DOI:
10.1039/C5RA28114H
(Paper)
RSC Adv., 2016,
6, 31367-31373
Corrosion resistance of a tungsten modified AISI 430 stainless steel bipolar plate for proton exchange membrane fuel cells†
Received
31st December 2015
, Accepted 15th March 2016
First published on 17th March 2016
Abstract
In order to improve the corrosion resistance of AISI 430 stainless steel (430 SS) as a bipolar plate for proton exchange membrane fuel cells (PEMFCs), a tungsten diffusion layer has been successfully prepared on the AISI 430 SS samples using a plasma surface diffusion alloying technique. The tungsten diffusion-modified 430 SS (W-modified 430 SS) has a black surface and a tungsten diffusion layer with a thickness of 7–8 μm. X-ray diffraction data shows that there is only the body-centered-cubic tungsten phase on the surface of the W-modified 430 SS. In addition, the average contact angle with water for W-modified 430 SS is 93.5°, demonstrating the better hydrophobicity of its surface compared with the untreated one with an average contact angle of 69.6°. The electrochemical behavior of W-modified 430 SS is investigated in the simulated anode environment of a PEMFC (0.05 M H2SO4 + 2 ppm HF + 0.01 M NaCl solution at 70 °C). Potentiodynamic polarization, potentiostatic polarization and electrochemical impedance spectroscopy measurements reveal that the W-modified diffusion layer considerably improves the corrosion resistance of the 430 SS specimen compared with the untreated one. The corrosion current density of W-modified 430 SS is maintained at 2–3 μA cm−2 under the simulated anode conditions. Moreover, no obvious pitting generates on the surface of W-modified 430 SS after 4 h of potentiostatic testing, while serious corrosion occurs on the surface of untreated 430 SS.
1 Introduction
Proton exchange membrane fuel cells (PEMFCs) have drawn the attention of many countries and investigators due to their high efficiency, noiselessness, zero pollution emission and lower operation temperature.1 Therefore, they could be used as promising substitutes for the traditional fossil fuels and major power sources for electrical vehicles and portable applications. In PEMFC stacks, the bipolar plates, as important multi-functional components, not only distribute fuel gas and oxygen to the anode and cathode, respectively, but also connect the individual fuel cells into stacks and conduct the current from one cell to another.2 One of the primary properties of bipolar plates is corrosion resistance which is directly related to their service life.3 The corrosion of bipolar plates is mostly caused by two factors. In general, the bipolar plates are always exposed to a highly acidic solution containing anions such as F−, SO42−, SO32−, HSO4−, HSO3−, CO32− and HCO3− at higher operating temperatures.4 These ions result from the dissolution of the membrane electrode assembly (MEA) and the process technology of the electrode. Therefore, the bipolar plates are faced with inevitable electrochemical corrosion.5 Otherwise, aggressive ions such as Cl−, SO42− and OH− contained in the fuel gas and oxygen would increase the electrochemical corrosion of the bipolar plates.
In general, the Cl− ions introduced through the fabrication of the MEA and catalysts have little effect on the properties of PEMFCs. However, Cl− could be taken into the highly acidic solution and this produces a more serious challenge to the corrosion resistance of the bipolar plates, especially metallic bipolar plates, because Cl− can increase the pitting corrosion of many metals in highly acidic solutions.6–8 Furthermore, for some special methods of hydrogen production and purification, such as the utilization of exhaust gases from fertilizer plants or the chlor-alkali industry,9 the raw material contains large amounts of impurities especially Cl− which could not be eliminated thoroughly in the whole process. And when hydrogen which comes from either of these sources is used as the anode fuel of a PEMFC, the contained Cl− will be taken into the electrolyte through the anode side and accumulate gradually. Though the concentration of accumulated Cl− is limited, it will generate severe pitting corrosion on the anode plate.10 On the contrary, the cathode plate is not affected by Cl− during the working process due to the resistance of the proton exchange membrane.11 Therefore, the corrosion resistance of the anode plate becomes more important to the performance of PEMFCs.
