Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical conditions on Hycean worlds†
Nikku
Madhusudhan
 *a,
Julianne I.
Moses
*a,
Julianne I.
Moses
 b,
Frances
Rigby
b,
Frances
Rigby
 a and
Edouard
Barrier
a and
Edouard
Barrier
 a
a
aInstitute of Astronomy, University of Cambridge, Cambridge, UK. E-mail: nmadhu@ast.cam.ac.uk
bSpace Science Institute, Boulder, CO, USA
First published on 2nd August 2023
Abstract
Traditionally, the search for life on exoplanets has been predominantly focused on rocky exoplanets. The recently proposed Hycean worlds have the potential to significantly expand and accelerate the search for life elsewhere. Hycean worlds are a class of habitable sub-Neptunes with planet-wide oceans and H2-rich atmospheres. Their broad range of possible sizes and temperatures lead to a wide habitable zone and high potential for discovery and atmospheric characterization using transit spectroscopy. Over a dozen candidate Hycean planets are already known to be transiting nearby M dwarfs, making them promising targets for atmospheric characterization with the James Webb Space Telescope (JWST). In this work, we investigate possible chemical conditions on a canonical Hycean world, focusing on (a) the present and primordial molecular composition of the atmosphere, and (b) the inventory of bioessential elements for the origin and sustenance of life in the ocean. Based on photochemical and kinetic modeling for a range of conditions, we discuss the possible chemical evolution and observable present-day composition of its atmosphere. In particular, for reduced primordial conditions the early atmospheric evolution passes through a phase that is rich in organic molecules that could provide important feedstock for prebiotic chemistry. We investigate avenues for delivering bioessential metals to the ocean, considering the challenging lack of weathering from a rocky surface and the ocean separated from the rocky core by a thick icy mantle. Based on ocean depths from internal structure modelling and elemental estimates for the early Earth's oceans, we estimate the requirements for bioessential metals in such a planet. We find that the requirements can be met for plausible assumptions about impact history and atmospheric sedimentation, and supplemented by other steady state sources. We discuss the observational prospects for atmospheric characterisation of Hycean worlds with JWST and future directions of this new paradigm in the search for life on exoplanets.
1 Introduction
The search for life elsewhere is the holy grail of exoplanet science. While the detection of an atmospheric signature of an Earth-like planet orbiting a sun-like star remains a difficult goal,1–3 habitable-zone sub-Neptunes transiting nearby M dwarfs are more accessible; we refer to sub-Neptunes as planets with sizes between those of Earth and Neptune. Their bright and small host stars lead to larger planet–star size contrasts, making them more conducive to atmospheric characterisation using transit spectroscopy. Traditionally, the search for biosignatures in exoplanets has been focused on rocky exoplanets in the terrestrial habitable zone.4–6 Like terrestrial planets in the solar system, such planets are expected to have predominantly rocky interior compositions but their atmospheres may be more diverse, ranging from heavy CO2-rich or N2-rich atmospheres to lighter H2-rich atmospheres.4,6,7 Recent transit surveys have discovered a number of super-Earths in the habitable zones of their host stars, notably around nearby M dwarfs, e.g. TRAPPIST-1,8 LHS 1140,9–11 and TOI-700.12,13 Theoretical studies show that the James Webb Space Telescope (JWST) will have the capability to detect potential atmospheric biosignatures in such planets, albeit with significant investment of observing time.14–16Recently, a new class of sub-Neptune exoplanets, Hycean worlds, has been proposed to significantly expand and accelerate the search for life elsewhere. Hycean worlds are temperate sub-Neptunes with ocean-covered surfaces underneath H2-rich atmospheres.17 The temperatures and pressures in their oceans allow for habitable conditions similar to those known to sustain life in Earth's oceans. Hycean planets offer significant advantages in the search for life compared to traditionally favoured habitable super-Earths with terrestrial-like atmospheres. Firstly, due to the possibility of a large water fraction such planets can be significantly larger in size for their mass compared to rocky planets, up to 2.6 R⊕ for 10 M⊕. Several recent studies have shown temperate sub-Neptunes in this size range to be capable of hosting surface liquid water and deep oceans for a range of atmospheric and interior conditions.18–20 Secondly, the H2-rich atmospheres allow for a much wider habitable-zone for Hycean worlds compared to the terrestrial habitable zone.17 The potential of H2-rich atmospheres to sustain surface habitable temperatures for large orbital separations has also been suggested previously in the context of rocky exoplanets.21,22 Hycean planets at large orbital separations are also expected to be conducive for long-term habitability.23
Thanks to their physical properties Hycean worlds are more detectable and more accessible to atmospheric characterisation with current facilities compared to rocky habitable planets. The low mean molecular weight of their hydrogen-rich atmospheres and lower bulk gravity lead to larger atmospheric scale heights for Hycean planets relative to rocky planets of comparable mass. The extended atmospheres along with the large radii make them more favourable for transmission spectroscopy and searching for spectroscopic biomarkers.17 The size range of Hycean planets (∼1–2.6 R⊕) lies in the sub-Neptune regime (∼1–4 R⊕), the radius range that dominates the currently known exoplanet population.24,25 In particular, the Kepler26 and TESS27 missions have discovered tens of temperate sub-Neptunes orbiting nearby M dwarfs, which are excellent targets for transit spectroscopy. Over a dozen such planets have already been identified as candidate Hycean worlds with high potential for atmospheric follow-up.17,28–31 Simulation studies show that such planets are indeed excellent candidates for detections of key volatile species and potential biomarkers in their atmospheres.17,32–34,124
Characterising the atmospheric composition is essential to identify a Hycean world. A degenerate set of internal structures can generally explain the observed mass, radius and equilibrium temperature of a candidate Hycean world.18 As shown in Fig. 1, these solutions include rocky worlds with large H2-rich envelopes or mini-Neptunes which contain both a rocky layer and an icy mantle along with a significant H2-rich envelope, similar to the ice giants Uranus and Neptune in the solar system. Neither of these two scenarios would support a habitable surface, given the high temperature and pressure expected underneath the thick H2 envelope. Hycean worlds would lie between these two extremes, with a thin H2-rich atmosphere that allows for a liquid water layer underneath at habitable temperatures and pressures.17 Recent studies have suggested using atmospheric compositions to infer the presence of surfaces in sub-Neptunes with H2-rich atmospheres.35–37 Thus, resolving the degeneracies in internal structure and reliably identifying a Hycean world would require an understanding of the expected atmospheric chemical processes and the observable compositions.
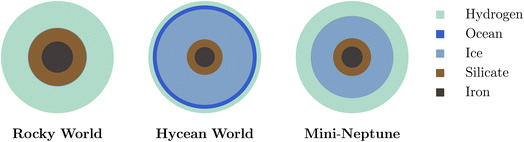 | ||
| Fig. 1 Schematic of degeneracy between internal structures of Hycean worlds with those of other possible planet types with H2-rich atmospheres. | ||
While Hycean worlds offer the required thermodynamic conditions for oceanic life their possible chemical conditions have not been explored in detail. A central question in determining the possibility of life on a planet is whether its environment has the potential for the origin and sustenance of life. The minimum ingredients required for life on Earth are known to be:38,39 (a) an energy source, (b) liquid water, and (c) various bioessential elements. Hycean worlds clearly satisfy the requirements for the presence of an energy source and liquid water with the presence of the host star and an abundant source of water. However, as is common to all ocean worlds, including icy moons in the solar system,40 the availability of chemical inventory accessible for seeding and sustaining life is a key challenge.41–44 In particular, Hycean worlds, with their planet-wide oceans, hinder access to bioessential elements resulting from geochemical cycles involving weathering of rocky surfaces that would be natural for a rocky planet. The large water fraction possible on such planets, as for other ocean worlds and sub-Neptunes in general, can result in a thick layer of high-pressure ice disconnecting the ocean from the rocky core and limiting access to bioessential nutrients.41,42,45,46 At the same time, other possible sources of nutrient delivery remain possible, including delivery through impacts,39,46 extraterrestrial dust39,46 and potential transport of nutrients from the inner core through a permeable ice layer.47–51 In addition to bioessential elements, the availability of pre-biotic organic molecules also plays an essential role in the origin of life. Pre-biotic molecules such as HCN are thought to have been critical for abiogenesis in the Early Earth.52 Whether such molecules can be delivered through extraterrestrial impactors or need to be created in situ, or both, remains an open debate.53
In the present work, we explore the chemical conditions possible on Hycean worlds. Using a canonical model of a Hycean world, we investigate the possible molecular inventory resulting from photochemical and kinetic processes in the atmosphere. We also explore sources of bioessential elements possible on such planets. In what follows, we describe our methods in Section 2, including the atmospheric structure, photochemistry, and internal structure models. We present our results and discuss the chemical conditions possible for life on Hycean worlds in Section 3. We discuss the observational prospects for identifying and characterising Hycean atmospheres with JWST in Section 4. We summarise our findings and discuss future directions in Section 5.
2 Methods
We explore the possible chemical conditions in a canonical Hycean planet focusing on the molecular composition of its atmosphere and the availability of bioessential elements in its ocean. We model the atmospheric pressure–temperature structure using a self-consistent radiative–convective equilibrium model, the atmospheric composition using a photochemical model, and the ocean properties using an internal structure model. Here we describe the model considerations.2.1 Atmospheric modelling
We consider a plane-parallel H2-rich atmosphere in radiative–convective equilibrium under Hycean conditions. We model the atmosphere using the GENESIS self-consistent modelling framework55 adapted for sub-Neptune atmospheres.17–19 This model self-consistently computes the temperature structure in radiative–convective equilibrium using the Rybicki scheme considering external irradiation, internal flux, possible scattering due to clouds/hazes, opacities from different chemical species (in chemical equilibrium or for a given composition), and day–night energy transport. The model computes line-by-line radiative transfer using the Feautrier method56 and the discontinuous finite element method.57 For given system parameters and chemical composition, the model self-consistently computes the temperature profile, flux profile, and the emergent spectrum of a planet.We assume a canonical Hycean planet with the bulk properties of the habitable-zone sub-Neptune K2-18 b, which has been suggested as a candidate Hycean planet.17,18 We adopt the system parameters reported by Benneke et al.,58 with a planet mass of 8.63 M⊕ (ref. 59) and radius of 2.61 R⊕ (ref. 58) orbiting an M3V host star. The thermodynamic conditions on Hycean worlds can span a large range, with the ocean surface temperatures of 273–400 K and surface pressures of 1–103 bar. For our canonical model, we consider a limiting case of a warm Hycean world in which the ocean surface is at a temperature (T) of ∼400 K and a pressure (P) of 100 bar. This choice is governed by the fact that most of the Hycean candidates currently identified are at zero-albedo equilibrium temperatures around 400 K. The bottom of the atmosphere is set at 100 bar, denoting the pressure at the ocean–atmosphere boundary, and the top at 10−6 bar. We derive the temperature profile with the required temperature and pressure at the bottom boundary by a combination of model parameters, which involve the incident irradiation, internal temperature, and scattering; an exploration of this parameter space is discussed in recent studies.17–19
We first consider our canonical planet to be irradiated with the maximal flux received by K2-18 b, i.e. at the sub-stellar point with no day–night energy redistribution (fr), which corresponds to an irradiation temperature of 331 K. Alternately, assuming a nominal fr of 0.35 this flux would correspond to an equivalent irradiation temperature of 369 K. We assume a nominal internal temperature of 30 K. The primary source of molecular opacity in this canonical model atmosphere is due to H2O.60,61 We also include H2–H2 and H2–He collision-induced absorption.62–65 We assume the H2O abundance to be saturated in the lower atmosphere until a pressure of 0.1 bar at which point the H2O abundance is assumed to be quenched higher up. We explore atmospheric pressure–temperature (P–T) profiles obtained using different levels of scattering in the atmosphere. Various sources of scattering can influence the P–T profile, including clouds and hazes of different compositions.17–19 Here, we follow the approach of recent studies17,19 and consider extinction due to hazes modelled as an enhanced Rayleigh scattering, with H2 Rayleigh scattering enhanced by a multiplicative factor which is a free parameter in the model. We also consider P–T profiles from other recent studies36,54 that assumed cloud/haze-free atmospheres for Hycean worlds. We discuss the specific P–T profiles used in this work in Section 3.1.
