Efficient visible-light photocatalytic H2 evolution over metal-free g-C3N4 co-modified with robust acetylene black and Ni(OH)2 as dual co-catalysts†
Guican Bi‡
ab,
Jiuqing Wen‡ab,
Xin Li*ab,
Wei Liua,
Jun Xie*ab,
Yueping Fanga and
Weiwei Zhangab
aCollege of Materials and Energy, South China Agricultural University, Guangzhou 510642, PR China. E-mail: Xinliscau@yahoo.com; xiejun@scau.edu.cn
bKey Laboratory of Energy Plants Resource and Utilization, Ministry of Agriculture, Key Laboratory of Biomass Energy of Guangdong Regular Higher Education Institutions, South China Agricultural University, Guangzhou 510642, PR China
First published on 16th March 2016
Abstract
In this work, a novel g-C3N4/acetylene black (AB)/Ni(OH)2 ternary composite photocatalyst with dual robust electron co-catalysts was successfully synthetized using a facile two-step strategy: ultrasonic dispersion treatment and a subsequent precipitation process. The photocatalytic H2-production activity over the composite photocatalyst was also evaluated using an aqueous solution containing triethanolamine under visible light (λ ≥ 420 nm). For the first time, it was revealed that the robust AB can be utilized as a co-catalyst to significantly enhance the photocatalytic H2-evolution activity of g-C3N4. The results also demonstrated that the ternary g-C3N4/AB/Ni(OH)2 nanocomposite exhibited enhanced photocatalytic H2-evolution activity as compared to bulk g-C3N4 and binary hybrids. The g-C3N4–0.5% AB–1.0% Ni(OH)2 (weight ratio) composite shows the highest H2 evolution rate of 240 μmol g−1 h−1 under visible light irradiation, which is 320, 100 and 3.31 times higher than that of pure g-C3N4, g-C3N4–0.5% AB and g-C3N4–1.0% Ni(OH)2, respectively. It is believed that the excellent synergetic effect between the robust AB and Ni(OH)2 as dual electron co-catalysts on the surface of g-C3N4 can achieve the effectively promoted separation of photo-generated electron–hole pairs and enhance the following H2-evolution kinetics, thus resulting in a significant enhancement of the photocatalytic H2 evolution activity over g-C3N4. It is expected that the combination of nano-carbons such as AB and other earth-abundant co-catalysts can become a general strategy to improve the H2-evolution activity over various kinds of conventional semiconductors.
1. Introduction
The energy crisis and environmental pollution have been widely regarded as two major challenges facing human society in the 21st century. Considering its high-energy capacity, environmental benignancy, and recycling utilization, hydrogen energy as an ultimate green and promising fuel has attracted tremendous attention from scientists over the past four decades.1 Among various types of technologies for hydrogen generation, direct and sustainable solar water splitting using semiconductor photocatalysts has been considered to be one of the most fascinating, promising and economic approaches to produce hydrogen energy. Since the discovery of photo-induced water splitting in a photoelectrochemical (PEC) cell composed of an n-type rutile TiO2 photoanode and a Pt counter electrode in 1972,2 many achievements in the development of novel semiconductor photocatalysts for splitting water to generate green H2 fuel have been made.1,3 To date, although a range of semiconductor photocatalysts, such as TiO2,4,5 SiC,6,7 Ta3N5,8 TaON,9,10 (Ga1−xZnx)(N1−xOx),11 and sulfides and their solid solutions12,13 (such as CdS14 and ZnxCd1−xS15) have been extensively explored for hydrogen generation through splitting water, none of them can meet all the requirements for practical large-scale application to photocatalytic H2 evolution, including high efficiency, stability, safety, low cost and suitable band gap, significantly restricting their practical application under visible light.1 To some extent, water splitting under visible light has been long considered to be one of the “Holy Grails” of chemistry.16 Consequently, developing efficient, stable, and cheap H2-production photocatalysts that operate under visible-light is still a challenging and daunting task in this area.In 2009, Wang et al. pioneeringly reported that photocatalytic H2 evolution over the metal-free graphitic carbon nitride (g-C3N4) semiconductor could be realized under visible-light irradiation.17 Since then, the promisingly robust g-C3N4 has attracted multitudinous attention in the fields of photocatalytic H2 evolution,18–21 the degradation of liquid/gas pollutants,22 and CO2 photoreduction.23–25 Unfortunately, pure g-C3N4 generally suffers from obvious shortcomings such as a rapid carrier recombination rate, a small specific surface area, low electronic conduction, poor water oxidation ability, insufficient H2-evolution active sites and a large optical band gap, which greatly limit its practical application in photocatalytic H2 generation.26,27 To overcome the shortcomings of g-C3N4, several modification strategies, such as doping with metal or nonmetal species,28,29 constructing mesoporous/micro-nanostructured structures,30–33 loading with H2-evolution co-catalysts,18,34–36 sensitizing with organic dyes,37,38 exploiting liquid-state/all-solid-state Z-scheme systems,39–43 and fabricating heterojunctions,26,44–47 have been widely employed to enhance the photocatalytic H2-evolution performance of g-C3N4 under visible light. However, up to now, it still remains a major challenge to fabricate highly efficient, persistently stable, and earth-abundant g-C3N4-based composite semiconductors for photocatalytic H2 evolution.
