduction.348,593 There are various signalling pathways reported to be involved in the ROS-mediated tissue regeneration response, such as Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases (MAPK).596 ROS also play a role in regulating vasoconstriction and vasodilation, the important events in vascularization, an event that accompanies tissue regeneration.593 Nitric oxide, which belongs to reactive nitrogen species, reacts with a number of molecules including ROS to regulate a vascular relaxation.593 Essentially cells and molecules including macrophages, fibroblasts, platelets, keratinocytes and endothelial cells utilize these radicals to facilitate a healing response. Initial release of ROS activates vasoconstriction and reduces local cell signalling to enable thrombus formation. An antibacterial environment is then created after blood vessel-bound neutrophils are recruited to the injury site as shown in Fig. 17. Subsequently more ROS are released by phagocytosis to prevent the growth of bacteria and induce vital signals to other immunocytes in response to the pathogens at the wound site, including monocytes.593 These further stimulate endothelial cell division and migration for new ECM formation, which subsequently promotes keratinocyte proliferation and migration by activating angiogenesis and fibroblast division and migration.593
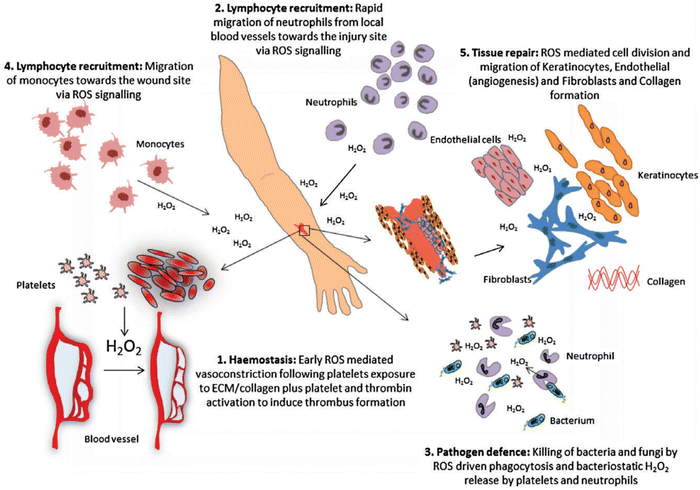 | ||
| Fig. 17 Schematic diagram illustrates the various role of ROS throughout severe wound healing conditions (i.e., homeostatic, level of ROS); (i) at early-stage, ROS help to reduce the blood flow and cell-signalling for thrombus formation, (ii) activated local neutrophils associated to blood vessels accumulate at the wound site for protection from bacteria, (iii) ROS produced by phagocytosis processes inhibit bacterial growth and help signal support for wound response, (iv) this helps to recruit immunocytes (monocytes) and help them to migrate towards to injury site to prevent attack invading pathogens, (v) at final stage, ROS stimulate endothelial cell differentiation and migration for blood vessel formation, and fibroblast differentiation and migration for new extracellular matrix formation and tissue regeneration. Reproduced with permission from Dunnill et al.593 [Copyright 2015, Wiley]. | ||
ROS responsive nanoparticles can play a vital role in responding to oxidative stress in a physiological environment. Under such conditions, the nanoparticles are targeted to the injury site as they exhibit either switchable solubility or the ability to degrade chemical bonds to create an oxidative microenvironment.596 Their functionalities are highly dependent on the types of ROS being targeted, material structure/characteristics, and exposure time.596 Wang et al. have reported a novel bioactive chitosan nanoparticle-loaded calcium alginate hydrogel for wound healing.597 Apart from inducing an antibacterial environment even at a very low dosage, the formulation also facilitated proliferation and migration of vascular endothelial cells (VEC) via massive production of ROS that subsequently assisted in metastasis and neovascularization creating an effective wound healing effect.597 Tang et al. have demonstrated a ROS-reactive nanomaterial poly-(1,4-phenyleneacetone dimethylene thioketal) loaded with stromal cell-derived factor-1α (SDF-1α). The loaded nanoparticle is reported able to respond to ROS by delivering drugs to inflammatory tissues accumulated with large amount of ROS, which subsequently attracts BMSCs, triggering vascularisation and healing.598 The role of RANs in various cellular functions including DNA damage and cytotoxicity are presented (Table 4).
| RANs | Application methods/surface modifications | Brief outcomes | Ref. |
|---|---|---|---|
| Fe3O4 and Ag NPs | Low-toxic concentration of deferoxamine (DFO) pre- treatment – applied in vitro HepG2 cells | • The toxicity of AgNPs was reduced by using iron as a chelator. | 565 |
| • AgNPs induced mitochondrial collapse, and O2˙− was produced and quickly dismutated to H2O2. | |||
| • In the presence of iron ions, H2O2 underwent a Fenton reaction, resulting in the production of an extremely reactive hydroxyl radical (˙OH). | |||
| • However, pre-treating AgNP-exposed cells with DFO significantly reduced the induction of DNA damage. | |||
| TiOxNPs and (PAA-TiOxNPs) | Polyacrylic acid-(PAA) modification | • In vitro, the absorption of PAA-TiOxNPs increased DNA damage and led to greater cytotoxicity when exposed to X-ray irradiation. | 599 |
| • In combination with X-ray treatment PAA-TiOxNPs had a significantly more powerful effect on inhibiting tumor growth than either treatment alone. | |||
| Polyvinylpyrrolidone (PVP) + iron oxide nanoparticles | S. aromaticum flower bud extract loading | • The application of nanoparticles to the MCF-7 breast cancer cell line has resulted in a notable increase in the accumulation within the sub-G1 phase, hence leading to the initiation of apoptosis. | 600 |
| • The augmentation in the quantity of nanoparticles resulted in a concomitant elevation in the enzymatic activity of caspase-3, caspase-8, and caspase-9. | |||
| • Besides, the introduction of functionalized iron nanoparticles (FeNPs) led to the initiation of oxidative stress, which subsequently triggered the creation of ROS and promoted death in MCF-7 cells. | |||
| • These findings underscore the promising prospects of using functionalized FeNPs in the field of cancer therapy. | |||
| Cu/CuONPs | Produced by chemical reductions | • The toxicity of Cu/CuONPs was assessed by evaluating their effects on human lung carcinoma cell line (A549) and human lung normal cell line (WI-38). | 601 |
| • The IC50 values of Cu/CuONPs were applied to both cell types, resulting in DNA damage and the generation of reactive oxygen species. | |||
| • The potential utility of investigating the toxicological impacts of Cu/CuONPs on mature A549 cells lies in the development of targeted therapeutic delivery systems for certain cancer cell types. | |||
| SiNPs | Suspended in DI and applied to murine neuroblastoma cell line: Neuro-2a | • The research conducted demonstrated that exposure to SiNPs resulted in a notable elevation of ROS levels within cellular structures. | 602 |
| • Nevertheless, prior administration of the antioxidant N-acetylcysteine (NAC) successfully mitigated the generation of ROS and the subsequent decline in cell viability, occurrence of apoptotic events, and activation of molecules associated with endoplasmic reticulum (ER) stress. | |||
| • The findings provide evidence that ROS plays a pivotal role in the harmful effects generated by silicon nanoparticles (SiNPs). | |||
| • This involvement of ROS leads to the activation of ER stress and subsequent initiation of apoptosis, finally culminating in the demise of neuronal cells. | |||
| ZnONPs | Dispersed into the culture media and up-taken by HEK-293 cells | • The concentration of ZnONPs has caused an excessive increase in ROS and O2 species, leading to impaired cellular oxidative stress management. | 603 |
| • The expression of TET-methylcytosine dioxygenase genes has significantly increased, while DNA-methyltransferases (DNMTs) expression remains unchanged. | |||
| • ZnONPs can cause a significant increase in ROS, resulting in various structural and functional abnormalities in cells. | |||
| CeO2NPs | Cellular internalization of nanoceria to the THP-1 cells | • The study found that nanoceria has powerful antioxidant properties, as shown by its ability to scavenge radical in mammalian cells. | 566 |
| • This suggests that nanoceria may be a promising treatment for conditions related to inflammation and oxidative stress. | |||
| AuNPs | Three types of 3–4 nm AuNPs on human A549 cells | • After 24 hours, cells were found to have agglomerated AuNP, but this did not affect cell viability or inflammation. | 604 |
| • Although no increase in ROS production was observed, however, intracellular glutathione levels decreased over time, indicating oxidative stress. | |||
| • Furthermore, all three types of AuNPs caused DNA damage, with the strongest genotoxic effect observed for positively charged AuNP I. | |||
| • However, despite the significant DNA damage caused by single exposure to AuNP I, A549 cells were able to recover and repair the damage over time. | |||
| TiO2NPs | Different concentration NPs orally presented to male rats | • The administration of escalating doses of TiO2NPs was observed to induce elevated levels of cytotoxicity, oxidative stress, and apoptosis in both Caco-2 and HepG2 cell lines. | 605 |
| • Additionally, it was shown that the inclusion of TiO2NPs resulted in a notable reduction in the activity of antioxidant enzymes and the expression of related genes, such as SOD, CAT, and GPx. | |||
| • Furthermore, the levels of glutathione (GSH) and the overall capacity for antioxidant activity (TAC) were also found to be diminished. | |||
| • The investigation revealed that the presence of TiO2NPs induced gene expressions associated with apoptosis and inflammatory pathways, along with caspase-3 activity in both the intestinal and hepatic tissues. | |||
| SeNPs | Hepatocellular carcinoma rat model (HCC) | • The co-administration of doxorubicin and SeNPs resulted in a notable decrease in chromosomal abnormalities, micronuclei formation, and DNA damage in male rats with hepatocellular carcinoma (HCC) when compared to the administration of doxorubicin alone. | 606 |
| AgNPs and AgNO3 | Nematode Caenorhabditis elegans | • The research demonstrated that the presence of NM300K AgNPs resulted in heightened production of ROSand elevated levels of oxidative stress, predominantly inside the intestinal lumen. | 607 |
| • To conduct a more comprehensive examination of the relative toxicity of these NPs in comparison to AgNO3, the research employed SOD-1 and two biosensors, namely HyPer and GRX. | |||
| • The findings of the study demonstrated that even at low concentrations, NM300K AgNPs induced cellular redox-potential instability and suggested the occurrence of oxidative stress. | |||
| • Furthermore, the data consistently demonstrated discernible variations in behavior between the two forms of Ag, namely in relation to uptake, retention, toxic effects, and patterns of oxidative stress. | |||
| Bi2O3NPs | In vitro human breast cancer (MCF-7) cells | • This study aimed to examine the impact of Bi2O3NPs on MCF-7 cells, with a specific focus on evaluating its cytotoxicity and induction of apoptosis. | 608 |
| • The findings indicated that the presence of Bi2O3NPs resulted in a decrease in cell viability and induced damage to the cell membrane, with the extent of these effects being directly proportional to the dosage administered, and cell cycle was observed, along with the induction of oxidative stress responses. | |||
| • This was demonstrated by the heightened production of ROS, elevated levels of lipid peroxidation, decreased GSH levels, and diminished activity of the SOD enzyme. | |||
| • Additionally, the investigation revealed that Bi2O3NPs triggered apoptosis via the mitochondrial route, as evidenced by a decrease in mitochondrial membrane potential (MMP) and an elevated expression ratio of bax/bcl-2 genes. | |||
| • In general, the results underscore the possible cytotoxic properties of Bi2O3NPs on MCF-7 cells. |
5. Conclusions and current and potential future clinical directions
The unique features of RANs such as intrinsic ROS production as well as their physicochemical properties such as particle shape, size, dimensions, surface chemical functionality, surface area, and imaging ability make them a promising nanoplatform for future application in biomedicine. The physical and chemical engineering of RANs has significantly changed their ability to generate ROS and provide control over pathological conditions. Furthermore, recent engineering of NPs has also helped to reduce their potential cytotoxicity, thereby improving the biosafety of NPs for therapeutic applications. Thanks to the advancement of nanoscience and nanotechnology, there are now many immense tools to synthesize various types of NPs and explore them as ROS-based therapeutic candidates in in vitro and in vivo applications.ROS is an essential biochemical component in various pathological conditions, ranging from tissue injury/damage, infection/inflammation, tissue regeneration, cancer, OA, and neurodegenerative disease. Furthermore, ROS also plays a crucial multifunctional role in cell biology including cell signalling, cell viability, proliferation, differentiation, and cell apoptosis. As a result, controlling and treating diseases by regulating the excess or deficiency of ROS in tissue/organ/body using RANs in the form of ROS generating or scavenging NPs, is possible. However, concerns about potential toxicities and the ability to target specific cells are significant. Some RANs may not be inherently biocompatible, and their introduction into the body could lead to adverse reactions such as inflammation, oxidative stress, or cellular damage. Moreover, some RANs release toxic ions or molecules during their redox-reactions, causing harm to cells or tissues. For example, certain metals used in nanoparticles formulations could pose toxicity risks. Additionally, if these nanoparticles are not effectively cleared from the body, they can accumulate in the organs such as the liver, spleen, kidneys, and leads to the potential toxicity over time. Furthermore, RANs face biological barriers, such as the blood brain or endothelial barriers in tumors, which can limit their ability to reach target cells or tissues. Therefore, precisely targeting RANs to specific cells is also a challenge for RANs.
Targeted application of redox-active nanoparticles in cells, tissues, or within the body requires advanced design and engineering to lower the energy barriers of ROS associated catalytic reactions within location specific conditions to regulate the favorable in vivo ROS-based therapeutic effect. However, these local in vivo physiological conditions (pH, temperature) can also trigger the ROS catalytic reactions by exciting the energy level of NPs, and thus can have severe consequences on health. Further exploration of the generation or scavenging of ROS by NPs and their associated cellular and molecular mechanisms is therefore required. A thorough understanding of these mechanisms will help significantly in efforts to engineer NPs with appropriate physicochemical functionalities.
Advancements in nanotechnology, biomaterials, and biomedical engineering are pushing towards overcoming challenges in medicine and unlocking the full potential of RANs. For instance, multifunctional nanoparticles adorned with targeting ligands and responsive moieties are making precision delivery of therapeutic agents to diseased tissues a reality. These strategies could enhance treatment efficacy while reducing the off-target effects and systemic toxicity. In future, developed RANs may respond to specific stimuli, prompting controlled drug release at the target site, which could improve treatment efficacy while minimizing side effects. Multi-therapeutic agent delivery is also promising, holding the potential to overcome drug resistance and improve treatment outcomes. Lastly, RANs with intrinsic fluorescence or luminescence properties could be used for minimally invasive in vivo bioimaging and biosensing, enabling early disease detection, monitoring, and personalized treatment approaches. Future redox-active nanomaterials will likely exhibit improved biodistribution, biodegradability, and clearance from the body, reducing the long-term accumulation and potential toxicity. Biocompatible degradation products could also be engineered to ensure safe elimination via renal or hepatobiliary pathways.
A common concern across the various nanomedicine modalities is their potential adverse effects on human health and safety. While RANs hold promises in preclinical studies for disease prevention and treatment, however their clinical applications remain limited, with research predominantly focused on in vitro studies and animal experiments. These models do not fully replicate human pathological conditions, particularly metastatic tumors, posing challenges in accurately mimicking the cellular or tissue damage caused by oxidative stress in physiological conditions.609 Moreover, the lack of clinical trial results is a major factor restricting the clinical translation of RANs. One of the key challenges includes inadequate methods for evaluating biocompatibility, which require a deeper understanding of toxicity mechanisms and enhanced in vivo cytotoxicity detection. This is mainly due to conventional antioxidants possess a double-edged sword effect.610 They suffer from low bioavailability due to poor gut absorption and nonspecific distribution, which reduces their local concentration and often leads to a failure in neutralizing excess free radicals.611 Conversely, high local concentrations can disrupt crucial ROS-mediated signalling pathways necessary for cell function and, in some cases, act as prooxidants, exacerbating oxidative damage.610,611 Additionally, they lack reusability, as they become inactive after neutralizing ROS through self-oxidation.611 Hence, to develop a comprehensive biocompatibility evaluation index, systemic toxicity testing must be performed, emphasizing the retention of nanomaterials in vital organs like the liver and kidneys, coupled with in vitro biosafety evaluations using human cell lines, which are also essential for advancing clinical trials.609
Advancements in nanotechnology offer various methods for modifying nanomaterials, such as using small molecules, polymers, antibodies, or nucleic acids, for targeted delivery by attaching cell-specific ligands that bind to receptors overexpressed in particular tissues.612 For example, nanomaterials capped with folate can precisely target cancer cells, which have higher folate receptor expression than normal cells, allowing for controlled local concentrations and maintaining cellular redox balance.611 Although nanomaterials may exhibit inherent properties such as ideal size, shape, hydrophobicity, rigidity, and composition, influencing biodistribution within the body, another important challenge is that redox-active nanodrugs, despite their longer-lasting effects and reduced side effects compared to traditional drugs, currently lack sufficient therapeutic efficacy for widespread clinical use, partly due to high design costs.609 These costs are associated with the complex structures of redox nanodrugs, especially those involved in multifunctional nanoplatform therapy systems. Additionally, RANs with strong permeability and retention effects often have low drug delivery efficiencies, highlighting the need for precise controlled release capabilities.609 While these nanoparticles show promise in diagnostics and bioassays, their therapeutic application faces challenges, including their use in topical or injectable solutions and implants. Some, like citrate-functionalized Mn3O4NPs, have shown success in preclinical trials, but others need further animal testing to confirm the efficacy observed in vitro.611 Injected nanoparticles must have minimal side effects and efficient excretion pathways, as certain materials like gold cannot be metabolized and must be excreted via urine or faeces.613 Their interaction with biological fluids and the need for precise targeting without causing toxicity are crucial considerations. Although simpler nanoparticle designs have paved the way for initial attempts, more complex structures pose significant translational challenges. Despite these complexities, progress has led to FDA-approved nanomedicines.614 However, more research on catalytic activity, therapeutic efficacy, and safety is needed to advance clinical applications effectively.611
In essence, addressing these challenges by selecting more cost-effective redox-responsive nanomaterials and improving synthesis methods can enhance their practicality and efficacy. Ultimately, it is crucial to understand comprehensively how these nanomaterials induce unintended effects on the immune and nervous systems. This necessitates a shift from descriptive toxicology to predictive toxicology.615 Given the diverse array of nanomaterial types and formulations, individual assessments for each are not feasible. Therefore, employing predictive toxicology models alongside high-content cellular screening emerges as a practical and promising approach to evaluate nanomaterial toxicity in humans, gradually making them applicable in clinical medicine.616
Overall, the field of redox-active nanomaterials in medicine will rely heavily on cross-disciplinary collaboration, inventive nanomaterial design, fabrication techniques, and thorough preclinical and clinical testing to guarantee both safety and effectiveness. With ongoing improvements in our comprehension of nanoscale phenomena and biological mechanisms, RANs are poised to transform personalized medicine in areas such as diagnostics tools, imaging technologies, drug administration, and therapeutic interventions.
