Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mixed-linker approach in designing porous zirconium-based metal–organic frameworks with high hydrogen storage capacity†
Ayesha
Naeem
a,
Valeska P.
Ting
b,
Ulrich
Hintermair
c,
Mi
Tian
b,
Richard
Telford
a,
Saaiba
Halim
a,
Harriott
Nowell
d,
Małgorzata
Hołyńska
e,
Simon J.
Teat
f,
Ian J.
Scowen
g and
Sanjit
Nayak
 *a
*a
aSchool of Chemistry and Forensic Sciences, University of Bradford, Richmond Road, Bradford, West Yorkshire BD7 1DP, UK. E-mail: s.nayak@bradford.ac.uk
bDepartment of Chemical Engineering, University of Bath, Claverton Down, Bath, BA2 7AY, UK
cCentre for Sustainable Chemical Technologies, University of Bath, Caverton Down, Bath, BA2 7AY, UK
dDiamond Light Source, Didcot, Oxon OX11 0DE, UK
eFachbereich Chemie and Wissenschaftliches Zentrum für Materialwissenschaften, Philipps Universität Marburg, Hans-Meerwein-Straße, 35043 Marburg, Germany
fAdvanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
gSchool of Chemistry, University of Lincoln, Brayford Pool, Lincoln, Lincolnshire LN6 7TS, UK
First published on 17th May 2016
Abstract
Three highly porous Zr(IV)-based metal–organic frameworks, UBMOF-8, UBMOF-9, and UBMOF-31, were synthesized by using 2,2′-diamino-4,4′-stilbenedicarboxylic acid, 4,4′-stilbenedicarboxylic acid, and combination of both linkers, respectively. The mixed-linker UBMOF-31 showed excellent hydrogen uptake of 4.9 wt% and high selectivity for adsorption of CO2 over N2 with high thermal stability and moderate water stability with permanent porosity and surface area of 2552 m2 g−1.
Hydrogen has gained enormous attention as a promising energy vector in renewable energy conversion schemes due to its environmentally friendly nature; it can be cleanly produced by electrolysis of water, and water is the main by-product of H2 combustion.1 However, especially for applications in automobiles, storage and transportation of hydrogen is a major challenge due to its very low boiling point and low volumetric density in gaseous form.2–4 Metal–organic frameworks (MOFs) with very high surface areas are promising candidates for tackling this problem by adsorptive storage of hydrogen to achieve higher hydrogen densities under acceptable conditions.5–8 Research in this area has indicated that this class of materials has the potential for meeting the systems’ H2 storage capacity benchmark of 5.5 wt% set for 2017 by the US Department of Energy (DOE).9 Despite the fact that a large number of MOFs have been studied for hydrogen storage properties,2,6,8,10,11 the problem of low thermal and water stabilities of many MOFs is a major issue for practical applications.12 Newly developed Zr-based MOFs with archetypical [Zr6O4(OH)4(CO2)] secondary building units (SBUs) are highly promising for this purpose, due to their exceptional thermal and water stabilities,13–16 and recent studies have shown that UiO-67 (UiO: University of Oslo) can attain up to 4.6 wt% H2 storage at 3.8 MPa.14
Here we report three Zr(IV)-based MOFs using 4,4′-stilbenedicarboxylic acid and 2,2′-diamino-4,4′-stilbenedicarboxylic acid as linkers, with one of them synthesized using a non-traditional mixed-linker approach, showing one of the highest (up to 4.9 wt%) hydrogen uptakes measured among the Zr-based MOFs reported to date.14,15,17,18 In addition, high selectivity of CO2 adsorption over nitrogen was also observed for UBMOF-31. Solvothermal reactions of 2,2′-diamino-4,4′-stilbenedicarboxylic acid (DASDCAH2) and 4,4′-stilbenedicarboxylic acid (SDCAH2) with ZrCl4 and benzoic acid (modulator) in a mixture of dimethylformamide (dmf) and N-methyl-2-pyrrolidone (NMP) (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
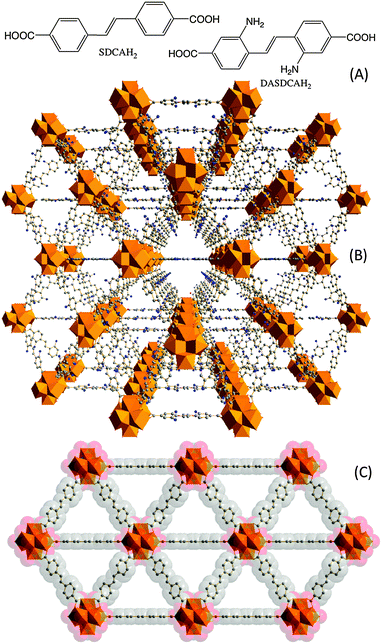
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) produced [Zr6O4(OH)4(DASDCA)6](PhCOOH)m(dmf)x(H2O)y(NMP)z (UBMOF-8) and [Zr6O4(OH)4(SDCA)6](PhCOOH)n(dmf)p(H2O)q(NMP)r (UBMOF-9), respectively, where UBMOF refers to University of Bradford Metal–Organic Framework (Fig. 1).19 The actual amount of solvent in the lattice varies, and it was not determined experimentally for these compounds. Both these syntheses resulted in crystals suitable for structural analysis by single crystal X-ray diffraction. Both these MOFs are formed of octahedral [Zr6O4(OH)4(CO2)] cluster units connected by twelve DASDCA or SDCA linkers similar to UiO-66.13 Use of these longer linkers results in large diagonal cage dimensions of approximately 25 Å and equilateral triangular faces with side lengths of 17.7 Å (Fig. 1 and Fig. S3.1 in ESI†). Structural analyses using the OLEX-2 program suite indicate solvent accessible volumes of 71.9% and 75.3% for UBMOF-8 and UBMOF-9, respectively, with a probe of radius 1.2 Å.20
1, v/v) produced [Zr6O4(OH)4(DASDCA)6](PhCOOH)m(dmf)x(H2O)y(NMP)z (UBMOF-8) and [Zr6O4(OH)4(SDCA)6](PhCOOH)n(dmf)p(H2O)q(NMP)r (UBMOF-9), respectively, where UBMOF refers to University of Bradford Metal–Organic Framework (Fig. 1).19 The actual amount of solvent in the lattice varies, and it was not determined experimentally for these compounds. Both these syntheses resulted in crystals suitable for structural analysis by single crystal X-ray diffraction. Both these MOFs are formed of octahedral [Zr6O4(OH)4(CO2)] cluster units connected by twelve DASDCA or SDCA linkers similar to UiO-66.13 Use of these longer linkers results in large diagonal cage dimensions of approximately 25 Å and equilateral triangular faces with side lengths of 17.7 Å (Fig. 1 and Fig. S3.1 in ESI†). Structural analyses using the OLEX-2 program suite indicate solvent accessible volumes of 71.9% and 75.3% for UBMOF-8 and UBMOF-9, respectively, with a probe of radius 1.2 Å.20
Unlike UBMOF-9, UBMOF-8 did not produce a phase pure material despite extensive efforts to optimise the synthetic conditions. An unidentified amorphous phase was present alongside the crystalline material for UBMOF-8 (Fig. 2), as evidenced by a noticeable amorphous halo in the PXRD pattern (Fig. 3A). As an alternate route to access the pure isostructural MOF with amino groups, we took advantage of the similar coordination behaviours of both linkers and experimentally optimised the ratio of the two linkers. This mixed linker approach resulted in a crystalline phase pure material (UBMOF-31) isostructural to UBMOF-8 and UBMOF-9 when DASDCAH2 and SDCAH2 were used in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 molar ratio. The empirical formula for desolvated UBMOF-31, [Zr6O4(OH)4(DASDCA)1.4(SDCA)4.6] (PhCOOH)0.78, was determined from the combination of data obtained from elemental analysis, thermogravimetric analysis (TGA) and NMR spectroscopy (see the ESI†).
2.5 molar ratio. The empirical formula for desolvated UBMOF-31, [Zr6O4(OH)4(DASDCA)1.4(SDCA)4.6] (PhCOOH)0.78, was determined from the combination of data obtained from elemental analysis, thermogravimetric analysis (TGA) and NMR spectroscopy (see the ESI†).
 | ||
| Fig. 2 SEM images of bulk phases of (A) UBMOF-8, (B) UBMOF-9, and (C) UBMOF-31, and their closeup views D, E, and F, respectively. | ||
The calculated and measured PXRD patterns match closely, suggesting a phase pure crystalline material for the pristine sample UBMOF-9 (Fig. 3B). The single crystals of UBMOF-8 produced along with powdery amorphous materials can be observed in the SEM images (see the ESI†). The discrepancy of elemental analysis (see the ESI†) for UBMOF-8 further suggests the presence of impurity in the bulk material. The residual amounts of modulator (PhCOOH) inside the MOFs were estimated for the solvent exchanged and dried samples (see the ESI†). In the IR spectra, the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency (1685 cm−1) for non-coordinate benzoic acid indicates their origin as guest molecules, not from defects. The flexibility of the linkers possibly plays a role in minimizing the defects for this class of MOFs, as was observed for another family of isostructural Zr-based MOFs with varied flexibility.21 In addition, the presence of extensive disorder in the crystal structures restricts any further investigation on the accurate determination of defects in these materials.22
O stretching frequency (1685 cm−1) for non-coordinate benzoic acid indicates their origin as guest molecules, not from defects. The flexibility of the linkers possibly plays a role in minimizing the defects for this class of MOFs, as was observed for another family of isostructural Zr-based MOFs with varied flexibility.21 In addition, the presence of extensive disorder in the crystal structures restricts any further investigation on the accurate determination of defects in these materials.22
It is evident from the literature that the presence of amino groups on the linkers facilitates hydrogen adsorption in MOFs.23 This motivated us to explore an alternate route to produce a phase pure amino functionalized isostructural MOF by a mixed-linker approach with controlled use of both linkers to synthesize UBMOF-31 with the DASDCAH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SDCAH2 ratio of 1.4
SDCAH2 ratio of 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.6. A further increase of DASDCAH2 resulted in amorphous impurity as originally observed for UBMOF-8. The infrared spectra (see the ESI†) of all three fresh compounds indicate the presence of a large amount of dmf with an intense peak at 1654 cm−1, which diminished upon washing with acetone and activation by supercritical CO2 during gas adsorption studies. The shoulder peaks around 3670 cm−1 possibly originate from the OH groups as suggested in the literature.24 Thermal stabilities of the three MOFs were studied by TGA in air (see the ESI†) at a heating ramp of 5 °C per minute. All three freshly prepared, air-dried MOFs show a large initial drop in weight (40–50%) due to solvent loss, followed by a stable plateau until they decompose at approximately 494 °C (UBMOF-8), 522 °C (UBMOF-9), and 508 °C (UBMOF-31), indicating thermal stabilities comparable with UiO-66.13,14 TGAs of de-solvated and vacuum treated samples were carried out to estimate the linker ratios for UBMOF-31 and the guest modulator, in combination with elemental analysis and NMR spectroscopy (see the ESI†). The PXRD pattern in Fig. 3A suggests that UBMOF-31 is phase pure and isostructural to UBMOF-8 and UBMOF-9 as expected. PXRD analyses of the samples revealed the stability of UBMOF-9 and UBMOF-31 under different conditions as shown in Fig. 3. Both these materials retain their original structures when treated with acetone for 24 hours at 20 °C, after activation using supercritical CO2, and after high-pressure adsorption studies with N2, H2, and CO2. When treated with water at 20 °C for 24 hours, both (UBMOF-8 and UBMOF-31) retain their shiny crystalline morphology to some extent as visually inspected (see the ESI†) and evidenced by PXRD (Fig. 3), with a small shift in 2θ for the low-angle peaks for UBMOF-9. However, UBMOF-31 mostly retains its original PXRD pattern, indicating better water stability compared to UBMOF-9. The hydrothermal treatment (100 °C) of both the MOFs over 24 hours resulted in broadening of the low-angle peaks leaving no visible high-angle peaks, indicating significant loss of crystallinity of the materials. The surface areas of the materials were determined by Brunauer–Emmett–Teller (BET) analysis with N2 at 77 K using a Micromeritics 3-Flex volumetric gas sorption analyser (P/Po range: 0.05–0.3) following the British Standard guidelines.25 A sample of UBMOF-9 that had been solvent-exchanged with acetone and degassed at 150 °C for 8 hours under dynamic high vacuum (10−7 mbar) prior to analysis displayed a surface area of 1169 m2 g−1. In order to access the true intrinsic porosity of these materials, the samples were solvent exchanged and subsequently subjected to supercritical CO2 (scCO2) extraction.26,27 The scCO2-activated samples showed BET surface areas of 2667 m2 g−1 and 2552 m2 g−1 for UBMOF-9 and UBMOF-31, respectively, more than twice the value obtained for the thermally dried samples. High pressure hydrogen sorption analysis was carried out for both scCO2-activated MOFs. A maximum loading of ∼4 wt% was observed for UBMOF-9 at a measured range of 13 MPa without reaching saturation (Fig. 4A). In contrast, UBMOF-31 attains a maximum uptake of 4.9 wt% at 4.6 MPa and 77 K (Fig. 4A), making it one of the best Zr-based MOFs for hydrogen storage capacity to date.14 The presence of a maximum observed for UBMOF-31 is usual and expected where the surface interacts positively for the adsorption process, and the adsorption efficiency drops after covering the surface with the first layer of guest molecules.8,28 For a brief overview and comparison of hydrogen uptakes by different Zr-based MOFs, see Table S8.1 in the ESI.† The increased hydrogen storage capacity of UBMOF-31 can be explained by attractive interactions between the H2 molecules and the amino substituted benzene rings as suggested by theoretical calculations.23
4.6. A further increase of DASDCAH2 resulted in amorphous impurity as originally observed for UBMOF-8. The infrared spectra (see the ESI†) of all three fresh compounds indicate the presence of a large amount of dmf with an intense peak at 1654 cm−1, which diminished upon washing with acetone and activation by supercritical CO2 during gas adsorption studies. The shoulder peaks around 3670 cm−1 possibly originate from the OH groups as suggested in the literature.24 Thermal stabilities of the three MOFs were studied by TGA in air (see the ESI†) at a heating ramp of 5 °C per minute. All three freshly prepared, air-dried MOFs show a large initial drop in weight (40–50%) due to solvent loss, followed by a stable plateau until they decompose at approximately 494 °C (UBMOF-8), 522 °C (UBMOF-9), and 508 °C (UBMOF-31), indicating thermal stabilities comparable with UiO-66.13,14 TGAs of de-solvated and vacuum treated samples were carried out to estimate the linker ratios for UBMOF-31 and the guest modulator, in combination with elemental analysis and NMR spectroscopy (see the ESI†). The PXRD pattern in Fig. 3A suggests that UBMOF-31 is phase pure and isostructural to UBMOF-8 and UBMOF-9 as expected. PXRD analyses of the samples revealed the stability of UBMOF-9 and UBMOF-31 under different conditions as shown in Fig. 3. Both these materials retain their original structures when treated with acetone for 24 hours at 20 °C, after activation using supercritical CO2, and after high-pressure adsorption studies with N2, H2, and CO2. When treated with water at 20 °C for 24 hours, both (UBMOF-8 and UBMOF-31) retain their shiny crystalline morphology to some extent as visually inspected (see the ESI†) and evidenced by PXRD (Fig. 3), with a small shift in 2θ for the low-angle peaks for UBMOF-9. However, UBMOF-31 mostly retains its original PXRD pattern, indicating better water stability compared to UBMOF-9. The hydrothermal treatment (100 °C) of both the MOFs over 24 hours resulted in broadening of the low-angle peaks leaving no visible high-angle peaks, indicating significant loss of crystallinity of the materials. The surface areas of the materials were determined by Brunauer–Emmett–Teller (BET) analysis with N2 at 77 K using a Micromeritics 3-Flex volumetric gas sorption analyser (P/Po range: 0.05–0.3) following the British Standard guidelines.25 A sample of UBMOF-9 that had been solvent-exchanged with acetone and degassed at 150 °C for 8 hours under dynamic high vacuum (10−7 mbar) prior to analysis displayed a surface area of 1169 m2 g−1. In order to access the true intrinsic porosity of these materials, the samples were solvent exchanged and subsequently subjected to supercritical CO2 (scCO2) extraction.26,27 The scCO2-activated samples showed BET surface areas of 2667 m2 g−1 and 2552 m2 g−1 for UBMOF-9 and UBMOF-31, respectively, more than twice the value obtained for the thermally dried samples. High pressure hydrogen sorption analysis was carried out for both scCO2-activated MOFs. A maximum loading of ∼4 wt% was observed for UBMOF-9 at a measured range of 13 MPa without reaching saturation (Fig. 4A). In contrast, UBMOF-31 attains a maximum uptake of 4.9 wt% at 4.6 MPa and 77 K (Fig. 4A), making it one of the best Zr-based MOFs for hydrogen storage capacity to date.14 The presence of a maximum observed for UBMOF-31 is usual and expected where the surface interacts positively for the adsorption process, and the adsorption efficiency drops after covering the surface with the first layer of guest molecules.8,28 For a brief overview and comparison of hydrogen uptakes by different Zr-based MOFs, see Table S8.1 in the ESI.† The increased hydrogen storage capacity of UBMOF-31 can be explained by attractive interactions between the H2 molecules and the amino substituted benzene rings as suggested by theoretical calculations.23
Although there are a large number of MOFs with high selectivity towards CO2 adsorption, only a few of them are stable under air and moisture, restricting their possible applications in CO2 capture and separation.12 In addition, the presence of amino groups on the linkers often facilitates CO2 adsorption by the chemisorption process. The presence of both of these features motivated us to further study CO2 adsorption properties of these MOFs.29 The CO2 uptake was recorded to be 8 wt% and 8.6 wt% for UBMOF-9 and UBMOF-31, respectively, at 0 °C and 1 bar pressure. Slightly lower uptakes of 2.06 wt% and 2.86 wt% were observed for UBMOF-9 and UBMOF-31, respectively, at 20 °C and 1 bar pressure (Fig. S8.9 in the ESI†). Adsorption of CO2 under high pressure was also studied. Notably UBMOF-31 shows much greater affinity towards CO2 reaching 73 wt% compared to 63 wt% of UBMOF-9 at 1 MPa (Fig. 4B).29 However, at a higher pressure, the CO2 uptake for UBMOF-9 exceeds the value for UBMOF-31 (see the ESI†). Therefore, the first part of the adsorption isotherm is most likely to be dominated by chemisorption at the amine sites and the latter part by physisorption on the internal surface of the highly porous framework. Consistent with this interpretation, a high selectivity towards CO2 adsorption over nitrogen was observed (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for UBMOF-31 in the chemisorption regime at 0 °C and 1 MPa, making it a promising material for CO2 capture and separation.
1) for UBMOF-31 in the chemisorption regime at 0 °C and 1 MPa, making it a promising material for CO2 capture and separation.
In summary, we report three new Zr-based MOFs, UBMOF-8, UBMOF-9, and UBMOF-31, with the latter synthesized using a mixed-linker strategy. Because of the presence of amino groups in UBMOF-31, it shows enhanced H2 adsorption capacity with 4.9 wt% of loading at 4.6 MPa, one of the highest uptakes for Zr-based MOFs reported to date. In addition, UBMOF-31 shows high affinity towards CO2 adsorption over nitrogen, with a selectivity of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 0 °C under 1 MPa pressure. In addition to its exceptional hydrogen uptake and selectivity towards CO2 adsorption, UBMOF-31 shows good hydrolytic stability, making it a promising candidate for further studies and practical applications for gas storage and separation. We are currently working on synthesizing phase pure UBMOF-8 and isostructural materials with an increased proportion of the DASDCAH2 linker to systematically investigate the effect of amino groups on the hydrogen storage capacity of this family of Zr-based MOFs.
1 at 0 °C under 1 MPa pressure. In addition to its exceptional hydrogen uptake and selectivity towards CO2 adsorption, UBMOF-31 shows good hydrolytic stability, making it a promising candidate for further studies and practical applications for gas storage and separation. We are currently working on synthesizing phase pure UBMOF-8 and isostructural materials with an increased proportion of the DASDCAH2 linker to systematically investigate the effect of amino groups on the hydrogen storage capacity of this family of Zr-based MOFs.
SN thanks the Royal Society of Chemistry for financial support. VPT and UH thank the University of Bath for funding through a Prize Research Fellowship and Whorrod Research Fellowship, respectively. MT thanks support from the H2FC SUPERGEN Hub (EP/E040071/1). The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
References
- A. Bocarsly and D. M. P. Mingos, Structure and Bonding (Fuel Cells and Hydrogen Storage), Springer, Heidelberg, Germany, 2011 Search PubMed.
- M. P. Suh, H. J. Park, T. K. Prasad and D. W. Lim, Chem. Rev., 2012, 112, 782–835 CrossRef CAS PubMed.
- M. Dincǎ and J. R. Long, Angew. Chem., Int. Ed., 2008, 47, 6766–6779 CrossRef PubMed.
- C. Stockford, N. Brandon, J. Irvine, T. Mays, I. Metcalfe, D. Book, P. Ekins, A. Kucernak, V. Molkov, R. Steinberger-Wilckens, N. Shah, P. Dodds, C. Dueso, S. Samsatli and C. Thompson, Int. J. Hydrogen Energy, 2015, 40, 5534–5543 CrossRef CAS.
- J. L. C. Rowsell and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4670–4679 CrossRef CAS PubMed.
- H. W. Langmi, J. Ren, B. North, M. Mathe and D. Bessarabov, Electrochim. Acta, 2014, 128, 368–392 CrossRef CAS.
- S. S. Han, J. L. Mendoza-Cortes and W. A. Goddard Iii, Chem. Soc. Rev., 2009, 38, 1460–1476 RSC.
- L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294–1314 RSC.
- See the hydrogen storage target set by DOE in the United States http://energy.gov/eere/fuelcells/downloads/fuel-cell-technologies-office-multi-year-research-development-and-22.
- M. Dincǎ, A. Dailly, Y. Liu, C. M. Brown, D. A. Neumann and J. R. Long, J. Am. Chem. Soc., 2006, 128, 16876–16883 CrossRef PubMed.
- Z. Wang, K. K. Tanabe and S. M. Cohen, Chem. – Eur. J., 2010, 16, 212–217 CrossRef CAS PubMed.
- S. S. Kaye, A. Dailly, O. M. Yaghi and J. R. Long, J. Am. Chem. Soc., 2007, 129, 14176–14177 CrossRef CAS PubMed.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- S. Chavan, J. G. Vitillo, D. Gianolio, O. Zavorotynska, B. Civalleri, S. Jakobsen, M. H. Nilsen, L. Valenzano, C. Lamberti, K. P. Lillerud and S. Bordiga, Phys. Chem. Chem. Phys., 2012, 14, 1614–1626 RSC.
- L. Li, S. Tang, C. Wang, X. Lv, M. Jiang, H. Wu and X. Zhao, Chem. Commun., 2014, 50, 2304–2307 RSC.
- Y. Han, H. Xu, Y. Liu, H. Li, H. Hou, Y. Fan and S. R. Batten, Chem. – Eur. J., 2012, 18, 13954–13958 CrossRef CAS PubMed.
- J. Ren, N. M. Musyoka, H. W. Langmi, B. C. North, M. Mathe, X. Kang and S. Liao, Int. J. Hydrogen Energy, 2015, 40, 10542–10546 CrossRef CAS.
- J. Ren, H. W. Langmi, B. C. North, M. Mathe and D. Bessarabov, Int. J. Hydrogen Energy, 2014, 39, 890–895 CrossRef CAS.
- Isostructural compound to UBMOF-9 reported during reviewing process: R. J. Marshall, C. L. Hobday, C. F. Murphie, S. L. Griffin, C. A. Morrison, S. A. Moggach and R. S. Forgan, J. Mater. Chem. A, 2016, 4, 6955–6963 CAS ; and in an MSc thesis: http://handle.ncl.edu.tw/11296/ndltd/48318579606892167335.
- O. V. Dolomanov, A. J. Blake, N. R. Champness and M. Schroder, J. Appl. Crystallogr., 2003, 36, 1283–1284 CrossRef CAS.
- S. B. Kalidindi, S. Nayak, M. E. Briggs, S. Jansat, A. P. Katsoulidis, G. J. Miller, J. E. Warren, D. Antypov, F. Corà, B. Slater, M. R. Prestly, C. Martí-Gastaldo and M. J. Rosseinsky, Angew. Chem., Int. Ed., 2015, 54, 221–226 CrossRef CAS PubMed.
- C. A. Trickett, K. J. Gagnon, S. Lee, F. Gándara, H.-B. Bürgi and O. M. Yaghi, Angew. Chem., Int. Ed., 2015, 54, 11162–11167 CrossRef CAS PubMed.
- R. C. Lochan and M. Head-Gordon, Phys. Chem. Chem. Phys., 2006, 8, 1357–1370 RSC.
- L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater., 2011, 23, 1700–1718 CrossRef CAS.
- Determination of the specific surface area of powders – Part 1: BET method of gas adsorption for solids (including porous materials): BS 4359-1:1996, ISO 9277:1995.
- Aerogels Handbook, Springer-Verlag, New York, 2011 Search PubMed.
- U. Hintermair, C. Roosen, M. Kaever, H. Kronenberg, R. Thelen, S. Aey, W. Leitner and L. Greiner, Org. Process Res. Dev., 2011, 15, 1275–1280 CrossRef CAS.
- N. Bimbo, J. E. Sharpe, V. P. Ting, A. Noguera-Díaz and T. J. Mays, Adsorption, 2013, 20, 373–384 CrossRef.
- T. M. McDonald, D. M. D'Alessandro, R. Krishna and J. R. Long, Chem. Sci., 2011, 2, 2022–2028 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, single-crystal structure determination, materials characterization (IR, TGA, NMR), and details of gas sorption experiments. CCDC 1440758 and 1440759. For the ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc03787a |
| This journal is © The Royal Society of Chemistry 2016 |