A traditional material for the bipolar plates of a PEMFC is graphite, which shows excellent corrosion resistance and low contact resistance in the low pH environment. However, its poor mechanical strength and high permeability make it difficult to fabricate a low cost thin plate, which is an important challenge for wide commercial use.12 Candidate metallic bipolar plate materials, including stainless steels,13,14 Ni–Cr alloys,15 aluminum16 and so on, are currently under consideration as substitutes for traditional graphite. Among them, stainless steel is widely accepted as one of the main PEMFC bipolar plate material candidates due to its suitable physical and mechanical properties as well as relatively low cost.13,17 However, bare stainless steel cannot be effectively applied into PEMFCs in terms of corrosion resistance. In order to improve the corrosion resistance of bare stainless steel in the PEMFC electrolyte, our laboratory has used transition metals and their nitrides or carbides as protective layers for 304 SS bipolar plates via a plasma surface diffusion alloying technique.18,19 Nonetheless, compared with the inevitable corrosion environment of a PEMFC, the stainless steel bipolar plate in the anode with hydrogen, which contains Cl−, will also face more severe pitting corrosion,6,8 which is caused by increased Cl− in the electrolyte. This situation has never been considered in all of our finished works and thus a stronger corrosion resistance layer must be prepared on the surface of bare stainless steel to extend its service life in the simulated anode environment of a PEMFC (0.05 M H2SO4 + 2 ppm HF + 0.01 M NaCl solution at 70 °C).
In this work, we prepared a W-modified layer on a stainless steel substrate via a plasma surface diffusion alloying technique, to improve the corrosion resistance of stainless steel in the simulated anode environment of a PEMFC (0.05 M H2SO4 + 2 ppm HF + 0.01 M NaCl). In this PEMFC environment, the pitting occurs mainly on the surface of the specimen which will be perfectly covered by the layer after the plasma surface diffusion alloying process, and thus the corrosion resistance of the substrate has no-effect on the pitting of the treated bipolar plate. Therefore, we chose AISI 430 SS, which has a low nickel content, as the substrate to reduce the cost of the PEMFC, although it is seen as being easily corroded in acidic solution. Furthermore, compared with other transition metals, tungsten shows good pitting resistance as the coating raw material for improving the pitting resistance of stainless steel bipolar plates especially against Cl−, because the WO3 film and inhibitor ion (WO42−) generated during the working process exhibit a protective effect in the PEMFC environment.20 Finally, compared with the different crystal structure of the austenite stainless steels which were used in our previous works,5,18,19 the similar crystal structure of AISI 430 SS and tungsten, which are both body-centered-cubic, is beneficial for forming better metallurgical adhesion.
2 Experimental section
2.1. Specimen preparation
AISI 430 SS sheets (thickness of 1.5 mm) were cut into specimens of 10 mm × 10 mm via electric discharge machining. The composition of AISI 304 SS is 0.082 C, 16.22 Cr, Ni < 1, 0.268 Si, 0.338 Mn, 0.004 S and 0.026 P (wt%), with Fe comprising the balance. Specimens were ground with #360, #500, #800, #1000, #1500 and #2000 grit SiC abrasive papers, polished mechanically with diamond paste, cleaned with ethanol in an ultrasonic cleaner and then dried at room temperature.
The W-modified layer was formed on the surface of 430 SS in a double glow plasma alloying furnace. This furnace has three electrodes: an anode, a sputtering source cathode (W plate) and a negative cathode (the specimen). The 430 SS specimens were firstly heated at 1173 K using a work power supply and their surfaces were cleaned via particle bombardment in the chamber, which was filled with pure argon, at a pressure of 40 Pa with a bias voltage of −900 V. After keeping the specimens at 1173 K for 30 min, the working voltage of the specimens was decreased to −580 to −620 V and then the sputtering source electrode power supply system was loaded and kept at −800 to −900 V, which allows more tungsten atoms to move from the sputtering source electrode onto the surface of the specimens. The specimens were treated at 1173 K for 2 h during the tungsten diffusion process.