2.2 Chemical kinetics modeling
To investigate the possible atmospheric composition of our canonical Hycean world, we use the Caltech/JPL 1D KINETICS photochemical model66–68 to solve the coupled continuity equations for the chemical production, loss, and vertical transport of atmospheric constituents. First, we consider models with a zero-flux boundary condition at the bottom of the atmosphere to investigate how the composition evolves with time chemically, when the atmospheric temperature at the ocean surface is too low to allow full chemical recycling of the photochemical products back into their original equilibrium constituents. Similar to previous investigations,35,37 these toy models start with a thermochemical equilibrium composition for different assumptions about bulk elemental abundances, and then the composition is allowed to evolve with time under stellar UV irradiation, assuming zero flux boundary conditions for all species (i.e., no atmospheric escape or external input at the top of the atmosphere, and no chemical exchange with or outgassing from the surface). The zero-flux assumption allows for a quick look at the timing and behavior of the transition from the assumed reduced initial atmosphere accreted from the protoplanetary disk—with H2O, CH4, and NH3 containing the bulk of the oxygen, carbon, and nitrogen in this cool, H2-rich atmosphere—to the CO2, CO, H2O, N2 dominant end state (also still within a H2-rich atmosphere) that occurs when the more photochemically fragile reduced forms of the elements are converted to molecules with stronger chemical bonds.35,37 Of course, chemical exchange between the surface and atmosphere will occur on a real planet, with outgassing of dissolved CO2 being particularly important for ocean worlds,36,69 which we also consider as discussed below. Table 1 shows the cases reported in this work, with the zero-flux models corresponding to cases 1–6.| Case | P–T profile | P b/bar | Star | Boundary condition | H2O | CH4 | NH3 | CO2 | CO |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PT1 | 100 | Old | Zero flux | 2.4 × 10−2 | 1.1 × 10−2 | 4.1 × 10−5 | 1.3 × 10−2 | 1.6 × 10−5 |
| 2 | PT2 | 100 | Old | Zero flux | 1.0 × 10−2 | 6.6 × 10−3 | 1.5 × 10−4 | 4.5 × 10−3 | 5.6 × 10−5 |
| 3 | PT3 | 100 | Old | Zero flux | 2.8 × 10−3 | 2.5 × 10−3 | 3.2 × 10−4 | 3.5 × 10−4 | 2.5 × 10−4 |
| 4 | PT1 | 1 | Old | Zero flux | 2.4 × 10−2 | 6.8 × 10−6 | 3.0 × 10−9 | 4.1 × 10−2 | 1.9 × 10−3 |
| 5 | PT3 | 1 | Old | Zero flux | 2.3 × 10−3 | 8.2 × 10−5 | 3.9 × 10−13 | 4.2 × 10−2 | 9.3 × 10−4 |
| 6 | PT4 | 1 | Old | Zero flux | 3.1 × 10−10 | 7.8 × 10−5 | 2.2 × 10−7 | 9.4 × 10−15 | 3.0 × 10−9 |
| 7* | PT1 | 100 | Young | Fixed H2O, CH4, NH3 | 2.5 × 10−2 | 5.0 × 10−2 | 1.5 × 10−2 | 8.5 × 10−5 | 7.9 × 10−5 |
| 8* | PT4 | 1 | Young | Fixed H2O, CH4, NH3 | 3.0 × 10−10 | 4.9 × 10−2 | 4.7 × 10−3 | 1.8 × 10−19 | 2.4 × 10−10 |
| 9 | PT1 | 100 | Old | Fixed N2, H2O; CO2 = 1.4 × 10−2 | 2.4 × 10−2 | 1.2 × 10−4 | 1.7 × 10−10 | 1.3 × 10−2 | 4.9 × 10−6 |
| 10 | PT1 | 100 | Old | Fixed N2, H2O; CO2 = 1.0 × 10−6 | 2.4 × 10−2 | 3.5 × 10−6 | 2.8 × 10−10 | 1.0 × 10−6 | 2.5 × 10−8 |
| 11 | PT4 | 1 | Old | Fixed N2, H2O; CO2 = 1.0 × 10−1 | 3.1 × 10−10 | 5.5 × 10−8 | 6.3 × 10−13 | 1.0 × 10−1 | 6.4 × 10−3 |
These zero-flux models use the fully reversed reaction mechanism of Moses et al.70 that includes 92 species containing the elements C, N, O, and H that interact with each other via ∼1650 reactions; further model details are provided in Moses et al.70 and Yu et al.35 As is discussed by Hu et al.,36 sulfur species are expected to be largely sequestered in the ocean on such planets, and volcanic outgassing is expected to be shut down by the overlying high pressures at the rock–ocean boundary.69,71 The eddy diffusion coefficient Kzz in our model atmospheres is assumed to vary with pressure P according to Kzz = 5.6 × 104P−0.5 cm2 s−1, with P in bar (see ref. 72), with Kzz capped at 1010 cm2 s−1 in the upper atmosphere, and Kzz = 106 cm2 s−1 at P > 0.5 bar. The stellar flux is taken from the HAZMAT database,73 and we investigate results assuming both a young (45 Myr) and old (5 Gyr) M dwarf of 0.45 solar mass, with a medium EUV level for that age/mass; the younger star has a higher EUV flux. One change from Yu et al.35 is that we consider H2O condensation in these models, following procedures outlined in Moses et al.74 Water vapor is saturated at the bottom (ocean) boundary. With some of the thermal profiles, H2O will condense again higher up in the atmosphere if the H2O vapor mixing ratio exceeds local saturation. Condensation/evaporation is assumed to be zero above the minimum in the saturation vapor mixing ratio near the tropopause (if one exists), such that the water vapor at higher altitudes never exceeds that minimum mixing ratio; i.e., we assume condensed water droplets or ice particles are not carried to higher altitudes where they might re-evaporate. Model results are presented at various points in time as the composition evolves.
We use these zero-flux models to gain insight into the potential atmospheric composition at various stages in the planet's history. All the models pass initially through an organic-rich phase, whose lifetime depends on the atmospheric thermal structure, the pressure at the ocean surface, and the UV flux from the star. All models end up with at least partial conversion of the original CH4 and NH3 to CO2, CO, N2, and heavier organic molecules. We use the output from these simple models to define boundary conditions for more realistic photochemical models designed to explore the atmospheric composition of a Hycean planet with a surface ocean.
The atmospheric composition of these more realistic Hycean-world models will depend strongly on boundary conditions, which are not known a priori for these planets. Guided by our zero-flux model results and other discussions in the literature,35–37,75–77 we examine an early organic-rich scenario where the atmosphere is assumed to start with a Neptune-like 100× solar metallicity composition in thermochemical equilibrium, with H2O controlled by saturation at the bottom of the model. Mixing ratios of H2O, NH3, CH4, and He are fixed at the lower boundary at these equilibrium (or saturated for H2O) abundances. Fixing the mixing ratio in these models in combination with photochemical loss results in an upward flux of O, C, N that in steady state must end up being balanced by a loss of photochemical products through the oceanic lower boundary. Zero flux is assumed for all species at the top of the atmosphere. Based on several early Earth models and/or ocean-world models,36,75–77 we have chosen deposition velocities vd at the lower boundary of zero for O2, N2, and several long-lived C2Hx–C4Hx hydrocarbons; vd = 10−8 cm s−1 for CO; vd = 10−5 cm s−1 for CO2, C2H6, C3H8, C4H10, and stable hydrocarbons with 5 or more carbon atoms (precursors to organic hazes); vd = 10−3 cm s−1 for H2CO, CH3OH, H2CCO, CH3CHO, HCN, CH3CN, HC3N, CH3NH2, NO, HNCO; all other species are assumed to flow through the boundary at the maximum possible rate, given by Kzz divided by the atmospheric scale height. For our canonical Hycean world, the scale height at the surface is larger than that on Earth, and the maximum vd is ∼0.22 cm s−1. We use the stellar flux from the younger star for this organic-rich “early-era” model. This model is used for cases 7 and 8 in Table 1.
We also consider “late-era” scenarios where the reduced parent species have already been depleted through conversion to more stable photochemical products, such that the mixing ratios of CO2, N2, H2O, and He are fixed at the lower boundary. These cases are similar to the thin-atmosphere ocean-world models presented by Hu et al.,36 where dissolved CO2 is expected to be the dominant carbon phase in the ocean,69 and the amount of atmospheric CO2 is controlled by its equilibrium with the ocean, which in turn depends on the oceanic pH. Hu et al.36 explored a range of possible fixed CO2 mixing ratios in their model, and we also consider three cases: (1) a 100 bar, low-CO2 case with a CO2 partial pressure of 10−4 bar (i.e., a mixing ratio of 10−6 for our 100 bar surface pressure), within the range of the lower bound calculated for a pH of 9–10 by Hu et al.,36 (2) a 100 bar, intermediate CO2 mixing ratio (along with N2) that matches the mixing ratios at the lower boundary of our zero-flux model runs at the 5 Gyr time period (i.e., 7.2 × 10−3 for N2 and 1.37 × 10−2 for CO2 in our warmest model), and (3) a 1 bar, high-CO2 case (10% CO2, 1% N2) that aligns with the high-CO2 case from Hu et al.36 The H2O mixing ratios in these models are fixed at saturation values at the lower boundary. The other boundary conditions are the same as in the organic-rich early-era model, except that CH4 is assumed to have vd = 0 and NH3 diffuses through the bottom boundary at the maximum possible rate. These “late era” models use the stellar flux from the older M dwarf star and are run to steady state. These models are represented by cases 9–11 in Table 1. We discuss our results in Section 3.
2.3 Internal structure modelling
The internal structure is modelled following the approach used for Hycean candidates in previous studies.18,20,78 For a given planet mass (Mp) and the mass fractions of the constituent layers (xi), the planet radius (Rp) is obtained by solving the structure equations, i.e. of mass continuity and hydrostatic equilibrium. We assume four-layers – an H2-rich atmosphere, an H2O layer, a silicate outer core and an Fe inner core. For each layer, the equation of state (EOS) and temperature profile are specified, as described below. The structure equations, the adopted EOSs and the P–T profiles form a set of coupled differential equations which are solved using a fourth-order Runge–Kutta method.In the H2-rich atmosphere the pressures and temperatures required to allow habitable surface conditions are adequately low that the ideal gas EOS is sufficiently accurate. More generally, for the H2-rich layer we use the EOS from Chabrier et al.79 given the atmospheric P–T profile. The surface is taken to be at the ocean–atmosphere boundary, or H2O–H2/He boundary (HHB).18 In the H2O layer, we adopt an adiabatic P–T profile, with the initial pressure and temperature set by the HHB. The temperature-dependent EOS for H2O is compiled from multiple sources – see previous studies20,80 for a full description of these. The EOSs we adopt for the core81 are in the form of a Birch–Murnaghan EOS82 for MgSiO3 perovskite83 and Fe,84 which are temperature independent. The core is assumed to be Earth-like in composition, with an Fe mass fraction of 0.33.