Among these different modification strategies, fabricating Schottky-type nano-heterojunctions and loading with suitable H2-evolution co-catalysts have been widely recognized as two promising and effective strategies to improve photocatalytic H2 generation over g-C3N4.1,19,27 From a sustainability point of view, it has been well demonstrated that earth-abundant nanocarbon based materials (such as heteroatom-doped or undoped carbon nanotubes (CNTs), carbon fiber, graphene, and carbon black)48–50 and noble metal-free electrocatalysts (such as cobaloxime,51 CoS,52 Cu(OH)2,53 NiSx,54,55 MoS2,56,57 and WS2,58,59) are two of the most important types of robust electron co-catalysts for boosting the photocatalytic H2-evolution activity of g-C3N4. In general, loading these two kinds of robust co-catalysts could not only greatly improve the electrical conductivity, but also increase the surface H2-evolution active sites in g-C3N4, thus significantly enhancing the efficiency in the separation and transport of photo-generated carriers and improving the H2-evolution kinetics.60–62 As a result, enhanced H2-evolution activity was achieved. More interestingly, the loading of both cheap nanocarbon materials and noble metal-free robust co-catalysts seems to be more promising in achieving significant H2-evolution activity over g-C3N4 due to the positive synergistic effects between them. In our previous work, it was demonstrated that loading 0.5 wt% conductive carbon black between g-C3N4 and 1.5 wt% amorphous NiS co-catalyst can enhance the visible-light photocatalytic H2-evolution activity by a factor of 2.51.63 It is believed that the excellent synergetic effect between the cheap carbon black and noble metal-free NiS co-catalyst on the surface of g-C3N4 achieved the improved harvesting capacity of visible light, promoted charge separation and enhanced the H2-evolution kinetics, thus resulting in the significant enhancement of the photocatalytic activity of g-C3N4. Similarly, it was also evidenced that a hybrid of CNTs and NiS can achieve greatly enhanced H2-evolution activity over mesoporous g-C3N4, due to the synergistic effect of the effectively promoted separation of photo-generated electron–hole pairs and enhanced H2-evolution kinetics.34 However, to the best of our knowledge, there have been no investigations into developing metal-free conductive acetylene black (AB) based co-catalysts for photocatalytic H2-generation applications up to now. On the one hand, compared to larger-size CNTs and graphene, the much cheaper AB with a smaller particle size of several tens of nanometers is most desirable for its application in enhancing the H2-generation activity of g-C3N4, as a uniform distribution of AB on the surface of g-C3N4 with a much larger contact area and the efficient transport of photo-generated carriers between AB and g-C3N4 could be readily achieved.64 On the other hand, Ni(OH)2 and Ni(OH)2/graphene hybrids have turned out to serve as efficient co-catalysts to greatly enhance the H2-evolution rate over different semiconductor photocatalysts.65,66 Thus, it is naturally expected that the combination of cheap metal-free AB with noble metal-free Ni(OH)2 as a robust co-catalyst can greatly boost the photocatalytic H2-evolution activity of g-C3N4 because the conductive AB plays a key role in promoting the charge transfer from g-C3N4 to the p-type Ni(OH)2 co-catalyst, thus significantly accelerating the H2-evolution rate on the Ni(OH)2 sites.
Herein, for the first time, the robust AB is chosen to act as an efficient co-catalyst for enhancing the photocatalytic H2-evolution activity of g-C3N4. The ternary hybrid photocatalyst combining g-C3N4, AB and Ni(OH)2 was synthesized using a facile two-step strategy: ultrasonic dispersion treatment and a subsequent precipitation process (as shown in Scheme 1). The photocatalytic H2-production performance of the composite photocatalysts was also evaluated using an aqueous solution containing triethanolamine under visible light (λ ≥ 420 nm). The results revealed that ternary g-C3N4/AB/Ni(OH)2 nanocomposite exhibited enhanced photocatalytic H2-evolution activity as compared to bulk g-C3N4 and binary hybrids of g-C3N4/AB and g-C3N4/Ni(OH)2. The photocatalytic enhancement in H2-evolution activity is mainly ascribed to the promoted separation and transfer of photo-generated electron–hole pairs in g-C3N4 as well as the accelerated H2-evolution kinetics on Ni(OH)2, resulting from the key role of the conductive AB as a super electron transfer channel. It is expected that our work could inspire ongoing interest in utilizing the integrative effect of conductive nanocarbons as well as other noble metal-free co-catalysts to boost the activity of semiconductor-based materials for photocatalytic H2 generation.
2. Experimental
2.1 Synthesis of g-C3N4/AB/Ni(OH)2 composite photocatalysts
All chemical reagents were used as purchased without further purification. The pristine g-C3N4 was prepared by calcining melamine in a muffle furnace at 550 °C for 4 h with a ramping rate of 5 °C min−1. After cooling to room temperature, g-C3N4 was obtained in powder form.The binary g-C3N4/AB hybrid was synthesized using a simple sonochemical approach. The typical procedure was as follows: 3 g of g-C3N4 and 15 mg of AB were dissolved in 100 mL of absolute ethyl alcohol. The suspension was ultrasonicated for approximately 2 hours. The resultant product was filtered and dried at 80 °C in an oven overnight. The as-obtained g-C3N4/AB nanocomposite with a 0.5 wt% percentage of AB was denoted as g-C3N4–0.5% AB.
Binary g-C3N4/Ni(OH)2 was prepared via a direct precipitation method at room temperature, using Ni(NO3)2 and NaOH as the precursors for Ni(OH)2. Ni(NO3)2 was firstly mixed with an ultrasonically dispersed g-C3N4 sheet, and then Ni2+ ions were bonded to the surface of g-C3N4 due to chemical absorption between the Ni2+ ions and the heptazine rings of g-C3N4 under constant stirring at room temperature. With the addition of NaOH solution, those Ni2+ ions anchored on the surface of the g-C3N4 sheet were then converted into Ni(OH)2 nanoparticles. The obtained g-C3N4/Ni(OH)2 composite with a 1.0 wt% percentage of Ni(OH)2 were denoted as g-C3N4–1.0% Ni(OH)2.
To fabricate the ternary g-C3N4/AB/Ni(OH)2 hybrid, a similar process was followed as above. In a typical synthesis, 600 mg of g-C3N4–0.5% AB was dispersed in 40 mL of deionized water using ultrasound, and then 3.764 mL of 0.0172 M Ni(NO3)2 solution was dropped into the dispersion. The suspension was ultrasonicated for 30 min, and then 2 mL of 0.125 M NaOH solution was dropped into the above mixed solution. After stirring vigorously for another 2 h at room temperature, powder samples were collected and washed with distilled water, and dried at 80 °C for 24 h. The obtained sample was denoted as g-C3N4–0.5% AB–1.0% Ni(OH)2.