2.2. Characterization
Scanning electron microscopy (SEM) (SUPRA 55 SAPPHIRE, CARL ZEISS, Germany) was used to observe the surface morphology of untreated and W-modified 430 SS and the cross-section of W-modified 430 SS. The cross-sectional composition of W-modified 430 SS was determined using energy-dispersive X-ray spectroscopy (EDS) (X-Max, OXFORD, UK) adjunct to the SEM. In addition, the surface contact angle with water for untreated and W-modified 430 SS was also measured using a contact angle system (SL200B, Kino, USA). The surface roughness of untreated and W-modified 430 SS was obtained using a roughness tester (HW-T6000, Hommelwerke, Germany). The X-ray diffraction (XRD) (RigakuD/MAX-3A, Rigaku Corporation, Japan) patterns of untreated and W-modified 430 SS were obtained using Cu Kα radiation (λ = 1.5406 Å) at an operating voltage of 40 kV and a filament current of 40 mA. The diffraction angle was scanned from 30° to 90°, at a rate of 6° min−1.
2.3. Electrochemical measurements
Potentiodynamic and potentiostatic tests were performed to measure the corrosion resistance of untreated and W-modified 430 SS, respectively. A solution (0.05 M H2SO4 + 2 ppm HF + 0.01 M NaCl solution) at 70 °C with H2 bubbling was used as an electrolyte in order to simulate the aggressive anode environment of a PEMFC. The temperature of the corrosion test was maintained using an isothermal water bath during the electrochemical tests. Before each test, specimens were cleaned with ethanol and then embedded in the corrosion test cells. The specimens were covered with insulating epoxy, and one side was exposed for electrochemical measurements. The electrochemical measurements were conducted in an electrochemical workstation (CHI660C, Huachen Instrument CO., China) controlled with a computer. A typical three-electrode system was used for the electrochemical measurements, in which a platinum sheet acted as the counter electrode, a saturated calomel electrode (SCE) connected to a Luggin capillary acted as the reference electrode and the specimens acted as the working electrode. All the specimens were stabilized at open circuit potential for 30 min before the electrochemical test. Potentiodynamic polarization curves were measured at a scanning rate of 1 mV s−1 starting from a specific voltage (a 0.2 V shift below the open circuit voltage) and the potentiostatic tests were performed at a −0.1 V (vs. SCE) potential in the simulated anode environment for 4 hours. After the 4 hour potentiostatic test, the surface morphology of untreated and W-modified 430 SS was observed using SEM. The electrochemical impedance spectroscopy (EIS) tests of untreated and W-modified 430 SS were carried out at open circuit potential in a frequency range from 100 kHz to 0.01 Hz with an amplitude of 10 mV.
3 Results and discussion
3.1. Microstructure
Fig. 1a and b show the surface morphology of untreated and W-modified 430 SS, respectively. The surfaces of untreated and W-modified 430 SS were both uniform, smooth and dense without surface micropores and other common surface defects.21 Compared with untreated 430 SS, the surface of W-modified 430 SS was much blacker (Fig. 1b). The thickness of the tungsten diffusion layer was approximately 7–8 μm (Fig. 1c). The cross-section of the tungsten diffusion layer had a dense microstructure without pinholes and microcracks. In addition, the EDS microanalysis of different positions on the cross-section showed a different W concentration, the higher concentration of 55.49 wt% W corresponded to the outer layer and 28.39 wt% W was determined for the inner one. These results suggest that there is a good metallurgical adhesion between the tungsten diffusion layer and 430 SS substrate with no interfacial defects, which is beneficial for increasing the corrosion resistance of W-modified 430 SS as bipolar plates for PEMFCs.
 |
| | Fig. 1 The SEM micrographs of the untreated 430 SS surface (a) and W-modified 430 SS surface (b), and the SEM image and composition of the cross-section of W-modified 430 SS (c). | |
X-ray diffraction (XRD) was carried out to identify the crystal structure of the tungsten diffusion layer and the XRD patterns of untreated and W-modified 430 SS are shown in Fig. 2. According to the Joint Committee on Powder Diffraction Standards (JCPDS) cards, the tungsten diffusion coating of the body-centered-cubic W phase was identified in the XRD pattern. Distinctly, there was no other impure phase of possible elements such as Fe or Cr in the XRD pattern of W-modified 430 SS, which demonstrated that tungsten uniformly distributed on the surface of W-modified 430 SS.
 |
| | Fig. 2 The XRD patterns of untreated and W-modified 430 SS. | |
3.2. Hydrophobicity
In the PEMFC stacks, water would be generated owing to the hydrogen and oxygen electrochemical reaction. In addition, to prevent the proton exchange membrane from dehydrating, the inlet gases need to be humidified. Meanwhile, the exhaust gases are often mixed with the resultant water. The liquid water could adhere to the bipolar plates, which not only blocks the reaction gases from accessing the electrode, but also accelerates the corrosion of the bipolar plates. From this view, the hydrophobicity of the bipolar plates is directly relevant to the properties of the PEMFC. Bipolar plates with a superior hydrophobicity would be helpful for the timely removal of liquid water from the PEMFC stack and beneficial for the simplification of water management. The hydrophobicity of untreated and W-modified 430 SS is described by the contact angle with water, which is shown in Fig. 3. The average contact angles of untreated and W-modified 430 SS were 69.6° and 93.5°, respectively. Usually, a hydrophobic material displays a contact angle more than 90° between liquid–vapor and liquid–solid surfaces as a water droplet is placed onto its surface.22 Therefore, the significantly increased contact angle indicates that W-modified 430 SS is more hydrophobic than untreated 430 SS. The better hydrophobic property of W-modified 430 SS is beneficial to the water management of the PEMFC stacks and reduces the corrosion of the bipolar plates.
 |
| | Fig. 3 The contact angle of the specimens with water: untreated 430 SS (a) and W-modified 430 SS (b). | |
3.3. Corrosion characteristics
The corrosion potential and corrosion current density, which can be estimated from the potentiodynamic polarization curves of the specimens, are approximate indicators of the corrosion resistance of the materials. A higher corrosion potential and lower corrosion current density typically imply a better corrosion resistance.23 The potentiodynamic polarization curves of untreated and W-modified 430 SS in the simulated environment of a PEMFC are shown in Fig. 4. In general, all the studied specimens showed a passivation zone (Fig. 4). Obviously, the corrosion potential (Ecorr) of W-modified 430 SS shifted from −398 mV to −358 mV, whilst the corrosion current density decreased from 50.1 μA cm−2 to 2.2 μA cm−2 (Fig. 4), due to the protection of the tungsten diffusion layer for the 430 SS substrate. Compared with untreated 430 SS, W-modified 430 SS displays an evident decrease in the corrosion current density, a slight improvement in the Ecorr and an apparent widening in the passivation zone in the simulated anode environment of a PEMFC, which demonstrates that the corrosion resistance of W-modified 430 SS is greatly ameliorated. The reason for this phenomenon may be that tungsten probably passes directly from the metal into the passive film (WO3) through interaction with water and the formation of insoluble WO3 provides enhanced stability against aggressive ion attack.19 Otherwise, we could observe a yellow precipitate in the electrolyte, which may be sparingly soluble tungstic acid (H2WO4),20 after the potentiodynamic polarization test of W-modified 430 SS. WO42−, as an inhibitive ion in the electrolyte, competes with aggressive ions (H2SO4, F− and Cl−) to adsorb onto the surface of the passive film. When the inhibitor ion adsorbs onto the surface of the passive film it exhibits a protective effect, while the adsorption of aggressive ions exhibits a corrosive effect.20 As shown in Fig. 1a and b, the surfaces of untreated and W-modified 430 SS are all uniform, smooth and dense without surface micropores and other common surface defects. The surface roughnesses of untreated and W-modified 430 SS were nearly equal in value (see the ESI, 1S†). These results demonstrate that the improvement of the corrosion resistance of W-modified 430 SS has no obvious relationship with its surface morphology. In summary, the corrosion resistance of these specimens is mainly determined by the chemical composition of their surface.
