Hetero aromatic donors as effective terminal groups for DPP based organic solar cells†
Marri Anil Reddya,
CH. Pavan Kumarab,
Akudari Ashoka,
Abhishek Sharmac,
G. D. Sharma*d and
Malapaka Chandrasekharam*ab
aNetwork of Institutes for Solar Energy, CSIR-Indian Institute of Chemical Technology, I&PC Division, Uppal Road, Tarnaka, Hyderabad-500 007, India. E-mail: chandra@iict.res.in; Fax: +91 4027193186; Tel: +91 4027193186
bAcademy of Scientific and Innovative Research, CSIR-IICT, India
cDepartment of Electronics and Communication Engineering, LNMIIT, Jaipur, India
dR & D Center for Science and Engineering, JEC Group of Colleges, Jaipur Engineering College Campus, Kukas, Jaipur 302028, India. E-mail: gdsharma273@gmail.com; sharmagd_in@yahoo.com
First published on 15th January 2016
Abstract
Four new solution-processable donor–acceptor–donor (D–A–D) structured low bandgap small molecules (CSDPP5, CSDPP6, CSDPP7 and CSDPP8) with diketopyrrolopyrrole as central acceptor unit and phenoxazine (POZ) or carbazole (CBZ) as terminal units were designed, synthesized and characterized. The new small molecules have been employed as donors along with the PC71BM as electron acceptor in solution processed BHJ organic solar cells, showed broad absorption bands with suitable electrochemical energy levels. When the BHJ active layer was cast from THF solvent, the optimal power conversion efficiencies obtained with CSDPP5, CSDPP6, CSDPP7 and CSDPP8 are 2.97% 3.06%, 2.42% and 2.43% respectively. The PCE of the devices when processed with DIO/THF solvent, have been further enhanced to 4.69%, 4.14% for CSDPP6:PC71BM and CSDPP8:PC71BM active layers respectively. The enhancement in PCE has been attributed to change in nanoscale morphology and more balanced charge transport resulting from increased hole mobility.
1. Introduction
Recently, organic solar cells (OSCs) have been evolving into promising clean and renewable energy sources, owing to their low-cost, flexibility and applicability in large area devices.1–6 In general, OSCs feature a bulk hetero junction (BHJ) structure, where conjugated polymers and a fullerene derivative serve as the electron donor (D) and acceptors (A) respectively. Significant recent advancements in the power conversion efficiency (PCE) of OSCs have been reported and a PCE of over 10% has been achieved through careful material design and device optimization7–14 for single junction while for a tandem junction OSCs over 11% have been achieved.15,16 However, conjugated polymers suffer from batch to batch variation in terms of molecular weight and polydispersity that affect the processability and thus semiconducting properties.16–18 BHJ solar cells based on solution processed small molecule have been recently investigated19–23 and achieved a PCE of 8% for single junction solar cells24–26 and 10.1% for tandem solar cells.27 Recently, Chen et al. have achieved a record PCE of about 10% for single junction solution processed small molecule organic solar cell28 which is very close to the best polymer organic solar cells. To date an impressive PCE of 12% has been achieved for a vacuum processed triple junction tandem solar cell based on small molecules.29Among the various donor building blocks investigated, diketopyrrolopyrrole (DPP) based molecules are promising candidates due to the favorable properties of the DPP unit, e.g., strong light absorption, photochemical stability, excellent charge carrier mobility, and easy synthesis.30–39 Alkyl substitution on the nitrogen atoms in the DPP unit can improve the solubility of the DPP molecules. Thiophene functionalized DPPs (TDPP) have attracted extensive applications in organic optoelectronics. This is due to the fact that the electron richness of the thiophene moiety can induce strong intramolecular charge transfer with the electron deficient DPP core and π–π stacking of conjugating units improves the optical and electrochemical properties. The highly efficient solution processed small molecule donor materials based on DPP can be classified into two categories according to their D/A structures, i.e. either DPP as the electron acceptor unit capped with two donor units (D–A–D), or donor unit end capped with two DPP as the acceptor. The overall PCEs of DPP donor molecules reported so far in literature is less than 5%. However, the extension of conjugated backbone to DPP-containing polymers achieved the PCE of about 6.5%.40–42 More recently, the highest efficiency of about 7% has been reported for OSCs using solution processed DPP based small molecules.43 Thus DPP based materials functionalized with D–A structures will have potential in high performance solar cells. In this context, phenoxazine (POZ) is known to be a good conjugated heterocyclic compound with electron rich oxygen and nitrogen heteroatoms. Recently, Sun et al. synthesized A–π–D–π–A small molecule employing POZ as core unit and dicyanovinyl as electron withdrawing end group for BHJ solar cell and achieved a PCE of about 5.6%.44
In this line of work, our group demonstrated the use of thiophene diketopyrrolopyrrole (TDPP) based molecular semiconductors as donor materials in BHJ solar cells and achieved moderate PCE.45,46 Recently, we have reported four new simple D–A–D structured DPP molecules (CSDPP9–CSDPP12) with DPP as central core unit and different electron donating groups as terminal units. The BHJ devices, based on CSDPP11:PC71BM and CSDPP12:PC71BM subjected to solvent and thermal annealing showed PCEs of 5.47% and 4.88% respectively.47 To further examine the effect of hetero aromatic donor units on the properties of TDPP based molecules, we have successfully synthesized four D–A–D structured small molecules, all of which contain thiophene capped DPP, i.e. with ethyl-hexyl (CSDPP6 and CSDPP8) and n-decyl long chain (CSDPP5 and CSDPP7) as acceptor core and different donor carbazole (CBZ) CSDPP7 and CSDPP8 and phenoxazine (POZ) CSDPP5 and CSDPP6 (Fig. 1) as arms. These small molecule donors exhibit excellent solubility in common organic solvents. In addition, their photophysical and electrochemical properties show that they harvest sunlight across the visible spectrum range and have appropriate HOMO and LUMO energy levels to satisfy the requirement of donor component for solution processed BHJ OSCs. Therefore, we have explored the photovoltaic properties of these new donors in BHJ solar cells with the structure ITO/PEDOT:PSS/donors:PC71BM/Al. The PCE for the devices from CSDPP5, CSDPP6, CSDPP7 and CSDPP8 as donor approached 2.97%, 3.06%, 2.42% and 2.43%, respectively and further improved up to 4.69% and 4.14% for the device using CSDPP6:PC71BM and CSDPP8:PC71BM active layers, respectively, processed with (1,8-diiodooctane) DIO/THF solvent.
2. Experimental details
2.1 Materials and instrumentation
The starting materials and reagents, carbazole, phenoxazine, thiophene carbonitrile, di-n-ethylsuccinate, tert-BuOK, tert-amyl alcohol, bromodecane, 2-ethyl hexyl bromide, bis(pinacalato)diboron and N-bromosuccinimide were purchased from Sigma-Aldrich. The solvents were purified by standard procedures and purged with nitrogen before use. All other chemicals used in this work were analytical grade and were used without further purification, and all reactions were performed under argon atmosphere unless and otherwise mentioned. Chromatographic separations were carried out on silica gel (60–120 mesh). 1HNMR spectra were recorded on Bruker 300 MHz spectrometer using TMS as an internal standard. Mass spectra were recorded on Shimadzu LCMS-2010 EV model with ESI probe. Absorption spectra were recorded on a Shimadzu UV-vis to near IR region 3600 spectrometer. Electrochemical data were obtained by cyclic voltammetry using a conventional three-electrode cell and a BAS100 electrochemical analyzer. C, H, N, S data was recorded on Elementar (variomicrotube) instrument.2.2 Synthesis of 10-hexyl-10H-phenoxazine (1)
To an ice-cooled suspension of NaH (0.314 g, 13.099 mmol) in DMF (30 mL) was added phenoxazine (2.00 g, 10.916 mmol) and stirred for 30 min at room temperature. Hexyl bromide (1.964 mL, 13.099 mmol) was added drop wise to the reaction mixture and then stirred for 16 h under N2. After removing the solvent, the resulting mixture was washed with ice water and extracted with EtOAC and dried over Na2SO4. After evaporation, the crude compound obtained was purified by silica gel column chromatography. The desired compound was eluted using n-hexane/ethyl acetate (9/1; v/v) to give viscous liquid 1 (82%). 1H NMR (300 MHz, CDCl3, δ): 6.68–6.73 (m, 2H), 6.56 (d, 4H), 6.38 (d, 2H), 3.44 (t, 2H), 1.60–1.70 (m, 2H), 1.34–1.43 (m, 6H), 092 (t, 3H). 13C NMR (75 MHz, CDCl3, δ): 144.97, 133.37, 123.51, 120.57, 115.23, 111.19, 44.05, 31.53, 26.55, 24.84, 22.62, 13.97. ESI-MS: m/z [M]+: 268.2.3 Synthesis of 3-bromo-10-hexyl-10H-phenoxazine (2)
To a solution of compound 1 (0.700 g, 2.621 mmol) in anhydrous chloroform (40 mL) was added N-bromosuccinimide (0.560 g, 3.146 mmol) portion wise in an ice-water bath. After complete addition, the solution mixture was warmed to room temperature and stirred for 6 h. The resulting solution was washed with water and brine. The organic phase was dried over Na2SO4 and the solvent was removed. The residue was purified by silica gel chromatography using ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane (1
hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as eluent to afford viscous liquid 2 (75%). 1H NMR (300 MHz, CDCl3, δ): 6.84–6.89 (m, 1H), 6.76–6.82 (m, 1H), 6.71–6.73 (m, 1H), 6.58–6.66 (m, 2H), 6.45 (d, 1H), 6.28 (d, 1H), 3.35–3.48 (brs, 2H), 1.56–1.69 (m, 2H), 1.33–1.43 (m, 6H), 0.91 (t, 3H). 13C NMR (75 MHz, CDCl3, δ): 145.65, 144.50, 132.77, 126.48, 126.08, 123.92, 120.90, 118.50, 118.31, 115.42, 112.15, 111.36, 44.13, 31.52, 26.52, 24.71, 22.63, 13.99. ESI-MS: m/z [M + 2H]+: 348.