3 Results
The potential for life on a habitable-zone planet depends on a wide range of properties, including the thermodynamic and chemical conditions in both the atmosphere as well as the interior. It has been suggested previously that Hycean planets allow for a wide range of thermodynamic conditions in their atmospheres and oceans, similar to conditions known to be conducive for life in Earth's oceans.17 Here we explore the possible chemical composition of a canonical Hycean atmosphere and the availability of bioessential elements in its ocean.3.1 Atmospheric and interior structure
We consider a canonical Hycean planet and investigate the possible chemical inventory in its atmosphere for a range of physical conditions. The temperature structure of the atmosphere is arguably the central factor influencing the habitability at the ocean surface. A wide range of atmospheric temperature structures are possible in temperate sub-Neptunes, including Hycean worlds, depending on the stellar irradiation, internal temperature, atmospheric composition, and scattering due to clouds/hazes.17–19,54,85–88 In particular, an adequate albedo due to clouds/hazes is required to maintain a liquid water surface for most candidate Hycean worlds.17–19 An atmosphere without stong scattering due to clouds/hazes would generally render the surface too hot to maintain a habitable ocean.19,54,85,86,88 The model considerations in this work are discussed in Section 2.1, based on the bulk properties of the candidate Hycean planet K2-18 b. We consider four P–T profiles for our models. First, we construct three profiles (PT1, PT2, and PT3) shown in Fig. 2, considering scattering due to hazes modelled with Rayleigh enhancement factors of 900, 1100 and 1300, respectively. These profiles represent a range of possible atmospheric temperatures but all have a maximum temperature of ∼400 K and pressure of 100 bar at the ocean surface. As discussed in Section 2.1, our choice of this limiting case is driven by expectations for most of the candidate Hycean worlds currently known. This surface temperature is also close to the maximum ocean temperature of 395 K at which life is known to survive in Earth's oceans.89,90 Any cooler Hycean worlds would be even more conducive to habitability, making the present case a conservative choice. We explore additional cases in which the PT1 and PT3 profiles are limited to the 1 bar pressure level, as shown in Table 1, representing shallower atmospheres with surfaces at a pressure of 1 bar and temperatures of ∼310–330 K.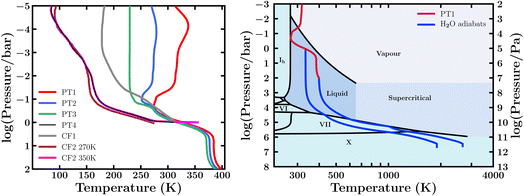 | ||
| Fig. 2 Left: Model pressure–temperature (P–T) profiles of a canonical Hycean world considered in this study (see Section 3.1). The profiles PT1–PT3 consider scattering due to hazes modelled as enhanced Rayleigh scattering. CF1 and CF2 refer to P–T profiles from previous studies36,54 assuming cloud/haze free (CF) model atmospheres for different conditions as discussed in Section 3.1. Two profiles are shown for CF2 (ref. 54) with different surface temperatures, and we consider a profile PT4 which has an intermediate surface temperature between the two CF2 profiles. Right: Internal temperature structure and the H2O phase diagram. The black curves denote the different phase boundaries. The blue curves show H2O adiabats starting at ocean surface pressures of 1 bar and 100 bar. The red curve shows the PT1 temperature profile of the canonical atmosphere from the left panel for reference. | ||
We also consider a P–T profile based on a cloud/haze-free (CF) model atmosphere of K2-18 b as pursued in recent works.36,54 Hu et al.36 consider an H2-rich atmosphere of K2-18 b with Earth-like equivalent insolation, achieved assuming a Bond albedo of 0.3, but otherwise considering no clouds/hazes in the model. Innes et al.54 report CF model P–T profiles for a K2-18 b-like planet with surface temperatures of 270 K and 350 K at 1 bar pressure, as shown in Fig. 2 and denoted by “CF2 270 K” and “CF2 350 K”, respectively. These correspond to the inner edge of the CF Hycean habitable zone around an M dwarf similar to K2-18, which for a 1 bar atmosphere is at 0.28 au,54i.e. corresponding to the runaway greenhouse limit for a steam-dominated lower atmosphere. We note that for these profiles a significant part of the atmosphere lies below freezing temperatures, making the presence of clouds/hazes and, hence, a high albedo arguably inevitable and inherently inconsistent with the assumption of a CF atmosphere. Nevertheless, it is still instructive to investigate the effect of photochemistry in such cool Hycean atmospheres. We consider a variant of the CF2 350 K profile, denoted as PT4, restricting the surface temperature to 310 K, which is intermediate between the two CF2 profiles, avoids steam domination, and is close to the other profiles shown in Fig. 2 at 1 bar, enabling a comparative study. Overall, we consider four P–T profiles (PT1–PT4) for our photochemical models as shown in Table 1.
We also use the atmospheric temperature profiles to define the outer boundary condition for the internal structure model as pursued previously for K2-18 b.18 The internal structure model is described in Section 2. The goal of the internal structure modelling is to estimate the range of ocean depths possible in our canonical Hycean world, which is then used to estimate the inventory of bioessential elements required in the ocean. For this purpose, we use the hottest of the four PT profiles (PT1), which leads to somewhat deeper oceans20 and therefore requires a higher nutrient budget, i.e. our conservative case. We consider two different surface pressures for the ocean and estimate the range of ocean depths possible given the 1σ bounds on the mass and radius of the planet. For a 1 bar surface, we obtain a minimum ocean depth of 120 km, for an upper bound mass for K2-18 b of 9.98 M⊕.58,59 From the atmospheric P–T profile, the surface temperature in this case is 328 K. Conversely, for the 100 bar case, we take the lower mass limit of 7.28 M⊕, obtaining a maximum ocean depth of 370 km. This is consistent with previous ocean depth estimates for the corresponding surface gravity.20 In this case the surface lies at 398 K. In both cases, the total H2O mass fraction in the interior is adjusted to minimise/maximise the ocean depth, with an upper limit of 90%.17 The H2O adiabats, showing the temperature structures in the H2O mantle, for both cases are shown in Fig. 2. Therefore, based on our above estimates we nominally consider the ocean depths of our canonical Hycean world to range between 100–400 km, which we use in Section 3.4.
3.2 Atmospheric composition
Hycean worlds are characterised by their bulk atmospheric composition being dominated by H2. However, the remaining molecular inventory of a Hycean atmosphere would depend on a number of factors, including the temperature structure, metallicity (or more generally, the bulk elemental abundances), surface pressure, the incident flux from the host star, potential atmospheric escape, and potential chemical fluxes to and from the ocean surface beneath the atmosphere. Many of these factors are unknown for Hycean worlds, particularly the initial conditions set by planet formation and the boundary conditions set by the ocean surface, which makes an ab initio modeling of their atmospheric chemistry challenging. Some recent studies have investigated the atmospheric chemistry of temperate sub-Neptunes with thin H2-rich atmospheres for different assumptions about the surface and atmospheric parameters, which are also relevant for Hycean worlds.35–37 Here we focus on our canonical Hycean atmosphere with temperature structures as discussed above and explore the possible atmospheric compositions for different assumptions about the initial and boundary conditions.As discussed in Section 2.2, we pursue a two-step approach. We first explore models assuming a chemically inactive (zero-flux) lower boundary to assess the sensitivity of the chemical processes to the assumed atmospheric properties, with no influence from the ocean, as pursued in recent work.35,37 We then consider cases with fixed mixing ratio and deposition velocity boundary conditions for the ocean–atmosphere interface and assess their effect on the chemistry. We consider different types of boundary conditions based on expectations for a primordial atmosphere in thermochemical equilibrium or with significant CO2 contribution from the ocean, as discussed in Section 2.2. We note that in this study we do not consider chemical fluxes resulting from potential life in the ocean which may significantly impact the atmospheric composition; we discuss this in Section 5.
The results for the photospheric (1 mbar) abundances of prominent molecules for the different model scenarios discussed in Section 2.2 are shown in Table 1. The vertical abundance profiles for some representative cases are shown in Fig. 3–5. Fig. 3 shows the abundance profiles for the models with zero-flux boundary conditions corresponding to cases 1, 3, 4 and 6 in Table 1. The abundances of the prominent molecules at 10 mbar and of other less abundant molecules are shown in the ESI.† In what follows, we present our findings on the prominent C, N, O molecules in the model atmospheres.
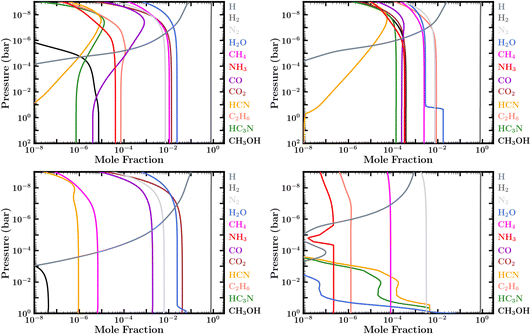 | ||
| Fig. 3 Fully evolved (steady state) volume mixing-ratio profiles of prominent volatile species with zero-flux boundary conditions (inactive surface) for a 100× solar composition atmosphere and surface pressure of (top) 100 bar and (bottom) 1 bar: (top left) for the PT1 temperature profile in Fig. 2, corresponding to case 1 in Table 1; (top right) for the PT3 temperature profile, corresponding to case 3; (bottom left) for the PT1 temperature profile, corresponding to case 4; (bottom right) for the PT4 temperature profile, corresponding to case 6. Note that the sharp decline in H2O, HCN, HC3N, and NH3 with altitude in case 6 is due to condensation. | ||
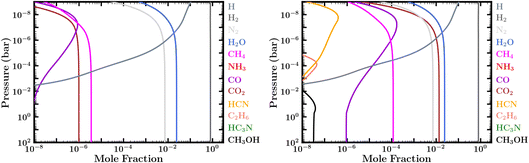 | ||
| Fig. 4 Steady-state mixing-ratio profiles considering an atmosphere with lower boundary conditions of an active ocean surface with prescribed N2, H2O, and CO2 mixing ratios and assuming the PT1 temperature profile in Fig. 2. Left: Abundances assuming a low CO2 mixing ratio of 10−6 at the atmosphere–ocean boundary, corresponding to case 10 in Table 1. Right: Abundances assuming a high CO2 mixing ratio of ∼1.4 × 10−2 at the atmosphere–ocean boundary, corresponding to Case 9 in Table 1. | ||
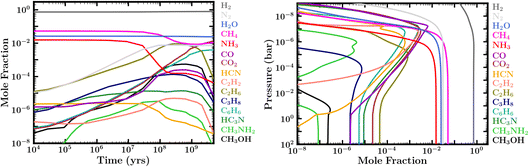 | ||
| Fig. 5 Left: Evolution of atmospheric molecular composition at 0.01 bar for case 1 in Table 1. Right: Abundance profiles corresponding to Case 7 in Table 1, based on initial conditions of a reduced atmosphere in thermochemical equilibrium (similar to case 1) and fixed boundary conditions for H2O, CH4 and NH3 (see Section 2.2). The abundances reflect the composition at 28 Myr, showing the availability of organic species early in the evolution. | ||
However, for the coolest Hycean worlds it is possible that the temperature at some point in the atmosphere is cold enough to lie within the ice sublimation regime causing H2O to freeze out and further restricting the water vapor abundance at higher altitudes. This can be seen for our case 3 and case 6, using profiles PT3 and PT4, in Table 1 and in Fig. 3. This “cold trap” effect is seen both in the Earth's dry stratosphere and in the solar system giant planets where H2O is negligible at the 0.1 bar level. In such cases it is possible that the H2O may not be detectable in transmission spectra, which typically probe pressures below ∼0.1 bar. Thus, whether H2O is observable or not depends on the temperature structure of the atmosphere, which in turn depends on the various factors discussed above.
