Open Access Article
Open Access ArticleHeavily Eu2O3-doped yttria-aluminoborate glasses for red photoconversion with a high quantum yield: luminescence quenching and statistics of cluster formation
Guojun
Gao
a,
Jingxue
Wei
a,
Yang
Shen
a,
Mingying
Peng
bc and
Lothar
Wondraczek
*ac
aOtto Schott Institute of Materials Research, University of Jena, 07743 Jena, Germany. E-mail: lothar.wondraczek@uni-jena.de
bState Key Laboratory of Luminescent Materials and Devices, School of Materials Science and Technology, South China University of Technology, 510641 Guangzhou, China
cThe Chinese-German Research Center for Photonic Materials and Devices at South China University of Technology, 510641 Guangzhou, China
First published on 21st August 2014
Abstract
We report on the photoluminescence (PL) properties of heavily Eu2O3 doped Y2O3–Al2O3–B2O3 glasses as a high-gain red-emitting material for photoconversion. A UV-to-red conversion efficiency of up to 60% is achieved in this material. Concentration quenching is related to the formation of –{Eu–O–Me–O–Eu}– (Me = B, Y, Al) and –{Eu–O–Eu}– linkages in the first and second coordination shell, respectively, of Eu3+. For a quantitative consideration, we employ a statistical approach to estimate the number of those species as a function of europium concentration. In this way, a crossover composition is obtained at a dopant concentration of ∼3 mol% of Eu2O3 where further addition of europium directly increases the number of –{Eu–O–Eu}–. In the latter species, the critical distance for concentration quenching of PL is achieved so that for concentrations above the crossover, PL quantum efficiency decreases. This observation is confirmed experimentally through lifetime analyses as well as direct measurements of internal quantum efficiency. The proposed tool can now be used to predict and optimize cluster formation and concentration quenching in rare-earth doped glasses.
Introduction
Inorganic materials doped with trivalent europium have been of strong interest for photo-conversion due to the 4f6 electronic configuration of Eu3+ which results in the characteristic red photoluminescence (PL) with often high quantum efficiency.1 In detail, the PL spectrum of Eu3+ usually exhibits a variety of sharp PL lines in the orange to red spectral region arising from the intra-configurational parity-forbidden 4f6 → 4f6 electronic transitions from the non-degenerated 5D0 level to the ground levels of 7FJ(J = 0, 1, 2, 3 and 4).1–4 While the band energy of these f–f transitions is usually not dependent on ligand field strength and, hence, the host material, the electric-dipole allowed transition of Eu3+:5D0 → 7F2 depends strongly on local symmetry. As a consequence, this transition can be used to probe the local structure in the considered host material. For this, the relative PL intensity ratio R of the electric- and magnetic-dipole-allowed transitions (usually denoted asymmetry ratio) is calculated, R = IED/IMD. For example, a high value of R reflects low ligand symmetry and high bond covalency of the Eu3+ species.5–7 The intrinsic excitation bands of Eu3+ are located in the near-ultraviolet spectral range (NUV), i.e., between ∼360 and 420 nm, and in the blue (∼464 nm). However, the parity-forbidden nature of Eu3+ absorption results in only low absorption cross section. Enhancing the Eu3+ doping concentration in the host material is one way to overcome this problem, but only as far as concentration quenching of photoluminescence can be avoided. This results in a trade-off between absorption efficiency, quantum efficiency and re-absorption.8 In particular, glasses with a high quantum yield of photoluminescence are desirable for applications where high transparency for light other than the excitation light is required to enable