9) as eluent to afford viscous liquid 2 (75%). 1H NMR (300 MHz, CDCl3, δ): 6.84–6.89 (m, 1H), 6.76–6.82 (m, 1H), 6.71–6.73 (m, 1H), 6.58–6.66 (m, 2H), 6.45 (d, 1H), 6.28 (d, 1H), 3.35–3.48 (brs, 2H), 1.56–1.69 (m, 2H), 1.33–1.43 (m, 6H), 0.91 (t, 3H). 13C NMR (75 MHz, CDCl3, δ): 145.65, 144.50, 132.77, 126.48, 126.08, 123.92, 120.90, 118.50, 118.31, 115.42, 112.15, 111.36, 44.13, 31.52, 26.52, 24.71, 22.63, 13.99. ESI-MS: m/z [M + 2H]+: 348.
2.4 Synthesis of 10-hexyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-10H-phenoxazine (3)
A solution of compound 2 (0.500 g, 1.272 mmol), bis(pinacolato)diboron (0.388 g, 1.399 mmol), KOAc (0.374 g, 3.817 mmol), and Pd(dppf)Cl2 (0.056 g, 0.076 mmol) in dry dimethoxy ethane (8 mL) was refluxed at 85 °C under a nitrogen atmosphere for 16 h. After being cooled to room temperature, the mixture was poured into water (20 mL) and the organic layer was separated. The aqueous layer was extracted with dichloromethane (3 × 20 mL) and the combined organic layers were dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure and the crude product was pre-adsorbed onto silica gel and chromatographed (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 n-hexane/ethyl acetate) to give viscous liquid 3 (75%). 1H NMR (300 MHz, CDCl3, δ): 7.23–7.25 (m, 1H), 7.02 (s, 1H), 6.77 (t, 1H), 6.57–6.64 (m, 2H), 6.42–6.51 (m, 2H), 3.35–3.58 (brs, 2H), 1.64 (q, 2H), 1.34–1.42 (m, 6H), 1.31 (s, 12H), 0.91 (t, 3H). 13C NMR (75 MHz, CDCl3, δ): 145.20, 144.20, 134.66, 131.02, 127.64, 123.35, 121.10, 120.83, 115.32, 111.39, 110.56, 83.44, 43.93, 31.50, 26.49, 24.77, 22.59, 13.99. ESI-MS: m/z [M + H]+: 394.
2 n-hexane/ethyl acetate) to give viscous liquid 3 (75%). 1H NMR (300 MHz, CDCl3, δ): 7.23–7.25 (m, 1H), 7.02 (s, 1H), 6.77 (t, 1H), 6.57–6.64 (m, 2H), 6.42–6.51 (m, 2H), 3.35–3.58 (brs, 2H), 1.64 (q, 2H), 1.34–1.42 (m, 6H), 1.31 (s, 12H), 0.91 (t, 3H). 13C NMR (75 MHz, CDCl3, δ): 145.20, 144.20, 134.66, 131.02, 127.64, 123.35, 121.10, 120.83, 115.32, 111.39, 110.56, 83.44, 43.93, 31.50, 26.49, 24.77, 22.59, 13.99. ESI-MS: m/z [M + H]+: 394.
2.5 Synthesis of 9-hexyl-9H-carbazole (4)
Compound 4 was synthesized according to the procedure as described above for synthesis of 1. Nature: white solid yield: 90%. 1H NMR (300 MHz, CDCl3, δ): 8.03 (d, 2H), 7.32–7.41 (m, 4H), 7.16 (t, 2H), 4.28 (t, 2H), 1.86 (q, 2H), 1.26–1.41 (m, 6H), 0.86 (t, 3H). 13C NMR (75 MHz, CDCl3, δ): 140.345, 125.497, 122.733, 120.274, 118.618, 108.594, 42.986, 31.553, 28.883, 26.934, 22.522, 14.003. ESI-MS: m/z [M + H]+: 252.2.6 Synthesis of 3-bromo-9-hexyl-9H-carbazole (5)
Compound 5 was synthesized according to the procedure as described above for synthesis of 2. Nature: light yellow color solid yield: 75%. 1H NMR (300 MHz, CDCl3, δ): 8.18 (s, 1H), 8.02 (d, 1H), 7.44–7.53 (m, 2H), 7.37 (d, 1H), 7.19–7.26 (m, 2H), 4.23 (t, 2H), 1.82 (q, 2H), 1.25–1.36 (m, 6H), 0.84 (t, 3H). 13C NMR (75 MHz, CDCl3, δ): 140.573, 138.921, 128.857, 128.086, 126.225, 124.421, 122.937, 121.680, 120.428, 119.068, 110.014, 108.835, 43.057, 31.480, 28.790, 26.854, 22.484, 13.977. ESI-MS: m/z [M + H]+: 330.2.7 Synthesis of 9-hexyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole (6)
Compound 6 was synthesized according to the procedure as described above for synthesis of 3. Nature: yellow color solid yield: 73%. 1H NMR (300 MHz, CDCl3, δ): 8.59 (s, 1H), 8.12 (d, 1H), 7.91 (d, 1H), 7.45 (t, 1H), 7.37–7.40 (m, 2H), 7.23 (t, 1H), 4.29 (t, 2H), 1.86 (q, 2H), 1.39 (s, 12H), 1.25–1.33 (m, 6H), 0.85 (t, 3H). 13C NMR (75 MHz, CDCl3, δ): 142.549, 140.456, 132.038, 127.699, 125.519, 123.063, 122.540, 120.475, 119.119, 108.653, 108.011, 83.479, 43.008, 31.503, 28.828, 26.862, 24.886, 22.459, 13.938. ESI-MS: m/z [M + H]+: 378.2.8 Synthesis of 3,6-dithiophen-2-yl-2,5-dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione (7)
3,6-Dithiophen-2-yl-2,5-dihydro-pyrrolo[3,4-c]pyrrole-1,4-dione was synthesized according to a reported procedure.48–502.9 Synthesis of 2,5-di-n-decyl-3,6-dithiophen-2-yl-pyrrolo[3,4-c]pyrrole-1,4-dione (8)
Compound 8 was synthesized according to a reported procedure to give purple brown shiny crystalline powder (yield: 75.4%).182.10 Synthesis of 2,5-bis(2-ethylhexyl)-3,6-dithiophen-2-yl-pyrrolo[3,4-c]pyrrole-1,4-dione (9)
In a three-necked, oven-dried 250 mL round-bottom flask, compound 7 (3.00 g, 10.0 mmol) and anhydrous K2CO3 (4.15 g, 30.0 mmol) in 100 mL of anhydrous DMF were heated to 120 °C under N2 for 1 h. 2-Ethylhexyl bromide (4.80 mL, 25.0 mmol) was then added dropwise, and the reaction mixture was further stirred overnight at 130 °C. After cooling to room temperature, 400 mL of distilled water was added and stirred for 1 h, the mixture was filtered. The solid obtained was washed with several portions of distilled water, methanol, and then air-dried. The crude product was purified by flash chromatography using chloroform as eluent, and the solvent was removed in vacuum to obtain a brown color solid (yield: 75%). 1H NMR (300 MHz, CDCl3, δ): 8.90 (d, 2H), 7.64 (d, 2H), 7.29 (t, 2H), 3.98–4.08 (m, 4H), 1.88–1.83 (m, 2H), 1.40–1.20 (m, 16H), 0.90 (m, 12H).2.11 Synthesis of 3,6-bis-(5-bromo-thiophen-2-yl)-2,5-di-decyl-pyrrolo[3,4-c]-pyrrole-1,4-dione (10)
The compound 10 was synthesized according to the reported procedure to give dark purple brown powder (yield: 64%).182.12 Synthesis of 3,6-bis-(5-bromo-thiophen-2-yl)-2,5-bis(2-ethylhexyl)-pyrrolo[3,4-c]-pyrrole-1,4-dione (11)
In a three-necked, oven-dried, 150 mL round-bottom flask, compound 9 (1 g, 1.908 mmol) was dissolved in 40 mL of anhydrous CHCl3, covered with aluminum foil, and stirred at room temperature under N2 for 15 min. N-Bromosuccinimide (0.781 g, 4.389 mmol) was then added, and the reaction mixture was stirred at room temperature for 12 h. The mixture was then poured into 100 mL of methanol, and the resulting suspension was further stirred at room temperature for 1 h. The solid precipitated was collected by vacuum filtration and washed with several portions of hot distilled water and hot methanol to obtain brown color solid (65%). 1H NMR (300 MHz, CDCl3, δ): 8.84 (d, 2H), 7.62 (d, 2H), 3.98–4.08 (m, 4H), 1.90–1.80 (m, 2H), 1.40–1.20 (m, 16H), 0.90 (m, 12H). ESI-MS: m/z (M + 4H)+: 685.2.13 General procedure for the synthesis of CSDPP5 and CSDPP7