This potential cold-trapping effect is relevant to the ongoing debate regarding the observed transmission spectrum of the candidate Hycean planet K2-18 b, which has an insolation similar to that received by the Earth.58,91 The transmission spectrum of the planet observed with HST has been explained by a degenerate set of solutions including H2O and/or CH4.18,58,86,92 A non-detection or underabundance of H2O in such an atmosphere could imply a cool and dry photosphere irrespective of the presence of an ocean underneath. This, in turn, could also imply the potential presence of H2O clouds below the observable atmosphere. On the other hand, for hotter Hycean candidates an unambiguous detection of H2O would be expected, as reported with the HST transmission spectrum of the planet TOI-270 d.30 Thus, the detection or non-detection of H2O in a Hycean atmosphere can place important constraints on the Bond albedo of the planet, which strongly dictates the temperature structure in the observable upper atmosphere. It is useful to note that besides the temperature profile, H2O is relatively unaffected by other factors in a Hycean atmosphere, e.g. photochemistry does not significantly alter the H2O abundance.
NH3 is also highly soluble in liquid water, thereby increasing its depletion potential due to dissolution in the Hycean ocean, which acts as a sink. This loss of NH3 to the ocean is considered in our more realistic boundary condition models (see also ref. 36), where we find that the steady-state NH3 mixing ratio becomes negligible; cases 9–11 in Table 1. Therefore, a substantial depletion or a lack of NH3 may be considered as a characteristic signature of a Hycean atmosphere. We note, however, the possibility that the presence of life in a Hycean ocean may itself be a source of NH3, as has been suggested previously for habitable rocky exoplanets with H2-rich atmospheres.95,96
The abundance of CH4 is less discriminating compared to NH3. Even though CH4 is also susceptible to photodissociation and other chemical loss, it is expected to be more abundant than NH3 due to multiple factors. Firstly, considering cosmic abundances,97 C is more than twice as abundant as N, and CH4 is more thermochemically stable than NH3 at the warmer temperatures near the ocean surfaces of Hycean planets. Secondly, in a H2-rich atmosphere CH4 is replenished by kinetic processes; that is, by photodissociation of other C bearing species such as CO and CO2 in the upper atmosphere, and—more significantly—by recycling reactions deeper in the atmosphere, where higher pressures and temperatures allow photochemical products such as CO to be at least partially converted back to CH4. We find significant CH4 abundances for models evolved up to 5 Gyr for different surface pressures and metallicities, as shown in Table 1 and Fig. 3, consistent with previous studies.35–37 We note that CH4 is comparatively less abundant for lower metallicity and lower surface pressure, as also seen in previous work with zero-flux boundary conditions.35
Paradoxically, we find that warmer atmospheres in our studied range for 100 bar surface pressures retain the most CH4, due to these aforementioned thermochemical recycling reactions being more efficient at higher temperatures. Atmospheres with ocean surfaces at lower pressures, on the other hand, lose more CH4 as a result of missing out on these thermochemical reactions at greater depths, and they lose the methane on faster time scales. Depending on atmospheric temperatures, surface pressure, and planetary age, it is possible that CH4 could be severely depleted and the C occupied primarily within CO2 for atmospheres with high initial metallicities, shallow surfaces, older evolved compositions, or low carbon inventories (including low CO2 dissolution scenarios, where CO2 from the ocean is the only source of carbon). This latter scenario can be seen from our cases 10 and 11 in Table 1 and Fig. 4. Thus, a Hycean atmosphere might be expected to have observable amounts of CH4 (≳10−4) under most conditions, but several factors can affect those expectations.
An important metric could be the relative abundance of CH4vs. NH3. If both molecules are present in high abundances consistent with expectations from thermochemical equilibrium, a Hycean ocean may be confidently ruled out. However, if NH3 is absent or substantially depleted relative to CH4 the presence of an ocean would be more likely.
We note the important difference that the CO2 abundance in our zero-flux boundary condition models is primarily due to chemical-kinetic processes in combination with initial metallicity assumptions, whereas that in cases 9, 10, & 11 and in the models of Hu et al.36 is an input to the model based on the assumption for how much CO2 is released from the ocean. In the Hu et al.36 models, the abundance of atmospheric CO2 is controlled by whatever fixed mixing ratio they adopt for CO2 at the lower boundary, which they nominally assume to be between 4 × 10−4 and 0.1; their estimate for the lower-bound ranges between 5 × 10−5 and 7 × 10−4 bar partial pressure of CO2. We also examine cases where the only carbon present in the atmosphere is that released through equilibrium with CO2 dissolved in the ocean, with an assumed CO2 mixing ratio at the ocean surface of 10−6 (i.e., CO2 partial pressure of 10−4 bar for our 100 bar atmosphere case), ∼1.4%, or 10%. These correspond to cases 9–11 in Table 1 and Fig. 4.
As with the Hu et al.36 study, we find that adopting fixed mixing-ratio lower-boundary conditions for CO2, N2, and H2O in a canonical ocean model results in an atmosphere with much larger CO2/CO ratios than for otherwise similar planets without ocean interaction. We agree that the CO2/CO ratio could be a useful indicator to distinguish between high-metallicity deep atmospheres and planets with shallower atmospheres and liquid-water oceans. However, our high CO2 ocean-release cases produce much less CH4 than is derived for the Hu et al.36 models, which end up with more CH4 than CO2, despite CO2 being the only incoming source of carbon in the model.
While the presence of substantial atmospheric CO2 and high CO2/CO ratios may be strong indicators of a Hycean ocean, the absence of CO2—while less likely on a warm Hycean planet—might also be less informative. For example, following the approach of Hu et al.36 a low CO2 abundance could indicate (a) a high efficiency of dissolution of CO2 in the ocean and less availability in the atmosphere, or (b) more C sequestered in the core, making it less available in the ocean, or a generally low C content in the planetary interior as a whole. Secondly, the abundance of CO2 in the atmosphere can also depend on the initial availability of CH4 and H2O in the atmosphere through the CH4 + H2O → CO2 conversion process (i.e., assuming that the primordial atmosphere contained CH4 not in immediate chemical equilibrium with the liquid ocean, such that the oceanic CO2 does not control the entire atmospheric carbon budget). For cooler Hycean planets where H2O can be frozen out in the observable atmosphere, CO2 could also be underabundant from this process and hence less observable. Finally, it is also possible that microorganisms in a Hycean ocean could efficiently use dissolved CO2 for biological processes and subsequently release other gases such as CH4 or O2. Microbial methanogenesis is known to be a significant source of CO2 consumption in the Earth's oceans98–100 and it is not inconceivable that life in a Hycean ocean may find efficient ways to do the same.
Similarly, CH3OH becomes very abundant in some of our zero-flux boundary condition cases (e.g. cases 2 & 3 in Table 1 and Fig. 3) but is more depleted in our models with ocean interaction, due to dissolution in the ocean. In that way, CH3OH can become a good discriminator of ocean versus solid surfaces, as suggested originally by Tsai et al.37 Note also that the CH3OH abundance in our zero-flux models has a high sensitivity to atmospheric temperatures, being less abundant in some atmospheres because it becomes a casualty of the more efficient conversion of CO into CH4 at depth in warmer atmospheres (as in cases 1 and 4, see e.g.Fig. 3), or becomes a casualty of the low atmospheric oxygen abundance that results from H2O freeze out in colder atmospheres (as in case 6, e.g., Fig. 3).
With our cooler zero-flux models with a 100 bar surface, we find that C2H6 could also be an excellent indicator of a dry, unreactive planetary surface on a sub-Neptune planet. This can be seen in Fig. 3. If H2O is moderately depleted through condensation/sublimation near a tropopause cold trap (e.g., with our PT2 & PT3 profiles, cases 2 and 3), such that H2O has a reduced abundance in the middle and upper atmosphere, conversion of CH4 into CO and CO2 becomes less effective than conversion of CH4 into heavier hydrocarbons. In that situation, the overall photochemistry becomes more like that of Jupiter, except the hydrocarbons are not recycled efficiently back to CH4 at depth. The eventual steady-state abundance of photochemically stable hydrocarbons such as C2H6 and other alkanes (e.g., C3H8 and C4H10) becomes very large in cases with no surface loss, such that C2H6 actually contains the bulk of the carbon in the atmosphere. We are also finding a significant C6H6 abundance in our zero-flux models, but that result seems less robust, as we end the carbon chemistry at C6H6 and do not consider further loss of benzene to PAHs and other heavy organics. In our ocean models with fixed CO2, N2, and H2O boundary conditions, we assume a moderate deposition velocity loss of C2H6 through the ocean boundary, and the steady-state C2H6 mixing ratio is less dramatic. Therefore, C2H6 should provide a better observational marker than CH3OH to distinguish ocean planets from solid-surface planets on sub-Neptunes that are cold enough for significant atmospheric water depletion. Another species of potential note is HC3N, which becomes relatively abundant for some of our models.
3.3 Prebiotic molecular inventory
The molecular inventory of a habitable planet in the early stages of its evolution is expected to play an important role in its propensity for seeding life. On the Earth, key pre-biotic molecules such as HCN are thought to have played a pivotal role in abiogenesis.52 Here, we investigate the molecular abundances over the lifetime of our canonical Hycean planet. At the outset, we find that most of the models considered undergo a phase that is rich in organic molecules early in the evolution, typically within the first ∼1–300 Myr. The relative abundances of the different molecules depend on the boundary conditions and other factors discussed above. However, a common outcome in our zero-flux models is the prevalence of important organics such as CH4, C2H6, C6H6, and nitriles over the early evolutionary history, as shown in Fig. 5, left panel.We find that our models with fixed boundary conditions corresponding to an initially reduced atmosphere result in abundant organics starting very early in the evolution. We explored this in cases 7 and 8 in Table 1; the abundance profiles for case 7 are shown in Fig. 5, right panel. We find that hydrocarbons, nitriles, alcohols, and other organics can become extremely abundant in the first few million years of atmospheric evolution, if we assume the original starting composition were reduced (e.g., CH4, NH3, and H2O being the main carriers of C, N, and O, as would be expected for a cool H2-rich accretion atmosphere in thermochemical equilibrium in the gaseous envelope, but not the ocean); see also ref. 35 and 37. High abundances of C2H6, C3H8, C4H10, C6H6, C2H2, HCN, H3CN, CH3NH2, CH3CN, and CH3OH are particularly worth mentioning from our model results. While it may be unlikely that we manage to catch this early evolutionary stage in observations of Hycean planets, these photochemically produced molecules could provide pre-biotic molecules to the ocean. The abundance of HCN, an important prebiotic molecule on Earth,52 is strongly correlated with the presence of NH3 in the atmosphere. As NH3 is photochemically destroyed over time, and is readily soluble in the ocean, most of the N is locked in N2 thereby reducing the abundance of HCN at later times. However, other hydrocarbons with lesser or no dependence on N continue to be abundant. Heavier organic species are present but not particularly abundant in our final steady-state atmospheres with ocean interaction included, due to relatively low abundances of the primary parent molecule CH4. However, if CH4 continues to be abundant in the present-day atmosphere due to the reasons described above, a rich organic molecular inventory may continue as well.