2.2 Characterization
The X-ray diffraction (XRD) patterns were obtained at room temperature using a MSAL-XD2 diffractometer with Cu Kα radiation (operated at 36 kV and 30 mA, λ = 0.15406 nm). Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were obtained on a JEM-2100HR (200 kV, Japan), using an accelerating voltage of 120 kV. X-ray photoelectron spectroscopy (XPS) was performed with a VG ESCALAB250 surface analysis system using a monochromatized Al Kα X-ray source (300 W, 5 mA, and 15 kV). The base pressure was about 3 × 10−9 mbar. The shift of the binding energy owing to relative surface charging was corrected using the C 1s level at 284.6 eV as an internal standard. The UV-Vis spectroscopy in the 200–800 nm region was performed with a Daojin UV-2550PC Diffuse Reflectance Spectrometer. Nitrogen adsorption–desorption isotherms were measured on a Gemini-2360 analyzer (Micromeritics Co., USA) at 77 K. The Brunauer–Emmett–Teller (BET) method was used to determine the specific surface area. The pore-size distributions were derived from the desorption branches of the isotherms using the Barrett–Joyner–Halenda (BJH) method. The photoluminescence (PL) spectra were measured using an LS 50B (Perkin Elmer, Inc., USA) with an excitation wavelength of 385 nm at room temperature.2.3 Photocatalytic reaction testing
Photocatalytic water splitting was carried out in a LabSolar H2 photocatalytic hydrogen evolution system (Perfectlight, Beijing) including a 300 W Xe lamp (PLS-SXE300, Beijing Trusttech). In a typical photocatalytic reaction, 50 mg of powder sample was dispersed in a Pyrex glass reactor containing a mixed solution of 85 mL of water and 15 mL of triethanolamine (TEOA). Then the system was sealed and vacuumized to keep the pressure at ∼ 0.1 MPa. Afterwards, a circular cooling water system was turned on and the reactor was irradiated with a Xe lamp (300 W) with a UV cut-off filter (λ ≥ 420 nm) under magnetic stirring. The gases evolved were analyzed on line with a gas chromatograph (GC-7900, TCD, with N2 as the carrier gas) after 1 h of illumination. The reaction was continued for 3 h. A cyclic experiment was carried out to investigate the photocatalytic stability of the g-C3N4/AB/Ni(OH)2 composite. After 3 h of reaction, the H2 produced was evacuated and then the experiment was run for another 3 h.2.4 Photoelectrochemical measurement experiments
The working electrodes were prepared as follows: 5 mg of photocatalyst powder was added into 2 mL of ethanol to make a slurry, and the powders were dispersed using ultrasonication. 500 μL of the solution was injected onto a 2 × 3.5 cm2 fluorine-doped tin-oxide (FTO) glass substrate using a drop casting method. The resulting electrodes were then dried under infrared light for 1 hour. Transient photocurrent measurements were performed on an electrochemical analyzer (CHI660E, CHI Shanghai, Inc.) using a standard three-electrode configuration, using the as-prepared working electrodes as the working electrode, a Pt wire as the counter electrode and Ag/AgCl (saturated KCl) as a reference electrode. A 300 W Xe arc lamp with a sharp cut-off filter served as a visible-light source (λ ≥ 420 nm). The electrochemical impedance spectra (EIS) of the above-mentioned working electrodes in the three-electrode system were also recorded via a computer controlled IM6e impedance measurement unit (Zahner Elektrik, Germany) over a frequency range of 0.01–105 Hz with an AC amplitude of 0 mV in the dark or under visible light. 0.1 M Na2SO4 aqueous solution was used as the electrolyte.3. Results and discussion
3.1 The structure and composition of the photocatalysts
The phase structure and composition of pure g-C3N4 and various composite photocatalysts with different AB and Ni(OH)2 content were initially investigated using powder XRD patterns. Fig. 1 shows the typical XRD patterns for various kinds of semiconductor photocatalysts. It can be seen that the XRD patterns of all samples feature two distinct diffraction peaks at 27.4° (the high-intensity diffraction peak) and 13.08° (the weak peak), which be indexed as the (002) and (100) diffraction planes observed for the graphitic hexagonal phase of g-C3N4, respectively (JCPDS # 87-1526).17 These two diffraction peaks are consistent with previous reports on g-C3N4 prepared through the polymerization of different precursors such as urea, cyanamide, dicyandiamide, and melamine.48,55,67 The strong (002) peak at 2θ = 27.4° is due to the characteristic interlayer stacking reflection of conjugated aromatic systems, corresponding to the interlayer distance d = 0.326 nm in g-C3N4, indicating that the pure g-C3N4 was well crystallized. The small (100) peak at 13.08°, corresponding to an interplanar separation of 0.681 nm, is associated with an in-plane structural packing motif.17,66,68 Furthermore, it was also seen from Fig. 1 that all the different peaks in the XRD pattern of the pure Ni(OH)2 are readily assigned to those in the pattern of hexagonal Ni(OH)2 (JCPDS # 14-0117), indicating the presence of Ni(OH)2.5,66,69 For the pure AB sample, the broad characteristic peaks in its patterns can be also well matched with those in the reference patterns (JCPDS # 75-2078), which represents that the AB sample is an amorphous carbon material.70 Moreover, for those samples containing AB and Ni(OH)2, no obvious shift of the g-C3N4 diffraction peaks was observed, implying no Ni- and C-doping in the lattice of g-C3N4. Thus, the Ni(OH)2 or AB should be only uniformly dispersed on the surface of g-C3N4, which can be further supported by the TEM micrographs. In addition, no significant diffraction peaks of any other phases or impurities can be detected, which may be possibly attributed to the low content, weak crystallization, ultrafine particle size and high uniform dispersion of AB and Ni(OH)2 in the ternary hybrid samples.70,71 It is therefore difficult to draw any conclusions regarding the introduction of AB and Ni(OH)2 co-catalysts from the XRD measurements only.The morphology and micro-structure of the ternary composite photocatalyst were further investigated using TEM measurements. Fig. 2 presents typical TEM images of pure g-C3N4, AB and the g-C3N4–0.5% AB–1.0% Ni(OH)2 composite sample. It is obviously seen from Fig. 2a that the pure g-C3N4 used in current study shows an obvious wrinkled sheet-like structure, which is in good agreement with our previous reports.34,44 It was also revealed from Fig. 2b that the AB nanoparticles exhibit a uniform size of ca. 20–50 nm. Clearly, the AB nanoparticles are very unstable and linked to each other to form a unique linear chain-like aggregated structure, which is in good agreement with previous reports in the literature.71,72 Fig. 2c further confirms that the linear chain-like aggregated AB nanoparticles are well distributed on the surface of the g-C3N4. Moreover, as observed in Fig. 2d and e, some AB and Ni(OH)2 nanoparticles are readily identified in the ternary composite sample. To further confirm the intimate interface contact of AB, Ni(OH)2 and g-C3N4 in the ternary nanohybrid, high resolution TEM (HRTEM) imaging of the g-C3N4–0.5% AB–1.0% Ni(OH)2 was performed, as shown in Fig. 2f. It is obviously that the inter-planar distance of 0.374 nm contributes to the (301) plane of hexagonal g-C3N4 (JCPDS # 87-1526). Furthermore, the HRTEM images also show a well distinguishable crystalline structure and the distance between two adjacent lattice fringes is about 0.214 nm, which is attributed to the (101) plane of hexagonal Ni(OH)2 (JCPDS # 14-0117). These results obviously reveal that highly dispersed Ni(OH)2 nanocrystallites are uniformly and closely attached on the surface of g-C3N4 or AB, which should be helpful for rapid charge separation in the electron-transfer process and provide additional reactive sites for H2 evolution over g-C3N4. It should be also noted that the amorphous AB nanoparticles don’t have the corresponding lattice fringes, and these cannot be observed through performing HRTEM. Additionally, energy dispersive X-ray (EDX) analysis was carried out to obtain the quantitative analysis of elements. Fig. S1† displays the EDX spectrum of a g-C3N4–0.5% AB–1.0% Ni(OH)2 sample. Based on the obvious signals of O and Ni elements in the EDS results of the g-C3N4–0.5% AB–1.0% Ni(OH)2 sample, the calculated atomic ratio of O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni was determined to be 2.11
Ni was determined to be 2.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, further demonstrating the presence of Ni(OH)2 in the composite photocatalyst. It should be noted that the Cu signal comes from the copper grid used in the measurement. Nevertheless, all these more detailed observations indicate that Ni(OH)2 nanoparticles are intimately deposited on the surface of g-C3N4 or AB, which will facilitate the rapid vectorial transfer of photogenerated electrons from g-C3N4 to Ni(OH)2, thus leading to the enhancement of the charge separation and photocatalytic efficiency.