Data availability
The data that support the finding of this review study are openly available and collected from the published research articles, review papers with permission of publishers.Conflicts of interest
The authors declare that they have no financial interests or personal conflict of interest.Acknowledgements
The authors would like to acknowledge the Engineering and Physical Science Research Council (EPSRC), the UK for empower program at University of Bristol under grant no. EP/T020792/1, and the National Research Foundation (NRF; 2021R1A5A2022318, RS-2024-00348908, and RS-2023-00220408) of Republic of Korea. The Biotechnology and Biological Science Research Council (BBSRC) London Interdisciplinary Bioscience PhD Consortium (LIDo; BB/M009513/1).References
- B. Perillo, M. Di Donato, A. Pezone, E. Di Zazzo, P. Giovannelli, G. Galasso, G. Castoria and A. Migliaccio, Exp. Mol. Med., 2020, 52, 192–203 CrossRef CAS PubMed.
- H. Sies and D. P. Jones, Nat. Rev. Mol. Cell Biol., 2020, 21, 363–383 CrossRef CAS PubMed.
- C.-S. Lee, R. K. Singh, H. S. Hwang, N.-H. Lee, A. G. Kurian, J.-H. Lee, H. S. Kim, M. Lee and H.-W. Kim, Prog. Mater. Sci., 2023, 135, 101087 CrossRef CAS.
- J. Bi, J. Zhang, Y. Ren, Z. Du, Q. Li, Y. Wang, S. Wei, L. Yang, J. Zhang, C. Liu, Y. Lv and R. Wu, Redox Biol., 2019, 20, 296–306 CrossRef CAS.
- F. Pagliari, C. Mandoli, G. Forte, E. Magnani, S. Pagliari, G. Nardone, S. Licoccia, M. Minieri, P. Di Nardo and E. Traversa, ACS Nano, 2012, 6, 3767–3775 CrossRef CAS.
- T. Zhou, E. R. Prather, D. E. Garrison and L. Zuo, Int. J. Mol. Sci., 2018, 19(2), 417 CrossRef PubMed.
- N. Solovyev, E. Drobyshev, G. Bjørklund, Y. Dubrovskii, R. Lysiuk and M. P. Rayman, Free Radicals Biol. Med., 2018, 127, 124–133 CrossRef CAS PubMed.
- M. P. Murphy, H. Bayir, V. Belousov, C. J. Chang, K. J. A. Davies, M. J. Davies, T. P. Dick, T. Finkel, H. J. Forman, Y. Janssen-Heininger, D. Gems, V. E. Kagan, B. Kalyanaraman, N.-G. Larsson, G. L. Milne, T. Nyström, H. E. Poulsen, R. Radi, H. Van Remmen, P. T. Schumacker, P. J. Thornalley, S. Toyokuni, C. C. Winterbourn, H. Yin and B. Halliwell, Nat. Metab., 2022, 4, 651–662 CrossRef PubMed.
- A. Marino, M. Battaglini, N. Moles and G. Ciofani, ACS Omega, 2022, 7, 25974–25990 CrossRef CAS.
- S. Mollazadeh, M. Mackiewicz and M. Yazdimamaghani, Mater. Sci. Eng., C, 2021, 118, 111536 CrossRef CAS PubMed.
- L. Han, X.-Y. Zhang, Y.-L. Wang, X. Li, X.-H. Yang, M. Huang, K. Hu, L.-H. Li and Y. Wei, J. Controlled Release, 2017, 259, 40–52 CrossRef CAS PubMed.
- Y. Liu, P. Bhattarai, Z. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 2053–2108 RSC.
- M. Hao, B. Chen, X. Zhao, N. Zhao and F.-J. Xu, Mater. Chem. Front., 2020, 4, 2571–2609 RSC.
- A. Moazzen, N. Öztinen, E. Ak-Sakalli and M. Koşar, Heliyon, 2022, 8, e10467 CrossRef CAS PubMed.
- Q. Guo, S. Ji, Q. Yue, L. Wang, J. Liu and J. Jia, Anal. Chem., 2009, 81, 5381–5389 CrossRef CAS PubMed.
- G. Goncalves, P. A. A. P. Marques, C. M. Granadeiro, H. I. S. Nogueira, M. K. Singh and J. Grácio, Chem. Mater., 2009, 21, 4796–4802 CrossRef CAS.
- J. Bai Aswathanarayan, R. Rai Vittal and U. Muddegowda, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 1444–1451 CrossRef CAS.
- S. Franco-Ulloa, D. Guarnieri, L. Riccardi, P. P. Pompa and M. De Vivo, J. Chem. Theory Comput., 2021, 17, 4512–4523 CrossRef CAS PubMed.
- Z. Nie, K. J. Liu, C.-J. Zhong, L.-F. Wang, Y. Yang, Q. Tian and Y. Liu, Free Radicals Biol. Med., 2007, 43, 1243–1254 CrossRef CAS PubMed.
- S. M. Ansar, S. Chakraborty and C. L. Kitchens, Nanomaterials, 2018, 8, 339 CrossRef.
- T. Yasukawa, H. Miyamura and S. Kobayashi, ACS Catal., 2016, 6, 7979–7988 CrossRef CAS.
- K. N. L. Hoang, S. M. McClain, S. M. Meyer, C. A. Jalomo, N. B. Forney and C. J. Murphy, Chem. Commun., 2022, 58, 9728–9741 RSC.
- P. Vardakas, I. A. Kartsonakis, I. D. Kyriazis, P. Kainourgios, A. F. A. Trompeta, C. A. Charitidis and D. Kouretas, Environ. Res., 2023, 220, 115156 CrossRef CAS PubMed.
- Y. González-García, E. R. López-Vargas, G. Cadenas-Pliego, A. Benavides-Mendoza, S. González-Morales, A. Robledo-Olivo, Á. G. Alpuche-Solís and A. Juárez-Maldonado, Int. J. Mol. Sci., 2019, 20, 5858 CrossRef.
- K. D. Patel, R. K. Singh and H.-W. Kim, Mater. Horiz., 2019, 6, 434–469 RSC.
- I. L. Medintz, H. T. Uyeda, E. R. Goldman and H. Mattoussi, Nat. Mater., 2005, 4, 435–446 CrossRef CAS.
- C. M. Sims, S. K. Hanna, D. A. Heller, C. P. Horoszko, M. E. Johnson, A. R. Montoro Bustos, V. Reipa, K. R. Riley and B. C. Nelson, Nanoscale, 2017, 9, 15226–15251 RSC.
- B. Yang, Y. Chen and J. Shi, Chem. Rev., 2019, 119, 4881–4985 CrossRef CAS PubMed.
- Y. Nosaka and A. Y. Nosaka, Chem. Rev., 2017, 117, 11302–11336 CrossRef CAS.
- X. Shi, Y. Tian, S. Zhai, Y. Liu, S. Chu and Z. Xiong, Front. Chem., 2023, 11, 1115440 CrossRef CAS PubMed.
- P. Chen, W. Zhang, X. Fan, X. Shi, Y. Jiang, L. Yan, H. Li, C. Wang, L. Han, X. Lu and C. Ou, Nano Today, 2024, 55, 102157 CrossRef CAS.
- P. H. Hoet, B. Nemery and D. Napierska, Nano Today, 2013, 8, 223–227 CrossRef CAS.
- H. Sies, Redox Biol., 2015, 4, 180–183 CrossRef CAS.
- A. Costa, A. Scholer-Dahirel and F. Mechta-Grigoriou, Semin. Cancer Biol., 2014, 25, 23–32 CrossRef CAS PubMed.
- S. H. Yoo, H.-W. Kim and J. H. Lee, J. Tissue Eng., 2022, 13, 1–14, DOI:10.1177/20417314221083414.
- Y. Vakrat-Haglili, L. Weiner, V. Brumfeld, A. Brandis, Y. Salomon, B. McLlroy, B. C. Wilson, A. Pawlak, M. Rozanowska, T. Sarna and A. Scherz, J. Am. Chem. Soc., 2005, 127, 6487–6497 CrossRef CAS.
- M. Wang, M. Chang, C. Li, Q. Chen, Z. Hou, B. Xing and J. Lin, Adv. Mater., 2022, 34, 2106010 CrossRef CAS.
- Y. Lin, X. Chen, C. Yu, G. Xu, X. Nie, Y. Cheng, Y. Luan and Q. Song, Acta Biomater., 2023, 159, 300–311 CrossRef CAS PubMed.
- M. Sharifi-Rad, N. V. Anil Kumar, P. Zucca, E. M. Varoni, L. Dini, E. Panzarini, J. Rajkovic, P. V. Tsouh Fokou, E. Azzini, I. Peluso, A. Prakash Mishra, M. Nigam, Y. El Rayess, M. E. Beyrouthy, L. Polito, M. Iriti, N. Martins, M. Martorell, A. O. Docea, W. N. Setzer, D. Calina, W. C. Cho and J. Sharifi-Rad, Front. Physiol., 2020, 11, 694 CrossRef.
- E. Birben, U. M. Sahiner, C. Sackesen, S. Erzurum and O. Kalayci, World Allergy Organ. J., 2012, 5, 9–19 CrossRef CAS.
- C. M. C. Andrés, J. M. Pérez de la Lastra, C. Andrés Juan, F. J. Plou and E. Pérez-Lebeña, Int. J. Mol. Sci., 2023, 24, 1841 CrossRef PubMed.
- J. Fujii, T. Homma and T. Osaki, Antioxidants, 2022, 11, 501 CrossRef CAS.
- B. Lipinski and E. Pretorius, Hematology, 2012, 17, 241–247 CrossRef CAS PubMed.
- B. Lipinski, Oxid. Med. Cell. Longevity, 2011, 2011, 809696 Search PubMed.
- S. Adhikari, R. Crehuet, J. M. Anglada, J. S. Francisco and Y. Xia, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 18216–18223 CrossRef CAS.
- P. Chaudhary, P. Janmeda, A. O. Docea, B. Yeskaliyeva, A. F. Abdull Razis, B. Modu, D. Calina and J. Sharifi-Rad, Front. Chem., 2023, 11, 1158198 CrossRef CAS.
- L. M. Smith, H. M. Aitken and M. L. Coote, Acc. Chem. Res., 2018, 51, 2006–2013 CrossRef CAS.
- J. Helberg and D. A. Pratt, Chem. Soc. Rev., 2021, 50, 7343–7358 RSC.
- C. von Sonntag, in Mechanisms of DNA Damage and Repair: Implications for Carcinogenesis and Risk Assessment, ed. M. G. Simic, L. Grossman, A. C. Upton and D. S. Bergtold, Springer, US, Boston, MA, 1986, pp. 51–59 DOI:10.1007/978-1-4615-9462-8_6.
- K. Jomova, R. Raptova, S. Y. Alomar, S. H. Alwasel, E. Nepovimova, K. Kuca and M. Valko, Arch. Toxicol., 2023, 97, 2499–2574 CrossRef CAS PubMed.
- K. Wang, W. Mao, X. Song, M. Chen, W. Feng, B. Peng and Y. Chen, Chem. Soc. Rev., 2023, 52, 6957–7035 RSC.
- V. Nayak, K. R. B. Singh, A. K. Singh and R. P. Singh, New J. Chem., 2021, 45, 2849–2878 RSC.
- F. Collin, Int. J. Mol. Sci., 2019, 20(10), 2407 CrossRef PubMed.
- D. D. Saraev, Z. Wu, H.-Y. H. Kim, N. A. Porter and D. A. Pratt, ACS Chem. Biol., 2023, 18, 2073–2081 CrossRef CAS.
- L. K. Folkes, S. Bartesaghi, M. Trujillo, P. Wardman and R. Radi, Int. J. Mol. Sci., 2022, 23, 1797 CrossRef CAS.
- E. Illés, A. Mizrahi, V. Marks and D. Meyerstein, Free Radicals Biol. Med., 2019, 131, 1–6 CrossRef PubMed.
- R. Luc and C. Vergely, Int. J. Biomed. Sci., 2008, 4, 255–259 CrossRef CAS.
- A. M. Fleming and C. J. Burrows, Chem. Soc. Rev., 2020, 49, 6524–6528 RSC.
- R. Xiao, Y. Meng, Y. Fu, S. Wacławek, Z. Wei, R. Spinney, D. D. Dionysiou, W. Zeng and W. P. Hu, Chem. Eng. J., 2023, 474, 145245 CrossRef CAS.
- L. Wojnárovits, T. Tóth and E. Takács, Sci. Total Environ., 2020, 717, 137219 CrossRef.
- S. M. Andrabi, N. S. Sharma, A. Karan, S. M. S. Shahriar, B. Cordon, B. Ma and J. Xie, Adv. Sci., 2023, 10, 2303259 CrossRef CAS.
- M. K. Chug and E. J. Brisbois, ACS Mater. Au, 2022, 2, 525–551 CrossRef CAS.
- Y. Deng, F. Jia, S. Chen, Z. Shen, Q. Jin, G. Fu and J. Ji, Biomaterials, 2018, 187, 55–65 CrossRef CAS PubMed.
- E. Lubos, D. E. Handy and J. Loscalzo, Front. Biosci.-Landmark, 2008, 13, 5323–5344 CrossRef CAS PubMed.
- C. Schöneich, Molecules, 2019, 24(23), 4357 CrossRef.
- Y. M. Lee, W. He and Y.-C. Liou, Cell Death Dis., 2021, 12, 58 CrossRef CAS PubMed.
- T. Nauser, W. H. Koppenol and C. Schöneich, Free Radicals Biol. Med., 2015, 80, 158–163 CrossRef CAS.
- D. M. Lynch and E. M. Scanlan, Molecules, 2020, 25, 3094 CrossRef CAS.
- M. Janků, L. Luhová and M. Petřivalský, Antioxidants, 2019, 8, 105 CrossRef.
- T. Kietzmann, A. Petry, A. Shvetsova, J. M. Gerhold and A. Görlach, Br. J. Pharmacol., 2017, 174, 1533–1554 CrossRef CAS PubMed.
- B. Chance, H. Sies and A. Boveris, Physiol. Rev., 1979, 59, 527–605 CrossRef CAS.
- J. F. Turrens, J. Physiol., 2003, 552, 335–344 CrossRef CAS PubMed.
- R. Z. Zhao, S. Jiang, L. Zhang and Z. B. Yu, Int. J. Mol. Med., 2019, 44, 3–15 CAS.
- H. Y. Chung, G. S. Lee, S. H. Nam, J. H. Lee, J. P. Han, S. Song, G.-D. Kim, C. Jung, D. Y. Hyeon, D. Hwang, B.-O. Choi and S. C. Yeom, Brain, 2024, 147, 2114–2127 CrossRef.
- S. Lenzen, V. I. Lushchak and F. Scholz, Arch. Toxicol., 2022, 96, 1915–1920 CrossRef CAS.
- J. P. Crow and J. S. Beckman, in The Role of Nitric Oxide in Physiology and Pathophysiology, ed. H. Koprowski and H. Maeda, Springer Berlin Heidelberg, Berlin, Heidelberg, 1995, pp. 57–73 DOI:10.1007/978-3-642-79130-7_7.
- R. Radi, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 5839–5848 CrossRef CAS.
- C. M. C. Andrés, J. M. Pérez de la Lastra, C. Andrés Juan, F. J. Plou and E. Pérez-Lebeña, Int. J. Mol. Sci., 2023, 24, 1841 CrossRef PubMed.
- R. Campos Chisté, M. Freitas, A. Zerlotti Mercadante and E. Fernandes, Curr. Med. Chem., 2015, 22, 4234–4256 CrossRef.
- J. H. Kramer, B. F. Dickens, V. Mišík and W. B. Weglicki, J. Mol. Cell. Cardiol., 1995, 27, 371–381 CrossRef CAS.
- N. A. Popova, G. M. Sysoeva, V. P. Nikolin, V. I. Kaledin, E. V. Tretyakov, M. V. Edeeva, S. M. Balakhnin, E. L. Lushnikova, G. Audran and S. Mark, Bull. Exp. Biol. Med., 2017, 164, 49–53 CrossRef CAS PubMed.
- D. S. Dimić, D. A. Milenković, E. H. Avdović, J. Nakarada, J. M. Dimitrić Marković and Z. S. Marković, Chem. Eng. J., 2021, 424, 130331 CrossRef.
- Z. Hao, J. Ma, C. Miao, Y. Song, L. Lian, S. Yan and W. Song, Environ. Sci. Technol., 2020, 54, 10118–10127 CrossRef CAS PubMed.
- D. B. Medinas, G. Cerchiaro, D. F. Trindade and O. Augusto, IUBMB Life, 2007, 59, 255–262 CrossRef CAS PubMed.
- B. N. Gantner, K. M. LaFond and M. G. Bonini, Redox Biol., 2020, 34, 101550 CrossRef CAS.
- Y. Liu, G. Fiskum and D. Schubert, J. Neurochem., 2002, 80, 780–787 CrossRef CAS PubMed.