3.4 Availability of bioessential elements
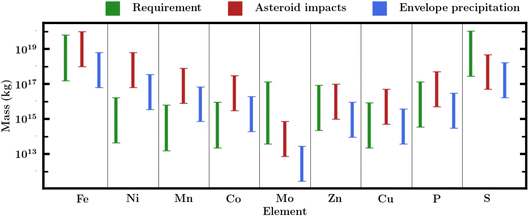
A central challenge to the habitability of Hycean worlds, as for all ocean worlds, is the availability of bioessential elements. Bioessential elements include the common volatile elements (CHNOPS) as well as essential metals such as Fe, Na, K, Mg, Mn, Ni, Ca, Cu, Zn, Co, and Mo,39,40,46,101 which perform key functions in biochemical processes in terrestrial life. The challenge lies in the fact that ocean worlds would not have ready access to these elements which are typically acquired through interaction between the hydrological cycle and the silicate portion of the planet. This problem is particularly acute for planets with deep oceans where high-pressure ice at the bottom of the liquid water column can preclude access to elements from the rocky core. This challenge is well recognised both for volatile-rich sub-Neptunes39,46 as well as for icy moons in the solar system.40 For planets with H2O-rich interiors and H2-rich atmospheres, including Hycean worlds, H and O are naturally present in abundance while C and N are also found in large quantities in volatile ices (e.g. Rubin et al.102) and so could be present in the required abundances. However, this is not necessarily the case for P, S, and refractory metals. Here we assess possible sources of these elements for our canonical Hycean world.We use Robbins et al.101 estimates of Archean ocean metal concentrations. These originate from geochemical modelling by Saito et al.,106 except for the Mo values which are adopted from Anbar and Knoll.107 We take Jones et al.105 and Crowe et al.104 values for the P and S concentrations respectively. These estimates are obtained through a variety of methods. The Saito et al.106 results come from geochemical modelling of the concentrations of various metal species under anoxic and iron-rich conditions representative of Archean oceans.101,103,106,108,109 The modelling finds that the relative availability of various metals follows Fe > Ni,Mn,Co ≫ Zn,Cu. It is assumed that there is sufficient nutrient influx (e.g. from weathering) for the actual Earth ocean concentrations to match these modelling estimates. The P, S, and Mo values, in contrast, are taken from proxy sedimentary records. This can offer a more nuanced view of changes over time but can be limited by imperfect sorption models and post-deposition alteration of the sediments.101,110,111
A proportion of the elemental mass budget will be present in a precipitate instead of a dissolved form. Since the former will not be bioavailable, we adopt a dissolved fraction104–107 to scale our Hycean mass budget for each element. This increases the required mass budget for each element. We note that we have not modelled self-consistent Hycean ocean chemistry or any kind of (geo)chemical cycle and so there is still significant uncertainty in this dissolved fraction. The Hycean ocean considered here would be warmer than the Earth ocean, meaning that the solubilities of various species would be different than on Earth. To reflect these unknowns, we introduce uncertainties of 1 dex in the primitive ocean concentrations, except for Mo for which we consider an increased uncertainty of 1.5 dex as its Archean abundance is relatively less accessible. The mass requirements are shown in Table 2 and Fig. 6.
| Element | C PO | M req | f CC | F diss | M imp |
|---|---|---|---|---|---|
| Fe | −4.6 | 3.2 × 1018 | 2.0 × 10−1 | 3.0 × 10−1 | 4.0 × 1017 |
| Ni | −8.2 | 8.5 × 1014 | 1.3 × 10−2 | 3.0 × 10−1 | 2.6 × 1017 |
| Mn | −8.3 | 3.2 × 1014 | 1.6 × 10−3 | 6.0 × 10−1 | 3.2 × 1016 |
| Co | −8.1 | 4.6 × 1014 | 6.0 × 10−4 | 7.0 × 10−1 | 1.2 × 1016 |
| Mo | −9.0 | 2.1 × 1015 | 1.5 × 10−6 | 3.0 × 10−2 | 3.0 × 1013 |
| Zn | −14.0 | 4.5 × 1015 | 2.0 × 10−4 | 1.0 × 10−7 | 4.0 × 1015 |
| Cu | −22.0 | 4.4 × 1014 | 1.0 × 10−4 | 1.0 × 10−14 | 2.0 × 1015 |
| P | −6.5 | 6.9 × 1015 | 1.0 × 10−3 | 9.8 × 10−1 | 2.0 × 1016 |
| S | −5.0 | 5.7 × 1018 | 1.0 × 10−2 | 4.0 × 10−2 | 2.0 × 1017 |
Only a small fraction of the 2 × 1021 kg will end up dissolved in the Hycean oceans. Large impacts can travel through the ocean and deliver mass to the ice layers or even the core. Hydrodynamic modelling of asteroid impacts into Earth's oceans115–117 suggests impactors will form impact craters if the ocean depth is less than 5–7 times the impactor diameter. If this factor applies to a 200 km deep Hycean ocean, only objects with diameters below 29 km would break apart completely in the ocean. Considering a chondritic asteroid density of 1600 kg m−3118 this would mean only objects lighter than ∼2 × 1016 kg, and so only ∼0.5% of the total mass would be in impactors small enough to completely break apart in the ocean. Larger collisions that do reach a solid surface will throw up material back into the atmosphere, which will rain back down as small particles.53,119 On the Earth, large collisions excavate material with a total mass nearly as large as the impacting mass,120 significantly increasing the amount of mass deposited on the planet surface.
However, as little work on large impacts in deep oceans has been done, we are cautious about this additional amount of mass delivered to the oceans. We assume that between 0.25% and 25% of the total impact mass stays in the oceans, with a median of 1% or 2 × 1019 kg, and provides the required source of bioessential elements for seeding life. This fraction will change depending both on ocean depth and the precise physics of impact. The mass requirements and asteroid nutrient delivery rates are shown in Table 2 as well as Fig. 6. We find that in general asteroid impacts can plausibly deliver enough mass for the bioessential elements with the possible exception of molybdenum and sulphur.
Another avenue for nutrient delivery is through steady state accretion of extraterrestrial dust. The possibility of extraterrestrial dust being a sustained source of bioessential nutrients has been considered in the context of both the Earth121 and sub-Neptunes.46 For example, in the context of the Earth, the amount of Fe obtained through this source is estimated to be 4 × 106 kg per year,122 compared to a global phytoplankton Fe assimilation of 7 × 108 kg per year.123 So this flux would only be able to support a small biosphere by itself, and it would take of order 1012 years for it to raise the overall ocean Fe budget to the required ∼1018 kg. While it may help life continue once it has started, it may not be a substantial contributing factor to the start of life, unless the dust accretion rates are significantly higher at early times. On the other hand, depending on the system parameters the impactor and dust influx on Hycean worlds may be significantly higher than assumed here based on Earth values. Future work can investigate if the larger planet sizes of Hycean worlds and their closer proximity to their host stars, among other system properties, may enhance the mass influx rate.
| Element | X/H | f atm | M req | M cond |
|---|---|---|---|---|
| Fe | 3.0 × 10−5 | 1.3 × 10−3 | 3.2 × 1018 | 6.6 × 1017 |
| Ni | 1.6 × 10−6 | 7.2 × 10−5 | 8.5 × 1014 | 3.7 × 1016 |
| Mn | 3.3 × 10−7 | 1.1 × 10−5 | 3.2 × 1014 | 7.1 × 1015 |
| Co | 8.5 × 10−8 | 4.2 × 10−6 | 4.6 × 1014 | 2.0 × 1015 |
| Mo | 7.6 × 10−11 | 5.4 × 10−9 | 2.1 × 1015 | 2.9 × 1012 |
| Zn | 3.6 × 10−8 | 1.7 × 10−6 | 4.5 × 1015 | 9.3 × 1014 |
| Cu | 1.5 × 10−8 | 7.2 × 10−7 | 4.4 × 1015 | 3.8 × 1014 |
| P | 2.6 × 10−7 | 5.9 × 10−6 | 6.9 × 1015 | 3.1 × 1015 |
| S | 1.3 × 10−5 | 3.2 × 10−4 | 5.7 × 1018 | 1.7 × 1016 |
We consider a nominal atmospheric mass of our canonical Hycean planet to range between 10−6 and 10−4 Mp, informed by our internal structure model and previous estimates for K2-18 b.18 Considering a solar composition for the atmosphere, we estimate the elemental budget of key metal species, as shown in Table 3. For P and S, which are not expected to condense out in the same fashion, we take a Neptune-like 100× solar metallicity atmosphere and assume that 1% of the atmospheric species end up in the ocean, very likely an underestimate.36 We find that condensation in our primordial atmosphere provides comparable elemental inventory to the ocean requirements. Fig. 6 shows these estimates alongside the masses of required nutrients and possible asteroid impacts.
