Facile and universal photo-induced living radical polymerization system mediated by iniferter agent and copper(II) acetate at ambient temperature
Liangfang Fan,
Hongjuan Jiang,
Lifen Zhang*,
Zhenping Cheng* and
Xiulin Zhu
Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Department of Polymer Science and Engineering, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: chengzhenping@suda.edu.cn; zhanglifen@suda.edu.cn; Fax: +86-512-65882787
First published on 27th March 2015
Abstract
A facile and universal photo-induced living radical polymerization system suitable for various types of monomers, such as oil-soluble methyl methacrylate (MMA), n-butyl acrylate (n-BA) and styrene (St) as well as water-soluble poly(ethylene glycol) monomethyl ether methacrylate (PEGMA) and 2-(dimethylamino)ethyl methacrylate (DMAEMA), was successfully developed with iniferter agent 1-cyano-1-methylethyl diethyldithiocarbamate (MANDC) and organic catalyst copper(II) acetate (Cu(OAc)2) under UV irradiation at ambient temperature. The polymerization kinetics with different molar ratios ([MMA]0/[MANDC]0/[Cu(OAc)2]0 = 500/1/x (x = 0.1 (200 ppm), 0.01 (20 ppm), 0.0025 (5 ppm))) indicated that the novel polymerization system showed typical “living”/controlled radical polymerization features, indicated by a linear increase of molecular weights with monomer conversion while keeping relatively narrow molecular weight distributions (Mw/Mn = 1.19–1.45) for the resultant polymers. Even when the amount of Cu(OAc)2 was minimized to only 1 ppm level, the polymerization system still showed living character. The living features of the obtained polymers were further confirmed by a successful chain-extension experiment. In addition, a possible polymerization mechanism was discussed in this work.
1. Introduction
Living radical polymerization (LRP), defined by IUPAC as reversible deactivation radical polymerization (RDRP), such as initiator-transfer agent-terminator (iniferter) polymerization,1 nitroxide-mediated radical polymerization (NMP),2 atom transfer radical polymerization (ATRP)3 and reversible addition–fragmentation chain transfer (RAFT) polymerization,4 has been developed as an effective and convenient way for the synthesis of well-defined (co)polymers with designable molecular weights and narrow molecular weight distributions. Recently, photo-induced LRP has been paid much more extensive attention due to its more facile way to synthesize various controlled (co)polymers under mild conditions,5 and many excellent photo-induced LRP systems including photo-induced NMP,6 ATRP,7 and RAFT polymerization8 have been reported.For more than 30 years, photo-induced polymerization has been widely used in coatings, inks, adhesives and printing plates. Compared to thermally based polymerization, photo-induced polymerization has some particular advantages. For instance, it can be performed at or below room temperature without released volatile organic compounds;9 and the photo-induced polymerization is usually fast, efficient, environmental and low energy consumption. It is well known that iniferter is the earliest photoinitiated LRP method reported by Otsu and co-workers in 1982.1a,b Although it can be used to prepare well-defined polymers with various macromolecular structures, the polymerizations are relatively poor-controlled because of slow exchange between dormant species and propagating radicals. In order to improve the performance of iniferter, the iniferter agents are used as the pseudohalogen ATRP initiators in the presence of transition metal catalysts and ligands in various ATRP systems. For example, Qiu and coworkers added metal salt catalyst to prepare well-defined polymers with narrow molecular weight distributions,10 and Zhu's group carried out further researches to successfully improve the functionality.11 However, those systems were implemented at a high temperature. Afterward, Matyjaszewski and coworkers employed CuBr(I)/1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA) as the catalyst and 2-(N,N-diethyldithiocarbamyl)ethyl isobutyrate (EMADC) as pseudohalogen ATRP initiator to successfully prepare well-defined polymers with narrow molecular weight distributions under UV irradiation at mild temperature.12 Recently, they employ organic copper(II) species (copper(II) acetylacetonate (Cu(acac)2) or copper(II) hexafluoroacetylacetonate (Cu(hfa)2)) and inifeter agents (1-cyano-1-methylethyl diethyldithiocarbamate (MANDC) or EMADC) to successfully construct the LRP system in the absence of any ligands and reducing agents at 80 °C or 120 °C.13 Furthermore, Cheng and co-workers used cheaper organic copper(II) acetate (Cu(OAc)2) instead of Cu(hfa)2 and MANDC as the initiator to effectively establish a facile LRP system with wide range of monomer generality at 70 or 80 °C.14
Considering the advantages of photo-induced polymerization, in this work, we developed a facile LRP system using Cu(OAc)2 as the catalyst and MANDC as initiator/transfer agent in the absence of any ligand, reducing agent and photoinitiator under UV irradiation at ambient temperature. It is interesting to find that the resultant polymer (Mw/Mn = 1.43) is narrower than those without Cu(OAc)2 (Mw/Mn = 1.56) even when the amount of Cu(OAc)2 is minimized to only 1 ppm level. Furthermore the possible polymerization mechanism was discussed.
2. Experimental section
2.1. Materials
Methyl methacrylate (MMA, >99%), styrene (St, >99%), n-butyl acrylate (n-BA, >99%), t-butyl acrylate (t-BA, >99%), and vinyl acetate (VAc, >99%) were obtained from Shanghai Chemical Reagents Co. Ltd (Shanghai, China) and passed through a neutral alumina column before use. Poly(ethylene glycol) monomethyl ether methacrylate (PEGMA, average molecular weight 500 g mol−1, 99%, Sigma-Aldrich), 2-(dimethylamino)ethyl methacrylate (DMAEMA, 99%, Energy Chemical), were purified by passing through a neutral alumina column before use. Copper(II) acetate (Cu(OAc)2) (>98%) was purchased from Alfa Aesar; copper chloride dihydrate (CuCl2·2H2O) (>99%), copper bromide (CuBr2) (>99%) and tetrahydrofuran (THF) (analytical reagent) were purchased from Shanghai Chemical Reagents Co. Ltd (Shanghai, China) and used as-received. 1-Cyano-1-methylethyl diethyldithiocarbamate (MANDC) (98%) was prepared according to a previously reported literature.15 All the other chemicals were obtained from Shanghai Chemical Reagents Co. Ltd and used as received unless mentioned.2.2. Typical polymerization procedure of MMA
The polymerization procedure for the molar ratio of [MMA]0/[MANDC]0/[Cu(OAc)2]0 = 500/1/0.1 is as follows: a mixture was obtained by adding solid Cu(OAc)2 (3.4 mg, 0.0187 mmol), THF (1.0 mL) to a dried ampoule under stirring; after all the Cu(OAc)2 were fully dissolved, transferring 0.1 mL of the mixed solution into another ampoule in which MANDC (4.2 mg, 0.0193 mmol) was added in advance; finally, 1.0 mL (9.44 mmol) of MMA was added into the ampoule. The reaction mixture was degassed by argon to eliminate the dissolved oxygen for 10 min, flame-sealed quickly and then transferred into a reaction tank under UV irradiation (240 μw cm−2 at 365 nm) at 25 °C. After the desired polymerization time, the ampoule was cooled by iced water. Afterwards, it was opened and the contents were dissolved in THF (∼3 mL), and precipitated into a large amount of methanol (∼300 mL). The polymer obtained by filtration was dried under vacuum until constant weight at 35 °C. The monomer conversion was determined gravimetrically.2.3. Chain extension of resultant PMMA
A predetermined quantity of PMMA was added into a dried ampoule, then the predetermined quantity of PEGMA and Cu(OAc)2 dissolved with THF were added. The ampoule was bubbled with argon for 10 min to eliminate the dissolved oxygen in the solution, and then flame-sealed and transferred into a reaction tank under UV irradiation (240 μw cm−2 at 365 nm) at 25 °C. The rest of the procedure was the similar with that for the polymerization of MMA described above.2.4. Characterization
The number-average molecular weight (Mn,GPC) values and molecular weight distribution (Mw/Mn) values of the polymers were determined using a TOSOH HLC-8320 gel permeation chromatograph (GPC) equipped with a refractive-index detector (TOSOH), using TSKgel guardcolumn SuperMP-N (4.6 × 20 mm) and two TSKgel SupermultiporeHZ-N (4.6 × 150 mm) with measurable molecular weights ranging from 5 × 102 to 5 × 105 g mol−1. THF was used as the eluent at a flow rate of 0.35 mL min−1 and 40 °C. GPC samples were injected using a TOSOH plus autosampler and calibrated with PMMA standards purchased from TOSOH. 1H NMR spectrum was recorded on a Bruker 300 MHz nuclear magnetic resonance (NMR) instrument using CDCl3 as the solvent and tetramethylsilane (TMS) as the internal standard at ambient temperature.3. Results and discussion
3.1. Effect of the amount of copper(II) acetate on polymerization of MMA
It is well known that MANDC is a typical iniferter agent. As shown in entry 1 in Table 1, when the polymerization of MMA was conducted just with MANDC, a broad molecular weight distribution (Mw/Mn = 1.56) was achieved, indicating a typical feature of photo-iniferter polymerization. As mentioned in introduction, in order to improve the controllability over polymerization, an organic catalyst copper(II) acetate was used as an additive to establish a novel LRP system. Therefore, the polymerization of MMA in the presence of iniferter agent MANDC and catalyst Cu(OAc)2 was performed under UV irradiation at room temperature (25 °C). The results are shown in Table 1. It can be seen that adding Cu(OAc)2 into the polymerization system can enhance the controllability over polymerization significantly. The polydispersities of the resultant PMMAs can be reduced to 1.09–1.36 (entries 2–10) from 1.56 (entry 1) which depend on the amount of catalyst. Even the amount of catalyst Cu(OAc)2 reduced to 1 ppm (entry 11 in Table 1), PMMA with a narrower molecular weight distribution (Mw/Mn = 1.43) than those without Cu(OAc)2 can also be obtained, indicating high effectiveness of the organic catalyst under UV irradiation. On the other hand, we also conducted the reference polymerization of MMA with the molar ratio of [MMA]0/[MANDC]0/[Cu(OAc)2]0 = 500/1/0.1 in the dark at 25 °C (entry 12); however, no polymers could be obtained even after 36 h, indicating indispensable role of UV irradiation. In addition, it is found that from Table 1 increasing the amount of catalyst results in the decrease of polymerization rate although enhances the controllability over polymerization. This is contributed to the fact that the addition of Cu(OAc)2 facilitates to re-establish a dynamic equilibrium of active and dormant species and therefore reduces the concentration of propagating radicals in the polymerization system (vide infra). It is noted that the molecular weights of the resultant PMMAs are much higher than the corresponding theoretical ones (Table 1). This may be because the free radical decomposed from MANDC by UV irradiation can also initiate the polymerization before the establishment of a dynamic equilibrium between the dormant and active species, which caused the deviation of the actual molecular weights from the theoretical ones.| Entry | Rb | Cu (ppm) | Time (h) | Con. (%) | Mn,thd (g mol−1) | Mn,GPC (g mol−1) | Mw/Mn |
|---|---|---|---|---|---|---|---|
| a Polymerization conditions: VMMA = 1.0 mL, VTHF = 0.1 mL, under UV irradiation (240 μw cm−2 at 365 nm), temperature = 25 °C.b R = [MMA]0/[MANDC]0/[Cu(OAc)2]0.c Polymerization was conducted in the dark without UV irradiation at 25 °C.d Mn,th = ([M]0/[MANDC]0) × Mw,MMA × conversion%. | |||||||
| 1 | 500/1/0 | 0 | 4 | 56.5 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.56 |
| 2 | 500/1/1 | 2000 | 25 | 20.7 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.10 |
| 3 | 500/1/0.5 | 1000 | 25 | 20.7 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.09 |
| 4 | 500/1/0.2 | 400 | 25 | 33.9 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.12 |
| 5 | 500/1/0.1 | 200 | 25 | 44.8 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.13 |
| 6 | 500/1/0.05 | 100 | 22 | 28.3 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.13 |
| 7 | 500/1/0.02 | 40 | 22 | 28.0 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.22 |
| 8 | 500/1/0.01 | 20 | 22 | 36.8 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.25 |
| 9 | 500/1/0.005 | 10 | 22 | 36.5 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.26 |
| 10 | 500/1/0.001 | 2 | 12 | 40.6 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.36 |
| 11 | 500/1/0.0005 | 1 | 9 | 53.1 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.43 |
| 12 | 500/1/0.1c | 200 | 36 | NA | NA | NA | NA |
3.2. Polymerization kinetics
In order to further study the polymerization behaviors with various amount of catalyst, polymerization kinetics of MMA under UV irradiation was investigated in detail. Fig. 1(a) shows the polymerization kinetics for polymerization of MMA with the molar ratio of [MMA]0/[MANDC]0/[Cu(OAc)2]0 = 500/1/x (x = 0 (0 ppm), 0.1 (200 ppm), 0.01 (20 ppm), 0.0025 (5 ppm)). As we can see from Fig. 1(a), the linearity of the plots indicated that the concentration of propagating radicals remained constant during the polymerization. At the same time, it can be observed that the polymerization rate increased with the decrease of the amount of catalyst Cu(OAc)2. In addition, an induction period (≈2.9 h) was observed in 4 cases, which resulted from that much time was needed to establish a dynamic equilibrium between active and dormant species at room temperature. Furthermore, the apparent rate constant kappp (Rp = −d[M]/dt = kp[Pn˙][M] = kappp[M]) can be calculated from the slope of the plot.16 A value of kappp of 4.09 × 10−5 s−1, 1.89 × 10−5 s−1, 0.78 × 10−5 s−1, and 0.66 × 10−5 s−1 was obtained for the case x = 0 (typical iniferter polymerization), 0.0025, 0.01 and 0.1, respectively. From Fig. 1(b), it can be seen that Mn,GPC values increased linearly with monomer conversion in all cases. However, without the addition of catalyst copper(II) acetate (i.e., typical iniferter polymerization), the Mn,GPC values are far away from the theoretical molecular weight (Mn,th) and Mw/Mn values are more than 1.5; with the increasing amount of copper(II) acetate, the polymerization rate decreased while keeping narrow polydispersities for the resultant polymers, which is consistent with the results observed in Table 1. Therefore, the addition of Cu(OAc)2 can enhance the controllability over the polymerization significantly.3.3. Effect of type of catalysts on polymerization of MMA
Typical ATRP catalysts (e.g., CuBr2 and CuCl2) were used in place of organic catalyst Cu(OAc)2 to carry out the similar polymerization of MMA. The results are shown in Table 2. As we can see from Table 2, not only the polymerizations with CuBr2 or CuCl2·2H2O as the catalyst but also the polymerization catalyzed by copper(II) acetate without MANDC were unable to obtain polymers after 48 h, indicating that typical ATRP catalyst is not suitable for this case. One possible explanation for the unsuccessful catalyst of CuBr2 and CuCl2·2H2O is due to the exchange between halogen (Br and Cl) and the DC group from MANDC. Because of the good stability carbon–halogen bond may be hard to be activated under UV irradiation in our cases.| Entry | Catalyst | Time (h) | Con. (%) | Mn,thb (g mol−1) | Mn,GPC (g mol−1) | Mw/Mn |
|---|---|---|---|---|---|---|
a Polymerization conditions: [MMA]0/[MANDC]0/[catalyst]0 = 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, VMMA = 1.0 mL, VTHF = 0.1 mL, under UV irradiation (240 μw cm−2 at 365 nm), temperature = 25 °C.b Mn,th = ([M]0/[MANDC]0) × Mw,MMA × conversion%.c [MANDC]0 = 0. 0.1, VMMA = 1.0 mL, VTHF = 0.1 mL, under UV irradiation (240 μw cm−2 at 365 nm), temperature = 25 °C.b Mn,th = ([M]0/[MANDC]0) × Mw,MMA × conversion%.c [MANDC]0 = 0. |
||||||
| 1 | Cu(OAc)2 | 35 | 76.2 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.16 |
| 2 | CuBr2 | 48 | NA | NA | NA | NA |
| 3 | CuCl2·2H2O | 48 | NA | NA | NA | NA |
| 4c | Cu(OAc)2 | 48 | NA | NA | NA | NA |
3.4. Effect of monomer concentration on polymerization of MMA
Subsequently, we investigated the effect of the concentration of monomer on polymerization of MMA under UV irradiation. As shown in Table 3, the resultant PMMAs have narrow molecular weight distributions (Mw/Mn = 1.12–1.43) with all the molar ratios of [MMA]0/[MANDC]0 from 100/1 to 2000/1, and the corresponding molecular weights can be up to more than 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. In addition, from Table 3, the polymerization rate slightly increases with the molar ratio of [MMA]0/[MANDC]0. This may be contributed to the following issues: (1) increasing the molar ratio indicates decreases the concentration of initiator MANDC which will result in the decrease of the polymerization rate; and (2) the increase of the molar ratio also means the decrease of catalyst concentration of Cu(OAc)2 in the polymerization system which facilitates increasing of polymerization rate as discussed above. Anyway, we can design various target molecular weights by changing molar ratios of [MMA]0/[MANDC]0 and monomer conversion.
000 g mol−1. In addition, from Table 3, the polymerization rate slightly increases with the molar ratio of [MMA]0/[MANDC]0. This may be contributed to the following issues: (1) increasing the molar ratio indicates decreases the concentration of initiator MANDC which will result in the decrease of the polymerization rate; and (2) the increase of the molar ratio also means the decrease of catalyst concentration of Cu(OAc)2 in the polymerization system which facilitates increasing of polymerization rate as discussed above. Anyway, we can design various target molecular weights by changing molar ratios of [MMA]0/[MANDC]0 and monomer conversion.
| Entry | Rb | Time (h) | Con. (%) | Mn,thc (g mol−1) | Mn,GPC (g mol−1) | Mw/Mn |
|---|---|---|---|---|---|---|
| a Polymerization conditions: VMMA = 1.0 mL, VTHF = 0.1 mL, under UV irradiation (240 μw cm−2 at 365 nm), temperature = 25 °C.b R = [MMA]0/[MANDC]0/[Cu(OAc)2]0.c Mn,th = ([M]0/[MANDC]0) × Mw,MMA × conversion%. | ||||||
| 1 | 100/1/0.1 | 20 | 14.2 | 1400 | 8200 | 1.13 |
| 2 | 200/1/0.1 | 20 | 15.0 | 3000 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.12 |
| 3 | 500/1/0.1 | 20 | 26.8 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.16 |
| 4 | 800/1/0.1 | 20 | 28.2 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.21 |
| 5 | 1500/1/0.1 | 20 | 28.7 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.26 |
| 6 | 2000/1/0.1 | 13 | 38.9 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.43 |
3.5. Generality of monomers
In order to extend the applicability of this novel polymerization system, we choose different types of monomers (e.g., St, n-BA, t-BA, VAc, PEGMA and DMAEMA) to conduct the polymerization mediated by MANDC and copper(II) acetate under UV irradiation at ambient temperature. Table 4 shows the results of different monomers on the polymerization with the molar ratio of [Monomer]0/[MANDC]0/[Cu(OAc)2]0 = 500/1/0.01 (20 ppm). It can be seen that all the monomers can be successfully carried out at 20 ppm level catalyst and the resultant polymers have relatively narrow molecular weight distributions (Mw/Mn = 1.20–1.36), indicating that the polymerization system composed of MANDC and Cu(OAc)2 is an universal and facile strategy for controlled synthesis of polymers with wide range of monomer structures.| Entry | Monomer | Time (h) | Con. (%) | Mn,thb (g mol−1) | Mn,GPC (g mol−1) | Mw/Mn |
|---|---|---|---|---|---|---|
a Polymerization conditions: [Monomer]0/[MANDC]0/[Cu(OAc)2]0 = 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, Vmonomer = 1.0 mL, VTHF = 0.1 mL, under UV irradiation (240 μw cm−2 at 365 nm), temperature = 25 °C.b Mn,th = ([M]0/[MANDC]0) × Mw,monomer × conversion%. 0.01, Vmonomer = 1.0 mL, VTHF = 0.1 mL, under UV irradiation (240 μw cm−2 at 365 nm), temperature = 25 °C.b Mn,th = ([M]0/[MANDC]0) × Mw,monomer × conversion%. |
||||||
| 1 | St | 120 | 35.9 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.20 |
| 2 | n-BA | 12 | 40.2 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.36 |
| 3 | t-BA | 20 | 38.8 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.35 |
| 4 | VAc | 120 | 3.5 | 1500 | 2300 | 1.23 |
| 5 | PEGMA | 3 | 15.5 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.27 |
| 6 | DMAEMA | 5 | 39.7 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.34 |
3.6. Chain extension and feasible polymerization mechanism
The chain end of PMMA (Mn,GPC = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, Mw/Mn = 1.28) was analyzed by 1H NMR spectroscopy. Fig. 2 shows the 1H NMR spectrum of PMMA. The chemical shift at 3.60 ppm (b in Fig. 2) corresponded to the methyl ester groups in PMMA. The chemical shift at 3.90 ppm (a in Fig. 2) was attributed to the methylene protons of the DC (–SC(S)N(CH2CH3)2)2 end group,14a but another peak of the methylene protons of DC end group was overlapped with peak b. Besides, the molecular weight (Mn,NMR) calculated by integral of a and b was 16
100 g mol−1, Mw/Mn = 1.28) was analyzed by 1H NMR spectroscopy. Fig. 2 shows the 1H NMR spectrum of PMMA. The chemical shift at 3.60 ppm (b in Fig. 2) corresponded to the methyl ester groups in PMMA. The chemical shift at 3.90 ppm (a in Fig. 2) was attributed to the methylene protons of the DC (–SC(S)N(CH2CH3)2)2 end group,14a but another peak of the methylene protons of DC end group was overlapped with peak b. Besides, the molecular weight (Mn,NMR) calculated by integral of a and b was 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 g mol−1, which is close to the Mn,GPC value (13
400 g mol−1, which is close to the Mn,GPC value (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1). To further confirm the living feature of the obtained polymers, chain extension with fresh PEGMA from the PMMA macroinitiator (Mn,GPC = 13
100 g mol−1). To further confirm the living feature of the obtained polymers, chain extension with fresh PEGMA from the PMMA macroinitiator (Mn,GPC = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, Mw/Mn = 1.28) was conducted in the presence of catalyst Cu(OAc)2 under UV irradiation at room temperature. As shown in Fig. 3, block copolymer (PMMA-b-PPEGMA) with molecular weight of 30
100 g mol−1, Mw/Mn = 1.28) was conducted in the presence of catalyst Cu(OAc)2 under UV irradiation at room temperature. As shown in Fig. 3, block copolymer (PMMA-b-PPEGMA) with molecular weight of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and Mw/Mn = 1.15 was achieved successfully. Therefore, all these results manifested the typical living features of the novel polymerization system.
000 g mol−1 and Mw/Mn = 1.15 was achieved successfully. Therefore, all these results manifested the typical living features of the novel polymerization system.
 | ||
Fig. 2 1H NMR spectrum of PMMA (Mn,GPC = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, Mw/Mn = 1.28) obtained from photo-induced LRP of MMA with CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard. 100 g mol−1, Mw/Mn = 1.28) obtained from photo-induced LRP of MMA with CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard. | ||
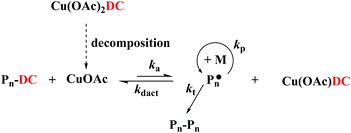
According to the reported references of Matyjaszewski's group13 and Cheng's group,14 the Cu(II) species could form the relatively stable Cu(III) species under the function of DC (–SC(S)N(CH2CH3)2)2 group. In this case, the DC group can be generated under UV irradiation and may form the resultant Cu(III) species Cu(OAc)2DC by reaction with Cu(OAc)2 in situ. Unfortunately, we tried to obtain the Cu(III) species but failed due to its unstability as discussed in our previous work.14a However, it may facilitate to decompose to Cu(I) species and therefore establish a dynamic equilibrium between Cu(II) species and Cu(I) species, as shown in Scheme 1. Under the equilibrium, the concentration of propagating radicals was minimized to control the polymerization over with molecular weights and molecular weight distributions.
4. Conclusions
Adding catalytic amount of organic catalyst Cu(OAc)2 to the photo-induced iniferter system can enhance the polymerization controllability significantly, and therefore developing a novel, facile and universal LRP strategy for controlled synthesis of polymers with various types of monomer structures (e.g., methacrylates, acrylates and styrene) under UV irradiation at ambient temperature. Due to the homogeneous catalysis in the presence of organic catalyst Cu(OAc)2, even if the amount of Cu(OAc)2 was reduced to 1 ppm, the resultant polymers still have narrower polydispersity (Mw/Mn = 1.43) than those obtained via conventional iniferter mechanism.Acknowledgements
The financial support from the National Natural Science Foundation of China (no. 21174096, 21274100) and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) is gratefully acknowledged.Notes and references
- (a) T. Otsu and M. Yoshida, Makromol. Chem., Rapid Commun., 1982, 3, 127 CrossRef CAS; (b) T. Otsu, M. Yoshida and T. Tazaki, Makromol. Chem., Rapid Commun., 1982, 3, 133 CrossRef CAS; (c) X. M. Yang and K. Y. Qiu, J. Appl. Polym. Sci., 1996, 61, 513 CrossRef CAS; (d) S. H. Qin, K. Y. Qiu, G. Swift, D. G. Westmoreland and S. Wu, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 4610 CrossRef CAS; (e) T. Otsu, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2121 CrossRef CAS; (f) N. A. Peppase and J. H. Ward, Adv. Drug Delivery Rev., 2004, 56, 1587 CrossRef PubMed.
- (a) M. K. Georges, R. P. N. Veregin, P. M. Kazmaier and G. K. Hamer, Macromolecules, 1993, 26, 2987 CrossRef CAS; (b) C. J. Hawker, A. W. Bosman and E. Harth, Chem. Rev., 2001, 101, 3661 CrossRef CAS PubMed; (c) V. Sciannamea, R. Jérôme and C. Detrembleur, Chem. Rev., 2008, 108, 1104 CrossRef CAS PubMed; (d) D. Yang, C. Feng and J. H. Hu, Polym. Chem., 2013, 4, 2384 RSC; (e) J. Nicolas, Y. Guillaneuf, C. Lefay, D. Bertin, D. Gigmes and B. Charleux, Prog. Polym. Sci., 2013, 38, 63 CrossRef CAS PubMed.
- (a) J. S. Wang and K. Matyjaszewski, J. Am. Chem. Soc., 1995, 117, 5614 CrossRef CAS; (b) M. Kato, M. Kamigaito, M. Sawamoto and T. Higashimura, Macromolecules, 1995, 28, 1721 CrossRef CAS; (c) M. Kamigaito, T. Ando and M. Sawamoto, Chem. Rev., 2001, 101, 3689 CrossRef CAS PubMed; (d) K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921 CrossRef CAS PubMed; (e) M. Ouch, T. Terashim and M. Sawamoto, Chem. Rev., 2009, 109, 4963 CrossRef PubMed; (f) F. di Lena and K. Matyjaszewski, Prog. Polym. Sci., 2010, 35, 959 CrossRef CAS PubMed; (g) L. J. Bai, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Polym. Chem., 2012, 3, 2685 RSC; (h) W. W. He, H. J. Jiang, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Polym. Chem., 2013, 4, 2919 RSC; (i) Z. Xue, D. He and X. Xie, Polym. Chem., 2015, 6, 1660 RSC.
- (a) J. Chiefari, Y. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. Le, R. T. Mayadunne, G. F. Meijs, C. L. Moad and G. Moad, Macromolecules, 1998, 31, 5559 CrossRef CAS; (b) G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2009, 62, 1402 CrossRef CAS; (c) C. Boyer, V. Bulmus, T. P. Davis, V. Ladmiral, J. Q. Liu and S. Perrier, Chem. Rev., 2009, 109, 5402 CrossRef CAS PubMed; (d) D. J. Keddie, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 2012, 45, 5321 CrossRef CAS; (e) G. Graeme, E. Rizzardo and S. H. Thang, Chem.–Asian J., 2013, 8, 1634 CrossRef PubMed; (f) D. J. Keddie, Chem. Soc. Rev., 2014, 43, 496 RSC.
- (a) Y. Yagci, Macromol. Symp., 2000, 161, 19 CrossRef CAS; (b) A. M. I. Ali and A. G. Mayes, Macromolecules, 2010, 43, 8376 CrossRef; (c) M. A. Tasdelen, M. Uygun and Y. Yagci, Macromol. Rapid Commun., 2011, 32, 58 CrossRef CAS PubMed; (d) Y. Nakamura, T. Arima, S. Tomita and S. Yamago, J. Am. Chem. Soc., 2012, 134, 5536 CrossRef CAS PubMed; (e) N. V. Alfredo, N. E. Jalapa, S. L. Morales, A. D. Ryabov, R. Le Lagadec and L. Alexandrov, Macromolecules, 2012, 45, 8135 CrossRef CAS; (f) A. Ohtsuki, A. Goto and H. Kaji, Macromolecules, 2013, 46, 96 CrossRef CAS; (g) Y. G. Zhao, M. M. Yu, S. L. Zhang, Y. C. Liu and X. F. Fu, Macromolecules, 2014, 47, 6238 CrossRef CAS; (h) A. Nastasaki, V. Nikolaou, G. S. Pappas, Q. Zhang, C. Wan, P. Wilson, T. P. Davis, M. R. Whittaker and D. M. Haddleton, Chem. Sci., 2014, 5, 3536 RSC; (i) J. T. Xu, K. Jung, N. A. Corrigan and C. Boyer, Chem. Sci., 2014, 5, 3568 RSC; (j) J. T. Xu, K. Jung, A. Atme, S. Shanmugam and C. Boyer, J. Am. Chem. Soc., 2014, 136, 5508 CrossRef CAS PubMed; (k) A. Anastasaki, V. Nikolaou, Q. Zhang, S. R. Samanta, C. Waldron, A. J. Haddleton, R. McHale, D. Fox, V. Percec, P. Wilson and D. M. Haddleton, J. Am. Chem. Soc., 2014, 136, 1141 CrossRef CAS PubMed.
- (a) J. C. Scaiano, T. J. Connolly, N. Mohtat and C. N. Pliva, Can. J. Chem., 1997, 75, 92 CrossRef CAS; (b) X. X. Liu, X. H. Zhang, X. H. Zhang, G. G. Wu, J. W. Yang, L. X. Pang, Z. H. Zeng and Y. L. Chen, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 2659 CrossRef CAS; (c) Y. Guillaneuf, D. Bertin, D. Gigmes, D. L. Versace, J. Lalevée and J. P. Fouassier, Macromolecules, 2010, 43, 2204 CrossRef CAS.
- (a) Z. Guan and B. Smart, Macromolecules, 2000, 33, 6904 CrossRef CAS; (b) K. Ishizu and K. Ochi, Macromolecules, 2006, 39, 3238 CrossRef CAS; (c) D. Konkolewicz, K. Schroeder, J. Buback, S. Memhard and K. Matyjaszewski, ACS Macro Lett., 2012, 1, 1219 CrossRef CAS; (d) T. Zhang, T. Chen, I. Amin and R. Jordan, Polym. Chem., 2014, 5, 4790 RSC; (e) A. Anastassaki, V. Nikolaou, A. Simula, J. Godfrey, M. X. Li, G. Nurumbetov, P. Wilson and D. M. Haddleton, Macromolecules, 2014, 47, 3852 CrossRef; (f) X. W. Jiang, J. Wu, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Macromol. Rapid Commun., 2014, 35, 1879 CrossRef CAS PubMed.
- (a) L. C. Lu, N. F. Yang and Y. L. Cai, Chem. Commun., 2005, 42, 5287 RSC; (b) L. C. Lu, H. J. Zhang, N. F. Yang and Y. L. Cai, Macromolecules, 2006, 39, 3770 CrossRef CAS; (c) W. D. Jiang, L. C. Lu and Y. L. Cai, Macromol. Rapid Commun., 2007, 28, 725 CrossRef CAS; (d) H. J. Zhang, J. J. Deng, L. C. Lu and Y. L. Cai, Macromolecules, 2007, 40, 9252 CrossRef CAS; (e) Y. Shi, H. Gao, L. C. Lu and Y. L. Cai, Chem. Commun., 2009, 11, 1368 RSC; (f) Y. Shi, G. H. Liu, L. C. Gao and Y. L. Cai, Macromolecules, 2009, 42, 3917 CrossRef CAS; (g) G. H. Liu, H. Shi, Y. R. Cui, J. Y. Tong, Y. Thao, D. J. Wang and Y. L. Cai, Polym. Chem., 2013, 4, 1176 RSC; (h) J. Y. Tong, Y. Shi, G. H. Liu, T. Huang, N. Xu, Z. G. Zhu and Y. L. Cai, Macromol. Rapid Commun., 2013, 34, 1827 CrossRef CAS PubMed; (i) S. Shanmugam, J. T. Xu and C. Boyer, Macromolecules, 2014, 47, 4930 CrossRef CAS; (j) J. T. Xu, K. Jung and C. Boyer, Macromolecules, 2014, 47, 4217 CrossRef CAS; (k) C. K. Fu, J. T. Xu, L. Tao and C. Boyer, ACS Macro Lett., 2014, 3, 633 CrossRef CAS.
- (a) Y. Yagci, S. Jockusch and N. J. Turro, Macromolecules, 2010, 43, 6245 CrossRef CAS; (b) M. A. Tasdelen, Y. Y. Durmaz, B. Karagoz, N. Bicak and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3387 CrossRef CAS; (c) J. F Quinn, L. Barner, C. Barner-Kowollik, E. Rizzardo and T. P. Davis, Macromolecules, 2002, 35, 7620 CrossRef; (d) L. C. Lu, N. F. Yang and Y. L. Cai, Chem. Commun., 2005, 5287 RSC.
- (a) X. P. Chen and K.-Y. Qiu, Chem. Commun., 2000, 233 RSC; (b) P. Li and K.-Y. Qiu, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 2093 CrossRef CAS; (c) P. Li, S. H. Qin, D. Q. Qin and K.-Y. Qiu, Polym. Int., 2004, 53, 756 CrossRef CAS.
- (a) W. Zhang, N. C. Zhou, J. Zhu, B. Sun and X. L. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 510 CrossRef CAS; (b) W. Zhang, X. L. Zhu, J. Zhu and J. Y. Chen, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 32 CrossRef CAS; (c) W. Zhang, X. L. Zhu, Z. P. Cheng and J. Zhu, J. Appl. Polym. Sci., 2007, 106, 230 CrossRef CAS.
- Y. Kwak and K. Matyjaszewski, Macromolecules, 2010, 43, 5180 CrossRef CAS.
- Y. Z. Zhang, K. Schröder, Y. W. Kwak, P. Krys, A. N. Morin, T. Pintauer, R. Poli and K. Matyjaszewski, Macromolecules, 2013, 46, 5512 CrossRef CAS.
- (a) H. J. Jiang, L. F. Zhang, X. W. Jiang, X. G. Bao, Z. P. Cheng and X. L. Zhu, Macromol. Rapid Commun., 2014, 35, 1332 CrossRef CAS PubMed; (b) H. J. Jiang, C. Tian, L. F. Zhang, Z. P. Cheng and X. L. Zhu, RSC Adv., 2014, 4, 52430 RSC.
- Y. Kwak and K. Matyjaszewski, Macromolecules, 2008, 41, 6627 CrossRef CAS.
- (a) Z. P. Cheng, X. L. Zhu, G. J. Chen, W. J. Xu and J. M. Lu, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 3823 CrossRef CAS; (b) M. Q. Ding, X. W. Jiang, J. Y. Peng, L. F. Zhang, Z. P. Cheng and X. L. Zhu, Green Chem., 2015, 17, 271 RSC.
| This journal is © The Royal Society of Chemistry 2015 |