visually transparent emitters.8 Such target applications are, for example, luminescent concentrators,9–11 bulk spectral converters for photochemical12 or photovoltaic solar energy harvesting,13,14 or three-dimensional luminescent displays.15 Compared to (single-) crystalline hosts, glasses provide the major advantage of facile processing into almost any size and shape.In the present study, we consider the borate system of Y2O3–Al2O3–B2O3 (YAB).16 On the one hand, borate glasses generally offer a comparably high solubility for rare-earth ions.8,17 In addition to this, the crystalline YAl3(BO3)4 is known as an excellent nonlinear optical crystal and host for various rare-earth dopants (e.g., Nd3+ and Yb3+).18,19 However, especially the incongruent melting of the constituting oxides strongly complicates the synthesis of single-crystalline YAl3(BO3)4, which puts a limit on possible applications. On the other hand, due to the similarity of ionic radii (0.90 vs. 0.947 Å), the octahedral site of Y3+ can readily be occupied by Eu3+.20 While there are no clear data available, in the glassy matrix, we expect a 6 to 8-fold coordination situation for both Y3+ and Eu3+, with a tendency to lower coordination with higher Y/Eu content.21,22 Due to the supposedly less symmetric environment of the Y3+/Eu3+-sites, however, we also expect that the red emission band at ∼612 nm will be more dominant in the PL spectrum of Eu3+-doped yttria-aluminoborate glasses.23–25 Finally, rare earth species (e.g., Eu3+) tend to be better dispersed in glasses as compared to crystals, which may be advantageous with respect to dopant concentration and concentration quenching of PL.26
Experimental section
Precursor glasses were chosen at an excess of B2O3versus (Eu, Y, Al)2O3 as compared to the stoichiometric YAl3(BO3)4, and also at an excess of (Eu3+,Y3+) over the Al3+-ion with higher-field strength in order to ensure facile glass formation and high rare-earth content. Nominal compositions (mol%) were (20−x)Y2O3–20Al2O3–60B2O3–xEu2O3 (YAB–xEu with x = 0, 0.25, 0.5, 1, 2, 4, 8 and 20). Corresponding mixtures of Y2O3 (99.99%), Al2O3 (99.9%), H3BO3 (99.9%) and Eu2O3 (99.999%) were prepared in batches of ∼30 g and melted conventionally in alumina crucibles at 1400 °C for 2 h in air. Melts were poured into preheated stainless steel moulds and annealed at 600 °C for 2 h to obtain stress-free glass slabs. These were subsequently cut into disks of ∼30 × 20 × 5 mm3 and polished on both sides for further characterization.UV-vis absorption spectra were recorded with a double-beam photospectrometer (Cary 5000) over the spectral range of 300 to 700 nm with a step size of 1 nm. Static photoexcitation (PLE) and luminescence (PL) spectra and dynamic decay curves of Eu3+-related PL were collected with a high-resolution spectrofluorometer (Horiba Jobin Yvon Fluorolog FL3-22). PLE spectra were corrected over the lamp intensity with a silicon photodiode. PL spectra were corrected by the spectral response of the employed photomultiplier tube. Absorbance, internal (ηIQE) and external quantum efficiency (ηEQE) were obtained through measuring excitation and emission spectra on samples and a blank reference with a BaSO4-coated integration sphere. The values of absorbance (a), ηIQE and ηEQE were then determined as follows:
| a = (∫ER − ∫ES)/∫ER | (1) |
| ηIQE = (∫LS − ∫LR)/(∫ER−∫ES) | (2) |
| ηEQE = ηIQEa | (3) |
In eqn (1)–(3), ∫LS and ∫LR are the integrated PL intensities with and without the sample, and ∫ER and ∫ES are the integrated intensities of excitation light without and with the sample in the integration sphere, respectively. CIE 1931 (Commission International de I'Eclairage) chromaticity coordinates were determined by integrating the PL spectra. All analyses were performed at room temperature.