4 Observational prospects with JWST
A central promise of Hycean planets is their accessibility to atmospheric characterisation with current facilities, particularly the JWST.17 Their low gravities and low atmospheric mean molecular weight make Hycean worlds more conducive to atmospheric spectroscopy compared to rocky exoplanets of comparable mass and temperature. Several recent studies have demonstrated the feasibility of detecting key molecular signatures, including biomarkers, in candidate Hycean worlds using transit spectroscopy with JWST.17,34,124 Our goal here is to assess (a) the spectral features of relevant chemical species observable with JWST transit spectroscopy, and (b) chemical diagnostics for identifying a Hycean planet.4.1 Atmospheric spectroscopy with JWST
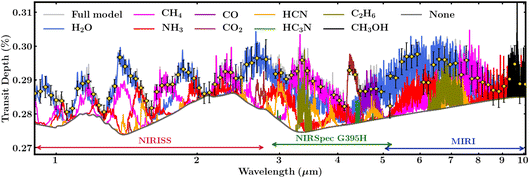
The high sensitivity and broad spectral range of JWST provide a promising avenue to detect key molecular species expected in Hycean atmospheres using transit spectroscopy. Here we assess the spectral features of the molecules predicted in our photochemical calculations, in Section 3, that may be observable with JWST. We model a transmission spectrum of K2-18 b using an adaptation of the AURA exoplanet atmospheric modeling and retrieval code.125 We refer the reader to previous works17,124 for details on the model setup using the AURA code and for the same planet bulk parameters as considered here. The model considers a plane parallel atmosphere at the day–night terminator of the planet in hydrostatic equilibrium and computes the transmission spectrum for a given temperature structure and composition. Besides the molecules considered in previous works using the AURA model,17,124,125 we consider three additional molecules C2H6,126–128 HC3N,126,127,129 and CH3OH.126,127,130,131 As seen in Section 3, the atmospheric composition can vary widely depending on a number of factors, including the temperature structure, atmospheric metallicity, ocean surface pressure, and ocean–atmosphere interactions. We consider an isothermal temperature structure at 300 K and uniform volume mixing ratios of 10−2 for H2O, 10−4 for CH4, and 10−5 for all other species. While these values lie within the wide range of abundances predicted by our chemical models, we note that some of the species may be significantly less or more abundant in a Hycean atmosphere than assumed here for illustration.The model transmission spectrum along with the relative contributions of the molecules and synthetic JWST observations are shown in Fig. 7. Following previous work,17 we assume 1 transit with JWST NIRISS132 and 3 transits with NIRSpec G395H,133,134 and an additional 2 transits with the MIRI135 spectrograph to obtain a broad 1–10 μm spectral coverage. The simulated spectra were generated using Pandexo.136 The resultant 6 transits of K2-18 b can be observed in ∼50 hours with JWST. As can be seen in Fig. 7, and consistent with previous studies,17,36,37,124 the prominent molecules H2O, CH4, NH3, CO2 and CO have strong spectral features across the 1–10 μm range and are readily discernible with the expected JWST data quality if present at the atmospheric abundances assumed in the model. Conversely, if any of them are underabundant then robust upper-limits can be placed on their abundances. For example, simulated retrieval studies17,124 have shown that the abundances of H2O, CH4, and NH3 can be retrieved to precision better than 0.3 dex with JWST quality data. Among the minor species, HCN and C2H6 have the stronger features, whereas CH3OH and HC3N would be more challenging to detect, as previously reported for CH3OH.37
4.2 Chemical diagnostics of a Hycean world
As discussed in Section 1, chemical characterisation of the atmosphere is essential to identify a Hycean world. The bulk properties of a candidate Hycean world, i.e. the mass, radius, and temperature, can generally be consistent with a degenerate set of internal structures,18 as shown in Fig. 1. Depending on the specific bulk properties, the possibilities could include (a) a Hycean world with a shallow H2-rich atmosphere overlying a habitable ocean, (b) a sub-Neptune with a shallow H2-rich atmosphere but with a solid surface underneath, or (c) a sub-Neptune with a deep H2-rich atmosphere with temperatures and pressures at the bottom of the H2 layer too high to be habitable. Several recent studies have investigated the possibility of atmospheric chemical signatures to identify the presence of solid or ocean surfaces in sub-Neptunes,35–37 considering the possibility of a shallow H2-rich atmosphere18 in K2-18 b as a canonical example. Here we revisit this avenue based on the chemical calculations in the present work and motivated by previous works.35–37 A flowchart of the chemical diagnostics of a Hycean world is shown in Fig. 8.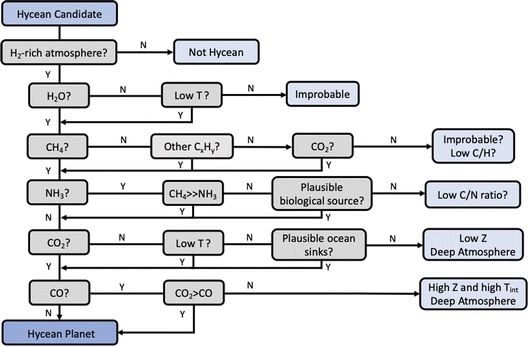 | ||
| Fig. 8 Chemical diagnostics for Hycean atmospheres based on our photochemical models and motivated by recent works.35–37 The relevance of each prominent molecule for the identification of a Hycean atmosphere is shown. For each molecule, the implications of its detection or non-detection are indicated, considering a minimal detectability threshold of 1 ppm abundance. Low T refers to cases where the atmospheric temperature is expected to be cold enough for H2O condensation. CxHy refers to hydrocarbons. Z refers to atmospheric metallicity in a deep H2-rich atmosphere, and Tint refers to the internal temperature. The pathways reflect the results of our photochemical calculations across the range of Hycean conditions considered and are generally consistent with previous works.35–37 See Section 4. | ||
Given a candidate Hycean world,17 the first requirement is to confirm the presence of an H2-rich atmosphere using spectroscopic observations. An H2-rich atmosphere is usually inferred indirectly through the detection of a spectral feature of one of the prominent molecules, e.g. H2O, the spectral amplitude of which provides a constraint on the mean molecular mass of the atmosphere.30,58 As discussed above, JWST will be able to robustly detect spectral features and constrain H2-rich atmospheres for several nearby Hycean planets.
H2O is naturally expected to be the dominant molecule observable in a Hycean atmosphere. However, if the temperature structure is too cool in the observable photosphere, H2O can be depleted due to condensation, as in the Earth's stratosphere. As discussed in Section 3, for some of the P–T profiles of K2-18 b considered, H2O can indeed be underabundant. On the other hand, warmer Hycean candidates would be expected to have discernible H2O features.
The abundance of CH4 in a Hycean atmosphere depends strongly on the boundary conditions. While CH4 is susceptible to loss from photochemical processes, especially for low ocean surface pressures (e.g. 1 bar), it can still be present in significant abundances due to other production pathways. The underabundance of CH4 would normally indicate the C present in other molecules such as CO2 or, in the case of low-temperature Hyceans, higher-order hydrocarbons. The simultaneous depletion of all these molecules would be unlikely for a Hycean atmosphere, and may instead indicate a deep atmosphere with an unusually low C/H ratio.
A key predictor of a Hycean atmosphere is the NH3 abundance. Our results confirm previous findings36,37 that NH3 is expected to be significantly underabundant in a Hycean atmosphere due to both its photochemical destruction in a shallow atmosphere and its high solubility in the ocean underneath. NH3 is also expected to be significantly underabundant compared to CH4, making a high CH4/NH3 ratio a useful diagnostic of a Hycean atmosphere. Conversely, a significant abundance of NH3 in a Hycean candidate would need to be investigated for its potential biological origins95 which are not accounted for in our models. In the absence of such a scenario, a low CH4/NH3 ratio may be indicative of a deep atmosphere with a low C/N ratio.
A significant abundance of CO2 is another strong predictor of a Hycean atmosphere, consistent with previous studies.36 However, we argue that the underabundance of CO2 does not robustly rule out a Hycean candidate, as it can occur due to various factors, including a cold and dry atmosphere, low C content in the interior, high dissolution in the ocean and/or consumption by marine biota.99,100 The lack of CO2, in the presence of CH4 and H2O, would also be consistent with a deep atmosphere with a low metallicity.
Finally, while the CO abundance on its own is not a critical diagnostic, the CO2/CO ratio is expected to be higher for a Hycean atmosphere compared to that expected in the absence of an ocean (see also ref. 36). Conversely, a CO/CO2 ratio above unity would indicate a deep atmosphere with a high metallicity and high internal temperature as suggested previously.36
In summary, considering the major molecules, the key diagnostics of a candidate Hycean world would be (a) abundance of H2O, CH4, other hydrocarbons and/or CO2, (b) underabundance of NH3, particularly a high CH4/NH3 ratio, and (c) CO2/CO > 1. Other molecules can also be abundant depending on the specific atmospheric conditions. HCN and CH3OH are expected to be underabundant in Hycean atmospheres, as also found in previous studies.37 In particular, HCN can be underabundant due to the absence of NH3 and CH3OH can be depleted due to dissolution in the ocean. We also find that other hydrocarbons such as C2H6 can be abundant for cool Hycean worlds where H2O may be moderately depleted in the observable atmosphere through condensation; in this case CH4 photodestruction leads primarily to other hydrocarbons rather than CO and CO2.
Therefore, robustly establishing the presence of an ocean on a candidate Hycean world would require precise abundance estimates of multiple molecules discussed above and using their relative abundances to systematically constrain the key chemical pathways given the bulk properties of the planet in question. Previous work has also suggested other organic molecules as potentially observable biomarkers in Hycean worlds.17 These include molecules such as methyl chloride, dimethyl sulphide, carbonyl sulphide, nitrous oxide or carbon sulphide, which are produced in relatively low quantities by life on Earth but can be promising biomarkers in H2-rich atmospheres, depending on the biomass present and UV environment.95
5 Summary and discussion
Hycean worlds open a new paradigm in the search for life on exoplanets. Hycean planets are expected to be more detectable in transits and more amenable to atmospheric characterisation using transit spectroscopy than habitable rocky exoplanets. Previous work has shown that the thermodynamic conditions in the atmospheres and oceans of Hycean worlds can be similar to those known to be capable of hosting life in Earth's oceans.17 In the present work, we investigate the chemical conditions possible on a canonical Hycean world, focusing on the present and prebiotic molecular inventory in the atmosphere and the availability of bioessential elements in the ocean. The observable atmospheric composition of a Hycean planet depends on a number of factors, including the temperature structure, atmospheric metallicity, ocean surface pressure, and ocean–atmosphere interactions. However, based on the results of several recent works17,35–37 and the present study, the atmospheric compositions of Hycean worlds may be expected to include a range of prominent molecules that are detectable with modest investment of JWST time. In particular, the relative proportions of some of the key molecules, e.g. a high CH4/NH3 and/or a high CO2/CO, could help distinguish a Hycean world from a non-habitable mini-Neptune or super-Earth with a thicker H2-rich atmosphere.5.1 Chemical inventory for life
We also investigate the question of whether a Hycean world would be conducive for the origin and sustenance of life. A number of factors affect the habitability of a planet, including its geophysical conditions, evolutionary history, architecture of the planetary system and astrophysical conditions.137 From an ecological perspective, it is commonly agreed that life as we know on Earth has three essential requirements:38,39 (a) energy sources, radiative and/or chemical, (b) liquid water, and (c) various bio-essential elements. In addition to these requirements, life also needs the right environmental conditions, e.g. temperature, pressure, pH, and UV radiation.At the outset, by definition, the thermodynamic conditions (pressure and temperature) in a Hycean ocean would not be dissimilar to those experienced by microbial life in Earth's oceans.17 The requirements of an energy source and presence of liquid water are also naturally met, more so than most solar system environments for which a liquid water surface is a key limitation.39,40 The energy source includes both the radiant energy from the star as well as the chemical free energy from chemical reactions, e.g. between abundant H2 and CO2 in the ocean. In particular, as discussed above, the presence of abundant H2 may be more conducive to processes such as methanogenesis that are prevalent in microbial communities in anoxic environments on Earth.99,100 Previous studies have also discussed the feasibility of life in H2-rich environments, as also seen on Earth.95,138
Considering atmospheric evolution starting from reduced primordial conditions the atmosphere undergoes a phase rich in organic molecules potentially conducive for prebiotic chemistry. In particular, several hydrocarbons and nitriles including C2H2, C2H6, C3H8, C6H6, HCN, and HC3N become abundant (above ∼1 ppm) starting between 1–100 Myr with some remaining abundant for over a Gyr. We note however that the abundances of these molecules are strongly dependent on the initial conditions and boundary conditions describing the ocean–atmosphere interactions. Future studies can investigate the timescales on which the primordial ocean can equilibrate with a H2-rich atmosphere139,140 and how it would influence the primordial composition and chemical evolution of the atmosphere.