1, further demonstrating the presence of Ni(OH)2 in the composite photocatalyst. It should be noted that the Cu signal comes from the copper grid used in the measurement. Nevertheless, all these more detailed observations indicate that Ni(OH)2 nanoparticles are intimately deposited on the surface of g-C3N4 or AB, which will facilitate the rapid vectorial transfer of photogenerated electrons from g-C3N4 to Ni(OH)2, thus leading to the enhancement of the charge separation and photocatalytic efficiency.
 | ||
| Fig. 2 TEM image of pure g-C3N4 (a), AB (b), and a g-C3N4–0.5% AB–1.0% Ni(OH)2 sample (c–e) and the corresponding HRTEM image (f) (the scale bars in (c–f) were 200, 20, 10 and 2 nm, respectively). | ||
XPS measurements were carried out to further study the chemical composition and oxidation state of AB, Ni(OH)2 and g-C3N4 in the ternary composite, as displayed in Fig. 3. The XPS survey spectrum of a g-C3N4–0.5% AB–1.0% Ni(OH)2 sample is shown in Fig. 3a, obviously indicating the co-existence of the elements C, N, Ni and a small amount of O. The corresponding high resolution XPS spectra of C 1s, N 1s and Ni 2p of the g-C3N4–0.5% AB–1.0% Ni(OH)2 sample are displayed in Fig. 3b–d, respectively. As shown in Fig. 3b, it is obvious that the two C 1s core-level peaks are obtained with binding energies of 284.9 and 288.3 eV. The peak with a binding energy of 284.9 eV was attributed to carbon impurities, which may be identified as sp2 C–C bonds from graphitic carbon on the surface of the g-C3N4–0.5% AB–1.0% Ni(OH)2 sample or C–C bonds from AB, demonstrating the formation of an interaction between AB and g-C3N4. Whereas, the main peak located at 288.3 eV, ascribed to the sp2-bonded carbon in the triazine rings (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonding), indicates the major carbon environment in g-C3N4.24 The N 1s XPS spectrum displayed in Fig. 3c can be deconvoluted into one distinct peak and three small peaks with BEs of 398.6, 399.1, 400.7 and 405.1 eV, which are assigned to C
N bonding), indicates the major carbon environment in g-C3N4.24 The N 1s XPS spectrum displayed in Fig. 3c can be deconvoluted into one distinct peak and three small peaks with BEs of 398.6, 399.1, 400.7 and 405.1 eV, which are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C (398.6 eV), tertiary nitrogen (C)3–N (399.1 eV), N–H (400.7 eV), and π-excitation (405.1 eV).63,66,73 In addition, Fig. 3d shows the high-resolution XPS spectra of Ni 2p3/2 for the g-C3N4–0.5% AB–1.0% Ni(OH)2 sample. The binding energy of Ni 2p3/2 centered at 856.5 eV is the typical Ni2+ phase of Ni(OH)2, which is consistent with previous reports.5,66,69 The peak at 862.33 eV is a corresponding satellite peak of Ni. All the above results further confirm the formation of a ternary hybrid composite composed of g-C3N4, AB and Ni(OH)2.
N–C (398.6 eV), tertiary nitrogen (C)3–N (399.1 eV), N–H (400.7 eV), and π-excitation (405.1 eV).63,66,73 In addition, Fig. 3d shows the high-resolution XPS spectra of Ni 2p3/2 for the g-C3N4–0.5% AB–1.0% Ni(OH)2 sample. The binding energy of Ni 2p3/2 centered at 856.5 eV is the typical Ni2+ phase of Ni(OH)2, which is consistent with previous reports.5,66,69 The peak at 862.33 eV is a corresponding satellite peak of Ni. All the above results further confirm the formation of a ternary hybrid composite composed of g-C3N4, AB and Ni(OH)2.
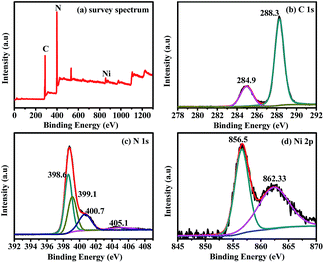 | ||
| Fig. 3 XPS survey spectrum (a), and high-resolution XPS spectra of the C 1s region (b), N 1s region (c), and Ni 2p region (d) of a g-C3N4–0.5% AB–1.0% Ni(OH)2 sample. | ||
N2 adsorption–desorption isotherms of some typical nanocomposites were obtained to further investigate information about their porous structures. Fig. S2† shows the liquid nitrogen adsorption–desorption isotherms and corresponding pore size distribution curves (inset) for g-C3N4, g-C3N4–1.0% Ni(OH)2 and g-C3N4–0.5% AB–1.0% Ni(OH)2. As observed in Fig. S2,† it is obvious that all the composites and pure g-C3N4 display robust mesoporous structures due to the presence of a classical type IV curve with a H3 adsorption hysteresis loop, according to International Union of Pure and Applied Chemistry (IUPAC) classification.74 It is generally believed that the type H3 hysteresis loop is related to the formation of slit-shaped mesopores, which may originate from the aggregation of plate-like g-C3N4 sheets. The pore size distribution curves of pure g-C3N4, g-C3N4–1.0% Ni(OH)2 and g-C3N4–0.5% AB–1.0% Ni(OH)2 show broad peaks centered at approximately 2–100 nm, further demonstrating the presence of mesopores and macropores (inset in Fig. S2†). The textural parameters of the three samples are summarized in Table S1.† As seen from Table S1,† the BET specific surface areas of g-C3N4, g-C3N4–1.0% Ni(OH)2 and g-C3N4–0.5% AB–1.0% Ni(OH)2 are 79.20, 74.30 and 68.19 m2 g−1, respectively. It is obvious that the introduction of AB and Ni(OH)2 nanoparticles could slightly lead to a decrease in specific surface area and an increase in pore diameter. This is due to loaded AB and Ni(OH)2 nanoparticles, maybe causing the partial filling/blocking of micropores and mesopores in g-C3N4. These data suggest that improvements in the surface area and pore volume are not the decisive factors for determining the photocatalytic activity of g-C3N4–0.5% AB–1.0% Ni(OH)2.