Results and discussion
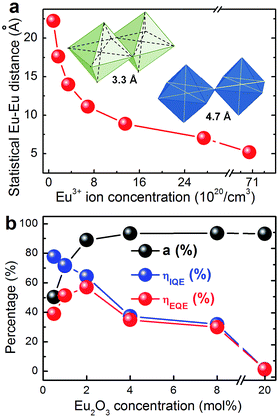
Fig 1a shows the UV-vis optical absorption spectra of YAB–xEu glasses for various concentrations of Eu2O3. For Eu3+-doped samples, seven characteristic absorption bands of Eu3+ with maxima at 362, 376, 394, 414, 465, 532 and 579 nm arising from the intrinsic 4f ↔ 4f transitions of Eu3+ from the ground state 7F0 to the labeled excited states can be identified, as illustrated schematically in the energy level diagram of Eu3+ (inset of Fig. 1a).8,26,27 The increased intensity of absorption bands of Eu3+ with Eu2O3 concentration especially in the UV region results in a shift of the absorption edge to longer wavelength, which is reflected by the changing visual color of the samples from colorless to dark orange with increasing Eu2O3 concentration (Fig. 1e). Fig. 1b present PLE spectra of YAB–xEu glasses by monitoring Eu3+ PL at 612 nm. In full agreement with the absorption bands of Eu3+ (Fig. 1a), six typical PLE lines of Eu3+ peaking at 318, 362, 376, 394, 414 and 464 nm are observed. They are assigned to the aforementioned transitions of Eu3+ from the ground state 7F0 to the labeled excited states (Fig. 1b).25 The sharp PLE line at 394 nm (Eu3+:7F0 → 5L6) which shows the strongest intensity is chosen as the PLE wavelength to record the following PL spectra (Fig. 1c).Six characteristic PL lines of Eu3+ peaking at 578, 591, 612, 652, 700 and 748 nm are observed. These bands are attributed to the 4f6 ↔ 4f6 transitions of Eu3+ from the lowest excited state 5D0 to the ground state multiplet of 7FJ (J = 0, 1, 2, 3, 4 and 5).25 The intensity of all Eu3+ PL lines increases linearly with increasing Eu2O3 concentration up to x = 2. A plateau is found for further increasing concentration up to x ≤ 8 mol%, reflecting the interplay of the negative effect of beginning concentration quenching and the positive effect of further increasing absorbance on PL intensity. Strong quenching is found for the sample doped with 20 mol% Eu2O3. This observation is confirmed through dynamic PL analyses. The decay curves of Eu3+ PL (at 612 nm) follow a single-exponential function for all studied dopant concentrations. The effective emission lifetime τ1/e of Eu3+ remains at a constant value of ∼1.90 ms up to x = 2. For a higher doping concentration up to x = 8, it decreases to ∼1.35 ms, and decreases more for x = 20, i.e., to ∼0.092 ms (Fig. 2a). This indicates that concentration quenching sets-in at a concentration of ∼x = 2: at this concentration, the distance between neighbouring Eu3+-species becomes critical so that energy transfer and non-radiative relaxation may occur. As previously noted, unfortunately, clear data on Eu3+ (or Y3+) coordination in the considered glass are not available. For a first approximation, we may assume two principle coordination environments, i.e., 6-fold and 8-fold which are typically found for Eu3+ in oxide glasses.22,28,29
At room pressure, the minimum Eu–Eu-distance for corner-sharing polyhedra through a linear Eu–O–u group corresponds to twice the Eu–O-distance which is about 2.34 Å in Eu2O3, i.e., ∼4.7 Å. For edge-sharing, it is ∼3.3 Å (inset of Fig. 3a). A somewhat higher Eu–O distance would occur in 8-fold oxygen coordinated Eu3+, i.e., ∼2.4 Å, corresponding to ∼4.8 and ∼3.4 Å for corner and edge-sharing groups, respectively.22 A first estimate of the average statistical distance between all Eu3+ species can be obtained directly from the volumetric ion concentration. This is illustrated in Fig. 3a. In this consideration, the critical average Eu–Eu distance at which the onset of notable concentration quenching is observed corresponds to ∼11.1 Å for x = 2. Even though we are considering a glassy lattice, however, it is clear that within the length scale regime of <20 Å, the average value of interionic distances has only little meaning because the mid- and short-range structure is more and more specified. That is, within the first and second coordination shell, the distribution of potential Eu–Eu distances converges around two values which correspond to the bridging species of –{Eu–O–Eu}– (first coordination shell, Fig. 4b) and –{Eu–O–Me–O–Eu}– (second coordination shell, Fig. 4a, with Me = B, Y, Al according to the chemical composition of the considered glass). If we neglect the potential impact of local chemical heterogeneity (preferential ion clustering, dynamic heterogeneity, etc.), the speciation into either of the two configurations is given by the probability at which they occur in the glass lattice. For the –{Eu–O–Eu}– species, this probability is approximately,