We investigated the availability of bioessential nutrients in a canonical Hycean ocean building on elemental estimates for oceans of the early Earth.101,106 Life on Earth requires a range of bioessential elements, including key volatile elements (CHNOPS) and heavier metallic elements. While C, H, N, O and some heavier elements such as Na and K are naturally expected to be abundant in H2-rich atmospheres,46,93,94,141 the availability of P, S, and refractory metals is a bigger challenge. In particular, for ocean worlds, the presence of a thick icy mantle below a planet-wide ocean hinders ready access to weathering of rocky surfaces that is the dominant source of bioessential metals for life in Earth's oceans.41,42,45,46
Based on internal structure models for our canonical Hycean planet, we estimate the ocean depths to range between 100–400 km, consistent with previous studies for similar planets.20,42 Assuming concentrations similar to those estimated for the early Earth's oceans101,106 we estimate the mass requirements of P, S, and key bioessential refractory metals: Fe, Ni, Mn, Co, Mo, Zn and Cu. We find that the requirements can be reasonably met for plausible assumptions about the delivery of nutrients through asteroid impacts similar to the impact history of the Hadean Earth, considering a representative asteroid composition based on carbonaceous chondrites. We also consider nutrient availability in the planetary atmosphere/envelope accreted during formation assuming solar composition, consistent with the typical ∼1% dust mass fraction in protoplanetary and interstellar environments. The metals are present in condensates which sediment out of the relatively cold Hycean atmosphere post-formation, and can meet the abundance requirement of the Hycean ocean. Making conservative assumptions about the sequestration of P and S species in the Hycean ocean, we find that atmospheric species may also make a substantial contribution to these elemental budgets. Finally, extraterrestrial dust and potential convective transport of core material through the ice layer, may also provide supplemental steady-state sources of nutrients though unlikely to meet the entire primordial requirement. Overall, our results suggest that Hycean worlds provide adequate avenues to meet the chemical requirements of potential life similar to those estimated for the early Earth.
5.2 Future directions
Our initial investigation suggests that Hycean worlds have the potential to provide a promising environment for the origin and prevalence of life. However, the novelty of these worlds also open a wide range of open questions which affect their potential habitability. Here we outline some key directions for future research in this area.• What are the atmospheric conditions at formation? In particular, a key question is about the timescale over which the primordial Hycean atmosphere equilibrates with the ocean underneath and what the resultant atmospheric composition could be. This sets the initial conditions for the later molecular evolution of the Hycean atmosphere, including the potential for prebiotic molecular chemistry.
• What are the boundary conditions at the ocean–atmosphere interface of a Hycean world? The presence of life in a Hycean ocean could add sources and sinks of prominent molecules which can significantly impact the observable chemistry. For example, methanogenesis as prevalent in anoxic environments on Earth can be a significant sink for CO2 and source for additional CH4 in a Hycean atmosphere.
• What is the possible impact history for a Hycean planet and how does the planetary system architecture influence it? Higher/lower impact fluxes or a different impactor size distribution would significantly change the bioessential element contributions. Furthermore, what happens when large bodies (10s or 100s km radius) land in very deep oceans? What percentage of the impact mass ends up in the hydrosphere?
• Can convection of the core material through the icy mantle in a Hycean interior contribute to the bioessential element budget in the ocean? The amount of material transport through an ice VII layer is uncertain, let alone through ice X. The element flux will depend on many other factors such as the heat flux from the core, the size of the ice layer, the presence of liquid water at the bottom of the ice layer, and a host of poorly known chemical and physical properties of high-pressure ices.
• How do (bio)(geo)chemical cycles operate on Hycean worlds? The lack of rocky surfaces makes an Earth-like cycle impossible. There could be some kind of equilibrium between precipitation onto the seafloor and these precipitates having their minerals leached back into the ocean.
• How do dynamical processes affect Hycean worlds? Accurate modeling of the atmospheric circulation is needed to properly establish the climates of Hycean planets, which in turn would have effects on the chemistry, ocean–atmosphere interactions and observable features of the atmospheres. Similarly, modeling of ocean dynamics is also needed to assess the homogeneity and turbulence of the ocean, which can have significant effects on nutrient profiles, sedimentation, and leaching rates, which in turn can affect biochemical processes.
Overall, in this work we find that the chemical conditions in Hycean atmospheres and nutrient availability in their oceans have the potential to meet the requirements for oceanic microbial life similar to those in the early Earth. As discussed above, several important questions remain and motivate new directions for further theoretical investigations on the potential habitability of Hycean worlds. Observationally, Hycean worlds show significant promise to expand and accelerate the search for signatures of extraterrestrial life with current and upcoming facilities within this decade.
Author contributions
NM conceived, planned and led the project and writing of the manuscript with contributions from JM, FR and EB. NM conducted the modelling of the atmospheric structure and JM conducted the photochemical modelling with inputs from NM. JM, NM and FR processed the outputs of the photochemical calculations. FR and NM conducted the interior modelling. EB and NM conducted the estimations of bioessential elements. NM and FR computed the atmospheric spectra and synthetic JWST observations.Conflicts of interest
There are no conflicts to declare.Acknowledgements
NM, FR and EB acknowledge support from UK STFC and UKRI towards the PhD studies of FR and EB. JM acknowledges support from the NASA Exoplanet Research Program grant 80NSSC23K0281. NM thanks Alexandra Turchyn, Mark Wyatt, Siddharth Gandhi, Anjali Piette, and Andrew Sellek for helpful discussions. EB thanks Skyla White and Richard Anslow for helpful discussions.Notes and references
- L. Arnold, et al. , Astron. Astrophys., 2014, 564, A58 CrossRef.
- F. Rodler and M. López-Morales, Astrophys. J., 2014, 781, 54 CrossRef.
- Y. K. Feng, et al. , Astron. J., 2018, 155, 200 CrossRef.
- J. F. Kasting, D. P. Whitmire and R. T. Reynolds, Icarus, 1993, 101, 108–128 CrossRef CAS PubMed.
- F. Selsis, et al. , Astron. Astrophys., 2007, 476, 1373–1387 CrossRef CAS.
- R. K. Kopparapu, et al. , Astrophys. J., 2013, 765, 131 CrossRef.
- L. T. Elkins-Tanton and S. Seager, Astrophys. J., 2008, 685, 1237–1246 CrossRef.
- M. Gillon, et al. , Nature, 2017, 542, 456–460 CrossRef CAS PubMed.
- J. A. Dittmann, et al. , Nature, 2017, 544, 333–336 CrossRef CAS PubMed.
- K. Ment, et al. , Astron. J., 2019, 157, 32 CrossRef CAS.
- J. Lillo-Box, et al. , Astron. Astrophys., 2020, 642, A121 CrossRef.
- E. A. Gilbert, et al. , Astron. J., 2020, 160, 116 CrossRef CAS.
- J. E. Rodriguez, et al. , Astron. J., 2020, 160, 117 CrossRef.
- J. K. Barstow and P. G. Irwin, Mon. Not. R. Astron. Soc.: Lett., 2016, 461, L92–L96 CrossRef CAS.
- I. A. G. Snellen, et al. , Astron. J., 2017, 154, 77 CrossRef.
- J. Lustig-Yaeger, et al. , Astron. J., 2019, 158, 27 CrossRef CAS.
- N. Madhusudhan, A. A. A. Piette and S. Constantinou, Astrophys. J., 2021, 918, 1 CrossRef CAS.
- N. Madhusudhan, et al. , Astrophys. J., 2020, 891, L7 CrossRef CAS.
- A. A. A. Piette and N. Madhusudhan, Astrophys. J., 2020, 904, 154 CrossRef.
- M. C. Nixon and N. Madhusudhan, Mon. Not. R. Astron. Soc., 2021, 505, 3414–3432 CrossRef CAS.
- D. J. Stevenson, Nature, 1999, 400, 32 CrossRef CAS PubMed.
- R. Pierrehumbert and E. Gaidos, Astrophys. J., Lett., 2011, 734, L13 CrossRef.
- M. Mol Lous, R. Helled and C. Mordasini, Nat. Astron., 2022, 6, 819–827 CrossRef.
- F. Fressin, et al. , Astrophys. J., 2013, 766, 81 CrossRef.
- B. J. Fulton and E. A. Petigura, Astrophys. J., 2018, 156, 264 CAS.
- W. J. Borucki, et al. , Science, 2010, 327, 977 CrossRef CAS PubMed.
- G. R. Ricker, et al. , J. Astron. Telesc. Instrum. Syst., 2014, 1, 014003 CrossRef.
- A. Fukui, et al. , Publ. Astron. Soc. Jpn., 2022, 74, L1–L8 CrossRef CAS.
- K. Kawauchi, et al. , Astron. Astrophys., 2022, 666, A4 CrossRef CAS.
- T. Mikal-Evans, et al. , Astron. J., 2023, 165, 84 CrossRef.
- C. Piaulet, et al. , Nat. Astron., 2023, 7, 206–222 Search PubMed.
- C. L. Phillips, et al. , Astrophys. J., 2021, 923, 144 CrossRef CAS.
- C. Phillips, et al., arXiv, 2022, preprint, arXiv:2209.12919, DOI:10.48550/arXiv.2209.12919.
- M. Leung, et al. , Astrophys. J., 2022, 938, 6 CrossRef.
- X. Yu, et al. , Astrophys. J., 2021, 914, 38 CrossRef CAS.
- R. Hu, et al. , Astrophys. J., Lett., 2021, 921, L8 CrossRef CAS.
- S.-M. Tsai, et al. , Astrophys. J., Lett., 2021, 922, L27 CrossRef CAS.
- C. P. McKay, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 12628–12633 CrossRef CAS PubMed.
- C. S. Cockell, et al. , Astrobiology, 2016, 16, 89–117 CrossRef PubMed.
- A. R. Hendrix, et al. , Astrobiology, 2019, 19, 1–27 CrossRef PubMed.
- H. Lammer, et al. , Astron. Astrophys. Rev., 2009, 17, 181–249 CrossRef.
- L. Noack, et al. , Icarus, 2016, 277, 215–236 CrossRef CAS.
- M. Lingam and A. Loeb, Astron. J., 2018, 156, 151 CrossRef.
- B. Journaux, et al. , Space Sci. Rev., 2020, 216, 7 CrossRef.
- S. Maruyama, et al. , Geosci. Front., 2013, 4, 141–165 CrossRef CAS.
- S. Seager, et al. , Universe, 2021, 7, 172 CrossRef CAS.
- G. Choblet, et al. , Icarus, 2017, 285, 252–262 CrossRef CAS.
- K. Kalousová, et al. , Icarus, 2018, 299, 133–147 CrossRef.
- K. Kalousová and C. Sotin, Geophys. Res. Lett., 2018, 45, 8096–8103 CrossRef.
- J.-A. Hernandez, R. Caracas and S. Labrosse, Nat. Commun., 2022, 13, 3303 CrossRef CAS PubMed.
- L. Lebec, et al. , Icarus, 2023, 396, 115494 CrossRef CAS.
- P. B. Rimmer, et al. , Sci. Adv., 2018, 4, eaar3302 CrossRef CAS PubMed.