A 50 mL of Schlenk tube was charged with compound 3 or 6 (0.679 mmol), Pd(PPh3)4 (0.062 g, 0.054 mmol). Dimethoxy ethane (8 mL) and 2 M aqueous sodium carbonate (2 mL) were added, and the tube was purged with argon gas with 5 evacuate/refill cycles. Compound 10 (0.200 g, 0.271 mmol) was subsequently added as a neat liquid. The tube was sealed and heated at 90 °C vigorously for 18 h. Upon cooling to ambient temperature, the organics were extracted into dichloromethane (3 × 30 mL) from 30 mL water. The combined organics were washed with water (1 × 30 mL) and brine (1 × 30 mL), dried over Na2SO4, filtered and the solvent was removed under reduced pressure to give CSDPP5 and CSDPP7.2.14 2,5-Didecyl-3,6-bis(5-(10-hexyl-10H-phenoxazin-3-yl)thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (CSDPP5) (dark blue solid, 46%)
1H NMR (300 MHz, CDCl3, δ): 8.88–9.02 (brs, 2H), 7.20–7.35 (m, 4H), 6.96–7.10 (m, 2H), 6.75–6.80 (m, 4H), 6.61–6.68 (m, 4H), 6.34–6.43 (m, 4H), 4.05 (t, J = 7.74 Hz, 4H), 3.33–3.47 (brs, 4H), 1.75 (br, 4H), 1.57–1.68 (m, 6H), 1.25–1.37 (m, 38H), 0.84–0.94 (m, 12H). FT-IR (KBr), cm−1: 3059, 2921, 2850, 1654, 1592, 1551, 1491, 1430, 1380, 1271, 1072, 1024, 908, 854, 789, 728. Anal. cal.: C70H86N4O4S2. C: 75.64, H: 7.80, N: 5.04, S: 5.77 found-C: 75.32, H: 7.82, N: 5.03, S: 5.73.2.15 2,5-Didecyl-3,6-bis(5-(9-hexyl-9H-carbazol-3-yl)thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (dark blue solid, CSDPP7) (58%)
1H NMR (300 MHz, CDCl3, δ): 9.02 (d, J = 4.20 Hz, 2H), 8.36 (s, 2H), 8.16 (d, J = 7.80 Hz, 2H), 7.77 (d, J = 6.90 Hz, 2H), 7.48–7.53 (m, 4H), 7.38–7.41 (m, 4H), 7.29 (d, J = 7.20 Hz, 2H), 4.28 (t, J = 7.80 Hz, 4H), 4.16 (t, J = 7.50 Hz, 4H), 1.78–1.92 (m, 8H), 1.25–1.53 (m, 40H), 0.82–0.89 (m, 12H). FT-IR (KBr), cm−1: 3051, 2919, 2850, 1650, 1597, 1544, 1467, 1428, 1336, 1273, 1216, 1186, 1147, 1071, 1018, 958, 860, 787, 725, 622, 489. Anal. cal.: C70H86N4O2S2. C: 77.88, H: 8.03, N: 5.19, S: 5.94 found-C: 77.84, H: 8.02, N: 5.17, S: 5.92.2.16 General procedure for the synthesis of CSDPP6 and CSDPP8
The synthetic procedure followed for CSDPP6 or CSDPP8 is similar to the preparation of the compounds CSDPP5 or CSDPP7 employing compound 11 (0.200 g, 0.293 mmol) in place of compound 10. The products were purified by column chromatography on silica (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dichloromethane/hexane) to afford either CSDPP6 or CSDPP8.
6 dichloromethane/hexane) to afford either CSDPP6 or CSDPP8.
2.17 2,5-Bis(2-ethylhexyl)-3,6-bis(5-(10-hexyl-10H-phenoxazin-3-yl)thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (CSDPP6) (dark blue solid, 48%)
1H NMR (300 MHz, CDCl3, δ): 8.93–9.00 (brs, 2H), 7.27–7.29 (m, 2H), 7.08–7.10 (d, J = 8.10 Hz, 2H), 6.88 (s, 2H), 6.77–6.83 (m, 2H), 6.63–6.70 (m, 4H), 6.42–6.49 (m, 4H), 4.04 (d, J = 7.20 Hz, 4H), 3.42–3.52 (brs, 4H), 1.90–1.99 (m, 2H), 1.66 (q, J = 6.60 Hz, J = 14.70 Hz, 4H), 1.24–1.42 (m, 28H), 0.86–0.97 (m, 18H). FT-IR (KBr), cm−1: 3076, 2955, 2924, 2854, 1653, 1589, 1552, 1489, 1420, 1382, 1277, 1227, 1156, 1072, 1025, 863, 834, 794, 734. Anal. cal.: C66H78N4O4S2. C: 75.10, H: 7.45, N: 5.31, S: 6.08 found-C: 75.12, H: 7.41, N: 5.29, S: 6.02.2.18 2,5-Bis(2-ethylhexyl)-3,6-bis(5-(9-hexyl-9H-carbazol-3-yl)thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (dark blue solid, CSDPP8) (56%)
1H NMR (300 MHz, CDCl3, δ): 9.03 (d, J = 4.80 Hz, 2H), 8.36 (s, 2H), 8.15 (d, J = 8.40 Hz, 2H), 7.77 (d, J = 6.90 Hz, 2H), 7.47–7.53 (m, 4H), 7.39–7.42 (m, 4H), 7.29 (d, J = 7.50 Hz, 2H), 4.28 (t, J = 6.60 Hz, 4H), 4.12 (dd, J = 3.90 Hz, J = 3.60 Hz, 4H), 1.99–2.03 (m, 2H), 1.87 (q, J = 7.80 Hz, J = 8.10 Hz, 4H), 1.25–1.46 (m, 28H), 0.84–0.97 (m, 18H). FT-IR (KBr), cm−1: 3057, 2923, 2854, 1654, 1596, 1548, 1485, 1427, 1328, 1265, 1229, 1149, 1077, 1022, 873, 794, 732, 623, 418. Anal. cal.: C66H78N4O2S2. C: 77.45, H: 7.65, N: 5.47, S: 6.27 found-C: 77.43, H: 7.62, N: 5.45, S: 6.25.2.19 Device fabrication and characterizations
The BHJ OSCs were prepared using the indium tin oxide (ITO) coated glass substrate as anode, Al as cathode and a blended film of CSDPP5 or CSDPP6 or CSDPP7 or CSDPP8 (CSDPP:PC71BM) as photoactive layer as follows: firstly, ITO coated glass substrates were cleaned with detergent, ultrasonicated in acetone and isopropyl alcohol, and subsequently dried in an oven for 12 h. An aqueous solution of PEDOT:PSS (Heraeus, Clevious P VP, Al 4083) was spin cast on the ITO substrates obtaining a film of about 40 nm thick. The PEDOT:PSS film was then dried for 10 min at a temperature of 120 °C in ambient conditions. Then, a 15 mg mL−1 solution of CSDPP/PC71BM blends in different solvents were prepared with different weight ratio and then spun cast on the top of PEDOT:PSS layer and then dried at 80 °C for 10 min. THF and DIO/THF (1, 2, 3 and 4% (v%) of DIO) were used as solvents for spun cost. The thickness of the photoactive layer is about 100 ± 10 nm. Finally ∼90 nm thick Al electrode was deposited on the top of BHJ film under reduced pressure (<10−6 Torr). All the devices were fabricated and tested in ambient atmosphere without encapsulation. The active area of the devices is about 20 mm2.The current–voltage characteristics of the devices were measured using a computer controlled Keithley 238 source meter in dark as well as under illumination intensity of 100 mW cm−2. A xenon light source coupled with AM1.5 optical filter was used as light source. The incident photon to current efficiency (IPCE) of the devices was measured illuminating the device through the light source and monochromator and resulting current was measured using Keithley electrometer under short circuit condition.