| PEu–Eu = 1 − (1 − x/100)CNEu | (4) |
with the Eu-coordination number CNEu and assuming an infinitely large ensemble of atoms. For the –{Eu–O–Me–O–Eu}– species, i.e., for having no Eu-neighbour on the first coordination shell but one on the second, it is
| PEu–Me–Eu = (1 − PEu–Eu)(1 − (1 − x/100)(CNAv−1)CNEu. | (5) |
In eqn (5), CNAv is the average coordination number of the cation species on the first coordination shell. As an approximation, we use IVB, VIAl and VIY and average according to the glass composition and the value of x. The result of this calculation is shown in Fig. 4c. As the accurate average coordination number of Eu3+ is not known for the present glass system beyond that it lies between six and eight, we plot data for VIEu and VIIIEu. We also plot the sum of PEu–Eu and PEu–Me–Eu as a measure of at least how many of the Eu species are found within the first or second coordination shells of each other. That is, when this sum approaches unity, each Eu has at least one Eu within its first or second coordination shell. At the nominal Eu2O3 dopant concentration of ∼3 mol%, we find a compositional crossover where the statistical number of –{Eu–O–Me–O–Eu}– species starts to decrease in favour of –{Eu–O–Eu}–. Interestingly, this crossover composition corresponds well to the observed onset of notable concentration quenching. At the crossover point, we may assume that about 60% of the Eu species lie within ∼10 Å of each other, and about 25% are first-shell neighbours which lie within ∼4.7 Å of each other.
This observed correlation confirms that the Eu–Eu linkages form mostly statistically with no notable contribution of selective cluster formation or even phase separation. At the crossover composition, newly added Eu2O3 does not contribute anymore to the number of –{Eu–O–Me–O–Eu}– or farther separated Eu species which act as efficient luminescence centers. Instead, from this composition onwards, every Eu-ion which is added to the material increases the number of –{Eu–O–Eu}– in which the critical interaction distance for PL quenching is achieved. Hence, notable PL quenching is observed for higher dopant concentrations. We believe that this approach can be used to predict and optimize the concentration quenching properties of similar photoluminescent glasses.
As expected, the PL spectra are dominated by the electronic-dipole allowed transition of Eu3+:5D0 → 7F2 at 612 nm for all samples which suggests an environment of lower symmetry for the Eu3+ species. As already noted, the intensity of the electronic-dipole allowed transition is strongly dependent on the symmetry of the local environment while the magnetic-dipole allowed transition of Eu3+:5D0 → 7F1 is independent of symmetry. Here, we obtain an asymmetry ratio of R ∼ 3.55, which is a relatively high value.5–8 This confirms the distortion of local symmetry in YAB glasses. Expectedly, R does not notably depend on the Eu3+ concentration x, which reflects the very similar structural role of Y3+ and Eu3+.30,31
As already noted, there is no notable variation in the intensity ratios of all emission peaks. The corresponding CIE 1931 chromaticity coordinates of all sample are (∼0.650 ± 0.003, ∼0.349 ± 0.001), close to the NTSC standard red of (0.670, 0.330). For illustration, we show photographs of the samples UV-A light (Fig. 1f) and under local excitation with a green laser beam (532 nm, Fig. 1c). Besides the PL lines from the lowest excited state of Eu3+, 5D0, the PL lines which originate from higher lying levels such as 5D1 are very weak or even absent in all samples (inset of Fig. 1d). This is due to the relatively high maximum phonon energy of the considered borate glass system (∼1400 cm−1).32–34 The forbidden transition of Eu3+:5D0 → 7F0 at 580 nm can be observed in all samples. Noteworthily, Eu2+-related photoluminescence cannot be observed in any of the samples.