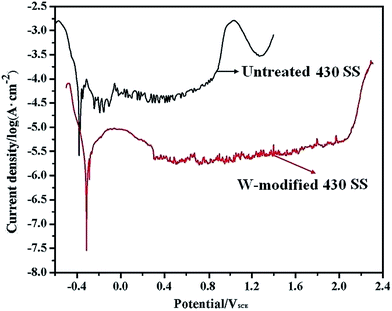 |
| | Fig. 4 The polarization curves of untreated and W-modified 430 SS in 0.05 M H2SO4 + 2 ppm F− + 0.01 M NaCl at 70 °C bubbled with H2. | |
In order to further study the corrosion behavior of W-modified 430 SS in the simulated anode environment of a PEMFC, potentiostatic tests were conducted by monitoring the current density at −0.1VSCE in 0.05 M H2SO4 + 2 ppm HF + 0.01 M NaCl solution at 70 °C with H2 bubbling. The current density curves as a function of time for untreated and W-modified 430 SS are shown in Fig. 5. The current density gradually reached a stable value for untreated 430 SS after approximately 30 min and there was an obvious fluctuation before reaching this value. Meanwhile, the current density of W-modified 430 SS also reached a stable value, which was faster and more direct. Otherwise, the current density of W-modified 430 SS was much smaller than that of untreated 430 SS. The stable current density of W-modified 430 SS might be related to the fact that the applied potential (−0.1 V) is nearly in the passivation region according to the potentiodynamic polarization curve (Fig. 4) and the fluctuation of untreated 430 SS is caused by its unstable surface film. During the 4 h potentiostatic test, the current density of untreated 430 SS stabilizes at 1–2 mA cm−2. For W-modified 430 SS, the current density gradually stabilizes at 2–3 μA cm−2 after 30 min demonstrating the better stability and corrosion resistance of the passive film formed on the surface of W-modified 430 SS. The surface morphology of untreated and W-modified 430 SS after the potentiostatic tests is shown in Fig. 6. It is obvious to see that serious corrosion occurred on the surface of untreated 430 SS, accompanied by substantial evidence of material dissolution (Fig. 6a). However, the corroded surface of W-modified 430 SS was relatively smooth without any obvious pitting (Fig. 6b), suggesting that the passive film formed on the tungsten diffusion layer is relatively dense and tough and offers better corrosion resistance in the simulated anode environment of a PEMFC.