3.2 The optical properties of photocatalysts
The optical properties of all photocatalysts were measured using UV-vis diffuse reflectance spectroscopy. The comparison of the UV-vis spectra and the corresponding color (inset) of all samples are shown in Fig. 4, respectively. It can be observed from Fig. 4 that all samples show strong absorption intensities from the UV through the visible range up to 462 nm, indicating that the visible light absorption is ascribed to the intrinsic band gap transition of 2.7 eV for g-C3N4.75 As compared with pure g-C3N4 in the visible region, the ternary hybrid sample shows a similar absorption edge in shape, suggesting that the loading of AB and Ni(OH)2 cannot cause the red-shift of the absorption edge of g-C3N4 in this study. The AB and Ni(OH)2 co-catalysts are free on the surface of the g-C3N4 instead of being incorporated into the lattice of g-C3N4. However, as compared to pure g-C3N4, all ternary composites exhibit slightly enhanced absorption intensities in the visible light region ranging from 440 to 800 nm. Moreover, the absorption intensity of the as-prepared samples increased with increasing AB content, which agrees with the color change of the prepared samples that vary from light yellow to dark gray (insert of Fig. 4). Therefore, it can be concluded that the incorporation of Ni(OH)2 and AB as co-catalysts resulted in the noticeable enhancement of the absorption ability of visible light in the range from 440 to 800 nm without affecting the band-gap properties of g-C3N4, which would facilitate more light harvesting and the generation and separation of photo-generated charge carriers, thus resulting in the enhanced photocatalytic activity of the samples. | ||
| Fig. 4 UV-vis absorption spectra of all photocatalysts. (a) g-C3N4, (b) g-C3N4–0.5% AB, (c) g-C3N4–1.0% Ni(OH)2, and (d) g-C3N4–0.5% AB–1.0% Ni(OH)2. | ||
3.3 The activity and stability of photocatalysts
The photocatalytic hydrogen-production activity of the composite photocatalysts was evaluated using TEOA as a hole sacrificial reagent under visible-light irradiation (>420 nm). To rule out the possibility of mechanocatalytic water splitting, some typical control experiments such as those involving no photocatalyst or non-irradiation have to be performed.76 In this study, no appreciable hydrogen was produced in the absence of either irradiation or a photocatalyst, indicating that hydrogen was generated by photocatalytic reactions. Fig. 5A shows the H2-evolution rate over samples with different loading of Ni(OH)2 and AB under visible light irradiation. As can be seen from Fig. 5A, no remarkable decrease in the H2 evolution rate was observed for all samples in the 3 h reaction, implying that the photocatalysts are relatively stable under light illumination. A further comparison of the average photocatalytic H2-evolution rates over various photocatalysts is shown in Fig. 5B. As observed in Fig. 5B, the pure g-C3N4 sample only shows a very low visible light photocatalytic H2-evolution activity with a rate of 0.75 μmol g−1 h−1, because of the fast charge recombination and lack of reactive sites on g-C3N4. It was demonstrated that pure g-C3N4 is inactive for photocatalytic H2 production under visible light irradiation under the current conditions. However, the introduction of 0.5% AB and 1.0% Ni(OH)2 on the surface of g-C3N4 can achieve H2 evolution rates of 2.4 and 72.5 μmol g−1 h−1, which are 3.2 and 96.7 times higher than that of bulk g-C3N4, respectively. Clearly, the AB and Ni(OH)2 co-catalysts play the role of promoting charge separation and increasing the H2-evolution active sites, respectively. In this regard, the function of the AB co-catalyst is similar to those of other carbons such as CNTs and graphene, all of which can only boost the photocatalytic H2-evolution activity of g-C3N4 by a factor of 3–5, due to the absence of reactive sites on the surface of these carbons.48,75 In contrast, it is generally accepted that the Ni(OH)2 co-catalyst can act as the reactive sites,5,15,65,66,69 thus leading to an almost 100-fold enhancement in the H2-evolution rate over g-C3N4. Importantly, the low quantum efficiency of semiconductor photocatalysts is generally due to insufficient H2-evolution active sites on their surfaces.1 Accordingly, it is expected that the combination of these two kinds of co-catalyst could not only promote charge separation through the high electrical conductivity of AB, but also introduce abundant H2-evolution active sites on g-C3N4, thus significantly enhancing the H2-evolution rate over g-C3N4. As expected, among the as-prepared photocatalysts, the highest H2 evolution rate (240 μmol g−1 h−1, as shown in Fig. 5B) was observed over the optimized g-C3N4–0.5% AB–1.0% Ni(OH)2 composite, which is 320 times higher than that of pure g-C3N4. More importantly, under the same reaction conditions, the photocatalytic activity of the g-C3N4–0.5% AB–1.0% Ni(OH)2 composite is approximately 100 and 3.31 times higher than that of the binary g-C3N4–0.5% AB and g-C3N4–1.0% Ni(OH)2 samples, respectively. The results further indicate that each co-catalyst in the ternary composite could play an important role in determining their visible-light photocatalytic activity. Therefore, the positive synergistic effects of the Ni(OH)2 and AB co-catalysts resulted in the remarkable photoactivity enhancement of the g-C3N4–AB–Ni(OH)2 ternary hybrid.Apart from activity, the stability of a photocatalyst is very crucial for practical application. To confirm the stability of our composite catalysts, we performed four consecutive hydrogen evolution runs over the best sample, g-C3N4–0.5% AB–1.0% Ni(OH)2, under the same conditions. Each cycle was performed under visible light irradiation for 3 h. After each run, the reaction system was re-evacuated. Fig. 5C displays the recycling measurements of photocatalytic H2 evolution over the g-C3N4–0.5% AB–1.0% Ni(OH)2 sample under visible light irradiation (λ ≥ 420 nm). As seen in Fig. 5C, there is only a small activity loss (almost 20%) after four recycling runs. It is believed that the decreased catalytic activity over the g-C3N4–0.5% AB–1.0% Ni(OH)2 sample mainly originated from the slow falling off of AB and Ni(OH)2 nanoparticles from g-C3N4.63 These results indicate that the ternary hybrid photocatalyst is relatively stable, thus favoring its application in sustainable reuse.