2.20 Computational details
All the calculations have been performed using the Gaussian 09 program package.51 Geometrical optimization was performed in vacuo using B3LYP52,53 exchange–correlation functional and a 6-311G(d,p) basis set.54 The optimized geometries were then used to obtain frontier molecular orbitals (FMOs). To simulate the optical spectra, the lowest spin allowed singlet–singlet transitions were computed on the ground state geometry. TDDFT calculations of the lowest singlet–singlet excitations were performed in THF solution, on the optimized geometries using the B3LYP/6-311G(d,p) level of theory. The integral equation formalism polarizable continuum model (PCM)55 within self-consistent reaction field (SCRF) theory, has been used to describe the solvation of the molecules. The software GaussSum 2.2.5 (ref. 56) was utilized to simulate the major portion of absorption spectrum and to analyze the nature of transitions. The percentage contributions of individual moieties of the dyes to the respective molecular orbitals have also been calculated.3. Results and discussion
3.1 Synthesis of CSDPP5–CSDPP8
The synthetic route to CSDPP5–CSDPP8 sensitizers were depicted in Scheme 1. The commercially available starting materials phenoxazine and carbazole were N-alkylated using NaH as base in DMF followed by bromination in presence of N-bromosuccinimide. The Miyaura borylation of the bromo substituted compounds 2 and 4 with bis(pinacolato)-diborane (B2Pin2) under PdCl2(dppf) catalysed reaction in presence of KOAc and 1,2-dimethoxyethane, resulted in compounds 3 and 6. The DPP derivative 7 was synthesized according to a reported procedure,49–51 followed by N-alkylation with 1-bromodecane and 2-ethylhexylbromide to afford pure N-alkylated products 8 and 9. Compounds 8 and 9 were further subjected to bromination with NBS to afford dibromo compounds 10 and 11. The target sensitizers CSDPP5–CSDPP8 were prepared employing the Pd(PPh3)4 assisted Suzuki coupling reaction of 3 or 6 with compound 10 and 11 in yields of 46–58% respectively.3.2 Optical and electrochemical properties
The UV-visible absorption spectra of the DPP small molecules (CSDPP5–CSPP8) in THF solution and in film (THF solution) are shown in Fig. 2a and b respectively and the corresponding maximum absorption wavelengths (λmax) are compiled in Table 1. The λmax of CSDPP5 and CSDPP6 solution was observed at 647 nm (molar extinction coeff. (ε), 6.3899 M−1 cm−1 and 6.5494 M−1 cm−1), while λmax for CSDPP7 and CSDPP8 was observed at 623 nm (ε, 6.9098 M−1 cm−1 and 6.5046 M−1 cm−1), resulting from the intramolecular charge transfer (ICT) transition between the donor and acceptor units in the conjugated small molecules. The small molecules CSDPP5 and CSDPP6 exhibited red shift in the absorption band compared to CSDPP7 and CSDPP8 indicating an enhanced ICT transition band which is resulting from the better donating capacity of phenoxazine. The thin films of these small molecules exhibited similar absorption trend with broader and red shifted which is attributed to π–π intermolecular interactions in the solid state. The optical bandgap for these small molecules was estimated from the onset absorption wavelength in thin film and are 1.65 eV, 1.66 eV, 1.77 eV, and 1.78 eV for CSDPP5, CSDPP6, CSDPP7 and CSDPP8, respectively. The lower value of optical bandgap for CSDPP5 and CSDPP6 reflects a higher amount of charge transfer between the DPP acceptor core and conjugated arms for phenoxazine donor as compared to carbazole. It is well known that the broader absorption and lower bandgap are beneficial to improve the light harvesting efficiency of the active layer, resulting higher photocurrent generation. We expect higher photocurrent for CSDPP5 and CSDPP6 as compared to CSDPP7 and CSDPP8.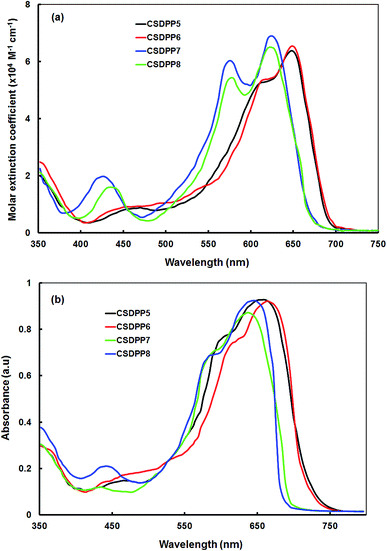 | ||
| Fig. 2 Optical absorption spectra of CSDPP5, CSDPP6, CSDPP7 and CSDPP8 in (a) dilute THF solution and (b) thin film cast from THF solvent. | ||
| Dye | λmaxa (ε × 10−4 M−1 cm−1) [nm] | Eoxb (V)/EHOMO (eV) | E0–0c (eV) | Eg(opt)d (eV) | ELUMOd (V)/ELUMO (eV) |
|---|---|---|---|---|---|
| a Absorption spectra were recorded in THF solutions at 298 K.b The oxidation potentials (Eox vs. NHE) of these dyes are corresponding to the HOMO levels.c E0–0, was derived from the intersection of the absorption and emission spectra.d Optical bandgap estimated from the onset absorption edge of absorption spectra in thin film.e ELUMO = Eox − E0–0. | |||||
| CSDPP5 | 647 (6.3899) | 0.546 (−5.24) | 1.846 | 1.65 | −1.30 (−3.39) |
| CSDPP6 | 647 (6.5494) | 0.534 (−5.23) | 1.845 | 1.66 | −1.31 (−3.39) |
| CSDPP7 | 623 (6.9098) | 0.603 (−5.30) | 1.937 | 1.77 | −1.33 (−3.36) |
| CSDPP8 | 623 (6.5046) | 0.623 (−5.32) | 1.933 | 1.78 | −1.31 (−3.39) |
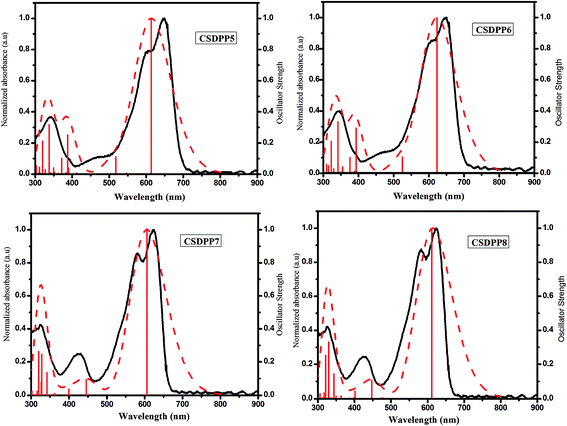
In order to provide in-depth insight into the electronic structures of CSDPPs, theoretical calculations based on the DFT method at the B3LYP/6-311G(d,p) level were carried out, in which the N-hexyl group was replaced by N-methyl for simplicity. The optimized geometries of CSDPP5–CSDPP8 at B3LYP/6-311G(d,p) level theory are shown in Fig. S1.† The TDDFT studies of these molecules in THF are in nearly good agreement with experimental UV-vis spectra. The spectra have been produced by convoluting Gaussian functions with FWHM = 0.30 eV. The normalized plots of simulated and experimental UV-vis spectra of CSDPPs are depicted in Fig. 3. The respective summarized data of important allowed transitions is reported in ESI.† The wavelength of the excitations with the largest oscillator strengths (f) within these bands are given in Table 2. The most intense singlet transition is a HOMO to LUMO transition at 615 nm (f = 1.2162) for CSDPP5, 624 nm (f = 1.2145) for CSDPP6, 606 nm (f = 1.5051) for CSDPP7 and 615 nm (f = 1.4894) for CSDPP8. The optimized geometry parameters, and detailed list of the calculated energy levels at the B3LYP/6-311G(d,p) level of theory are shown in ESI.†
| CSDPPs | λmaxa (nm) | λmax (nm) | HOMO | LUMO | HLG | f | Main contribution | μ (D) |
|---|---|---|---|---|---|---|---|---|
| a Absorption values obtained from experimental data. | ||||||||
| CSDPP5 | 647 | 615 | −5.18 | −2.88 | 2.30 | 1.216 | H → L (99%) | 0.89 |
| CSDPP6 | 647 | 624 | −5.16 | −2.91 | 2.25 | 1.214 | H → L (99%) | 1.47 |
| CSDPP7 | 623 | 606 | −5.06 | −2.75 | 2.31 | 1.505 | H → L (99%) | 0.29 |
| CSDPP8 | 623 | 615 | −5.04 | −2.77 | 2.27 | 1.489 | H → L (99%) | 0.21 |
The highest occupied molecular orbital (HOMO) energy levels of these small molecules in thin films have been determined from the cyclic voltammetry (CV) (Fig. 4a) and compiled in Table 1. The CV of these compounds exhibited a reversible oxidation wave where the HOMO level of these compounds were estimated from the onset oxidation potential under the assumption that energy level of Fc/Fc+ was 4.7 eV below the vacuum level. The lowest unoccupied molecular orbital (LUMO) energy level of these small molecules was estimated from the difference of HOMO energy level and E0–0, where E0–0 derived from the intersection of the absorption and emission spectra and compiled in Table 1. It can be seen from the Table 1 that the deeper HOMO energy level for CSDPP5 (−5.24 eV) CSDPP6 (−5.23 eV) CSDPP7 (−5.30 eV) and CSDPP8 (−5.32 eV) is beneficial for high Voc. The difference in HOMO energy level of CSDPP5, CSDPP6 and CSDPP7, CSDPP8 are attributed to the different donor units, i.e. POZ and CBZ respectively, while the LUMO energy level of four DPPs is almost same. The HOMO level of CSDPP5 is identical to that of CSDPP6 and CSDPP7 showed almost similar to CSDPP8. The deeper lying HOMO levels and sufficient LUMO offsets (>0.6 V), (Fig. 4b) allow these small molecules to serve as electron donors in combination with PC71BM as an acceptor, which has HOMO and LUMO levels of −6.1 eV and −4.1 eV, respectively.
To understand the nature of charge transfer in the electronic transitions, we have plotted the isodensity surface plots (isovalue = 0.02) of the HOMO, LUMO and important nearest molecular orbitals (Fig. S2†) which are involved in transitions with strong contributions to the first excitation as well as to next two excitations with strong oscillator strength. To gain further insight into the electron density distribution in each molecule, partial electron density contribution and terminal moieties in each molecule from the respective frontier orbitals have been generated and depicted in Fig. S3.† For all the CSDPPs the HOMO and LUMO extend at least over all of their main inner body up to thiophene moieties. The HOMO−2 is majorly localized over the terminating groups while LUMO+2 localized at various degrees over the whole of the structure. We partition the structures into the diketopyrrolopyrrole (DKPP), the thiophene moieties (Th), and terminal groups phenothiazine (PTZ), carbazole (CBZ) for CSDPP5, CSDPP6, CSDPP7 and CSDPP8 respectively. The HOMO of CSDPPs has contributions from DKPP by (42%, 43%, 48% and 47%), Th (25%, 26%, 30%, 30%) and the terminal groups (33%, 32%, 23%, 22%) respectively. The LUMO of CSDPPs is dominated by contributions from the DKPP and Th groups by 52%, 52%, 54%, 54% and 37%, 37%, 37%, 37% respectively, with only minor contribution 11%, 11%, 9%, and 9% from the PTZ and CBZ moieties, respectively.
The calculated HOMO, LUMO energies and the HOMO–LUMO gap (HLG) at a B3LYP/6-311G(d,p) level in THF solvent is given in Fig. 5. The HOMO–LUMO gaps are shown in Table 2.
 | ||
| Fig. 5 Calculated HOMO–LUMO gaps at B3LYP/6-311G(d,p) level of theory and respective HOMO and LUMO orbital pictures at B3LYP/6-311G(d,p) level of CSDPPs in THF solvent. | ||
The dipole moment of the organic molecules depends on the charge transfer process within the molecule, which in turn depends on the availability of electron donating group.57 From Table 2, PTZ (electron donor) containing CSDPPs showed higher dipole moment compared to CBZ containing molecules, due to higher electron donating capacity of PTZ moiety. The higher dipole moment of CSDPP6 might be attributed to more electron donating nature and structural arrangement of 2-ethylhexyl moiety on nitrogen.
3.3 Photovoltaic properties
In BHJ OSCs, the relative quantities of donor and acceptor materials employed in the active layer is of great importance for the photovoltaic performance, since there should be a balance between the absorbance and charge transporting network of the active layer. When the acceptor content is low, the electron transporting ability will be limited, while when the acceptor content is too high, the absorbance and hole transporting ability of the active layer will be decreased. In the present investigation, PC71BM has been employed as acceptor, since it possesses high absorption in the visible region as compared to PC61BM. BHJ active layers with a mixture of DPPs and PC71BM in THF in different weight ratios were tested and optimum ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. We are discussing our results with the optimum blend ratio. The optical absorption spectra of the blends were shown in Fig. 6 (for CSDPP5:PC71BM and CSDPP7:PC71BM blend films showed similar absorption profile to CSDPP6:PC71BM and CSDPP8:PC71BM blends respectively). The broad absorption covering from 350 to 700 nm with two absorption bands, corresponding to the PC71BM (shorter wavelength region) and DPPs (longer wavelength region), indicate that both donor and acceptor are contributing to the exciton generation and thereby photocurrent generation.
2. We are discussing our results with the optimum blend ratio. The optical absorption spectra of the blends were shown in Fig. 6 (for CSDPP5:PC71BM and CSDPP7:PC71BM blend films showed similar absorption profile to CSDPP6:PC71BM and CSDPP8:PC71BM blends respectively). The broad absorption covering from 350 to 700 nm with two absorption bands, corresponding to the PC71BM (shorter wavelength region) and DPPs (longer wavelength region), indicate that both donor and acceptor are contributing to the exciton generation and thereby photocurrent generation.
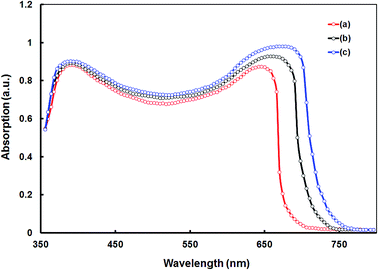 | ||
| Fig. 6 Absorption spectra of (a) CSDPP8:PC71BM (THF cast), (b) CSDPP6:PC71BM (THF cast), and (c) CSDPP6:PC71BM (DIO/THF cast) active layers. | ||
Solution processed BHJ solar cells based on these DPP small molecules were fabricated with a conventional structure glass/ITO/PEDOT:PSS/active layer/Al and the J–V characteristics of the devices are shown in Fig. 7a (CSDPP5 and CSDPP7) and Fig. 8a (CSDPP6 and CSDPP8), under illumination at AG1.5 G, 100 mW cm−2 and the photovoltaic parameters are summarized in Table 3. With optimized weight ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the device based on CSDPP5:PC71BM and CSDPP6:PC71BM (cast with THF solvent) showed a PCE of 2.97% (Jsc = 7.88 mA cm−2, Voc = 0.84 V and FF = 0.47) and 3.06% (Jsc of 7.92 mA cm−2, Voc of 0.84 V and FF of 0.46), respectively. In contrast device fabricated with CSDPP7:PC71BM, CSDPP8:PC71BM processed under same conditions produced lower PCE of 2.42% (6.34 mA cm−2, Voc = 0.91 V and FF = 0.42) and 2.43% (Jsc of 6.42 mA cm−2, Voc of 0.90 V and FF of 0.42), respectively. CSDPP5 and CSDPP7 have DPP core with n-decyl long side chain, whereas CSDPP6 and CSDPP8 have same DPP core with ethyl-hexyl side chain indicates that the alkyl modification in the DPP core does not have much influence in the solar cells performance. The difference in the photovoltaic performance is mainly due to the different end capping donor unit. Therefore we are discussing the detailed analysis of CSDPP6 and CSDPP8 based devices. The higher value of Voc for CSDPP8 based device as compared to the CSDPP6 counter part is attributed to its deeper HOMO level than CSDPP6. However, the larger value of PCE of CSDPP6 based device as compared to CSDPP8 is due to the higher values of Jsc and FF. The enhancement in Jsc is mainly due to the better light harvesting property of CSDPP6 compared to CSDPP8 (as shown in the absorption spectra Fig. 2).
2), the device based on CSDPP5:PC71BM and CSDPP6:PC71BM (cast with THF solvent) showed a PCE of 2.97% (Jsc = 7.88 mA cm−2, Voc = 0.84 V and FF = 0.47) and 3.06% (Jsc of 7.92 mA cm−2, Voc of 0.84 V and FF of 0.46), respectively. In contrast device fabricated with CSDPP7:PC71BM, CSDPP8:PC71BM processed under same conditions produced lower PCE of 2.42% (6.34 mA cm−2, Voc = 0.91 V and FF = 0.42) and 2.43% (Jsc of 6.42 mA cm−2, Voc of 0.90 V and FF of 0.42), respectively. CSDPP5 and CSDPP7 have DPP core with n-decyl long side chain, whereas CSDPP6 and CSDPP8 have same DPP core with ethyl-hexyl side chain indicates that the alkyl modification in the DPP core does not have much influence in the solar cells performance. The difference in the photovoltaic performance is mainly due to the different end capping donor unit. Therefore we are discussing the detailed analysis of CSDPP6 and CSDPP8 based devices. The higher value of Voc for CSDPP8 based device as compared to the CSDPP6 counter part is attributed to its deeper HOMO level than CSDPP6. However, the larger value of PCE of CSDPP6 based device as compared to CSDPP8 is due to the higher values of Jsc and FF. The enhancement in Jsc is mainly due to the better light harvesting property of CSDPP6 compared to CSDPP8 (as shown in the absorption spectra Fig. 2).
 | ||
| Fig. 7 (a) Current–voltage (J–V) characteristics and (b) IPCE spectra of the BHJ solar cells based on CSDPP5:PC71BM (THF cast) (black color), CSDPP7:PC71BM (THF cast) (red color). | ||
 | ||
| Fig. 8 (a) Current–voltage (J–V) characteristics and (b) IPCE spectra of the BHJ solar cells based on CSDPP6:PC71BM (THF cast) (black color) and CSDPP8:PC71BM (THF cast) (red color). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in each device
2 in each device
| Blend | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) | μh (cm2 V−1 s−1) | μe/μh |
|---|---|---|---|---|---|---|
| a Processed with THF.b Processed with DIO/THF. | ||||||
| CSDPP5:PC71BMa | 7.88 | 0.84 | 0.47 | 2.97 | 9.48 × 10−6 | 25 |
| CSDPP6:PC71BMa | 7.92 | 0.84 | 0.46 | 3.06 | 9.56 × 10−6 | 22 |
| CSDPP7:PC71BMa | 6.34 | 0.91 | 0.42 | 2.42 | 6.38 × 10−6 | 38 |
| CSDPP8:PC71BMa | 6.42 | 0.90 | 0.42 | 2.43 | 6.44 × 10−6 | 32 |
| CSDPP6:PC71BMb | 10.48 | 0.80 | 0.56 | 4.69 | 3.56 × 10−5 | 6.2 |
| CSDPP8:PC71BMb | 8.92 | 0.86 | 0.52 | 4.14 | 2.63 × 10−5 | 8.9 |
The IPCE values of the optimized devices based on CSDPPs are illustrated in Fig. 7b (CSDPP5 and CSDPP7) and Fig. 8b (CSDPP6 and CSDPP8). The IPCE response for four devices in the lower wavelength region corresponds to absorption spectra of PC71BM and is almost same. The device based on CSDPP6:PC71BM exhibit a broad and high IPCE values from 520 nm to 720 nm exceeding 47%. In comparison, the IPCE values of the device fabricated from CSDPP8:PC71BM are lower and also the response range is relatively narrower than that of the device based on CSDPP6:PC71BM. The Jsc values estimated from integrating the IPCE spectra are 7.82 mA cm−2 and 6.36 mA cm−2 for CSDPP6:PC71BM and CSDPP8:PC71BM, respectively, which is in good agreement with the experimentally observed Jsc values obtained from J–V characteristics under illumination. These IPCE spectra of the devices from CSDPP6 and CSDPP8 are consistent with the corresponding UV-visible absorption spectra and confirm that CSDPP6 having phenoxazine donor units can broaden the IPCE spectra and hence improve the PCE.
The electron and hole mobilities of the active layers were measured by space charge limited current (SCLC) method32,58 using J–V characteristics of the electron only and hole only devices in dark (Fig. 9). The CSDPP8:PC71BM based device exhibits a hole and electron mobility of 6.44 × 10−6 cm2 V−1 s−1 and 2.18 × 10−4 cm2 V−1 s−1, respectively. The device based on CSDPP6:PC71BM shows a high hole mobility of 9.56 × 10−6 cm2 V−1 s−1 and almost same electron mobility as for CSDPP8:PC71BM based device. The electron to hole mobility ratio of the devices based on CSDPP5:PC71BM and CSDPP7:PC71BM is 25 and 38, and that of CSDPP6:PC71BM and CSDPP8:PC71BM are 22 and 32 respectively. The more balanced charge transport of the device based on CSDPP6:PC71BM active layer is reflected from the higher PCE.
 | ||
| Fig. 9 Current–voltage (J–V) characteristics in dark for hole-only based on (a) CSDPP6:PC71BM (THF cast) active layer (black color) and (b) CSDPP8:PC71BM (THF cast) (red color). | ||
The low PCEs for the above devices based on the active layer processed with THF solvent are mainly due to the low values of Jsc and FF, although the Voc is quite high. The series resistance (Rs) derived from the J–V characteristics of the devices, under illumination at voltage equivalent to Voc, is very large (234 Ω cm2 and 168 Ω cm2 for CSDPP8:PC71BM and CSDPP6:PC71BM, respectively). In general, Rs is mainly arising from bulk resistance of active layer, electron and hole transporting layers. Bulk resistance of the active layer can be easily tunable via improving the film morphology using different treatments, i.e. thermal annealing,59,60 solvent annealing61,62 and solvent additives63–65 that would enhance the charge carrier mobilities. The higher boiling point solvent additives are known to improve the morphology and charge carrier mobilities by reducing the molecular aggregation. Moreover, the relatively large exciton binding energy (0.3 eV) and short diffusion length (10–20 nm) of organic semiconductors warrants a large D/A interfacial area for efficient exciton dissociation and charge transport in BHJ OSCs. However, if the scale phase separated domains in BHJ layer is smaller than the coulomb capture radius, it would increase either germinate or bimolecular recombination probability. Therefore, an optimum nanomorphology and phase separation is required for efficient OSCs. Particularly, the choice of the solvent plays an important role and greatly influence the morphology of the active layer, since the morphology of the active layer depends upon the evaporation rate of the solvents. In an effort to improve the performance of the BHJ OSC based on CSDPP6:PC71BM and CSDPP8:PC71BM active layer, we employed solvent additive (DIO) method and optimized the concentration of DIO as 3% v, i.e. DIO (3 v%)/THF solvent. The current–voltage characteristics of the resulting devices are shown in Fig. 10a (CSDPP6:PC71BM (black color) and CSDPP8:PC71BM (red color)) and corresponding photovoltaic parameters are compiled in Table 3. Remarkably, the PCE of the solar cells were improved from 3.06% to 4.69% and 2.43% to 4.14% for CSDPP6:PC71BM and CSDPP8:PC71BM based devices, respectively.
 | ||
| Fig. 10 (a) Current–voltage (J–V) characteristics and (b) IPCE spectra of the BHJ solar cells based on CSDPP6:PC71BM (DIO/THF cast) (black color) and CSDPP8:PC71BM (DIO/THF cast) (red color). | ||
The superior performance of OSC based on CSDPP6:PC71BM processed with solvent additive is attributed to its improved Jsc (10.48 mA cm−2) and FF (0.56) values. The improved Jsc value is ascribed to the enhanced IPCE response of the solar cell. As shown in Fig. 10b, the devices with active layer processed with DIO/THF solvent exhibit a stronger and broader IPCE response compared to THF processed active layer. The Jsc depends upon the light harvesting capability of the active layer. As can be seen from the absorption spectra, (Fig. 6) the active layer cast from the DIO/THF showed higher absorption coefficient, particularly in the longer wavelength region, indicating that the active layer improved light harvesting efficiency, resulting high value of FF.
The photovoltaic performance of the organic BHJ solar cells is closely related with the nanomorphology of the active layer, we have investigated the film morphologies of CSDPP6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) blend film cast from THF and DIO/THF solvents, from the atomic force microscopy (AFM), in tapping mode. Fig. 11 shows AFM height images of the blend films processed with THF and DIO/THF. The blend film cast from THF solvent showed a relatively homogeneous and flat surface with a root mean square (RMS) surface roughness of 0.62 nm and poor phase separation. However, the spin coated film from DIO/THF has slightly more aggregated domains and a phase separated surface with larger RMS roughness of 1.64 nm. Similar results for CSDPP8:PC71BM were observed with surface roughness of 0.54 nm and 1.46 nm for the film cast from THF and DIO/THF, respectively. The aggregated domains may be likely originated from the enhanced intermolecular interaction of SM (solvent medium), during the film formation.66,67 A higher surface roughness is expected to increase the internal light scattering and enhance the light absorption,68 consistent with the absorption spectra displayed in Fig. 6. The blend film with larger domain size suggests a good phase separation and well connected domains allow efficient charge generation and charge transfer within the active layer. The sum of all these effects enhances the Jsc and PCE for the device processed with DIO/THF solvent than that exhibited by device processed with THF only.
2) blend film cast from THF and DIO/THF solvents, from the atomic force microscopy (AFM), in tapping mode. Fig. 11 shows AFM height images of the blend films processed with THF and DIO/THF. The blend film cast from THF solvent showed a relatively homogeneous and flat surface with a root mean square (RMS) surface roughness of 0.62 nm and poor phase separation. However, the spin coated film from DIO/THF has slightly more aggregated domains and a phase separated surface with larger RMS roughness of 1.64 nm. Similar results for CSDPP8:PC71BM were observed with surface roughness of 0.54 nm and 1.46 nm for the film cast from THF and DIO/THF, respectively. The aggregated domains may be likely originated from the enhanced intermolecular interaction of SM (solvent medium), during the film formation.66,67 A higher surface roughness is expected to increase the internal light scattering and enhance the light absorption,68 consistent with the absorption spectra displayed in Fig. 6. The blend film with larger domain size suggests a good phase separation and well connected domains allow efficient charge generation and charge transfer within the active layer. The sum of all these effects enhances the Jsc and PCE for the device processed with DIO/THF solvent than that exhibited by device processed with THF only.
 | ||
Fig. 11 AFM height images of CSDPP6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) films cast from THF and DIO/THF, scan sizes are 3 μm × 3 μm. 2) films cast from THF and DIO/THF, scan sizes are 3 μm × 3 μm. | ||
For the efficient OSCs, the mobility of electrons and hole in the BHJ active layer is also of great importance, as these should be balanced in order to achieve efficient charge transport and collection at the different electrodes. The mobility of holes and electrons in the CSDPP6:PC71BM active layer film cast from THF and DIO/THF were estimated from hole only device (ITO/PEDOT:PSS/CSDPP6:PC71BM/Au) and electron only device (ITO/Al/CSDPP6:PC71BM/Al), respectively, by means of space charge limited current (SCLC) measurements.69 The current–voltage characteristics of the hole only devices are shown in Fig. 12. The hole and electron mobilities were estimated by fitting the experimental data in the following equation.
 | ||
| Fig. 12 Current–voltage (J–V) characteristics in dark for hole-only based on (a) CSDPP6:PC71BM (DIO/THF cast) active layer (black color) and (b) CSDPP8:PC71BM (DIO/THF cast) (red color). | ||
4. Conclusions
Four solution processed D–A–D small molecules based on DPP as central acceptor core and different terminals, i.e. carbazole (CSDPP7, CSDPP8) and phenoxazine (CSDPP5, CSDPP6) donor units were synthesized and their optical and electrochemical properties investigated. The LUMO energy level of these small molecules is almost same as that of DPP acceptor, but exhibit different HOMO levels due to the presence of different donor units. The BHJ OSCs CSDPP5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w), CSDPP6
2 w/w), CSDPP6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) CSDPP7
2 w/w) CSDPP7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) and CSDPP8
2 w/w) and CSDPP8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/w) processed with THF showed overall PCE of about 2.97%, 3.06%, 2.42% and 2.43%, respectively. The incorporation of 3% v DIO as solvent additive enhanced the PCE of CSDPP6:PC71BM and CSDPP8:PC71BM up to 4.69% and 4.14%, attributed to the better phase separation nanomorphology of the active layer and enhanced charge transportation in the active layer. From above results, we conclude that the terminal donor significantly affect the PCEs of the BHJ OSCs.
2 w/w) processed with THF showed overall PCE of about 2.97%, 3.06%, 2.42% and 2.43%, respectively. The incorporation of 3% v DIO as solvent additive enhanced the PCE of CSDPP6:PC71BM and CSDPP8:PC71BM up to 4.69% and 4.14%, attributed to the better phase separation nanomorphology of the active layer and enhanced charge transportation in the active layer. From above results, we conclude that the terminal donor significantly affect the PCEs of the BHJ OSCs.
Acknowledgements
Ch. P. K. thanks UGC, New Delhi for a senior research fellowship.References
- Y. J. Cheng, S. H. Yang and C. S. Hsu, Chem. Rev., 2009, 109, 5868–5923 CrossRef CAS PubMed.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS.
- K. Li, Z. Li, K. Feng, X. Xu, L. Wang and Q. Peng, J. Am. Chem. Soc., 2013, 135, 13549–13557 CrossRef CAS PubMed.
- H. L. Yip and A. K. Y. Jen, Energy Environ. Sci., 2012, 5, 5994–6011 CAS.
- J. Chen and Y. Cao, Acc. Chem. Res., 2009, 42, 1709–1718 CrossRef CAS PubMed.
- L. Dou, J. You, Z. Hong, Z. Xu, G. Li, R. A. Street and Y. Yang, Adv. Mater., 2013, 25, 6642–6671 CrossRef CAS PubMed.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591–595 Search PubMed.
- S. H. Liao, H. J. Jhuo, Y. S. Cheng and S. A. Chen, Adv. Mater., 2013, 25, 4766–4771 CrossRef CAS PubMed.
- A. M. Abakumov, A. a. Tsirlin, I. Bakaimi, G. V. Tendel and A. Lappas, Chem. Mater., 2014, 26, 3306–3315 CrossRef CAS.
- X. Guo, M. Zhang, W. Ma, L. Ye, S. Zhang, S. Liu, H. Ade, F. Huang and J. Hou, Adv. Mater., 2014, 26, 4043–4049 CrossRef CAS PubMed.
- W. Zhang, Y. Wu, Q. Bao, F. Gao and J. Fang, Adv. Energy Mater., 2014 DOI:10.1002/aenm.201400359.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293–5301 CrossRef CAS PubMed.
- B. Kan, M. Li, Q. Zhang, F. Liu, X. Wan, Y. Wang, W. Ni, G. Long, X. Yang, H. Feng, Y. Zuo, M. Zhang, F. Huang, Y. Cao, T. P. Russell and Y. Chen, J. Am. Chem. Soc., 2015, 137, 3886–3893 CrossRef CAS PubMed.
- A. R. B. M. Yusoff, D. Kim, H. P. Kim, F. K. Shneider, W. J. da Silva and J. Jang, Energy Environ. Sci., 2015, 8, 303–316 CAS.
- C.-C. Chen, W.-H. Chang, K. Yoshimura, K. Ohya, J. You, J. Gao, Z. Hong and Y. Yang, Adv. Mater., 2014, 26, 5670–5677 CrossRef CAS PubMed.
- A. A. Virkar, S. Mannsfeld, Z. N. Bao and N. Stingelin, Adv. Mater., 2010, 22, 3857–3875 CrossRef CAS PubMed.
- Z. He, H. Wu and Y. Cao, Adv. Mater., 2014, 26, 1006–1024 CrossRef CAS PubMed.
- X. Gao and Y. Hu, J. Mater. Chem. C, 2014, 2, 3099–3117 RSC.
- A. Mishra and P. Bäuerle, Angew. Chem., Int. Ed., 2012, 51, 2020–2067 CrossRef CAS PubMed.
- Y. Lin, Y. Li and X. Zhan, Chem. Soc. Rev., 2012, 41, 4245–4272 RSC.
- Y. Sun, G. C. Welch, W. L. Leong, C. J. Takacs, G. C. Bazan and A. J. Heeger, Nat. Mater., 2012, 11, 44–48 CrossRef CAS PubMed.
- J. E. Coughlin, Z. B. Henson, G. C. Welch and G. C. Bazan, Acc. Chem. Res., 2014, 47, 257–270 CrossRef CAS PubMed.
- J. Zhou, X. Wan, Y. Liu, Y. Zuo, Z. Li, G. He, G. Long, W. Ni, C. Li, X. Su and Y. Chen, J. Am. Chem. Soc., 2012, 134, 16345–16351 CrossRef CAS PubMed.
- A. K. K. Kyaw, D. H. Wang, D. Wynands, J. Zhang, T. Q. Nguyen, G. C. Bazan and A. J. Heeger, Nano Lett., 2013, 13, 3796–3801 CrossRef CAS PubMed.
- J. Zhou, Y. Zuo, X. Wan, G. Long, Q. Zhang, W. Ni, Y. Li, Z. Li, G. Su, C. Li, B. Kan, M. Li and Y. Chen, J. Am. Chem. Soc., 2013, 135, 8484–8487 CrossRef CAS PubMed.
- Y. Chen, X. Wan and G. Long, Acc. Chem. Res., 2013, 46, 2645–2655 CrossRef CAS PubMed.
- Y. Liu, C. Chen, Z. Hong, J. Gao, Y. Yang, H. Zhou, L. Dou, G. Li and Y. Yang, Sci. Rep., 2013, 3, 3356/1–8 Search PubMed.
- B. Kan, Q. Zhang, M. Li, X. Wan, W. Ni, G. Long, Y. Wang, X. Yang, H. Feng and Y. Chen, J. Am. Chem. Soc., 2014, 136, 15529–15532 CrossRef CAS PubMed.
- Heliatek consolidates its technology leadership by establishing a new world record for organic solar technology with a cell efficiency of 12%, available at http://www.heliatek.com/en/press/press-releases/details/heliatek-consolidates-its-technology-leadership-by-establishing-a-new-world-record-for-organic-solar-technology-with-a-cell-effi, 2013 accessed July 22, 2014.
- B. Tamayo, M. Tantiwiwat, B. Walker and T. Q. Nguyen, J. Phys. Chem. C, 2008, 112, 15543–15552 Search PubMed.
- A. B. Tamayo, X. D. Dang, B. Walker, J. Seo, T. Kent and T. Q. Nguyen, Appl. Phys. Lett., 2009, 94, 103301–103302 CrossRef.
- B. Walker, A. B. Tamayo, X. D. Dang, P. Zalar, J. H. Seo, A. Garcia, M. Tantiwiwat and T. Q. Nguyen, Adv. Funct. Mater., 2009, 19, 3063–3069 CrossRef CAS.
- J. Liu, B. Walker, A. Tamayo, Y. Zhang and T. Q. Nguyen, Adv. Funct. Mater., 2013, 23, 47–56 CrossRef CAS.
- S. Qu and H. Tian, Chem. Commun., 2012, 48, 3039–3051 RSC.
- O. P. Lee, A. T. Yiu, P. M. Beaujuge, C. H. Woo, T. W. Holcombe, J. E. Millstone, J. D. Douglas, M. S. Chen and J. M. Fréchet, Adv. Mater., 2011, 23, 5359–5363 CrossRef CAS PubMed.
- C. Kim, J. Liu, J. Lin, A. B. Tamayo, B. Walker, G. Wu and T. Q. Nguyen, Chem. Mater., 2012, 24, 1699–1709 CrossRef CAS.
- B. Zhao, K. Sun, F. Xue and J. Ouyang, Org. Electron., 2012, 13, 2516–2524 CrossRef CAS.
- M. Chen, W. Fu, M. Shi, X. Hu, J. Pan, J. Ling, H. Li and H. Chen, J. Mater. Chem. A, 2013, 1, 105–111 CAS.
- J. Liu, Y. Sun, P. Moonsin, M. Kuik, C. M. Proctor, J. Lin, B. B. Hsu, V. Promarak, A. J. Heeger and T. Q. Nguyen, Adv. Mater., 2013, 25, 5898–5903 CrossRef CAS PubMed.
- W. Li, W. S. C. Roelofs, M. M. Wienk and R. A. J. Janssen, J. Am. Chem. Soc., 2012, 134, 13787–13795 CrossRef CAS PubMed.
- H. Bronstein, Z. Chen, R. S. Ashraf, W. Zhang, J. Du, J. R. Durrant, P. S. Tuladhar, K. Song, S. E. Watkins, Y. Geerts, M. M. Weink, R. A. J. Janssen, T. Anthopoulos, H. Sirringhaus, M. Heeney and I. McCulloch, J. Am. Chem. Soc., 2011, 133, 3272–3275 CrossRef CAS PubMed.
- L. Dou, J. Gao, E. Richard, J. You, C. C. Chen, K. C. Cha, Y. He, G. Li and Y. Yang, J. Am. Chem. Soc., 2012, 134, 10071–10079 CrossRef CAS PubMed.
- H. Qin, L. Li, F. Guo, S. Su, J. Peng, Y. Cao and X. Peng, Energy Environ. Sci., 2014, 7, 1397–1401 CAS.
- M. Cheng, X. Yang, C. Chen, Q. Tan and L. Sun, J. Mater. Chem. A, 2014, 2, 10465–10469 CAS.
- G. D. Sharma, M. Anil Reddy, K. Ganesh, S. P. Singh and M. Chandrasekharam, RSC Adv., 2014, 4, 732–742 RSC.
- M. Chandrasekharam, M. Anil Reddy, K. Ganesh, G. D. Sharma, S. P. Singh and J. Laxmikanth Rao, Org. Electron., 2014, 15, 2116–2125 CrossRef CAS.
- C. H. Pavan Kumar, K. Ganesh, T. Suresh, A. Sharma, K. Bhanuprakash, G. D. Sharma and M. Chandrasekharam, RSC Adv., 2015, 5, 93579–93590 RSC.
- A. Iqbal, M. Jost, R. Kirchmayr, J. Pfenninger, A. Rochat and O. Wallquist, Bull. Soc. Chim. Belg., 1988, 97, 615–643 CrossRef CAS.
- A. C. Rochat, L. Cassar and A. Iqbal, European Pat., 0094911, 1983.
- N. Avcibasi, M. Smet, B. Metten, W. Dehaen, F. C. De Schryver, G. Bultynck, G. Callewaert, H. De Smedt, L. Missiaen and N. Boens, Int. J. Photoenergy, 2004, 6, 159–167 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci and G. A. Petersson, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford, CT, 2010 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1996, 104, 1040 CrossRef CAS.
- G. A. Petersson, A. Bennett, T. G. Tensfeldt, M. A. Al-Laham, W. A. Shirley and J. Mantzaris, J. Chem. Phys., 1988, 89, 2193–2198 CrossRef CAS.
- M. Cossi, V. Barone, R. Cammi and J. Tomasi, Chem. Phys. Lett., 1996, 255, 327–335 CrossRef CAS.
- N. M. O'Boyle, A. L. Tenderholt and K. M. Langner, J. Comput. Chem., 2008, 29, 839–845 CrossRef PubMed.
- K. Chandrasekhar, L. R. Naik, H. M. Suresh Kumar and N. N. Math, Indian J. Pure Appl. Phys., 2006, 44, 292–299 CAS.
- M. A. Khan, W. Xu, H. Khizarul, Y. Bai, X. Y. Jiang, Z. L. Zhang, W. Q. Zhu, Z. L. Zhang and W. Q. Zhu, J. Appl. Phys., 2008, 103, 014509 CrossRef.
- W. Ma, C. Yang, X. Gong, K. Lee and A. J. Heeger, Adv. Funct. Mater., 2005, 15, 1617–1622 CrossRef CAS.
- J. A. Mikroyannidis, A. N. Kabanakis, S. S. Sharma and G. D. Sharma, Adv. Funct. Mater., 2011, 21, 746–755 CrossRef CAS.
- G. Li, V. Shrotriya, J. S. Huang, Y. Yao, T. Moriarty, K. Emery and Y. Yang, Nat. Mater., 2005, 4, 864–868 CrossRef CAS.
- Y. Zhao, Z. Xie, Y. Qu, Y. Geng and L. Wang, Appl. Phys. Lett., 2007, 90, 043504 CrossRef.
- A. Tamayo, T. Kent, M. Tantitiwat, M. A. Dante, J. Rogers and T. Q. Nguyen, Energy Environ. Sci., 2009, 2, 1180–1186 CAS.
- C. Min, J. Li, G. Veronis, J. Y. Lee, S. Fan and P. Peumans, Appl. Phys. Lett., 2010, 97, 133302 CrossRef.
- B. Walker, C. Kim and T. Q. Nguyen, Chem. Mater., 2010, 23, 470–482 CrossRef.
- Q. Shi, P. Cheng, Y. Li and X. Zhan, Adv. Energy Mater., 2012, 2, 63–67 CrossRef CAS.
- A. K. K. Kyaw, D. H. Wang, C. Luo, Y. Cao, T. Q. Nguyen, G. C. Bazan and A. J. Heerger, Adv. Energy Mater., 2014, 4 DOI:10.1002/aenm.201301469.
- J. D. Zimmermen, X. Xiao, C. K. Renshaw, S. Wang, V. V. Diev, M. E. Thompson and S. R. Forrest, Nano Lett., 2012, 12, 4366–4371 CrossRef PubMed.
- G. G. Malliaras, J. R. Salem, P. J. Brock and C. Scott, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 13411–13414 CrossRef.
- A. J. Moule and K. Meerholz, Adv. Mater., 2008, 20, 240–245 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Structures of optimized geometries, molecular orbitals, percentage contributions of the orbital density of the individual groups, calculated excitation energies, calculated HOMOs and LUMOs energy levels, optimized geometry parameters of CSDPP5–CSDPP8 calculated at B3LYP/6-311G(d,p) level theory. See DOI: 10.1039/c5ra24610e |
| This journal is © The Royal Society of Chemistry 2016 |