- S. Chenna, W. J. H. Koopman, J. H. M. Prehn and N. M. C. Connolly, Am. J. Physiol.: Cell Physiol., 2022, 323, C69–C83 CrossRef CAS PubMed.
- Z. Yang and D. J. Klionsky, Curr. Opin. Cell Biol., 2010, 22, 124–131 CrossRef CAS.
- V. P. Tan and S. Miyamoto, J. Mol. Cell. Cardiol., 2016, 95, 31–41 CrossRef CAS.
- C. C. Dibble and B. D. Manning, Nat. Cell Biol., 2013, 15, 555–564 CrossRef CAS.
- G. Filomeni, D. De Zio and F. Cecconi, Cell Death Differ., 2015, 22, 377–388 CrossRef CAS PubMed.
- G. Filomeni, D. De Zio and F. Cecconi, Cell Death Differ., 2015, 22, 377–388 CrossRef CAS.
- B. Groitl and U. Jakob, Biochim. Biophys. Acta, Proteins Proteomics, 2014, 1844, 1335–1343 CrossRef CAS.
- T. M. Buttke and P. A. Sandstrom, Immunol. Today, 1994, 15, 7–10 CrossRef CAS PubMed.
- A. Nugud, D. Sandeep and A. T. El-Serafi, J. Adv. Res., 2018, 14, 73–79 CrossRef CAS PubMed.
- Z. Ghanbari Movahed, M. Rastegari-Pouyani, M. H. Mohammadi and K. Mansouri, Biomed. Pharmacother., 2019, 112, 108690 CrossRef CAS.
- R. Bhowmick and R. R. Sarkar, PLoS One, 2020, 15, e0235204 CrossRef CAS PubMed.
- C. E. Murry, M. H. Soonpaa, H. Reinecke, H. Nakajima, H. O. Nakajima, M. Rubart, K. B. S. Pasumarthi, J. Ismail Virag, S. H. Bartelmez, V. Poppa, G. Bradford, J. D. Dowell, D. A. Williams and L. J. Field, Nature, 2004, 428, 664–668 CrossRef CAS PubMed.
- R. D. Unwin, D. L. Smith, D. Blinco, C. L. Wilson, C. J. Miller, C. A. Evans, E. Jaworska, S. A. Baldwin, K. Barnes and A. Pierce, Blood, 2006, 107, 4687–4694 CrossRef CAS PubMed.
- J. M. Funes, M. Quintero, S. Henderson, D. Martinez, U. Qureshi, C. Westwood, M. O. Clements, D. Bourboulia, R. B. Pedley and S. Moncada, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 6223–6228 CrossRef CAS.
- J.-H. Paik, Z. Ding, R. Narurkar, S. Ramkissoon, F. Muller, W. S. Kamoun, S.-S. Chae, H. Zheng, H. Ying and J. Mahoney, Cell Stem Cell, 2009, 5, 540–553 CrossRef CAS PubMed.
- F. Kocabas, J. Zheng, S. Thet, N. G. Copeland, N. A. Jenkins, R. J. DeBerardinis, C. Zhang and H. A. Sadek, Blood, 2012, 120, 4963–4972 CrossRef CAS.
- T. Suda, K. Takubo and G. L. Semenza, Cell Stem Cell, 2011, 9, 298–310 CrossRef CAS PubMed.
- J. M. Ryu, H. J. Lee, Y. H. Jung, K. H. Lee, D. I. Kim, J. Y. Kim, S. H. Ko, G. E. Choi, I. I. Chai, E. J. Song, J. Y. Oh, S.-J. Lee and H. J. Han, Int. J. Stem Cells, 2015, 8, 24–35 CrossRef CAS PubMed.
- M. L. Circu and T. Y. Aw, Free Radical Biol. Med., 2010, 48, 749–762 CrossRef CAS.
- S. V. Avery, Biochem. J., 2011, 434, 201–210 CrossRef CAS PubMed.
- P. Storz, Front. Biosci., 2005, 10, 1881–1896 CrossRef CAS PubMed.
- D. Trachootham, Y. Zhou, H. Zhang, Y. Demizu, Z. Chen, H. Pelicano, P. J. Chiao, G. Achanta, R. B. Arlinghaus, J. Liu and P. Huang, Cancer Cell, 2006, 10, 241–252 CrossRef CAS PubMed.
- M. Azmanova and A. Pitto-Barry, ChemBioChem, 2022, 23, e202100641 CrossRef CAS.
- E. Panieri and M. M. Santoro, Cell Death Dis., 2016, 7, e2253–e2253 CrossRef CAS.
- R. K. Singh, K. D. Patel, C. Mahapatra, S. P. Parthiban, T.-H. Kim and H.-W. Kim, ACS Appl. Mater. Interfaces, 2019, 11, 288–299 CrossRef CAS.
- G. Y. Liou and P. Storz, Free Radical Res., 2010, 44, 479–496 CrossRef CAS.
- P. Storz, in Oxidative Stress and Redox Regulation, ed. U. Jakob and D. Reichmann, Springer, Netherlands, Dordrecht, 2013, pp. 427–447 DOI:10.1007/978-94-007-5787-5_15.
- S. Shibuya, Y. Ozawa, K. Watanabe, N. Izuo, T. Toda, K. Yokote and T. Shimizu, PLoS One, 2014, 9, e109288 CrossRef PubMed.
- D. Yang, S. G. Elner, Z.-M. Bian, G. O. Till, H. R. Petty and V. M. Elner, Exp. Eye Res., 2007, 85, 462–472 CrossRef CAS.
- J. Krstic, D. Trivanovic, S. Mojsilovic and J. F. Santibanez, Oxid. Med. Cell. Longevity, 2015, 2015, 654594 Search PubMed.
- L. E. Huang, J. Gu, M. Schau and H. F. Bunn, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 7987–7992 CrossRef CAS PubMed.
- M. R. Abid, K. C. Spokes, S.-C. Shih and W. C. Aird, J. Biol. Chem., 2007, 282, 35373–35385 CrossRef CAS PubMed.
- Y. Higaki, T. Mikami, N. Fujii, M. F. Hirshman, K. Koyama, T. Seino, K. Tanaka and L. J. Goodyear, Am. J. Physiol.: Endocrinol. Metab., 2008, 294, E889–E897 CrossRef CAS.
- T. Minamoto, M. Mai and Z. Ronai, Cancer Detect. Prev., 2000, 24, 1–12 CAS.
- T. Minamoto, A. V. Ougolkov and M. Mai, Expert Rev. Mol. Diagn., 2002, 2, 565–575 CrossRef CAS.
- D. Ferraro, S. Corso, E. Fasano, E. Panieri, R. Santangelo, S. Borrello, S. Giordano, G. Pani and T. Galeotti, Oncogene, 2006, 25, 3689–3698 CrossRef CAS.
- V. Chavda, B. Chaurasia, K. Garg, H. Deora, G. E. Umana, P. Palmisciano, G. Scalia and B. Lu, Brain Disord., 2022, 5, 100029 CrossRef CAS.
- B. S. Berlett and E. R. Stadtman, J. Biol. Chem., 1997, 272, 20313–20316 CrossRef CAS.
- M. Mittal, M. R. Siddiqui, K. Tran, S. P. Reddy and A. B. Malik, Antioxid. Redox Signaling, 2014, 20, 1126–1167 CrossRef CAS.
- S. Salzano, P. Checconi, E.-M. Hanschmann, C. H. Lillig, L. D. Bowler, P. Chan, D. Vaudry, M. Mengozzi, L. Coppo, S. Sacre, K. R. Atkuri, B. Sahaf, L. A. Herzenberg, L. A. Herzenberg, L. Mullen and P. Ghezzi, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 12157–12162 CrossRef CAS.
- M. Schäfer and S. Werner, Pharmacol. Res., 2008, 58, 165–171 CrossRef.
- I. Liguori, G. Russo, F. Curcio, G. Bulli, L. Aran, D. Della-Morte, G. Gargiulo, G. Testa, F. Cacciatore, D. Bonaduce and P. Abete, Clin. Interventions Aging, 2018, 13, 757–772 CrossRef CAS.
- M. Cano Sanchez, S. Lancel, E. Boulanger and R. Neviere, Antioxidants, 2018, 7(8), 98 CrossRef PubMed.
- R. Moseley, J. E. Stewart, P. Stephens, R. J. Waddington and D. W. Thomas, Br. J. Dermatol., 2004, 150, 401–413 CrossRef CAS.
- C. Caspersen, N. Wang, J. Yao, A. Sosunov, X. Chen, J. W. Lustbader, H. W. Xu, D. Stern, G. McKhann and S. D. Yan, FASEB J., 2005, 19, 2040–2041 CrossRef CAS PubMed.
- C. S. Casley, L. Canevari, J. M. Land, J. B. Clark and M. A. Sharpe, J. Neurochem., 2002, 80, 91–100 CrossRef CAS PubMed.
- L. S. Forno, J. Neuropathol. Exp. Neurol., 1996, 55, 259–272 CrossRef CAS PubMed.
- M. G. Spillantini, R. A. Crowther, R. Jakes, M. Hasegawa and M. Goedert, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 6469–6473 CrossRef CAS PubMed.
- E. M. Valente, P. M. Abou-Sleiman, V. Caputo, M. M. K. Muqit, K. Harvey, S. Gispert, Z. Ali, D. Del Turco, A. R. Bentivoglio, D. G. Healy, A. Albanese, R. Nussbaum, R. González-Maldonado, T. Deller, S. Salvi, P. Cortelli, W. P. Gilks, D. S. Latchman, R. J. Harvey, B. Dallapiccola, G. Auburger and N. W. Wood, Science, 2004, 304, 1158–1160 CrossRef CAS PubMed.
- W. C. Nichols, N. Pankratz, D. Hernandez, C. Paisán-Ruíz, S. Jain, C. A. Halter, V. E. Michaels, T. Reed, A. Rudolph, C. W. Shults, A. Singleton and T. Foroud, Lancet, 2005, 365, 410–412 CAS.
- B. Xiao, J.-Y. Goh, L. Xiao, H. Xian, K.-L. Lim and Y.-C. Liou, J. Biol. Chem., 2017, 292, 16697–16708 CrossRef CAS.
- W. J. Szlachcic, P. M. Switonski, W. J. Krzyzosiak, M. Figlerowicz and M. Figiel, Dis. Models Mech., 2015, 8, 1047 CAS.
- F. O. Walker, Lancet, 2007, 369, 218–228 CrossRef CAS PubMed.
- B. Rotblat, A. L. Southwell, D. E. Ehrnhoefer, N. H. Skotte, M. Metzler, S. Franciosi, G. Leprivier, S. P. Somasekharan, A. Barokas, Y. Deng, T. Tang, J. Mathers, N. Cetinbas, M. Daugaard, B. Kwok, L. Li, C. J. Carnie, D. Fink, R. Nitsch, J. D. Galpin, C. A. Ahern, G. Melino, J. M. Penninger, M. R. Hayden and P. H. Sorensen, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 3032–3037 CrossRef CAS.
- A. Johri and M. F. Beal, Biochim. Biophys. Acta, Mol. Basis Dis., 2012, 1822, 664–674 CrossRef CAS PubMed.
- C. Giorgi, S. Marchi, I. C. M. Simoes, Z. Ren, G. Morciano, M. Perrone, P. Patalas-Krawczyk, S. Borchard, P. Jędrak, K. Pierzynowska, J. Szymański, D. Q. Wang, P. Portincasa, G. Węgrzyn, H. Zischka, P. Dobrzyn, M. Bonora, J. Duszynski, A. Rimessi, A. Karkucinska-Wieckowska, A. Dobrzyn, G. Szabadkai, B. Zavan, P. J. Oliveira, V. A. Sardao, P. Pinton and M. R. Wieckowski, Int. Rev. Cell Mol. Biol., 2018, 340, 209–344 CAS.
- A. N. Kolodkin, R. P. Sharma, A. M. Colangelo, A. Ignatenko, F. Martorana, D. Jennen, J. J. Briedé, N. Brady, M. Barberis, T. D. G. A. Mondeel, M. Papa, V. Kumar, B. Peters, A. Skupin, L. Alberghina, R. Balling and H. V. Westerhoff, NPJ Syst. Biol. Appl., 2020, 6, 34 CrossRef PubMed.
- G.-Y. Liou and P. Storz, Free Radical Res., 2010, 44, 479–496 CrossRef CAS PubMed.
- M. I. Anik, N. Mahmud, A. A. Masud, M. I. Khan, M. N. Islam, S. Uddin and M. K. Hossain, ACS Appl. Bio Mater., 2022, 5, 4028–4054 CrossRef CAS.
- J. M. Lü, P. H. Lin, Q. Yao and C. Chen, J. Cell. Mol. Med., 2010, 14, 840–860 CrossRef.
- L. W. Zheng, H. Chung and Y.-S. Kim, Food Res. Int., 2015, 75, 328–336 CrossRef CAS PubMed.
- K. U. Ingold and D. A. Pratt, Chem. Rev., 2014, 114, 9022–9046 CrossRef CAS PubMed.
- N. R. Perron and J. L. Brumaghim, Cell Biochem. Biophys., 2009, 53, 75–100 CrossRef CAS PubMed.
- J. Lu and A. Holmgren, Free Radicals Biol. Med., 2014, 66, 75–87 CrossRef CAS.
- M. D. Brand, C. Affourtit, T. C. Esteves, K. Green, A. J. Lambert, S. Miwa, J. L. Pakay and N. Parker, Free Radical Biol. Med., 2004, 37, 755–767 CrossRef CAS.
- R. Amorati and L. Valgimigli, Free Radical Res., 2015, 49, 633–649 CrossRef CAS.
- L. Valgimigli, A. Baschieri and R. Amorati, J. Mater. Chem. B, 2018, 6, 2036–2051 RSC.
- A. Aranda, L. Sequedo, L. Tolosa, G. Quintas, E. Burello, J. V. Castell and L. Gombau, Toxicol. In Vitro, 2013, 27, 954–963 CrossRef CAS PubMed.
- D. Yu, Y. Zha, Z. Zhong, Y. Ruan, Z. Li, L. Sun and S. Hou, Sens. Actuators, B, 2021, 339, 129878 CrossRef CAS.
- A. Kessler, P. Huang, E. Blomberg and I. Odnevall, Chem. Res. Toxicol., 2023, 36, 1891–1900 Search PubMed.
- T. M. Potter, B. W. Neun and S. T. Stern, in Characterization of Nanoparticles Intended for Drug Delivery, ed. S. E. McNeil, Humana Press, Totowa, NJ, 2011, pp. 181–189 DOI:10.1007/978-1-60327-198-1_19.
- S. Sadeghian, G. A. Kojouri and A. Mohebbi, Biol. Trace Elem. Res., 2012, 146, 302–308 CrossRef CAS PubMed.
- W. Zhang, J. Gao, L. Lu, T. Bold, X. Li, S. Wang, Z. Chang, J. Chen, X. Kong, Y. Zheng, M. Zhang and J. Tang, NanoImpact, 2021, 23, 100338 CrossRef CAS.
- C. Jiang, C. Zhang, J. Song, X. Ji and W. Wang, Spectrochim. Acta, Part A, 2021, 250, 119316 CrossRef CAS.
- Y. Jv, B. Li and R. Cao, Chem. Commun., 2010, 46, 8017–8019 RSC.
- W. He, X. Han, H. Jia, J. Cai, Y. Zhou and Z. Zheng, Sci. Rep., 2017, 7, 40103 CrossRef CAS PubMed.
- S.-B. He, L. Yang, X.-L. Lin, L.-M. Chen, H.-P. Peng, H.-H. Deng, X.-H. Xia and W. Chen, Talanta, 2020, 211, 120707 CrossRef CAS.
- G.-J. Cao, X. Jiang, H. Zhang, T. R. Croley and J.-J. Yin, RSC Adv., 2017, 7, 52210–52217 RSC.
- S. V. Otari, M. Kumar, M. Z. Anwar, N. D. Thorat, S. K. S. Patel, D. Lee, J. H. Lee, J.-K. Lee, Y. C. Kang and L. Zhang, Sci. Rep., 2017, 7, 10980 CrossRef.
- A. K. Keshari, R. Srivastava, P. Singh, V. B. Yadav and G. Nath, J. Ayurveda Integr. Med., 2020, 11, 37–44 CrossRef PubMed.
- R. Prasad, J. Nanopart., 2014, 2014, 1–8 CrossRef.
- P. V. AshaRani, G. Low Kah Mun, M. P. Hande and S. Valiyaveettil, ACS Nano, 2009, 3, 279–290 CrossRef CAS.
- A. Manke, L. Wang and Y. Rojanasakul, BioMed Res. Int., 2013, 2013, 942916 Search PubMed.
- K. D. Patel, A. K. Patel, P. Sawadkar, B. Singh and A. W. Perriman, in Multifunctional And Targeted Theranostic Nanomedicines: Formulation, Design And Applications, ed. K. Jain and N. K. Jain, Springer Nature, Singapore, 2023, pp. 97–117 DOI:10.1007/978-981-99-0538-6_5.
- A. Onodera, F. Nishiumi, K. Kakiguchi, A. Tanaka, N. Tanabe, A. Honma, K. Yayama, Y. Yoshioka, K. Nakahira, S. Yonemura, I. Yanagihara, Y. Tsutsumi and Y. Kawai, Toxicol. Rep., 2015, 2, 574–579 CrossRef CAS.
- C. Carlson, S. M. Hussain, A. M. Schrand, L. K. Braydich-Stolle, K. L. Hess, R. L. Jones and J. J. Schlager, J. Phys. Chem. B, 2008, 112, 13608–13619 CrossRef CAS PubMed.
- M. A. Elfaky, A. Sirwi, S. H. Ismail, H. H. Awad and S. S. Gad, Curr. Issues Mol. Biol., 2022, 44, 2923–2938 CrossRef CAS.