3.4 The charge-separation performances of photocatalysts
To further deeply understand the photocatalytic enhancement mechanism for H2 evolution, the recombination of photo-generated electrons and holes in semiconductors was also investigated through PL analysis. PL spectra are commonly employed to reveal information about the migration, transfer and recombination processes of photo-generated electron–hole pairs in composite samples, which are the most key factors determining the overall photo-activity associated with semiconductor-based materials.75 PL spectra of different photocatalysts at room temperature with an excitation wavelength of 385 nm are shown in Fig. 6. Obviously, it is evident that all samples exhibit similar emission trends, with the one main emission peak at about 460 nm, which can be attributed to the recombination of electron–hole pairs in the g-C3N4.34,77 The samples modified with acetylene black and/or Ni(OH)2 were found to have a much lower PL intensity as compared with pure g-C3N4 under visible-light irradiation, which can mainly be ascribed to the suppressed direct recombination of electrons and holes. Especially, the g-C3N4–0.5% AB–1.0% Ni(OH)2 composite photocatalyst exhibited the weakest PL intensity, suggesting the longest lifetime of photo-generated charge carriers in the ternary nanocomposite compared to those of the binary hybrids and pure g-C3N4, which is qualitatively in good agreement with the trend in photo-activity enhancement. It is not surprising, therefore, that AB, as a good electron-acceptor carbon material, can rapidly transfer photogenerated electrons from the CB of g-C3N4 to Ni(OH)2 active sites through the intimate interface contacts between different components in the ternary nanohybrid. The experimental results further revealed that more efficient transfer and separation of charge carriers can be realized under visible-light illumination, owing to the coexistence of robust AB and Ni(OH)2 as dual co-catalysts. | ||
| Fig. 6 PL spectra of different photocatalysts at room temperature with an excitation wavelength of 385 nm. | ||
To further deeply understand the photocatalytic enhancement mechanism for H2 evolution, PEC analysis under visible light was also performed, which is one of the most powerful techniques to study interfacial charge transfer and recombination rates occurring in three-electrode systems.34,44,63 The transient photocurrent–time (I–t) curves recorded for g-C3N4, g-C3N4–0.5% AB, g-C3N4–1.0% Ni(OH)2, and the g-C3N4–0.5% AB–1.0% Ni(OH)2 composite photocatalyst under intermittent visible light irradiation (λ > 420 nm) are demonstrated in Fig. 7. It is obvious that the g-C3N4–0.5% AB–1.0% Ni(OH)2 photocatalyst exhibits the highest photocurrent density, which is obviously higher than those of pure g-C3N4, and the binary g-C3N4–0.5% AB and g-C3N4–1.0% Ni(OH)2 photocatalysts. It is well known that the photocurrent is generated mainly through the transport of photo-induced electrons to the back contact, and at the same time, the photogenerated holes are utilized by the hole acceptor in the electrolyte.1 As the photocurrent is a long-term process and is limited by the carrier mobility,78 the continuous enhancement in photocurrent can be attributed to the relatively higher carrier mobility, more efficient separation of photo-induced charge carriers and longer lifetime of the photogenerated electron–hole pairs in g-C3N4–0.5% AB–1.0% Ni(OH)2, as compared with pure g-C3N4. More importantly, it is noted from Fig. 7 that the decay of the transient photocurrent for the ternary sample is much slower than those of other binary hybrids and pure g-C3N4, further indicating that the ternary hybrid is relatively stable due to more efficiently improved interfacial charge transfer. Clearly, these transient photocurrent results demonstrate that the ternary hybrids could achieve a remarkably increased intensity of carrier mobility and improved separation and transfer of photo-excited electron–hole pairs due to the introduction of the AB and Ni(OH)2 co-catalysts, thus greatly contributing to the photoactivity enhancement.
 | ||
| Fig. 7 Transient photocurrent response (I–t curves) of different photocatalysts in 0.1 M Na2SO4 aqueous solution under visible light irradiation at 0.2 V vs. Ag/AgCl. | ||
To further confirm the enhanced charge separation rate, EIS in the dark was also performed, which is one of the most powerful techniques to study the interfacial charge migration, and transfer and recombination rates occurring in three-electrode systems.34 The EIS Nyquist plots of g-C3N4–0.5% AB and g-C3N4–0.5% AB–1.0% Ni(OH)2 samples are shown in Fig. 8. As shown in Fig. 8, the diameter of the arc radius in the EIS Nyquist plots of the g-C3N4–0.5% AB–1.0% Ni(OH)2 composite electrode is smaller than those of the g-C3N4–0.5% AB in the dark and under visible light irradiation, indicating that metal-free AB could more efficiently boost the interfacial charge transfer. This obvious enhancement of interfacial electron transfer also indicates a slower recombination and a longer lifetime of the photo-generated charge carriers in g-C3N4.
 | ||
| Fig. 8 Nyquist plots of g-C3N4–0.5% AB and g-C3N4–0.5% AB–1.0% Ni(OH)2 samples in 0.1 M Na2SO4 aqueous solution. | ||
3.5 Proposed photocatalytic mechanism
Based on all the above results, a possible mechanism for the separation and transport of the electron–hole pairs over the g-C3N4/AB/Ni(OH)2 under visible-light irradiation is proposed in Scheme 2. The g-C3N4 could be excited by visible light and generate the photo-generated electron–hole pairs. Normally, in these charge carriers only a fraction of electrons could participate in the photocatalytic reaction because of quick recombination. However, when inserting an AB layer between the g-C3N4 photocatalyst and Ni(OH)2 co-catalyst, these electrons excited from the conduction band of g-C3N4 can rapidly transfer to AB due to its excellent electronic conductivity, bringing about the rapid separation of the photogenerated electron and hole pairs. Therefore, the photo-induced electrons on the conduction band of g-C3N4 can be faster injected to the Ni(OH)2 co-catalyst through the conductive AB as the electron transfer channels due to the intimate interface contacts between AB, Ni(OH)2 and g-C3N4 in the ternary nanohybrid, thus leading to a higher enhancement of photocatalytic H2 evolution activity compared with the g-C3N4/Ni(OH)2 photocatalysts. Meanwhile, under visible light irradiation, the holes in the valence band of g-C3N4 can be directly consumed by the surface oxidation reaction of the sacrificial reagent (TEOA) adsorbed on the surface of g-C3N4. As a result, the excellent synergetic effect between the AB and Ni(OH)2 as dual co-catalysts on the surface of g-C3N4 can achieve the effectively promoted separation of photo generated electron–hole pairs and enhance the H2-evolution kinetics, therefore leading to the significant enhancement of the photocatalytic H2 evolution activity over the ternary hybrid photocatalyst. | ||
| Scheme 2 Proposed photocatalytic H2 production and charge transfer mechanisms in the g-C3N4–0.5% AB–1.0% Ni(OH)2 composite under visible light irradiation. | ||
4. Conclusions
To summarize, a new g-C3N4/AB/Ni(OH)2 composite photocatalyst with dual robust electron co-catalysts was successfully prepared using a facile two-step strategy: ultrasonic dispersion treatment and a subsequent precipitation process. For the first time, it was demonstrated that the robust AB can be utilized as a co-catalyst to greatly enhance the H2-evolution activity over the g-C3N4 semiconductor. More importantly, it was also found that the optimized g-C3N4–0.5% AB–1.0% Ni(OH)2 composite shows the highest H2 evolution rate of 240 μmol g−1 h−1 under visible light irradiation, which is 320, 100 and 3.31 times higher than that of pure g-C3N4, g-C3N4–0.5% AB and g-C3N4–1.0% Ni(OH)2, respectively. It is believed that the excellent synergetic effect between the robust AB and Ni(OH)2 as dual electron co-catalysts on the surface of g-C3N4 can achieve the effectively promoted separation and transportation of charge carriers and enhance the H2-evolution kinetics, thus achieving a significant enhancement of the photocatalytic H2 evolution activity over the ternary composite photocatalyst. This study signifies that the highly efficient and stable ternary g-C3N4/AB/Ni(OH)2 nanocomposite could be a promising photocatalyst for application in photocatalytic hydrogen generation. It is hoped that the combination of AB and other earth-abundant co-catalysts can become a general strategy to improve the H2-evolution activity over various kinds of conventional semiconductors.Acknowledgements
The work was supported by the industry and research collaborative innovation major projects of Guangzhou (201508020098), the National Natural Science Foundation of China (20906034, 21173088, and 21207041), and the State Key Laboratory of Advanced Technology for Material Synthesis and Processing (Wuhan University of Technology) (2015-KF-7).Notes and references
- X. Li, J. Yu, J. Low, Y. Fang, J. Xiao and X. Chen, J. Mater. Chem. A, 2015, 3, 2485–2534 CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- X. Chen, S. Shen, L. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- J. Wen, X. Li, W. Liu, Y. Fang, J. Xie and Y. Xu, Chin. J. Catal., 2015, 36, 2049–2070 CrossRef CAS.