- D. Parkos, et al. , J. Geophys. Res.: Planets, 2018, 123, 892–909 CrossRef CAS.
- H. Innes, S.-M. Tsai and R. T. Pierrehumbert, arXiv, 2023, preprint, arXiv:2304.02698, DOI:10.48550/arXiv.2304.02698.
- S. Gandhi and N. Madhusudhan, Mon. Not. R. Astron. Soc., 2017, 472, 2334–2355 CrossRef CAS.
- I. Hubeny, Mon. Not. R. Astron. Soc., 2017, 469, 841–869 CrossRef CAS.
- J. I. Castor, P. G. Dykema and R. I. Klein, Astrophys. J., 1992, 387, 561 CrossRef CAS.
- B. Benneke, et al. , Astrophys. J., Lett., 2019, 887, L14 CrossRef CAS.
- R. Cloutier, et al. , Astron. Astrophys., 2019, 621, A49 CrossRef CAS.
- R. J. Barber, J. Tennyson, G. J. Harris and R. N. Tolchenov, Mon. Not. R. Astron. Soc., 2006, 368, 1087–1094 CrossRef CAS.
- L. Rothman, et al. , J. Quant. Spectrosc. Radiat. Transfer, 2010, 111, 2139–2150 CrossRef CAS.
- J. Borysow, L. Frommhold and G. Birnbaum, Astrophys. J., 1988, 326, 509 CrossRef CAS.
- G. S. Orton, et al. , Icarus, 2007, 189, 544–549 CrossRef CAS.
- M. Abel, L. Frommhold, X. Li and K. L. C. Hunt, J. Phys. Chem. A, 2011, 115, 6805–6812 CrossRef CAS PubMed.
- C. Richard, et al. , J. Quant. Spectrosc. Radiat. Transfer, 2012, 113, 1276–1285 CrossRef CAS.
- M. Allen, Y. L. Yung and J. W. Waters, J. Geophys. Res., 1981, 86, 3617–3627 CrossRef CAS.
- Y. L. Yung, M. Allen and J. P. Pinto, Astrophys. J., Suppl. Ser., 1984, 55, 465–506 CrossRef CAS PubMed.
- J. I. Moses, et al. , Astrophys. J., 2011, 737, 15 CrossRef.
- E. S. Kite and E. B. Ford, Astrophys. J., 2018, 864, 75 CrossRef.
- J. I. Moses, et al. , Astrophys. J., 2013, 777, 34 CrossRef PubMed.
- E. S. Kite, M. Manga and E. Gaidos, Astrophys. J., 2009, 700, 1732–1749 CrossRef CAS.
- J. I. Moses, et al. , Exp. Astron., 2022, 53, 279–322 CrossRef.
- S. Peacock, et al. , Astrophys. J., 2020, 895, 5 CrossRef CAS.
- J. I. Moses, et al. , Icarus, 2000, 145, 166–202 CrossRef CAS.
- J. F. Kasting, Origins Life Evol. Biosphere, 1990, 20, 199–231 CrossRef CAS PubMed.
- G. Arney, et al. , Astrobiology, 2016, 16, 873–899 CrossRef CAS PubMed.
- S. Ranjan, et al. , Astrophys. J., 2020, 896, 148 CrossRef CAS.
- N. Madhusudhan, K. K. M. Lee and O. Mousis, Astrophys. J., Lett., 2012, 759, L40 CrossRef.
- G. Chabrier, S. Mazevet and F. Soubiran, Astrophys. J., 2019, 872, 51 CrossRef CAS.
- S. W. Thomas and N. Madhusudhan, Mon. Not. R. Astron. Soc., 2016, 458, 1330–1344 CrossRef CAS.
- S. Seager, et al. , Astrophys. J., 2007, 669, 1279–1297 CrossRef CAS.
- F. Birch, J. Geophys. Res., 1952, 57, 227–286 CrossRef CAS.
- B. B. Karki, et al. , Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 14750–14756 CrossRef CAS.
- T. J. Ahrens, Mineral Physics & Crystallography: A Handbook of Physical Constants, American Geophysical Union, Washington, DC, 2000 Search PubMed.
- M. Scheucher, F. Wunderlich, J. L. Grenfell, M. Godolt, F. Schreier, D. Kappel, R. Haus, K. Herbst and H. Rauer, Astrophys. J., 2020, 898, 44 CrossRef CAS.
- D. Blain, B. Charnay and B. Bézard, Astron. Astrophys., 2021, 646, A15 CrossRef CAS.
- B. Charnay, D. Blain, B. Béard, J. Leconte, M. Turbet and A. Falco, Astron. Astrophys., 2021, 646, A171 CrossRef CAS.
- R. T. Pierrehumbert, Astrophys. J., 2023, 944, 20 CrossRef.
- L. J. Rothschild and R. L. Mancinelli, Nature, 2001, 409, 1092–1101 CrossRef CAS PubMed.
- N. Merino, et al. , Front. Microbiol., 2019, 10, 780 CrossRef PubMed.
- K. K. Hardegree-Ullman, et al. , Astrophys. J., Suppl. Ser., 2020, 247, 28 CrossRef CAS.
- A. Tsiaras, et al. , Nat. Astron., 2019, 3, 1086–1091 CrossRef.
- K. Lodders and B. Fegley, Icarus, 2002, 155, 393–424 CrossRef CAS.
- J. I. Moses, et al. , Astrophys. J., 2013, 777, 34 CrossRef PubMed.
- S. Seager, et al. , Astrophys. J., 2013, 777, 95 CrossRef.
- S. Ranjan, et al. , Astrophys. J., 2022, 930, 131 CrossRef.
- M. Asplund, et al. , Annu. Rev. Astron. Astrophys., 2009, 47, 481–522 CrossRef CAS.
- T. M. Hoehler, et al. , FEMS Microbiol. Ecol., 2001, 38, 33–41 CrossRef CAS.
- W. Bains, S. Seager and A. Zsom, Life, 2014, 4, 716–744 CrossRef PubMed.
- B. A. Berghuis, et al. , Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 5037–5044 CrossRef CAS PubMed.
- L. J. Robbins, et al. , Earth-Sci. Rev., 2016, 163, 323–348 CrossRef CAS.
- M. Rubin, et al. , Mon. Not. R. Astron. Soc., 2019, 489, 594–607 CrossRef CAS.
- A. D. Anbar, Science, 2008, 322, 1481–1483 CrossRef CAS PubMed.
- S. A. Crowe, et al. , Science, 2014, 346, 735–739 CrossRef CAS PubMed.
- C. Jones, et al. , Geology, 2015, 43, 135–138 CrossRef CAS.
- M. A. Saito, D. M. Sigman and F. M. Morel, Inorg. Chim. Acta, 2003, 356, 308–318 CrossRef CAS.
- A. D. Anbar and A. H. Knoll, Science, 2002, 297, 1137–1142 CrossRef CAS PubMed.
- E. D. Swanner, et al. , Earth-Sci. Rev., 2020, 211, 103430 CrossRef CAS.
- N. J. Tosca, et al. , Geol. Soc. Am. Bull., 2016, 128, 511–530 CrossRef CAS.
- L. J. Robbins, et al. , GeoBiology, 2013, 11, 295–306 CrossRef CAS PubMed.
- I. Mukherjee and R. R. Large, Geology, 2020, 48, 1018–1022 CrossRef CAS.
- K. Lodders, Oxford Research Encyclopedia of Planetary Science, 2020 Search PubMed.
- K. J. Zahnle, et al. , Planet. Sci. J., 2020, 1, 11 CrossRef.
- D. Nesvorný, F. V. Roig, D. Vokrouhlický, W. F. Bottke, S. Marchi, A. Morbidelli and R. Deienno, Icarus, 2023, 399, 115545 CrossRef.
- G. Gisler, R. Weaver and M. Gittings, Pure Appl. Geophys., 2011, 168, 1187–1198 CrossRef.
- T. Davison and G. S. Collins, Meteorit. Planet. Sci., 2007, 42, 1915–1927 CrossRef CAS.
- M. Nishizawa, et al. , J. Geophys. Res.: Planets, 2020, 125, e06291 Search PubMed.
- B. Carry, Planet. Space Sci., 2012, 73, 98–118 CrossRef.
- H. Genda, R. Brasser and S. Mojzsis, Earth Planet. Sci. Lett., 2017, 480, 25–32 CrossRef CAS.
- B. C. Johnson and H. J. Melosh, Icarus, 2012, 217, 416–430 CrossRef CAS.
- P. W. Reiners and A. V. Turchyn, Geology, 2018, 46, 863–866 CrossRef CAS.
- B. Peucker-Ehrenbrink, G. Ravizza and G. Winckler, Elements, 2016, 12, 191–196 CrossRef.
- I. Y. Fung, et al. , Global Biogeochem. Cycles, 2000, 14, 281–295 CrossRef CAS.
- S. Constantinou and N. Madhusudhan, Mon. Not. R. Astron. Soc., 2022, 514, 2073–2091 CrossRef CAS.
- A. Pinhas, et al. , Mon. Not. R. Astron. Soc., 2018, 480, 5314–5331 CrossRef CAS.
- I. E. Gordon, et al. , J. Quant. Spectrosc. Radiat. Transfer, 2022, 277, 107949 CrossRef CAS.
- F. Lattanzi, C. D. Lauro and J. V. Auwera, J. Mol. Spectrosc., 2011, 267, 71–79 CrossRef CAS.
- J. J. Harrison, N. D. C. Allen and P. F. Bernath, J. Quant. Spectrosc. Radiat. Transfer, 2010, 111, 357–363 CrossRef CAS.
- A. Jolly, Y. Benilan and A. Fayt, J. Mol. Spectrosc., 2007, 242, 46–54 CrossRef CAS.
- L. Xu, et al. , J. Mol. Spectrosc., 2004, 228, 453–470 CrossRef CAS.
- H. Müller, et al. , Astron. Astrophys., 2001, 370, L49–L52 CrossRef.
- R. Doyon, et al., Space Telescopes and Instrumentation 2012: Optical, Infrared, and Millimeter Wave, 2012, p. 84422R Search PubMed.
- P. Ferruit, et al., Space Telescopes and Instrumentation 2012: Optical, Infrared, and Millimeter Wave, 2012, p. 84422O Search PubMed.
- S. M. Birkmann, et al., Space Telescopes and Instrumentation 2014: Optical, Infrared, and Millimeter Wave, 2014, p. 914308 Search PubMed.
- G. H. Rieke, et al. , Publ. Astron. Soc. Pac., 2015, 127, 584 CrossRef.
- N. E. Batalha, et al. , Publ. Astron. Soc. Pac., 2017, 129, 064501 CrossRef.
- V. S. Meadows and R. K. Barnes, Handbook of Exoplanets, Springer International Publishing AG, 2018, p. 57 Search PubMed.
- S. Seager, et al. , Nat. Astron., 2020, 4, 802–806 CrossRef.
- E. S. Kite, et al. , Astrophys. J., 2020, 891, 111 CrossRef CAS.
- W. Misener, H. E. Schlichting and E. D. Young, Mon. Not. R. Astron. Soc., 2023, 524, 981–992 CrossRef.
- N. Madhusudhan, et al. , Space Sci. Rev., 2016, 205, 285–348 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fd00075c |
| This journal is © The Royal Society of Chemistry 2023 |