- B. Dąbrowska-Bouta, G. Sulkowski, W. Strużyński and L. Strużyńska, Neurotoxic. Res., 2019, 35, 495–504 CrossRef.
- Y. Qing, L. Cheng, R. Li, G. Liu, Y. Zhang, X. Tang, J. Wang, H. Liu and Y. Qin, Int. J. Nanomed., 2018, 13, 3311–3327 CrossRef CAS.
- Y.-H. Hsin, C.-F. Chen, S. Huang, T.-S. Shih, P.-S. Lai and P. J. Chueh, Toxicol. Lett., 2008, 179, 130–139 CrossRef CAS PubMed.
- M. J. Piao, K. A. Kang, I. K. Lee, H. S. Kim, S. Kim, J. Y. Choi, J. Choi and J. W. Hyun, Toxicol. Lett., 2011, 201, 92–100 CrossRef CAS.
- D. He, A. M. Jones, S. Garg, A. N. Pham and T. D. Waite, J. Phys. Chem. C, 2011, 115, 5461–5468 CrossRef CAS.
- D. He, S. Garg and T. D. Waite, Langmuir, 2012, 28, 10266–10275 CrossRef CAS.
- E. Mendis, N. Rajapakse, H.-G. Byun and S.-K. Kim, Life Sci., 2005, 77, 2166–2178 CrossRef CAS PubMed.
- Q. Chang, L. Yan, M. Chen, H. He and J. Qu, Langmuir, 2007, 23, 11197–11199 CrossRef CAS PubMed.
- Y. Inoue, M. Hoshino, H. Takahashi, T. Noguchi, T. Murata, Y. Kanzaki, H. Hamashima and M. Sasatsu, J. Inorg. Biochem., 2002, 92, 37–42 CrossRef CAS.
- J. R. Hom, R. A. Quintanilla, D. L. Hoffman, K. L. de Mesy Bentley, J. D. Molkentin, S.-S. Sheu and G. A. Porter Jr., Dev. Cell, 2011, 21, 469–478 CrossRef CAS PubMed.
- A. Henglein, J. Phys. Chem., 1979, 83, 2209–2216 CrossRef CAS.
- A. Henglein and J. Lilie, J. Am. Chem. Soc., 1981, 103, 1059–1066 CrossRef CAS.
- L. Z. Flores-López, H. Espinoza-Gómez and R. Somanathan, J. Appl. Toxicol., 2019, 39, 16–26 CrossRef.
- M. E. Hamdy, M. Del Carlo, H. A. Hussein, T. A. Salah, A. H. El-Deeb, M. M. Emara, G. Pezzoni and D. Compagnone, J. Nanobiotechnol., 2018, 16, 48 CrossRef PubMed.
- M. U. Farooq, V. Novosad, E. A. Rozhkova, H. Wali, A. Ali, A. A. Fateh, P. B. Neogi, A. Neogi and Z. Wang, Sci. Rep., 2018, 8, 2907 CrossRef PubMed.
- Y. Liu, W. Ma and J. Wang, Curr. Pharm. Des., 2018, 24, 2719–2728 CrossRef CAS.
- P. C. Nagajyothi, T. V. M. Sreekanth, C. O. Tettey, Y. I. Jun and S. H. Mook, Bioorg. Med. Chem. Lett., 2014, 24, 4298–4303 CrossRef CAS PubMed.
- P. Lau, N. Bidin, S. Islam, W. Shukri, N. Zakaria, N. Musa and G. Krishnan, Lasers Surg. Med., 2017, 49, 380–386 CrossRef PubMed.
- M. G. Arafa, R. F. El-Kased and M. M. Elmazar, Sci. Rep., 2018, 8, 13674 CrossRef.
- D. A. Giljohann, D. S. Seferos, W. L. Daniel, M. D. Massich, P. C. Patel and C. A. Mirkin, Angew. Chem., Int. Ed., 2010, 49, 3280–3294 CrossRef CAS.
- R. G. Saratale, I. Karuppusamy, G. D. Saratale, A. Pugazhendhi, G. Kumar, Y. Park, G. S. Ghodake, R. N. Bharagava, J. R. Banu and H. S. Shin, Colloids Surf., B, 2018, 170, 20–35 CrossRef CAS.
- N. A. Sutan, D. S. Manolescu, I. Fierascu, A. M. Neblea, C. Sutan, C. Ducu, L. C. Soare, D. Negrea, S. M. Avramescu and R. C. Fierascu, Mater. Sci. Eng., C, 2018, 93, 746–758 CrossRef CAS.
- M. I. Din, F. Arshad, Z. Hussain and M. Mukhtar, Nanoscale Res. Lett., 2017, 12, 638 CrossRef.
- H. Joy Prabu and I. Johnson, Karbala Int. J. Mod. Sci., 2015, 1, 237–246 CrossRef.
- K. B. Ayaz Ahmed, S. Subramanian, A. Sivasubramanian, G. Veerappan and A. Veerappan, Spectrochim. Acta, Part A, 2014, 130, 54–58 CrossRef CAS.
- N. Y. Stozhko, M. A. Bukharinova, E. I. Khamzina, A. V. Tarasov, M. B. Vidrevich and K. Z. Brainina, Nanomaterials, 2019, 9, 1655 CrossRef CAS PubMed.
- M. Hamelian, K. Varmira and H. Veisi, J. Photochem. Photobiol., B, 2018, 184, 71–79 CrossRef CAS.
- F. G. Milanezi, L. M. Meireles, M. M. de Christo Scherer, J. P. de Oliveira, A. R. da Silva, M. L. de Araujo, D. C. Endringer, M. Fronza, M. C. C. Guimarães and R. Scherer, Saudi Pharm. J., 2019, 27, 968–974 CrossRef CAS.
- L. M. Nieves, K. Mossburg, J. C. Hsu, A. D. A. Maidment and D. P. Cormode, Nanoscale, 2021, 13, 19306–19323 RSC.
- Y. Mantri, B. Davidi, J. E. Lemaster, A. Hariri and J. V. Jokerst, Nanoscale, 2020, 12, 10511–10520 RSC.
- N. Sarfraz and I. Khan, Chem. – Asian J., 2021, 16, 720–742 CrossRef CAS.
- I. B. Becerril-Castro, I. Calderon, N. Pazos-Perez, L. Guerrini, F. Schulz, N. Feliu, I. Chakraborty, V. Giannini, W. J. Parak and R. A. Alvarez-Puebla, Analysis Sensing, 2022, 2, e202200005 CrossRef CAS.
- J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. D. P. Rodriguez-Torres, L. S. Acosta-Torres, L. A. Diaz-Torres, R. Grillo, M. K. Swamy, S. Sharma, S. Habtemariam and H.-S. Shin, J. Nanobiotechnol., 2018, 16, 71 CrossRef.
- G. Ajnai, A. Chiu, T. Kan, C.-C. Cheng, T.-H. Tsai and J. Chang, J. Exp. Clin. Med., 2014, 6, 172–178 CrossRef CAS.
- T. Xia, M. Kovochich, J. Brant, M. Hotze, J. Sempf, T. Oberley, C. Sioutas, J. I. Yeh, M. R. Wiesner and A. E. Nel, Nano Lett., 2006, 6, 1794–1807 CrossRef CAS PubMed.
- T. Xia, M. Kovochich, M. Liong, L. Madler, B. Gilbert, H. Shi, J. I. Yeh, J. I. Zink and A. E. Nel, ACS Nano, 2008, 2, 2121–2134 CrossRef CAS.
- A. Sasidharan and N. A. Monteiro-Riviere, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2015, 7, 779–796 CAS.
- M. Holzinger, A. Le Goff and S. Cosnier, Front. Chem., 2014, 2, 63 Search PubMed.
- I. Willner, R. Baron and B. Willner, Biosens. Bioelectron., 2007, 22, 1841–1852 CrossRef CAS.
- K. Lee, H. Lee, K. W. Lee and T. G. Park, Biomaterials, 2011, 32, 2556–2565 CrossRef CAS PubMed.
- Y. Higashi, J. Mazumder, H. Yoshikawa, M. Saito and E. Tamiya, Anal. Chem., 2018, 90, 5773–5780 CrossRef CAS.
- H. Niu, R. Yuan, Y. Chai, L. Mao, Y. Yuan, Y. Zhuo, S. Yuan and X. Yang, Biosens. Bioelectron., 2011, 26, 3175–3180 CrossRef CAS.
- L. Mao, R. Yuan, Y. Chai, Y. Zhuo and Y. Xiang, Biosens. Bioelectron., 2011, 26, 4204–4208 CrossRef CAS.
- Y. Cui, Y. Zhao, Y. Tian, W. Zhang, X. Lü and X. Jiang, Biomaterials, 2012, 33, 2327–2333 CrossRef CAS.
- H. Lee and D. G. Lee, Colloids Surf., B, 2018, 167, 1–7 CrossRef CAS.
- Q. Zhang, V. M. Hitchins, A. M. Schrand, S. M. Hussain and P. L. Goering, Nanotoxicology, 2011, 5, 284–295 CrossRef CAS PubMed.
- D. Mateo, P. Morales, A. Ávalos and A. I. Haza, Toxicol. Mech. Methods, 2014, 24, 161–172 CrossRef CAS.
- A. Watanabe, M. Kajita, J. Kim, A. Kanayama, K. Takahashi, T. Mashino and Y. Miyamoto, Nanotechnology, 2009, 20, 455105 CrossRef.
- K. Tahir, S. Nazir, A. Ahmad, B. Li, S. A. Ali Shah, A. U. Khan, G. M. Khan, Q. U. Khan, Z. U. Haq Khan and F. U. Khan, RSC Adv., 2016, 6, 85903–85916 RSC.
- N. A. S. Ismail, J. X. Lee and F. Yusof, Antioxidants, 2022, 11, 986 CrossRef CAS PubMed.
- A. F. Elhusseiny and H. H. A. M. Hassan, Spectrochim. Acta, Part A, 2013, 103, 232–245 CrossRef CAS.
- A. J. Kora and L. Rastogi, Arabian J. Chem., 2018, 11, 1097–1106 CrossRef CAS.
- L. N. Lewis and N. Lewis, J. Am. Chem. Soc., 1986, 108, 7228–7231 CrossRef CAS.
- A. Roucoux, J. Schulz and H. Patin, Chem. Rev., 2002, 102, 3757–3778 CrossRef CAS PubMed.
- Y. Yoshihisa, Q.-L. Zhao, M. A. Hassan, Z.-L. Wei, M. Furuichi, Y. Miyamoto, T. Kondo and T. Shimizu, Free Radical Res., 2011, 45, 326–335 CrossRef CAS.
- Y. Yoshihisa, A. Honda, Q.-L. Zhao, T. Makino, R. Abe, K. Matsui, H. Shimizu, Y. Miyamoto, T. Kondo and T. Shimizu, Exp. Dermatol., 2010, 19, 1000–1006 CrossRef CAS PubMed.
- P. K. Gupta and L. Mishra, Nanoscale Adv., 2020, 2, 1774–1791 RSC.
- C. Mu, K. E. Prosser, S. Harrypersad, G. A. MacNeil, R. Panchmatia, J. R. Thompson, S. Sinha, J. J. Warren and C. J. Walsby, Inorg. Chem., 2018, 57, 15247–15261 CrossRef CAS.
- S. K. Srivastava and M. Constanti, J. Nanopart. Res., 2012, 14, 831 CrossRef.
- G. J. Cao, X. Jiang, H. Zhang, J. Zheng, T. R. Croley and J. J. Yin, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2017, 35, 223–238 CrossRef CAS.
- R. Boyd, Nat. Chem., 2011, 3, 570 CrossRef CAS.
- M. E. Weeks, J. Chem. Educ., 1932, 9, 474 CrossRef CAS.
- M. Kieliszek, I. Bano and H. Zare, Biol. Trace Elem. Res., 2022, 200, 971–987 CrossRef CAS PubMed.
- J. Zhao, R. Zhou, K. Hui, Y. Yang, Q. Zhang, Y. Ci, L. Shi, C. Xu, F. Huang and Y. Hu, Oncotarget, 2017, 8, 18832–18847 CrossRef PubMed.
- B. Lipinski, Anticancer Agents Med. Chem., 2017, 17, 658–661 CrossRef CAS.
- F. Maiyo and M. Singh, Nanomedicine, 2017, 12, 1075–1089 CrossRef CAS.
- J. C. Avery and P. R. Hoffmann, Nutrients, 2018, 10, 1203 CrossRef PubMed.
- R. A. Sunde and A. M. Raines, Adv. Nutr., 2011, 2, 138–150 CrossRef CAS.
- Y. Wang, W. Kong, L. Wang, J. Z. Zhang, Y. Li, X. Liu and Y. Li, Phys. Chem. Chem. Phys., 2019, 21, 1336–1343 RSC.
- O. K. Chun, A. Floegel, S. J. Chung, C. E. Chung, W. O. Song and S. I. Koo, J. Nutr., 2010, 140, 317–324 CrossRef CAS PubMed.
- A. P. Kipp, D. Strohm, R. Brigelius-Flohe, L. Schomburg, A. Bechthold, E. Leschik-Bonnet and H. Heseker, J. Trace Elem. Med. Biol., 2015, 32, 195–199 CrossRef CAS.
- Z. Huang, A. H. Rose and P. R. Hoffmann, Antioxid. Redox Signaling, 2012, 16, 705–743 CrossRef CAS.
- H. Gill and G. Walker, Nutr. Diet., 2008, 65, S41–S47 CrossRef.
- K. H. Winther, M. P. Rayman, S. J. Bonnema and L. Hegedüs, Nat. Rev. Endocrinol., 2020, 16, 165–176 CrossRef CAS PubMed.
- T. Nauser, S. Dockheer, R. Kissner and W. H. Koppenol, Biochemistry, 2006, 45, 6038–6043 CrossRef CAS.
- N. K. Kildahl, J. Chem. Educ., 1995, 72, 423 CrossRef CAS.
- S. Zhai, X. Hu, Y. Hu, B. Wu and D. Xing, Biomaterials, 2017, 121, 41–54 CrossRef CAS PubMed.
- J. Mu, L. Zhang, M. Zhao and Y. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 7090–7098 CrossRef CAS.
- X. Miao, W. Cao, W. Zheng, J. Wang, X. Zhang, J. Gao, C. Yang, D. Kong, H. Xu, L. Wang and Z. Yang, Angew. Chem., Int. Ed., 2013, 52, 7781–7785 CrossRef CAS.
- O. Brodin, S. Eksborg, M. Wallenberg, C. Asker-Hagelberg, E. H. Larsen, D. Mohlkert, C. Lenneby-Helleday, H. Jacobsson, S. Linder, S. Misra and M. Björnstedt, Nutrients, 2015, 7, 4978–4994 CrossRef CAS PubMed.
- S. J. Knox, P. Jayachandran, C. A. Keeling, K. J. Stevens, N. Sandhu, S. L. Stamps-DeAnda, R. Savic, L. Shura, M. K. Buyyounouski and K. Grimes, Transl. Oncol., 2019, 12, 1525–1531 CrossRef.
- H. K. Rooprai, I. Kyriazis, R. K. Nuttall, D. R. Edwards, D. Zicha, D. Aubyn, D. Davies, R. Gullan and G. J. Pilkington, Int. J. Oncol., 2007, 30, 1263–1271 CAS.
- S. Y. Cui, H. Jin, S. J. Kim, A. P. Kumar and Y. I. Lee, J. Biochem., 2008, 143, 685–693 CrossRef CAS.
- H. Amani, R. Habibey, F. Shokri, S. J. Hajmiresmail, O. Akhavan, A. Mashaghi and H. Pazoki-Toroudi, Sci. Rep., 2019, 9, 6044 CrossRef PubMed.
- E. H. Lo, T. Dalkara and M. A. Moskowitz, Nat. Rev. Neurosci., 2003, 4, 399–414 CrossRef CAS.
- S. Yang, J. Sun, P. He, X. Deng, Z. Wang, C. Hu, G. Ding and X. Xie, Chem. Mater., 2015, 27, 2004–2011 CrossRef CAS.
- H. W. Tan, H.-Y. Mo, A. T. Y. Lau and Y.-M. Xu, Int. J. Mol. Sci., 2018, 20, 75 CrossRef.
- S. Khandelwal, M. Boylan, J. E. Spallholz and L. Gollahon, Int. J. Mol. Sci., 2018, 19(11), 3352 CrossRef.
- C. M. Weekley, J. B. Aitken, S. Vogt, L. A. Finney, D. J. Paterson, M. D. de Jonge, D. L. Howard, P. K. Witting, I. F. Musgrave and H. H. Harris, J. Am. Chem. Soc., 2011, 133, 18272–18279 CrossRef CAS PubMed.
- Y. Wang, J. Wang, H. Hao, M. Cai, S. Wang, J. Ma, Y. Li, C. Mao and S. Zhang, ACS Nano, 2016, 10, 9927–9937 CrossRef CAS.
- T. Li, F. Li, W. Xiang, Y. Yi, Y. Chen, L. Cheng, Z. Liu and H. Xu, ACS Appl. Mater. Interfaces, 2016, 8, 22106–22112 CrossRef CAS.
- W. Zheng, T. Yin, Q. Chen, X. Qin, X. Huang, S. Zhao, T. Xu, L. Chen and J. Liu, Acta Biomater., 2016, 31, 197–210 CrossRef CAS.
- W. Zheng, C. Cao, Y. Liu, Q. Yu, C. Zheng, D. Sun, X. Ren and J. Liu, Acta Biomater., 2015, 11, 368–380 CrossRef CAS.
- J. Sun, C. Wei, Y. Liu, W. Xie, M. Xu, H. Zhou and J. Liu, Biomaterials, 2019, 197, 417–431 CrossRef CAS.
- M. S. Wason, J. Colon, S. Das, S. Seal, J. Turkson, J. Zhao and C. H. Baker, Nanomedicine, 2013, 9, 558–569 CrossRef CAS PubMed.
- J.-D. Cafun, K. O. Kvashnina, E. Casals, V. F. Puntes and P. Glatzel, ACS Nano, 2013, 7, 10726–10732 CrossRef CAS.
- N. Sutradhar, A. Sinhamahapatra, S. K. Pahari, P. Pal, H. C. Bajaj, I. Mukhopadhyay and A. B. Panda, J. Phys. Chem. C, 2011, 115, 12308–12316 CrossRef CAS.
- R. Dobrucka, Iran. J. Sci. Technol., Trans. A: Sci., 2018, 42, 547–555 CrossRef.
- N. John Sushma, D. Prathyusha, G. Swathi, T. Madhavi, B. Deva Prasad Raju, K. Mallikarjuna and H.-S. Kim, Appl. Nanosci., 2016, 6, 437–444 CrossRef CAS.
- G. Kiranmai and A. R. Reddy, Toxicol. Ind. Health, 2013, 29, 897–903 CrossRef CAS.
- Y.-C. Kuo, S.-P. Lin, C.-W. Lin, Y.-C. Tsai, T.-S. Wu and I. L. Hsiao, ACS Appl. Nano Mater., 2023, 6, 19915–19925 CrossRef CAS.
- A. Sinha and S. K. Khare, Bioresour. Technol., 2011, 102, 4281–4284 CrossRef CAS.
- A. M. Allahverdiyev, E. S. Abamor, M. Bagirova and M. Rafailovich, Future Microbiol., 2011, 6, 933–940 CrossRef CAS PubMed.
- J. Matena, S. Petersen, M. Gieseke, A. Kampmann, M. Teske, M. Beyerbach, H. Escobar, H. Haferkamp, N.-C. Gellrich and I. Nolte, Int. J. Mol. Sci., 2015, 16, 7478 CrossRef CAS.
- O. Lucaciu, O. Soriţău, D. Gheban, D. R. Ciuca, O. Virtic, A. Vulpoi, N. Dirzu, R. Câmpian, G. Băciuţ, C. Popa, S. Simon, P. Berce, M. Băciuţ and B. Crisan, BMC Biotechnol., 2015, 15, 1–18 CrossRef PubMed.
- D. Kuroda, M. Niinomi, M. Morinaga, Y. Kato and T. Yashiro, Mater. Sci. Eng., A, 1998, 243, 244–249 CrossRef.
- B. J. Farrell, B. I. Prilutsky, J. M. Ritter, S. Kelley, K. Popat and M. Pitkin, J. Biomed. Mater. Res., Part A, 2014, 102, 1305–1315 CrossRef PubMed.
- T. K. Ahn, D. H. Lee, T. S. Kim, G. C. Jang, S. Choi, J. B. Oh, G. Ye and S. Lee, Adv. Exp. Med. Biol., 2018, 1077, 355–368 CrossRef CAS.
- F. U. Rehman, C. Zhao, H. Jiang and X. Wang, Biomater. Sci., 2016, 4, 40–54 RSC.
- M. Song, R. Zhang, Y. Dai, F. Gao, H. Chi, G. Lv, B. Chen and X. Wang, Biomaterials, 2006, 27, 4230–4238 CrossRef CAS.
- J. Wei, H. Shi, M. Zhou, D. Song, Y. Zhang, X. Pan, J. Zhou and T. Wang, Appl. Catal., A, 2015, 499, 101–108 CrossRef CAS.
- R. Cai, Y. Kubota, T. Shuin, H. Sakai, K. Hashimoto and A. Fujishima, Cancer Res., 1992, 52, 2346–2348 CAS.
- N. Lagopati, E.-P. Tsilibary, P. Falaras, P. Papazafiri, E. A. Pavlatou, E. Kotsopoulou and P. Kitsiou, Int. J. Nanomed., 2014, 9, 3219–3230 CAS.
- P. Boffetta, V. Gaborieau, L. Nadon, M. F. Parent, E. Weiderpass and J. Siemiatycki, Scand. J. Work, Environ. Health, 2001, 27, 227–232 CrossRef CAS.
- Y. Wang, H. Cui, J. Zhou, F. Li, J. Wang, M. Chen and Q. Liu, Environ. Sci. Pollut. Res., 2015, 22, 5519–5530 CrossRef CAS.
- K. Niska, K. Pyszka, C. Tukaj, M. Wozniak, M. W. Radomski and I. Inkielewicz-Stepniak, Int. J. Nanomed., 2015, 10, 1095–1107 CAS.
- S. K. Verma, E. Jha, P. K. Panda, A. Thirumurugan, S. K. S. Parashar, S. Patro and M. Suar, ACS Omega, 2018, 3, 1244–1262 CrossRef CAS PubMed.
- C. Jin, Y. Tang, F. G. Yang, X. L. Li, S. Xu, X. Y. Fan, Y. Y. Huang and Y. J. Yang, Biol. Trace Elem. Res., 2011, 141, 3–15 CrossRef CAS.
- C. Angelé-Martínez, C. Goodman and J. Brumaghim, Metallomics, 2014, 6, 1358–1381 CrossRef.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- P.-C. Maness, S. Smolinski, D. M. Blake, Z. Huang, E. J. Wolfrum and W. A. Jacoby, Appl. Environ. Microbiol., 1999, 65, 4094–4098 CrossRef CAS PubMed.
- J. Cai, J. Peng, J. Feng, R. Li, P. Ren, X. Zang, Z. Wu, Y. Lu, L. Luo, Z. Hu, J. Wang, X. Dai, P. Zhao, J. Wang, M. Yan, J. Liu, R. Deng and D. Wang, Nat. Commun., 2023, 14, 3643 CrossRef CAS PubMed.
- J. Chu, Z. Kong, D. Lu, W. Zhang, X. Wang, Y. Yu, S. Li, X. Wang, S. Xiong and J. Ma, Mater. Lett., 2016, 166, 179–182 CrossRef CAS.
- J. Kysar and P. K. Sekhar, Appl. Nanosci., 2016, 6, 959–964 CrossRef CAS.
- A. Liu, M. Ichihara, I. Honma and H. Zhou, Electrochem. Commun., 2007, 9, 1766–1771 CrossRef CAS.
- R. Berenguer, M. O. Guerrero-Pérez, I. Guzmán, J. Rodríguez-Mirasol and T. Cordero, ACS Omega, 2017, 2, 7739–7745 CrossRef CAS.
- K. K. Dey, S. Jha, A. Kumar, G. Gupta, A. K. Srivastava and P. P. Ingole, Electrochim. Acta, 2019, 312, 89–99 CrossRef CAS.
- R. K. Sharma, P. Kumar and G. B. Reddy, J. Alloys Compd., 2015, 638, 289–297 CrossRef CAS.
- X. Zhou, G. Wu, J. Wu, H. Yang, J. Wang and G. Gao, Phys. Chem. Chem. Phys., 2014, 16, 3973–3982 RSC.
- S. Pang, G. Li and Z. Zhang, Macromol. Rapid Commun., 2005, 26, 1262–1265 CrossRef CAS.
- J. Zhu, L. Cao, Y. Wu, Y. Gong, Z. Liu, H. E. Hoster, Y. Zhang, S. Zhang, S. Yang, Q. Yan, P. M. Ajayan and R. Vajtai, Nano Lett., 2013, 13, 5408–5413 CrossRef CAS.
- A. Makarevich, O. Makarevich, A. Ivanov, D. Sharovarov, A. Eliseev, V. Amelichev, O. Boytsova, A. Gorodetsky, M. Navarro-Cía and A. Kaul, CrystEngComm, 2020, 22, 2612–2620 RSC.
- A. Pan, H. B. Wu, L. Yu, T. Zhu and X. W. Lou, ACS Appl. Mater. Interfaces, 2012, 4, 3874–3879 CrossRef CAS.
- F. Natalio, R. André, A. F. Hartog, B. Stoll, K. P. Jochum, R. Wever and W. Tremel, Nat. Nanotechnol., 2012, 7, 530–535 CrossRef CAS.
- A. A. Vernekar, D. Sinha, S. Srivastava, P. U. Paramasivam, P. D’Silva and G. Mugesh, Nat. Commun., 2014, 5, 5301 CrossRef CAS.
- S. Das, A. Roy, A. K. Barui, M. M. A. Alabbasi, M. Kuncha, R. Sistla, B. Sreedhar and C. R. Patra, Nanoscale, 2020, 12, 7604–7621 RSC.
- B. L. Zhu, Q. Qiherima, L. Lv and X. J. Wang, Key Eng. Mater., 2012, 492, 264–267 CAS.
- Y. Du and Y. Zhang, J. Exp. Nanosci., 2014, 9, 120–125 CrossRef CAS.
- S. Rong, P. Zhang, F. Liu and Y. Yang, ACS Catal., 2018, 8, 3435–3446 CrossRef CAS.
- T. Soejima, K. Nishizawa and R. Isoda, J. Colloid Interface Sci., 2018, 510, 272–279 CrossRef CAS.
- V. Kumar, K. Singh, S. Panwar and S. K. Mehta, Int. Nano Lett., 2017, 7, 123–131 CrossRef CAS.
- J. Shin, R. M. Anisur, M. K. Ko, G. H. Im, J. H. Lee and I. S. Lee, Angew. Chem., Int. Ed., 2009, 48, 321–324 CrossRef CAS PubMed.
- Y. Chen, H. Chen, S. Zhang, F. Chen, S. Sun, Q. He, M. Ma, X. Wang, H. Wu, L. Zhang, L. Zhang and J. Shi, Biomaterials, 2012, 33, 2388–2398 CrossRef CAS PubMed.
- H. Zhang, F. Xu, J. Xue, S. Chen, J. Wang and Y. Yang, Sci. Rep., 2020, 10, 6067 CrossRef CAS PubMed.
- S. Fairose, S. Ernest and Ashna, Mater. Today: Proc., 2017, 4, 12085–12093 Search PubMed.
- Y. Zhang, L. Chen, R. Sun, R. Lv, T. Du, Y. Li, X. Zhang, R. Sheng and Y. Qi, ACS Biomater. Sci. Eng., 2022, 8, 638–648 CrossRef CAS PubMed.
- J. Kim, H. Y. Kim, S. Y. Song, S.-H. Go, H. S. Sohn, S. Baik, M. Soh, K. Kim, D. Kim, H.-C. Kim, N. Lee, B.-S. Kim and T. Hyeon, ACS Nano, 2019, 13, 3206–3217 CrossRef CAS.
- Y. Wu, Z. Zhou, M. Zhang, S. Li, M. Sun and Z. Song, J. Nanobiotechnol., 2023, 21, 432 CrossRef CAS PubMed.
- F. Qian, Z. Huang, W. Liu, Y. Liu and X. He, J. Biomed. Mater. Res., Part A, 2023, 111, 1678–1691 CrossRef CAS.
- S. Alarifi, D. Ali and S. Alkahtani, BioMed Res. Int., 2017, 2017, 5478790 Search PubMed.
- N. Singh, M. Geethika, S. M. Eswarappa and G. Mugesh, Chem. – Eur. J., 2018, 24, 8393–8403 CrossRef CAS.
- N. Singh, M. A. Savanur, S. Srivastava, P. D'Silva and G. Mugesh, Nanoscale, 2019, 11, 3855–3863 RSC.
- M. H. Tootoonchi, M. Hashempour, P. L. Blackwelder and C. A. Fraker, Acta Biomater., 2017, 59, 327–337 CrossRef CAS.
- C. R. Gordijo, K. Koulajian, A. J. Shuhendler, L. D. Bonifacio, H. Y. Huang, S. Chiang, G. A. Ozin, A. Giacca and X. Y. Wu, Adv. Funct. Mater., 2011, 21, 73–82 CrossRef CAS.
- C. R. Gordijo, A. J. Shuhendler and X. Y. Wu, Adv. Funct. Mater., 2010, 20, 1404–1412 CrossRef CAS.
- P. Prasad, C. R. Gordijo, A. Z. Abbasi, A. Maeda, A. Ip, A. M. Rauth, R. S. DaCosta and X. Y. Wu, ACS Nano, 2014, 8, 3202–3212 CrossRef CAS.
- I. Rabani, J. Yoo, H.-S. Kim, D. V. Lam, S. Hussain, K. Karuppasamy and Y.-S. Seo, Nanoscale, 2021, 13, 355–370 RSC.
- R. Shanmuganathan, S. Sathiyavimal, Q. Hoang Le, M. M. Al-Ansari, L. A. Al-Humaid, G. K. Jhanani, J. Lee and S. Barathi, Environ. Res., 2023, 236, 116747 CrossRef CAS PubMed.
- D. Das and B. J. Saikia, Chem. Phys. Impact, 2023, 6, 100137 CrossRef.
- L. Díez-Tercero, L. M. Delgado, E. Bosch-Rué and R. A. Perez, Sci. Rep., 2021, 11, 11707 CrossRef.
- N. Mandakhbayar, Y. Ji, A. El-Fiqi, K. D. Patel, D. S. Yoon, K. Dashnyam, O. Bayaraa, G. Jin, K. Tsogtbaatar, T.-H. Kim, J.-H. Lee and H.-W. Kim, Bioact. Mater., 2024, 31, 298–311 CAS.
- S. E. Kim, L. Zhang, K. Ma, M. Riegman, F. Chen, I. Ingold, M. Conrad, M. Z. Turker, M. Gao, X. Jiang, S. Monette, M. Pauliah, M. Gonen, P. Zanzonico, T. Quinn, U. Wiesner, M. S. Bradbury and M. Overholtzer, Nat. Nanotechnol., 2016, 11, 977–985 CrossRef CAS PubMed.
- L. Jiang, N. Kon, T. Li, S. J. Wang, T. Su, H. Hibshoosh, R. Baer and W. Gu, Nature, 2015, 520, 57–62 CrossRef CAS.
- C. Luo, Y. Li, L. Yang, X. Wang, J. Long and J. Liu, Arch. Toxicol., 2015, 89, 357–369 CrossRef CAS.
- Y. Ichikawa, M. Ghanefar, M. Bayeva, R. Wu, A. Khechaduri, S. V. Naga Prasad, R. K. Mutharasan, T. J. Naik and H. Ardehali, J. Clin. Invest., 2014, 124, 617–630 CrossRef CAS PubMed.
- L. S. Lin, J. Song, L. Song, K. Ke, Y. Liu, Z. Zhou, Z. Shen, J. Li, Z. Yang, W. Tang, G. Niu, H. H. Yang and X. Chen, Angew. Chem., Int. Ed., 2018, 57, 4902–4906 CrossRef CAS.
- S. Niu, L. K. Zhang, L. Zhang, S. Zhuang, X. Zhan, W. Y. Chen, S. Du, L. Yin, R. You, C. H. Li and Y. Q. Guan, Theranostics, 2017, 7, 344–356 CrossRef CAS PubMed.
- B. Torres-Herrero, I. Armenia, M. Alleva, L. Asín, S. Correa, C. Ortiz, Y. Fernández-Afonso, L. Gutiérrez, J. M. de la Fuente, L. Betancor and V. Grazú, ACS Nano, 2023, 17, 12358–12373 CrossRef CAS PubMed.
- Y. Zhang, Y. Wang, Q. Zhou, X. Chen, W. Jiao, G. Li, M. Peng, X. Liu, Y. He and H. Fan, ACS Appl. Mater. Interfaces, 2021, 13, 52395–52405 CrossRef CAS.
- Y. Hao, Z. Dong, M. Chen, Y. Chao, Z. Liu, L. Feng, Y. Hao, Z. L. Dong, M. C. Chen, Y. Chao, Z. Liu and L. Z. Feng, Biomaterials, 2020, 228, 119568 CrossRef CAS.
- A. K. Hauser, M. I. Mitov, E. F. Daley, R. C. McGarry, K. W. Anderson and J. Z. Hilt, Biomaterials, 2016, 105, 127–135 CrossRef CAS.
- M. I. Khan, A. Mohammad, G. Patil, S. A. H. Naqvi, L. K. S. Chauhan and I. Ahmad, Biomaterials, 2012, 33, 1477–1488 CrossRef CAS.
- J. Liu, L. Wang, J. Cao, Y. Huang, Y. Lin, X. Wu, Z. Wang, F. Zhang, X. Xu and G. Liu, Nanoscale, 2014, 6, 9025–9033 RSC.
- E.-J. Park, D.-H. Choi, Y. Kim, E.-W. Lee, J. Song, M.-H. Cho, J.-H. Kim and S.-W. Kim, Toxicol. In Vitro, 2014, 28, 1402–1412 CrossRef CAS PubMed.
- H. Y. Tan, N. Wang, S. Li, M. Hong, X. Wang and Y. Feng, Oxid. Med. Cell. Longevity, 2016, 2016, 2795090 Search PubMed.
- J. Sarkar, N. Chakraborty, A. Chatterjee, A. Bhattacharjee, D. Dasgupta and K. Acharya, Nanomaterials, 2020, 10(2), 312 CrossRef CAS PubMed.
- S. Sukumar, A. Rudrasenan and D. Padmanabhan Nambiar, ACS Omega, 2020, 5, 1040–1051 CrossRef CAS PubMed.
- A. Ananth, S. Dharaneedharan, M.-S. Heo and Y. S. Mok, Chem. Eng. J., 2015, 262, 179–188 CrossRef CAS.
- M. Nasrollahzadeh, M. Maham and S. Mohammad Sajadi, J. Colloid Interface Sci., 2015, 455, 245–253 CrossRef CAS PubMed.
- D. Rehana, D. Mahendiran, R. S. Kumar and A. K. Rahiman, Biomed. Pharmacother., 2017, 89, 1067–1077 CrossRef CAS PubMed.
- C. Angelé-Martínez, K. V. T. Nguyen, F. S. Ameer, J. N. Anker and J. L. Brumaghim, Nanotoxicology, 2017, 11, 278–288 CrossRef.
- A. M. Studer, L. K. Limbach, L. Van Duc, F. Krumeich, E. K. Athanassiou, L. C. Gerber, H. Moch and W. J. Stark, Toxicol. Lett., 2010, 197, 169–174 CrossRef CAS PubMed.
- H. L. Karlsson, P. Cronholm, J. Gustafsson and L. Möller, Chem. Res. Toxicol., 2008, 21, 1726–1732 Search PubMed.
- J. P. Kehrer, Toxicology, 2000, 149, 43–50 CrossRef CAS.
- H. Ashrafizadeh, S. R. Abtahi and A. A. Oroojan, Diabetes Metab. Syndr., 2020, 14, 443–445 CrossRef PubMed.
- B. Stieberova, M. Zilka, M. Ticha, F. Freiberg, P. Caramazana-González, J. McKechnie and E. Lester, ACS Sustainable Chem. Eng., 2017, 5, 2493–2500 CrossRef CAS.
- S. Y. Kim, S. H. Jeong, E. Y. Lee, Y.-H. Park, H. C. Bae, Y. S. Jang, E. H. Maeng, M.-K. Kim and S. W. Son, J. Toxicol. Environ. Health Sci., 2011, 3, 258–261 CrossRef.
- C. García-Gómez, A. Obrador, D. González, M. Babín and M. D. Fernández, Sci. Total Environ., 2017, 589, 11–24 CrossRef.
- Y. K. Mishra and R. Adelung, Mater. Today, 2018, 21, 631–651 CrossRef CAS.
- M. Martínez-Carmona, Y. Gun’ko and M. Vallet-Regí, Nanomaterials, 2018, 8, 268 CrossRef.
- J. W. Rasmussen, E. Martinez, P. Louka and D. G. Wingett, Expert Opin. Drug Delivery, 2010, 7, 1063–1077 CrossRef CAS.
- R. Lahri, M. Rahman, M. Wright, P. Kosmas and M. Thanou, Med. Phys., 2018, 45, 3820–3830 CrossRef CAS.
- P. Ruenraroengsak, D. Kiryushko, I. G. Theodorou, M. M. Klosowski, E. R. Taylor, T. Niriella, C. Palmieri, E. Yagüe, M. P. Ryan, R. C. Coombes, F. Xie and A. E. Porter, Nanoscale, 2019, 11, 12858–12870 RSC.
- J. Wang, J. S. Lee, D. Kim and L. Zhu, ACS Appl. Mater. Interfaces, 2017, 9, 39971–39984 CrossRef CAS PubMed.
- S. Chaudhary, A. Umar, K. K. Bhasin and S. Baskoutas, Materials, 2018, 11(2), 287 CrossRef PubMed.
- J. Singh, T. Dutta, K.-H. Kim, M. Rawat, P. Samddar and P. Kumar, J. Nanobiotechnol., 2018, 16, 84 CrossRef CAS.
- J. A. Hernandez-Viezcas, H. Castillo-Michel, A. D. Servin, J. R. Peralta-Videa and J. L. Gardea-Torresdey, Chem. Eng. J., 2011, 170, 346–352 CrossRef CAS PubMed.
- G. Chichiriccò and A. Poma, Nanomaterials, 2015, 5(2), 851–873 CrossRef.
- P. C. Nagajyothi, S. J. Cha, I. J. Yang, T. V. M. Sreekanth, K. J. Kim and H. M. Shin, J. Photochem. Photobiol., B, 2015, 146, 10–17 CrossRef CAS.
- M. Shoeb, B. R. Singh, J. A. Khan, W. Khan, B. N. Singh, H. B. Singh and A. H. Naqvi, Adv. Nat. Sci., 2013, 4, 035015 CAS.
- J.-H. Li, X.-R. Liu, Y. Zhang, F.-F. Tian, G.-Y. Zhao, Q.-L.-Y. Yu, F.-L. Jiang and Y. Liu, Toxicol. Res., 2012, 1, 137–144 CrossRef CAS.
- W. M. U. Daniels, J. Hendricks, R. Salie and S. J. van Rensburg, Metab. Brain Dis., 2004, 19, 79–88 CrossRef CAS.
- X. Xu, D. Chen, Z. Yi, M. Jiang, L. Wang, Z. Zhou, X. Fan, Y. Wang and D. Hui, Langmuir, 2013, 29, 5573–5580 CrossRef CAS PubMed.
- J. Gupta and D. Bahadur, ACS Omega, 2018, 3, 2956–2965 CrossRef CAS.
- M. Ahrén, L. Selegård, A. Klasson, F. Söderlind, N. Abrikossova, C. Skoglund, T. Bengtsson, M. Engström, P.-O. Käll and K. Uvdal, Langmuir, 2010, 26, 5753–5762 CrossRef.
- S. J. Seo, S. M. Han, J. H. Cho, K. Hyodo, A. Zaboronok, H. You, K. Peach, M. A. Hill and J. K. Kim, Radiat. Environ. Biophys., 2015, 54, 423–431 CrossRef CAS PubMed.
- D. J. Todd and J. Kay, Curr. Rheumatol. Rep., 2008, 10, 195–204 CrossRef CAS.
- P. Sharma, S. Brown, G. Walter, S. Santra and B. Moudgil, Adv. Colloid Interface Sci., 2006, 123–126, 471–485 CrossRef CAS PubMed.
- A. Trovarelli and P. Fornasiero, Catalysis by Ceria and Related Materials, 2013 DOI:10.1142/9781848169647_fmatter.
- J. M. Dowding, S. Das, A. Kumar, T. Dosani, R. McCormack, A. Gupta, T. X. T. Sayle, D. C. Sayle, L. von Kalm, S. Seal and W. T. Self, ACS Nano, 2013, 7, 4855–4868 CrossRef CAS.
- I. Celardo, J. Z. Pedersen, E. Traversa and L. Ghibelli, Nanoscale, 2011, 3, 1411–1420 RSC.
- M. S. Lord, J. F. Berret, S. Singh, A. Vinu and A. S. Karakoti, Small, 2021, 17, 2102342 CrossRef CAS.
- F. Corsi, F. Caputo, E. Traversa and L. Ghibelli, Front. Oncol., 2018, 8, 309 CrossRef PubMed.
- D. Damatov and J. M. Mayer, Chem. Commun., 2016, 52, 10281–10284 RSC.
- B. C. Nelson, M. E. Johnson, M. L. Walker, K. R. Riley and C. M. Sims, Antioxidants, 2016, 5(2), 15 CrossRef PubMed.
- J. M. Dowding, S. Seal and W. T. Self, Drug Delivery Transl. Res., 2013, 3, 375–379 CrossRef CAS.
- W. He, Y. Liu, W. G. Wamer and J.-J. Yin, J. Food Drug Anal., 2014, 22, 49–63 CrossRef CAS PubMed.
- Y. Xue, Q. Luan, D. Yang, X. Yao and K. Zhou, J. Phys. Chem. C, 2011, 115, 4433–4438 CrossRef CAS.
- V. Patel, M. Singh, E. L. H. Mayes, A. Martinez, V. Shutthanandan, V. Bansal, S. Singh and A. S. Karakoti, Chem. Commun., 2018, 54, 13973–13976 RSC.
- A. Srinivas, P. J. Rao, G. Selvam, P. B. Murthy and P. N. Reddy, Toxicol. Lett., 2011, 205, 105–115 CrossRef CAS PubMed.
- A. Filippi, F. Liu, J. Wilson, S. Lelieveld, K. Korschelt, T. Wang, Y. Wang, T. Reich, U. Pöschl, W. Tremel and H. Tong, RSC Adv., 2019, 9, 11077–11081 RSC.
- C. Mahapatra, R. K. Singh, J.-H. Lee, J. Jung, J. K. Hyun and H.-W. Kim, Acta Biomater., 2017, 50, 142–153 CrossRef CAS PubMed.
- F. Corsi, F. Caputo, E. Traversa and L. Ghibelli, Front. Oncol., 2018, 8, 309 CrossRef.
- I. Celardo, M. De Nicola, C. Mandoli, J. Z. Pedersen, E. Traversa and L. Ghibelli, ACS Nano, 2011, 5, 4537–4549 CrossRef CAS PubMed.
- F. Caputo, M. De Nicola, A. Sienkiewicz, A. Giovanetti, I. Bejarano, S. Licoccia, E. Traversa and L. Ghibelli, Nanoscale, 2015, 7, 15643–15656 RSC.
- S.-H. Shin, O. Purevdorj, O. Castano, J. A. Planell and H.-W. Kim, J. Tissue Eng., 2012, 3, 2041731412443530 Search PubMed.
- A. Thill, O. Zeyons, O. Spalla, F. Chauvat, J. Rose, M. Auffan and A. M. Flank, Environ. Sci. Technol., 2006, 40, 6151–6156 CrossRef CAS.
- M. Culcasi, L. Benameur, A. Mercier, C. Lucchesi, H. Rahmouni, A. Asteian, G. Casano, A. Botta, H. Kovacic and S. Pietri, Chem. – Biol. Interact., 2012, 199, 161–176 CrossRef CAS PubMed.
- L. De Marzi, A. Monaco, J. De Lapuente, D. Ramos, M. Borras, M. Di Gioacchino, S. Santucci and A. Poma, Int. J. Mol. Sci., 2013, 14, 3065–3077 CrossRef CAS PubMed.
- G. Pota, B. Silvestri, G. Vitiello, N. Gallucci, R. Di Girolamo, S. Scialla, M. G. Raucci, L. Ambrosio, M. Di Napoli, A. Zanfardino, M. Varcamonti, A. Pezzella and G. Luciani, Biomater. Adv., 2023, 153, 213558 CrossRef CAS PubMed.
- K. D. Patel, A. K. Patel, A. G. Kurian, R. K. Singh and H.-W. Kim, in Nanotheranostics for Treatment and Diagnosis of Infectious Diseases, ed. K. Jain and J. Ahmad, Academic Press, 2022, pp. 319–352 DOI:10.1016/B978-0-323-91201-3.00011-6.
- X. Yuan, X. Zhang, L. Sun, Y. Wei and X. Wei, Part. Fibre Toxicol., 2019, 16, 18 CrossRef PubMed.
- J. Meng, X. Li, C. Wang, H. Guo, J. Liu and H. Xu, ACS Appl. Mater. Interfaces, 2015, 7, 3180–3188 CrossRef CAS.
- E. J. Comparetti, V. d A. Pedrosa and R. Kaneno, Bioconjugate Chem., 2018, 29, 709–718 CrossRef CAS.
- B. D. Holt, P. A. Short, A. D. Rape, Y.-L. Wang, M. F. Islam and K. N. Dahl, ACS Nano, 2010, 4, 4872–4878 CrossRef CAS.
- B. D. Holt, H. Shams, T. A. Horst, S. Basu, A. D. Rape, Y.-L. Wang, G. K. Rohde, M. R. K. Mofrad, M. F. Islam and K. N. Dahl, J. Funct. Biomater., 2012, 3, 398–417 CrossRef CAS PubMed.
- B. D. Holt, K. N. Dahl and M. F. Islam, Small, 2011, 7, 2348–2355 CrossRef CAS.
- M. R. McDevitt, D. Chattopadhyay, B. J. Kappel, J. S. Jaggi, S. R. Schiffman, C. Antczak, J. T. Njardarson, R. Brentjens and D. A. Scheinberg, J. Nucl. Med., 2007, 48, 1180–1189 CrossRef CAS PubMed.
- B. L. Allen, P. D. Kichambare, P. Gou, I. I. Vlasova, A. A. Kapralov, N. Konduru, V. E. Kagan and A. Star, Nano Lett., 2008, 8, 3899–3903 CrossRef CAS.
- V. E. Kagan, N. V. Konduru, W. Feng, B. L. Allen, J. Conroy, Y. Volkov, I. I. Vlasova, N. A. Belikova, N. Yanamala, A. Kapralov, Y. Y. Tyurina, J. Shi, E. R. Kisin, A. R. Murray, J. Franks, D. Stolz, P. Gou, J. Klein-Seetharaman, B. Fadeel, A. Star and A. A. Shvedova, Nat. Nanotechnol., 2010, 5, 354–359 CrossRef CAS PubMed.
- F. T. Andón, A. A. Kapralov, N. Yanamala, W. Feng, A. Baygan, B. J. Chambers, K. Hultenby, F. Ye, M. S. Toprak, B. D. Brandner, A. Fornara, J. Klein-Seetharaman, G. P. Kotchey, A. Star, A. A. Shvedova, B. Fadeel and V. E. Kagan, Small, 2013, 9, 2721–2729 CrossRef PubMed.
- K. Bhattacharya, R. El-Sayed, F. T. Andón, S. P. Mukherjee, J. Gregory, H. Li, Y. Zhao, W. Seo, A. Fornara, B. Brandner, M. S. Toprak, K. Leifer, A. Star and B. Fadeel, Carbon, 2015, 91, 506–517 CrossRef CAS.
- M. Yang and M. Zhang, Front. Mater., 2019, 6, 225 CrossRef.
- B. L. Allen, G. P. Kotchey, Y. Chen, N. V. K. Yanamala, J. Klein-Seetharaman, V. E. Kagan and A. Star, J. Am. Chem. Soc., 2009, 131, 17194–17205 CrossRef CAS.
- M. Zhang, M. Yang, H. Nakajima, M. Yudasaka, S. Iijima and T. Okazaki, ACS Appl. Nano Mater., 2019, 2, 4293–4301 CrossRef CAS.
- J. Ju, S. Regmi, A. Fu, S. Lim and Q. Liu, J. Biophotonics, 2019, 12, e201800367 CrossRef PubMed.
- Z. Jin, N. Dridi, G. Palui, V. Palomo, J. V. Jokerst, P. E. Dawson, Q.-X. A. Sang and H. Mattoussi, Anal. Chem., 2023, 95, 2713–2722 CrossRef CAS PubMed.
- I. L. Christensen, Y.-P. Sun and P. Juzenas, J. Biomed. Nanotechnol., 2011, 7, 667–676 CrossRef CAS.
- V. Ruiz, L. Yate, I. García, G. Cabanero and H.-J. Grande, Carbon, 2017, 116, 366–374 CrossRef CAS.
- L. Nilewski, K. Mendoza, A. S. Jalilov, V. Berka, G. Wu, W. K. A. Sikkema, A. Metzger, R. Ye, R. Zhang, D. X. Luong, T. Wang, E. McHugh, P. J. Derry, E. L. Samuel, T. A. Kent, A.-L. Tsai and J. M. Tour, ACS Appl. Mater. Interfaces, 2019, 11, 16815–16821 CrossRef CAS PubMed.
- Y. Li, S. Li, Y. Wang, J. Wang, H. Liu, X. Liu, L. Wang, X. Liu, W. Xue and N. Ma, Phys. Chem. Chem. Phys., 2017, 19, 11631–11638 RSC.
- W. Stahl and H. Sies, Mol. Aspects Med., 2003, 24, 345–351 CrossRef CAS PubMed.
- K. Okuda, T. Hirota, M. Hirobe, T. Nagano, M. Mochizuki and T. Mashino, Fullerene Sci. Technol., 2000, 8, 127–142 CrossRef CAS.
- K. D. Patel, T.-H. Kim, N. Mandakhbayar, R. K. Singh, J.-H. Jang, J.-H. Lee and H.-W. Kim, Acta Biomater., 2020, 108, 97–110 CrossRef CAS.
- Y.-M. Li, K. D. Patel, Y.-K. Han, S.-M. Hong, Y.-X. Meng, H.-H. Lee, J. H. Park, J. C. Knowles, J. K. Hyun, J.-H. Lee and H.-W. Kim, J. Chem. Eng., 2023, 466, 143125 CrossRef CAS.
- I. Fenoglio, M. Tomatis, D. Lison, J. Muller, A. Fonseca, J. B. Nagy and B. Fubini, Free Radicals Biol. Med., 2006, 40, 1227–1233 CrossRef CAS.
- R. M. Lucente-Schultz, V. C. Moore, A. D. Leonard, B. K. Price, D. V. Kosynkin, M. Lu, R. Partha, J. L. Conyers and J. M. Tour, J. Am. Chem. Soc., 2009, 131, 3934–3941 CrossRef CAS.
- Y. Qiu, Z. Wang, A. C. E. Owens, I. Kulaots, Y. Chen, A. B. Kane and R. H. Hurt, Nanoscale, 2014, 6, 11744–11755 RSC.
- G. de la Torre, G. Bottari and T. Torres, Adv. Energy Mater., 2017, 7, 1601700 CrossRef.
- I. L. Christensen, Y. P. Sun and P. Juzenas, J. Biomed. Nanotechnol., 2011, 7, 667–676 CrossRef CAS.
- W. Zhang, J. Chavez, Z. Zeng, B. Bloom, A. Sheardy, Z. Ji, Z. Yin, D. H. Waldeck, Z. Jia and J. Wei, ACS Appl. Nano Mater., 2018, 1, 2699–2708 CrossRef CAS PubMed.
- G. Huang, Y. Lin, L. Zhang, Z. Yan, Y. Wang and Y. Liu, Sci. Rep., 2019, 9, 19651 CrossRef CAS PubMed.
- Y. Chong, C. Ge, G. Fang, X. Tian, X. Ma, T. Wen, W. G. Wamer, C. Chen, Z. Chai and J. J. Yin, ACS Nano, 2016, 10, 8690–8699 CrossRef CAS.
- S. S. Maktedar, G. Avashthi and M. Singh, RSC Adv., 2016, 6, 114264 RSC.
- H.-Y. Chou, H.-M. D. Wang, C.-H. Kuo, P.-H. Lu, L. Wang, W. Kang and C.-L. Sun, ACS Omega, 2020, 5, 6588–6597 CrossRef CAS.
- G. Choe, S.-W. Kim, J. Park, J. Park, S. Kim, Y. S. Kim, Y. Ahn, D.-W. Jung, D. R. Williams and J. Y. Lee, Biomaterials, 2019, 225, 119513 CrossRef CAS PubMed.