- J. Yu, Y. Hai and B. Cheng, J. Phys. Chem. C, 2011, 115, 4953–4958 CAS.
- X. Zhou, X. Li, Q. Gao, J. Yuan, J. Wen, Y. Fang, W. Liu, S. Zhang and Y. Liu, Catal. Sci. Technol., 2015, 5, 2798–2806 CAS.
- X. Zhou, Q. Gao, X. Li, Y. Liu, S. Zhang, Y. Fang and J. Li, J. Mater. Chem. A, 2015, 3, 10999–11005 CAS.
- G. Hitoki, A. Ishikawa, T. Takata, J. N. Kondo, M. Hara and K. Domen, Chem. Lett., 2002, 31, 736–737 CrossRef.
- S. S. K. Ma, K. Maeda and K. Domen, Catal. Sci. Technol., 2012, 2, 818–823 CAS.
- M. Hara, J. Nunoshige, T. Takata, J. N. Kondo and K. Domen, Chem. Commun., 2003, 39, 3000–3001 RSC.
- K. Maeda, K. Teramura, D. Lu, T. Takata, N. Saito, Y. Inoue and K. Domen, Nature, 2006, 440, 295 CrossRef CAS PubMed.
- Z. Kai and G. Liejin, Catal. Sci. Technol., 2013, 3, 1672–1690 Search PubMed.
- Q. Li, X. Li, S. Wageh, A. A. Al-Ghamdi and J. Yu, Adv. Energy Mater., 2015, 5, 1500010 Search PubMed.
- J. Yuan, J. Wen, Q. Gao, S. Chen, J. Li, X. Li and Y. Fang, Dalton Trans., 2015, 44, 1680–1689 RSC.
- J. Ran, J. Zhang, J. Yu and S. Z. Qiao, ChemSusChem, 2014, 7, 3426–3434 CrossRef CAS PubMed.
- A. Bard and M. Fox, Acc. Chem. Res., 1995, 28, 141–145 CrossRef CAS.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- J. Hong, Y. Wang, Y. Wang, W. Zhang and R. Xu, ChemSusChem, 2013, 6, 2263–2268 CrossRef CAS PubMed.
- S. Cao and J. Yu, J. Phys. Chem. Lett., 2014, 5, 2101–2107 CrossRef CAS PubMed.
- S. Yin, J. Han, T. Zhou and R. Xu, Catal. Sci. Technol., 2015, 5, 5048–5061 CAS.
- S. Ye, R. Wang, M.-Z. Wu and Y.-P. Yuan, Appl. Surf. Sci., 2015, 358, 15–27 CrossRef CAS.
- J. Yu, S. Wang, J. Low and W. Xiao, Phys. Chem. Chem. Phys., 2013, 15, 16883–16890 RSC.
- X. Li, J. Wen, J. Low, Y. Fang and J. Yu, Sci. China Mater., 2014, 57, 70–100 CrossRef.
- J. Yu, K. Wang, W. Xiao and B. Cheng, Phys. Chem. Chem. Phys., 2014, 16, 11492–11501 RSC.
- J. Low, B. Cheng, J. Yu and M. Jaroniec, Energy Storage Materials, 2016, 3, 24–35 CrossRef.
- Z. Zhao, Y. Sun and F. Dong, Nanoscale, 2014, 7, 15–37 RSC.
- S. Cao, J. Low, J. Yu and M. Jaroniec, Adv. Mater., 2015, 27, 2150–2176 CrossRef CAS PubMed.
- J. Hong, X. Xia, Y. Wang and R. Xu, J. Mater. Chem., 2012, 22, 15006–15012 RSC.
- G. Zhang, M. Zhang, X. Ye, X. Qiu, S. Lin and X. Wang, Adv. Mater., 2014, 26, 805–809 CrossRef CAS PubMed.
- J. Hong, S. Yin, Y. Pan, J. Han, T. Zhou and R. Xu, Nanoscale, 2014, 6, 14984–14990 RSC.
- S. Yang, Y. Gong, J. Zhang, L. Zhan, L. Ma, Z. Fang, R. Vajtai, X. Wang and P. M. Ajayan, Adv. Mater., 2013, 25, 2452–2456 CrossRef CAS PubMed.
- Z. Huang, F. Li, B. Chen and G. Yuan, RSC Adv., 2015, 5, 102700–102706 RSC.
- X. Li, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2016 10.1039/c5cs00838g.
- Y. Zhong, J. Yuan, J. Wen, X. Li, Y. Xu, W. Liu, S. Zhang and Y. Fang, Dalton Trans., 2015, 44, 18260–18269 RSC.
- G. Zhang, G. Li and X. Wang, ChemCatChem, 2015, 7, 2864–2870 CrossRef CAS.
- S. Liang, Y. Xia, S. Zhu, S. Zheng, Y. He, J. Bi, M. Liu and L. Wu, Appl. Surf. Sci., 2015, 358, 304–312 CrossRef CAS.
