Nanosized surface films on brass alloys by XPS and XAES†
Federica Cocco a,
Bernhard Elsenerab,
Marzia Fantauzzia,
Davide Atzeia and
Antonella Rossi*a
a,
Bernhard Elsenerab,
Marzia Fantauzzia,
Davide Atzeia and
Antonella Rossi*a
aDipartimento di Scienze Chimiche e Geologiche, Università degli Studi di Cagliari, INSTM, UdR, Campus di Monserrato S.S. 554-Italy, Cagliari, Italy. E-mail: rossi@unica.it
bETH Zurich, Institute for Building Materials, ETH Hönggerberg, CH-8093 Zurich, Switzerland
First published on 17th March 2016
Abstract
Chemical state identification and quantification based on photoelectron spectra is challenging in the case of copper and zinc and their alloys. In this work an analytical strategy for simultaneous chemical state identification and quantification of copper and zinc chemical states in complex layered systems is presented. This approach is based on the curve fitting of the multicomponent X-ray excited Auger spectra, CuL3M4.5M4.5 and ZnL3M4.5M4.5, that clearly distinguish metallic and oxide components and result in separated ILMM,met and ILMM,ox peak areas. On reference copper and zinc compounds, showing only a single chemical state, the intensity ratio R between photoelectron I2p and Auger intensity ILMM was determined. Rmet was obtained using pure metals and a sputtered brass alloy Cu37Zn. Rox was calculated using the pure oxides. Based on these experimental intensity ratios, R, a quantification factor, k = Rox/Rmet, is calculated for both copper and zinc. This quantification factor k is independent of the instrument employed for the analysis, as proved here by using different spectrometers. The factor k is then used to transfer the experimental Auger intensity ratio (ILMM,met/ILMM,ox) into the I2p,met/I2p,ox intensity ratio, which is required for the quantitative analysis by XPS. The potential of this approach based on XPS and XAES for the patina studies on copper alloys, relevant in various fields including corrosion and cultural heritage, is presented for Cu37Zn model brass alloy after different surface pre-treatments. This approach has proven to be successful.
1. Introduction
The identification of the chemical environment for most elements can be routinely based on the measurement of the binding energy (BE) of the X-ray photoelectron signals and on the comparison with the values provided in databases such as the NIST Database,1 PHI Handbook2 or in book data compilations.3 It is however well known that this approach is very difficult for copper and zinc compounds due to the fact that the chemical shift between the elemental state and the oxidized one is very small. Recently,4 BE data from the NIST database has been revised. Metallic copper Cu(0) is reported at a BE of 932.61 eV (std. dev. = 0.21 eV) while the same signal in the case of copper oxide Cu2O, is at a BE of 932.43 eV (std. dev. = 0.24 eV). These two chemical states of copper have thus statistically the same BE and cannot be differentiated setting the experimental conditions commonly used by laboratory spectrometers. In the case of zinc the situation is similar: metallic zinc Zn(0) has a BE of 1021.65 eV (std. dev. = 0.01 eV) whereas in zinc oxide ZnO, Zn(II) has a BE of 1021.00 eV (std. dev. = 0.04 eV). Also in this case a univocal identification of the zinc chemical state is challenging. In more complex systems such as copper/zinc alloys with nanometre-thick oxide over-layers the peaks will largely overlap and make identification of the chemical state and quantification of the species in the various layers even more difficult. A close inspection of the shape of the X-ray induced Auger signals CuL3M4.5M4.5 and ZnL3M4.5M4.5 together with the calculation of the Auger parameter greatly improve the assignment of the chemical state as it has been shown in several works for both copper5–8,11 and zinc compounds.9–11 Despite the fact that Auger signals are sensitive to changes in the chemical state, the quantification when both chemical states of copper and zinc are simultaneously present is made difficult by the presence of a large number of signals. In few studies the quantification based on the X-ray excited Auger signals have been undertaken: a first attempt of curve fitting of the X-ray induced Auger signals of Cu and Cu2O was reported in 1988.12 The same group13 reported CuL3M4.5M4.5 spectra of Cu, Cu2O, Cu(OH)2 and CuO with an empirically-based curve fitting of the Auger signals. The ratio between Cu2p and CuL3M4.5M4.5 intensity was discussed and a cross section σL3M4.5M4.5 for the Auger signals identical for oxidized and metallic compounds was experimentally deduced. A later work14 on copper–zinc alloys in borate buffer solutions exploited the same approach to fit CuL3M4.5M4.5 and ZnL3M4.5M4.5 Auger spectra of Cu, Zn, Cu2O and ZnO. The assumption of a constant σL3MM was maintained and no complex Auger spectra with simultaneous presence of metal and oxide components (e.g. Cu2O on Cu, ZnO on Zn) were reported so that also the quantitative results are provided for a simple system.In this work an analytical strategy for simultaneous chemical state identification and full quantification of copper and zinc, when occurring in complex nanostructured systems such as brass alloys with thin oxide – hydroxide surface films or patinas, is presented. The approach relies on the curve-fitting based on the theoretical description of the multicomponent XAES signals CuL3M4.5M4.5 and ZnL3M4.5M4.5 aiming to carefully determine the intensity (percentage) of the metallic and oxidized copper and/or zinc components. This percentage, after the correction for the intensity ratio 2p/LMM calculated on pure zinc and copper metal and zinc and copper oxides, is then used for converting them to the areas of Cu2p3/2 or Zn2p3/2 signals used for the subsequent quantification. The approach has been tested on Cu37Zn model brass alloy after different surface pre-treatments and has proven to be successful.
This approach has been developed in the framework of a wide research project15 aiming to control the conditions that might cause damage on historical brass musical instruments and represents the starting point to relate non-destructive electrochemical tests to their surface chemistry.