- N. Baali, A. Khecha, A. Bensouici, G. Speranza and N. Hamdouni, C, 2019, 5, 75 CAS.
- C. S. Sharma, S. Sarkar, A. Periyakaruppan, J. Barr, K. Wise, R. Thomas, B. L. Wilson and G. T. Ramesh, J. Nanosci. Nanotechnol., 2007, 7, 2466–2472 CrossRef CAS.
- S. K. Manna, S. Sarkar, J. Barr, K. Wise, E. V. Barrera, O. Jejelowo, A. C. Rice-Ficht and G. T. Ramesh, Nano Lett., 2005, 5, 1676–1684 CrossRef CAS.
- S. Alarifi and D. Ali, Int. J. Toxicol., 2015, 34, 258–265 CrossRef CAS.
- S. Rasras, H. Kalantari, M. Rezaei, M. A. Dehghani, L. Zeidooni, K. Alikarami, F. Dehghani and S. Alboghobeish, Toxicol. Ind. Health, 2019, 35, 497–506 CrossRef CAS.
- W.-C. Hou, S. BeigzadehMilani, C. T. Jafvert and R. G. Zepp, Environ. Sci. Technol., 2014, 48, 3875–3882 CrossRef CAS PubMed.
- A. V. Singh, K. K. Mehta, K. Worley, J. S. Dordick, R. S. Kane and L. Q. Wan, ACS Nano, 2014, 8, 2196–2205 CrossRef CAS PubMed.
- J. Zhang, H. Yang, G. Shen, P. Cheng, J. Zhang and S. Guo, Chem. Commun., 2010, 46, 1112–1114 RSC.
- G. P. Kotchey, B. L. Allen, H. Vedala, N. Yanamala, A. A. Kapralov, Y. Y. Tyurina, J. Klein-Seetharaman, V. E. Kagan and A. Star, ACS Nano, 2011, 5, 2098–2108 CrossRef CAS.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- M. C. Duch, G. R. S. Budinger, Y. T. Liang, S. Soberanes, D. Urich, S. E. Chiarella, L. A. Campochiaro, A. Gonzalez, N. S. Chandel, M. C. Hersam and G. M. Mutlu, Nano Lett., 2011, 11, 5201–5207 CrossRef PubMed.
- X. Yang, Y. Wang, X. Huang, Y. Ma, Y. Huang, R. Yang, H. Duan and Y. Chen, J. Mater. Chem., 2011, 21, 3448–3454 RSC.
- M. Kalbacova, A. Broz, J. Kong and M. Kalbac, Carbon, 2010, 48, 4323–4329 CrossRef CAS.
- T. R. Nayak, H. Andersen, V. S. Makam, C. Khaw, S. Bae, X. Xu, P.-L. R. Ee, J.-H. Ahn, B. H. Hong, G. Pastorin and B. Özyilmaz, ACS Nano, 2011, 5, 4670–4678 CrossRef CAS.
- Z. Liu, J. T. Robinson, X. Sun and H. Dai, J. Am. Chem. Soc., 2008, 130, 10876–10877 CrossRef CAS PubMed.
- J. T. Robinson, S. M. Tabakman, Y. Liang, H. Wang, H. Sanchez Casalongue, D. Vinh and H. Dai, J. Am. Chem. Soc., 2011, 133, 6825–6831 CrossRef CAS PubMed.
- T. Parasassi, R. Brunelli, G. Costa, M. De Spirito, E. Krasnowska, T. Lundeberg, E. Pittaluga and F. Ursini, Sci. World J., 2010, 10, 1192–1202 CrossRef CAS PubMed.
- L. Feng, S. Zhang and Z. Liu, Nanoscale, 2011, 3, 1252–1257 RSC.
- K. Yang, S. Zhang, G. Zhang, X. Sun, S. T. Lee and Z. Liu, Nano Lett., 2010, 10, 3318–3323 CrossRef CAS.
- H. Hong, K. Yang, Y. Zhang, J. W. Engle, L. Feng, Y. Yang, T. R. Nayak, S. Goel, J. Bean, C. P. Theuer, T. E. Barnhart, Z. Liu and W. Cai, ACS Nano, 2012, 6, 2361–2370 CrossRef CAS PubMed.
- Y. Wang, J. Lu, L. Tang, H. Chang and J. Li, Anal. Chem., 2009, 81, 9710–9715 CrossRef CAS.
- Z. Zhu, J. Qian, X. Zhao, W. Qin, R. Hu, H. Zhang, D. Li, Z. Xu, B. Z. Tang and S. He, ACS Nano, 2016, 10, 588–597 CrossRef CAS.
- B. Tian, C. Wang, S. Zhang, L. Feng and Z. Liu, ACS Nano, 2011, 5, 7000–7009 CrossRef CAS.
- H.-S. Hsieh and R. G. Zepp, Environ. Sci.: Nano, 2019, 6, 3734–3744 RSC.
- X. Niu, J. Chen and J. Gao, Asian J. Pharm. Sci., 2019, 14, 480–496 CrossRef.
- Y. Liu, G. Li, L. Huan and S. Cao, Nanoscale, 2024, 16, 504–526 RSC.
- T. K. Nguyen, S. Aberoumand and D. V. Dao, Small, 2021, 17, 2101775 CrossRef CAS.
- X. Li, W. Li, Q. Liu, S. Chen, L. Wang, F. Gao, G. Shao, Y. Tian, Z. Lin and W. Yang, Adv. Funct. Mater., 2021, 31, 2008901 CrossRef CAS.
- F. Chen, G. Li, E. R. Zhao, J. Li, G. Hableel, J. E. Lemaster, Y. Bai, G. L. Sen and J. V. Jokerst, Biomaterials, 2018, 179, 60–70 CrossRef CAS PubMed.
- A. Borkowski, M. Szala, P. Kowalczyk, T. Cłapa, D. Narożna and M. Selwet, Chemosphere, 2015, 135, 233–239 CrossRef CAS PubMed.
- J. Pourchez, V. Forest, N. Boumahdi, D. Boudard, M. Tomatis, B. Fubini, N. Herlin-Boime, Y. Leconte, B. Guilhot, M. Cottier and P. Grosseau, J. Nanopart. Res., 2012, 14, 1143 CrossRef.
- M. Schieber and N. S. Chandel, Curr. Biol., 2014, 24, R453–R462 CrossRef CAS.
- Z. Yi, H. I. Hussain, C. Feng, D. Sun, F. She, J. E. Rookes, D. M. Cahill and L. Kong, ACS Appl. Mater. Interfaces, 2015, 7, 9937–9946 CrossRef CAS.
- L. Chen, X. Zhou, W. Nie, Q. Zhang, W. Wang, Y. Zhang and C. He, ACS Appl. Mater. Interfaces, 2016, 8, 33829–33841 CrossRef CAS.
- Q. Zhao, H. Geng, Y. Wang, Y. Gao, J. Huang, Y. Wang, J. Zhang and S. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 20290–20299 CrossRef CAS.
- R. Cheng, F. Feng, F. Meng, C. Deng, J. Feijen and Z. Zhong, J. Controlled Release, 2011, 152, 2–12 CrossRef CAS.
- S. Zanganeh, G. Hutter, R. Spitler, O. Lenkov, M. Mahmoudi, A. Shaw, J. S. Pajarinen, H. Nejadnik, S. Goodman, M. Moseley, L. M. Coussens and H. E. Daldrup-Link, Nat. Nanotechnol., 2016, 11, 986–994 CrossRef CAS.
- J.-W. Kim, C. Mahapatra, J.-Y. Hong, M. S. Kim, K. W. Leong, H.-W. Kim and J. K. Hyun, Adv. Sci., 2017, 4, 1700034 CrossRef.
- C. Nathan and A. Cunningham-Bussel, Nat. Rev. Immunol., 2013, 13, 349–361 CrossRef CAS.
- T.-C. Cheong, E. P. Shin, E.-K. Kwon, J.-H. Choi, K.-K. Wang, P. Sharma, K. H. Choi, J.-M. Lim, H.-G. Kim, K. Oh, J.-H. Jeon, I. So, I.-G. Kim, M.-S. Choi, Y. K. Kim, S.-Y. Seong, Y.-R. Kim and N.-H. Cho, ACS Chem. Biol., 2015, 10, 757–765 CrossRef CAS.
- B. Zhu, Y. Li, Z. Lin, M. Zhao, T. Xu, C. Wang and N. Deng, Nanoscale Res. Lett., 2016, 11, 198 CrossRef PubMed.
- R. Pal, B. Chakraborty, A. Nath, L. M. Singh, M. Ali, D. S. Rahman, S. K. Ghosh, A. Basu, S. Bhattacharya, R. Baral and M. Sengupta, Int. Immunopharmacol., 2016, 38, 332–341 CrossRef CAS PubMed.
- Y. Lu, Y. Yang, Z. Gu, J. Zhang, H. Song, G. Xiang and C. Yu, Biomaterials, 2018, 175, 82–92 CrossRef CAS PubMed.
- M. Schieber and N. S. Chandel, Curr. Biol., 2014, 24, R453–R462 CrossRef CAS.
- C. Gorrini, I. S. Harris and T. W. Mak, Nat. Rev. Drug Discovery, 2013, 12, 931–947 CrossRef CAS.
- G. Cui, H. Zhang, Q. Guo, S. Shan, S. Chen, C. Li, X. Yang, Z. Li, Y. Mu, H. Shao and Z. Du, Toxicol. Mech. Methods, 2020, 30, 646–655 CrossRef CAS.
- G. N. Joshi, A. M. Goetjen and D. A. Knecht, Mol. Biol. Cell, 2015, 26, 3150–3164 CrossRef CAS PubMed.
- Y.-J. Lin, C.-C. Yang, I.-T. Lee, W.-B. Wu, C.-C. Lin, L.-D. Hsiao and C.-M. Yang, Biomedicines, 2023, 11, 2628 CrossRef CAS PubMed.
- S. N. Petrache Voicu, D. Dinu, C. Sima, A. Hermenean, A. Ardelean, E. Codrici, M. S. Stan, O. Zărnescu and A. Dinischiotu, Int. J. Mol. Sci., 2015, 16, 29398–29416 CrossRef.
- C. Marques, P. Maroni, L. Maurizi, O. Jordan and G. Borchard, Int. J. Biol. Macromol., 2024, 256, 128339 CrossRef CAS.
- S. Tenzer, D. Docter, J. Kuharev, A. Musyanovych, V. Fetz, R. Hecht, F. Schlenk, D. Fischer, K. Kiouptsi, C. Reinhardt, K. Landfester, H. Schild, M. Maskos, S. K. Knauer and R. H. Stauber, Nat. Nanotechnol., 2013, 8, 772–781 CrossRef CAS PubMed.
- Y.-M. Go and D. P. Jones, J. Biol. Chem., 2013, 288, 26512–26520 CrossRef CAS.
- R. Kumar, G. R. Pulikanti, K. R. Shankar, D. Rambabu, V. Mangili, L. R. Kumbam, P. S. Sagara, N. Nakka and M. Yogesh, in Metal Oxides for Biomedical and Biosensor Applications, ed. K. Mondal, Elsevier, 2022, pp. 205–231 DOI:10.1016/B978-0-12-823033-6.00007-7.
- R. K. Singh, K. D. Patel, K. W. Leong and H.-W. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 10309–10337 CrossRef CAS.
- A. Luchini and G. Vitiello, Front. Chem., 2019, 7, 343 CrossRef CAS.
- S. F. Lim and R. H. Austin, in Applications of Nanoscience in Photomedicine, ed. M. R. Hamblin and P. Avci, Chandos Publishing, Oxford, 2015, pp. 377–391 DOI:10.1533/9781908818782.377.
- S. Wen, J. Zhou, K. Zheng, A. Bednarkiewicz, X. Liu and D. Jin, Nat. Commun., 2018, 9, 2415 CrossRef PubMed.
- A. Priyam, N. M. Idris and Y. Zhang, J. Mater. Chem., 2012, 22, 960–965 RSC.
- Y. He, S. Guo, L. Wu, P. Chen, L. Wang, Y. Liu and H. Ju, Biomaterials, 2019, 225, 119501 CrossRef CAS.
- W.-H. Chen, G.-F. Luo, W.-X. Qiu, Q. Lei, S. Hong, S.-B. Wang, D.-W. Zheng, C.-H. Zhu, X. Zeng, J. Feng, S.-X. Cheng and X.-Z. Zhang, Small, 2016, 12, 733–744 CrossRef CAS PubMed.
- L. Diebold and N. S. Chandel, Free Radicals Biol. Med., 2016, 100, 86–93 CrossRef CAS.
- S. Kumari, A. K. Badana, M. M. G, S. G and R. Malla, Biomarker Insights, 2018, 13, 1177271918755391 CrossRef.
- A. D. Pandya, E. Jager, S. Bagheri Fam, A. Hocherl, A. Jager, V. Sincari, B. Nystrom, P. Stepanek, T. Skotland, K. Sandvig, M. Hruby and G. M. Maelandsmo, Int. J. Nanomed., 2019, 14, 6269–6285 CrossRef CAS PubMed.
- P. Sonkusre and S. S. Cameotra, J. Nanobiotechnol., 2017, 15, 43 CrossRef PubMed.
- A. Khurana, S. Tekula, M. A. Saifi, P. Venkatesh and C. Godugu, Biomed. Pharmacother., 2019, 111, 802–812 CrossRef CAS PubMed.
- A. P. Bidkar, P. Sanpui and S. S. Ghosh, Nanomedicine, 2017, 12, 2641–2651 CrossRef CAS PubMed.
- C.-W. Chen, Y.-C. Chan, M. Hsiao and R.-S. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 32108–32119 CrossRef CAS.
- H. Luo, F. Wang, Y. Bai, T. Chen and W. Zheng, Colloids Surf., B, 2012, 94, 304–308 CrossRef CAS PubMed.
- L. Kong, Q. Yuan, H. Zhu, Y. Li, Q. Guo, Q. Wang, X. Bi and X. Gao, Biomaterials, 2011, 32, 6515–6522 CrossRef CAS PubMed.
- Y. Zhuang, L. Li, L. Feng, S. Wang, H. Su, H. Liu, H. Liu and Y. Wu, Nanoscale, 2020, 12, 1389–1396 RSC.
- L. Chan, L. He, B. Zhou, S. Guan, M. Bo, Y. Yang, Y. Liu, X. Liu, Y. Zhang, Q. Xie and T. Chen, Chem. – Asian J., 2017, 12, 3053–3060 CrossRef CAS.
- F. L. Heppner, R. M. Ransohoff and B. Becher, Nat. Rev. Neurosci., 2015, 16, 358–372 CrossRef CAS.
- F. Fanizza, M. Campanile, G. Forloni, C. Giordano and D. Albani, J. Tissue Eng., 2022, 13, 1–20, DOI:10.1177/2041731422109533.
- O. Devinsky, A. Vezzani, S. Najjar, N. C. De Lanerolle and M. A. Rogawski, Trends Neurosci., 2013, 36, 174–184 CrossRef CAS.
- D. Kempuraj, R. Thangavel, P. A. Natteru, G. P. Selvakumar, D. Saeed, H. Zahoor, S. Zaheer, S. S. Iyer and A. Zaheer, J. Neurol. Neurosurg. Spine, 2016, 1, 1003 Search PubMed.
- M. T. Lin and M. F. Beal, Nature, 2006, 443, 787–795 CrossRef CAS.
- S. Gandhi and A. Y. Abramov, Oxid. Med. Cell. Longevity, 2012, 2012, 428010 Search PubMed.
- E. Radi, P. Formichi, C. Battisti and A. Federico, J. Alzheimer's Dis., 2014, 42(Suppl 3), S125–S152 Search PubMed.
- A. Federico, E. Cardaioli, P. Da Pozzo, P. Formichi, G. N. Gallus and E. Radi, J. Neurol. Sci., 2012, 322, 254–262 CrossRef CAS.
- P. J. Landrigan, B. Sonawane, R. N. Butler, L. Trasande, R. Callan and D. Droller, Environ. Health Perspect., 2005, 113, 1230–1233 CrossRef CAS PubMed.
- L. M. Billings, S. Oddo, K. N. Green, J. L. McGaugh and F. M. LaFerla, Neuron, 2005, 45, 675–688 CrossRef CAS PubMed.
- E. D. Roberson, K. Scearce-Levie, J. J. Palop, F. Yan, I. H. Cheng, T. Wu, H. Gerstein, G.-Q. Yu and L. Mucke, Science, 2007, 316, 750–754 CrossRef CAS PubMed.
- T. Yin, L. Yang, Y. Liu, X. Zhou, J. Sun and J. Liu, Acta Biomater., 2015, 25, 172–183 CrossRef CAS PubMed.
- L. Yang, Q. Chen, Y. Liu, J. Zhang, D. Sun, Y. Zhou and J. Liu, J. Mater. Chem. B, 2014, 2, 1977–1987 RSC.
- N. Huang, X. Chen, X. Zhu, M. Xu and J. Liu, Biomaterials, 2017, 141, 296–313 CrossRef CAS PubMed.
- S. Mukherjee, V. S. Madamsetty, D. Bhattacharya, S. Roy Chowdhury, M. K. Paul and A. Mukherjee, Adv. Funct. Mater., 2020, 30, 2003054 CrossRef CAS.
- G. H. Kim, J. E. Kim, S. J. Rhie and S. Yoon, Exp. Neurobiol., 2015, 24, 325–340 CrossRef PubMed.
- H. E. Moon and S. H. Paek, Exp. Neurobiol., 2015, 24, 103–116 CrossRef PubMed.
- J. Blesa, I. Trigo-Damas, A. Quiroga-Varela and V. R. Jackson-Lewis, Front. Neuroanat., 2015, 9, 91 Search PubMed.
- W. J. Szlachcic, P. M. Switonski, W. J. Krzyzosiak, M. Figlerowicz and M. Figiel, Dis. Models Mech., 2015, 8, 1047–1057 CAS.