The absorbance (a), internal quantum efficiency (ηIQE) and external quantum efficiency (ηEQE) are reported in Fig. 3b. For x = 0.5 mol%, we obtain a value of ηIQE of ∼80%. In correspondence with PL lifetime observations, this value decreases to ∼32% for a Eu2O3 concentration of 8 mol%. In parallel, the absorbance increases, which leads to the observed plateau in the emission intensity. The absorbance at 394 nm is ∼50% for x = 0.5, and increases to ∼94% for x = 4. The quantum yield therefore increases from ∼40% to ∼60% for a Eu2O3 concentration of up to 2 mol%. At higher concentration, it commences to decrease (Fig. 3b). This means that at the optimal Eu2O3 doping concentration of ∼2 mol%, about two thirds of the incoming photons are absorbed and re-emitted through Eu3+ photoluminescence in the red spectral range.
Conclusions
In conclusion, heavily Eu2O3 doped Y2O3–Al2O3–B2O3 represents a high-gain red-emitting material for UV-to-red photoconversion with a conversion efficiency of up to 60%. We related concentration quenching to the occurrence of –{Eu–O–Me–O–Eu}– (Me = B, Y, Al) and –{Eu–O–Eu}– entities in the first and second coordination shell, respectively, of Eu3+. For a quantitative consideration, we proposed a statistical approach to estimate the number of those species as a function of europium concentration. In this way, a crossover was found for a dopant concentration of ∼3 mol% of Eu2O3 where further addition of europium directly increases the number of –{Eu–O–Eu}– in which the critical distance for concentration quenching of PL is achieved. This observation is confirmed experimentally through lifetime analyses as well as quantum efficiency measurements. The proposed tool can now be used to predict and optimize cluster formation and concentration in rare-earth doped glasses.Acknowledgements
Parts of this work have been supported financially by the German Science Foundation through its priority program 1594 (grant no. WO1220/10-1). The authors further acknowledge financial support from the Department of Education of Guangdong Province (Grant no. 2013gjhz0001).Notes and references
- G. Blasse and B. C. Grabmaier, Luminescent materials, Springer-Verlag, 1994 Search PubMed.
- G. Gao, N. Da, S. Reibstein and L. Wondraczek, Opt. Express, 2010, 18, A575–A583 CrossRef CAS PubMed.
- G. Gao, S. Reibstein, M. Peng and L. Wondraczek, J. Mater. Chem., 2011, 21, 3156–3161 RSC.
- D. K. Williams, B. Bihari, B. M. Tissue and J. M. McHale, J. Phys. Chem. B, 1998, 102, 916–920 CrossRef CAS.
- J. C. Boyer, F. Vetrone, J. A. Capobianco, A. Speghini and M. Bettinelli, J. Phys. Chem. B, 2004, 108, 20137–20143 CrossRef CAS.
- Z. Qi, C. Shi, W. Zhang, W. Zhang and T. Hu, Appl. Phys. Lett., 2002, 81, 2857–2859 CrossRef CAS PubMed.
- C.-H. Yan, L.-D. Sun, C.-S. Liao, Y.-X. Zhang, Y.-Q. Lu, S.-H. Huang and S.-Z. Lu, Appl. Phys. Lett., 2003, 82, 3511–3513 CrossRef CAS PubMed.
- G. Gao and L. Wondraczek, J. Mater. Chem. C, 2013, 2, 691–695 RSC.
- A. Goetzberger and W. Greube, Appl. Phys., 1977, 14, 123–139 CAS.
- H. Lin, D. Hou, L. Li, Y. Tao and H. Liang, Dalton Trans., 2013, 42, 12891–12897 RSC.