2. Material and methods
2.1. Material and surface preparation
Pure copper and zinc metals (foils, purity > 99.9%, purchased from Goodfellow Cambridge Ltd, UK) and copper and zinc oxides (CuO 99.999%, Cu2O ≥ 99.99% anhydrous and ZnO ≥ 99.0%) supplied by (Sigma Aldrich, Sant Louis, USA) were analysed in this work as reference materials.A model brass alloy Cu37Zn (Goodfellow Cambridge Ltd, UK) in the “as received” state, after mechanical polishing and after Ar+ sputtering was used for testing the analytical approach. Its certified composition is reported to be 63% Cu, 37% Zn.
The mechanical polishing procedure is required for a well-defined starting point of the investigated surfaces. The sample was first delicately ground with silica paper (grit sizes of 2400 and 4000) and then polished using a DP Dur cloth and diamond paste with grain diameters of 3, 1 and 1/4 μm (Struers, Ballerup DK). Ethanol (p.a.), supplied by J. T. Baker® Chemicals (Avantor Performance Materials, Inc. Global Headquarters, USA) was used for cooling, lubricating and for cleaning the samples in ultra-sonic bath for five minutes. The surface was dried under an argon stream and immediately introduced in the spectrometer.
Pure copper, zinc and brass samples were also investigated after ion sputtering using an acceleration voltage of 3 kV and an ion current of 1 μA for a maximum of 300 s. These conditions were chosen in order to remove the contamination and the oxidized layers until the complete disappearance of C1s and O1s signals.
2.2. X-ray photoelectron spectroscopy and data processing
Theta probe. Survey and high-resolution spectra were acquired in fixed analyser transmission mode (FAT) setting the pass energy equal to 200 eV and to 100 eV respectively selecting the standard lens mode. Step-size was 1 eV and 0.05 eV, respectively. The full-width at half-maximum (FWHM) of the silver Ag3d5/2 signal for the high-resolution spectra was 0.84 eV. The spectrometer was calibrated according to ISO 15472:2001 with an accuracy of ±0.1 eV.
PHI Quantera. Survey and high-resolution spectra were acquired in fixed analyser transmission mode (FAT) setting the pass energy equal to 280 eV and to 69 eV respectively. Step-size was 1 eV and 0.125 eV, respectively. The FWHM of the silver Ag3d5/2 signal for the high-resolution spectra was 0.79 eV. The spectrometer was calibrated according to ISO 15472:2010 [Surface Chemical Analysis – X-ray Photoelectron Spectrometers – Calibration of energy scales]. The accuracy was found to be equal to ±0.1 eV.
When necessary, the BE values were referenced to the aliphatic carbon at 285.0 eV. Data were acquired under computer control (Thetaprobe: Avantage v. 3.45; Quantera: Compass v. 4). Three different regions were analysed on each sample. BE values and atomic percentages are reported in this work as mean values on three points with their corresponding standard deviations.
Data processing. The high-resolution spectra were processed using CASAXPS software.16 The background was subtracted according to the Shirley-Sherwood background subtraction routine17 prior to the curve fitting. This background subtraction routine was selected since this model is the most popular in practical surface analysis. It is worth to emphasise that Tougaard's background was also applied to the sputtered cleaned alloy and its composition was in excellent agreement with the one obtained by Shirley's background subtraction. Gaussian and Lorentzian (GL) product functions were used for curve fitting. Quantitative analysis of “as received” and sputter-cleaned brass surfaces were performed on the basis of the integrated intensity using the first-principle model3 assuming the sample homogeneity. The atomic concentrations of the etched samples were calculated using the equation:
 | (1) |
Quantitative analysis of the brass surfaces covered with a thin oxide film (e.g. after mechanical polishing) was performed according to the three-layer model,22 the thickness and composition of the oxide layer and the composition of the metal phase beneath the film could be calculated from a single XPS/XAES measurement.
3. Results
In the first part of this section the XPS results of reference materials are presented. In the second part, the XPS results of the Cu37Zn brass model alloy are shown.3.1. Reference materials
Copper. The Cu2p3/2 region is shown in Fig. ESI 1a (ESI†) and the data are listed in Table ESI 1.† The maximum of the signal was found at 932.5(0.1) eV in agreement with the literature.8,11–14 The CuL3M4.5M4.5 XAES signal (Fig. ESI 1b†) shows a complex fine structure. Peaks related to the 1S, 3P, 1D, 3F and 1G final states could be identified in agreement with literature.23–25 The most intense peak at 918.6 eV (labelled with A in Table ESI 1†) was assigned to the 1G multiplet of the two-localized-hole d8 final state,23,25 which splits in various multiplet states corresponding to structures seen in Fig. ESI 1b (ESI†). The peaks assigned to the 3P and 1D final state transitions were not resolved and contributed to one signal at 919.8(0.1) eV (peak labelled with D in Table ESI 1†) in agreement with literature.24,26,27 An additional peak detected at 916.5(0.1) eV could not be explained on the basis of theoretical calculations carried out on the structure of the copper Auger multiplet signals but according to some authors might be assigned to the presence of satellites.28,29 Their origin is still debated but this topic is behind the scope of this investigation. Parameters used for the curve fitting of this signal are provided in Table ESI 1.† Earlier, curve fitting of X-ray induced Auger signals was based on empirical approach.12–14
Zinc. The Zn2p3/2 signal acquired on sputtered zinc (Fig. ESI 2a†) showed a single peak at 1021.6(0.1) eV, the fit parameters are listed in Table ESI 2.† Similarly to copper also the ZnL3M4.5M4.5 Auger signal (Fig. ESI 2b†) exhibited a complex fine structure and the signals related to 1S, 3P, 1D, 3F and 1G final states were identified. The most intense peak was ascribed to 1G final state transition (KE = 992.1(0.1) eV), which is the most probable transition.24 The peaks assigned to the 3P and 1D final state transitions are not resolved and they form one signal at 993.5(0.1) eV. In agreement with the literature24,26 between the 1G and 1S signals an additional peak (KE = 989.6(0.1) eV) is present that could not be explained on the basis of theoretical calculations carried out on the structure of the zinc Auger multiplet signals and also in this case might be tentatively assigned to the satellite structure.28 Parameters used for the curve fitting of the spectra are provided in Table ESI 2.†
Copper oxide CuO. The survey spectra of CuO (not shown) showed all the characteristic signals of copper and oxygen. C1s peak was observed due to an adventitious contamination from air exposure. The most intense photoelectron peak of copper, Cu2p3/2, was found at a BE of 933.8(0.1) eV (Fig. ESI 3a†) and it was accompanied by two strong satellites at the high BE side of the main peak, due to a d9 characteristic configuration of compounds containing Cu(II) (3d9).23,28,29 The differences between the BE of the satellites and of the main peak were found to be +7.7(0.1) eV as also reported by other authors.5,13 The curve fitting parameters of Cu2p3/2 are listed in Table ESI 3.†
The CuL3M4.5M4.5 Auger signal (Fig. ESI 3b†) is quite different from those of the pure copper (Fig. ESI 1b†) due to the difference in the ground state, d9 for Cu(II) and d10 for Cu(0) and to the presence of the oxygen in the structure. It was found to be multicomponent and the kinetic energy (KE) of most intense peak (1G, labelled with A in Table ESI 3†) was 917.8(0.1) eV. The KE values of all the components and the curve fitting parameters of the Auger signal of CuO are presented in Table ESI 3.† The O1s high-resolution signal is provided in Fig. ESI 3c.† The O1s signal of the copper(II) oxide appears at 530.1(0.1) eV; an additional signal was observed at 531.7(0.1) eV due to the presence of hydroxide on the surface of the powder according to Chawla et al.30 In Table ESI 3† peak energy and fit parameters for oxygen are listed.
Copper oxide Cu2O. Besides the photoelectron and the Auger lines due to copper, also the signals attributable to oxygen and carbon were detected in the survey spectra of Cu2O. The carbon signal is likely due to the ambient exposure during the sample preparation and transfer into the spectrometer. The maximum of the Cu2p3/2 high-resolution spectrum (Fig. ESI 4a†) was found at 932.4(0.1) eV in agreement with ref. 13; curve-fitting parameters are provided in Table ESI 4.† Unlike CuO, the Cu2p3/2 spectrum of Cu2O did not show a satellite structure and the FWHM of the peak height is much lower than that of the signal of CuO. The CuL3M4.5M4.5 Auger spectra (Fig. ESI 4b†) exhibited a complex shape as a result of different final states. The KE values and the parameters used for the curve fitting of Cu2O Auger signal are presented in Table ESI 4.† The curve fitting was performed using four components in agreement with ref. 12–14 while for metallic copper five components were used. The main peak related to the 1G final state transition, which is the most probable transition, showed a KE of 916.8(0.1) eV. The O1s signal (Fig. ESI 4c†) was located at ca. 530 eV (Table ESI 4†). The fit resulted to be the convolution of three components: the most intense one at 530.3(0.1) eV due to the copper(I) oxide and the other two components which might be related to the presence of a thin organic contamination layer and to adsorbed water in the outer part of the sample surface.
Zinc oxide ZnO. The Zn2p3/2 signal (Fig. ESI 5a†) was found at 1021.6(0.1) eV, curve-fitting parameters are given in Table ESI 5.† The ZnL3M4.5M4.5 Auger peak (Fig. ESI 5b†) showed a general broadening and the satellite structure at low KE was less pronounced than in the metallic state, in agreement with ref. 9 and 11. The KE values and curve-fitting parameters for the individual signals are given in Table ESI 5.† The oxygen O1s signal of ZnO (Fig. ESI 5c†) showed three peaks, the curve-fitting parameters are given in Table ESI 5.†
The main result of this section on copper and zinc reference compounds is the theory based curve-fitting of the XAES signals of both metallic (Fig. ESI 1b and 2b†) and oxide compounds (Fig. ESI 3b and 4b†) of copper and zinc. Differences in kinetic energies and area ratios of the individual XAES signals were maintained constant, so XAES envelopes characterized by the KE and the intensity of the main peak could then be used in curve fitting of more complex systems such as brass alloys.
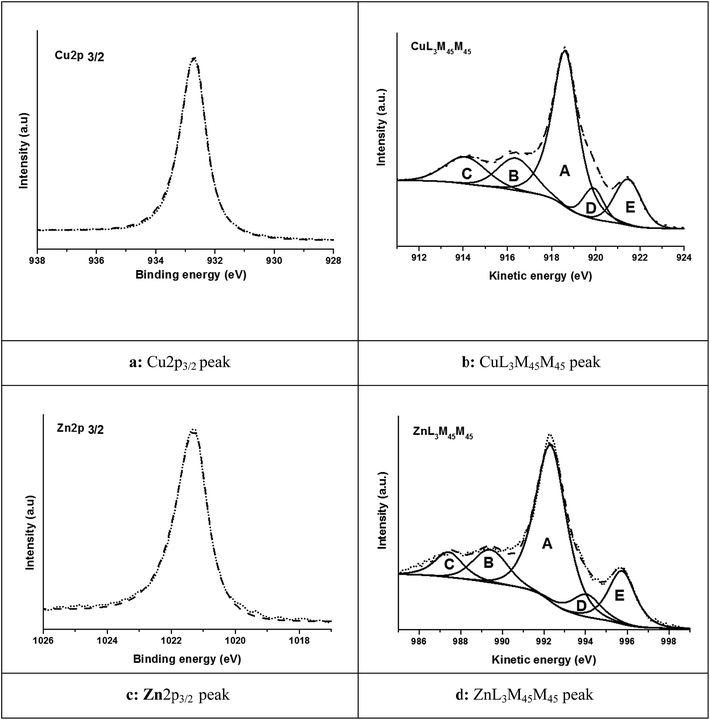
3.2. Brass model alloy Cu37Zn
The high-resolution spectra of Cu2p3/2, Zn2p3/2, CuL3M4.5M4.5, ZnL3M4.5M4.5 and O1s are shown in Fig. 1; the energy values and the fit parameters are listed in Table 1. The Cu2p3/2 spectrum (Fig. 1a) showed the characteristic shake-up satellites of a Cu2+ compound. The BE of 934.9(0.1) eV is 1.0 eV higher compared to CuO (Table ESI 3†). The reason might be ascribed to the presence of copper(II) hydroxide in agreement with other authors.11,13,30,31 The Zn2p3/2 signal at 1022.4(0.1) eV (Fig. 1c) is 0.8 eV more positive compared to the pure ZnO (Table ESI 5†); this might be due to the presence of zinc hydroxide as reported by ref. 1 and 2.
| Peak | BE (eV ±0.1) | FWHM (eV ±0.1) | Line shape |
|---|---|---|---|
| Cu2p3/2 | 934.8 | 2.4 | GL(70)T(1.5) |
| Sat 1 | 941.1 | 2.6 | GL(30) |
| Sat 2 | 944.3 | 2.6 | GL(30) |
| Zn2p3/2 | 1022.4 | 2.1 | GL(70) |
| O1s | 530.7 | 1.7 | GL(40) |
| O1s | 532.0 | 1.7 | GL(40) |
| O1s | 533.3 | 1.7 | GL(40) |
| Signal | KE (eV ±0.1) | Assignment | Intensity ratio | FWHM (eV ±0.1) | Line shape |
|---|---|---|---|---|---|
| Cu LMM | |||||
| A | 916.2 | 1G | 1 | 3.0 | GL(30) |
| B | 914.1 | 0.3 | 3.9 | GL(30) | |
| C | 911.7 | 1S | 0.1 | 3.9 | GL(30) |
| D | 917.5 | 3P/1D | 0.1 | 3.1 | GL(30) |
| E | 919.0 | 3F | 0.3 | 3.0 | GL(30) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Zn LMM | |||||
| A | 987.5 | 1G | 1 | 4.0 | GL(30) |
| B | 984.8 | 0.1 | 3.0 | GL(30) | |
| C | 982.3 | 1S | 0.03 | 2.0 | GL(30) |
| D | 989.0 | 3P/1D | 0.2 | 3.3 | GL(30) |
| E | 990.9 | 3F | 0.3 | 4.4 | GL(30) |
The Auger spectra of both copper CuL3M4.5M4.5 (Fig. 1b) and zinc ZnL3M4.5M4.5 (Fig. 1d) showed five components. Those spectra differ from those of the oxides of copper (Fig. ESI 3†) and zinc (Fig. ESI 4†): the spectra are noisier and their components are broader (higher FWHM). The KE of the main peak (A) of the copper CuL3M4.5M4.5 signal is shifted by 1.6 eV to lower values compared to CuO; the shift of ZnL3M4.5M4.5 with respect to ZnO is found to be −0.7 eV. Thus, approximately, the shift to more positive BE values of the 2p signals are equal to the shift to more negative kinetic energies of the Auger signals, resulting in the same Auger parameter (see discussion Section 4.3).
| Peak | BE (eV ±0.1) | FWHM (eV ±0.1) | Line shape |
|---|---|---|---|
| Cu2p3/2 | 932.7 | 0.9 | GL(97)T(2) |
| Zn2p3/2 | 1021.3 | 0.9 | GL(92)T(1.5) |
| KE (eV ±0.1) | Assignment | Intensity ratio | FWHM (eV ±0.1) | Line shape | |
|---|---|---|---|---|---|
| Cu LMM | |||||
| A | 918.6 | 1G | 1 | 1.4 | GL(80) |
| B | 916.4 | Sat. | 0.3 | 1.9 | GL(30) |
| C | 914.1 | 1S | 0.3 | 2.2 | GL(30) |
| D | 920.0 | 3P/1D | 0.1 | 1.0 | GL(30) |
| E | 921.4 | 3F | 0.3 | 1.4 | GL(30) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Zn LMM | |||||
| A | 992.3 | 1G | 1 | 1.7 | GL(70) |
| B | 989.5 | Sat. | 0.1 | 1.9 | GL(70) |
| C | 987.4 | 1S | 0.1 | 1.6 | GL(70) |
| D | 993.6 | 3P/1D | 0.2 | 1.6 | GL(70) |
| E | 995.7 | 3F | 0.4 | 1.5 | GL(70) |
The high-resolution spectra of copper (Fig. 2b) and zinc (Fig. 2d) Auger signals exhibited a complex fine structure similar to those of the metal ones (Fig. ESI 1b and 2b†). Table 2 presents the electron energy values, and the fit parameters related to the acquired spectra.
| Peak | BE (eV ±0.1) | FWHM (eV ±0.1) | Line shape |
|---|---|---|---|
| Cu2p3/2 | 932.5 | 1.3 | GL(70) |
| Zn2p3/2 | 1021.7 | 1.7 | GL(70) |
| O1s | 530.4 | 1.6 | GL(45) |
| O1s | 531.8 | 1.6 | GL(45) |
| O1s | 533.3 | 1.6 | GL(45) |
| Signal | KE (eV ±0.1) | Attribution |
|---|---|---|
| CuLMM metal | 918.5 | Envelope |
| CuLMM oxide | 916.8 | Envelope |
| ZnLMM metal | 988.0 | Envelope |
| ZnLMM oxide | 992.3 | Envelope |
Cu2p3/2 and Zn2p3/2 showed only one single peak (Fig. 3a and c) with the same BE as in pure copper and zinc. The XAES spectra of CuL3M4.5M4.5 (Fig. 3b) and ZnL3M4.5M4.5 (Fig. 3d) have a clearly different shape from those of pure metals and pure oxide compounds. In fact the copper and zinc Auger signals showed the simultaneous presence of the components assigned to the metal and to the oxides due to the presence of a thin oxide layer. The curve fitting of the CuL3M4.5M4.5 and ZnL3M4.5M4.5 Auger peaks of copper and zinc was performed using the envelopes of the metallic states (shown in Fig. ESI 1b and 2b†) and the oxidized ones (Fig. ESI 4c and 5c†). This is to our knowledge the first time that Cu and Zn Auger signals of complex layered systems have been resolved in their components.
4. Discussion
XPS surface analysis usually relies on the most intense photoelectron signals. As it is well known, copper Cu2p and zinc Zn2p photoelectron signals do not exhibit a chemical shift between metallic and oxidized state. The BE values of metallic and oxidized zinc are reported at 1021.6 eV, and for metallic copper and Cu2O the BE (932.5 eV in both cases) do not allow the univocal identification of the two states. Several papers4,6,11–14 proposed thus the use of X-ray induced Auger signals (XAES) where clear shifts in KE and changes in the shape of the spectra are found between metals and oxides. Regarding the quantitative analysis of copper–zinc alloys (brasses) with an oxidized surface layer in the nano-meter range literature only reports few examples.11–14 In the following a novel approach for univocal assignment to the various copper and zinc chemical states when they are simultaneously present is described together with the description of a quantitative analysis method to be followed when a thin-layer of copper and zinc oxy-hydroxides is present on the surface of the alloy.4.1. Curve fitting of X-ray induced Auger spectra
The XAES signals of pure copper and zinc metals and oxides are composed of several peaks that were here assigned following the results of the theoretical approach reported in literature predicting the differences in KE and intensity ratio (Table ESI 1–5†).7,9,23,26,29 In this work the spectra of metallic copper and zinc (Fig. ESI 1b and 2b†) as well as copper and zinc oxide reference compounds (Fig. ESI 3b, 4b and 5b†) have been measured and processed. Each spectrum (envelope) could be reproduced based on the individual components and then used for the subsequent curve fitting of complex signals. A similar but empirically based approach for curve fitting of X-ray induced Auger signals of copper and zinc was proposed by other authors but the best results were reported by ref. 12–14 where reference spectra of various compounds were characterized under controlled conditions.Also the signals of pure zinc (Fig. ESI 2b†) and zinc in the brass alloy (Fig. 2d) are similar. A slight shift of the KE of the main peak (A) assigned to 1G of +0.2 eV is found (compare Table ESI 2† with Table 2). The energy difference and the intensity ratios between the individual signals are reasonably identical for pure zinc (Table ESI 2†) and zinc in the sputtered alloy (Table 2).
Regarding the binding energies, BE of copper Cu2p3/2 is shifted by +0.2 eV in the alloy and BE of zinc Zn2p3/2 by −0.3 eV. The same results were found in studies of Cu–Zn alloys.11,32 These small differences can be attributed to changes in the electronic environment going from the pure metal to the alloy. The lattice parameter of the cubic face centred lattice increases from 3.608 Å for pure copper to 3.696 Å for alloys containing 37% of zinc. Hence, the electron distribution on each atom can be expected different for pure copper and for the Cu37Zn alloy. Positive shifts for the BE and (nearly equal) negative shifts for the KE result in the same Auger parameter α′, thus in similar final state effects.
A comparison of the XAES signals for copper oxide Cu2O (Fig. ESI 4b†), CuO (Fig. ESI 3b†) and the copper oxi-hydroxides of the “as received” brass alloy (Fig. 1b) showed clear differences, thus at the surface of the “as received” alloy a different copper-compound is likely to be present. Based both on the Cu2p3/2 (Fig. 1a) and the CuL3M4.5M4.5 signal (Fig. 1b) the presence of mainly copper hydroxide in the outer part of the surface film can be envisaged in agreement with what already reported in ref. 13. Using the chemical state plot (Section 4.3) the species present at the surface of the as received alloy will be identified.
4.2. Quantitative analysis
Quantitative analysis in a system such as brass alloy with a thin oxidized surface film is not possible because the copper and zinc 2p signals do not distinguish between metallic and oxidized states. Only the X-ray excited Auger signals allow a clear and quantitative distinction between metallic and oxidized state (Fig. 3b and d). Here a procedure and a formula that allows transfer the experimental intensity ratio from the LMM to the (unknown) intensity ratio of the 2p signals is presented.The experimentally determined intensity ratio R (Table 4) is, as expected, different for copper and zinc, but it also results different for the metallic (Rmet) and the oxidized (Rox) state. Thus, the common assumption of an element specific sensitivity factor S or cross section σ for a given transition independent on its chemical environment cannot be here applied. In addition the values of R depend on the geometry of the instrument (Table 4). In order to calculate the surface composition independently on the spectrometer geometry, it is here proposed a correction factor k, defined as k = Rox/Rmet (Table 4). Indeed, the correction factors k are independent on the instrument and k = 1.5 for copper and k = 0.8 for zinc have been found (Table 4).
| System | Source | Intensity ratio R 2p/LMM | k = Rox/Rmet | ||
|---|---|---|---|---|---|
| Theta | Quantera | Theta | Quantera | ||
| Cu metal | Pure metal | 3.4 | 2.0 | 1.56 | 1.5 |
| Cu metal | Sputtered alloy | — | 1.9 | — | 1.55 |
| CuO | Pure oxide | 5.3 | 3.0 | 1.56 | 1.5 |
| Cu-oxide | As rec. corrected | 5.1 | 3.0 | 1.5 | 1.5 |
| Cu-oxide | As received alloy | 2.5 | 1.5 | 0.74 | 0.75 |
| Zn metal | Pure metal | 3.8 | 2.3 | 0.79 | 0.74 |
| Zn metal | Sputtered alloy | — | 2.0 | — | 0.87 |
| ZnO | Pure oxide | 3.0 | 1.7 | 0.79 | 0.74 |
| ZnO | As rec. corrected | 3.2 | 0.83 | ||
| Zn-oxide | As received alloy | 1.4 | 1.2 | 0.36 | 0.52 |
The experimentally determined ratios R between the photoelectron intensity I2p and the Auger signal intensity ILMM for the “as received” Cu37Zn alloy were found to be very different from CuO (Table 4). This is due to the different attenuation of the photoelectrons (ca. KE = 552 eV) and of the X-ray excited Auger electrons (ca. KE = 917 eV) by the thick contamination layer on the as-received alloy. Assuming 5 nm contamination (Table 8), the attenuation of the 2p photoelectrons is twice as strong as for the X-ray excited Auger. The attenuation corrected ratio R is within the range of the pure oxide and, as a consequence, also the k value is identical (Table 4). The same reasoning holds for the zinc signals.
For brass alloys with a thin surface film (as example the mechanically polished sample, Fig. 3) the CuL3M4.5M4.5 (Fig. 3b) and ZnL3M4.5M4.5 (Fig. 3d) spectra allow determining the intensity of the metallic and the oxide contribution. From these intensities the intensity ratio r = LMMox/LMMmet is calculated. For copper this was found r equal to 1 while for zinc it was found equal to 4 (Table 5). The experimentally determined intensity ratio in the X-ray induced Auger signals, r, and the correction factor k (from Table 4) allow calculate the corresponding intensity ratio for the 2p signals. The equation derived (appendix A) for the calculation is:
 | (2) |
| Metal | % ox LMM | % met LMM | Ratio r ox/met | Correction factor k | % ox in 2p | Iox 2p | Imet 2p |
|---|---|---|---|---|---|---|---|
| Copper | 50 | 50 | 1 | 1.55 | 61 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 066 066 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 206 206 |
| Zinc | 80 | 20 | 4 | 0.8 | 76 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 269 269 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 068 068 |
The calculated percentage of oxidized and metallic compound in the 2p signal is shown in Fig. 4 for both copper and zinc as a function of the input data r = LMMox/LMMmet.
 | ||
| Fig. 4 Percentage of oxidized component in the 2p signal versus percentage of the oxidized component in the LMM signal for copper and zinc. | ||
Table 5 reports the input parameters and the corresponding calculated results. It can be noted that for copper the intensity of the oxide increases by ca. 20% (from 50 to 60%) whereas for zinc the intensity of the metal increases by 20% (from 20 to 24%).
The theoretical background of this empirical approach, especially of the intensity ratio R = I2p/ILMM (Table 4) can be discussed. While the intensity ratios of different X-ray excited Auger signals, e.g. L23M4.5M4.5/L23M4.5M4.5 for Ni, Cu and Zn could be related to the effective charge Δq on the metal atom in the different compounds,32,33 such a theoretical basis for the intensity ratio between 2p photoelectron and LMM X-ray excited Auger electrons signals is lacking. An attempt to use intensity ratio between 2p photoelectrons and LMM Auger electrons was made to quantify Cu2O on Cu.12 In later studies13,14 the ratio of experimental intensities I2p/ILMM was used to determine the sensitivity factor for the Auger signals σLMM of copper and zinc, assuming this factor to be the same for metallic and oxidized compounds. As in this work Rox and Rmet are different both for copper and zinc (see Table 4) meaning that also the calculated cross sections σLMM,ox and σLMM,met are different. The assumption of equal cross section12–14 does not hold for complex layered systems.
Only the experimentally determined factor k = Rox/Rmet as proposed in this work, is characteristic for an element (copper, zinc), reasonably constant and independent on the instrument used for the analysis. Thus the factor k can be used to convert ratios LMMox/LMMmet resulted from the curve fitting of X-ray excited Auger signals (Fig. 3b and d) to intensity ratios of the photoelectron signal 2pox/2pmet needed for obtaining the intensities of each metallic and oxidized component. These data are required for the quantitative analysis of nano-structured layered systems (see Section 4.2.3.).
Sputtered Cu37Zn model brass alloy. After slight Ar+ sputtering no oxides were detected on the surface of the alloy here examined: both Cu2p and Zn2p photoelectron signals and corresponding XAES signals (Fig. 2) refer to the metallic state only. Quantitative analyses were performed following the first principle model and the results are summarized in Table 6. The difference with the nominal composition can be ascribed to a preferential sputtering effect that was already reported in ref. 34.
| Point | Contamination | Cu met | Zn met |
|---|---|---|---|
| 1 | nd | 74% | 26% |
| 2 | nd | 80% | 20% |
Cu37Zn model alloy “as received”. In the as received state, even after a very slight sputtering to only remove the contamination layer, no signals from the underlying alloy were detected. Thus both the Cu and Zn2p photoelectron signals and the XAES signals (Fig. 1) refer to the oxidized state only. Quantitative analysis has been performed following the first principle approach. The results are presented in Table 7.
| Point | Oxide film | Contamination | Cu ox | Zn ox |
|---|---|---|---|---|
| 1 | >10 nm | 4.5 nm | 83 | 17 |
| 2 | >10 nm | 3.8 nm | 84 | 16 |
| 3 | >10 nm | 4.5 nm | 85 | 15 |
| 4 | >10 nm | 4.0 nm | 83 | 17 |
Mechanically polished Cu37Zn model brass alloy. The XAES spectra of both copper (Fig. 3b) and zinc (Fig. 3d) show the presence of metallic and oxidized components, indicating a thin oxide layer on the brass surface. XAES areas were used to calculate the ratio r of metallic and oxidized copper and zinc respectively (Table 5). The intensities of the metallic and oxidized components in the 2p photoelectron signal were calculated following the approach outlined above in Section 4.2.1., results of one example are provided in Table 5.
Once the photoelectron intensities ICu,met, ICu,ox, IZn,met and IZn,ox and of the contamination Ic are known (Table 5), the quantitative analysis of such a thin-layered system was performed following the three-layer model,22,35,36 assuming the presence of a contamination layer and of a homogeneous oxide film on top of the alloy. The density for Cu37Zn alloy was taken as 8.5 g cm−3, the density of the oxide film was assumed to be 6 g cm−3 (6.14 g cm−3 for Cu2O and 5.61 g cm−3 for ZnO). The results of the quantitative analysis of the mechanically polished Cu37Zn alloy obtained for three different analysis points are illustrated in Table 8.
| Area of analysis | Oxide film thickness (nm) | Contamination layer thickness (nm) | Cu ox wt% | Zn ox wt% | Cu met wt% | Zn met wt% |
|---|---|---|---|---|---|---|
| 1 | 1.9 | 0.7 | 61 | 39 | 63.4 | 36.6 |
| 2 | 1.5 | 1.7 | 70 | 30 | 62.6 | 37.4 |
| 3 | 0.5 | 2.4 | 69 | 31 | 65.8 | 34.2 |
They are consistent: a thin oxide film is present on the surface of the alloy. The oxide film is rich in copper oxide (Cu2O) and contains about 33% of zinc oxide (ZnO). The alloy composition beneath the oxide film is in average very close to the nominal composition, 64.5(1.5)% copper and 35.5(1.5)% zinc. One of three samples even shows 37% zinc.
4.3. Chemical state plot
The chemical state identification using XPS surface analysis has became routine for most of the elements in the periodic table.4 However, for copper and zinc the chemical state identification based on the BE only is challenging since it is not possible distinguish between Zn(II) and Zn(0) in the Zn2p and between Cu(I) and Cu(0) in the Cu2p spectra.4 While the photoelectron signals of copper and zinc showed the same BE, the Auger signals of both elements are different from their pure elements (Fig. ESI 1–5†). Moretti11 has shown that the chemical state plot and the modified Auger parameter concept are particularly important for copper and zinc compounds because the two dimensional representation of photoelectron BE versus XAES KE, Wagner chemical state plot, allows a more accurate assignment. Other examples of successful application are sulphur compounds in sulphur containing minerals,8 the chemical state of phosphorus in amorphous alloys,37 phosphate glasses38 or in nickel–phosphorus coatings.39| BEphotoelectron | KEAuger | α′ | α′a | |
|---|---|---|---|---|
| Copper | ||||
| Cu(0) | 932.5 | 918.6 | 1851.1 | 1851.1 |
| Cu2O | 932.4 | 916.8 | 1849.2 | 1849.2 |
| CuO | 933.9 | 917.8 | 1851.7 | 1851.7 |
| Cu37Zn mech pol met | 932.5 | 918.5 | 1851.0 | 1851.0 |
| Cu37Zn mech pol ox | 932.5 | 916.8 | 1849.3 | — |
| Cu37Zn as received | 935.0 | 916.2 | 1851.2 | 1851.2 |
| Cu37Zn sputtered | 932.7 | 918.6 | 1851.3 | 1851.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Zinc | ||||
| Zn(0) | 1021.6 | 992.1 | 2013.7 | 2013.7 |
| ZnO | 1021.3 | 988.1 | 2009.4 | 2009.4 |
| Cu37Zn mech pol ox | 1021.7 | 988.0 | 2009.7 | 2009.7 |
| Cu37Zn mech pol met | 1021.7 | 992.3 | 2014.1 | — |
| Cu37Zn as received | 1022.4 | 987.7 | 2010.1 | 2010.1 |
| Cu37Zn sputtered | 1021.7 | 992.3 | 2014.1 | 2014.1 |
The copper signal of the sputtered alloy Cu37Zn coincides with that of the metallic copper. Copper in the “as received” alloy is found close to the Cu(OH)2 standard, confirming that the outmost surface is mainly of copper hydroxide. The mechanically polished alloy showed two points: one that coincides with metallic copper, the other with Cu2O. This indicates that on top of the alloy a thin oxide film is present (see Section 4.2.3.). If, as very often is done, only the most intense Auger line of copper (918.5(0.1) eV, Fig. 3b) were used for speciation, the presence of Cu2O would not have been revealed.
5. Conclusions
The following conclusions can be drawn from this surface analytical work:(1) A new analytical strategy for simultaneous chemical state identification and quantification of copper and zinc in complex, thin-layered systems has been established.
(2) Curve fitting of the multicomponent X-ray excited Auger spectra of copper CuL3M4.5M4.5 and zinc ZnL3M4.5M4.5 allows the discrimination of the different chemical states (e.g. metallic and oxide components) in complex systems.
(3) The kinetic energies of the curve fitted Auger signals allow an unambiguous assignment of the chemical state of copper and zinc compounds with the chemical state plot.
(4) Standards of pure metals and oxides are essential to accurately determine the envelopes of the complex Auger signals for curve fitting and to calculate the experimental intensity ratios R = I2p/ILMM between photoelectron and Auger intensity required for quantification.
(5) The experimentally determined quantification factor k = Rox/Rmet as proposed in this work is characteristic for an element (copper, zinc), reasonably constant and independent on the instrument used for the analysis.
(6) The composition of the alloy beneath the thin oxide film of mechanically polished samples of Cu37Zn calculated with the new analytical strategy presented in this paper is found to be very close to the nominal composition.
(7) The new analytical approach for quantification has been tested on Cu37Zn model brass alloys after different surface pre-treatments and has proven to be successful.
Appendix A
Derivation of eqn (2)Definitions
R experimentally determined intensity ratio 2p/LMM (see Table 4)
k experimentally determined constant k = Rox/Rmet (see Table 4)
rLMM experimentally determined intensity ratio in the Auger signal rLMM = I(LMM)ox/I(LMM)met
r2p unknown ratio in the 2p photoelectron signal r2p = I(2p)ox/I(2p)met
| r2p = I(2p)ox/I(2p)met |
| % (2p)ox = 100 × r2p/(1 + r2p) |
| r2p = k × rLMM |
| % (2p)ox = 100 × k × rLMM/(1 + k × rLMM) thus eqn (2) in the paper. |
Acknowledgements
The Italian Ministry of University and Research (MIUR) is thanked for financing the PRIN project prot. 2010329WPF_005 “Sustainability in cultural heritage: from diagnosis to the development of innovative systems for consolidation, cleaning and protection”. The authors wish to express their gratitude to Istituto Nazionale di Previdenza Sociale (National Social Security Institution), for its support of this work. Sardinia Regional Government is gratefully acknowledged for the financial support (P.O.R. Sardegna F.S.E. Operational Program of the Regione Autonoma della Sardegna, European Social Fund 2007–2013 – Axis IV Human Resources, Objective 1.3, Line of Activity 1.3.1 “Avviso di chiamata per il finanziamento di Assegni di Ricerca”). The authors wish to express their gratitude to Prof. Nicholas D. Spencer (Laboratory for Surface Science and Technology ETH Zürich, Switzerland) for the access to the Quantera. Mr Cossu is acknowledged for the technical maintenance of the spectrometers.References
- NIST Standard Reference Database 20 Version 4.1, accessed November 2015, http://srdata.nist.gov/xps Search PubMed.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-ray Photoelectron Spectroscopy, Perkin-Elmer Corp., Physical electronics, Minnesota, 1992 Search PubMed.
- D. Briggs and J. T. Grant, Surface Analysis by Auger and X-ray Photoelectron Spectroscopy, IM Publications and Surface Publication Data, West Sussex, 2003 Search PubMed.
- M. C. Biesinger, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2010, 257, 887–898 CrossRef CAS.
- D. C. Frost, A. Ishitani and C. A. McDowell, Mol. Phys., 1972, 24, 861–877 CrossRef CAS.
- P. E. Larson, J. Electron Spectrosc. Relat. Phenom., 1974, 4, 213–218 CrossRef CAS.
- I. Platzman, R. Brener, H. Haick and R. Tannenbaum, J. Phys. Chem. C, 2008, 112, 1101–1108 CAS.
- M. Fantauzzi, D. Atzei, B. Elsener, P. Lattanzi and A. Rossi, Surf. Interface Anal., 2006, 38, 922–930 CrossRef CAS.
- G. Deroubaix and P. Marcus, Surf. Interface Anal., 1992, 18, 39–46 CrossRef CAS.
- E. Antonides and G. A. Sawatzky, J. Phys. C: Solid State Phys., 1976, 9, 547–552 CrossRef.
- G. Moretti, J. Electron Spectrosc. Relat. Phenom., 1998, 95, 95–144 CrossRef CAS.
- H. D. Speckmann, S. Haupt and H. H. Strehblow, Surf. Interface Anal., 1988, 11, 148–155 CrossRef CAS.
- P. Druska and H. H. Strehblow, Surf. Interface Anal., 1995, 23, 440–450 CrossRef CAS.
- I. Milosev and H. H. Strehblow, J. Electrochem. Soc., 2003, 150, 517–524 CrossRef.
- B. Elsener, M. Alter, T. Lombardo, M. Ledergerber, M. Wörle, F. Cocco, S. Palomba, M. Fantauzzi and A. Rossi, Microchem. J., 2016, 124, 757–764 CrossRef CAS.
- N. Fairley, CASAXPS, Version 2.3.15, Casa Software Ltd, Wilmslow, UK, 2010 Search PubMed.
- D. A. Shirley, Phys. Rev. B: Solid State, 1972, 5, 4709–4714 CrossRef.
- J. H. Scofield, J. Electron Spectrosc. Relat. Phenom., 1976, 8, 129–137 CrossRef CAS.
- R. F. Reilman, A. Msezane and S. T. Manson, J. Electron Spectrosc. Relat. Phenom., 1976, 8, 389–394 CrossRef CAS.
- M. Fantauzzi, A. Pacella, J. Fournier, A. Gianfagna, G. B. Andreozzi and A. Rossi, Anal. Bioanal. Chem., 2012, 404, 821–833 CrossRef CAS PubMed.
- S. Tanuma, C. J. Powell and D. R. Penn, Surf. Interface Anal., 2003, 35, 268–275 CrossRef CAS.
- A. Rossi and B. Elsener, Surf. Interface Anal., 1992, 18, 499–504 CrossRef CAS.
- S. R. Barman and D. D. Sarma, J. Phys.: Condens. Matter, 1992, 4, 7607–7616 CrossRef CAS.
- S. P. Kowalczyk, R. A. Pollak, F. R. McFeely, L. Ley and D. A. Shirley, Phys. Rev. B: Solid State, 1973, 8, 2387–2391 CrossRef CAS.
- J. Ghijsen, L. H. Tjeng, J. van Elp, H. Eskes, J. Westerink, G. A. Sawatzky and M. T. Czyzyk, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 38, 11322–11330 CrossRef CAS.
- E. Antonides, E. C. Janse and G. A. Sawatzky, Phys. Rev. B: Solid State, 1977, 15, 1669–1679 CrossRef CAS.
- E. D. Roberts, P. Weightman and C. E. Johnson, J. Phys. C: Solid State Phys., 1975, 8, L301–L304 CrossRef CAS.
- G. G. Kleiman, J. Electron Spectrosc. Relat. Phenom., 1999, 100, 17–34 CrossRef CAS.
- G. G. Kleiman, R. Landers, P. A. P. Nascente and S. G. C. de Castro, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 4405–4413 CrossRef CAS.
- S. K. Chawla, N. Sankarraman and J. H. Payer, J. Electron Spectrosc. Relat. Phenom., 1992, 61, 1–18 CrossRef CAS.
- N. S. McIntyre and M. G. Cook, Anal. Chem., 1975, 47, 2208–2213 CrossRef CAS.
- J. A. Rodriguez and J. Hrbek, J. Vac. Sci. Technol., 1993, 11, 1998–2002 CrossRef CAS.
- S. Yashonath and M. S. Hedge, J. Chem. Sci., 1980, 89, 489–494 CAS.
- G. E. Hammer and R. M. Shemenski, Surf. Interface Anal., 1987, 10, 355–359 CrossRef CAS.
- D. De Filippo, A. Rossi, B. Elsener and S. Virtanen, Surf. Interface Anal., 1990, 15, 668–674 CrossRef CAS.
- A. Rossi and B. Elsener, Mater. Sci. Forum, 1995, 185–188, 337–346 CrossRef CAS.
- B. Elsener and A. Rossi, Electrochim. Acta, 1992, 37, 2269–2276 CrossRef CAS.
- M. Crobu, A. Rossi, F. Mangolini and N. D. Spencer, Anal. Bioanal. Chem., 2012, 403, 1415–1432 CrossRef CAS PubMed.
- B. Elsener, D. Atzei, A. Krolikowski, V. Rossi Albertini, C. Sadun, R. Caminiti and A. Rossi, Chem. Mater., 2004, 16, 4216–4225 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23135c |
| This journal is © The Royal Society of Chemistry 2016 |