- Y. Wang, J. Hong, W. Zhang and R. Xu, Catal. Sci. Technol., 2013, 3, 1703–1711 CAS.
- S. Min and G. Lu, J. Phys. Chem. C, 2012, 116, 19644–19652 CAS.
- X. Ding, Y. Li, J. Zhao, Y. Zhu, Y. Li, W. Deng and C. Wang, APL Mater., 2015, 3, 104410 CrossRef.
- D. J. Martin, P. J. T. Reardon, S. J. A. Moniz and J. Tang, J. Am. Chem. Soc., 2014, 136, 12568–12571 CrossRef CAS PubMed.
- H. Katsumata, Y. Tachi, T. Suzuki and S. Kaneco, RSC Adv., 2014, 4, 21405–21409 RSC.
- J. Lang, M. Liu, Y. Su, L. Yan and X. Wang, Appl. Surf. Sci., 2015, 358, 252–260 CrossRef CAS.
- F. Shi, L. Chen, M. Chen and D. Jiang, Chem. Commun., 2015, 51, 17144–17147 RSC.
- J. Yuan, J. Wen, Y. Zhong, X. Li, Y. Fang, S. Zhang and W. Liu, J. Mater. Chem. A, 2015, 3, 18244–18255 CAS.
- D. Jiang, J. Li, C. Xing, Z. Zhang, S. Meng and M. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 19234–19242 CAS.
- H. Liu, Z. Jin, Z. Xu, Z. Zhang and D. Ao, RSC Adv., 2015, 5, 97951–97961 RSC.
- W. Chen, T.-Y. Liu, T. Huang, X.-H. Liu, G.-R. Duan, X.-J. Yang and S.-M. Chen, RSC Adv., 2015, 5, 101214–101220 RSC.
- Q. Xiang, J. Yu and M. Jaroniec, J. Phys. Chem. C, 2011, 115, 7355–7363 CAS.
- J. Zhang and F. Huang, Appl. Surf. Sci., 2015, 358, 287–295 CrossRef CAS.
- S. Patnaik, S. Martha, S. Acharya and K. M. Parida, Inorg. Chem. Front., 2016, 3, 336–347 RSC.
- S.-W. Cao, X.-F. Liu, Y.-P. Yuan, Z.-Y. Zhang, J. Fang, S. C. J. Loo, J. Barber, T. C. Sum and C. Xue, Phys. Chem. Chem. Phys., 2013, 15, 18363–18366 RSC.
- Y. Zhu, Y. Xu, Y. Hou, Z. Ding and X. Wang, Int. J. Hydrogen Energy, 2014, 39, 11873–11879 CrossRef CAS.
- X. Zhou, Z. Luo, P. Tao, B. Jin, Z. Wu and Y. Huang, Mater. Chem. Phys., 2014, 143, 1462–1468 CrossRef CAS.
- Y. Xu and R. Xu, Appl. Surf. Sci., 2015, 351, 779–793 CrossRef CAS.
- L. Yin, Y.-P. Yuan, S.-W. Cao, Z. Zhang and C. Xue, RSC Adv., 2014, 4, 6127–6132 RSC.
- L. Ge, C. Han, X. Xiao and L. Guo, Int. J. Hydrogen Energy, 2013, 38, 6960–6969 CrossRef CAS.
- Y. Hou, A. B. Laursen, J. Zhang, G. Zhang, Y. Zhu, X. Wang, S. Dahl and I. Chorkendorff, Angew. Chem., Int. Ed., 2013, 52, 3621–3625 CrossRef CAS PubMed.
- Y. Hou, Y. Zhu, Y. Xu and X. Wang, Appl. Catal., B, 2014, 156, 122–127 CrossRef.
- M. S. Akple, J. Low, S. Wageh, A. A. Al-Ghamdi, J. Yu and J. Zhang, Appl. Surf. Sci., 2015, 358, 196–203 CrossRef CAS.
- J. Ran, J. Zhang, J. Yu, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- R. Cao, W. Lai and P. Du, Energy Environ. Sci., 2012, 5, 8134–8157 CAS.
- J. Wen, X. Li, H. Li, S. Ma, K. He, Y. Xu, Y. Fang, W. Liu and Q. Gao, Appl. Surf. Sci., 2015, 358, 204–212 CrossRef CAS.
- Z. Wu, H. Gao, S. Yan and Z. Zou, Dalton Trans., 2014, 43, 12013–12017 RSC.
- Z. Yan, X. Yu, A. Han, P. Xu and P. Du, J. Phys. Chem. C, 2014, 118, 22896–22903 CAS.
- J. Yu, S. Wang, B. Cheng, Z. Lin and F. Huang, Catal. Sci. Technol., 2013, 3, 1782–1789 CAS.
- X. Bai, R. Zong, C. Li, D. Liu, Y. Liu and Y. Zhu, Appl. Catal., B, 2014, 147, 82–91 CrossRef CAS.
- X. Bai, L. Wang, Y. Wang, W. Yao and Y. Zhu, Appl. Catal., B, 2014, 152, 262–270 CrossRef.
- J. Ran, J. Yu and M. Jaroniec, Green Chem., 2011, 13, 2708–2713 RSC.
- W. Ahmad, L. Chu, M. R. Al-Bahrani, X. Ren, J. Su and Y. Gao, Mater. Res. Bull., 2015, 67, 185–190 CrossRef CAS.
- K.-J. Huang, J.-Z. Zhang, Y.-L. Jia, K. Xing and Y.-M. Liu, J. Alloys Compd., 2015, 641, 119–126 CrossRef CAS.
- W. P. Tang, J. Mater. Chem., 2004, 14, 3457–3461 RSC.
- B. Zhu, P. Xia, W. Ho and J. Yu, Appl. Surf. Sci., 2015, 344, 188–195 CrossRef CAS.
- K. Sing, D. Everett, R. Haul, L. Moscou, R. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- L. Ge and C. Han, Appl. Catal., B, 2012, 117–118, 268–274 CrossRef CAS.
- S. Ikeda, T. Takata, T. Kondo, G. Hitoki, M. Hara, J. N. Kondo, K. Domen, H. Hosono, H. Kawazoe and A. Tanaka, Chem. Commun., 1998, 34, 2185–2186 RSC.
- D. Jiang, L. Chen, J. Zhu, M. Chen, W. Shi and J. Xie, Dalton Trans., 2013, 42, 15726–15734 RSC.
- M. Zhang, W. Yao, Y. Lv, X. Bai, Y. Liu, W. Jiang and Y. Zhu, J. Mater. Chem. A, 2014, 2, 11432–11438 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03118h |
| ‡ Guican Bi and Jiuqing Wen contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |