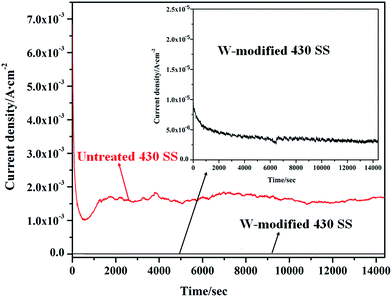 |
| | Fig. 5 The time–current density relationship of untreated and W-modified 430 SS in 0.05 M H2SO4 + 2 ppm F− + 0.01 M NaCl at 70 °C bubbled with H2 at −0.1VSCE. | |
 |
| | Fig. 6 The surface morphology of untreated (a) and W-modified 430 SS (b) at −0.1 V for 4 h in 0.05 M H2SO4 + 2 ppm F− + 0.01 M NaCl at 70 °C bubbled with H2. | |
3.4. Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy (EIS) is an effective method for studying the corrosion process of the electrical conductors in electrolytes under the low amplitude of its perturbation signal, which allows for the monitoring of the electrode electrochemical behavior with time without altering its surface properties. Fig. 7 shows the Nyquist plots of untreated and W-modified 430 SS acquired under the simulated anode PEMFC conditions after 1 h under the open circuit voltage. Within the frequency range of the measurement, the Nyquist plots for W-modified 430 SS display an arc shape, while those for untreated 430 SS present a Warburg diffusion tail in the low frequency range. Therefore, the impedance spectra were analyzed using two different equivalent circuit models, as illustrated in Fig. 8a (for untreated 430 SS) and Fig. 8b (for W-modified 430 SS), where Rs denotes the solution resistance between the reference and working electrode; Rc denotes the charge transfer resistance; Rf and Cf denote the resistance and capacitance of the passive film layer, respectively,24 and W denotes the Warburg impedance. Due to the nonideal capacitive response of the interface between the specimens and solution, a constant phase element (CPE) was introduced to the fitting procedure. The impedance of the CPE can be represented as:where Q is the admittance magnitude of the CPE, ω is the angular frequency and n is the exponential term. When n = 1, ZCPE is equal to the pure capacitance impedance. Contrarily when n = 0 it presents the pure resistor impedance. Actually, n is in the range from 0 to 1, and Q can be approximated by the capacitance.25 It can be observed that the physical models provided accurate fits to the experimental data, especially with respect to the shape of the impedance spectra in the Nyquist plots (Fig. 7). Therefore, it can be concluded that the models provide a reliable representation of the corrosion system. The fitted results are summarized in Table 1. The solution resistance (Rs) of the two samples was nearly equal in value, suggesting a similar ion conductivity in the test solutions. The charge transfer resistance for W-modified 430 SS was 1.723 × 105 Ω cm2, while for untreated 430 SS it was 219.5 Ω cm2 (Table 1). The values of the charge transfer resistance show that the corrosion rate of untreated 430 SS is close to three orders of magnitude higher than that of W-modified 430 SS.26
 |
| | Fig. 7 The Nyquist plots of untreated and W-modified 430 SS measured using EIS in 0.05 M H2SO4 + 2 ppm F− + 0.01 M NaCl at 70 °C bubbled with H2. | |
 |
| | Fig. 8 Equivalent circuits of the corrosion system for untreated 430 SS/solution (a) and W-modified 430 SS/solution (b). | |
Table 1 The fitted results of the EIS spectra for untreated and W-modified 430 SS
| Sample |
Circuit model |
Rs (Ω cm2) |
Cf (Ω−1 cm−2 S−1) |
Rf (Ω cm2) |
Q (Ω−1 cm−2 S−n) |
n |
Rc (Ω cm2) |
W (Ω−1 S−0.5 cm−2) |
| Untreated |
a |
2.314 |
7.820 × 10−7 |
2.687 |
7.163 × 10−3 |
0.8449 |
2.19 × 102 |
3.88 × 10−3 |
| W-Modified |
b |
2.920 |
3.558 × 10−5 |
1801.3 |
7.692 × 10−5 |
0.9122 |
1.72 × 105 |
4.92 × 10−4 |
4 Conclusions
A W-modified layer with a thickness of about 7–8 μm was fabricated on the surface of 430 SS via a plasma surface diffusion alloying method. The microstructure of the coating was dense and uniform, and it was well bonded metallurgically with the substrate. The larger contact angle with water suggested a better hydrophobicity for W-modified 430 SS, which is beneficial for water management in the PEMFC stacks and anticorrosion of the bipolar plate. The potentiodynamic polarization, potentiostatic polarization and EIS results provided evidence that the corrosion resistance of the 430 SS substrate was significantly improved by the tungsten diffusion layer. The self-corrosion potential of W-modified 430 SS increased from −398 mV to −358 mV (vs. SCE), while the corrosion current densities decreased from 1–2 mA cm−2 to 2–3 μA cm−2 when being observed in the simulated anode environment of a PEMFC. Furthermore, W-modified 430 SS exhibited a charge transfer resistance of 1.723 × 105 Ω cm2, three orders of magnitude higher than that of untreated 430 SS (219.5 Ω cm2). After a 4 h potentiostatic test, serious corrosion on the surface of untreated 430 SS occurred and there was scarcely any pitting on the surface of W-modified 430 SS. The overall performance suggests that the W-modified 430 SS bipolar plate developed in this work will exhibit excellent properties in the anode environment of PEMFCs.
Acknowledgements
Work is supported by the Natural Science Foundation of China (No. 51479019, 21476035 and 21506198) and Fundamental Research Funds for Central Universities (3132014323).
Notes and references
- P. Ekdunge and M. Råberg, Int. J. Hydrogen Energy, 1998, 23, 381 CrossRef CAS; J. Hackney and R. de Neufville, Transport. Res. Pol. Pract., 2001, 35, 243 CrossRef; R. Devanathan, Energy Environ. Sci., 2008, 1, 101 Search PubMed.
- H. Tsuchiya and O. Kobayashi, Int. J. Hydrogen Energy, 2004, 29, 985 CrossRef CAS.
- P. L. Hentall, J. B. Lakeman, G. O. Mepsted, P. L. Adcock and J. M. Moore, J. Power Sources, 1999, 80, 235 CrossRef CAS.
- V. Mehta and J. S. Cooper, J. Power Sources, 2003, 114, 32 CrossRef CAS.
- R. J. Tian, J. C. Sun and L. Wang, Int. J. Hydrogen Energy, 2006, 31, 1874 CrossRef CAS.
- S. A. M. Refaey, F. Taha and A. M. Abd El-Malak, Appl. Surf. Sci., 2005, 242, 114 CrossRef CAS; Y. Tsutsumi, A. Nishikata and T. Tsuru, Corros. Sci., 2007, 49, 1394 CrossRef.
- R. H. Zhang, L. P. Wang and W. Shi, RSC Adv., 2015, 5, 95750 RSC; B. Zaid, D. Saidi, A. Benzaid and S. Hadji, Corros. Sci., 2008, 50, 1841 CrossRef CAS; M. H. Shao, Y. Fu, R. G. Hu and C. J. Lin, Mater. Sci. Eng., 2003, A344, 323 CrossRef.
- Y. J. Ren and C. L. Zeng, J. Power Sources, 2008, 182, 524 CrossRef CAS.
- I. Moussallem, J. Jörissen, U. Kunz, S. Pinnow and T. Turek, J. Appl. Electrochem., 2008, 38, 1177 CrossRef CAS.
- S.-J. Lee, J.-J. Lai and C.-H. Huang, J. Power Sources, 2005, 145, 362 CrossRef CAS.
- M. A. Meltser and S. A. Grot, Proton exchange membrane, US Pat., 5763113. 9, June, 1998.
- E. A. Cho, U. S. Jeon, H. Y. Ha, S. A. Hong and I. H. Oh, J. Power Sources, 2004, 125, 178 CrossRef CAS; X. Q. Yan, M. Hou, H. F. Zhang, F. N. Jing, P. W. Ming and B. L. Yi, J. Power Sources, 2006, 160, 252 CrossRef.
- H. L. Wang, M. A. Sweikart and J. A. Turner, J. Power Sources, 2003, 115, 243 CrossRef CAS.
- H. L. Wang and J. A. Turner, J. Power Sources, 2004, 128, 193 CrossRef CAS; H. L. Wang, G. Teeter and J. Turner, J. Electrochem. Soc., 2005, 152, B99 CrossRef; A. K. Iversen, Corros. Sci., 2006, 48, 1036 CrossRef; D. P. Davies, P. L. Adcock, M. Turpin and S. J. Rowen, J. Power Sources, 2000, 86, 237 CrossRef; D. P. Davies, P. L. Adcock, M. Turpin and S. J. Rowen, J. Appl. Electrochem., 2000, 30, 101 CrossRef; S. P. Mani, C. Anandan and N. Rajendran, RSC Adv., 2015, 5, 64466 RSC.
- M. P. Brady, K. Weisbrod, C. Zawodzinski, I. Paulauskas, R. A. Buchanan and L. R. Walker, Electrochem. Solid-State Lett., 2002, 5, A245 CrossRef CAS; H. Wang, M. P. Brady, G. Teeter and J. A. Turner, J. Power Sources, 2004, 138, 86 CrossRef; H. Wang, M. P. Brady, K. L. More, H. M. Meyer III and J. A. Turner, J. Power Sources, 2004, 138, 79 CrossRef; M. P. Bradya, K. Weisbrod, I. Paulauskas, R. A. Buchanan, K. L. More, H. Wang, M. Wilson, F. Garzon and L. R. Walker, Scr. Mater., 2004, 50, 1017 CrossRef.
- S.-J. Lee, C.-H. Huang, Y.-P. Chen and C.-T. Hsu, J. Fuel Cell Sci. Technol., 2005, 2, 290 CrossRef CAS; S.-J. Lee, C.-H. Huang, Y.-P. Chen and Y.-M. Chen, J. Fuel Cell Sci. Technol., 2005, 2, 208 CrossRef.
- Y. Wang and D. O. Northwood, J. Power Sources, 2006, 163, 500 CrossRef CAS.
- L. X. Wang, J. C. Sun, P. B. Li, B. Jing, S. Li, Z. S. Wen and S. J. Ji, J. Power Sources, 2012, 208, 397 CrossRef CAS; L. X. Wang and J. C. Sun, J. Renewable Sustainable Energy, 2013, 5, 021407 CrossRef; L. X. Wang, J. C. Sun, J. Sun, Y. Lv, S. Li, S. J. Ji and Z. S. Wen, J. Power Sources, 2012, 199, 195 CrossRef; L. X. Wang, J. C. Sun, B. Kang, S. Li, S. J. Ji, Z. S. Wen and X. C. Wang, J. Power Sources, 2014, 246, 775 CrossRef; L. X. Wang, J. C. Sun, P. B. Li, J. Sun, Y. Lv, B. Jing, S. Li, S. J. Ji and Z. S. Wen, Int. J. Hydrogen Energy, 2012, 37, 5876 CrossRef.
- L. X. Wang, J. C. Sun, S. Li, S. J. Ji, Z. S. Wen and B. Jing, Fuel Cells, 2013, 6, 1131 CrossRef CAS.
- R. M. Saleh, M. M. Badran, A. A. Elhosary and H. A. Eldahan, Br. Corros. J., 1988, 23, 105 CrossRef CAS.
- K. S. Weil, G. Xia, Z. G. Yang and J. Y. Kim, Int. J. Hydrogen Energy, 2007, 32, 3724 CrossRef CAS.
- C.-Y. Chung, S.-K. Chen, T.-S. Chin, T.-H. Ko, S.-W. Lin, W.-M. Chang and S.-N. Hsiao, J. Power Sources, 2009, 186, 393 CrossRef CAS.
- L. Wang, J. C. Sun and X. L. Xu, Surf. Coat. Technol., 2001, 145, 31 CrossRef.
- K. Jüttner, W. J. Lorenz, M. W. Kendig and F. Mansfeld, J. Electrochem. Soc., 1988, 135, 332 CrossRef CAS; I. Frateur, C. Deslouis, M. E. Orazem and B. Tribollet, Electrochim. Acta, 1999, 44, 4345 CrossRef.
- Z. He and F. Mansfeld, Energy Environ. Sci., 2009, 2, 215 Search PubMed; X. J. Wu, H. Y. Ma, S. H. Chen, Z. Y. Xu and A. F. Sui, J. Electrochem. Soc., 1999, 146, 1847 CrossRef CAS.
- W. Trabelsi, E. Triki, L. Dhouibi, M. G. S. Ferreira, M. L. Zheludkevich and M. F. Montemor, Surf. Coat. Technol., 2006, 200, 4240 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra28114h |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 