- G. Cenini, A. Lloret and R. Cascella, Oxid. Med. Cell. Longevity, 2019, 2019, 2105607 Search PubMed.
- S. Kausar, F. Wang and H. Cui, Cells, 2018, 7(12), 274 CAS.
- A. Grzelak, M. Wojewodzka, S. Meczynska-Wielgosz, M. Zuberek, D. Wojciechowska and M. Kruszewski, Redox Biol., 2018, 15, 435–440 CrossRef CAS PubMed.
- A. Gupta, S. Das and S. Seal, Nanomedicine, 2014, 9, 2725–2728 CrossRef CAS.
- S. Naz, J. Beach, B. Heckert, T. Tummala, O. Pashchenko, T. Banerjee and S. Santra, Nanomedicine, 2017, 12, 545–553 CrossRef CAS.
- H. Zhou, Y. Gong, Y. Liu, A. Huang, X. Zhu, J. Liu, G. Yuan, L. Zhang, J.-A. Wei and J. Liu, Biomaterials, 2020, 237, 119822 CrossRef CAS.
- H. J. Kwon, D. Kim, K. Seo, Y. G. Kim, S. I. Han, T. Kang, M. Soh and T. Hyeon, Angew. Chem., Int. Ed., 2018, 57, 9408–9412 CrossRef CAS.
- L. Zhang, P.-F. Wei, Y.-H. Song, L. Dong, Y.-D. Wu, Z.-Y. Hao, S. Fan, S. Tai, J.-L. Meng, Y. Lu, J. Xue, C.-Z. Liang and L.-P. Wen, Biomaterials, 2019, 216, 119248 CrossRef CAS PubMed.
- X. Li, J. Tsibouklis, T. Weng, B. Zhang, G. Yin, G. Feng, Y. Cui, I. N. Savina, L. I. Mikhalovska, S. R. Sandeman, C. A. Howel and S. V. Mikhalovsky, J. Drug Targeting, 2017, 25, 17–28 CrossRef CAS PubMed.
- D. K. Sarko and C. E. McKinney, Front. Neurosci., 2017, 11, 82 Search PubMed.
- S. M. ElMorsy, D. A. Gutierrez, S. Valdez, J. Kumar, R. J. Aguilera, M. Noufal, H. Sarma, S. Chinnam and M. Narayan, J. Colloid Interface Sci., 2024, 670, 357–363 CrossRef CAS PubMed.
- R.-R. Lin, L.-L. Jin, Y.-Y. Xue, Z.-S. Zhang, H.-F. Huang, D.-F. Chen, Q. Liu, Z.-W. Mao, Z.-Y. Wu and Q.-Q. Tao, Adv. Sci., 2024, 11, 2306675 CrossRef CAS PubMed.
- H. Tan, Y. Huang, S. Dong, Z. Bai, C. Chen, X. Wu, M. Chao, H. Yan, S. Wang, D. Geng and F. Gao, Small, 2023, 19, 2303530 CrossRef CAS PubMed.
- X. Song, Q. Ding, W. Wei, J. Zhang, R. Sun, L. Yin, S. Liu and Y. Pu, Small, 2023, 19, 2206959 CAS.
- R. Ruotolo, G. De Giorgio, I. Minato, M. G. Bianchi, O. Bussolati and N. Marmiroli, Nanomaterials, 2020, 10(2), 235 CrossRef CAS PubMed.
- W. DeCoteau, K. L. Heckman, A. Y. Estevez, K. J. Reed, W. Costanzo, D. Sandford, P. Studlack, J. Clauss, E. Nichols, J. Lipps, M. Parker, B. Hays-Erlichman, J. C. Leiter and J. S. Erlichman, Nanomedicine, 2016, 12, 2311–2320 CrossRef CAS PubMed.
- W. Cong, R. Bai, Y.-F. Li, L. Wang and C. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 34725–34735 CrossRef CAS.
- A. M. Khan, B. Korzeniowska, V. Gorshkov, M. Tahir, H. Schrøder, L. Skytte, K. L. Rasmussen, S. Khandige, J. Møller-Jensen and F. Kjeldsen, Nanotoxicology, 2019, 13, 221–239 CrossRef CAS.
- H. J. Kwon, M.-Y. Cha, D. Kim, D. K. Kim, M. Soh, K. Shin, T. Hyeon and I. Mook-Jung, ACS Nano, 2016, 10, 2860–2870 CrossRef CAS PubMed.
- Z. Gao, Y. Nakanishi, S. Noda, H. Omachi, H. Shinohara, H. Kimura and Y. Nagasaki, J. Biomater. Sci., Polym. Ed., 2017, 28, 1036–1050 CrossRef CAS PubMed.
- Y. Shen, B. Cao, N. R. Snyder, K. M. Woeppel, J. R. Eles and X. T. Cui, J. Nanobiotechnol., 2018, 16, 13 CrossRef PubMed.
- P. Boonruamkaew, P. Chonpathompikunlert, L. B. Vong, S. Sakaue, Y. Tomidokoro, K. Ishii, A. Tamaoka and Y. Nagasaki, Sci. Rep., 2017, 7, 3785 CrossRef PubMed.
- L. Ling, Y. Jiang, Y. Liu, H. Li, A. Bari, R. Ullah and J. Xue, J. Photochem. Photobiol., B, 2019, 201, 111657 CrossRef CAS.
- A. V. Ivanov, B. Bartosch and M. G. Isaguliants, Oxid. Med. Cell. Longevity, 2017, 2017, 3496043 Search PubMed.
- U. Z. Paracha, K. Fatima, M. Alqahtani, A. Chaudhary, A. Abuzenadah, G. Damanhouri and I. Qadri, Virol. J., 2013, 10, 251 CrossRef.
- S. Li, H. Li, X. Xu, P. E. Saw and L. Zhang, Theranostics, 2020, 10, 1262–1280 CrossRef CAS PubMed.
- N. Thakur, P. Manna and J. Das, J. Nanobiotechnol., 2019, 17, 84 CrossRef.
- Y. Huang, M. Zhang, M. Jin, T. Ma, J. Guo, X. Zhai and Y. Du, Adv. Healthcare Mater., 2023, 12, 2300748 CrossRef CAS.
- M. G. Schappi, V. Jaquet, D. C. Belli and K. H. Krause, Semin. Immunopathol., 2008, 30, 255–271 CrossRef.
- Q. Ren, S. Sun and X.-D. Zhang, Nano Res., 2021, 14, 2535–2557 CrossRef CAS.
- T. Kuijpers and R. Lutter, Cell. Mol. Life Sci., 2012, 69, 7–15 CrossRef CAS.
- K. S. Kim, D. Lee, C. G. Song and P. M. Kang, Nanomedicine, 2015, 10, 2709–2723 CrossRef CAS PubMed.
- T. Hemnani and M. S. Parihar, Indian J. Physiol. Pharmacol., 1998, 42, 440–452 CAS.
- A. Chompoosor, K. Saha, P. S. Ghosh, D. J. Macarthy, O. R. Miranda, Z. J. Zhu, K. F. Arcaro and V. M. Rotello, Small, 2010, 6, 2246–2249 CrossRef CAS PubMed.
- U. S. Srinivas, B. W. Q. Tan, B. A. Vellayappan and A. D. Jeyasekharan, Redox Biol., 2019, 25, 101084 CrossRef CAS.
- A. Grzelak, M. Wojewódzka, S. Meczynska-Wielgosz, M. Zuberek, D. Wojciechowska and M. Kruszewski, Redox Biol., 2018, 15, 435–440 CrossRef CAS PubMed.
- P. Patel, K. Kansara, R. Singh, R. K. Shukla, S. Singh, A. Dhawan and A. Kumar, Int. J. Nanomed., 2018, 13, 39–41 CrossRef CAS.
- K. Mortezaee, M. Najafi, H. Samadian, H. Barabadi, A. Azarnezhad and A. Ahmadi, Chem.-Biol. Interact., 2019, 312, 108814 CrossRef CAS PubMed.
- Y. Li, S. Yu, Q. Wu, M. Tang, Y. Pu and D. Wang, J. Hazard. Mater., 2012, 219–220, 221–230 CrossRef CAS PubMed.
- K. R. Smith, L. R. Klei and A. Barchowsky, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2001, 280, L442–L449 CrossRef CAS.
- S. J. Soenen, P. Rivera-Gil, J.-M. Montenegro, W. J. Parak, S. C. De Smedt and K. Braeckmans, Nano Today, 2011, 6, 446–465 CrossRef CAS.
- K. Bedard, H. Attar, J. Bonnefont, V. Jaquet, C. Borel, O. Plastre, M. J. Stasia, S. E. Antonarakis and K. H. Krause, Hum. Mutat., 2009, 30, 1123–1133 CrossRef CAS PubMed.
- J. A. Nogueira-Machado and M. M. Chaves, Expert Opin. Ther. Targets, 2008, 12, 871–882 CrossRef CAS.
- Y. Zhang, T. Peng, H. Zhu, X. Zheng, X. Zhang, N. Jiang, X. Cheng, X. Lai, A. Shunnar, M. Singh, N. Riordan, V. Bogin, N. Tong and W. P. Min, J. Transl. Med., 2010, 8, 133 CrossRef CAS PubMed.
- R. F. Loeser, Trans. Am. Clin. Climatol. Assoc., 2017, 128, 44–54 Search PubMed.
- Y. Henrotin, G. Deby-Dupont, C. Deby, M. De Bruyn, M. Lamy and P. Franchimont, Br. J. Rheumatol., 1993, 32, 562–567 CrossRef CAS.
- M. L. Tiku, J. B. Liesch and F. M. Robertson, J. Immunol., 1990, 145, 690–696 CrossRef CAS.
- Y. E. Henrotin, P. Bruckner and J. P. L. Pujol, Osteoarthritis Cartilage, 2003, 11, 747–755 CrossRef CAS PubMed.
- M. A. Altay, C. Erturk, A. Bilge, M. Yapti, A. Levent and N. Aksoy, Rheumatol. Int., 2015, 35, 1725–1731 CrossRef CAS.
- C. Erturk, M. A. Altay, S. Selek and A. Kocyigit, Scand. J. Clin. Lab. Invest., 2012, 72, 433–439 CrossRef.
- C. M. Davies, F. Guilak, J. B. Weinberg and B. Fermor, Osteoarthritis Cartilage, 2008, 16, 624–630 CrossRef CAS.
- V. N.-S. Lucas José Sá da Fonseca, M. Oliveira Fonseca Goulart and L. Antas Rabelo, Oxid. Med. Cell. Longevity, 2019, 2019, 16 Search PubMed.
- R. Bordy, P. Totoson, C. Prati, C. Marie, D. Wendling and C. Demougeot, Nat. Rev. Rheumatol., 2018, 14, 404–420 CrossRef CAS.
- D. Chen, J. Shen, W. Zhao, T. Wang, L. Han, J. L. Hamilton and H.-J. Im, Bone Res., 2017, 5, 16044 CrossRef CAS.
- J. S. Lee, M. L. Cho, J. Y. Jhun, S. Y. Min, J. H. Ju, C. H. Yoon, J. K. Min, S. H. Park, H. Y. Kim and Y. G. Cho, J. Clin. Immunol., 2006, 26, 204–212 CrossRef CAS PubMed.
- D. Jawaheer, M. F. Seldin, C. I. Amos, W. V. Chen, R. Shigeta, C. Etzel, A. Damle, X. Xiao, D. Chen, R. F. Lum, J. Monteiro, M. Kern, L. A. Criswell, S. Albani, J. L. Nelson, D. O. Clegg, R. Pope, H. W. Schroeder, Jr., S. L. Bridges, Jr., D. S. Pisetsky, R. Ward, D. L. Kastner, R. L. Wilder, T. Pincus, L. F. Callahan, D. Flemming, M. H. Wener and P. K. Gregersen, Arthritis Rheum., 2003, 48, 906–916 CrossRef CAS PubMed.
- A. Mirshafiey and M. Mohsenzadegan, Iran. J. Allergy, Asthma Immunol., 2008, 7, 195–202 CAS.
- M. Hultqvist, P. Olofsson, J. Holmberg, B. T. Bäckström, J. Tordsson and R. Holmdahl, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12646–12651 CrossRef CAS.
- S. Mangialaio, H. Ji, A. S. Korganow, V. Kouskoff, C. Benoist and D. Mathis, Arthritis Rheum., 1999, 42, 2517–2523 CrossRef CAS PubMed.
- A. Corthay, A. S. Hansson and R. Holmdahl, Arthritis Rheum., 2000, 43, 844–851 CrossRef CAS.
- K. Kannan, R. A. Ortmann and D. Kimpel, Pathophysiology, 2005, 12, 167–181 CrossRef PubMed.
- K. A. Gelderman, M. Hultqvist, L. M. Olsson, K. Bauer, A. Pizzolla, P. Olofsson and R. Holmdahl, Antioxid. Redox Signaling, 2007, 9, 1541–1567 CrossRef CAS.
- S. Saxena, H. Vekaria, P. G. Sullivan and A. W. Seifert, Nat. Commun., 2019, 10, 4400 CrossRef PubMed.
- C. Dunnill, T. Patton, J. Brennan, J. Barrett, M. Dryden, J. Cooke, D. Leaper and N. T. Georgopoulos, Int. Wound J., 2017, 14, 89–96 CrossRef PubMed.
- H. Dou, S. Wang, J. Hu, J. Song, C. Zhang, J. Wang and L. Xiao, J. Tissue Eng., 2023, 14, 1–23, DOI:10.1177/20417314231172584.
- S. Zhou, M. Xie, J. Su, B. Cai, J. Li and K. Zhang, J. Tissue Eng., 2023, 14, 1–28, DOI:10.1177/20417314231185848.
- Y. Yao, H. Zhang, Z. Wang, J. Ding, S. Wang, B. Huang, S. Ke and C. Gao, J. Mater. Chem. B, 2019, 7, 5019–5037 RSC.
- T. Wang, Y. Zheng, Y. Shen, Y. Shi, F. Li, C. Su and L. Zhao, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 138–149 CrossRef.
- T. Tang, H. Jiang, Y. Yu, F. He, S. Z. Ji, Y. Y. Liu, Z. S. Wang, S. C. Xiao, C. Tang, G. Y. Wang and Z. F. Xia, Int. J. Nanomed., 2015, 10, 6571–6585 CAS.
- M. Nakayama, R. Sasaki, C. Ogino, T. Tanaka, K. Morita, M. Umetsu, S. Ohara, Z. Tan, Y. Nishimura, H. Akasaka, K. Sato, C. Numako, S. Takami and A. Kondo, Radiat. Oncol., 2016, 11, 91 CrossRef.
- T. Thenmozhi, 3 Biotech., 2020, 10, 82 CrossRef CAS.
- H. M. Fahmy, N. M. Ebrahim and M. H. Gaber, J. Trace Elem. Med. Biol., 2020, 60, 126481 CrossRef CAS PubMed.
- K.-I. Lee, J.-W. Lin, C.-C. Su, K.-M. Fang, C.-Y. Yang, C.-Y. Kuo, C.-C. Wu, C.-T. Wu and Y.-W. Chen, Toxicol. In Vitro, 2020, 63, 104739 CrossRef CAS.
- S. R. Choudhury, J. Ordaz, C. L. Lo, N. P. Damayanti, F. Zhou and J. Irudayaraj, Toxicol. Sci, 2017, 156, 261–274 Search PubMed.
- S. May, C. Hirsch, A. Rippl, N. Bohmer, J.-P. Kaiser, L. Diener, A. Wichser, A. Bürkle and P. Wick, Nanoscale, 2018, 10, 15723–15735 RSC.
- E. Abbasi-Oshaghi, F. Mirzaei and M. Pourjafar, Int. J. Nanomed., 2019, 14, 1919–1936 CrossRef CAS PubMed.
- O. M. Abd El-Moneim, A. H. Abd El-Rahim and N. A. Hafiz, Toxicol. Rep., 2018, 5, 771–776 CrossRef CAS PubMed.
- L. M. Rossbach, D. H. Oughton, E. Maremonti, C. Coutris and D. A. Brede, Sci. Total Environ., 2020, 721, 137665 CrossRef CAS.
- M. Ahamed, M. J. Akhtar, M. A. M. Khan, S. A. Alrokayan and H. A. Alhadlaq, Chemosphere, 2019, 216, 823–831 CrossRef CAS.
- X. Shi, Y. Tian, S. Zhai, Y. Liu, S. Chu and Z. Xiong, Front. Chem., 2023, 11, 1115440 CrossRef CAS PubMed.
- J. Bouayed and T. Bohn, Oxid. Med. Cell. Longevity, 2010, 3(4), 228–237 CrossRef.
- A. Adhikari, S. Mondal, S. Darbar and S. Kumar Pal, Biomol. Concepts, 2019, 10(1), 160–174 CAS.
- S. Hua, M. B. C. de Matos, J. M. Metselaar and G. Storm, Front. Pharmacol., 2018, 9, 790 CrossRef.
- P. C. Naha, K. C. Lau, J. C. Hsu, M. Hajfathalian, S. Mian, P. Chhour, L. Uppuluri, E. S. McDonald, A. D. A. Maidment and D. P. Cormode, Nanoscale, 2016, 8(28), 13740–13754 RSC.
- D. P. Cormode, L. Gao and H. Koo, Trends Biotechnol., 2018, 36(1), 15–29 CrossRef CAS PubMed.
- A. Nel, T. Xia, H. Meng, X. Wang, S. Lin, Z. Ji and H. Zhang, Acc. Chem. Res., 2013, 46(3), 607–621 CrossRef CAS PubMed.
- B. C. Nelson, C. W. Wright, Y. Ibuki, M. Moreno-Villanueva, H. L. Karlsson, G. Hendriks, C. M. Sims, N. Singh and S. H. Doak, Mutagenesis, 2017, 32(1), 215–232 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |