Hierarchical self-assembly of protoporphyrin IX-bridged Janus particles into photoresponsive vesicles†
Youqian Xu,
Liang Wang,
Xinyun Zhu and
Cai-Qi Wang*
School of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing 100049, P. R. China. E-mail: wang-caiqi@ucas.ac.cn
First published on 11th March 2016
Abstract
Control over the building blocks in supramolecule by hierarchical self-assembly techniques provides a flexible strategy in nanotechnology and material science. In this study, a protoporphyrin IX (PPIX)-bridged Janus particle, as an amphiphilic building block, is driven by host–guest recognition between di-β-cyclodextrin-modified PPIX (PPIX-2CD) and azobenzene (AZO)-focused hydrophobic/hydrophilic hyperbranched polymers. The supramolecular Janus particles were hierarchically constructed into photoresponsive vesicles, which disassembled under the irradiation of ultraviolet (UV) light because of the trans- to cis-isomerization of the AZO groups. Results show that the PPIX-inserted vesicles exhibited excellent photostability against intense infrared radiation and good singlet oxygen producing ability.
Introduction
Protoporphyrin IX (PPIX) that are capable of photo-induced electron transfer and charge transport or photochemical reactions are extensively used in the field of biomimetic catalysis,1 photodynamic therapy,2,3 molecular recognition,4 sensors5 and model precursors in photosynthesis.6,7 However, optical instability and poor water-solubility greatly restrict its service life and application.8 In recent years, biologically inspired construct strategies have been attracting attention in which the molecular building blocks establish a particular topological structure to self-assemble higher-order supramolecular complexes.9,10 Aliphatic polyether as one class of candidate smart material can aggregate into micelles, which increase the stability of the photosensitizer.11–13 In addition, aliphatic polyethers are biocompatible and have been investigated as drug carriers and for gene therapy.14–16 On the other hand, hyperbranched polymers (HBPs) can be synthesized easily using a one-step procedure, making them economic to apply drug delivery.17 The highly branched structure, ample terminal groups and internal cavities of HBPs lead to minimal entanglements, increased free volume and solubility, and lower viscosity in solution.18–20 Thus, the great challenge is producing the smart materials that fabricate predefined structures that respond to external stimuli in a predictable manner to improve photosensitizer stability.Hierarchical self-assembly techniques provide strategies to fabricate more complicated topologies and synthesize state-of-the-art supramolecular materials with dynamic and tuneable properties, which have been the focus of much attention in nanotechnology and material science.21 The pre-assembled motif is established in advance, which acts as the primary building blocks that further self-assemble into an ordered organization to achieve specific functionalities such as light-harvesting,22,23 sensing24,25 and catalysis.26–28 The design of porphyrin building blocks and noncovalent interactions can be utilized to achieve supramolecular organizations. The primary building blocks can be a pair of electrostatic attraction donor–acceptor molecules stacked via a charge-transfer interaction,29,30 a host–guest synergistic effect, a supramolecular structure based on a host–guest interaction,31,32 or a metal–ligand coordination interaction.33–35 The organized building blocks through a synergistic effect obtains them a higher degree self-organization to obtain prospective properties. Janus particles have been the focus of significant attention because of their interesting properties associated with their asymmetric structure and potential performance.36,37 As potential building blocks, only few studies reported Janus particles generating a more highly organized structure.38,39
Herein, we propose a feasible route to realize well defined photoresponsive vesicles based on PPIX-bridged Janus particles. Three pre-assembled motifs containing di-β-cyclodextrin-modified PPIX (PPIX-2CD) and azobenzene (AZO)-focused hydrophobic/hydrophilic hyperbranched molecule were established in advance. The hierarchical self-assembly behaviour of PPIX-2CD, azobenzene-focused hyperbranched polyglycerol (AZO-HPG), and azobenzene-focused hyperbranched poly(3-ethyl-3-oxetanemethanol) (AZO-HBPO), based on the synergistic effect of host–guest recognition of cyclodextrin (CD) and AZO groups and hydrophobic interaction and π–π stacking of PPIX, was investigated. The resulting hierarchical nanostructures, including PPIX-bridged Janus particles and vesicles, were investigated in detail (Scheme 1). The photoresponsibility and the photostability of the obtained vesicles were determined under UV light and xenon (Xe)-lamp continuous irradiation. Moreover, the singlet oxygen production of nanoparticles that indicate PPIX photocatalytic activity was examined in the presence of potassium iodide (KI) solution.
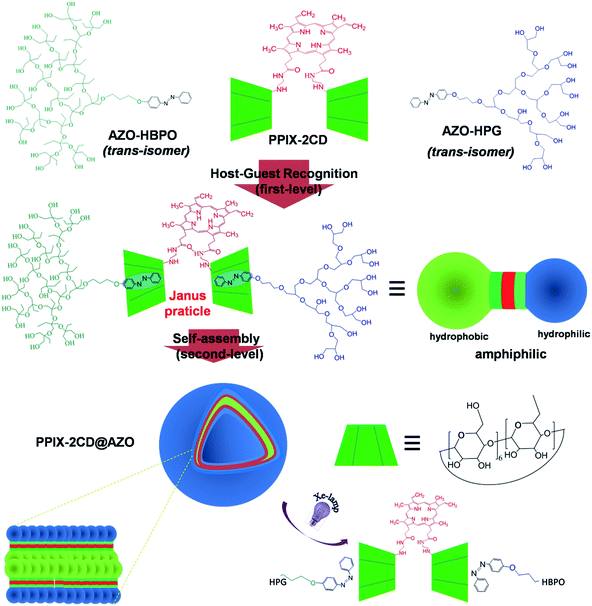 | ||
| Scheme 1 Preparation, self-assembly and disassembly processes of the photoresponsive vesicles constructed by PPIX-bridged Janus particles. | ||
Results and discussion
PPIX-2CD was synthesized via a single amino group substitution of β-CD conjugated with PPIX (Scheme S1†). The substitution of CD (DSCD) on PPIX quantified by the 1H NMR spectrum is close to 2, which indicated that PPIX-2CD was a suitable bridge linkage (Fig. S1–S3†).Two motifs containing the AZO group, namely, AZO-HPG (Mn,GPC = 814 Da, Mn,NMR = 970 Da, DPn = 11, PDI = 1.29, DB = 0.53) (Scheme S2 and S3; Fig. S4–S6 and S9†) and AZO-HBPO (Mn,GPC = 2121 Da, Mn,NMR = 3334 Da, DPn = 25, PDI = 1.38, DB = 0.20) (Scheme S4 and Fig. S7–S9†), were designed and synthesized through cationic ring-opening polymerization based on previous studies to construct amphiphilic Janus particles. The slow monomer addition technique was utilized to ensure that a high conversion and low monomer concentration were maintained at all reaction times for more dendritic segments. This method increased the possibility of monomers reacting with the end hydroxyl-terminated group and decreased HBPO or HPG homopolymers.
Hierarchical self-assembly behaviour was carried out among PPIX-2CD, AZO-HPG, and AZO-HBPO via the solvent-induced method, which was based on the synergistic effect of host–guest recognition of CD of PPIX-2CD, AZO of AZO-HBPO and AZO-HPG, hydrophobic interaction and π–π stacking. The self-assembled processes were conducted by adding water dropwise into a sequentially mixed DMF solution of PPIX-2CD, AZO-HPG and AZO-HBPO (molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
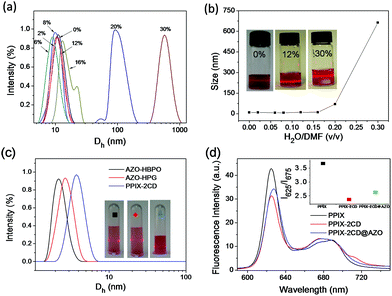
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then monitored by dynamic light scattering (DLS) measurements. The distribution with the increase in average hydrodynamic diameter (Dh) was observed when the volume ratio of water/DMF was gradually increased from 0% to 30% (water/DMF, v/v) (Fig. 1a and b).
1) and then monitored by dynamic light scattering (DLS) measurements. The distribution with the increase in average hydrodynamic diameter (Dh) was observed when the volume ratio of water/DMF was gradually increased from 0% to 30% (water/DMF, v/v) (Fig. 1a and b).
The average size of AZO-HPG, AZO-HBPO, and PPIX-2CD in dried DMF, as determined by DLS, was 2.5, 2.3 and 3.8 nm, respectively, indicating a narrow size distribution (Fig. 1c). When the three motifs were mixed in dried DMF, the resulting mixed solution exhibited weak Tyndall scattering. The average size of the mixture reached 11 nm, as determined by DLS. This size could be attributed to the formation of equatorial complexes driven by the interaction of the hydroxyl groups of the CD molecule and AZO groups in DMF, as reported by Denamor et al.40 The interaction process was also verified by UV-Vis spectroscopy. Compared with PPIX-2CD, the Soret band peak of PPIX-2CD in DMF mixed solution at 405 nm slightly decreased. No evident signal of the AZO group was observed because of its low concentration (Fig. S10†).
With water added dropwise into the DMF mixed solution, the aforementioned equatorial type of complexes changed into axial type with a smaller size because of the size match between trans-AZO and the hydrophobic cavity of CD rings. With the increase in the water/DMF ratio, Dh of established particles slightly decreased to approximately 9.5 nm, which was close to the size of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of PPIX-2CD, AZO-HPG and AZO-HBPO. This result showed that unique supramolecular PPIX-bridged Janus particles were constructed at this stage through AZO/CD host–guest interactions.
1 complex of PPIX-2CD, AZO-HPG and AZO-HBPO. This result showed that unique supramolecular PPIX-bridged Janus particles were constructed at this stage through AZO/CD host–guest interactions.
In the self-assembly experiment, AZO-HPG and PPIX-2CD in an equal molar ratio were mixed in dry DMF in advance, which facilitated coupling of a PPIX-2CD molecule to include an AZO-HPG molecule because of the steric hindrance effect of HPG branches. AZO-HBPO was added into the abovementioned mixed solution. The other CD molecule on PPIX-2CD could couple with the AZO group of AZO-HBPO to form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pre-assembled complexes (i.e., Janus particles). The mixed solutions were stirred for 4 h. A library of complexes could be obtained by recombination of motifs within a mixture of performed HPG-AZO@CD-PPIX-CD@AZO-HBPO. The use of steric hindrance could direct the formation of specific complexes.41 In this progress, it is highly possible that there is a mixture of symmetric PPIX-2CD@2AZO and Janus PPIX-2CD@2AZO. Once PPIX-2CD reacted with two AZO-HPG or two AZO-HBPO, the symmetric PPIX-2CD@2AZO would be dissolved in water or precipitate. Water was added dropwise to the DMF solution to obtain a mixed water/DMF solution. The dynamic nature of the supramolecular complexes was apparent, with the mixture changing over time and ultimately producing PPIX-bridged Janus particles through self-selectivity. The obtained Janus particles that possess an amphiphilic asymmetric structure could be used as building blocks to generate higher order structures.
1 pre-assembled complexes (i.e., Janus particles). The mixed solutions were stirred for 4 h. A library of complexes could be obtained by recombination of motifs within a mixture of performed HPG-AZO@CD-PPIX-CD@AZO-HBPO. The use of steric hindrance could direct the formation of specific complexes.41 In this progress, it is highly possible that there is a mixture of symmetric PPIX-2CD@2AZO and Janus PPIX-2CD@2AZO. Once PPIX-2CD reacted with two AZO-HPG or two AZO-HBPO, the symmetric PPIX-2CD@2AZO would be dissolved in water or precipitate. Water was added dropwise to the DMF solution to obtain a mixed water/DMF solution. The dynamic nature of the supramolecular complexes was apparent, with the mixture changing over time and ultimately producing PPIX-bridged Janus particles through self-selectivity. The obtained Janus particles that possess an amphiphilic asymmetric structure could be used as building blocks to generate higher order structures.
In the subsequent stage (H2O/DMF, 16–30%), Dh of the self-assembled system (PPIX-2CD/AZO-HPG/AZO-HBPO, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) rapidly increased to approximately 663 nm, which indicated that the Janus particles further self-assembled into second-level supramolecular aggregates. The mixed solution with a 30% (v/v) water/DMF ratio was dialyzed against water to remove DMF, and a khaki turbid assembled solution was obtained. The resulting aggregates, named PPIX-2CD@AZO, were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM) (Fig. 2). A spherical vesicle (approximately 694 nm) was observed and its size distribution was relatively narrow, which corresponded with the AFM results. The damaged vesicle confirmed the formation of a hollow structure by hydrophobic/hydrophilic interaction of Janus building blocks.
1) rapidly increased to approximately 663 nm, which indicated that the Janus particles further self-assembled into second-level supramolecular aggregates. The mixed solution with a 30% (v/v) water/DMF ratio was dialyzed against water to remove DMF, and a khaki turbid assembled solution was obtained. The resulting aggregates, named PPIX-2CD@AZO, were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM) (Fig. 2). A spherical vesicle (approximately 694 nm) was observed and its size distribution was relatively narrow, which corresponded with the AFM results. The damaged vesicle confirmed the formation of a hollow structure by hydrophobic/hydrophilic interaction of Janus building blocks.
 | ||
| Fig. 2 Self-assemblies captured by (a) TEM, (b) particle size distribution histograms by DLS, (c and d) SEM, (e and f) the height profile of PPIX-2CD@AZO particles and AFM images. | ||
The arrangement of PPIX in the PPIX-2CD@AZO vesicle was determined by UV-Vis spectroscopy (Fig. S10†) and fluorescence spectroscopy (Fig. 1d). The results showed that PPIX-2CD@AZO had a broader profile of the Soret band in UV-Vis spectra than that in the PPIX-2CD itself, which indicated that PPIX in the vesicle had a higher order aggregation.8
Compared with the fluorescence emission spectra of PPIX in an aqueous solution that of PPIX-2CD and PPIX-2CD@AZO revealed slight quenching at 625 nm and enhanced intensity at 675 nm. We used the ratio of I625/I675 to determine architectural difference, where I625 is the intensity at 625 nm and I675 is the intensity at 675 nm. This result showed that the intensity of PPIX inserted into PPIX-2CD@AZO was inferior to that of free PPIX, but exceeded the intensity of the PPIX component of PPIX-2CD. PPIX of PPIX-2CD and the hierarchical self-assembly underwent face-to-face stacking by π interactions between the aromatic macrocyclic cores.38 However, hyperbranched molecules were usually randomly distributed and formed a spherical cluster with a large steric hindrance effect. The aromatic macrocyclic cores could not stack so close, which enhanced the fluorescence efficiency. Thus, the vesicles constructed by hierarchical self-assembly of PPIX-bridged Janus particles might exhibit good performance for spectroscopic applications.
For photolabile PPIX, stability is a prerequisite for application in photochemistry and photochemical catalysis. The absorption spectra of the photosensitizer as a function of time were tested with light irradiation under ambient conditions to investigate the photostability of PPIX (inserted into PPIX-2CD@AZO vesicle) and the free state. Given that PPIX itself had a poor solubility in neutral water, the photostability of PPIX was tested in a weak alkaline aqueous solution (pH 7.4).
The photostability of PPIX and PPIX-2CD@AZO vesicles in an aqueous solution was determined under Xe-lamp (CEL-TCX250, 250 W) irradiation. The peak values of the Soret band of PPIX decreased by approximately 37% after 60 min of irradiation (Fig. 3a and c), which was mainly due to the reaction of reactive oxygen with the porphyrin ring during irradiation.42 By contrast, the peak values of the Soret band of PPIX-2CD@AZO decreased weakly under the same conditions, which indicated good photostability. The morphological transformation of the self-assembled vesicles was observed by TEM and SEM. After 20 min of irradiation, the vesicles had an intact spherical morphology (Fig. 5a and b). Only a small part of the vesicles fractured after 60 min of irradiation. In this test, a Xe-lamp without any cut-off filter was used and the entire 190–1100 nm wavelength region was utilized. Thus, the CD and AZO system may exhibit a dynamic balance of axial coordination and dissociation accompanied with trans–cis transformation of the AZO groups.43,44 Over time, the dynamic balance was destroyed, in which a small amount of AZO-HBPO or AZO-HPG exhibited incomplete host–guest recognition that led to the occurrence of disassembly. The results showed that the optimal photostability of PPIX-2CD@AZO under the Xe-lamp irradiation was obtained.
We further investigated the reversible trans–cis photoisomerization of AZO derivatives with UV light irradiation. The response of PPIX-2CD@AZO vesicles triggered by UV light (QVF135, 500 W) was observed by TEM (Fig. 4e). The vesicles were not observed after the solution was irradiated by UV light for 5 min. Therefore, UV light could divide the inclusion complex into three segments, leading to disassembly of the vesicles. However, some smaller nano-vesicles (30–50 nm) were observed in the solution. Thus, we implemented a long UV irradiation time to investigate the function of the nanovesicles and photostability of PPIX and PPIX-2CD@AZO in an aqueous solution.
The absorbance of PPIX dramatically decreased as the UV light irradiation time increased (Fig. 3d). After 10 min, an approximately 25.2% decrease in the absorbance of the major Soret band at 383 nm was observed. The light pink color of the solution faded after 60 min, and approximately 63% of the PPIX was degraded. In contrast, PPIX inserted into the PPIX-2CD@AZO vesicle exhibited good photostability to resist UV degradation. The absorbance of PPIX-2CD@AZO was decreased by only 8% after 10 min of UV irradiation (500 W). After 60 min, only 26% of the PPIX was degraded. The PPIX-2CD@AZO vesicles disassembled when subjected to UV irradiation. PPIX-2CD@2CD exhibited an excellent protective effect against UV irradiation, which might be attributed to protection from the CD and micelle structures. After disassembling, AZO-HBPO became insoluble and precipitated. AZO-HPG, an amphiphilic molecule, possibly converted into a micelle structure that could partially coat PPIX-2CD and some micelles or nanovesicles. Thus, the micellar effect provided partial photoprotection against PPIX degradation.
Singlet oxygen (1O2) is an important product of the photosensitization effect based on PPIX, which is a very reactive compound that reacts with biomolecules for use in photodynamic treatment and other fields.45,46 However, 1O2 possesses a rather short lifetime, especially an in aqueous solution. The iodide method was implemented to estimate 1O2 production. The amount of produced triiodide ion (I3−) was directly proportional to the concentration of 1O2 that was produced upon continuous irradiation.8,47–49 The ability of PPIX-2CD@AZO to generate 1O2 was investigated in phosphate buffer solution (pH 7.4). Fig. 5 shows the changes in the absorption spectra of PPIX-2CD@AZO with irradiation time in the iodide solution. Two new absorption bands at 287 and 351 nm were observed, which indicated I3− formation. The I3− conversion rate reached 86% after 60 min of irradiation.
 | ||
| Fig. 5 Changes in the absorption spectrum upon the irradiation of contained 0.1 mM KI self-assemble solutions. The inset is time-dependent measurement. | ||
The quantum yield of 1O2 production was relatively high because PPIX-2CD-bridged Janus amphiphilic particles constructed supramolecular vesicles that provided a relatively stable environment for PPIX as a result of steric hindrance. Aromatic macrocyclic cores with suitable stacking separation distance and continuous paths for transporting charge carriers can be formed and reduce quenching.50 The productivity of light-harvesting productivity was enhanced because of dynamic non-covalent bond combination of the hyperbranched polyether. Thus, the issue of close stacking based on the strong hydrophobic and π–π interactions to decrease the efficiency of light-harvesting was resolved.
Experimental section
PPIX-2CD was dissolved in DMF and mixed with AZO-HPG solution in DMF and stirred for 0.5 h. The AZO-HBPO solution was added to the resulting mixed solution, with a PPIX-2CD, AZO-HPG and AZO-HBPO molar ratio equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.5 mg mL−1 PPIX-2CD). After stirring for 3 h at ambient temperature, water was added dropwise to the DMF solution to obtain a mixed solution with a 30% (v/v) water/DMF volume ratio. The mixed solutions were stirred for 4 h and then dialyzed against water (MWCO = 3.5 kDa; Solarbio) for 48 h to remove DMF. The final solution had a slight yellow turbidity, indicating the formation of supramolecular self-assemblies. The morphology of the nanoparticles was evaluated by TEM, SEM, and AFM.
1 (0.5 mg mL−1 PPIX-2CD). After stirring for 3 h at ambient temperature, water was added dropwise to the DMF solution to obtain a mixed solution with a 30% (v/v) water/DMF volume ratio. The mixed solutions were stirred for 4 h and then dialyzed against water (MWCO = 3.5 kDa; Solarbio) for 48 h to remove DMF. The final solution had a slight yellow turbidity, indicating the formation of supramolecular self-assemblies. The morphology of the nanoparticles was evaluated by TEM, SEM, and AFM.
The optical response of PPIX-2CD@AZO nanoparticles was tested with UV radiation for 5 min. UV lamp power was up to 500 W (Philips, QVF135, Shanghai) to accelerate vesicle rupture. The optical response performance of PPIX-2CD@AZO nanoparticles was detected by TEM.
Conclusions
In summary, it was determined that a photoresponsive supramolecular vesicle possessed outstanding spectroscopic characteristics through hierarchical self-assembly techniques. The pre-made building blocks were assembled around di-CD-functionalized PPIX and AZO-focused hydrophilic/hydrophobic hyperbranched polymers to generate PPIX-bridged Janus particles, which further comprised supramolecular vesicles. The obtained self-assembly exhibited excellent photostability against UV light and Xe-lamp irradiation, and it could be disassembled under UV light irradiation (λ = 365 nm). The light-responsive supramolecular assembly provides a potential system for photodynamic therapy and targeted delivery.Acknowledgements
This study was supported by the Foundation of University of Chinese Academy of Sciences (Y25102CN00 and Y0JT017J01).Notes and references
- J. J. Warren and J. M. Mayer, J. Am. Chem. Soc., 2011, 133, 8544–8551 CrossRef CAS PubMed.
- B. Li, E. H. Moriyama, F. Li, M. T. Jarvi, C. Allen and B. C. Wilson, Photochem. Photobiol., 2007, 83, 1505–1512 CrossRef CAS PubMed.
- T. Nakanishi, T. Ogawa, C. Yanagihara and I. Tamai, J. Pharm. Sci., 2015, 104, 3092–3100 CrossRef CAS PubMed.
- M. Krieg and D. G. Whitten, J. Am. Chem. Soc., 1984, 106, 2477–2479 CrossRef CAS.
- W. Knoben, M. Crego-Calama and S. H. Brongersma, Sens. Actuators, B, 2012, 166, 349–356 CrossRef.
- S. V. Bhosale, S. V. Bhosale, G. V. Shitre, S. R. Bobe and A. Gupta, Eur. J. Org. Chem., 2013, 3939–3954 CrossRef CAS.
- E. M. Coccia, E. Perrotti, E. Stellacci, R. Orsatti, N. Del Russo, G. Marziali, U. Testa and A. Battistini, Eur. J. Org. Chem., 1997, 764–772 CAS.
- C. Aggelidou, T. A. Theodossiou and K. Yannakopoulou, Photochem. Photobiol., 2013, 89, 1011–1019 CrossRef CAS PubMed.
- J. M. Lehn, Science, 2002, 295, 2400–2403 CrossRef CAS PubMed.
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418–2421 CrossRef CAS PubMed.
- S. V. Bhosale, S. V. Bhosale, M. B. Kalyankar, S. J. Langford and C. H. Lalander, Aust. J. Chem., 2010, 63, 1326–1329 CrossRef CAS.
- M. B. Cardoso, D. Smolensky, W. T. Heller, K. Hong and H. O'Neill, Energy Environ. Sci., 2011, 4, 181–188 CAS.
- A. Z. Samuel and S. Ramakrishnan, Macromolecules, 2012, 45, 2348–2358 CrossRef CAS.
- G. A. Husseini and W. G. Pitt, Adv. Drug Delivery Rev., 2008, 60, 1137–1152 CrossRef CAS PubMed.
- M. Calderon, M. A. Quadir, S. K. Sharma and R. Haag, Adv. Mater., 2010, 22, 190–218 CrossRef CAS PubMed.
- G. Bonacucina, M. Cespi, G. Mencarelli, G. Giorgioni and G. F. Palmieri, Polymers, 2011, 3, 779–811 CrossRef CAS.
- H. Jin, W. Huang, X. Zhu, Y. Zhou and D. Yan, Chem. Soc. Rev., 2012, 41, 5986–5997 RSC.
- X.-p. Miao, Y.-s. Guo, L.-f. He, Y. Meng and X.-y. Li, Chin. J. Polym. Sci., 2015, 33, 1574–1585 CrossRef CAS.
- X. Feng, D. Taton, E. Ibarboure, E. L. Chaikof and Y. Gnanou, J. Am. Chem. Soc., 2008, 130, 11662–11676 CrossRef CAS PubMed.
- J. Pan, M. Wen, D. Yin, B. Jiang, D. He and L. Guo, Tetrahedron, 2012, 68, 2943–2949 CrossRef CAS.
- A. Wang, J. Huang and Y. Yan, Soft Matter, 2014, 10, 3362–3373 RSC.
- D. R. Abujetas, R. Paniagua-Dominguez and J. A. Sanchez-Gil, ACS Photonics, 2015, 2, 921–929 CrossRef CAS.
- L. Liu, D. Thang Duy, R. Kodiyath, Q. Kang, H. Abe, T. Nagao and J. Ye, Adv. Funct. Mater., 2014, 24, 7754–7762 CrossRef CAS.
- A. I. Campbell and S. J. Ebbens, Langmuir, 2013, 29, 14066–14073 CrossRef CAS PubMed.
- R. Villalonga, P. Diez, A. Sanchez, E. Aznar, R. Martinez-Manez and J. M. Pingarron, Chem.–Eur. J., 2013, 19, 7889–7894 CrossRef CAS PubMed.
- Q. Chen, L. Zheng, B. Chen and J. Lin, Eur. Polym. J., 2013, 49, 2610–2616 CrossRef CAS.
- A. Kirillova, C. Schliebe, G. Stoychev, A. Jakob, H. Lang and A. Synytska, ACS Appl. Mater. Interfaces, 2015, 7, 21218–21225 CAS.
- P. B. Landon, A. H. Mo, A. D. Printz, C. Emerson, C. Zhang, W. Janetanakit, D. A. Colburn, S. Akkiraju, S. Dossou, B. Chong, G. Glinsicy and R. Lal, Langmuir, 2015, 31, 9148–9154 CrossRef CAS PubMed.
- S. V. Bhosale, S. V. Nalage, J. M. Booth, A. Gupta, S. K. Bhargava and S. V. Bhosale, Supramol. Chem., 2012, 24, 779–786 CrossRef CAS.
- C. F. J. Faul, Acc. Chem. Res., 2014, 47, 3428–3438 CrossRef CAS PubMed.
- Y. Shi, Z. Yang, H. Liu, Z. Li, Y. Tian and F. Wang, ACS Macro Lett., 2015, 4, 6–10 CrossRef CAS.
- B. Jiang, W. Tao, X. Lu, Y. Liu, H. Jin, Y. Pang, X. Sun, D. Yan and Y. Zhou, Macromol. Rapid Commun., 2012, 33, 767–772 CrossRef CAS PubMed.
- D. S. Bohle, E. L. Dodd, T. B. J. Pinter and M. J. Stillman, Inorg. Chem., 2012, 51, 10747–10761 CrossRef CAS PubMed.
- Y. Yan and J. Huang, Coord. Chem. Rev., 2010, 254, 1072–1080 CrossRef CAS.
- J. Liu, T. Chen, X. Deng, D. Wang, J. Pei and L. J. Wan, J. Am. Chem. Soc., 2011, 133, 21010–21015 CrossRef CAS PubMed.
- J. Hu, S. Zhou, Y. Sun, X. Fang and L. Wu, Chem. Soc. Rev., 2012, 41, 4356–4378 RSC.
- X. Yu, S. Huang, K. Chen, Z. Zhou, X. Guo and L. Li, Ind. Eng. Chem. Res., 2015, 54, 2690–2696 CrossRef CAS.
- Y. Liu, C. Yu, H. Jin, B. Jiang, X. Zhu, Y. Zhou, Z. Lu and D. Yan, J. Am. Chem. Soc., 2013, 135, 4765–4770 CrossRef CAS PubMed.
- S. Chatterjee and S. Ramakrishnan, ACS Macro Lett., 2014, 3, 953–957 CrossRef CAS.
- A. F. D. Denamor, R. Traboulssi and D. F. V. Lewis, J. Am. Chem. Soc., 1990, 112, 8442–8447 CrossRef.
- Y. Ma, S. V. Kolotuchin and S. C. Zimmerman, J. Am. Chem. Soc., 2002, 124, 13757–13769 CrossRef CAS PubMed.
- E. Nagababu and J. M. Rifkind, Antioxid. Redox Signaling, 2004, 6, 967–978 CrossRef CAS PubMed.
- X. Zheng, D. Wang, Z. Shuai and X. Zhang, J. Phys. Chem. B, 2012, 116, 823–832 CrossRef CAS PubMed.
- W. S. Lee and A. Ueno, Macromol. Rapid Commun., 2001, 22, 448–450 CrossRef CAS.
- H. Homayoni, K. Jiang, X. Zou, M. Hossu, L. H. Rashidi and W. Chen, Photodiagn. Photodyn. Ther., 2015, 12, 258–266 CrossRef CAS PubMed.
- L. M. Rossi, P. R. Silva, L. L. R. Vono, A. U. Fernandes, D. B. Tada and M. S. Baptista, Langmuir, 2008, 24, 12534–12538 CrossRef CAS PubMed.
- J. Černý, M. Karásková, J. Rakušan and S. Nešpůrek, J. Photochem. Photobiol., A, 2010, 210, 82–88 CrossRef.
- J. Mosinger, M. Janoskova, K. Lang and P. Kubat, J. Photochem. Photobiol., A, 2006, 181, 283–289 CrossRef CAS.
- J. Mosinger and B. Mosinger, Experientia, 1995, 51, 106–109 CrossRef CAS PubMed.
- V. Borovkov, Symmetry, 2014, 6, 256–294 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00836d |
| This journal is © The Royal Society of Chemistry 2016 |