- B. Tian, B. Chen, Y. Tian, X. Li, J. Zhang, H. Zhong, L. Cheng, S. Fu, H. Zhong, Y. Wang, X. Zhang, H. Xia and R. Hua, J. Mater. Chem. C, 2013, 1, 2338–2344 RSC.
- L. Wondraczek, M. Batentschuk, M. A. Schmidt, R. Borchardt, S. Scheiner, B. Seemann, P. Schweizer and C. J. Brabec, Nat. Commun., 2013, 4, 2047 Search PubMed.
- X. Huang, S. Han, W. Huang and X. Liu, Chem. Soc. Rev., 2012, 42, 173–201 RSC.
- M. Peng and L. Wondraczek, J. Mater. Chem., 2009, 19, 627–630 RSC.
- E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185–1189 CAS.
- H. L. Rutz, D. E. Day and C. F. Spencer, J. Am. Ceram. Soc., 1990, 73, 1788–1790 CrossRef CAS PubMed.
- G. Gao and L. Wondraczek, Opt. Mater. Express, 2013, 3, 633–644 CrossRef CAS.
- D. R. S. Santos, C. N. Santos, A. S. S. de Camargo, W. F. Silva, W. Q. Santos, M. V. D. Vermelho, N. G. C. Astrath, L. C. Malacarne, M. S. Li, A. C. Hernandes, A. Ibanez and C. Jacinto, J. Chem. Phys., 2011, 134, 124503 CrossRef CAS PubMed.
- A. Brenier, D. Jaque and A. Majchrowski, Opt. Mater., 2006, 28, 310–323 CrossRef PubMed.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- O. Bouty, J. M. Delaye and S. Peuget, Procedia Chem., 2012, 7, 540–547 CrossRef CAS PubMed.
- Y. Shimizugawa, N. Umesaki, J. Qiu and K. Hirao, J. Synchrotron Radiat., 1999, 6, 624–626 CrossRef CAS PubMed.
- R. Balda, J. Fernández, J. L. Adam and M. A. Arriandiaga, Phys. Rev. B: Condens. Matter, 1996, 54, 12076–12086 CrossRef CAS.
- P. Babu and C. K. Jayasankar, Phys. B, 2000, 279, 262–281 CrossRef CAS.
- A. Ivankov, J. Seekamp and W. Bauhofer, J. Lumin., 2006, 121, 123–131 CrossRef CAS PubMed.
- H. Deters, J. F. de Lima, C. J. Magon, A. S. S. de Camargo and H. Eckert, Phys. Chem. Chem. Phys., 2011, 13, 16071–16083 RSC.
- G. Gao and L. Wondraczek, Opt. Mater. Express, 2014, 4, 476–485 CrossRef CAS.
- S. Iftekhar, B. Pahari, K. Okhotnikov, A. Jaworski, B. Stevensson, J. Grins and M. Edén, J. Phys. Chem. C, 2012, 116, 18394–18406 CAS.
- T. Murata, Y. Moriyama and K. Morinaga, Sci. Technol. Adv. Mater., 2000, 1, 139–145 CrossRef CAS.
- A. A. Reddy, S. Das, S. Ahmad, S. S. Babu, J. M. F. Ferreira and G. V. Prakash, RSC Adv., 2012, 2, 8768–8776 RSC.
- G. Gao and L. Wondraczek, J. Mater. Chem. C, 2013, 1, 1952–1958 RSC.
- C. Xu, Y. Li, Y. Huang, Y. M. Yu and H. J. Seo, J. Mater. Chem., 2012, 22, 5419–5426 RSC.
- B. S. Richards, Sol. Energy Mater. Sol. Cells, 2006, 90, 1189–1207 CrossRef CAS PubMed.
- G. Jia, M. Yang, Y. Song, H. You and H. Zhang, Cryst. Growth Des., 2009, 9, 301–307 CAS.
| This journal is © The Royal Society of Chemistry 2014 |